Abstract
Pelagic cephalopods have evolved a series of fascinating traits, such as excellent visual acuity, high-speed agility, and photophores for adaptation to open pelagic oceans. However, the genetic mechanisms underpinning these traits are not well understood. Thus, in this study, we obtained high-quality genomes of two purpleback flying squid species (Sthenoteuthis oualaniensis and Sthenoteuthis sp.), with sizes of 5450 Mb and 5651 Mb, respectively. Comparative genomic analyses revealed that the S-crystallin subfamily SL20-1 associated with visual acuity in the purpleback flying squid lineage was significantly expanded, and the evolution of high-speed agility for the species was accompanied by significant positive selection pressure on genes related to energy metabolism. These molecular signals might have contributed to the evolution of their adaptative predatory and anti-predatory traits. In addition, the transcriptomic analysis provided clear indications of the evolution of the photophores of purpleback flying squids, especially the recruitment of new genes and energy metabolism-related genes which may have played key functional roles in the process.
Keywords: Cephalopoda, Evolution, Flying squid, Genome, Photophore
Introduction
Cephalopods are a group of marine mollusk with remarkable morphology and behavior that play key ecological roles, are commercially important, and have been intensively studied [1]. Large populations of cephalopods inhabit depths ranging from shallow to abyssal [2], [3]. They are preyed upon by various apex predators (such as billfish, tuna, sharks, and cetaceans) that are generally the fastest and most efficient in the ocean [4], [5], [6]. In response to the predation, most cephalopods have developed excellent visual acuity [7], [8], [9], strong muscles, and morphological traits [10] that enable them to evade danger rapidly. They also have high metabolic levels and hence constantly high levels of energy supplies for their muscles [11]. The continuous adaptations and counter-adaptations induced by interactions between prey and predators — the ‘arms race’, is one of the most intense forms of co-evolution. This ‘arms race’ is particularly pronounced between the pelagic cephalopods (e.g., cuttlefishes and squids) and their predators [12]. These cephalopods have extremely strong muscles with obliquely-striated, quickly contractible, highly aerobic fibers, and morphological features, such as fins, which enable powerful swimming [10]. Some species of pelagic cephalopods have also repeatedly evolved photophores that assist in escape [13], predation [14], and mating [15]. However, although it has long been accepted that adaptative evolution has resulted in a series of fascinating traits in cephalopods [16], [17], [18], [7], the genetic mechanisms involved are much less clear.
Common pelagic cephalopods include members of the Ommastrephidae (Teuthida, Decapodiformes) called flying squids, which can jump out of the water and in some cases glide more than 30 m in the air [19]. They can achieve the fastest recorded speed (∼ 8 m/s) of any aquatic invertebrates [20], [21]. Important taxa with such capabilities include the purpleback flying squids (Sthenoteuthis spp.) [20], [22], [23]. These are the most abundant large squids in the tropical and subtropical Indo-Pacific ocean, found at depths from the surface to more than 600 m [2]. Moreover, the purpleback flying squids have a high degree of adaptation to their pelagic life and at least five morphological and ecological forms in terms of body size and possession or absence of photophores [2]. Thus, the purpleback flying squids are ideal models for studying the genetic mechanisms involved in the evolution of pelagic cephalopods’ adaptive traits.
In this study, we constructed high-quality genomes for two ‘forms’ of purpleback flying squids. One (S. oualaniensis) is the ‘medium’ or ‘typical’ form, which has a dorsal mantel length (at maturity) of > 120 mm and spherical photophores forming an oval patch in the anterodorsal mantle musculature [2], [24] (Figure S1). The other (Sthenoteuthis sp.) is the ‘dwarf form’, which is smaller and lacks the dorsal photophore patch (Figure S1). The dwarf form was previously treated as S. oualaniensis, but is now considered an undescribed species [25], [26]. Through comparative genomic analyses, we deeply profiled genomic features of these two purpleback flying squids and investigated molecular signals associated with adaptations of pelagic life in cephalopods, such as their excellent visual system, high behavioral flexibility, and photophore. The results suggest that the genomes of these two purpleback flying squids are important resources that can facilitate research not only on cephalopods but also on adaptive evolution and molecular genetics more generally.
Results and discussion
Assemblies and genomic characteristics
Using a combination of PacBio long reads, 10× Genomics short reads, RNA sequencing (RNA-seq), and Hi-C approaches, we obtained high-quality genomes of the two species of purpleback flying squids of the Ommastrephidae (S. oualaniensis and Sthenoteuthis sp.) (Tables S1–S4; Figures S2 and S3). The sizes of the genome assemblies were 5450 Mb and 5651 Mb for S. oualaniensis and Sthenoteuthis sp., respectively (Table 1), close to the genome sizes estimated by k-mer analysis (Figure S4). The S. oualaniensis genome was assembled to chromosome level, with 95.72% of contigs anchored to the 46 chromosomes (Figure 1A). The contig N50 and scaffold N50 values of the S. oualaniensis assembly were 1.52 Mb and 118.8 Mb, respectively, while the contig N50 value for the Sthenoteuthis sp. assembly was 1.3 Mb. The S. oualaniensis genome is the only squid genome that has been assembled to chromosome level (Figure 1A; Table 1). GC contents of the acquired S. oualaniensis and Sthenoteuthis sp. genomes were 33.70% and 33.00%, respectively, similar to those of other relatives (Table S5). Results of the Benchmarking Universal Single-Copy Orthologs (BUSCO) [27] assessment showed that the S. oualaniensis and Sthenoteuthis sp. assemblies had 89.8% and 93.6% genomic integrity, respectively (Table 1). RNA-seq analysis of S. oualaniensis and Sthenoteuthis sp. yielded 389,954 and 410,364 transcripts with total lengths of 200–25,632 bp and 200–31,105 bp, respectively (Table S6).
Table 1.
Summary of the assemblies and annotations of the two Sthenoteuthis species
| Sthenoteuthis oualaniensis | Sthenoteuthis sp. | |
|---|---|---|
| Genome size (Mb) | 5450 | 5651 |
| Contig N50 (Mb) | 1.52 | 1.3 |
| Scaffold N50 (Mb) | 118.8 | - |
| GC content | 33.07% | 33.00% |
| Chromosome number | 46 | - |
| Gene number | 26,646 | 28,715 |
| BUSCO for genomes | 89.4% | 93.6% |
| BUSCO for genes | 90.4% | 94.3% |
Note: BUSCO, Benchmarking Universal Single-Copy Orthologs.
Figure 1.
