Summary
Background
Functionally impaired variants of COQ2, encoding an enzyme in biosynthesis of coenzyme Q10 (CoQ10), were found in familial multiple system atrophy (MSA) and V393A in COQ2 is associated with sporadic MSA. Furthermore, reduced levels of CoQ10 have been demonstrated in MSA patients.
Methods
This study was a multicentre, randomised, double-blinded, placebo-controlled phase 2 trial. Patients with MSA were randomly assigned (1:1) to either ubiquinol (1500 mg/day) or placebo. The primary efficacy outcome was the change in the unified multiple system atrophy rating scale (UMSARS) part 2 at 48 weeks. Efficacy was assessed in all patients who completed at least one efficacy assessment (full analysis set). Safety analyses included patients who completed at least one dose of investigational drug. This trial is registered with UMIN-CTR (UMIN000031771), where the drug name of MSA-01 was used to designate ubiquinol.
Findings
Between June 26, 2018, and May 27, 2019, 139 patients were enrolled and randomly assigned to the ubiquinol group (n = 69) or the placebo group (n = 70). A total of 131 patients were included in the full analysis set (63 in the ubiquinol group; 68 in the placebo group). This study met the primary efficacy outcome (least square mean difference in UMSARS part 2 score (−1.7 [95% CI, −3.2 to −0.2]; P = 0.023)). The ubiquinol group also showed better secondary efficacy outcomes (Barthel index, Scale for the Assessment and Rating of Ataxia, and time required to walk 10 m). Rates of adverse events potentially related to the investigational drug were comparable between ubiquinol (n = 15 [23.8%]) and placebo (n = 21 [30.9%]).
Interpretation
High-dose ubiquinol was well-tolerated and led to a significantly smaller decline of UMSARS part 2 score compared with placebo.
Funding
Japan Agency for Medical Research and Development.
Keywords: Multiple system atrophy, COQ2, Ubiquinol, Clinical trial, Disease-modifying therapy
Research in context.
Evidence before this study
Multiple system atrophy (MSA) is a progressive neurodegenerative disease characterized by autonomic failure with various combinations of parkinsonism, cerebellar ataxia, and pyramidal dysfunction. Biallelic functionally impaired variants of COQ2, encoding an enzyme in biosynthesis of coenzyme Q10, were found in familial MSA and V393A in COQ2 and have been confirmed to be associated with sporadic MSA by meta-analysis. Furthermore, reduced levels of coenzyme Q10 have been demonstrated not only in patients with MSA carrying V393A but also in those not carrying V393A. These findings raised a possibility that coenzyme Q10 supplementation may slow down the progression of multiple system atrophy.
We searched PubMed with the terms “multiple system atrophy” OR “Shy-Drager syndrome” OR “olivo-ponto-cerebellar atrophy” OR “striato-nigral degeneration” AND filtered the article type with “Randomized Controlled Trial” for articles published in English up to March 31, 2022. We identified 12 randomised, placebo-controlled trials of putative disease-modifying therapies—including fluoxetine, repetitive transcranial magnetic stimulation, inosine 5′-monophosphate, epigallocatechin gallate, rasagiline, rifampicin, lithium, mesenchymal stem cells, minocycline, riluzole, paroxetine, and amantadine—in patients with MSA. Seven studies followed up participants for 1 year or longer, and no beneficial clinical effects have been established for any treatment.
Added value of this study
At 48 weeks, the ubiquinol group showed a significantly smaller decline of the Unified Multiple System Atrophy Rating Scale (UMSARS) part 2 score (motor examination scores) compared with placebo. The least square mean group difference (ubiquinol−placebo) in the UMSARS part 2 was −1.7, exceeding the previously reported minimum clinically important difference of 1.5 in patients with MSA. The ubiquinol group also showed better secondary efficacy outcomes, as assessed by Barthel index, Scale for the Assessment and Rating of Ataxia, and time required to walk 10 m.
Implications of all the available evidence
Orally administered ubiquinol have clinical benefits in patients with MSA. The clinical efficacy of ubiquinol is consistent with the previous findings that decreased CoQ10 levels were observed not only in patients with MSA carrying the V393A variant in COQ2 but also in those not carrying the variant.
Introduction
Multiple system atrophy (MSA) is a progressive neurodegenerative disease characterized by autonomic failure with various combinations of parkinsonism, cerebellar ataxia, and pyramidal dysfunction.1,2 MSA has two subtypes: MSA-cerebellar (C) and MSA-parkinsonism (P).1,2 Its estimated prevalence is 9.3 per 100,000, according to the national registry in Japan (Japan Intractable Diseases Information Center, https://www.nanbyou.or.jp/) and the median intervals from onset to confinement to a wheelchair and death are 5 and 9 years, respectively.3 Glial cytoplasmic inclusions (GCIs), composed of misfolded α-synuclein, have been demonstrated as the neuropathological hallmark,4 and it has been proposed that misfolded α-synuclein may spread in a prion-like manner to neighboring cells and functionally connected brain regions.5 The etiologies underlying the formation of GCIs, however, remain to be elucidated.
Although MSA has been considered to be a nongenetic disorder,6 we have identified multiplex MSA families7 and demonstrated biallelic variants in COQ2 in two multiple families including two autopsy-confirmed MSA cases.8 We further demonstrated a significant association of the V393A variant in COQ2 with sporadic MSA in Japan,8 which has subsequently been confirmed in East Asian populations by meta-analysis.9 Of note, V393A is present in East Asian populations with minor allele frequencies of 0.93–2.12%,9 whereas it is virtually absent in European descent populations. COQ2 encodes an enzyme involved in the biosynthesis of coenzyme Q10 (CoQ10) and the level of CoQ10 in the autopsied cerebellum of a patient carrying biallelic variants of COQ2 was substantially decreased.8 Furthermore, decreases in CoQ10 levels have also been observed in the blood,10, 11, 12 cerebrospinal fluid,13 fibroblasts,14 and cerebellar tissues15,16 of MSA patients who do not carry the V393A variant, indicating that decreased CoQ10 levels commonly occur in MSA patients. Intriguingly, decreased COQ2 mRNA expression levels have recently been demonstrated in the cerebellum, motor cortex, and putamen of autopsied MSA brains,17 which may underlie the decreased CoQ10 levels in MSA patients.
CoQ10 plays an essential role in mitochondrial electron transport. A decreased oxygen consumption rate was found in the yeast coq2 null strain harboring mutant human COQ2 cDNAs18 and in induced pluripotent stem cell (iPSC)-derived neurons from an MSA patient carrying biallelic COQ2 variants.19 Reductions in complex II/III activities and ATP levels in the disease-affected regions of MSA brain were reported.17,20 Furthermore, restoration of brain oxygen consumption rate was observed with administration of 1200 mg ubiquinol (the reduced form of CoQ10) in a patient at the advanced stage of familial MSA who had biallelic variants in COQ2.21
These findings raised a possibility that CoQ10 supplementation may slow down the progression of MSA. There are two redox states of CoQ10: oxidised form (ubiquinone) and reduced form (ubiquinol). Irrespective of the redox state of CoQ10 (ubiquinol or ubiquinone), when administered orally, administered CoQ10 is mostly present in the body as ubiquinol. Furthermore, the bioavailability of ubiquinol is known to be higher than that of ubiquinone.22 These are the rationale for selection of ubiquinol as the investigational drug. Various supplements containing ubiquinone or ubiquinol are commercially available, although the contents of CoQ10 (ubiquinone or ubiquinol) in supplements are far lower than the amount of ubiquinol employed in this clinical trial. We initially conducted a phase 1 trial (UMIN Clinical Trials Registry number, UMIN000016695), which confirmed that high-dose ubiquinol supplementation (900, 1200, or 1500 mg) was safe and tolerated by healthy participants and that the highest plasma ubiquinol levels were observed in the group with 1500 mg/day.23 Increased levels of total CoQ10 (sum of levels of ubiquinol and ubiquinone) were observed in the cerebrospinal fluid. On the basis of these findings, we designed a placebo-controlled phase 2 trial for MSA patients with a high dose of ubiquinol (1500 mg/day). Here, we report the results from the placebo-controlled, randomised phase 2 trial, which aimed to evaluate the efficacy and safety of a high dose of ubiquinol for the treatment of patients with MSA in this.
