ABSTRACT
BACKGROUND:
The aim of this study was to examine the effect of systemic immunoinflammatory index (SII), calculated on presentation to the emergency department (ED), on the prediction of clinical outcomes of patients who were diagnosed with acute pancreatitis (AP).
METHODS:
This research was designed as a single-center, cross-sectional, and retrospective study. Adult patients who were diagnosed with AP in the ED between October 2021 and October 2022 in the tertiary care hospital, whose diagnostic and therapeutic procedures were complete in the data recording system, have been included in the study.
RESULTS:
Mean age, respiratory rate, and length of stay of the non-survivors were significantly higher than the mean of the survivors (t-test, p=0.042, p=0.001, and p=0.001, respectively). Mean SII score of the patients with fatal outcome was higher than the survivors (t-test, p=0.001). ROC analysis of the SII score to predict mortality revealed that the area under the curve was found to be 0.842 (95%CI 0.772–0.898), and the Youden index was 0.614, (p=0.001). When the cutoff value of the SII score in determining mortality is >1243, the sensitivity of the score was found to be 85.0%, specificity 76.4%, positive predictive value 37.0%, and negative predictive value 96.9%.
CONCLUSION:
SII score was statistically significant in estimating mortality. SII calculated on presentation to the ED can be a useful scoring system to predict the clinical outcomes of patients who were admitted to the ED and were diagnosed with AP.
Keywords: Acute pancreatitis, biomarker, immunoinflammation, mortality, outcome, systemic immunoinflammatory index
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory condition of the pancreas that are clinically characterized by abdominal pain and high levels of pancreatic enzymes in the blood. While the incidence of AP increases over the years, its mortality decreases substantially. Gallstones and alcoholism constitute around 70% while iatrogenic pancreatitis makes around 10% of the cases.[1] The incidence of AP is rising worldwide due to obesity and increased prevalence of gallstones, medication use, and alcoholism.
The revised Atlanta classification lists criteria to diagnose AP as 1 abdominal pain consistent with AP (acute onset of a persistent, severe, and epigastric pain often radiating to the back); two serum lipase or amylase at least 3 times greater than the upper limit of normal; and three typical radiologic findings of acute pancreatic damage on contrast-enhanced computed tomography and/or on magnetic resonance imaging or ultrasonography.[2]
Mortality rates are much higher in certain subgroups of patients with severe course of AP. The ability to predict disease severity helps distinguish patients at high risk of serious morbidity and mortality. The greater the power to predict the severity of the disease, the easier it will be to apply appropriate early triage for intensive care units and special therapeutic interventions.[3] In routine examination, systemic immunoinflammatory index (SII), which is an easy and economical biomarker calculated from complete blood count (CBC), is the product of the formula “platelet count × neutrophil/lymphocyte count.”
Various scoring systems and biomarkers are used for rapid diagnosis and treatment in emergency department (ED) patients.[4,5] SII can be used for this purpose as. SII has been used to predict outcomes of the patients with AP in the past decade, especially in the field of oncology. It has also been employed by other disciplines such as ED due to its superiority in predicting inflammation as it contains three main parameters of CBC. As a new inflammatory marker, SII correlates positively with neutrophil and platelet counts and negatively with lymphocyte counts, and its clinical significance has been reported in malignant and inflammatory diseases.[6,7]
The primary objective of this study is to examine the effect of SII calculated on presentation to the ED on the clinical outcomes of patients who were admitted to the ED and were diagnosed with AP.
MATERIALS AND METHODS
This research was designed as a single-center, cross-sectional, and retrospective study. The institutional management approved the analysis and issued a waiver of consent (ethics committee ruling number: 3688, date: October 18, 2022). Adult patients who were diagnosed with AP in the ED between October 2021 and October 2022 in our tertiary care hospital, whose diagnostic and therapeutic procedures were completely available in the data recording system, have been included in the study. Excluded from the study were patients under 18 years of age, pregnant women, and those with incomplete data. The sample size was calculated as 136 patients, based on the number of patients in the studies conducted with a 95% Confidence Interval (CI), and a 0.5% margin of error. The study period is determined as 1 year between October 01, 2021, and October 01, 2022. It was planned to record the data obtained from the electronic files of the patients in the follow-up sheets to be created for each patient.
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 for Windows and MedCalc. Descriptive measures are presented as mean and standard deviation with percentage distribution. The conformity of the data to the normal distribution was checked with the Kolmogorov–Smirnov test. ROC analysis was used to determine the cutoff value of the SII score in predicting mortality. The significance level was taken as p<0.05.