Genome assemblies and annotations of two Sthenoteuthis species
S. oualaniensis and Sthenoteuthis sp. are referred to as the “typical form” and “dwarf form” of purpleback flying squids, respectively. A. Genome-wide Hi-C map of the 46 pseudo-chromosomes of S. oualaniensis. B. Syntenic comparison of S. oualaniensis and Sthenoteuthis sp. Numbers 1 to 46 refer to the chromosomes of S. oualaniensis. Each color block in the outermost layer represents a scaffold. Each line represents a syntenic block of five or more genes. Densities of specific kinds of TEs (ranging from 0 to 80%) were counted in 500-kb windows. C. Summary of gene clusters estimated from Orthofinder analysis based on sequences of eight Mollusca species and one annelid worm. D. UpSet plot of gene families. E. Divergence distribution of TEs of species in Teuthida. Latin binomials of the listed species are as follows: Polychaete worm, Capitella teleta; Owl limpet, Lottia gigantea; Japanese scallop, Mizuhopecten yessoensis; California two-spot octopus, Octopus bimaculoides; Octopus, Octopus minor; East Asian common octopus, Octopus vulgaris; Bobtail squid, Euprymna scolopes. TE, transposable element; LINE, long interspersed nuclear element; SINE, short interspersed nuclear element; LTR, long terminal repeat.
Based on the high-quality assemblies, we predicted a total of 26,646 and 28,715 protein-coding genes in the genomes of S. oualaniensis and Sthenoteuthis sp., respectively (Table S5). Of these, 19,913 (74.73%) and 21,853 (76.10%) are supported by corresponding transcripts, and 24,845 (93.24%) and 27,897 (97.13%) predictions could be functionally annotated using entries in at least one database (Figures S5 and S6). Results of BUSCO analysis suggested that the assembly completeness of S. oualaniensis and Sthenoteuthis sp. were 89.4% and 93.6%, respectively, and the annotation completeness of them were 90.4 and 94.3%, respectively (Table 1, Table S7). Basic metrics for the protein-coding genes of these two purpleback flying squids, including gene number/length, exon number/length, and codon usage, are close to those of other Mollusca (Table S5). Synteny analysis, based on the coding genes, identified 743 blocks with at least five syntenic genes between S. oualaniensis and Sthenoteuthis sp. and demonstrated the good colinearity between the two genomes, which also indicated that the two genomes were well assembled and annotated (Figure 1B). Finally, 22,162 (83%) S. oualaniensis genes and 25,991 (90%) Sthenoteuthis sp. genes were clustered into 16,235 gene clusters, with sizes close to those of other relatives (Figure 1C and D).
Repeats analysis indicated that close to half of the genomes of both S. oualaniensis and Sthenoteuthis sp. are composed of repetitive sequences: 53.90% and 42.37%, respectively (Tables S8 and S9), which is comparable to that of other cephalopods [28], [29]. Identification and classification of the transposable elements (TEs) of the two genomes (Figure 1E; Tables S8 and S9) showed that DNA and long interspersed nuclear element (LINE) were the two most abundant types of TEs in the S. oualaniensis genome (accounting for 25.47% and 22.05% of the total, respectively), but only accounted for 14.13% and 10.00% of the TEs, respectively, in the Sthenoteuthis sp. genome. There was a markedly smaller proportion of simple tandem repeats in the S. oualaniensis genome (6.33%) than in the Sthenoteuthis sp. genome (25.86%) (Tables S8 and S9). Thus, despite the very close relationship between the two purpleback flying squid species, they might have distinct patterns of TE activity.
Phylogenetic status and species validity of two purpleback flying squids
Maximum-likelihood (ML) gene tree and species tree analyses based on 334 one-to-one genes yielded consistent topologies (Figure S7). The Octopodiformes and Decapodiformes species each clustered into monophyletic lineages, which apparently diverged around 366.5 million years ago (MYA) (Figure 2; Figure S7). The two purpleback flying squid species are the most closely related of the included taxa, having diverged at approximately 41.0 MYA. These two forms of purpleback flying squids, S. oualaniensis and Sthenoteuthis sp., were considered to be members of the same species for a long time, but this was recently questioned [25], [26]. Morphological studies provided evidence that Sthenoteuthis sp. was distinguishable from S. oualaniensis concerning external features, including variables of the head, carcass, and arms, as well as the shape and size of fins [30], [31]. Our results corroborate the conclusion that Sthenoteuthis sp. should be regarded as a distinct species, as we detected clear differences in their genomes and derived a substantial divergence time.
Figure 2.
Coalescence tree of eight Mollusca species and one annelid worm based on 334 single-copy orthologs
Estimated divergence time and expanded/contracted gene families are marked at the nodes. Red, blue, and black numbers indicate the number of expanded gene families, the number of contracted gene families, and the estimated divergence time, respectively. MYA, million years ago.
Excellent vision of purpleback flying squids
Keen vision is regarded as a major product of evolutionary arms races in squids [7], which strongly contributes to the ability of purpleback flying squids (and others) to avoid predation and catch prey even in dim conditions [1], [7], [9]. Important components of their eyes include several soluble crystallins that play key roles in maintaining the transparency and optical clarity of the lens [32]. In particular, S-crystallins are present in the lenses of many cephalopods, and have refractive properties that strongly contribute to good vision (and hence cephalopod survival) in poor light [33], [34]. S-crystallins are even claimed to provide a “perfect medium”, forming gels of varying density, in the spherical lenses of cephalopods [34], [35], [36].
Cluster analysis detected 2489 gene families that were expanded in both purpleback flying squid species (under the criterion of both family-wide and viterbi P values < 0.01; Figure 2, Figures S8 and S9). The SL20-1 gene subfamily which encodes S-crystallins was most significantly expanded, with 39 and 99 gene members in S. oualaniensis and Sthenoteuthis sp., respectively (Figure 3A and B). Transcriptomic analysis revealed that most of these expanded SL20-1 genes were only highly expressed in eyes (Figure 3C, Figure S10), clearly indicating that expansion of this subfamily plays an important role in the emergence of purpleback flying squids’ excellent vision.
Figure 3.
Expanded gene families in the Sthenoteuthis lineage
A. The top 20 expanded gene families. B. Unrooted maximum-likelihood tree of the massive expansion of the S-crystallin subfamily SL20-1, with 39 and 99 gene members in S. oualaniensis and Sthenoteuthis sp., respectively. C. Expression patterns of coexisting gene members of the SL20-1 subfamily in S. oualaniensis and Sthenoteuthis sp. Only those expressed gene members were shown. These gene IDs were detailed in Figure S10. Most of the significantly expanded genes were highly expressed in the eyes. Commen octopus, Octopus sinensis; Wellington flying squid, Sepia pharaonis.
High behavioral flexibility
The mobility of organisms strongly affects their predatory and anti-predatory abilities, and thus their evolutionary fitness [37]. Purpleback flying squids are highly successful in these terms, as they are the fastest and most mobile aquatic invertebrates, with very high metabolic levels that enable powerful output at all times [11], [23].