Methods
Study design
The trial was a multicenter, randomised, double-blind, placebo-controlled phase 2 trial to assess the safety and efficacy of high-dose ubiquinol in MSA patients. The study consists of a 4-week screening-observation period to collect the demographic information and to evaluate the eligibility of participants, a 48-week treatment period to assess the efficacy outcomes and the safety, and a 4-week follow-up period to confirm safety after discontinuation of the investigational drug and to evaluate the clinical status of the participants. The trial protocol was developed by the research group members from 13 participating institutions. Funding was provided by the Japan Agency for Medical Research and Development (AMED). The trial was approved by the participating institutional review boards and was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent. Details of the study design are shown in the Study Protocol of the Supplementary Appendix.
Participants
Patients were recruited from among patients registered in the Japan MSA Registry (https://msajp.org/) who met all the following criteria: 1) probable or possible MSA1; 2) able to walk 10 m on their own or with walking aids; 3) had completed nucleotide sequence analysis of COQ2, and 4) 30–79 years of age. For facilitating enrollment of a sufficient number of MSA patients with COQ2 V393A variant, we initially established the Japan MSA Registry in 2016 prior to this clinical trial. MSA patients fulfilling possible or probable MSA were registered in this registry with prior COQ2 genotyping. Recruitment of MSA patients was accomplished based on the information including disease subtype, age at onset, sex, and the COQ2 genotypes that had been registered in the MSA registry. The exclusion criteria were 1) taking ubiquinone, ubiquinol, idebenone (a synthetic analog of CoQ10), or statins (because they were known to alter plasma CoQ10 levels) at the beginning of the screening-observation period; 2) severe liver disease (Child–Pugh classification B or C); 3) women who are pregnant, lactating, or of childbearing potential (a woman must be 2 years postmenopausal or have undergone sterilization to be considered not likely to become pregnant); 4) prescribed with any other investigational drug within 3 months prior to this trial; and 5) judged inappropriate for the trial by the investigators. We did not collect information on whether the patient was probable or possible at the time of entry into this trial.
The second inclusion criteria before the administration of the investigational drug after the screening-observation period were as follows: 1) the primary efficacy outcome measure [the unified multiple system atrophy rating scale (UMSARS) part 2 score]24 did not change substantially (within 3 points) during the screening-observation period; 2) did not receive concomitantly prohibited drugs during the screening-observation period; 3) no safety issues were identified during the screening-observation period; 4) judged inappropriate for the trial by the investigators (pp. 6–9, Supplementary Appendix).
Randomisation and masking
Patients were randomly assigned in a 1:1 ratio to receive ubiquinol or placebo. Randomisation was accomplished by the randomisation centre (DOT WORLD Co., Ltd., Tokyo, Japan). Randomisation was conducted using a stratified blocked randomisation method according to 1) the presence or absence of V393A in COQ2; 2) the baseline UMSARS part 2 score (≥19 or <19); and 3) the disease subtype (MSA-C or MSA-P).
Treatment
Ubiquinol was purchased from Kaneka Corporation. The active drug (ubiquinol) is a light-yellow granule preparation containing 300 mg of ubiquinol in a package. The active drug and matching placebo were manufactured by Bushu Pharmaceuticals Ltd. As additives, it contains gum arabic powder, dextrin, ascorbic acid, and light anhydrous silicic acid. The assigned investigatory drug was administered once daily after breakfast. The dosing schedule was titrated upward every 2 weeks to 300, 600, 900, 1200, and 1500 mg/day (pp. 3–6, Supplementary Appendix). The duration of treatment was set to 48 weeks.
Concomitant drugs (tartilelin, protilerin tartrate hydrate, drugs indicated for Parkinson disease, and drugs for the treatment of orthostatic hypotension) were not allowed to be started or changed from the baseline visit until the week 24 visit.
Primary and secondary efficacy outcomes
The primary efficacy outcome was the change from the baseline in the score on the UMSARS part 2 (range, 0–56, with higher scores indicating more severe symptoms)24 at 48 weeks. The UMSARS that had been translated into Japanese and validated for reliability and validity was used.25 Secondary efficacy outcomes included the changes from the baseline in the score of UMSARS part 1,24 Barthel index,26 and Scale for the Assessment and Rating of Ataxia (SARA),27 time required to walk 10 m, and the ubiquinol levels in plasma at 48 weeks (pp. 10–11, Supplementary Appendix). The UMSARS part 1 rates the functional status of daily living through patient and caregiver interviews, while the UMSARS part 2 rates clinician-assessed motor examination scale. All efficacy outcomes were assessed at the baseline and 12, 24, 36, 48, and 52 weeks. Adverse events were coded based on the Medical Dictionary for Regulatory Activities, version 23.0.
Laboratory assessment
Plasma samples were obtained and stored at −80 °C in each participating institution until measurement. Quantitation of ubiquinol and ubiquinone levels were conducted by Kaneka Techno Research Co., Japan. Plasma ubiquinol and ubiquinone were extracted with 2-propanol and measured by liquid chromatography–tandem-mass spectrometry using Nexera (Shimadzu, Kyoto, Japan) and TripleQuad5500 (AB SCIEX, Framingham, MA). Total CoQ10 levels were calculated as a sum of ubiquinol and ubiquinone levels. The genotypes of COQ2 were determined as previously described8 by individuals who did not perform patient assessment (H.N. and M.T.).
Oxygen PET and cerebrospinal fluid testing were offered as optional tests to participants at one site (The University of Tokyo Hospital), but none of the participants consented to participate in these optional tests.
Statistical analysis
By considering the mean 1-year changes in UMSARS part 2 score in the placebo group in the previous trials,28, 29, 30 we assumed the mean ± standard deviation of UMSARS part 2 score at the baseline and 48 weeks to be 20.0 ± 6.0 and 25.0 ± 6.0 for the placebo group and 20.0 ± 6.0 and 22.0 ± 6.0 for the ubiquinol group, respectively, with the correlation of 0.6 between the baseline and 48 weeks. On the basis of these values, we calculated that 104 participants would be required under two-sided alpha of 0.05 and 80% power. The planned sample size was set to be 120, assuming a 15% drop-out rate. Given the relatively low frequency of Japanese MSA patients carrying the V393A variant in COQ2 (9.1%),8 we set the number of MSA patients carrying V393A to be 20 as a maximum achievable number.
The primary population used for the efficacy analysis was the full analysis set (FAS), including all patients who had undergone randomisation and had completed efficacy assessment at least once after the baseline. The per protocol set (PPS) is defined as a population, where any of the following cases were excluded from the FAS: 1. Cases that did not meet the inclusion criteria, 2. Cases that met the exclusion criteria, 3. Cases that used prohibited concomitant drugs, 4. Cases with less than 80% medication compliance during the trial period, 5. Cases that had major protocol deviations, 6. Cases that did not start 1500 mg/day by Week 12, and 7. Cases in which plasma ubiquinol levels exceeded 1.98 μg/mL at the baseline. All patients who participated in the study and were treated with at least one dose of the investigational drug will be included in the safety analysis [safety analysis set (SAS)].
