Abstract
Background:
We aimed to compare the prevalence and severity of fatigue in survivors of Covid-19 versus non-Covid-19 critical illness, and to explore potential associations between baseline characteristics and worse recovery.
Methods:
We conducted a secondary analysis of two prospectively collected datasets. The population included was 92 patients who received invasive mechanical ventilation (IMV) with Covid-19, and 240 patients who received IMV with non-Covid-19 illness before the pandemic. Follow-up data were collected post-hospital discharge using self-reported questionnaires. The main outcome measures were self-reported fatigue severity and the prevalence of severe fatigue (severity >7/10) 3 and 12-months post-hospital discharge.
Results:
Covid-19 IMV-patients were significantly younger with less prior comorbidity, and more males, than pre-pandemic IMV-patients. At 3-months, the prevalence (38.9% [7/18] vs. 27.1% [51/188]) and severity (median 5.5/10 vs 5.0/10) of fatigue were similar between the Covid-19 and pre-pandemic populations, respectively. At 6-months, the prevalence (10.3% [3/29] vs. 32.5% [54/166]) and severity (median 2.0/10 vs. 5.7/10) of fatigue were less in the Covid-19 cohort. In the total sample of IMV-patients included (i.e. all Covid-19 and pre-pandemic patients), having Covid-19 was significantly associated with less severe fatigue (severity <7/10) after adjusting for age, sex and prior comorbidity (adjusted OR 0.35 (95%CI 0.15–0.76, p=0.01).
Conclusion:
Fatigue may be less severe after Covid-19 than after other critical illness.
Keywords: COVID-19, SARS-CoV-2, critical care, critical illness, intensive care units
Introduction
Post-Intensive Care Syndrome (PICS) describes the constellation of physical, psychological and cognitive symptoms affecting 25% of critical illness survivors, which include fatigue, muscle weakness and post-traumatic stress. 1 PICS may persist for longer than 5 years and is associated with high hospital resource use and readmission rates. 2 Implementation of post-ICU rehabilitation guidelines is inconsistent, and the best ways to support intensive care unit (ICU) survivors are uncertain.3–5 Identifying those at greatest risk of PICS and providing evidence-based strategies to enhance their recovery remain priorities for critical care research. 5 The Covid-19 pandemic heralded a dramatic increase in ICU admissions: in one study including over 20,000 UK hospitalised patients, 17% required admission to ICU or high dependency units, over half of whom survived to discharge; thus a vast new cohort of patients at risk of PICS has emerged.6,7 This new cohort is relatively novel in terms of younger age, less previous comorbidity and longer ICU stays than ICU admissions before the Covid-19 pandemic.8,9
Many patients who survive acute-Covid-19 experience persistent symptoms beyond 4 weeks, known as ‘long-Covid’. 10 This includes a wide range of symptoms, such as breathlessness, fatigue and muscle pain, many of which overlap with PICS and other post-viral syndromes.1,11–18 The most common symptom of long-Covid described following both community and hospital-managed acute-Covid-19 was fatigue: fatigue was reported in 97.7% of community-managed cases who reported symptoms lasting over 28 days, and in 83% and 98% of UK and China patients, respectively, greater than 3-months after hospital discharge following admission with acute-Covid-19.13,18,19 Recent studies have shown requiring invasive mechanical ventilation (IMV) is associated with experiencing worse fatigue and other recovery, but have not attempted to compare ‘long-Covid’ to recovery from all critical illness and were without control groups.11,13,14
The prevalence of fatigue in ICU-survivors ranges from 13.8% to 80.9%. 20 This has a profound negative impact on survivors’ quality of life (QOL), and is one of the most prevalent and debilitating challenges of ICU-survivorship.20,21 Fatigue may lead to delayed return to employment and pre-critical illness physical fitness, difficulty in completing activities of daily living, social isolation, depression and many other negative sequalae.20–22 It is unclear if recovery is different in survivors of Covid-19 critical illness compared to other critical illness, requiring distinct risk assessment tools or interventions to support their recovery.
We aimed to characterise the prevalence and severity of fatigue in Covid-19 ICU-survivors, compared to survivors of non-Covid-19 critical illness, and to explore potential associations between baseline characteristics and fatigue severity in these populations to identify potential groups at greater risk.
Methods
Study design and population
This was a secondary analysis of two prospectively collected datasets.
Covid-19 cohort: International Severe Acute Respiratory Infection Consortium Coronavirus Clinical Characterisation Protocol-United Kingdom (ISARIC-4C CCP-UK):
Patients aged 18 years and over, admitted to hospital between 17 January and 5 October 2020 with confirmed or highly suspected SARS-CoV-2 infection at 31 UK centres, who consented to be contacted for follow-up studies, who completed a follow-up questionnaire and who were discharged at least 90 days ago at the time of data collection were eligible for inclusion. Patients who did not survive to follow-up were excluded. We restricted this secondary analysis to patients who received IMV.