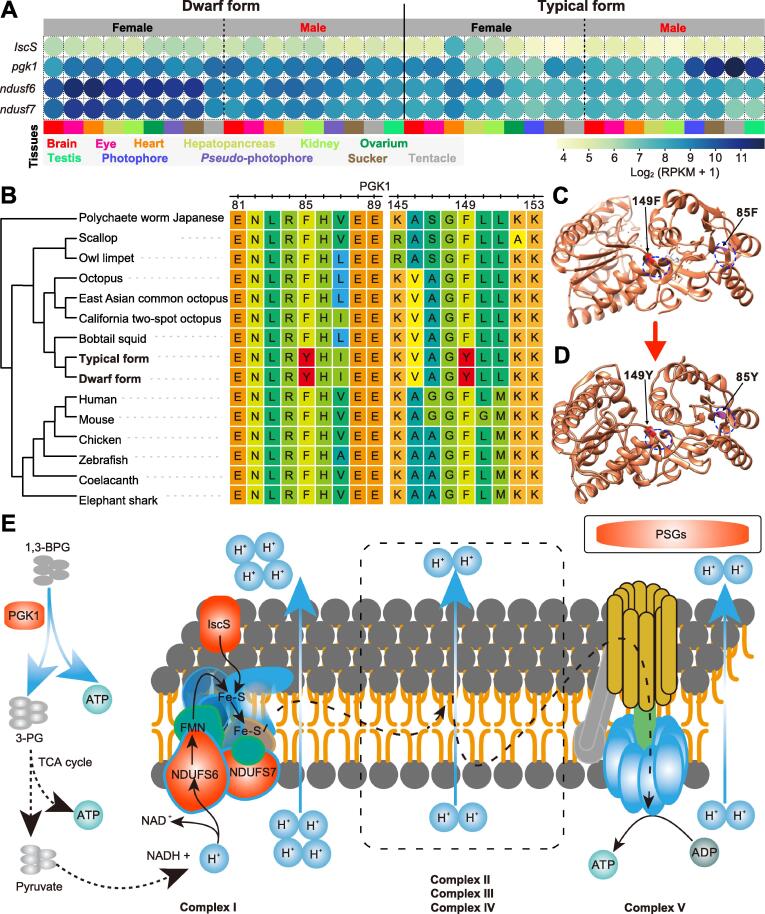
Our analysis identified 66 positively selected genes (PSGs) in the Sthenoteuthis lineage [false discovery rate (FDR)-adjusted P < 0.01; Table S10]. Gene Ontology (GO) enrichment analyses (FDR < 0.01) indicated that 23, 42, and 30 of these PSGs were associated with cellular components, molecular functions, and biological processes, respectively (Figures S11 and S12). The PSGs involved in energy metabolism were apparently under significant evolutionary selection pressure (FDR-adjusted P < 0.01). “Phosphoglycerate kinase activity” (GO:0004618, e.g., pgk1) and “[2Fe-2S] cluster assembly” (GO:0044571, e.g., IscS) were the two most significant GO terms (FDR < 0.05; Tables S11 and S12). The “quinone binding” term (GO:0048038, e.g., ndufa6 and ndufa7) was also significantly enriched (FDR < 0.05; Table S11). Transcriptomic analysis demonstrated that these genes were highly expressed in all the investigated tissues, implying that they play important roles (Figure 4A).
Figure 4.
Diagram ofPSGsassociated with energy metabolism
A. Expression patterns of four PSGs involved in energy metabolism that are highly expressed in all investigated tissues. B. Positively selected signals in two extremely conserved regions of PGK1. C. Three-dimensional structure of mouse PGK1 protein downloaded from the PDB database. Substructures of 85F and 149F are highlighted. D. Three-dimensional structure simulated by a homologous approach of mouse PGK1 with F85Y and F149Y substitutions. Structures adjacent to the substitute sites in (C) and (D) are signaled by the blue dashed circles. E. Schematic diagram of the glycolysis pathway and respiratory electron chain. PSG, positively selected gene; PGK1, phosphoglycerate kinase 1; PDB, Protein Data Bank; RPKM, reads per kilobase million.
Phosphoglycerate kinase 1 (PGK1), the first ATP-generating enzyme in the glycolytic pathway, both directly generates ATP and indirectly supplies fuel for the mitochondrial electron respiratory chain [38], [39]. Phylogenetic analysis by maximum likelihood (PAML) detected positive selection signals at two extremely conserved regions of the pgk1 gene (F85Y and F149Y; Figure 4B). Simulations showed that these two F-to-Y substitutions affect the structure of PGK1 protein resulting in the conversion of the original helix to a loop (Figure 4C and D). Thus, these substitutions were presumably highly important for purpleback flying squids. Similarly, a positive selection signal was detected at a conserved region of the IscS gene (Y67H; Figure S13). IscS participates in the synthesis of multiple iron-sulfur (Fe-S) proteins and the formation of Fe-S clusters in complex I (NADH: ubiquinone oxidoreductase; Figure 4E) [40]. Knockdown of the IscS gene leads to a decrease in mitochondrial activity [40], [41]. We also found positive selection signals in ndufa6 and ndufa7, which encode two important subunits of complex I that are essential for the catalytic activity of this complex (Table S10) [42], [43]. Complex I plays a key role in ATP synthesis driven by the mitochondrial electron respiratory chain [44] (Figure 4E). Therefore, the PSGs mentioned above are likely to promote ATP synthesis, which is important for the maintenance of high metabolic levels and the high behavioral flexibility of purpleback flying squids. Furthermore, the presence of these positively selected sites in the pelagic Architeuthis dux (Figure S14), which also possess high metabolic levels [2], [29], implies the importance of these selection signals for pelagic cephalopods. However, we should note that these results come from a small gene pool and therefore have some limitations.
Photophore transcriptome
Bioluminescence is a common feature of cephalopods, especially pelagic species [14], [45]. At least 63 genera of squid and cuttlefish have repeatedly evolved photophores that play important roles in defense, predation, and communication [13], [14], [15]. One of the species included in this study, S. oualaniensis, has a dorsal photophore patch, but not Sthenoteuthis sp. (Figure S1). Therefore, they are ideal models for studying the evolution of photophores. Principal component analysis (PCA) showed that S. oualaniensis photophores clustered with the pseudo-photophores (‘muscle tissue’ corresponding to the position of the photophore of S. oualaniensis) of Sthenoteuthis sp. (Figure 5A), and highly expressed genes of photophores had similar expression patterns (Figure 5B). Therefore, the pseudo-photophores of Sthenoteuthis sp. and photophores of S. oualaniensis seem to be homologous organs, and the pseudo-photophores may have some essential functions similar to those of the photophores.
Figure 5.
Results of photophore transcriptome analysis
A. PCA plot of 36 RNA-seq samples based on expression profiling of 23,082 orthologs. Pseudo-photophores of Sthenoteuthis sp. and photophores of S. oualaniensis clustered together (red dotted circle). B. Whole-tissue expression patterns of 16 highly expressed genes in the four “photophore” tissues. PCA, principal component analysis; PC, principal component.
Previous studies based on gene expression profiles have suggested that the massive recruitment of pre-existing gene modules plays an important role in the formation of cephalopod photophores [46], [47]. Here we specifically investigated the highly expressed genes in the “photophores” of these two squids, which represent genetic factors associated with the essential function of this organ. Among the 16 highly expressed genes, four were new genes (newgene1–4; Figure 5B). Although functional assignations of these genes were not supported by homologs in public databases, their specific expression patterns suggest that they might participate in the formation and some of the basic functions of luminophores. In addition, four of the highly expressed genes (phkg1, pygb, odh1, and obh1-like) are energy metabolism-related genes. All of these genes are involved in glucose metabolism. Both phkg1 and pygb are glycogen phosphorylases that regulate the catabolism of glycogen and provide glucose used in glycolysis [48], [49]. Octopine dehydrogenase (encoded by odh1), mainly found in mollusks [50], has a major function similar to that of lactate dehydrogenase, providing an important reducing agent for the glycolytic process [51]. These observations imply that these new genes and recruited energy metabolism-related genes might provide important support for the photophores of purpleback flying squids.