The primary efficacy outcome was analyzed using a restricted maximum likelihood-based repeated measures approach in combination with the Newton–Raphson algorithm. Analyses included the fixed, categorical effects of treatment, visit, and treatment-by-visit interaction, as well as the fixed covariates of the baseline UMSARS part 2 score, presence of the V393A variant in COQ2, and disease subtype (MSA-C or MSA-P). An autoregression (1) covariance structure with a random effect structure was used to model the within-patient errors. If this model does not converge, then the random effect was not included within-patient errors and an unstructured-covariance structure was used. Significance tests were based on the least squares (LS) mean using a two-sided alpha of 0.05. The same model was used for the secondary efficacy outcomes, including UMSARS part 1, Barthel index, SARA, and time required to walk 10 m. Sensitivity analyses were conducted to assess the impact of missing data due to drop-out and withdrawals by the last observation carried forward method, the worst observation carried forward method for both groups, imputation of the worst case for those in the ubiquinol group and the best case for the placebo group, multiple imputation method by fully conditional specification, and weighted generalized estimation equation method using observation-specific weighted approach (pp. 18–21, Supplementary Appendix).
The predefined subgroup analyses stratified by disease subtype (MSA-C or MSA-P), V393A variants in COQ2, baseline UMSARS part 2 score, gender, age, and duration of disease, and the secondary efficacy outcome analyses (UMSARS part 1, Barthel index, SARA, and time required to walk 10 m) were performed similarly to the primary analysis. Multiple comparisons for several pre-specified subgroups were not considered. For each subgroup, the model used in the primary efficacy outcome analysis was added to the treatment group × each subgroup to calculate the p-value of the interaction. All the statistical analyses were performed with SAS software, version 9.4 (SAS Institute). This study is registered with UMIN Clinical Trials Registry (UMIN-CTR, https://www.umin.ac.jp/ctr/), UMIN000031771.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patients
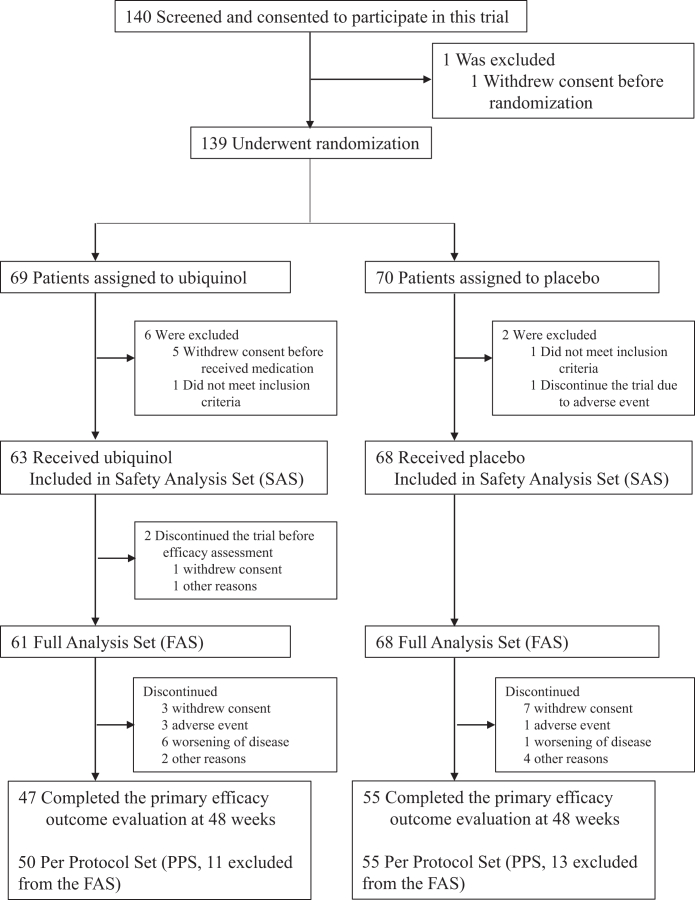
Between June 26, 2018, and May 27, 2019, a total of 140 patients provided written informed consent, among whom 139 were randomly assigned to the ubiquinol group (n = 69) or the placebo group (n = 70). After the screening-observation period, 131 patients were prescribed with ubiquinol (n = 63) or placebo (n = 68) (Fig. 1). Two in the ubiquinol group discontinued the trial before the first efficacy assessment, resulting in the FAS of 61 and 68 patients in the ubiquinol and placebo groups, respectively. Since the COVID-19 pandemic occurred during the clinical trial period, several patients withdrew their consent because they felt it was difficult to visit their medical institutions. Forty-seven patients in the ubiquinol group and 55 patients in the placebo group completed the 48-week evaluation. Baseline characteristics in the FAS were similar in the two groups (Table 1). All the carriers of V393A in COQ2 were heterozygous.
Fig. 1.
Clinical trial flow chart. Eight patients underwent randomisation but discontinued the trial before receiving medication: six withdrew their consent and two did not meet the inclusion criteria of the primary endpoint of no more than a 3-point change in UMSARS part 2 score during the screening-observation period as defined in the second inclusion criteria before the administration of the investigational drug after the screening-observation period. Two patients underwent randomisation but withdrew their consent before the first efficacy assessment and were not included in the full analysis set. No patients were lost to follow-up in the full analysis set. The per protocol set (PPS) is defined as a population, where any of the following cases were excluded from the FAS: 1. Cases that did not meet the inclusion criteria, 2. Cases that met the exclusion criteria, 3. Cases that used prohibited concomitant drugs, 4. Cases with less than 80% medication compliance during the trial period, 5. Cases that had major protocol deviations, 6. Cases that did not start 1500 mg/day by Week 12, and 7. Cases in which plasma ubiquinol levels exceeded 1.98 μg/mL at Week 0.
Table 1.
Demographic and clinical characteristics of patients at baseline.
| Ubiquinol N = 61 | Placebo N = 68 | |
|---|---|---|
| Age | ||
| Mean (SD) | 59.8 (8.5) | 62.0 (8.3) |
| Median (min, max) | 61.0 (43, 79) | 61.5 (42, 78) |
| Sex | ||
| Number (male/female) | 44/17 (72.1%/27.9%) | 38/30 (55.9%/44.1%) |
| UMSARS part 2 score | ||
| Mean (SD) | 23.2 (8.7) | 23.3 (7.9) |
| V393A in COQ2 | ||
| Number of carriers | 10 (16.4%) | 11 (16.2%) |
| Subtype | ||
| Number of MSA-C | 48 (78.7%) | 51 (75.0%) |
| Number of MSA-P | 13 (21.3%) | 17 (25.0%) |
| Body height (cm) | ||
| Mean (SD) | 163.4 (9.2) | 161.3 (9.8) |
| Body weight (kg) | ||
| Mean (SD) | 65.5 (13.1) | 61.6 (12.1) |
| Body mass index | ||
| Mean (SD) | 24.5 (4.2) | 23.6 (3.2) |
| Past medical history | ||
| Present | 30 (49.2%) | 40 (58.8%) |
| Absent | 31 (50.8%) | 28 (41.2%) |
| Comorbidity | ||
| Present | 59 (96.7%) | 61 (89.7%) |
| Absent | 2 (3.3%) | 7 (10.3%) |
| Disease duration (year) | ||
| Mean (SD) | 3.7 (1.8) | 3.8 (1.7) |
| Median (min, max) | 3.0 (1, 10) | 3.0 (1, 10) |
Among the FAS, 11 patients in the ubiquinol group and 13 patients in the placebo group were excluded from the PPS due to protocol deviations. The reasons including overlapping reasons were as follows: adherence rate of the investigational drug was less than 80% (1 in the ubiquinol group and 1 in the placebo group), the dose of 1500 mg/day of the investigational drug was not achieved (2 in the placebo group), and the plasma ubiquinol levels was higher than 1.98 μg/mL at Week 0 (5 in the ubiquinol group and 2 in the placebo group). Four in the ubiquinol group and nine in the placebo group initiated the concomitant drug (tartilelin, protilerin tartrate hydrate, drugs indicated for Parkinson disease, and drugs for the treatment of orthostatic hypotension) or changed the dose from the start of the screening-observation period to Week 24 of the treatment period. One in the ubiquinol group initiated a new rehabilitation program during the study period. At week 0, 26 of the 61 patients (42.6%) in the ubiquinol group and 26 of the 68 patients (38.2%) in the placebo group were taking antiparkinsonian drugs with mean levodopa equivalent daily doses (LEDD)31 of 347.0 mg/day and 360.5 mg/day in the ubiquinol and placebo groups, respectively, and at week 48, 20 of the 26 patients (76.9%) in the ubiquinol group and 19 of the 26 patients (73.1%) in the placebo group did not change the doses of their antiparkinsonian drugs throughout the 48-week treatment period. At week 48, 25 of the 47 patients (53.2%) in the ubiquinol group and 31 of the 55 patients (56.4%) in the placebo group were taking antiparkinsonian drugs with mean LEDD of 278.5 mg/day and 408.9 mg/day in the ubiquinol and placebo groups, respectively.
