Abstract
Background
With improved survival in patients with lymphoma, long-term toxicity and quality of life (QoL), including sexual health, have become increasingly important.
Aim
We aimed to (1) determine the prevalence of erectile dysfunction (ED) in adult male lymphoma survivors; (2) determine whether testosterone deficiency, comorbidities, or lifestyle factors were associated; and (3) evaluate their impact on QoL.
Methods
A cross-sectional study including 172 male survivors of Hodgkin lymphoma or diffuse large B cell lymphoma diagnosed in adulthood between 2008 and 2018 was performed. Patients were in complete metabolic remission after first-line treatment and remained in remission at follow-up (3-13 years after diagnosis). Participants completed 3 questionnaires measuring sexual health and general QoL. Serum concentrations of total testosterone were measured and thorough medical history and sociodemographic factors were obtained. The Danish SEXUS Project, European Male Ageing Study, and European Organization of Research and Treatment of Cancer (EORTC) Reference Manual were used as reference values of the general population.
Outcomes
Patient reported outcome measures including the 5-item International Index of Erectile Function, EORTC C30, and EORTC 22-item Sexual Health Questionnaire.
Results
ED was reported by 55.2%, which was higher than in an age-matched Danish population cohort (17.5%). Erectile function score (5-item International Index of Erectile Function) was negatively associated with comorbidity, body mass index, smoking, and age and positively with the number of children conceived before treatment and serum concentration of total testosterone. Overt testosterone deficiency in combination with ED was detected in 10 (5.7%) of 176 survivors, including excluded survivors in hormonal treatment, which is higher than for the general population (0.1%-3.2% for men <70 years of age). Mean EORTC C30 global health score for survivors with ED was lower (67.7) than for survivors without ED (80.1) but was comparable to the general population (71.2). Furthermore, a positive association was seen between sexual function and both sexual and general QoL.
Clinical implications
Sexual health is important for QoL and related to comorbidities. The focus on improving QoL requires that both sexual health and comorbidities are addressed in the follow-up of lymphoma patients.
Strengths and limitations
Despite the relatively high number of included survivors, the cross-sectional design of this study warrants longitudinal studies to clarify the specific underlying causes of sexual dysfunction.
Conclusion
ED was highly prevalent and associated with comorbidity in lymphoma survivors, and more focus on sexual health and treatment related comorbidity is needed to improve sexual and general QoL.
Keywords: neoplasms, erectile dysfunction, sexual health, testosterone, patient reported outcome measure, quality of life, comorbidity, drug-related side effects, adverse reactions
Introduction
With improved survival after treatment for malignant lymphoma,1 the need to manage long-term effects of disease and treatment, including sexual dysfunction (SD), is growing. Thus, SD has been described as highly prevalent affecting 20% to 54% of male survivors treated for lymphoma.2 However, comprehensive data on the extent, causes, and consequences of SD in long-term survivors after lymphoma treatment are scarce.
In 1992, Kornblith et al3 found a relationship between quality of life (QoL) and sexual health in survivors of Hodgkin lymphoma (HL). Survivors more often had decreased QoL when facing sexual health issues. SD can be a symptom of various aspects of life after cancer. Dealing with a new body image; anxiety for relapse; depression; and physical sequelae, including testosterone deficiency, neuropathy, and fatigue, can potentially affect the sexual health of cancer survivors.4–6
Patients might not relate sexual health issues, besides decreased fertility, to the cancer diagnosis or treatment and thereby escape diagnosis of SD. Physicians may also find the topic difficult to broach during a short outpatient visit, with other issues seeming more urgent, even though cancer patients report a need for more information about the risk of SD after treatment 7,8. Hence, focus on SD seems to be an unmet need of cancer survivorship. Earlier studies either have focused on SD in a cancer group of mixed diagnoses, pediatric cancer patients, both sexes or have left out important confounding factors.8–16 Behringer et al,9 compared data from 3 large randomized trials and found sexual function to be chronically impaired after late stage treatment for HL, whereas Eeltink et al17 found male non-HL survivors to have sexual function comparable to the general population. Because the largest study performed9 was designed to elucidate if there is a difference between treatment regimens and not to uncover the overall prevalence, the prevalence of SD in lymphoma survivors as an entity needs to be further elucidated. Our primary aim was to identify the extent of ED in a large population of long-term lymphoma survivors. Secondary aims were to elucidate relevant associations to testosterone level and sociodemographic and clinical factors and to evaluate whether ED affects QoL.
Methods
Patients
Danish-speaking male survivors of HL and diffuse large B cell lymphoma (DLBCL), treated with first-line chemotherapy between April 2008 and April 2018, with or without radiotherapy, were identified through the Danish Lymphoma Registry.18 First-line therapy did not change in Denmark during the treatment period. Survivors older than 18 years of age at treatment and between 18 and 65 years of age at follow-up, who were in complete metabolic remission, according to the Lugano classification, for a minimum of 1 year after completion of first line therapy, were invited to participate. Survivors treated with second-line treatment were excluded because the purpose was to investigate sexual dysfunction among a strictly homogeneous group of lymphoma patients perceived as cured of their malignant disease. Patients receiving second-line treatment have a poor prognosis and would introduce a possible confounder causing difficulties in differentiating between the independent effect of each variable on the outcome. Patients were identified from the population of the Capital Region and Region Zealand covering approximately 2.6 million citizens. Data were collected at follow-up between December 2020 and January 2022. Exclusion criteria were concurrent low-grade lymphoma, lymphoma affecting the central nervous system or testes, psychiatric disease expected to prevent compliance, prior or current abuse of anabolic steroids, current treatment of testosterone deficiency, and treatment with second-line therapy.