Pre-pandemic cohort: Evaluation of a Rehabilitation Complex Intervention for Patients Following Intensive Care Discharge (RECOVER) trial:
Patients aged 18 and over, discharged from ICU between 1 December 2010 and 31 January 2013 at two hospitals in Edinburgh, Scotland, who received a minimum of 48-h of IMV. 23 Patients randomised to the intervention group received enhanced hospital and community-based physical rehabilitation, and the control group received routine care. 23 Both groups were included in this analysis, as there were no significant differences at baseline or follow-up. 23
Full details of the populations included in CCP-UK and RECOVER, and study information, have previously been published.13,23
Clinical variables
Both datasets captured demographic information, including age, sex, pre-existing comorbidities, admission diagnosis, length of hospital stay and severity of acute illness. Comorbidities were defined in the ISARIC4C CCP-UK protocol for the Covid-19 cohort, 24 and by the Functional Comorbidity Index (FCI) for the pre-pandemic cohort. 23 For the pre-pandemic cohort, we combined ‘Angina’, ‘Congestive heart failure or heart disease’ and ‘Heart Attack’ from the FCI under the heading ‘Chronic Cardiac Disease’ to compare to the Covid-19 cohort (for which this was a category in the ISARIC4C protocol); similarly ‘Chronic Obstructive Pulmonary Disease’ on the FCI was compared to ‘Chronic Pulmonary Disease’. Diagnosis category was captured from the RECOVER data in order to stratify the RECOVER cohort by diagnosis, to allow those with Respiratory diagnoses only to be compared to the Covid-19 cohort. Variables indicating acute illness severity differed: CCP-UK included ISARIC-4C Mortality and World Health Organisation Severity Scores25,26; RECOVER included the Acute Physiology and Chronic Health Evaluation-II (APACHE-II) score, 27 duration of IMV, duration of ICU-admission, total and Respiratory Sequential Organ Failure Assessment (SOFA) scores. 28 Thus, and given the different underlying disease processes, acute illness severity, including duration of IMV and ICU admission, was not directly compared. RECOVER captured survival at 3, 6 and 12-months post-hospital discharge: patients alive at each time point were contacted for follow-up.
Outcomes
Both studies used patient-completed questionnaires to collect outcome data.13,23 CCP-UK completed follow-up assessment via postal questionnaires (or telephone where this was not possible), which patients completed once and returned. 13 RECOVER completed follow-up assessment face-to-face or by telephone at 3-months, and by post at 6 and 12-months post-hospital discharge. 23
In this secondary analysis, the primary outcome was patient-reported fatigue severity. Secondary outcomes were: ‘severe fatigue’, breathlessness and measures of health-related QOL.
In both studies, fatigue severity was measured using a 10-point Visual Analogue Scale (VAS), where zero is no fatigue and 10 is worst possible fatigue. ‘Severe fatigue’ was defined as fatigue of at least 7/10 on the VAS.
Statistical methods
Covid-19 IMV-patients were compared to the pre-pandemic IMV-patients at baseline, follow-up, and potential associations with outcomes within each group were compared.
Covid-19 and pre-pandemic IMV-patients, and the pre-pandemic subset with Respiratory diagnosis category, were compared at baseline in terms of age, sex, prior comorbidity, deprivation. Fatigue severity and the prevalence of severe fatigue were compared. Fatigue in Covid-19 IMV-patients was stratified by time since hospital discharge that patients responded to the CCP-UK questionnaire: approximately 3 (90–120 days) and 6-months (150–210 days), in order to compare fatigue at equivalent time points to that measured in the RECOVER study. Thus, a smaller subset of the Covid-19 sample was compared to the pre-pandemic group for fatigue at each time point. Fatigue severity (VAS scores) and ‘severe fatigue’ were compared as continuous and binary outcomes, respectively.
Where direct comparison was not possible, that is, where the same variables measured with the same tools were not available in the two datasets, outcome variables from both datasets were presented separately.
Categorical data were summarised as frequencies and percentages, continuous data as median and interquartile range (IQR). To test for differences across comparison groups in categorical data, we used Fisher’s exact test and for continuous data, using the Wilcoxon rank-sum test for two-sample testing and Kruskall–Wallis where there were more than two groups.
Using ordinal logistic regression and logistic regression, we explored if significant association existed between Covid-19 and severe fatigue in all patients included (Covid-19 and pre-pandemic group), after adjustment for patient demographics (age, sex and presence of comorbidity). With ordinal regression, we explored fatigue in categories: 0–2/10, 2–4/10, 4–6/10, 6–8/10, 8–10/10, and with logistic regression we explored more severe reported fatigue, taking scores less than 7/10 or not as a binary outcome. Pre-pandemic outcomes at 6 months were included. We repeated these regression analyses including the Covid-19 group and the pre-pandemic subset with Respiratory diagnosis category. Effect estimates are presented as mean differences alongside 95% confidence intervals (95%-CI). Statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, AUT) with the tidyverse, finalfit, eq5d and Hmisc packages. Statistical significance was taken at the level p≤0.05.