Primary efficacy outcome
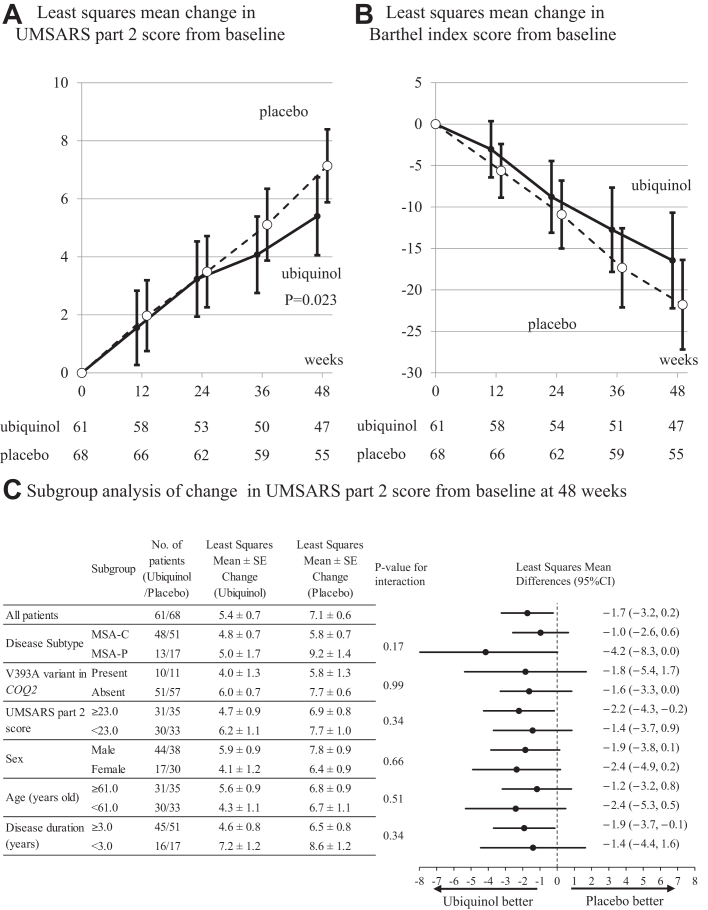
The LS mean changes in the UMSARS part 2 score from the baseline to 48 weeks were 5.4 in the ubiquinol group and 7.1 in the placebo group, which were significantly different (difference, −1.7; 95% confidence interval (CI), −3.2 to −0.2; P = 0.023) (Table 2 and Fig. 2A). The LS mean of the UMSARS part 2 score changes at each assessment (12, 24, 36, and 48 weeks) is shown in Fig. 2A and Table S1 in the Supplementary Appendix. Similar results were obtained in the PPS (Table S2 in the Supplementary Appendix).
Table 2.
Primary and secondary efficacy outcomesa.
| Efficacy outcomes | Ubiquinol | Placebo | Least squares mean differences (ubiquinol−placebo) (95% confidence interval) | P value |
|---|---|---|---|---|
| Number of patients | 61 | 68 | ||
| Primary efficacy outcomebc | ||||
| UMSARS part 2 | ||||
| Mean (±SD) baseline score | 23.2 ± 8.7 | 23.3 ± 7.9 | ||
| Change from baseline at 48 weeks (LS mean ± SE) | 5.4 ± 0.7 | 7.1 ± 0.6 | −1.7 (−3.2, −0.2) | 0.023 |
| Secondary efficacy outcomesbd | ||||
| UMSARS part 1 | ||||
| Mean (±SD) baseline score | 20.5 ± 7.0 | 20.5 ± 5.9 | ||
| Change from baseline at 48 weeks (LS mean ± SE) | 4.7 ± 0.8 | 4.9 ± 0.7 | −0.2 (−2.1, 1.7) | 0.864 |
| Barthel index | ||||
| Mean (±SD) baseline score | 79.4 ± 22.6 | 76.5 ± 20.3 | ||
| Change from baseline at 48 weeks (LS mean ± SE) | −16.5 ± 2.9 | −21.8 ± 2.7 | 5.3 (−2.0, 12.6) | 0.150 |
| SARA | ||||
| Mean (±SD) baseline score | 18.5 ± 6.8 | 18.0 ± 4.9 | ||
| Change from baseline at 48 weeks (LS mean ± SE) | 4.0 ± 0.6 | 5.3 ± 0.6 | −1.4 (−2.8, 0.1) | 0.067 |
| Time required to walk 10 m (sec) | ||||
| Mean (±SD) baseline (sec) | 35.7 ± 16.1 | 27.3 ± 42.8 | ||
| Change from baseline at 48 weeks (LS mean ± SE) | 31.9 ± 16.1 | 55.9 ± 15.0 | −24.1 (−67.7, 21.7) | 0.273 |
The full analysis set population included all the patients who had undergone randomisation and had completed efficacy assessment at least once after the baseline.
The least squares men changes from baseline between the ubiquinol and the placebo groups at week 48 were estimated using a mixed model for repeated measures. Treatment group, visit, and treatment-by-visit interaction were included as fixed effects and with adjustment for the baseline value of each endpoint, presence of the V393A variants in COQ2, and disease subtype (MSA-C or MSA-P). There was no imputation of missing data for the primary efficacy outcome or secondary efficacy outcome. The results for secondary efficacy outcomes were not corrected for multiple comparison.
Parameters were converged by the covariance structure AR (1) with random effects.
Parameters were converged by the covariance structure UN with no random effects.
Fig. 2.
Primary and subgroup analyses. Panel A shows the results for the primary efficacy outcome, the least squares mean changes from the baseline to 48 weeks in the score on the unified multiple-system atrophy rating scale part 2 (UMSARS part 2; range, 0–56, with higher scores indicating more severe symptoms) in the ubiquinol and placebo groups, analyzed with a restricted maximum likelihood (REML)-based repeated measures (MMRM) approach in combination with the Newton–Raphson algorithm. The difference between the ubiquinol and placebo groups in the primary outcome was −1.9 (95% confidence interval, −3.1 to −0.6; P = 0.003). Bars represent 95% confidence intervals. The number of participants evaluated is indicated below the figure. Panel B shows the results for secondary efficacy outcome, the least squares mean changes from the baseline at 48 weeks in the scores of the Barthel index (scores range from 0 to 18, with higher scores indicating greater impairment) in the ubiquinol and placebo groups, analyzed with a REML-based MMRM approach in combination with the Newton–Raphson algorithm. Bars represent standard errors. The number of participants evaluated is indicated below the figure. P-value in the Barthel index is not corrected for multiple comparison. Panel C shows forest plots of the difference in the change of UMSARS part 2 score from the baseline at 48 weeks between the ubiquinol and placebo groups in the full analysis set, stratified by disease subtype (MSA-C or MSA-P), presence or absence of the V393A variant in COQ2, above or below the median of the baseline UMSARS part 2 score, gender (male or female), above or below the median of age, and above or below the median of duration of disease. For each subgroup, the MMRM model used in the primary efficacy outcome analysis was added to the treatment group × each subgroup to calculate the P-value of the interaction.