We performed an observational cross-sectional study. After identification, relevant survivors from the eastern part of Denmark were sent a letter of invitation. Survivors who responded were included by electronic consent. Nonresponders were called by the research team and included upon consent. Information on height, weight, exercise, smoking status, offspring status, sexual activity status, sociodemographic characteristics, Cumulative Illness Rating Scale (CIRS) scores, and confirmation of clinical data obtained from the medical record were collected by telephone interview, conducted by the same investigator for all survivors included. Sexually active meant engaging in sexual activity, either alone or with a partner, within the last 6 months. Survivors were screened for testosterone deficiency with a single measurement of serum total testosterone. Fasting blood samples were drawn before 10 am at 2 sites, Copenhagen University Hospital – Rigshospitalet and Zealand University Hospital, and analyzed at the department of clinical biochemistry at Copenhagen University Hospital – Rigshsopitalet. Analyses were performed on Cobas e801 analytical unit, with Elecsys Testosterone II assays from Roche. Reference levels used were 8.6 to 29 nmol/L for the group 18 to 49 years of age and 6.7 to 26 nmol/L for men above the age of 49 years. Values below the limit of detection was reported as <0.087 nmol/L and values above the measuring range was reported as >52.0 nmol/L. The coefficient of variation was 6%.
QoL and sexual health were measured by the validated European Organization of Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Quality of Life Questionnaire C30 version 319 and EORTC 22-item Sexual Health Questionnaire (SHQ22)20 using the Danish versions. Sexual function was measured by 5-item International Index of Erectile Function (IIEF-5), also known as Sexual Health Inventory for Men (SHIM)21,22 in Danish. Description of questionnaire properties can be found in supplementary data (Table A1). Questionnaires were sent out digitally and filled in by the survivors. Comorbidity was evaluated by the CIRS score, which consists of 14 body system domains (originally 13, but adapted for elderly patients in 1991, now including hematology as a separate domain)23 and found to be a valid instrument.24–26 Each domain is scored between 0 and 4 points, resulting in a total score between 0 and 56. Zero points representing no comorbidity and 56 the highest comorbidity burden (not compatible with sustained life). Domains include cardiac; vascular; hematopoietic; respiratory; ear, nose, and throat; upper gastrointestinal tract; lower gastrointestinal tract; hepatic, renal; urethra-genital; musculoskeletal; neurologic; endocrinological; and psychiatric domains.
The Danish SEXUS Project,27 European Male Ageing Study,28 and EORTC Reference Manual29 were used as reference values of the general population.
Consent, questionnaires, and relevant data were registered digitally on the secure data capture platform REDCap (Research Electronic Data Capture). The study was performed in accordance with the Declaration of Helsinki and approved by the Danish Regional Committee on Health Research Ethics, Capital Region of Denmark (case number: H-20036508), and the Danish Capital Region Knowledge Center for Data Reviews (case number: P-2020-827).
Statistics
All statistical analyses were performed using R statistical software (version R-4.1.2; R Foundation for Statistical Computing). Descriptive statistics were used to characterize demographic data and patient treatment, using frequencies, percentages and means. t Tests were used to evaluate mean differences between survivors with and without ED for continuous data (dichotomized by IIEF-5 scores >21 and ≤21). Differences in categorical variables were evaluated by Fisher’s exact test. Univariate analyses were conducted using linear regression to examine the relationship between IIEF-5 scores and questionnaire scores, sociodemographics, comorbidity, and treatment variables. The analyses were done as a complete case analysis, and no missing data were present.
Results
Patient characteristics
Through the Danish Lymphoma Registry database, 333 survivors were identified (162 HL and 171 DLBCL), among which 238 eligible survivors were invited to participate, of which 64 declined. Thus, 174 (72.3%) survivors were initially included, and complete datasets were obtained from 172 survivors (Figure 1). There was an age difference between included and excluded survivors, with included survivors being older at diagnosis (median age 41 and 29 years of age, respectively) but younger at follow-up (median age 48 and 53 years of age, respectively). Follow-up time was not associated with erectile function in univariate analysis (Figure 2). Baseline clinical and epidemiological characteristics are outlined in Table 1 (Table A2 displays Table 1 divided by diagnoses) and treatment in Table 2.
Figure 1.
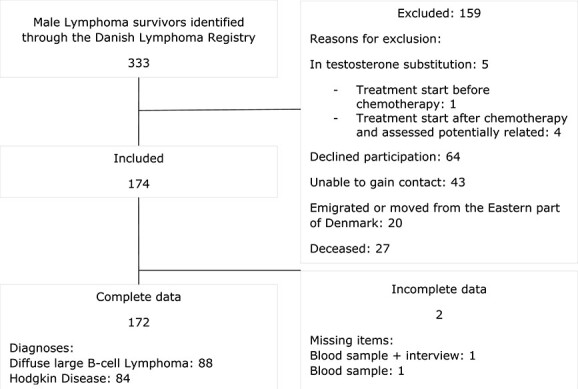
Flowchart of the study cohort.
Figure 2.
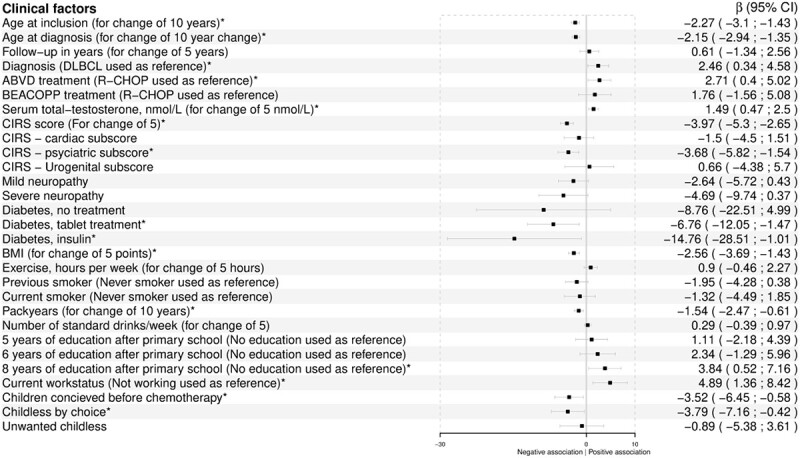
Univariate analyses by linear regression with 5-item International Index of Erectile Function (IIEF-5) score as the dependent variable. *Significant association. ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; BEACOPP, Bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone; BMI, body mass index; CIRS, Cumulative Illness Rating Scale; DLBCL, diffuse large B cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone.