Conclusion
In this study, we generated high-quality genomes of the two purpleback flying squid species. Comparative genomic analyses indicated that expansion of the S-crystallin subfamily SL20-1 and locus variation and expression patterns of genes related to energy metabolism are associated with adaptations of purpleback flying squids (such as excellent vision, high behavioral flexibility, and photophore) that have played important roles in the ‘arms race’ and other pelagic adaptations among marine organisms. Moreover, the study supports the validity of treating Sthenoteuthis sp. as a separate species at the genomic level. These findings advance our understanding of the genetic basis of pelagic cephalopods associated with predatory and anti-predatory traits and suggest that the two genomes could be important resources for studying not only cephalopods, but also co-evolution, bioluminescence, and other broader aspects of molecular genetics.
Materials and methods
Sampling and sequencing
Squid samples were caught by a commercial fishing vessel using a lit falling net at night in the South China Sea. One male individual and one female individual of both medium (S. oualaniensis) and dwarf (Sthenoteuthis sp.) purpleback flying squids were collected for sequencing. Muscles from the males were used for DNA extraction and genomic library preparation. A PacBio Sequel device was used for sequencing the long reads. Short-insert paired-ends libraries were prepared and sequenced according to the Illumina sequencing protocol. Sample indexing and partition barcoded libraries were prepared using a chromium genome reagent kit (Catalog No. PN-120229, 10× Genomics, CA) and sequenced by an Illumina HiSeq X-Ten system for Hi-C analysis of S. oualaniensis. To explore gene expression patterns of the species and aid gene annotation, RNA was extracted from nine tissues, including tentacle, brain, eye, heart, kidney, sucker, hepatopancreas, ovarium/testis, and photophore (for Sthenoteuthis sp., muscle tissue corresponding to the position of the photophore of S. oualaniensis was obtained), from each of the four S. oualaniensis and Sthenoteuthis sp. individuals for library preparation and sequenced using the Illumina HiSeq 2000 platform.
Estimation of genome sizes of the two Sthenoteuthis species
The genome sizes of the two Sthenoteuthis species were estimated by k-mer analysis using filtered Illumina reads. We used SOAPec v2 [52] to estimate the distribution of 17-mer depth, and then estimated the genome size from the total base and peak values of 17-mer depth.
Genome assembly
Based on the estimated genome sizes, we first assembled genomes of the two purpleback flying squid species to contig level with wtdbg2 v2.4.1 [53] and standard parameters. The arrow algorithm was used to polish the two draft genomes with the filtered PacBio reads. Then the filtered short paired-end reads were aligned to the draft genome by BWA-MEM v.0.7.12-r1039 [54] with standard parameters, and Pilon [55] was used to further polish the genomes in two rounds using the sorted bam files. Finally, the genome assembly of S. oualaniensis was anchored with the Hi-C reads by 3D-DNA [56] and Juicer v1.5 [57]. To improve the quality of the chromosome assembly, we used Juicebox assembly tools [58] to remove potential assembly errors. BUSCO v3.02 [27] with the “metazoa_odb9” library was used to evaluate the completeness of the two assemblies.
Repetitive sequence annotation
After obtaining a high-quality genome assembly, we used a combination of de novo and homologous predictions to annotate repetitive sequences, including tandem repeats and TEs. Firstly, tandem repeat finder v4.07 [59] was used to scan the tandem repeat elements with the parameter settings “2 7 7 80 10 50 500 -d -h -ngs”. Then we used RepeatModeler v1.0.8 [60] to build a de novo repeat library, and RepeatMasker v3.3.0 [61] to detect homologous repeat elements. After integrating the results of de novo and homologous predictions, Jukes-Cantor distances were calculated, and the R8s algorithm [62] was used to calculate rates of evolution from them.
Protein-coding gene prediction
A combination of ab initio, homologous, and transcript-based gene predictions was used to integrate the two genomes. The gene prediction pipeline was as follows. First, AUGUSTES v3.2.1 [63], GlimmerHMM v3.02 [64], and GeneID v1.4 [65] were used for de novo gene prediction. Second, we downloaded the non-redundant proteomes of Lottia gigantea (GCF_000327385.1), Mizuhopecten yessoensis (GCF_002113885.1), Octopus bimaculoides (GCF_001194135.1), Euprymna scolopes (GCA_004765925.1), O. minor (http://dx.doi.org/10.5524/100503), and O. vulgaris (GCA_003957725.1) for homologous gene prediction. We used TBLASTN v2.9 [66] to align the proteomes of the six relatives to the two purpleback flying squid genomes and extended 10,000 bp in both directions from the start and end of every TBLASTN hit. Then, all non-redundant transcripts of all tissues were aligned to the genome with TBLASTN and a 1000-bp extension was applied. Genewise v2.4 [67] was then used to resolve the gene structure according to the aforementioned hits. Next, we integrated results of the ab initio, homologous, and transcript-based predictions with 1:4:5 weights using EvidenceModeler [68]. Finally, for further functional annotation of these two gene sets, we scanned public databases, including Swiss-Prot, KOG, Nr, KEGG, GO, and Pfam to detect the best matches using Interproscan v5 [69].
Gene family clustering analysis
In addition to the two predicted gene sets and six Mollusca species mentioned above, we downloaded the genome of Capitella teleta (GCA_000328365.1) as an outgroup. Proteomes of these nine species were subjected to an all-vs-all BLAST search (E-value ≤ 1E−6) and then clustered by OrthoFinder [70] with default parameters. The shared gene clusters were visualized by the R package UpSetR [71]. The expanded and contracted gene families were investigated by CAFÉ v4.0.1 [72] using the result of the clustering analysis under the criterion of both family-wide and viterbi P values < 0.01.
Phylogeny and divergence time estimation
Based on the cluster analysis of the aforementioned nine species, the protein-coding sequences and corresponding codon sequences of 334 one-to-one homologous genes were picked out and aligned using MAFFT v7 [73]. The bad alignments were removed by trimAl [74]. Finally, we used RAxML v8.2.4 [75] with “-m GTRGAMMA -f a -x 271828 -N 100 -p 54321” parameter settings to construct phylogeny trees and ASTRAL [76] to infer a species tree. MCMCtree in the PAML package [77] was used to estimate divergence times in conjunction with two softbound calibration points from https://www.timetree.org, O. bimaculoides–C. teleta (585–679 MYA) and O. bimaculoides–L. gigantea (531–582 MYA).
