Abstract
The world's population is increasing and is anticipated to spread 10 billion by 2050, and the issue of food security is becoming a global concern. To maintain global food security, it is essential to increase crop productivity under changing climatic conditions. Conventional agricultural practices frequently use artificial/chemical fertilizers to enhance crop productivity, but these have numerous negative effects on the environment and people's health. To address these issues, researchers have been concentrating on substitute crop fertilization methods for many years, and biofertilizers as a crucial part of agricultural practices are quickly gaining popularity all over the globe. Biofertilizers are living formulations made of indigenous plant growth-promoting rhizobacteria (PGPR) which are substantial, environment-friendly, and economical biofertilizers for amassing crop productivity by enhancing plant development either directly or indirectly, and are the renewable source of plant nutrients and sustainable agronomy. The review aims to provide a comprehensive overview of the current knowledge on microbial inoculants as biofertilizers, including their types, mechanisms of action, effects on crop productivity, challenges, and limitations associated with the use of microbial inoculants. In this review, we focused on the application of biofertilizers to agricultural fields in plant growth development by performing several activities like nitrogen fixation, siderophore production, phytohormone production, nutrient solubilization, and facilitating easy uptake by crop plants. Further, we discussed the indirect mechanism of PGPRs, in developing induced system resistance against pest and diseases, and as a biocontrol agent for phytopathogens. This review article presents a brief outline of the ideas and uses of microbial inoculants in improving crop productivity as well as a discussion of the challenges and limitations to use microbial inoculants.
Keywords: Microbial inoculants, Biofertilizers, Crop productivity, Nitrogen fixation, Phytohormones, Nutrient solubilization
1. Introduction
Microorganisms in the soil play a crucial role in soil biodiversity and coordinated nutrient management. They are essential to the growth and evolution of plants. Recent years have seen the use of chemical fertilizers in agriculture, making the nation more self-sufficient in food production, but at the expense of the ecosystem and the well-being of all living things. The excessive use of these fertilizers in agriculture is expensive and has several negative impacts on soil fertility. To satisfy our agricultural requirements, beneficial microorganisms are better alternatives to conventional farming methods. Biofertilizers are safer than chemical fertilizers because they cause less environmental harm, have more focused activity, and are more efficient when used in lesser amounts. Additionally, they have the capacity to multiply while being concurrently regulated by the plant and local microbes. Additionally, microbial inoculants have quicker decomposition processes and are less likely to cause pathogens and pests to develop resilience [1].
Bioinoculants do not show any detrimental impact on the soil's plant and animal life as they are ecofriendly, highly efficient, and can be utilized as bio pesticides that do not affect any harmful influence on plant products. The plant requires mineral nutrients which can only be provided when chemical fertilizers are used directly or indirectly, along with organic manure and biofertilizers to increase the organic carbon in soil and uphold sustainability in a field and horticultural crops [2]. Microbial inoculants are described as organisms that are introduced into an environment for a particular purpose, such as biocontrol or promoting plant growth, such as bacteria, fungi, and other microorganisms [3]. The term bio-fertilizer refers to a wide range of products that contain living or dormant microorganisms, including bacteria, fungi, actinomycetes, and algae. Upon application, these microorganisms help to fix atmospheric nitrogen or solubilize/mobilize soil nutrients in addition to secreting substances that promote plant growth [4]. Now a day, biofertilizers and bio pesticides are currently available as substitutes for conventional inorganic fertilizers and synthetic pesticides respectively along with a variety of other products.
The market for biofertilizers, which was valued at USD 1.57 billion in 2018, is anticipated to expand at a compound annual growth rate of 12.1% between 2022 and 2027 [5]. Currently, there are a large number of small and fewer big companies operating across various geographical regions, creating a highly fragmented market. Currently, the market for biofertilizers is dominated by many small companies because it is largely unregulated; however, if regulations are implemented, as has happened in the market for biopesticides globally, it is possible that the market will become more consolidated [6].