Table 1.
Clinical and epidemiological characteristics of 172 adult male lymphoma survivors.
| Age at inclusion, y | 48 (24-65) |
| Age at diagnosis, y | 41 (19-61) |
| Follow-up time from diagnosis to inclusion, y | 7.2 (3-13.5) |
| Diagnosis | |
| Hodgkin disease | 84 (48.8) |
| Diffuse large B cell lymphoma | 88 (51.2) |
| Ann Arbor stage: Hodgkin disease | |
| I/II | 46 (54.8) |
| III/IV | 38 (45.2) |
| Ann Arbor stage: diffuse large B cell lymphoma | |
| I/II | 52 (59.1) |
| III/IV | 36 (40.9) |
| Relationship status | |
| Married/committed relationship | 144 (83.7) |
| Offspring status | |
| Child conceived before treatment | 89 (51.7) |
| Child conceived after treatment | 30 (17.4) |
| Childless, unwanted | 14 (8.1) |
| Childless, by choice | 39 (22.7) |
| Highest level of education | |
| 0-3 y after primary school | 26 (15.1) |
| 4-5 y after primary school | 58 (33.7) |
| 6-7 y after primary school | 34 (19.8) |
| 8-9 y after primary school | 54 (31.4) |
| Work status | |
| Currently employed | 155 (90.1) |
Values are median (range) or n (%).
Table 2.
Treatment regimens of 84 male Hodgkin lymphoma and 88 male diffuse large B cell lymphoma survivors.
| Patients | Cyclesa | Chemotherapy included | |
|---|---|---|---|
| Hodgkin Lymphoma | |||
| ABVD | 58 (93.6) | 4.6 (2-8) | Doxorubicin, bleomycin, vinblastine, dacarbazine |
| R-ABVD | 1 (1.6) | 6 | Rituximab, doxorubicin, bleomycin, vinblastine, dacarbazine |
| Brentuximab-ABVD | 1 (1.6) | 4 | Brentuximab, doxorubicin, bleomycin, vinblastine, dacarbazine |
| ABVD + R-CHOP | 2 (3.2) | 3.8 (2-5.5) + 4.0 (2-6)* | Doxorubicin, bleomycin, vinblastine, dacarbazine + rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone |
| BEACOPP | 10 (45.5) | 6.9 (6-8) | Bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone |
| BEACOPP escalated | 8 (36.4) | 6.8 (6-8) | Bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone with escalated doses of etoposide, doxorubicin and cyclophosphamide |
| BEACOPP + ABVD | 1 (4.6) | 2 + 4 | Bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone + doxorubicin, bleomycin, vinblastine, dacarbazine |
| BEACOPP escalated + ABVD | 3 (13.6) | 2.3 (2-3) + 2.3 (2-3)* | bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone with higher doses of etoposide, doxorubicin and cyclophosphamide + doxorubicin, bleomycin, vinblastine, dacarbazine |
| Diffuse large B cell lymphoma | |||
| CHOP | 1 (1.1) | 6 | Cyclophosphamide, doxorubicin, oncovin, prednisone |
| R-CHOP | 54 (61.4) | 5.1 (3-8) | Rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone |
| R-CHOEP | 27 (30.7) | 6.1 (6-8) | Rituximab, cyclophosphamide, doxorubicin, oncovin, etoposide, prednisone |
| R-CHOEP + DA EPOCH-R | 1 (1.1) | 1 + 5 | Rituximab, cyclophosphamide, doxorubicin, oncovin, etoposide, prednisone. |
| R-CHOP+R-CHOEP | 1 (1.1) | 3 + 3 | Rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone + rituximab, cyclophosphamide, doxorubicin, oncovin, etoposide, prednisone |
| R-COPE | 2 (2.3) | 4.5 (3-6) | Rituximab, cyclophosphamide, oncovin, prednisone, etoposide |
| R-CHOEP+High dose-Ara-C + high-dose MTX | 1 (1.1) | 7 | Rituximab, cyclophosphamide, doxorubicin, oncovin, etoposide, prednisone, cytarabine, methotrexate |
| R-CODOX-M/IVAC | 1 (1.1) | 2 + 2 | Rituximab, cyclophosphamide, doxorubicin, oncovin, methotrexate + rituximab, ifosfamide, etoposide, cytarabine |
| Patients | Radiation (Gy) | ||
| Radiotherapy, yes | 76 (44.2) | 30 (20-40) | |
| Methotrexate, yes | 18 (10.5) | — | |
Values are n (%) or mean (range), unless otherwise indicated.
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone; DA, dose adjusted; R-CHOP, rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone.
aResults are stated as (first regimen mean [range]) + (second regimen mean [range]) when more than 1 chemotherapy regimen was given.