Synteny between the genomes of two Sthenoteuthis species
To evaluate the conservation and quality of the two assemblies of Sthenoteuthis species, we used the S. oualaniensis assembly as a reference and Sthenoteuthis sp. assembly as the query in alignment analysis by LAST v942 [78] with the “-E 0.05” parameter setting. We also calculated the densities of repetitive elements and GC contents in 500-kb windows of the genomes. Finally, the results of these analyses were integrated into a circular layout by CIRCOS v0.69 [79].
Positive selection analysis
To evaluate the evolutionary pressure on Sthenoteuthis, we used the one-to-one homologous genes of the nine species listed above (see the “Gene family clustering analysis” section for details) to identify PSGs. We aligned codons of all the one-to-one homologous genes by PRANK v140603 [80] with “-codon -f = fasta”. All the gaps generated by alignments were removed by Gblocks v0.91b [81] with “-t = c”. Then we used an in-house Perl script to convert the aligned sequence to PAML format for use in PAML analysis. Finally, PAML 4.9i [77] was used to analyze the selection pressure on each gene with the ML method under the branch-site model. A species tree constructed from ASTRAL analysis was used as the input tree. The two Sthenoteuthis species were selected as foreground and the other seven species as background. The significance of the alternative model (estimated omega) against the null model (fixed omega) was assessed by likelihood ratio tests (LRTs), in which twice the log-likelihood difference (2DL) values were calculated and compared to a chi-squared distribution. Genes with P < 0.01 (with FDR correction) and carrying at least one site under positive selection with a Bayes empirical Bayes (BEB) posterior probability > 0.8, were identified as candidate PSGs.
Protein structure simulation
A homology-based approach was used to simulate structures of proteins encoded by PSGs. We first sought matches to the S. oualaniensis PGK1 protein sequence in the Protein Data Bank (PDB) database (https://www.rcsb.org/) and selected the hit with the highest score as a potential template for the simulation of the PGK1 protein structure. The corresponding positively selected sites (F85 and F149) of mouse PGK1 protein were replaced by those of Sthenoteuthis (85Y and 149Y). Then, the modified sequence was submitted to Phyre2 [82] for structure simulation. Finally, the Phyre2 result with the highest score was selected as the final structure and visualized by UCSF Chimera [83].
Transcriptomic analysis
Raw reads obtained from RNA-seq of the nine mentioned tissues of the four sampled individuals were filtered using fastp [84] with default parameters. The low-quality reads were removed by Sickle v1.33 (https://github.com/najoshi/sickle) with default parameters except for the “pe” setting. The cleaned reads were mapped to the reference genome with HISAT2 [85]. Then Trinity [86] was used to assemble these reads into transcripts. Next, we used TransDecoder [87] with default parameters to predict gene structures of the transcripts and CD-HIT [88] to remove redundant predictions. The numbers of reads and reads per kilobase of transcript per million mapped reads (RPKM) values for all genes in the 36 tissues were calculated by StringTie v2.1.4 [89] using the output of HISAT2 [85] analysis. Each gene with an RPKM value greater than 1 was considered a validly expressed gene. The Tau value of genes in all tissues was calculated using an in-house Perl script. Genes expressed in the photophore with Tau values ≥ 0.8 and higher RPKM values than in other tissues were regarded as being highly specifically expressed in the photophore. All the RNA-seq data for the 36 tissues were subjected to PCA, using normalized and logarithmically transformed RPKM values. Seaborn [90] was used to cluster and visualize clusters of the highly expressed genes in the photophore. Those genes shared only by the two Sthenoteuthis species and supported by transcripts were identified as new genes. To investigate the expression pattern of expanded genes of the SL20-1 subfamily across organs, RNA-seq reads of both species were mapped to the genomes of Sthenoteuthis sp. and subsequently assessed by RPKM values.
Code availability
The custom scripts related to this work have been deposited in BioCode at the National Genomics Data Center (NGDC), Beijing Institute of Genomics (BIG), Chinese Academy of Sciences (CAS) / China National Center for Bioinformation (CNCB), and are publicly accessible at https://ngdc.cncb.ac.cn/biocode/tools/BT007297/releases/1.0.
Data availability
The genomes and annotations of these two purpleback flying squid species, raw sequencing data, and acquired RNA-seq data have been deposited in the China National GeneBank (CNGB: CNP0001958), and are publicly accessible at https://db.cngb.org/cnsa. The raw sequencing data have also been deposited in the Genome Sequence Archive [91] at the NGDC, BIG, CAS / CNCB (GSA: CRA004867), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The genomes and annotations of these two purpleback flying squid species have also been deposited in the Genome Warehouse [92] at the NGDC, BIG, CAS / CNCB (GWH: GWHBECU00000000 for S. oualaniensis; GWHBFHL00000000 for Sthenoteuthis sp.), and are publicly accessible at https://ngdc.cncb.ac.cn/gwh.
CRediT author statement
Min Li: Conceptualization, Validation, Formal analysis, Investigation, Project administration, Writing - original draft. Baosheng Wu: Formal analysis, Data curation, Visualization, Writing - original draft. Peng Zhang: Validation, Resources, Investigation, Writing - review & editing. Ye Li: Formal analysis, Data curation, Visualization. Wenjie Xu: Formal analysis, Data curation, Visualization. Kun Wang: Writing - review & editing. Qiang Qiu: Writing - review & editing. Jun Zhang: Resources, Writing - review & editing. Jie Li: Resources, Investigation. Chi Zhang: Methodology, Software, Data curation. Jiangtao Fan: Resources, Writing - review & editing. Chenguang Feng: Conceptualization, Methodology, Validation, Project administration, Writing - original draft. Zuozhi Chen: Supervision, Project administration, Funding acquisition, Writing - review & editing. All authors have read and approved the final manuscript.
Competing interests
The authors have declared no competing interests.
Acknowledgments
This study was supported by grants from the Guangdong Major Project of Basic and Applied Basic Research, China (Grant No. 2019B030302004), the National Key R&D Program of China (Grant No. 2018YFC1406502), the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), China (Grant No. GML2019ZD0605), the Financial Fund of the Ministry of Agriculture and Rural Affairs of China (Grant No. NFZX2018), the Central Public-interest Scientific Basal Research Fund, Chinese Academy of Fishery Sciences (Grant Nos. 2019HY-XKQ03, 2020TD05, and 2021SD18), the China Postdoctoral Science Foundation (Grant No. 2021M693342), the Hubei Postdoctoral Innovation Post Project, China, the 1000 Talent Project of Shaanxi Province, China, and the Research Funds for Interdisciplinary subject of Northwestern Polytechnical University, China (Grant No. 19SH030408). We gratefully acknowledge colleagues at BGI-Shenzhen for data analyses. We also thank Prof. Shuigui Jiang at South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences for project administration and Dr. Shuai Zhang for reviewing the manuscript.
Handled by Yu Jiang
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gpb.2022.09.009.
Contributor Information
Chenguang Feng, Email: fengcg@nwpu.edu.cn.
Zuozhi Chen, Email: chenzuozhi@scsfri.ac.cn.
Supplementary material
The following are the Supplementary material to this article:
Supplementary Figure S1.