RESULTS
After exclusions, a total of 143 patients were enrolled in the final analysis. Among these, 123 patients (86%) survived while 20 (14%) died in the follow-up period. As a result of the statistical analysis, it was noted that the mean age of the non-survivors was significantly higher than the mean age of the survivors (t-test, p=0.042) (Table 1). Mean respiratory rate and length of stay of the non-survivors were also significantly higher than the mean of the survivors (t-test, p=0.001, and p=0.001). Table 1 also shows that mean ALT, AST, glucose, blood urea nitrogen, creatinine, Na, K, LDH, SO2, lactate, HCO3, CBC profiles including platelets, and neutrophil leukocytes levels of the non-survivors were found to be significantly higher than those of the survivors (p<0.05). On the other hand, SpO2, Ca, levels, and lymphocytes counts of the survivors were found to be significantly higher than the mean of the non-survivors (t-test, p=0.001, p=0.027, and p=0.008, respectively).
Table 1.
Comparison of survivors and non-survivors with respect to clinical, laboratory and radiological characteristics in patients with acute pancreatitis
| Survived (n=123) | Died (n=20) | p | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | ||
|
|
|
|
|
||
| N | % | N | % | ||
| Age | 60.7 | 16 | 68.7 | 14.5 | 0.042* |
| Sex | 0.796# | ||||
| Female | 70 | 569 | 12 | 60.0 | |
| Male | 53 | 43.1 | 8 | 40.0 | |
| Biliary pancreatitis | 52 | 51.0 | 10 | 52.6 | 0.895# |
| Hyperlipidemia | 8 | 10.4 | 3 | 18.8 | 0.346# |
| Alcoholic pancreatitis | 7 | 9,0 | 0 | 0 | 0.213# |
| Drug-related pancreatitis | 1 | 13 | 1 | 6.3 | 0.223# |
| Tumor-related pancreatitis | 2 | 2.6 | 1 | 5.9 | 0.486# |
| Idiopathic pancreatitis | 49 | 55.7 | 8 | 53.3 | 0.866# |
| Pancreatic edema | 4 | 5.3 | 5 | 31.3 | 0.002# |
| Pancreatic necrosis | 1 | 1.3 | 0 | 0 | 0.642# |
| Length of stay in hospital (days) | 6.8 | 4.9 | 15.7 | 16.5 | 0.001* |
| Systolic pressure (mmHg) | 134.2 | 16.5 | 130.9 | 26.9 | 0.456* |
| Diastolic pressure (mmHg) | 78.4 | 9.0 | 75.8 | 13.7 | 0.279* |
| Pulse rate (bpm) | 77.3 | 11.3 | 81.3 | 13.9 | 0.163* |
| Temperature (°C) | 36.3 | 0.7 | 36.7 | 1.0 | 0.054* |
| SpO2 (%) | 96.9 | 1.9 | 94.9 | 4.2 | 0.001* |
| Respiratory rate (bpm) | 14.6 | 2.3 | 17.7 | 7.3 | 0.001* |
| Albumin (g/L) | 19.4 | 15.3 | 14.9 | 15.1 | 0.237* |
| ALT (IU/L) | 68.3 | 78.0 | 277.8 | 615.3 | 0.001* |
| AST (IU/L) | 41.6 | 40.3 | 774.3 | 2261.9 | 0.001* |
| CRP (mg/L) | 116.7 | 84.3 | 142.6 | 89.9 | 0.210* |
| Glucose (mg/dL) | 104.8 | 44.3 | 138.8 | 77.6 | 0.006* |
| Urea (mg/dL) | 30.3 | 24.3 | 55.5 | 45.8 | 0.001* |
| Sodium (mEq/dL) | 137.8 | 3.3 | 139.7 | 6.0 | 0.040* |
| Potassium (mEq/dL) | 3.8 | 0.5 | 4.2 | 0.9 | 0.025* |
| LDH (IU/L) | 287.9 | 272.2 | 538.1 | 410.0 | 0.001* |
| Calcium (mg/dL) | 8.5 | 0.5 | 8.2 | 0.8 | 0.027* |
| Chloride (mg/dL) | 101.6 | 3.8 | 99.9 | 6.0 | 0.087* |
| Creatinine (mg/dL) | 0.85 | 0.96 | 1.3 | 1.0 | 0.047* |
| SO2 (mg/dL) | 63.9 | 21.7 | 70.3 | 23.5 | 0.226* |
| Lactate (mmol/dL) | 1.93 | 0.72 | 3.43 | 2.59 | 0.001* |
| HCO3 (mEq/dL) | 25.3 | 2.4 | 28.0 | 7.7 | 0.002* |
| PO2 (mmHg) | 37.6 | 13.5 | 58.5 | 44.8 | 0.001* |
| PCO2 (mmHg) | 43.7 | 8.2 | 44.9 | 6.0 | 0.539* |
| pH | 7.4 | 0.05 | 7.4 | 0.1 | 0.208* |
| PLT (109/L) | 211.6 | 82.1 | 272.3 | 137.8 | 0.007* |
| NE (103/mm3) | 6.4 | 3.5 | 11.1 | 4.9 | 0.001* |
| LY (103/mm3) | 1.5 | 0.6 | 1.1 | 0.5 | 0.008* |
| HGB (g/dL) | 11.7 | 1.7 | 12.1 | 2.6 | 0.450* |
| HCT (%) | 36.2 | 9.2 | 33 | 7.6 | 0.969* |
| WBC (103/mm3) | 8.8 | 3.6 | 13.1 | 5.1 | 0.001* |
| SII | 1005.6 | 791.5 | 3360.9 | 2485.0 | 0.001* |
Student t-test.