In addition, PGPRs are a special category of microbes that persuade plant defense mechanism and provide resistance to host through the extremely diverse mechanism for further pathogen attack, and considered more beneficial biocontrol agents (BCAs) than typical chemical fertilizers as they are non-pathogenic, naturally inhabitant of the rhizosphere, environment friendly, and enhancing plant yield directly. According to Gupta et al. [7], PGPRs can influence plant development either directly or indirectly and induce plant growth by deploying mineral nutrients in soils, regulating or inhibiting plants from phytopathogens, generating different plant growth regulators, ameliorating soil structure and bioremediating the soil through separation of noxious heavy metals and lowering chemical compounds such as pesticides, fungicides [[8], [9], [10]]. Besides, above mentioned functions of PGPRs, it also plays different defense actions in plants by producing antibiotics, siderophores, bio-surfactants and volatiles, and enzymes that vitiate cell wall and brought systemic resistance (ISR). Saharan and Nehra [11] suggested that a broad range of non-symbiotic and symbiotic bacterial species belonging to the genus Klebsiella, Azotobacter, Azospirillum, Bacillus, Enterobacter, and Serratia were considered as PGPRs. Many researchers are still working on knowing the diversity and significance of biofertilizers and their functions in the improvement of agricultural sustainability. The effects of PGPRs are due to plant age, plant species, soil factors, different stages of growth and various form of soil [12]. Kumar et al. [13,14] reported that PGPR's role in enhancing nutrient uptake for plants is an essential activity and appropriate for crop development. PGPRs overcome the reduction in plant growth generated by different forms of stresses [15] including, heavy metals stress [16], water logging stress [17], salt stress [18,19] drought stress [20], and various supplementary hostile environmental situations.
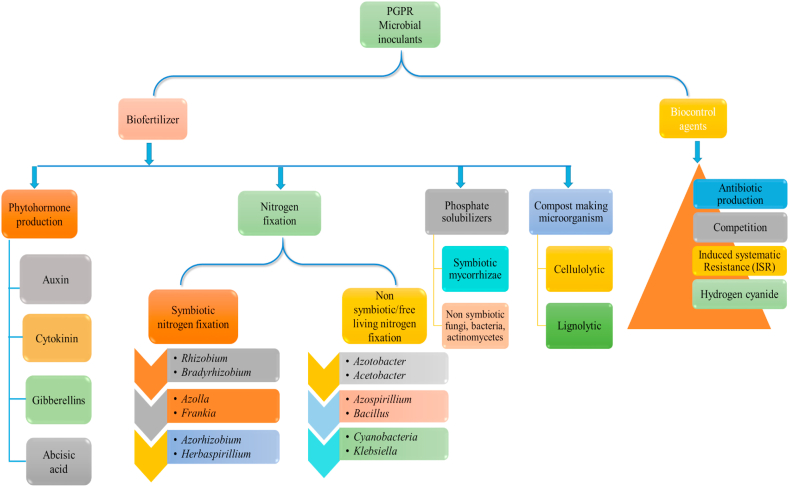
PGPRs inoculation soothes plant stress by promoting useful impacts on plant fitness, growth, increasing the production and assimilation of nutrients. Therefore, it is necessary to use PGPRs for ongoing, beneficial agricultural reasons to improve crop yields and soil fertility in challenging conditions. Over the last few decades, PGPRs are increasingly being used for secure and safe agriculture worldwide. The main obstacle to the farmers' success is a lack of high quality bioinoculants available to them. Azotobacter, Azolla, Acetobacter, Trichoderma, Bacillus thuriengensis, and Azospirillum need to receive the proper attention given to them and their use in different cereal and vegetable crops. To boost the soil's organic carbon and keep the sustainability of field and horticultural crops, these biofertilizers should be combined with organic manures and chemical fertilizers [2]. The schematic representation of different types of PGPRs or microbial inoculants and their significant contribution in crop improvements are documented in Fig. 1.
Fig. 1.
Schematic Illustration of PGPRs microbial inoculants.