Sociodemographic and clinical outcome characteristics
Of 172 survivors, 95 (55.2%) experienced ED: 39 (22.7%) had mild ED, 32 (18.6%) had moderate ED, and 24 (14.0%) had severe ED. The severe ED group (IIEF-5 score <8) included not only sexually active survivors with severe ED, but also sexually inactive survivors who automatically obtained a score ≤5. The influence of clinical factors on erectile function (IIEF-5 scores) is illustrated in Figure 2 and indicates that survivors of HL had a better erectile function compared with DLBCL survivors (P = .02). Severity of ED according to diagnosis is shown in Figure 3. A nonsignificant trend toward more severe ED could be seen among patients with DLBCL (P = .12). The same trend was seen when assessing sexually active survivors alone (data not shown). Survivors treated with an R-CHOP (rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone)–like regimen had worse erectile function than survivors treated with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) (P = .02). Eight (4.7%) survivors had serum total testosterone below the age adjusted reference level, and 6 of these reported ED (15% of men 24-39 years of age with ED, 5.9% of men 40-49 years of age with ED, 0% of men 50-59 years of age with ED, and 7.7% of men 60-65 years of age with ED) (Table 3). Despite mean serum concentration of total testosterone being within normal range for all age groups (for both survivors with and without ED), total testosterone concentration was positively associated with erectile function (P < .004) (Figure 4A). Survivors with ED had higher comorbidity (total CIRS) scores than survivors without ED (mean difference, 2.8; 95% CI, 1.8-3.8). While CIRS cardiac and urogenital subscores were comparable for the 2 groups, psychiatric diseases were more prevalent in the ED group (mean difference, 0.5; 95% CI, 0.2-0.8). Neuropathy and diabetes occurred equally in the 2 groups, but having diabetes severe enough to require medical treatment was associated with worse erectile function (P < .008). Mean body mass index (BMI) for both groups was just above the upper limit for a healthy weight and was higher for survivors with ED (mean difference, 2.7; 95% CI, 1.4-4.0; P < .001) (Table 3) and associated with erectile function (Figure 2). Both groups consumed the same amount of alcohol, exercised for an average of approximately 4 hours per week, and were equally often former or current smokers. However, number of pack-years was associated with erectile function (P = .001) (Table 3). Furthermore, good erectile function was associated with lower age (P < .001), long education (P = .02), current employment (P < .007), and having children before treatment (P < .02) (Figure 2). A small group of 5 survivors had used anabolic steroids during a maximum of 3 months 1.5 to 40 years ago. The use was sparse and hence disregarded (data not shown).
Figure 3.
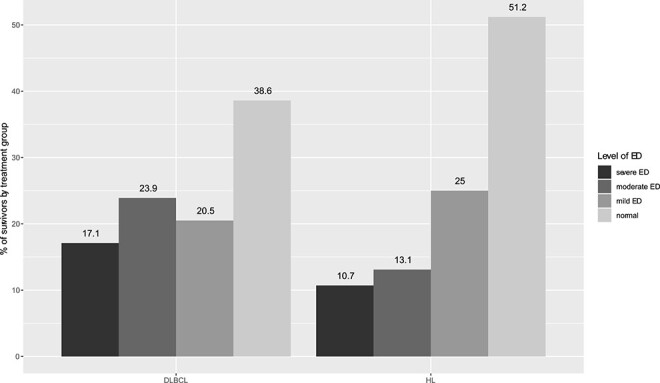
Distribution of erectile dysfunction (ED) severity in survivors with diffuse large B cell lymphoma (DLBCL) and Hodgkin lymphoma (HL) (P = .12).
Table 3.
Clinical outcome measures of 172 adult male lymphoma survivors.
| Survivors with IIEF-5 score <22 (ED) (n = 95) | Survivors with IIEF-5 score ≥22 (No ED) (n = 77) | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Sexually active | 78 (82.1) | 77 (100) | — | <.001a |
| Serum total testosterone, nmol/L | ||||
| Age 24-39 y | 15.1 | 16.1 | −1.0 (−4.4 to 2.3) | — |
| Age 40-49 y | 14.0 | 15.0 | −1.1 (−3.9 to 2.0) | — |
| Age 50-59 y | 15.5 | 15.1 | 0.4 (−3.3 to 4.2) | — |
| Age 60-65 y | 12.6 | 16.2 | −3.6 (−9.2 to 2.0) | — |
| Serum total testosterone below age-adjusted reference level | ||||
| Age 24-39 y | 3 (15.0) | 0 (0.0) | — | .05 |
| Age 40-49 y | 1 (5.9) | 1 (4.3) | — | 1.00 |
| Age 50-59 y | 0 (0.0) | 1 (6.7) | — | .32 |
| Age 60-65 y | 2 (7.7) | 0 (0.0) | — | 1.00 |
| CIRS | ||||
| Total score | 9.1 | 6.4 | 2.8 (1.8 to 3.8)a | — |
| Cardiac score | 2.1 | 1.9 | 0.2 (−0.5 to 0.9) | — |
| Psychiatric score | 1.6 | 1.1 | 0.5 (0.2 to 0.8)a | — |
| Urogenital score | 1.3 | 1.3 | 0.0 (−0.4 to 0.4) | — |
| Neuropathy | ||||
| None | 72 (75.8) | 68 (88.3) | — | .07 |
| Minor, no treatment | 16 (16.8) | 8 (10.4) | — | |
| Major, requiring treatment | 7 (7.4) | 1 (1.3) | — | |
| Diabetes | ||||
| No | 87 (91.6) | 77 (98.7) | — | .15 |
| Yes, no treatment | 1 (1.1) | 0 (0) | — | |
| Yes, treated with noninsulin | 6 (6.3) | 1 (1.3) | — | |
| Yes, treated with insulin | 1 (1.1) | 0 | — | |
| BMI, kg/m2 | 28.8 | 26.1 | 2.7 (1.4 to 4.0)a | — |
| Hours of exercise per week | 4.2 | 4.3 | −0.1 (−1.3 to 1.1) | — |
| Smoking status | ||||
| Never | 34 (35.8) | 37 (48.1) | — | .27 |
| Former smoker | 44 (46.3) | 30 (39.0) | — | |
| Current smoker | 17 (17.9) | 10 (13.0) | — | |
| Pack yearsb | 20.2 | 11.9 | 8.3 (2.9 to 13.6)a | — |
| Alcohol use | ||||
| Intake within limits | 81 (85.3) | 68 (88.3) | — | .46 |
| Above limitsc | 5 (5.3) | 6 (7.8) | — | |
| Former abuse | 9 (9.5) | 3 (3.9) | — | |
| Alcohol, standard drinks per weekd | 5.4 | 6.1 | −0.7 (−3.0 to 1.7) | — |
| Survivors with IIEF-5 score <22 (ED) (n = 95) | Survivors with IIEF-5 score ≥22 (no ED) (n = 77) | Mean difference (95% CI) | Reference scores | |
| EORTC SHQ22 function score | 44.1 | 56.2 | −12.1 (−15.5 to −8.8)a | — |
| EORTC SHQ22 symptom score | 12.9 | 6.3 | 6.6 (4.0 to 9.2)a | — |
| EORTC C30 function score | 81.8 | 93.8 | −12.0 (−15.7 to −8.4)a | 84.9e |
| EORTC C30 symptom score | 18.0 | 9.1 | 8.9 (5.1 to 12.6)a | 12.5e |
| EORTC C30 global health score | 67.7 | 80.1 | −12.4 (−17.5 to −7.3)a | 71.2e |
Values are n (%) for categorical variables with P values (t-tests) and mean for continuous variables with mean difference (95% CI) (Fisher’s exact test).