Chi-square analysis. SD: Standard deviation; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CRP: C-reactive protein; LD: Lactate dehydrogenase; PLT: Platelets; NE: Neutrophil leukocytes; LY: Lymphocytes; HGB: Hemoglobin; HCT: Hematocrit; WBC: White blood cell; SII: Systemic immunoinflammatory index.
It was recorded that mean SII score of the patients with fatal outcome was significantly higher than the survivors (t-test, p=0.001). As a result of the ROC analysis of the SII score to predict mortality, the area under the curve was found to be 0.842 (95%CI 0.772–0.898), and the Youden index was 0.614, (p=0.001) (Table 2 and Fig. 1). Statistical analysis revealed that the SII score was statistically significant in predicting mortality (p<0.001).
Table 2.
Diagnostic values and cut-off level of the SII score to predict mortality in patients with acute pancreatitis
| AUC | Cut-Off | Sensitivity (%) | Specificity (%) | +LR | -LR | PPV | NPV | Youden Index | |
|---|---|---|---|---|---|---|---|---|---|
| SII | 0.842 (0.772–0.898) | >1243 | 85.0 | 76.4 | 3.6 | 0.2 | 37.0 | 96.9 | 0.614 |
AUC: Area under the curve; LR: Likelihood ratio; PPV: Positive predictive value; NPV: Negative predictive value; SII: Systemic immunoinflammatory index.
Figure 1.
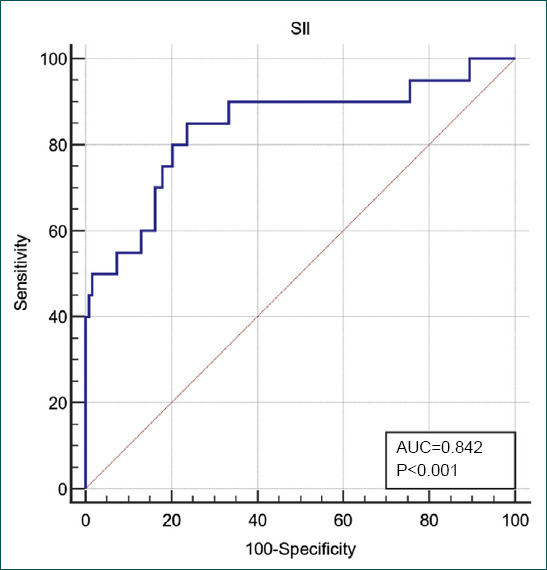
Areas under ROC curves based on the SII scores to predict mortality in patients with acute pancreatitis.
When the cutoff value of the SII score in determining mortality is >1243, the sensitivity of the score was found to be 85.0%, specificity 76.4%, positive predictive value 37.0%, and negative predictive value 96.9%.