Morphology and dissection of the two Sthenoteuthis species “Typical form” and “dwarf form” of purpleback flying squids are referred to as S. oualaniensis and Sthenoteuthis sp., respectively. A. Morphology and the dorsal photophore patch of the “typical form”. B. Morphology and the corresponding position of the photophore (pseudo-photophore) of the “dwarf form”. C. Dissection of the female individual. D. Dissection of the male individual.
Supplementary Figure S2.
Distribution of GC contents versus the sequencing depth for A The horizontal and vertical coordinates indicate the GC contents and sequencing depth respectively with 10 kb as a window. The GC contents of most windows were around 20%–50%, indicating high-quality raw reads without significant contamination.
Supplementary Figure S3.
Sequencing depth distribution for S. oualaniensis The horizontal coordinate indicates the reads depth of PacBio reads. The vertical coordinate indicates the cumulative ratio of base depth. The blue line indicates the reads depth, the yellow line indicates the accumulation of all the reads depth.
Supplementary Figure S4.
GenomeScope profiles for S. oualaniensis were estimated The result from jellyfish v2.2.6 with a k-mer size of 17 was used as the input file of GenomeScope v1.0. The second peak should be the major peak and has an estimated genome size of about 5.8 Gb. This is suggestive of the high heterozygosity of the species.
Supplementary Figure S5.
Venn diagram of S. oualaniensis to show the well-aligned gene sets from a different database, including NR, InterPro, KEGG, SwissProt, and Trembl database
Supplementary Figure S6.
Venn diagram of Sthenoteuthis sp. to show the well-aligned gene sets from a different database, including NR, InterPro, KEGG, SwissProt, and Trembl database
Estimation of phylogeny and divergence times among purpleback flying squid species and their relatives The divergence time was indicated by the blue number near each node, while the 95% CI was indicated by the blue brackets and its specific value was colored in blue under the corresponding divergence time. Two softbound calibration time points had been applied: Octopus bimaculoides – Capitella teleta (585–679 MYA) and Octopus bimaculoides – Lottia gigantea (531–582 MYA).
Top 20 KEGG enriched pathway of the significantly expanded gene families for both Sthenoteuthis species
The significantly enriched GO terms of the expanded gene families for both Sthenoteuthis species
Expression patterns of coexisting gene members of the SL20-1 subfamily in S. oualaniensis and Sthenoteuthis sp. Only those expressed gene copies were shown. Gene IDs and tissue information correspond to Figure 3C. Most of the significantly expanded genes were highly expressed in the eyes.
Classification of the three major GO function annotations for the 66 PSGs of the two Sthenoteuthis lineage
The significantly enriched KEGG pathway of the positively selected genes for both Sthenoteuthis species
Sequence alignments of IscS for the two Sthenoteuthis and other seven species from Mollusca and Annelida
Positive selection sites for genes PGK1 (Figure 4B) and IscS (Figure S13) shared by the two Sthenoteuthis were also detected from the giant squid (Architeuthis dux)
References
- 1.Hoving H.J.T., Perez J.A.A., Bolstad K.S.R., Braid H.E., Evans A.B., Fuchs D., et al. The study of deep-sea cephalopods. Adv Mar Biol. 2014;67:235–359. doi: 10.1016/B978-0-12-800287-2.00003-2. [DOI] [PubMed] [Google Scholar]
- 2.Jereb P., Roper C.F.E. FAO; Rome: 2010. Myopsid and oegopsid squids. Cephalopods of the world: An annotated and illustrated catalogue of cephalopod species known to date. [Google Scholar]
- 3.Jereb P., Roper C.F.E. Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. FAO; Rome: 2005. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae) [Google Scholar]
- 4.Lindberg D.R., Pyenson N.D. Things that go bump in the night: evolutionary interactions between cephalopods and cetaceans in the tertiary. Lethaia. 2007;40:335–343. [Google Scholar]
- 5.Allain V. What do tuna eat? A tuna diet study. SPC Fish Newsl. 2005:20–22. [Google Scholar]
- 6.Rosas-Luis R., Loor-Andrade P., Carrera-Fernández M., Pincay-Espinoza J.E., Vinces-Ortega C., Chompoy-Salazar L. Cephalopod species in the diet of large pelagic fish (sharks and billfishes) in Ecuadorian waters. Fish Res. 2016;173:159–168. [Google Scholar]
- 7.Partridge J.C. Sensory ecology: giant eyes for giant predators? Curr Biol. 2012;22:R268–R270. doi: 10.1016/j.cub.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson D.E., Warrant E.J., Johnsen S., Hanlon R., Shashar N. A unique advantage for giant eyes in giant squid. Curr Biol. 2012;22:683–688. doi: 10.1016/j.cub.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Thomas K.N., Robison B.H., Johnsen S. Two eyes for two purposes: in situ evidence for asymmetric vision in the cockeyed squids Histioteuthis heteropsis and Stigmatoteuthis dofleini. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160069. doi: 10.1098/rstb.2016.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochachka P.W., Moon T.W., Mustafa T., Storey K.B. Metabolic sources of power for mantle muscle of a fast swimming squid. Comp Biochem Physiol B. 1975;52:151–158. doi: 10.1016/0305-0491(75)90131-5. [DOI] [PubMed] [Google Scholar]
- 11.Shulman G.E., Chesalin M.V., Abolmasova G.I., Yuneva T.V., Kideys A. Metabolic strategy in pelagic squid of genus Sthenoteuthis (Ommastrephidae) as the basis of high abundance and productivity. An overview of the Soviet investigations. Bull Mar Sci. 2002;71:815–836. [Google Scholar]
- 12.Doubleday Z.A., Prowse T.A., Arkhipkin A., Pierce G.J., Semmens J., Steer M., et al. Global proliferation of cephalopods. Curr Biol. 2016;26:R406–R407. doi: 10.1016/j.cub.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Young Richard E., Roper Clyde F.E. Bioluminescent countershading in midwater animals: evidence from living squid. Science. 1976;191:1046–1048. doi: 10.1126/science.1251214. [DOI] [PubMed] [Google Scholar]
- 14.Johnsen S., Balser E.J., Fisher E.C., Widder E.A. Bioluminescence in the deep-sea cirrate octopod Stauroteuthis syrtensis Verrill (Mollusca: Cephalopoda) Biol Bull. 1999;197:26–39. doi: 10.2307/1542994. [DOI] [PubMed] [Google Scholar]
- 15.Robison B.H., Young R.E. Bioluminescence in pelagic octopods. Pac Sci. 1981;35:39–44. [Google Scholar]
- 16.York C.A., Bartol I.K., Krueger P.S., Thompson J.T. Squids use multiple escape jet patterns throughout ontogeny. Biol Open. 2020;9:bio054585. doi: 10.1242/bio.054585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanlon R.T., Messenger J.B. Cambridge University Press; Cambridge: 2018. Cephalopod behaviour. [Google Scholar]
- 18.York C.A., Bartol I.K. Anti-predator behavior of squid throughout ontogeny. J Exp Mar Biol Ecol. 2016;480:26–35. [Google Scholar]
- 19.Muramatsu K., Yamamoto J., Abe T., Sekiguchi K., Hoshi N., Sakurai Y. Oceanic squid do fly. Mar Biol. 2013;160:1171–1175. [Google Scholar]
- 20.Hou T.G., Yang X.B., Wang T.M., Liang J.H., Li S.W., Fan Y.B. Locomotor transition: how squid jet from water to air. Bioinspir Biomim. 2020;15:036014. doi: 10.1088/1748-3190/ab784b. [DOI] [PubMed] [Google Scholar]