Abbreviations: BMI, body mass index; CIRS, Cumulative Illness Rating Scale; ED, erectile dysfunction; EORTC, European Organization of Research and Treatment of Cancer; IIEF-5, 5-item International Index of Erectile Function; SHQ22, 22-item Sexual Health Questionnaire.
aSignificant difference.
bNumber of packs of cigarettes smoked per day multiplied by number of years smoked.
cA maximum of 14 standard drinks per week.
d12 g of alcohol.
eEORTC C30 reference scores (EORTC Quality of Life Questionnaire C30 Scoring Manual).
Figure 4.
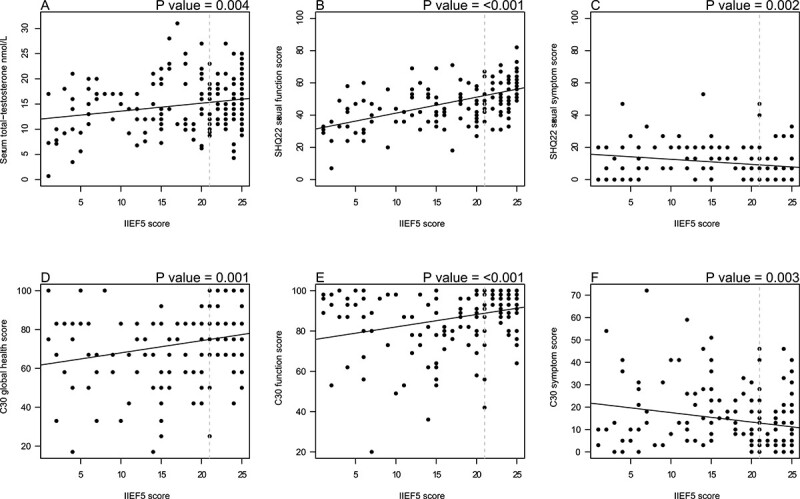
Five-item International Index of Erectile Function (IIEF-5) scores and associations with serum concentrations of total testosterone and quality-of-life (QoL) scores. (A-F) Dotted gray line representing cutoff score of 21 for erectile dysfunction. SHQ22, 22-item Sexual Health Questionnaire.
QoL characteristics
Sexual QoL was inferior in survivors with ED compared with sexual QoL in survivors without ED, with mean differences in sexual QoL scores (EORTC SHQ22) indicating significantly more sexual symptoms and worse sexual functioning in survivors with ED (P < .001) (Table 3). Furthermore, an association between erectile function and sexual QoL scores (SHQ22) was demonstrated (P ≤ .002) (Figure 4B, C). Mean general QoL scores (EORTC C30) for survivors with ED indicated inferior general QoL compared with both the general population29 and survivors without ED (P < .001) (Table 3), while general QoL scores for survivors without ED showed a population with good global health, a high level of functioning, and a low burden of general symptoms, with scores even better than in the general population (Table 3).29 Nevertheless, general QoL for all included survivors increased with higher erectile function scores (Figure 4D-4F). A comparison of sexual QoL scores (EORTC SHQ22) with general QoL scores (EORTC C30) showed that sexual health of male lymphoma survivors was more affected than general QoL (P ≤ .002 for comparison of both function and symptom scores) (Table 3). For survivors with ED, we found a mean C30 function score of 37.7 points higher than the mean SHQ22 function score (a difference of 37.6 points for survivors without ED). This difference was less pronounced in the symptom scores.
Discussion
We present data from a cross-sectional study of the frequency and significance of SD in lymphoma patients presumed to be cured. We detected a high prevalence of ED in male lymphoma survivors. ED was associated with not only inferior quality of their sexual life, but also an inferior general QoL. Even though overt testosterone deficiency was only found in a small fraction of survivors, we detected a significant positive association between erectile function (IIEF-5) and testosterone level. Age, comorbidity score, smoking, and BMI was negatively associated with erectile function, whereas longer education, current employment and having had children before treatment had a positive impact on erectile function.
ED was found in 55.2% of all lymphoma survivors, and 50.3% of sexually active survivors, comparable to earlier findings.14,15,30,31 A comprehensive study with more than 20 000 Danish men from the general population showed that 17.5% (25-64 years of age) of sexually active men had clinical ED according to the IIEF.27 In our study, we investigated a wide range of possible reasons for this increased prevalence, while earlier studies had a more limited focus. Due to the cross-sectional nature of our study, we could not detect causes for ED, but they were most likely multifactorial. For instance, we detected stable social status being important for maintaining a satisfying sexual function, indicating that factors like unemployment, lack of education, and decreased fertility may contribute negatively, all of which are highly relevant after a cancer diagnosis. Sexual health is associated with a high degree of complexity and is likely dependent on social, psychological, and physical factors with complex interactions. Factors such as robustness, coping strategies, and social support networks may all play a role in influencing sexual health outcomes.
Survivors of DLBCL had poorer sexual function than HL survivors, with a trend toward more severe ED. A likely explanation is the higher age of the DLBCL cohort, which may be accompanied by a higher incidence of comorbidity.32 As the survivors in the present study were treated with a heterogeneous group of chemotherapy regimens, analysis of the impact of treatment was difficult due to small numbers in each cohort. However, when categorizing treatment in 3 groups, R-CHOP–like regimen, ABVD and BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazapine, prednisone), we only found treatment with an R-CHOP–like regimen to be significantly associated with inferior ED compared with ABVD. Multivariate analysis using larger cohorts of uniformly treated patients is suggested to explore the relative importance of individual factors in relation to treatment. We did not perform multivariate analyses because of small subgroups arising from our univariate analyses.