Results
Patients included
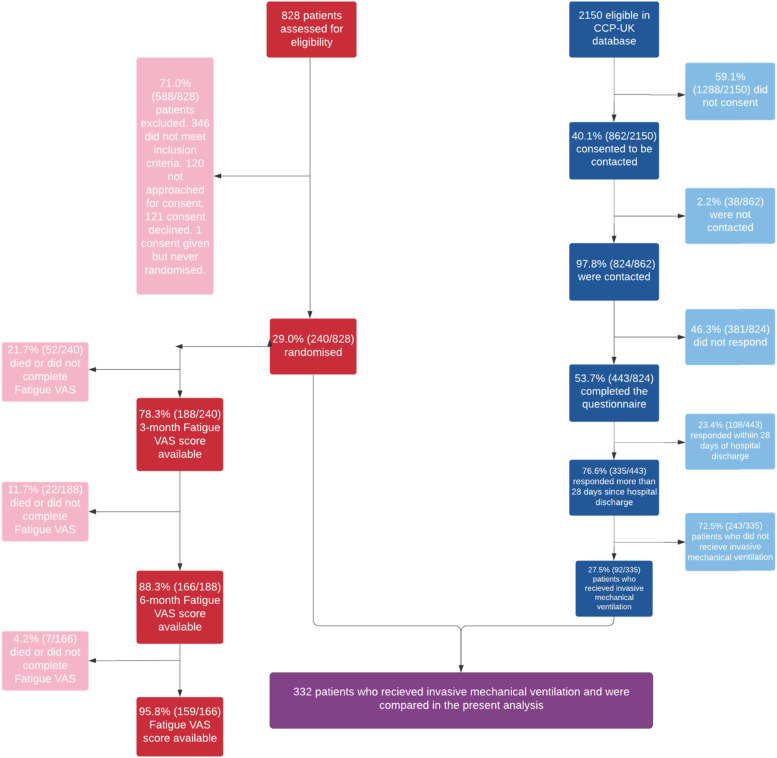
Three hundred-thirty five Covid-19 patients were included in the CCP-UK study: 27.5% (92/335) received IMV (Figure 1). Two-forty patients were included in RECOVER (pre-pandemic patients). A total of 332 patients (92 Covid-19, 240 pre-pandemic patients) who received IMV were included in this secondary analysis (Figure 1). 29.2% [70/240] and 35.0% [84/240] of the pre-pandemic cohort were admitted with Cardiovascular and Respiratory diagnosis categories, respectively (Table 1).
Figure 1.
Patient inclusion flowchart. Red: RECOVER trial participants, n=240; Blue: ISARIC4C participants, n=92.
Table 1.
Patient characteristics of Covid-19 (n = 92) and pre-pandemic IMV-patients (n = 240).
| Covid-19 cohort | Pre-pandemic cohort | p-value | ||
|---|---|---|---|---|
| Total N (%) | — | 92 (27.7) | 240 (72.3) | — |
| Age | Median (IQR) | 59.7 (51.1–64.5) | 62.0 (52.0–70.0) | 0.017 |
| Sex | Male | 65 (70.7) | 137 (57.1) | 0.032 |
| — | Female | 27 (29.3) | 103 (42.9) | — |
| Number of comorbidities | Median (IQR) | 1.0 (0.0–2.0) | 2.0 (1.0–4.0) | <0.001 |
| Comorbidities or not | No comorbidities | 35 (38.0) | 29 (12.1) | <0.001 |
| — | One or more comorbidities | 57 (62.0) | 211 (87.9) | — |
| Deprivation | Most deprived 1 | 10 (10.9) | 33 (13.8) | 0.277 |
| — | 2 | 19 (20.7) | 60 (25.0) | — |
| — | 3 | 26 (28.3) | 48 (20.0) | — |
| — | 4 | 22 (23.9) | 45 (18.8) | — |
| — | Least deprived 5 | 15 (16.3) | 54 (22.5) | — |
| Obesity | No | 62 (72.9) | 187 (77.9) | 0.434 |
| — | Yes | 23 (27.1) | 53 (22.1) | — |
| Diabetes | No | 66 (75.9) | 203 (84.6) | 0.097 |
| — | Yes | 21 (24.1) | 37 (15.4) | — |
| Asthma | No | 71 (82.6) | 197 (82.1) | 1.000 |
| — | Yes | 15 (17.4) | 43 (17.9) | — |
| Chronic pulmonary disease (not asthma) | No | 81 (94.2) | 203 (84.6) | 0.036 |
| Yes | 5 (5.8) | 37 (15.4) | ||
| Chronic cardiac disease | No | 78 (90.7) | 194 (80.8) | 0.052 |
| — | Yes | 8 (9.3) | 46 (19.2) | |
| ICU admission diagnosis category | Cardiovascular | - | 70 (29.2) | - |
| — | Respiratory | 92 (100.0) | 84 (35.0) | - |
| — | Gastrointestinal tract | - | 59 (24.6) | - |
| — | Renal | - | 3 (1.3) | - |
| — | Trauma | - | 8 (3.3) | - |
| — | Neurologic | — | 12 (5.0) | — |
| — | Miscellaneous diagnoses | - | 4 (1.7) | - |
| ISARIC-4C mortality score | Median (IQR) | 8.0 (5.0–10.0) | - | - |
| Length of hospital stay | Median (IQR) | 26.0 (15.5–40.0) | 32.0 (20.0–50.0) | 0.02 |
| ICU length of stay | Median (IQR) | - | 11.0 (6.8–18.0) | - |
| Duration of IMV | Median (IQR) | - | 8.0 (5.0–15.0) | - |
| Total SOFA score | Median (IQR) | - | 3.0 (2.0–4.0) | - |
| Respiratory SOFA score | 0 | - | 23 (9.6) | - |
| — | 1 | — | 73 (30.4) | — |
| — | 2 | — | 144 (60.) | — |
| APACHE-II score | Median (IQR) | - | 20.0 (16.0–25.0) | - |
| Time from discharge to completing CCP-UK questionnaire (days) | Median (IQR) | 185.0 (136.8–241.2) | - | - |
| 3-months survival | Yes | - | 216 (94.7) | - |
| — | No | — | 12 (5.3) | — |
| 6-month survival | Yes | - | 169 (89.4) | — |
| — | No | — | 20 (10.6) | — |
| 12-month survival | Yes | - | 163 (88.1) | — |
| — | No | — | 22 (11.9) | — |