Of the FAS of 61 and 68 patients in the ubiquinol and placebo groups, 47 and 55 patients were evaluated for UMSARS part 2 scores at weeks 48, and 44 and 52 patients were evaluated at weeks 52 after a 4-week wash-out period, respectively. The mean (standard deviation) changes in UMSARS part 2 scores between weeks 48 and 52 were 0.4 (2.3) and 0.5 (1.8) in the ubiquinol group and in the placebo group, respectively (the P-value for the paired-samples t-test was 0.87) (Fig. S1 in the Supplementary appendix). As shown in Fig. S1, the UMSARS part 2 scores continued to show similar trends of slight increases both in the ubiquinol and placebo groups and the UMSARS part 2 score in the ubiquinol group did not deteriorate toward that observed in the placebo group.
Secondary efficacy outcomes
The changes in the Barthel index score, SARA score, and time required to walk 10 m from the baseline to 48 weeks were better in the ubiquinol group than in the placebo group (Fig. 2B, Table 2, and Table S3 in the Supplementary Appendix). The LS mean differences of the Barthel index score and the SARA score at 48 weeks (ubiquinol−placebo) were 5.3 (95% CI, −2.0 to 12.6) and −1.4 (95% CI, −2.9 to 0.7), respectively. In the ubiquinol and placebo groups in the FAS, 30 (65.2%) and 34 (61.8%) patients completed the 10-m walk at 48 weeks; 3 (6.5%) and 3 (5.5%) patients attempted to walk but could not complete the 10-m walk at 48 weeks; 28 (45.9%) and 31 (45.6%) patients were unable to walk at 48 weeks in the same manner as they did at the baseline, respectively. The LS mean difference of the time required to walk 10 m at 48 weeks (ubiquinol−placebo) was −24.1 s (95% CI, −67.7 to 19.6), indicating that the time required to walk 10-m was shorter in the ubiquinol group than that in the placebo group. The LS mean difference of the UMSARS part 1 score at 48 weeks (ubiquinol−placebo) was −0.2 (95% CI, −2.1 to 1.7) (Table 2).
Subgroup analysis
The results of the prespecified subgroup analyses of the primary efficacy outcome are shown in Fig. 2C. The interaction between treatment effect and each subgroup was tested and P-values were shown in Fig. 2C. There was no significant interaction between treatment effect and any of the subgroups including the disease subtype (MSA-C and MSA-P). The LS mean (95% CI) differences of the UMSARS part 2 score (ubiquinol−placebo) in the MSA-C and MSA-P subgroups were −1.0 (−2.6 to 0.6) and −4.2 (−8.3 to 0.0), respectively. In the prespecified subgroup analyses of the secondary efficacy outcome, the Barthel index and SARA consistently showed favorable effect of ubiquinol both in the MSA-C and MSA-P subgroups (Tables S4 and S5 in the Supplementary Appendix). In the subgroup analysis with COQ2 genotypes, the LS mean (95% CI) differences of the UMSARS part 2 score in the carriers and non-carriers of the V393A variant in COQ2 were −1.8 (−5.4 to 1.7) and −1.6 (−3.3 to 0.0), respectively, and did not support a difference in the efficacy of ubiquinol between the carriers and non-carriers of the V393A variant (Table S6 in the Supplementary Appendix). Of note, subgroup analysis based on the disease duration (≥3.0 and <3.0 years) showed similar LS mean (95% CI) differences of the UMSARS part 2 score of −1.9 (−3.7 to 0.9) and −1.4 (−4.4 to 1.6), respectively.
Sensitivity analysis for primary efficacy outcome
Of the FAS (61 in the ubiquinol group and 68 in the placebo group), 23 (14 in the ubiquinol group and 13 in the placebo group) discontinued the trial without completing the 48-week evaluation (Fig. 1). The reasons for discontinuation were withdrawal of consent (3 in the ubiquinol group and 7 in the placebo group), adverse events (3 in the ubiquinol group and 1 in the placebo group), worsening of disease (6 in the ubiquinol group and 1 in the placebo group), and other reasons (2 in the ubiquinol group and 4 in the placebo group). The mean (standard deviation) baseline UMSARS part 2 scores in the discontinued patients were 27.9 (10.0) in the ubiquinol group and 26.8 (8.1) in the placebo group, which were higher than those in the FAS [23.2 (8.7) in the ubiquinol group and 23.3 (7.9) in the placebo group]. The mean (standard deviation) duration between baseline and the last assessment in the discontinuations were 157.2 days (88.7) in the ubiquinol group and 162.8 days (80.8) in the placebo group. In the sensitivity analysis, the results of the LS mean (95% CI) changes of the UMSARS part 2 score were −0.8 (−2.2, 0.6) in imputation of the worst case for those in the ubiquinol group and the best case for the placebo group, −1.6 (−3.5, 0.4) in multiple imputation method by fully conditional specification, and −1.8 (−3.6, 0.0) in weighted generalized estimation equation method using observation-specific weighted approach (Table S7 in the Supplementary Appendix).
Ubiquinol levels in plasma
The mean trough ubiquinol levels in plasma, in which the FAS was used, were 1.13 and 0.86 μg/mL at the baseline and 5.91 and 0.86 μg/mL at 48 weeks in the ubiquinol and placebo groups, respectively. Ratios of total CoQ10 to free cholesterol in peripheral mononuclear cells were also increased with ubiquinol supplementation (Figs. S2 and S3 in the Supplementary Appendix).
Safety
There were no remarkable differences in the frequency of adverse events between the ubiquinol and placebo groups (Table 3). The frequency (95% CI) of patients with serious adverse events was 31.7% (20.6%, 44.7%) in the ubiquinol group, which was higher than that in the placebo group [17.6% (9.5%, 28.8%)]. Two patients showed serious adverse events that could not be ruled out as causally related to the drug investigated in the ubiquinol group. One patient had vomiting but continued taking the drug, and one patient had worsening diarrhea, which was mild in severity and recovered after discontinuation of the investigational drug. Four deaths were observed in the ubiquinol group during the study period. Cause of each death in four patients (1, 2, 3, and 4) was assessed as 1. Sudden death due to MSA, 2. Upper airway obstruction, 3. Sudden death due to central sleep apnea, and 4). Asphyxiation due to aspiration of vomitus, respectively. Detailed information on the four patients is as follows: 1. Death occurred 234 days after taking ubiquinol, and the patient had been taking 1500 mg/day of ubiquinol until the day before death. The patient was noted to have bilateral vocal cord paralysis and was judged to have died of sudden death due to MSA (possibly due to upper airway obstruction). 2. Death occurred 313 days after taking ubiquinol, and the patient had been taking 1500 mg/day of ubiquinol until the day before death. Since aspiration and sputum was frequently observed due to dysphagia, death was presumed to be due to upper airway obstruction. 3. Death occurred 105 days after taking ubiquinol, and the patient had been taking 1500 mg/day of ubiquinol until the day before death. The patient had undergone a tracheostomy and was not on ventilatory management. Nocturnal apneas were frequently noted, and death due to central sleep apnea was presumed. 4. Death occurred 303 days after taking ubiquinol, and the patient had been taking 1500 mg/day of ubiquinol until the day before death. The patient vomited after breakfast, and the vomit caused asphyxiation. All causes of death were judged as related to MSA and not to be causally related to the investigational drug by investigators.
Table 3.