Besides social status, our data also confirmed an association between ED and overall comorbidity status. Sexual health in lymphoma patients has previously been shown to be affected by multiple factors including fatigue, mental disorders, testosterone deficiency, neuropathy, diabetes, and cardiovascular diseases.13,15,32,33 Furthermore, cancer survivors, including lymphoma survivors, have a higher frequency of comorbidity compared with the general population,34 which is probably a consequence of the lymphoma disease itself or of the treatment. Interestingly, our study only found significant association between ED and metabolic complications including high BMI and diabetes when analyzing subgroups of comorbidities. Our study did not show a significant association between cardiovascular illness, urogenital problems, neuropathy or exercise and ED, in contrast to earlier findings.35 This could be due to the relatively small size of the investigated subgroups. The younger age of our cohort with less comorbidity burden might also impact the lack of a significant association in our data. This is because with increasing age, comorbidity burden also increases. ED has been associated with cardiac morbidity, and even higher mortality rates compared with the general population.36,37 Monitoring of ED and the risk of accompanying comorbidity should therefore be included in the follow-up of lymphoma survivors.
Animal studies have shown that chemotherapeutic agents such as doxorubicin can affect the testosterone producing Leydig cells of the testes.38 Human studies have found a high prevalence of testosterone deficiency among survivors of lymphoma10,39 and testicular cancer.40 In our study, 8 (4.7%) of 172 survivors had serum total testosterone below the age-adjusted reference levels, with 6 (3.5%) in combination with ED. Including all relevant survivors screened for eligibility, 10 survivors suffered from testosterone deficiency and ED, corresponding to 10 (5.7%) of 176. Prevalence in the different age groups was higher than the earlier found prevalence for males from the general population (40-49 years of age: 0.1%, 50-59 years of age: 0.6% and 60-69 years of age: 3.2%)28 despite our cohort being younger, our lower cutoff for normal testosterone was more conservative. We also found a significant association between SD and total testosterone concentrations, indicating that even levels in the lower normal range affected sexual health, in accordance with earlier findings.10,28,41 Our survivors were screened with serum total testosterone alone as previously recommended.28 Imbalances in sex hormone binding globulin, the carrier protein of testosterone, can result in a normal serum total testosterone but a clinically relevant low serum free testosterone, although this is not highly prevalent.28 Thus, an altered testosterone-to-sex-hormone-binding-globulin ratio in lymphoma survivors should be considered in future studies.
Survivors in the current study had both high prevalence of ED and a decreased sexual QoL. Symptom scores were less affected than function scores, suggesting that survivors’ sexual health is influenced by other issues than symptoms alone such as psychological and social factors reported here. EORTC module for sexual health (SHQ22) has only been applied in a few studies,42,43 and comparison with the general population or other lymphoma groups is therefore not possible. However, it is an easy add-on module to the frequently used EORTC QLQ C30 investigating general QoL, which might increase the use in time. Interestingly, we found an association between the widely used IIEF-5 score and the SHQ22 score, suggesting that the SHQ22 is a valid tool. We also found an association between ED and general QoL. Previous research has shown a significant association between SD and health-related QoL in lymphoma survivors,30 and between testosterone levels and both sexual and general QoL.28 In our study, general QoL in lymphoma survivors without ED was not severely affected, in accordance with earlier findings.29 Previous studies hypothesized that cancer survivors are more appreciative of life after surviving cancer and therefore report high QoL despite of health-related problems.44 Survivors with ED had significantly lower QoL scores, indicating that ED was decisive for QoL in our study.
Some limitations to our study should be considered. Included survivors were older at diagnosis and had shorter mean follow-up time than excluded, which could represent selection bias. Perhaps survivors who had been in remission for many years thought their participation to be insignificant or did no longer suffer from problems. The calculated inclusion over 10 years based on incidence and inclusion criteria was slightly higher than the included cohort and may entail a risk of selection bias. However, this could also be due to underreporting in the register used to identify survivors. Inclusion and exclusion criteria were chosen to include a population representative of the general survivor in clinical practice. More stringent exclusion criteria could have resulted in a more homogeneous study population, thereby reducing the probability of confounders. However, if several factors with potential influence on sexual function had been part of the exclusion criteria, it would be contradictory to the aim of the study. Additionally, SD may be present before the cancer diagnosis and thereby not represent a newly introduced problem in cross-sectional studies like ours. Large cohort studies evaluating sexual health could identify decreasing satisfaction and function and follow the trajectory of sexual health from before the diagnosis, through the treatment and follow-up periods. However, a large longitudinal study with a required long follow-up period will be difficult to carry out. In observational studies like the current based on patient-reported outcome a risk of recall bias is present, especially for survivors with very little sexual activity. Furthermore, some questions in the patient-reported outcome measure can be difficult to answer for survivors who only engaged in sexual activity with themselves (eg, whether erections were rigid enough for penetration). This could introduce bias because results are based on the survivors’ assessment. Furthermore, ED can be a consequence of a wide range of health issues, such as pituitary illness and use of medication, which are not part of the present study. It is therefore possible that a relationship between health effects and earlier treatment has been overestimated. Our data should be validated in a longitudinal prospective study including all possible causes of ED.
Conclusion
Our data suggest that ED is a frequent problem in male long-term survivors of malignant lymphoma with an impact on sexual health and QoL. We found an association between ED and the serum concentration of total testosterone as well as the presence of morbidities at the time of follow-up. Our data suggest that monitoring SD in the follow-up of lymphoma patients merits increased awareness as part of the standard follow-up program to increase attention to potentially treatment related comorbidities and to improve sexual and general QoL.
Supplementary Material
Acknowledgments
This work received approval from the Danish Regional Committee on Health Research Ethics, Capital Region of Denmark (case number: H-20036508).