IQR: Interquartile range; presented as 25th to 75th centiles. Numbers are presented as N (%), unless otherwise denoted as a continuous variable. ICU: Intensive Care Unit; IMV: invasive mechanical ventilation; SOFA: Sequential Organ Failure Assessment; APACHE-II: Acute Physiology and Chronic Health-II.
Covid-19 IMV-patients were significantly younger (median 59.7 years, IQR:51.1 to 64.5, p=0.017) than pre-pandemic IMV-patients (median 62.0 years, IQR: 52.0 to 70.0, Table 1). A higher proportion of Covid-19 IMV-patients were males (70.7% [65/92]) than pre-pandemic (57.1% [137/240]). Significantly fewer Covid-19 IMV-patients had prior comorbidity (62.0% [57/92] vs. 87.9% [211/240], p<0.001), and the median number of comorbidities was significantly lower (1, IQR: 0 to 2 vs. 2, IQR: 1 to 4, p<0.001) Significantly fewer Covid-19 IMV-patients had chronic pulmonary disease (5.8% [5/86] vs. 15.4% [37/240], p=0.036). Both groups were similar in terms of socioeconomic status, the prevalence of obesity, diabetes, chronic cardiac disease and asthma. These associations were consistent when only Respiratory category pre-pandemic patients were included (Supplementary Table 1). Covid-19 IMV-patients responded a median 185 days, IQR: 137 to 241, after hospital discharge (Table 1).
Pre-pandemic IMV-patients received a median 8 days of IMV, IQR: 5 to 15; the median APACHE-II score was 20, IQR: 16 to 25 (Table 1).
Primary outcome
The median fatigue severity reported by Covid-19 IMV-patients at the time point they responded was 5.0, (n = 85), IQR: 2.0 to 7.0, Table 2). At 3-months, fatigue severity was similar between Covid-19 (n = 18) and pre-pandemic IMV-patients (n=188). At 6-months, pre-pandemic IMV-patients (n = 166) reported significantly greater fatigue than Covid-19 IMV-patients (n = 29), median 5.7/10, IQR: 3.5 to 7.3/10 vs. median 2/10, IQR: 1.0 to 5.0/10, p<0.001). These associations were again consistent when only Respiratory category pre-pandemic patients were included (Supplementary Table 2): at 3-months, fatigue severity was similar between Covid-19 (n = 18) and Respiratory pre-pandemic IMV-patients (n = 62); at 6-months, Respiratory pre-pandemic IMV-patients (n = 54) reported significantly greater fatigue than Covid-19 IMV-patients (n = 32), median 6.6/10, IQR: 4.6 to 7.8/10, p<0.001. Fatigue at 12-months was not compared because Covid-19 IMV-patients had not accrued follow-up to this time point.
Table 2.
Fatigue severity in Covid-19 and pre-pandemic IMV-patients.
| Covid-19 cohort | Pre-pandemic cohort | p-value | ||
|---|---|---|---|---|
| Total N | — | 18 | 188 | — |
| Fatigue severity at ∼3 months | Median (IQR) | 5.5 (2.0–6.2) | 5.0 (2.8–7.0) | 0.809 |
| Total N | — | 32 | 166 | — |
| Fatigue severity at ∼6 months | Median (IQR) | 2.0 (1.0–5.0) | 5.7 (3.5–7.3) | <0.001 |
| Total N | — | — | 159 | — |
| Fatigue severity at ∼12 months | — | — | 5.0 (3.0–7.0) | — |
| Total N | — | 85 | — | — |
| Fatigue severity across follow-up period | Median (IQR) | 5.0 (2.0–7.0) | — | — |
Fatigue severity according to Visual Analogue Scale in self-reported questionnaires. Covid-19 cohort: ∼3 months—90–120 days, ∼6 months—150–210 days, Fatigue at 12 months was not compared as the Covid-19 cohort had not accrued follow-up to this time point.