Safety outcomes.
| Event | Ubiquinol N = 63 |
Placebo N = 68 |
|---|---|---|
| Any adverse event | ||
| Number of patients (%) | 44 (69.8%) | 54 (79.4%) |
| Number of events | 147 | 151 |
| Potentially related to the investigational drug | ||
| Number of patients (%) | 15 (23.8%) | 21 (30.9%) |
| Number of events | 28 | 34 |
| Severe adverse event | ||
| Number of patients (%) | 20 (31.7%) | 12 (17.6%) |
| Number of events | 29 | 20 |
| Potentially related to the investigational drug | ||
| Number of patients (%) | 2 (3.2%) | 0 (0.0%) |
| Number of events | 2 | 0 |
| Adverse events leading to discontinuation of treatment | ||
| Number of patients (%) | 11 (17.5%) | 7 (10.3%) |
| Number of events | 13 | 8 |
| Potentially related to the investigational drug | ||
| Number of patients (%) | 1 (1.6%) | 1 (1.5%) |
| Number of events | 1 | 1 |
| Death | ||
| Number of patients (%) | 4 (6.3%) | 0 (0.0%) |
| Number of events | 4a | 0 |
| Potentially related to the investigational drug | ||
| Number of patients (%) | 0 (0.0%) | 0 (0.0%) |
| Number of events | 0 | 0 |
All of them were judged not to be causally related to the drug by investigators.
During the follow-up period after discontinuation of the investigational drug, the frequencies of adverse events were 11.1% and 17.6% in the ubiquinol and placebo groups, respectively. The frequency (95% CI) of patients with serious adverse events was 4.8% (1.0%, 13.3%) in the ubiquinol group, which was higher than that in the placebo group [0% (0.0%, 5.3%)].
Discussion
In this clinical trial, the difference in the UMSARS part 2 score from the baseline to 48 weeks as the primary efficacy outcome was significantly smaller in the ubiquinol group than in the placebo group, and the secondary efficacy outcomes including Barthel index, SARA score, and the 10-m walk time were consistently better in the ubiquinol group. The better outcomes observed in the Barthel index and the 10-m walk time that reflect activities of daily living, support the clinically meaningful efficacy of ubiquinol. When the correlation between the UMSARS part 2 scores at 0 and 48 weeks was examined in the FAS, the Pearson correlation coefficient was 0.83, which was higher than that we assumed at the design of the trial. This may have contributed to increased sensitivity in evaluating primary efficacy outcome.
The half-life of plasma CoQ10 in adult human was reported to be about 33 h.32 Given that the UMSARS part 2 score in the ubiquinol group did not deteriorate toward that observed in the placebo group at 52 weeks after a 4-week wash-out period, the results may support the role of ubiquinol as a disease-modifying effect rather than a symptomatic effect.
To investigate whether there are heterogeneities in the efficacy outcomes in the subgroup analysis, we conducted interaction test in the subgroup analysis. For each subgroup, the model used in the primary efficacy outcome analysis was added to the treatment group × each subgroup to calculate the P-value of the interaction, and none of the interactions was significant. In the subgroup analysis based on the UMSARS part 2 score, there seems to be a difference in the UMSARS part 2 scores between MSA-C and MSA-P (Fig. 2C), although no statistical tests considering multiple testing were conducted. The assessment of the MSA-C and MSA-P subgroups by SARA score, which is designed specifically for assessing cerebellar ataxia, showed LS mean (95% CI) differences of the SARA score (−1.3 [−2.4 to −0.2] at 48 weeks) in MSA-C and LS mean (95% CI) differences of the SARA score (−1.0 [−6.2 to 4.2] at 48 weeks) in MSA-P (Tables S4 and S5 in the Supplementary Appendix). These results supported the efficacy of the high dose ubiquinol in the MSA-C as well. As shown in Results, similar LS mean (95% CI) differences of the UMSARS part 2 score were observed in the carriers and non-carriers of the V393A variant in COQ2: −1.8 (−5.4 to 1.7) and −1.6 (−3.3 to 0.0) (Fig. 2C), respectively, supporting the efficacy of ubiquinol in MSA patients not carrying COQ2 variants as well. Since the carrier frequencies of V393A in patients with MSA and general population in the Japanese population were 9.1% and 4.2%, respectively,8,9 recruitment of a sufficient number of MSA patients with V393A was difficult. Although we increased the number of enrolled cases with carriers of V393A to 21 in this trial, the statistical power was limited due to the small sample size. Nonetheless, it is noteworthy that the similar LS means difference was observed in the carriers of V393A as observed in non-carriers. Of note, decreases in CoQ10 levels have also been observed in the blood,10, 11, 12 cerebrospinal fluid,13 fibroblasts,14 and cerebellar tissues15,16 of MSA patients who do not carry the V393A variant, indicating that decreased CoQ10 levels commonly occur in MSA patients irrespective of presence or absence of V393A, which is consistent with the findings that the similar LS means differences of the UMSARS part 2 score were observed in the carriers of V393A as well as in the non-carriers. Taken together, the present trial supported that a high-dose ubiquinol supplementation may mitigate the rate of progression of motor function impairment during the 48-week observation period.
Although the LS mean difference in the UMSARS part 2 score from the baseline to 48 weeks was 1.7 points, which is below the 3 points estimated for the target sample size, we consider this to be a clinically meaningful difference. First, using data from a placebo-controlled randomised controlled trial in patients with MSA, the minimum clinically important difference in the UMSARS part 2 was reported to be 1.5.33 This report included 132 MSA-P patients and showed that the optimal cut-offs, based on receiver operating characteristic curves, to distinguish patients rated minimally worse versus no change on the Clinical Global Impression of Improvement scale over 48 weeks was 1.5 points on the UMSARS part 2.33 Since similar analysis has not been conducted for MSA-C patients, caution needs to be taken in applying this value to the group largely consisting of MSA-C patients in this study Second, the LS mean difference in the Barthel index score was 5.3, well above the reported minimum clinically important difference in the Barthel index of 1.85.34 Third, the LS mean difference in the time required to walk 10 m was 24.1 s, which is a substantial difference.
Among the secondary efficacy outcomes, however, the LS mean (95% CI) difference in the UMSARS part 1 score, which reflects activities of daily living of MSA patients, between the ubiquinol and the placebo groups at 48 weeks was −0.2 (−2.1, 1.7) that is smaller than that of the UMSARS part 2 score. According to a report estimating the sample size required for a therapeutic trial with 1-year follow-up, it should be noted that the sample size to detect the effect of 25% reduction in the UMSARS part 1 score change with 80% power is estimated to be 645,35 which is larger than estimated sample size of 291 employing the UMSARS part 2 score. Another report from Japan, where MSA-C patients predominate MSA-P patients, estimated the number of samples to detect the effect of a 30% reduction in the UMSARS part 1 score with 80% power at 1002, much larger than the estimated sample size of 140, 136, and 249 employing the UMSARS part 2 score, the SARA score, and the Barthel index score, respectively.36 Although it would be of impact if the efficacy is confirmed by the UMSARS part 1 score that evaluates ADL of MSA patients, the number of patients and observation period in the clinical trial may not have been sufficient to demonstrate the efficacy based on the UMSARS part 1 score. The observation that changes in the Barthel index score from the baseline to 48 weeks were better in the ubiquinol group than in the placebo group supports the efficacy of ubiquinol on the activities of daily living of MSA patients.
The mean (standard deviation) total CoQ10 levels in plasma was 1.00 (0.86) μg/mL and 0.81 (0.25) μg/mL in the FAS and the PPS at the baseline. As defined in the study design, in the PPS, patients with the ubiquinol levels higher than 1.98 μg/mL at the baseline were excluded. The plasma total CoQ10 levels in the PPS group at the baseline were slightly higher than the 0.51 (0.22) μg/mL in MSA patients and 0.72 (0.42) μg/mL in controls that we previously reported.11 Although one of the exclusion criteria was the use of drugs or supplements containing CoQ10, there remains a possibility that a small number of patients may have taken CoQ10 as a supplement. The mean trough CoQ10 level at 48 weeks with 1500 mg/day of ubiquinol was 6.03 μg/mL. This was similar to 6.38 μg/mL in patients with Huntington's disease supplemented with 2400 mg/day of ubiquinone for 8 weeks and 7.49 μg/mL in those supplemented with 3600 mg/day of ubiquinone for 12 weeks,37 but tended to be lower than 10.23 μg/mL at 14 days of supplementation with ubiquinol (1500 mg/day) in healthy adult males in the phase 1 study (UMIN000016695).23 These differences may be due to the subjects' ages or underlying diseases. Although the dose–response was not investigated in the current trial, we considered that this dose (1500 mg/day of ubiquinol) is the maximum dose that has been shown to be safe and tolerated by patients, and even if the dose is increased further, the bioavailability is considered to remain the same.