- 21.Vogel S. Flow-assisted mantle cavity refilling in jetting squid. Biol Bull. 1987;172:61–68. [Google Scholar]
- 22.O'Dor R., Stewart J., Gilly W., Payne J., Borges T.C., Thys T. Squid rocket science: how squid launch into air. Deep-Sea Res Part II-Topical Stud Oceanogr. 2013;95:113–118. [Google Scholar]
- 23.Zuyev G., Nigmatullin C., Chesalin M., Nesis K. Main results of long-term worldwide studies on tropical nektonic oceanic squid genus Sthenoteuthis: an overview of the soviet investigations. Bull Mar Sci. 2002;71:1019–1060. [Google Scholar]
- 24.Young R.E. A brief review of the biology of the oceanic squid, Symplectoteuthis oualaniensis (Lesson) Comp Biochem Physiol B. 1975;52:141–143. doi: 10.1016/0305-0491(75)90129-7. [DOI] [PubMed] [Google Scholar]
- 25.Nesis K.N. In: Recent advances in fisheries biology. Okutani T., O'Dor R.K., Kubodera T., editors. Tokai University Press; Tokyo: 1993. Population structure of oceanic ommastrephids, with particular reference to Sthenoteuthis oualaniensis: a review; pp. 375–383. [Google Scholar]
- 26.Staaf D.J., Ruiz-Cooley R.I., Elliger C., Lebaric Z., Campos B., Markaida U., et al. Ommastrephid squids Sthenoteuthis oualaniensis and Dosidicus gigas in the eastern Pacific show convergent biogeographic breaks but contrasting population structures. Mar Ecol Prog Ser. 2010;418:165–178. [Google Scholar]
- 27.Simao F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 28.Li F., Bian L., Ge J., Han F., Liu Z., Li X., et al. Chromosome-level genome assembly of the East Asian common octopus (Octopus sinensis) using PacBio sequencing and Hi-C technology. Mol Ecol Resour. 2020;20:1572–1582. doi: 10.1111/1755-0998.13216. [DOI] [PubMed] [Google Scholar]
- 29.da Fonseca R.R., Couto A., Machado A.M., Brejova B., Albertin C.B., Silva F., et al. A draft genome sequence of the elusive giant squid, Architeuthis dux. Gigascience. 2020;9:giz152. doi: 10.1093/gigascience/giz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chembian J., Mathew S. Population structure of the purpleback squid Sthenoteuthis oualaniensis (Lesson, 1830) along the south-west coast of India. Indian Journal of Fisheries. 2014;61:20–28. [Google Scholar]
- 31.Zhao C., Kang B., He X., Yan Y. Morphological, molecular, and ecological evidence in population determination and fishery management of purpleback flying squid Sthenoteuthis oualaniensis in the South China Sea. Taiwania. 2021;66:241–250. [Google Scholar]
- 32.de Jong W.W., Hendriks W., Mulders J.W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989;14:365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- 33.Tan W.H., Cheng S.C., Liu Y.T., Wu C.G., Lin M.H., Chen C.C., et al. Structure of a highly active cephalopod S-crystallin mutant: new molecular evidence for evolution from an active enzyme into lens-refractive protein. Sci Rep. 2016;6:31176. doi: 10.1038/srep31176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madl T. Patchy proteins form a perfect lens. Science. 2017;357:546–547. doi: 10.1126/science.aao1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee A., Cerna Sanchez J.A., Yamauchi T., Taupin V., Couvrette J., Gorodetsky A.A. Cephalopod-inspired optical engineering of human cells. Nat Commun. 2020;11:2708. doi: 10.1038/s41467-020-16151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai J., Townsend J.P., Dodson T.C., Heiney P.A., Sweeney A.M. Eye patches: protein assembly of index-gradient squid lenses. Science. 2017;357:564–569. doi: 10.1126/science.aal2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bro-Jorgensen J. Evolution of sprint speed in African savannah herbivores in relation to predation. Evolution. 2013;67:3371–3376. doi: 10.1111/evo.12233. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein B.E., Hol W.G. Crystal structures of substrates and products bound to the phosphoglycerate kinase active site reveal the catalytic mechanism. Biochemistry. 1998;37:4429–4436. doi: 10.1021/bi9724117. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Jiang Y., Meisenhelder J., Yang W., Hawke D.H., Zheng Y., et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–719. doi: 10.1016/j.molcel.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urbina H.D., Silberg J.J., Hoff K.G., Vickery L.E. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J Biol Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- 41.Smid O., Horakova E., Vilimova V., Hrdy I., Cammack R., Horvath A., et al. Knock-downs of iron-sulfur cluster assembly proteins IscS and IscU down-regulate the active mitochondrion of procyclic Trypanosoma brucei. J Biol Chem. 2006;281:28679–28686. doi: 10.1074/jbc.M513781200. [DOI] [PubMed] [Google Scholar]
- 42.Angerer H., Radermacher M., Mankowska M., Steger M., Zwicker K., Heide H., et al. The LYR protein subunit NB4M/NDUFA6 of mitochondrial complex I anchors an acyl carrier protein and is essential for catalytic activity. Proc Natl Acad Sci U S A. 2014;111:5207–5212. doi: 10.1073/pnas.1322438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi X., Zhang Y., Chen R., Gong Y., Zhang M., Guan R., et al. ndufa7 plays a critical role in cardiac hypertrophy. J Cell Mol Med. 2020;24:13151–13162. doi: 10.1111/jcmm.15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J., Vinothkumar K.R., Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354–358. doi: 10.1038/nature19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munk O. The escal photophore of ceratioids (Pisces; Ceratioidei) – a review of structure and function. Acta Zoologica. 1999;80:265–284. [Google Scholar]
- 46.Belcaid M., Casaburi G., McAnulty Sarah J., Schmidbaur H., Suria Andrea M., Moriano-Gutierrez S., et al. Symbiotic organs shaped by distinct modes of genome evolution in cephalopods. Proc Natl Acad Sci U S A. 2019;116:3030–3035. doi: 10.1073/pnas.1817322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pankey M.S., Minin V.N., Imholte G.C., Suchard M.A., Oakley T.H. Predictable transcriptome evolution in the convergent and complex bioluminescent organs of squid. Proc Natl Acad Sci U S A. 2014;111:E4736–E4742. doi: 10.1073/pnas.1416574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philips K.B., Kurtoglu M., Leung H.J., Liu H., Gao N., Lehrman M.A., et al. Increased sensitivity to glucose starvation correlates with downregulation of glycogen phosphorylase isoform PYGB in tumor cell lines resistant to 2-deoxy-D-glucose. Cancer Chemother Pharmacol. 2014;73:349–361. doi: 10.1007/s00280-013-2358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burwinkel B., Hu B., Schroers A., Clemens P.R., Moses S.W., Shin Y.S., et al. Muscle glycogenosis with low phosphorylase kinase activity: mutations in PHKA1, PHKG1 or six other candidate genes explain only a minority of cases. Eur J Hum Genet. 2003;11:516–526. doi: 10.1038/sj.ejhg.5200996. [DOI] [PubMed] [Google Scholar]
- 50.Muller A., Janssen F., Grieshaber M.K. Putative reaction mechanism of heterologously expressed octopine dehydrogenase from the great scallop, Pecten maximus (L) FEBS J. 2007;274:6329–6339. doi: 10.1111/j.1742-4658.2007.06151.x. [DOI] [PubMed] [Google Scholar]