Severe fatigue at the time point they responded was reported in 28.2% [24/85] of Covid-19 IMV-patients who responded (Supplementary Table 3). At 3-months post-hospital discharge, the prevalence of severe fatigue was similar in Covid-19 (38.9%, [7/18]) and pre-pandemic IMV-patients (27.1%, [51/188]), and at 6-months significantly less Covid-19 IMV-patients experienced severe fatigue (10.3% [3/29] vs. 32.5% [54/166], p = 0.015). This pattern was again consistent when only Respiratory category pre-pandemic patients were included (Supplementary Table 4): at 3-months and 6-months, respectively, 35.5% [22/62] (p = 0.789) and 44.4% [24/54] (p = 0.0014) of Respiratory category pre-pandemic IMV-patients reported severe fatigue.
Predictors of fatigue
At a univariable level, in the Covid-19 group, females under 50 reported more severe fatigue (mean 5.20, 95%CI: 2.93–7.47); In pre-pandemic IMV-patients, sex did not affect outcomes (Supplementary Figure 1, Supplementary Table 5).
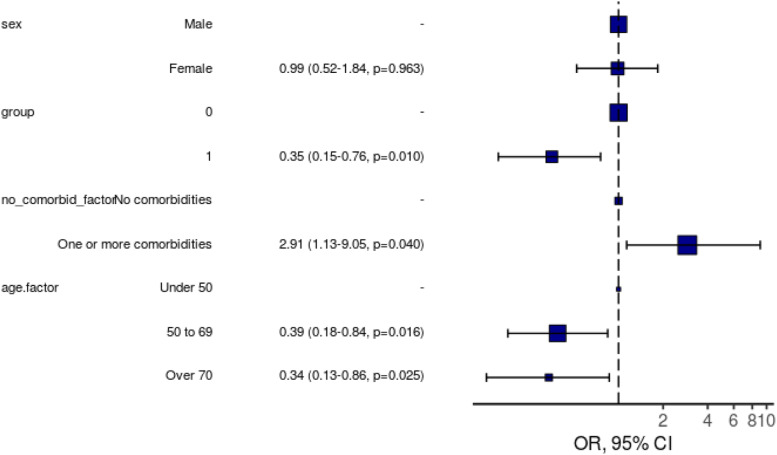
In the total sample included (Covid-19 and pre-pandemic patients), having Covid-19 was significantly associated with less severe fatigue (cut-off 7/10, adjusted OR 0.35 (95%CI:0.15–0.76, p=0.01) (Figure 2, Supplementary Table 5). When the Covid-19 IMV-patients and the Respiratory category pre-pandemic IMV-patients were included, having Covid-19 (adjusted OR 0.15, 95%CI: 0.05–0.41) and female sex (adjusted OR 0.34, 95%CI: 0.12–0.88) were significantly associated with less severe fatigue (Supplementary Figure 2, Supplementary Table 6). Ordinal logistic regression analysis showed that ventilated patients with Covid-19 (adjusted OR 0.50, 95%CI: 0.29–0.84) had less severe fatigue than patients ventilated due to other aetiologies (total pre-pandemic cohort). One or more comorbidities (adjusted OR 3.17, 95%CI: 1.75–5.81) was associated with greater fatigue, and age 50–69 (adjusted OR 0.56, 95%CI: 0.29–1.05), and age over 70 (adjusted OR 0.39, 95%CI: 0.18–0.84) were associated with less fatigue (Supplementary Table 7). When the Covid-19 IMV-patients and the Respiratory category pre-pandemic IMV-patients were included, ordinal logistic regression also showed that having Covid-19 (adjusted OR 0.29, 95%CI: 0.13–0.58) was significantly associated with less severe fatigue (Supplementary Table 8); having prior comorbidity was associated with greater fatigue (adjusted OR 2.18, 95%CI: 1.06–4.54).
Figure 2.
Logistic regression model for severe (≥7/10) fatigue at 6-months including all patients (Covid-19 and pre-pandemic group). OR, 95% CI, p-value. Pre-pandemic patients included at 6-months follow-up. Number in model = 251, AIC = 262.4, C-statistic = 0.676, H&L = Chi-sq (8) 2.26 (p=0.972) Group = Covid-19 status. 0= pre-pandemic group, non-Covid. 1= Covid-19 cohort. No_comorbid_factor = Number of comorbidities. Male, Group 0, No Comorbidities, Age Under 50 are marked with ‘-‘ as these were used as the reference level for each respective variable.