DISCUSSION
AP is an inflammatory disorder of the pancreas encompassing a cascade of cellular and molecular events. It starts from premature activation of zymogens with the involvement of innate immune system to a potential systemic inflammatory response and multiple organ failure.[8]
The same consensus report has divided the severity of AP into mild, moderately severe, and severe. The early phase of severe AP generally coincides with the 1st week and is characterized by the activation of the autoinflammatory cascade, gut dysbiosis, bacterial translocation, and alleviated immune responses. On the other hand, the late phase of the process is marked by the development of local and systemic complications.[9] The initial management of AP aims to define the clinical severity of the disease, and then to assess and treat the fluid losses and renal, cardiovascular, and respiratory failure. Thus, the evaluation of the vital signs and the organ functions as demonstrated by laboratory and radiological investigations are essential in the management of these patients.
The current understanding of the course of AP is yet to be completed, limiting management options to supportive care. A great deal of evidence has indicated modulation of the immune system for strategic therapeutic development, by blunting the inflammatory response and in turn, severity of AP. The clinical outcome for AP is bound with the degree of pancreatic necrosis and the severity of multisystem organ failure triggered by the systemic inflammatory response. Literature data suggest that there is a delicate balance between localized damage with inflammatory cytokine secretion and a systemic anti-inflammatory response that limits the inappropriate expression of proinflammatory agents. Implication of such mediators suggests that suppression of an inappropriate immune response may improve the prognosis of the patients with AP.[10]
Autodigestion of the pancreatic gland following secretion of the pancreatic enzymes activates local and systemic inflammation with boosted expression of several cytokines and acute phase reactants (TNF-alpha, IL-6, and CRP).[11,12] Overgrowth of gut bacteria and the related bacterial systemic translocation may cause more damage on this inflammatory pathways, which paves the way for the diminution of the immune system as a whole.[13,14] Likewise, the findings of the present study also supported that inflammatory biomarkers representing cellular immunity such as WBC and NE derived from non-survivors were significantly higher than the survivors. On the other hand, high levels of glucose, blood urea nitrogen, creatinine, liver enzymes, Na, K, LDH, and lactate in the non-survivors are suggestive of remarkably worse general condition and higher frequency of organ failures of non-survivors when compared to survivors.
SII can be used to determine the outcome of the inflammation-related diseases and to identify patients with poor clinical course. For example, SII was noted to be significantly higher in the non-survivor patients with acute cholecystitis than in the survivors in a Turkish study.[15] A high level of SII is an independent risk factor for short survival of patients with metastatic and unresectable pancreatic cancer. Patients with a high level of the inflammatory markers SII, neutrophil-to-lymphocyte count neutrophil/lymphocyte ratio (NLR), and the platelet-to-lymphocyte count, may be more prone to early liver metastasis.[16] Of note, findings of the present study conform with these previous information on the impact of SII with the severity of inflammation in the pancreatic tissue.
Schlanger et al.[17] studied on 312 patients with pancreatic ductal adenocarcinoma cited that the circulating immune cells and their values integrated in the assessed prognostic scores suffer statistically significant changes after curative pancreatic surgery. SII scores were compared between pre-operative and post-operative phases in this research which disclosed a significant increase in mean SII scores following surgery. Likewise, host systemic inflammatory response and local inflammatory responses, modified Glasgow Prognostic Score, Prognostic Index, and NLR were pointed out to play a role in stratification of outcomes of patients with pancreatic ductal adenocarcinoma.[18] All these findings implicate that immune scores can have a pivotal role as accurate prognosticators in patients with pancreatic inflammation. Studies in the literature have reported that an increased SII value may predict poor outcome in patients with AP.
Limitations
Retrospective and single-center design with a small sample size constitute major limitations of the study. Age limits and exclusions of the study should be taken into account before extrapolation of the findings to the general population.
Conclusion
SII is a marker of autoinflammatory cascade triggered by the acute inflammation by any cause. It can be a useful scoring system to predict the clinical outcomes of patients who were admitted to the ED and were diagnosed with AP when calculated on presentation to the ED. In addition, the SII score was statistically significant in estimating mortality. Future broad-based and multicentric studies should analyze the interaction of SII with specific subgroups, subpopulations with comorbidities, age groups, and other variables.