The frequency of patients who discontinued the trial without completing the evaluation at 48 weeks (20.9%) was similar to those in previous studies.30,38 There was no difference between the ubiquinol and placebo groups in the baseline UMSARS part 2 score and the change in the UMSARS part 2 score between baseline and the last assessment in the discontinued patients. The frequencies of reported adverse events, serious adverse events, discontinued treatment because of adverse events, and deaths were mainly consistent with those expected in MSA patients and were similar to those reported in the previous clinical trials.30,38 Two cases (one with vomiting and one with worsening diarrhea) were listed as serious adverse events for which a causal relationship could not be ruled out by the investigators. Since ubiquinol is lipid-soluble and non-dispersible in water, it may be necessary to pay attention to gastrointestinal symptoms in patients taking a high-dose ubiquinol, although the adverse events were infrequent. Death during the study period was observed in 4 of 63 patients (6.3%) in the ubiquinol group, but not in the placebo group, which was comparable to a previous study (6 deaths out of 92).38
Several clinical trials have been conducted for MSA,28, 29, 30,38 but this is the first to demonstrate the clinical efficacy as the disease-modifying therapy for MSA. The clinical efficacy of ubiquinol is consistent with the previous findings that decreased CoQ10 levels were observed in the blood,10, 11, 12 cerebrospinal fluid,13 fibroblasts,14 and cerebellar tissues15,16 of MSA patients including those from Europe and North America who do not carry the V393A variant, and, furthermore, that restoration of brain oxygen consumption rate was observed with administration of 1200 mg ubiquinol in a patient at the advanced stage of familial MSA who had biallelic variants in COQ2.21
There are several limitations in this study. Although this is a multicentre study, the participants were exclusively Japanese, and it is well recognized that relative frequencies of MSA-C and MSA-P patients considerably differ among various regions. Assessment of ADL is no doubt important to evaluate clinically meaningful effects, but the UMSARS part 1 score was reported to need a larger sample size when used to detect treatment effects than the part 2 score.35,36 In the current clinical trial, a sufficient evidence of efficacy on activities of daily living function was not obtained. Due to the relatively small number of the patients, the subgroup analyses are not sufficiently powered. Furthermore, in clinical trials for slowly progressive neurodegenerative diseases, long observation periods may be required to obtain clinically meaningful results. This is a challenge for the clinical trials for slowly progressive neurogenerative diseases like MSA. Moreover, any information on patient acceptability was not included in this study. In addition, because the time of assessment was not fixed, motor fluctuations may have influenced the assessment of motor symptoms. Because reproductive and developmental toxicity studies in experimental animals had not been completed at the start of this trial, we were advised by Pharmaceuticals and Medical Devices Agency (PMDA), a Government Agency responsible for approval of drugs in Japan, to exclude women with childbearing potential as one of the exclusion criteria in this trial.
Although the role of CoQ10 in the pathogenesis of MSA is not fully elucidated, we hope that the results of this clinical trial will accelerate the development of disease-modifying therapies for MSA. Further studies are warranted to investigate the clinical efficacy of supplementation of high doses of ubiquinol for patients with MSA.
Contributors
JM and ST conceived and designed the study. JM, TM, AC, HI, TT, HK, MH, IW, TG, TM, YT, HM, KI, TY, SK, NS, RT, KA, TI, OO, DM, RY, JK, MK, RH, KO, HT, MM, IY, HS, and ST were investigators who conducted the study and collected data. JM, TM, YU, TK, MT, HN, and ST directly accessed and verified the underlying data. JM, TM, YU, TK, KJL-P, and ST contributed to data analysis or data interpretation. All authors contributed to data interpretation and writing, review, and approval of the manuscript for submission.
Data sharing statement
The data underlying this Article will be shared with researchers on reasonable request to the corresponding author. Data will be shared through a secure online platform after signing a data access agreement. Data will be available at the time of publication and for a minimum of 5 years from the end of the trial.
Declaration of interests
JM reports honoraria from Kyowa Kirin, Alnylam Japan, Sanofi, Sumitomo Pharma, Pfizer, Daiichi Sankyo, and Takeda Pharmaceutical Company; grants from Japan Agency for Medical Research and Development (AMED), Japan Society for the Promotion of Science (JSPS), and Takeda Science Foundation. TM reports honoraria from Eisai and Sumitomo Pharma; grants from JSPS. HI reports honoraria from Takeda Pharmaceutical Company, Eisai, Biogen Japan, Sumitomo Pharma, FP Pharmaceutical Corporation, Kyowa Kirin, UCB Japan, Chugai Pharmaceutical, and Daiichi Sankyo Company; grants from AMED, JSPS, and Kato Memorial Trust for Nanbyo Research. TT reports honoraria from Sumitomo Pharma. OO reports honoraria from Kyowa Hakko Kirin Co., Ltd., Bristol-Myers Squibb, Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharm, Takeda, Daiichi-Sankyo, FUJIFILM, SANOFI, and FP-pharm; grants from AMED, JSPS, Japanese Ministry of Health, Labor, and Welfare, Takeda Science Foundation, and Life Science Foundation of Japan. ST reports consulting fees from Sanwakagaku Kenkyusho, and Ono Pharmaceutical; honoraria from Sanofi, Senju Pharmaceutical, Novartis, Kyowa Kirin, and Daiichisankyo; grants from AMED, JSPS, and Nobel Pharma. All other authors declare no competing interests.
Acknowledgments
This study was supported by grants (17lk1403008h0001, 18lk1403008h0002, 19lk1403008h0003, 20lm0203141h0001, 21lm0203141h0002) from the Japan Agency for Medical Research and Development (AMED).
We would like to express our sincere gratitude for the patients participated in this study and the support of the following trial site subinvestigators and staff members for logistic and technical support: Kosuke Kashiwabara, Kana Osawa, Chiyomi Uemura, Yuka Kawamata, Munenori Takata, Mariko Takeda, Yumi Tanaka, Ai Okazaki, Yukana Yanai, Fumiko Watanabe, Nahoko Yoshihara, Asaka Tanaka, Masami Kaneko, Maika Kissei, Miho Echizenya, Akihiko Furuya, Makoto Yoshimoto, Maki Kobayashi, Takashi Onodera, Yuko Kageyama, and other members of Clinical Research Promotion Center, Mio Takeyama, Zhenghong Wu (The University of Tokyo Hospital), Masaki Yamada, Noriaki Suzuki (CMIC CMO Co., Ltd.), Yuka Tejima, Ayako Kurumada (National Center Hospital, National Center of Neurology and Psychiatry), Kokoro Ozaki, MD, Yoichiro Nishida, MD, Taro Ishiguro, MD, Ryuji Koike, MD, Haruko Hiraki, Natsumi Ota (Tokyo Medical And Dental University Hospital), Yoshitaka Yamanaka, MD, Atsuhiko Sugiyama, MD, Kaoru Sakuma, Yoko Kaneko (Chiba University Hospital), Mami Iwasaki (Kyoto University Hospital), Toru Yamashita, MD, Yoshio Omote, MD (Okayama University Hospital), Masayoshi Tada, MD, Satomi Ikarashi, Ayumi Namekata, Miki Nagai (Niigata University Medical & Dental Hospital), Masako Fujise, Machiko Shimura (Kyushu University Hospital), Sachiko Ando (Minimal Co., Ltd.), Miwa Imai, Mariko Nabekura (Nagoya University Hospital), Hiroshi Takigawa, MD, Hiromi Mori, Chika Yamamoto (Tottori University Hospital), Mikiya Suzuki, MD, Sachiko Namatame, MD, Yuka Ogura, MD, Yoshie Sasai, Satomi Kamoda (National Hospital Organization Higashisaitama National Hospital), Keiko Higashi, MD, Yuji Okamoto, MD, Yusuke Sakiyama, MD, Mari Okuno (Kagoshima University Hospital), and Miho Deai (Hokkaido University Hospital).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101920.