Discussion
We found a high prevalence and severity of patient-reported persistent fatigue following hospital discharge in ventilated patients with both Covid-19 and non-Covid-19 critical illness. At 3-months post-hospital discharge, the prevalence and severity of fatigue reported were similar following Covid-19 and non-Covid-19 critical illness; however by 6-months, patients ventilated for Covid-19 had significantly less severe fatigue than patients ventilated for other critical illness.
Both Covid-19 and non-Covid-19 critical illness survivors experienced high levels of fatigue, adding to existing evidence that finding the best rehabilitation services to support ICU-survivors is a research priority.4,5 In our study, the difference in fatigue prevalence and severity at 6-months suggests that the recovery trajectory from Covid-19 critical illness may be different to general PICS. Covid-19 critical illness survivors are also more likely to suffer additional respiratory sequelae. 29 In our analysis, patients who survived Covid-19 were younger with less prior comorbidity than those surviving other critical illness – this may explain why less fatigue at 6-months was reported after Covid-19 critical illness, as coping with the challenges of comorbidity has been identified as a barrier to ICU-recovery.30,31 The lower fatigue in Covid-19 patients at 6-months is surprising as Covid-19 patients may have been expected to suffer worse fatigue than pre-pandemic patients if they were more likely to be discharged at a lower functional level due to greater high-demand for ICU beds throughout the pandemic and a focus on survival as opposed to rehabilitation. A multitude of different factors interact to drive fatigue in critical illness survivors, including deconditioning secondary to extended immobilisation, anaemia, poor sleep, depression, post-traumatic stress, respiratory illness (which may be due to prolonged ventilation), chronic disease and drugs.1,20,30,32–35 It is possible that once acute illness severity exceeds the threshold for requiring IMV and ICU-admission, further illness severity and underlying disease have little impact on persistent fatigue. This is consistent with recent research which found that post-ICU rehabilitation requirements were unaffected by Covid-19 infection status. 36
Our data provides important insights into these complex syndromes, which need far greater research to assess the true impact they have on patients. To our knowledge, no previous study has included an appropriate control group with similar illness severity to a Covid-19 cohort. There are several important limitations to our study that must be considered. Firstly, our findings may be subject to responder and survivor bias. The sample sizes included by both CCP-UK and RECOVER were relatively small, with the RECOVER sample being substantially larger, and individuals with milder symptoms may have felt less compelled to respond, and those with the most severe symptoms or who died would have been unable to respond. Self-reported fatigue may have been affected by patients’ perceptions of their function or fatigue level prior to ICU admission, which may be affected by their age or previous comorbidities. As the Covid-19 group was significantly younger with less comorbidity, and therefore may have had a self-perceived lower baseline fatigue, an ideal study would have compared more objective measurements of fatigue level. Secondly, the overall Covid-19 cohort was included in our logistic regression models, which may have introduced bias from differing questionnaire response times post-hospital discharge. However, we controlled for CCP-UK and RECOVER measuring outcomes at different time points by stratifying fatigue by time since hospital discharge when comparing fatigue prevalence and severity, which allowed equivalent time points to be compared but further restricted Covid-19 sample sizes, therefore limiting the external validity of our findings to the general ICU population. Thirdly, our results may not be fully representative of all survivors of Covid-19 or non-Covid critical illness, as only patients admitted to a few UK hospitals were included (31 hospitals were included in CCP-UK and only two in RECOVER; therefore, CCP-UK is likely to have captured a much wider variation in population and recovery services available to the patients included). Forthly, as CCP-UK and RECOVER did not use the same outcome measures, we were unable to explore differences in indicators of acute illness severity, including duration of IMV and length of ICU stay, between Covid-19 and pre-pandemic patients, which may have been an important risk factor for fatigue and therefore confounded our results; however, a recent systematic review found no significant difference in mortality, length of hospital stay or IMV-free days between Covid-19 and the general Acute Respiratory Distress Syndrome population. 37 Also, pre-critical illness factors, such as prior comorbidity, may be stronger predictors of hospital readmission and resource use than acute illness factors in ICU survivor patients. 31 An ideal study design would have used retrospective measurements of pre-critical illness functional levels, utilised repeated measures (much like the RECOVER study) of those with Covid-19, and would also feature a non-Covid-19 contemporaneous control group, to control for other factors which may impact post-ICU recovery, such as the ‘lockdown’ restrictions in place during CCP-UK’s study period; restrictions such as office closures may have meant survivors’ lives were less physically demanding, allowing for greater rest, and the pandemic has heralded the formalisation of ICU-recovery clinics in some areas. 38 As the RECOVER study was completed 8 years prior to CCP-UK, the level of follow-up care available is likely to have changed over this time prior to the Covid-19 pandemic, particularly as rehabilitation after critical illness was made a NICE quality standard in 2017, recommending a review at 2–3 months post-ICU-discharge. 4
We found high levels of fatigue following both Covid-19 and non-Covid-19 critical illness. Survivors of Covid-19 critical illness experienced less severe fatigue at 6-months post-hospital discharge than survivors of non-Covid-19 critical illness, potentially due in part to the comparatively younger and less comorbid Covid-19 ICU cohort versus the non-Covid-19 cohort. Research targeting interventions to best support critical illness survivors is required in order to optimise recovery.