Footnotes
Ethics Committee Approval: This study was approved by the University of Health Sciences, Şişli Hamidiye Etfal Training and Research Hospital (SUAM) Clinical Research Ethics Committee (Date: 18.10.2022, Decision No: 3688).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: M.K.; Design: M.K., H.A; Supervision: U.D.H; Resource: M.K.; Materials: H.A.; Data: H.A.; Analysis: M.K., U.D.H; Literature search: H.A., M.K.; Writing: H.A., M.K.; Critical revision: U.D.H, H.A.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Quinlan JD. Acute pancreatitis. Am Fam Physician. 2014;90:632–9. [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012:Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Wang T, Dong X, Sun L, Wu Q, Liu J, et al. Systemic immune-inflammation index for predicting the prognosis of critically ıll patients with acute pancreatitis. Int J Gen Med. 2021;14:4491–8. doi: 10.2147/IJGM.S314393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohat AK, Kurt E, Şenel Ç. The comparison of two prediction models for ureteral stones:CHOKAI and STONE scores. Am J Emerg Med. 2021;44:187–91. doi: 10.1016/j.ajem.2020.08.099. [DOI] [PubMed] [Google Scholar]
- 5.Ak R, Doğanay F, Akoğlu EU, Akoğlu H, Uçar AB, Kurt E, et al. Predictive value of scoring systems for the diagnosis of acute appendicitis in emergency department patients:Is there an accurate one? Hong Kong J Emerg Med. 2020;27:262–9. [Google Scholar]
- 6.Liu X, Guan G, Cui X, Liu Y, Liu Y, Luo F. Systemic immune-inflammation index (SII) can be an early indicator for predicting the severity of acute pancreatitis:A retrospective study. Int J Gen Med. 2021;14:9483–9. doi: 10.2147/IJGM.S343110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ergenç H, Ertürk Z, Eminler AT, Cinemre H. Diagnostic and prognostic value of neutrophil/lymphocyte ratio and platelet/lymphocyte ratios on acute pancreatitis patients. Online Türk Sağlık Bilimleri Dergisi. 2022;7:80–5. [Google Scholar]
- 8.Shamoon M, Deng Y, Chen YQ, Bhatia M, Sun J. Therapeutic implications of innate immune system in acute pancreatitis. Expert Opin Ther Targets. 2016;20:73–87. doi: 10.1517/14728222.2015.1077227. [DOI] [PubMed] [Google Scholar]
- 9.Pagliari D, Brizi MG, Saviano A, Mancarella FA, Dal Lago AA, Serricchio ML, et al. Clinical assessment and management of severe acute pancreatitis:A multi-disciplinary approach in the XXI century. Eur Rev Med Pharmacol Sci. 2019;23:771–87. doi: 10.26355/eurrev_201901_16892. [DOI] [PubMed] [Google Scholar]
- 10.Minkov GA, Halacheva KS, Yovtchev YP, Gulubova MV. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. 2015;44:713–7. doi: 10.1097/MPA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 11.Zhang MS, Zhang KJ, Zhang J, Jiao XL, Chen D, Zhang DL. Phospholipases A-II (PLA2-II) induces acute pancreatitis through activation of the transcription factor NF-kappaB. Eur Rev Med Pharmacol Sci. 2014;18:1163–9. [PubMed] [Google Scholar]
- 12.Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2013;17:349–55. [PubMed] [Google Scholar]
- 13.Pagliari D, Saviano A, Newton EE, Serricchio ML, Dal Lago AA, et al. Gut microbiota-immune system crosstalk and pancreatic disorders. Mediators Inflamm. 2018;2018:7946431. doi: 10.1155/2018/7946431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y, Xu H, Chen Y, Wu J, Jin F, Wu Q, et al. Therapeutic effect of Bifidobacterium combined with early enteral nutrition in the treatment of severe acute pancreatitis:A pilot study. Eur Rev Med Pharmacol Sci. 2018;22:4018–24. doi: 10.26355/eurrev_201806_15288. [DOI] [PubMed] [Google Scholar]
- 15.Özdemir S, Altunok İ, Özkan A, İslam MM, Algın A, Eroğlu SE, et al. The role of the hematological inflammatory index and systemic role of the hematological inflammatory index and systemic immuno-inflammation index in acute cholecystitis. Eur J Clin Exp Med. 2022;20:330–5. [Google Scholar]
- 16.Han R, Tian Z, Jiang Y, Guan G, Wang X, Sun X, et al. Prognostic significance of the systemic immune inflammation index in patients with metastatic and unresectable pancreatic cancer. Front Surg. 2022;9:915599. doi: 10.3389/fsurg.2022.915599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlanger D, Popa C, Paşca S, Seicean A, Al Hajjar N. The role of systemic immuno-inflammatory factors in resectable pancreatic adenocarcinoma:A cohort retrospective study. World J Surg Oncol. 2022;20:144. doi: 10.1186/s12957-022-02606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imrie CW. Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology. 2015;15:327–30. doi: 10.1016/j.pan.2015.04.004. [DOI] [PubMed] [Google Scholar]