Appendix A. Supplementary data
References
- 1.Gilman S., Wenning G.K., Low P.A., et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanciulli A., Wenning G.K. Multiple-system atrophy. N Engl J Med. 2015;372:249–263. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe H., Saito Y., Terao S., et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- 4.Wakabayashi K., Yoshimoto M., Tsuji S., Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 5.Woerman A.L., Watts J.C., Aoyagi A., Giles K., Middleton L.T., Prusiner S.B. α-Synuclein: multiple system atrophy prions. Cold Spring Harb Perspect Med. 2018;8:a024588. doi: 10.1101/cshperspect.a024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenning G.K., Wagner S., Daniel S., Quinn N.P. Multiple system atrophy: sporadic or familial? Lancet. 1993;342:681. doi: 10.1016/0140-6736(93)91789-o. [DOI] [PubMed] [Google Scholar]
- 7.Hara K., Momose Y., Tokiguchi S., et al. Multiplex families with multiple system atrophy. Arch Neurol. 2007;64:545–551. doi: 10.1001/archneur.64.4.545. [DOI] [PubMed] [Google Scholar]
- 8.The Multiple-System Atrophy Research Collaboration Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- 9.Porto K.J., Hirano M., Mitsui J., et al. COQ2 V393A confers high risk susceptibility for multiple system atrophy in East Asian population. J Neurol Sci. 2021;429 doi: 10.1016/j.jns.2021.117623. [DOI] [PubMed] [Google Scholar]
- 10.Kasai T., Tokuda T., Ohmichi T., et al. Serum levels of coenzyme Q10 in patients with multiple system atrophy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsui J., Matsukawa T., Yasuda T., Ishiura H., Tsuji S. Plasma coenzyme Q10 levels in patients with multiple system atrophy. JAMA Neurol. 2016;73:977–980. doi: 10.1001/jamaneurol.2016.1325. [DOI] [PubMed] [Google Scholar]
- 12.Du J., Wang T., Huang P., et al. Clinical correlates of decreased plasma coenzyme Q10 levels in patients with multiple system atrophy. Parkinsonism Relat Disord. 2018;57:58–62. doi: 10.1016/j.parkreldis.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Compta Y., Giraldo D.M., Muñoz E., et al. Cerebrospinal fluid levels of coenzyme Q10 are reduced in multiple system atrophy. Parkinsonism Relat Disord. 2018;46:16–23. doi: 10.1016/j.parkreldis.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Monzio Compagnoni G., Kleiner G., Bordoni A., et al. Mitochondrial dysfunction in fibroblasts of multiple system atrophy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3588–3597. doi: 10.1016/j.bbadis.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Schottlaender L.V., Bettencourt C., Kiely A.P., et al. Coenzyme Q10 levels are decreased in the cerebellum of multiple-system atrophy patients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barca E., Kleiner G., Tang G., et al. Decreased coenzyme Q10 levels in multiple system atrophy cerebellum. J Neuropathol Exp Neurol. 2016;75:663–672. doi: 10.1093/jnen/nlw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao J.H.T., Purushothuman S., Jensen P.H., Halliday G.M., Kim W.S. Reductions in COQ2 expression relate to reduced ATP levels in multiple system atrophy brain. Front Neurosci. 2019;13:1187. doi: 10.3389/fnins.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda T., Matsukawa T., Mitsui J., Tsuji S. Oxygen consumption rate for evaluation of COQ2 variants associated with multiple system atrophy. Neurogenetics. 2019;20:51–52. doi: 10.1007/s10048-018-0563-7. [DOI] [PubMed] [Google Scholar]
- 19.Nakamoto F.K., Okamoto S., Mitsui J., et al. The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci Rep. 2018;8 doi: 10.1038/s41598-018-32573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foti S.C., Hargreaves I., Carrington S., Kiely A.P., Houlden H., Holton J.L. Cerebral mitochondrial electron transport chain dysfunction in multiple system atrophy and Parkinson's disease. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-42902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsui J., Koguchi K., Momose T., et al. Three-year follow-up of high-dose ubiquinol supplementation in a case of familial multiple system atrophy with compound heterozygous COQ2 mutations. Cerebellum. 2017;16:664–672. doi: 10.1007/s12311-017-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosoe K., Kitano M., Kishida H., Kubo H., Fujii K., Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007;47:19–28. doi: 10.1016/j.yrtph.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Mitsui J., Matsukawa T., Tanaka M., et al. Randomized, double-blind, placebo-controlled phase 1 study to evaluate the safety and pharmacokinetics of high doses of ubiquinol in healthy adults. Neurol Clin Neurosci. 2022;10:14–24. [Google Scholar]
- 24.Wenning G.K., Tison F., Seppi K., et al. Development and validation of the unified multiple system atrophy rating scale (UMSARS) Mov Disord. 2004;19:1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 25.Chikada A., Mitsui J., Matsukawa T., et al. Reliability and validity of Japanese version of unified multiple system atrophy rating scale. Neurol Clin Neurosci. 2021;9:171–180. [Google Scholar]
- 26.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 27.Schmitz-Hübsch T., du Montcel S.T., Baliko L., et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 28.Dodel R., Spottke A., Gerhard A., et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial) Mov Disord. 2010;25:97–107. doi: 10.1002/mds.22732. [DOI] [PubMed] [Google Scholar]
- 29.Low P.A., Robertson D., Gilman S., et al. Efficacy and safety of rifampicin for multiple system atrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:268–275. doi: 10.1016/S1474-4422(13)70301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poewe W., Seppi K., Fitzer-Attas C.J., et al. Efficacy of rasagiline in patients with the parkinsonian variant of multiple system atrophy: a randomised, placebo-controlled trial. Lancet Neurol. 2015;14:145–152. doi: 10.1016/S1474-4422(14)70288-1. [DOI] [PubMed] [Google Scholar]
- 31.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 32.Tomono Y., Hasegawa J., Seki T., Motegi K., Morishita N. Pharmacokinetic study of deuterium-labelled coenzyme Q10 in man. Int J Clin Pharmacol. 1986;24:536–541. [PubMed] [Google Scholar]
- 33.Krismer F., Seppi K., Wenning G.K., Abler V., Papapetropoulos S., Poewe W. Minimally clinically important decline in the parkinsonian variant of multiple system atrophy. Mov Disord. 2016;31:1577–1581. doi: 10.1002/mds.26743. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh Y.W., Wang C.H., Wu S.C., Chen P.C., Sheu C.F., Hsieh C.L. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair. 2007;21:233–238. doi: 10.1177/1545968306294729. [DOI] [PubMed] [Google Scholar]
- 35.May S., Gilman S., Sowell B.B., et al. Potential outcome measures and trial design issues for multiple system atrophy. Mov Disord. 2007;22:2371–2377. doi: 10.1002/mds.21734. [DOI] [PubMed] [Google Scholar]
- 36.Matsushima M., Yabe I., Oba K., et al. Comparison of different symptom assessment scales for multiple system atrophy. Cerebellum. 2016;15:190–200. doi: 10.1007/s12311-015-0686-4. [DOI] [PubMed] [Google Scholar]
- 37.Hyson H.C., Kieburtz K., Shoulson I., et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. Mov Disord. 2010;25:1924–1928. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- 38.Levin J., Maaß S., Schuberth M., et al. Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019;18:724–735. doi: 10.1016/S1474-4422(19)30141-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.