Supplemental Material
Supplemental Material, sj-pdf-1-inc-10.1177_17511437211052226 for Recovery from Covid-19 critical illness: A secondary analysis of the ISARIC4C CCP-UK cohort study and the RECOVER trial by Ellen Pauley, Thomas M Drake, David M Griffith, Louise Sigfrid, Nazir I Lone, Ewen M Harrison, J Kenneth Baillie, Janet T Scott, Timothy S Walsh, Malcolm G Semple and Annemarie B Docherty in Journal of the Intensive Care Society
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work is supported by grants from the following: the National Institute for Health Research (NIHR) [award CO-CIN-01], the Medical Research Council [grant MC_PC_19059] and by the NIHR Health Protection Research Unit (HPRU) in Emerging and Zoonotic Infections at University of Liverpool in partnership with Public Health England (PHE), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford [award 200907], NIHR HPRU in Respiratory Infections at Imperial College London with PHE [award 200927], NIHR Biomedical Research Centre at Imperial College London [IS-BRC-1215–20013] and NIHR Clinical Research Network for providing infrastructure support for this research. The views expressed are those of the authors and not necessarily those of the NIHR, MRC or PHE.
Study registration ISRCTN66726260: The ISARIC WHO CCP-UK study was registered at https://www.isrctn.com/ISRCTN66726260 and designated an Urgent Public Health Research Study by NIHR.
Ethical approval: Ethical approval was given by the South Central – Oxford C Research Ethics Committee in England (Ref 13/SC/0149), the Scotland A Research Ethics Committee (Ref 20/SS/0028) and the WHO Ethics Review Committee (RPC571 and RPC572, 25 April 2013.
Data sharing: This work uses data provided by patients and collected by the NHS as part of their care and support #DataSavesLives. The CO-CIN data were collated by ISARIC4C Investigators. ISARIC4C welcomes applications for data and material access through our Independent Data and Material Access Committee (https://isaric4c.net).
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Ellen Pauley https://orcid.org/0000-0001-6994-9722
Nazir I Lone https://orcid.org/0000-0003-2707-2779
References
- 1.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: An overview. J Transl Int Med 2017; 5: 90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lone NI, Gillies MA, Haddow C, et al. Five-Year Mortality and Hospital Costs Associated with Surviving Intensive Care. Am J Respir Crit Care Med 2016; 194: 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly B, Douiri A, Steier J, et al. A UK survey of rehabilitation following critical illness: implementation of NICE Clinical Guidance 83 (CG83) following hospital discharge. BMJ Open 2014; 4: e004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Excellence NI for health and C . Rehabilitation after critical illness in adults. NICE guidance, 2017, https://www.nice.org.uk/guidance/qs158/resources/rehabilitation-after-critical-illness-in-adults-pdf-75545546693317 (accessed 15 April 2019). [PubMed] [Google Scholar]
- 5.James Lind Alliance . The Priority Setting Partnerships (Online). Intensive Care Top; 10. [Google Scholar]
- 6.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia 2020; 75: 1340–1349. [DOI] [PubMed] [Google Scholar]
- 8.ICNARC . ICNARC report on COVID-19 in cri?cal care: England, Wales and Northern Ireland, 2021. [Google Scholar]
- 9.SICSAG: Scottish Intensive Care Society Audit Group . Scottish Intensive Care Society Audit Group report on COVID-19, 2021. [Google Scholar]
- 10.NICE . COVID-19 rapid guideline: managing the long-term effects of COVID-19, 2020. NICE guideline [NG188], https://www.nice.org.uk/guidance/ng188 (accessed 10 March 2020). [PubMed] [Google Scholar]
- 11.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation—a multi-centre prospective cohort study. medRxiv 2021; 2021: 21254057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latronico N., Peli E., Rodella F., et al. Prepr with Lancet. Three-Month Outcome in Survivors of COVID-19 Associated Acute Respiratory Distress Syndrome, SSRN Journal. DOI: 10.2139/ssrn.3749226. [DOI]
- 13.Sigfrid L., Drake T. M., Pauley E., et al. , Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. DOI: 10.1101/2021.03.18.21253888.Epub ahead of print 2021 [DOI] [PMC free article] [PubMed]
- 14.Munblit D, Bobkova P, Spiridonova E, et al. Risk factors for long-term consequences of COVID-19 in hospitalised adults in Moscow using the ISARIC Global follow-up protocol: StopCOVID cohort study. medRxiv 2021: 2021. DOI: 02.17.21251895. [Google Scholar]