- 51.Philipp E.E., Wessels W., Gruber H., Strahl J., Wagner A.E., Ernst I.M., et al. Gene expression and physiological changes of different populations of the long-lived bivalve Arctica islandica under low oxygen conditions. PLoS One. 2012;7:e44621. doi: 10.1371/journal.pone.0044621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo R., Liu B., Xie Y., Li Z., Huang W., Yuan J., et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruan J., Li H. Fast and accurate long-read assembly with wtdbg2. Nat Methods. 2020;17:155–158. doi: 10.1038/s41592-019-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker B.J., Abeel T., Shea T., Priest M., Abouelliel A., Sakthikumar S., et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudchenko O., Batra S.S., Omer A.D., Nyquist S.K., Hoeger M., Durand N.C., et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science. 2017;356:92–95. doi: 10.1126/science.aal3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durand N.C., Shamim M.S., Machol I., Rao S.S., Huntley M.H., Lander E.S., et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 2016;3:95–98. doi: 10.1016/j.cels.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durand N.C., Robinson J.T., Shamim M.S., Machol I., Mesirov J.P., Lander E.S., et al. Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst. 2016;3:99–101. doi: 10.1016/j.cels.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saha S., Bridges S., Magbanua Z.V., Peterson D.G. Empirical comparison of ab initio repeat finding programs. Nucleic Acids Res. 2008;36:2284–2294. doi: 10.1093/nar/gkn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarailo-Graovac M., Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics. 2009;Chapter 4:4:10.1–14. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- 62.Sanderson M.J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics. 2003;19:301–302. doi: 10.1093/bioinformatics/19.2.301. [DOI] [PubMed] [Google Scholar]
- 63.Stanke M., Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33:W465–W467. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen J.E., Majoros W.H., Pertea M., Salzberg S.L. JIGSAW, GeneZilla, and GlimmerHMM: puzzling out the features of human genes in the ENCODE regions. Genome Biol. 2006;7:S9.1–13. doi: 10.1186/gb-2006-7-s1-s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alioto T., Blanco E., Parra G., Guigo R. Using geneid to identify genes. Curr Protoc Bioinformatics. 2018;64:e56. doi: 10.1002/cpbi.56. [DOI] [PubMed] [Google Scholar]
- 66.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birney E., Clamp M., Durbin R. GeneWise and genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haas B.J., Salzberg S.L., Zhu W., Pertea M., Allen J.E., Orvis J., et al. Automated eukaryotic gene structure annotation using evidencemodeler and the program to assemble spliced alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones P., Binns D., Chang H.Y., Fraser M., Li W., McAnulla C., et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emms D.M., Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conway J.R., Lex A., Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33:2938–2940. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Bie T., Cristianini N., Demuth J.P., Hahn M.W. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22:1269–1271. doi: 10.1093/bioinformatics/btl097. [DOI] [PubMed] [Google Scholar]
- 73.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–880. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirarab S., Reaz R., Bayzid M.S., Zimmermann T., Swenson M.S., Warnow T. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics. 2014;30:i541–i548. doi: 10.1093/bioinformatics/btu462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 78.Kielbasa S.M., Wan R., Sato K., Horton P., Frith M.C. Adaptive seeds tame genomic sequence comparison. Genome Res. 2011;21:487–493. doi: 10.1101/gr.113985.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loytynoja A. Phylogeny-aware alignment with PRANK. Methods Mol Biol. 2014;1079:155–170. doi: 10.1007/978-1-62703-646-7_10. [DOI] [PubMed] [Google Scholar]
- 81.Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 82.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 84.Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haas B.J., Papanicolaou A., Yassour M., Grabherr M., Blood P.D., Bowden J., et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W., Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 89.Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waskom M.L. Seaborn: statistical data visualization. J Open Source Softw. 2021;6:3021. [Google Scholar]
- 91.Chen T., Chen X., Zhang S., Zhu J., Tang B., Wang A., et al. The Genome Sequence Archive Family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics. 2021;19:578–583. doi: 10.1016/j.gpb.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen M., Ma Y., Wu S., Zheng X., Kang H., Sang J., et al. Genome Warehouse: a public repository housing genome-scale data. Genomics Proteomics Bioinformatics. 2021;19:584–589. doi: 10.1016/j.gpb.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimation of phylogeny and divergence times among purpleback flying squid species and their relatives The divergence time was indicated by the blue number near each node, while the 95% CI was indicated by the blue brackets and its specific value was colored in blue under the corresponding divergence time. Two softbound calibration time points had been applied: Octopus bimaculoides – Capitella teleta (585–679 MYA) and Octopus bimaculoides – Lottia gigantea (531–582 MYA).
Top 20 KEGG enriched pathway of the significantly expanded gene families for both Sthenoteuthis species
The significantly enriched GO terms of the expanded gene families for both Sthenoteuthis species
Expression patterns of coexisting gene members of the SL20-1 subfamily in S. oualaniensis and Sthenoteuthis sp. Only those expressed gene copies were shown. Gene IDs and tissue information correspond to Figure 3C. Most of the significantly expanded genes were highly expressed in the eyes.
Classification of the three major GO function annotations for the 66 PSGs of the two Sthenoteuthis lineage
The significantly enriched KEGG pathway of the positively selected genes for both Sthenoteuthis species
Sequence alignments of IscS for the two Sthenoteuthis and other seven species from Mollusca and Annelida
Positive selection sites for genes PGK1 (Figure 4B) and IscS (Figure S13) shared by the two Sthenoteuthis were also detected from the giant squid (Architeuthis dux)
Data Availability Statement
The genomes and annotations of these two purpleback flying squid species, raw sequencing data, and acquired RNA-seq data have been deposited in the China National GeneBank (CNGB: CNP0001958), and are publicly accessible at https://db.cngb.org/cnsa. The raw sequencing data have also been deposited in the Genome Sequence Archive [91] at the NGDC, BIG, CAS / CNCB (GSA: CRA004867), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The genomes and annotations of these two purpleback flying squid species have also been deposited in the Genome Warehouse [92] at the NGDC, BIG, CAS / CNCB (GWH: GWHBECU00000000 for S. oualaniensis; GWHBFHL00000000 for Sthenoteuthis sp.), and are publicly accessible at https://ngdc.cncb.ac.cn/gwh.