Contributor Information
Signe Micas Pedersen, Department of Hematology, Copenhagen University Hospital – Rigshospitalet, 2100 KBH Ø, Copenhagen, Denmark.
Torsten Holm Nielsen, Department of Hematology, Copenhagen University Hospital – Rigshospitalet, 2100 KBH Ø, Copenhagen, Denmark; Danish Medicines Agency, 2300 KBH S, Copenhagen, Denmark.
Anne Ortved Gang, Department of Hematology, Copenhagen University Hospital – Rigshospitalet, 2100 KBH Ø, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, 2200 KBH N, Denmark.
Christian Bjørn Poulsen, Department of Hematology, Zealand University Hospital, 4000 Roskilde, Denmark.
Peter de Nully Brown, Department of Hematology, Copenhagen University Hospital – Rigshospitalet, 2100 KBH Ø, Copenhagen, Denmark.
Niels Jørgensen, Department of Growth and Reproduction, Copenhagen University Hospital – Rigshospitalet, 2100 KBH Ø, Denmark.
Claus Larsen Feltoft, Department of Endocrinology, Herlev University Hospital, 2730 Herlev, Denmark.
Lars Møller Pedersen, Department of Clinical Medicine, University of Copenhagen, 2200 KBH N, Denmark; Department of Hematology, Zealand University Hospital, 4000 Roskilde, Denmark.
Author Contribution
Conceptualization: S.M.P., T.H.N., A.O.G., C.B.P., P.N.B., C.L.F., and L.M.P.; data curation: S.M.P., and L.M.P.; formal analysis: S.M.P. and L.M.P.; funding acquisition: S.M.P., T.H.N., A.O.G., and L.M.P.; investigation: S.M.P.; methodology: S.M.P., T.H.N., A.O.G., C.B.P., P.N.B., C.L.F., and L.M.P.; project administration: S.M.P. and L.M.P.; resources: S.M.P., T.H.N., A.O.G., C.B.P., C.L.F., and L.M.P.; supervision: L.M.P.; visualization: S.M.P., T.H.N., A.O.G., C.B.P., P.N.B., N.J., C.L.F., and L.M.P.; writing – original draft: S.M.P.; writing – review and editing: S.M.P., T.H.N., A.O.G., C.B.P., P.N.B., C.L.F., N.J., and L.M.P.
Funding
Funding was obtained from the Department of Hematology, Copenhagen University Hospital – Rigshospitalet Research Funds; an Einar Willumsens Scholarship; a Jens and Maren Thestrups Scholarship; and the Johannes Fog Fund.
Conflicts of Interest: SMP received study medicine for a subsequent study on the treatment of testosterone deficiency from Besins Healthcare. No payment was provided, and Besins Healthcare had no influence on content of this article and has no rights to publish data from the study. No other conflicts of interest.
References
- 1. Malignt Lymfom Og CLL National årsrapport 2021. Accessed May 2, 2022. https://www.lymphoma.dk/wp-content/uploads/2022/11/Aarsrapport-2021-LYFO-CLL.pdf.
- 2. Arden-Close E, Eiser C, Pacey A. Sexual functioning in male survivors of lymphoma: a systematic review. J Sex Med. 2011;8(7):1833–1840. [DOI] [PubMed] [Google Scholar]
- 3. Kornblith A, Anderson J, Cella DF, et al. Comparison of psychosocial adaptation and sexual function of survivors of advanced hodgkin disease treated by MOPP, ABVD, or MOPP alternating with ABVD. Cancer. 1992;70(10):2508–2516. [DOI] [PubMed] [Google Scholar]
- 4. Eikeland SA, Smeland KB, Mols F, et al. Chemotherapy-induced peripheral neuropathy after modern treatment of Hodgkin’s lymphoma; symptom burden and quality of life. Acta Oncol. 2021;60(7):911–920. [DOI] [PubMed] [Google Scholar]
- 5. Luigjes-Huizer TNM, Humphris G, Kasparian NA, Lam WWT, Lebel S. What is the prevalence of fear of cancer recurrence in cancer survivors and patients? A systematic review and individual participant data meta-analysis. Psychooncology. 2022;31(6):879–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olsson C, Sandin-Bojö A-K, Bjuresäter K, Larsson M. Changes in sexuality, body image and health related quality of life in patients treated for hematologic malignancies: a longitudinal study. Sex Disabil. 2016;34(4):367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frick M, Vachani C, Hampshire MK, et al. Patient-reported survivorship care practices and late effects after treatment of Hodgkin (HL) and non-Hodgkin lymphoma (NHL). J Clin Oncol. 2018;36(7_suppl):119. [DOI] [PubMed] [Google Scholar]
- 8. Mütsch J, Friedrich M, Leuteritz K, et al. Sexuality and cancer in adolescents and young adults - a comparison between reproductive cancer patients and patients with non-reproductive cancer. BMC Cancer. 2019;19(1):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Behringer K, Müller H, Flechtner H-H, et al. Sexual quality of life in Hodgkin lymphoma: a longitudinal analysis by the German Hodgkin study group. Br J Cancer. 2013;108(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiserud C, Fosså A, Bjøro T, Holte H, Cvancarova M, SD F. Gonadal function in male patients after treatment for malignant lymphomas, with emphasis on chemotherapy. Br J Cancer. 2009;100(3):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olsson C, Eklund AJ, Larsson M, Ringnér A. Sexuality after treatment of diffuse large B cell lymphoma: patients’ experiences and psychometric testing of the sexual adjustment questionnaire - Swedish version II. Cancer Nurs. 2021;44(6):499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiserud CE, Seland M, Holte H, et al. Fatigue in male lymphoma survivors differs between diagnostic groups and is associated with latent hypothyroidism. Acta Oncol. 2015;54(1):49–59. [DOI] [PubMed] [Google Scholar]
- 13. Seguin L, Touzani R, Bouhnik A-D, et al. Deterioration of sexual health in cancer survivors five years after diagnosis: data from the French national prospective VICAN survey. Cancers (Basel). 2020;12(11):3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aksoy S, Harputluoglu H, Kilickap S, et al. Erectile dysfunction in successfully treated lymphoma patients. Support Care Cancer. 2008;16(13):291–297. [DOI] [PubMed] [Google Scholar]