- 15.Carfì A, Bernabei R, Landi F, et al. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott JT, Sesay FR, Massaquoi TA, et al. Post-Ebola Syndrome, Sierra Leone. Emerg Infect Dis 2016; 22: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung TM, Wills B, Clapham HE, et al. The Uncertainty Surrounding the Burden of Post-acute Consequences of Dengue Infection. Trends Parasitol 2019; 35: 673–676. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. DOI: 10.1038/s41591-021-01292-y.Epub ahead of print 2021 [DOI] [PMC free article] [PubMed]
- 20.Bench S, Stayt L, Shah A, et al. Prevalence and experience of fatigue in survivors of critical illness: a mixed-methods systematic review, DOI: 10.1111/anae.15441 10.1111/anae.15441.Anaesthesia; n/a. Epub ahead of print 11 March 2021 [DOI] [PubMed] [Google Scholar]
- 21.Kean S, Salisbury LG, Rattray J, et al. 'Intensive care unit survivorship'—A constructivist grounded theory of surviving critical illness. J Clin Nurs 2017; 26: 3111–3124. [DOI] [PubMed] [Google Scholar]
- 22.Sidiras G, Patsaki I, Karatzanos E, et al. Long term follow-up of quality of life and functional ability in patients with ICU acquired Weakness—A post hoc analysis. J Crit Care 2019; 53: 223–230. [DOI] [PubMed] [Google Scholar]
- 23.Walsh TS, Salisbury LG, Merriweather JL, et al. Increased Hospital-Based Physical Rehabilitation and Information Provision After Intensive Care Unit Discharge: The RECOVER Randomized Clinical Trial. JAMA Intern Med 2015; 175: 901–10. [DOI] [PubMed] [Google Scholar]
- 24.ISARIC. ISARIC . Clinical Characterisation Protocol (CCP): UK version, 2020, https://isaric4c.net/protocols/(accessed 1 February 2020). [Google Scholar]
- 25.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20: e192–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta RK, Harrison EM, Ho A, et al. Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: a prospective cohort study. Lancet Respir Med 0, Epub ahead of print 5 March 2021. DOI: 10.1016/S2213-2600(20)30559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SK, Chun HJ, Kim DW, et al. Acute Physiology and Chronic Health Evaluation II and Simplified Acute Physiology Score II in predicting hospital mortality of neurosurgical intensive care unit patients. J Korean Med Sci 2009; 24: 420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–10. [DOI] [PubMed] [Google Scholar]
- 29.McWilliams D, Weblin J, Hodson J, et al. Rehabilitation Levels in COVID-19 Patients Admitted to Intensive Care Requiring Invasive Ventilation: An Observational Study. Ann Am Thorac Soc 2020; 18: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaghy E, Salisbury L, Lone NI, et al. Unplanned early hospital readmission among critical care survivors: a mixed methods study of patients and carers. BMJ Qual Saf 2018; 27: 915–927. [DOI] [PubMed] [Google Scholar]
- 31.Lone NI, Lee R, Salisbury L, et al. Predicting risk of unplanned hospital readmission in survivors of critical illness: A population-level cohort study, 2018. thoraxjnl-2017-210822.Thorax [DOI] [PubMed] [Google Scholar]
- 32.Docherty AB, Turgeon AF, Walsh TS. Best practice in critical care: anaemia in acute and critical illness. Transfus Med 2018; 28: 181–189. [DOI] [PubMed] [Google Scholar]
- 33.Hatch R, Young D, Barber V, et al. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Crit Care 2018; 22: 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009; 37: S299–308. [DOI] [PubMed] [Google Scholar]
- 35.Medrzycka-Dabrowska W, Lewandowska K, Kwiecień-Jaguś K, et al. Sleep Deprivation in Intensive Care Unit - Systematic Review. Open Med (Wars) 2018; 13: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puthucheary Z, Brown C, Corner E, et al. The Post-ICU presentation screen (PICUPS) and rehabilitation prescription (RP) for intensive care survivors part II: Clinical engagement and future directions for the national Post-Intensive care Rehabilitation Collaborative. J Intensive Care Soc 2021: 1751143720988708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dmytriw AA, Chibbar R, Chen PPY, et al. Outcomes of acute respiratory distress syndrome in COVID-19 patients compared to the general population: a systematic review and meta-analysis. Expert Rev Respir Med, 152021: 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NHS England. NHS launches 40 ‘long COVID’ clinics to tackle persistent symptoms, 2020, https://www.england.nhs.uk/2020/11/nhs-launches-40-long-covid-clinics-to-tackle-persistent-symptoms/%0A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-inc-10.1177_17511437211052226 for Recovery from Covid-19 critical illness: A secondary analysis of the ISARIC4C CCP-UK cohort study and the RECOVER trial by Ellen Pauley, Thomas M Drake, David M Griffith, Louise Sigfrid, Nazir I Lone, Ewen M Harrison, J Kenneth Baillie, Janet T Scott, Timothy S Walsh, Malcolm G Semple and Annemarie B Docherty in Journal of the Intensive Care Society