- 15. Bersvendsen HS, Sagstuen Haugnes H, Dahl AA, et al. Sexual function in long-term male lymphoma survivors after high-dose therapy with autologous stem cell transplantation. Bone Marrow Transplant. 2020;55(5):891–905. [DOI] [PubMed] [Google Scholar]
- 16. Beckjord EB, Arora NK, Bellizzi K, Hamilton AS, Rowland JH. Sexual well-being among survivors of non-Hodgkin lymphoma. Oncol Nurs Forum. 2011;38(5):E351–E359. [DOI] [PubMed] [Google Scholar]
- 17. Eeltink CM, Lissenberg-Witte BI, Incrocci L, et al. Self-reported sexual function in sexually active male Hodgkin lymphoma survivors. Sex Med. 2020;8(3):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arboe B, Josefsson P, Jørgensen J, et al. The Danish National Lymphoma Registry. Clin Epidemiol. 2016;8:577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 20. Oberguggenberger AS, Nagele E, Inwald EC, et al. Phase 1-3 of the cross-cultural development of an EORTC questionnaire for the assessment of sexual health in cancer patients: the EORTC SHQ-22. Cancer Med. 2018;7(3):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326. [DOI] [PubMed] [Google Scholar]
- 22. Cappelleri JC, Seigel RL, Glasser DB, Osterloh IH, Rosen RC. Relationship between patient self-assessment of erectile dysfunction and the sexual health inventory for men. Clin Ther. 2001;23(10):1707–1719. [DOI] [PubMed] [Google Scholar]
- 23. Miller MD, Rifai HA, Mulsant B, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. [DOI] [PubMed] [Google Scholar]
- 24. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. [DOI] [PubMed] [Google Scholar]
- 25. De Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. [DOI] [PubMed] [Google Scholar]
- 26. Hudon C, Fortin M, Vanasse A. Cumulative illness rating scale was a reliable and valid index in a family practice context. J Clin Epidemiol. 2005;58(6):603–608. [DOI] [PubMed] [Google Scholar]
- 27. Frisch M, Moseholm E, Andersson M, Andresen, Josefine B, Graugaard C. Sex in Denmark. Key findings from Project SEXUS 2017-2018. Accessed May 2, 2022. https://www.projektsexus.dk/. [Google Scholar]
- 28. Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. [DOI] [PubMed] [Google Scholar]
- 29. Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values. 419. Accessed May 2, 2022. https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf.
- 30. Kim IR, Kim SH, Ok ON, et al. Sexual problems in male vs. female non-Hodgkin lymphoma survivors: prevalence, correlates, and associations with health-related quality of life. Ann Hematol. 2017;96(5):739–747. [DOI] [PubMed] [Google Scholar]
- 31. Gan G, Ng DLC, Leong YC. Erectile dysfunction in male lymphoma survivors in a southeast Asian country. Singap Med J. 2022;63:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pellegrino F, Sjoberg DD, Tin AL, et al. Relationship between age, comorbidity, and the prevalence of erectile dysfunction. Eur Urol Focus. 2023;9(1):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Musicki B, Bella AJ, Bivalacquia TJ, et al. Basic science evidence for the link between erectile dysfunction and cardiometabolic dysfunction. J Sex Med. 2015;12(12):2233–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hvidberg MF, Frølich A, Lundstrøm SL, Larsen NK. Catalogue of multimorbidity mean based severity and associational prevalence rates between 199+ chronic conditions—a nationwide register-based population study. PLoS One. 2022;17(9):e0273850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitta RM, Kaufmann O, Louzada ACS, et al. The association between physical activity and erectile dysfunction: a cross-sectional study in 20,789 Brazilian men. PLoS One. 2022;17(11):e0276963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antonio L, Wu FCW, Moors H, et al. Erectile dysfunction predicts mortality in middle-aged and older men independent of their sex steroid status. Age Ageing. 2022;51:1–9. [DOI] [PubMed] [Google Scholar]
- 37. Fan Y, Hu B, Man C, Cui F. Erectile dysfunction and risk of cardiovascular and all-cause mortality in the general population: a meta-analysis of cohort studies. World J Urol. 2018;36(10):1681–1689. [DOI] [PubMed] [Google Scholar]
- 38. Akinjo O, Gant TW, Marczylo EL. Perturbation of microRNA signalling by doxorubicin in spermatogonial, Leydig and Sertoli cell lines in vitro. Toxicol Res. 2018;7:760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin study group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231–239. [DOI] [PubMed] [Google Scholar]
- 40. Bandak M, N J, Juul A, et al. Testosterone deficiency in testicular cancer survivors - a systematic review and meta-analysis. Andrology. 2016;4(3):382–388. [DOI] [PubMed] [Google Scholar]
- 41. Bandak M, Jørgensen N, Juul A, et al. Longitudinal changes in serum levels of testosterone and luteinizing hormone in testicular cancer patients after orchiectomy alone or bleomycin, etoposide, and cisplatin. Eur Urol Focus. 2018;4(4):591–598. [DOI] [PubMed] [Google Scholar]
- 42. Greimel E, Nagale E, Bliem B, Bjelic-Radisic V. Sexualität im Lebenszyklus-nach gynäkologischen Karzinomen Einleitung. J Urol Urogynäkol. 2019;26:29–32. [Google Scholar]
- 43. Casswell G, Gough K, Drosdowsky A, et al. Clinical investigation sexual health and interpersonal relationships after chemoradiation therapy for human papillomavirus-associated oropharyngeal cancer: a cross-sectional study. Int J Radiat Oncol Biol Phys. 2020;110(2):382–393. [DOI] [PubMed] [Google Scholar]
- 44. De Haes JC, van Knippenberg FCE. The quality of life of cancer patients: a review of the literature. Soc Sci Med. 1985;20(8):809–817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