The aim of this review is to summarize the importance of microbial inoculants used as biofertilizers, and their mechanism to enhance crop productivity. This review highlights a detailed study of direct and indirect mechanisms of bio inoculants, including biological nitrogen fixation (symbiotic and non-symbiotic), phytohormone production, nutrient solubilization (phosphate and potassium), siderophore production etc., and biocontrol of phytopathogen, chitinases, HCN, and others antifungal properties, as biofertilizer to increase crop yield.
2. Mode of actions or mechanism of PGPRs
2.1. Direct mechanism of PGPRs
2.1.1. Biological nitrogen fixation (BNF)
The primary nutrient for plant growth is nitrogen, considered the fourth significant component of plant dry biomass. It is an important part of genetic material, membranous lipids, and amino acids (enzymatic and structural proteins) [21]. It was described that most nitrogen is inaccessible to animals and plants in gaseous form. Nitrogen fixing PGPRs inoculation on plant yield leads to disease management, growth elevating functions, and retaining the nitrogen level [22]. BNF is a process that converts air elemental nitrogen into ammonia through a reduction cycle with the aid of microbes, some of which are eubacteria, actinomycetes, and blue-green algae. (Table-1). Asymbiotic or free-living nitrogen fixation and symbiotic or associative nitrogen fixation are the broad category of biological nitrogen fixation and are converted by a different mechanism in different crops, and have been presented in Table 1, Table 2. The volume of free nitrogen available in the atmosphere is about 4 × 10 21 g N, of which nearly 2.5 × 10 11 kg NH3 is fixed yearly by microorganisms [23]. Naturally, in total, 70% of the nitrogen is fixed by microbes referred to as biological nitrogen fixation, and 30% by chemical and physical processes [2]. Table 2 shows the amount of atmospheric nitrogen fixed by different microbial inoculants.
Table 1.
Showing different types of nitrogen fixing microorganisms in various crops.
| S. No. | Free living/non-symbiotic/symbiotic | Crops | Reference(s) | Free living/non-symbiotic/symbiotic | Crops | Reference(s) |
|---|---|---|---|---|---|---|
| 1. | Azotobacter, Azospirillum Azospirilliun sp., Pseudomonas sp., | Potato | [34] |
B. subtilis, B. lichenoformis P. agglomerans, B. cereus |
chickpea | [45] [46] |
| 2. |
Herbaspirillum sp., Bacillus sps. Pasteurella multicida, K. pneumonia, K. oxycota Acetobacter |
Sugarcane, | [35] [36] |
Bacillus sp. Klebsiella sp. B. majavensis, P. aeruginosa, |
Maize | [47] [48] |
| 3. |
Azospirillum brasilense Pseudomonas sp. P. mosselii Stenotrophomonas maltophilia, Chryseobacterium |
Wheat | [37] [38] [39] |
Bacillus, Pseudomonas sp. | Sorghum and chilli | [49] |
| 4. |
Pseudomonas sp, S. marcescens Anabaena, Azolla K. pneumonia, B. subtilis, Microbacterium |
Rice | [40] [41] |
Mesorhizobium Sinorhizobium Azorhizobium |
Lotus Alfaalfa Sesbania |
- |
| 5. |
Rhizobium sp. Bradyrhizobium |
Beans, Peas, Green gram (Vigna radiate) |
[42] [27] |
Cyanobacteria | Moss | – |
| 6. |
Rhizobium japonicum Bradyrhizobium |
Soybean (Glycine max) | [43] [44] |
Frankia | Actinorhizal plants | – |
Table 2.
Showing various inoculants' contributions to the amount of biological N2 fixation.
| S. No. | State | Aerobic/anaerobic | Bacteria | Crop | Amount of N2 fixed Kg/ha/year | Reference(s) |
|---|---|---|---|---|---|---|
| 1. | Free living | Aerobic | Azotobacter | 10–20 | [2] | |
| Azotobacter | Dry land crops | 20–25 | [4] | |||
| Anaerobic | Clostridia | 2–5 | ||||
| Facultative | Klebsiella | 5–10 | ||||
| 2. | Symbiotic | Legumes | Rhizobia | 50–500 | [2] | |
| Rhizobium strains | Groundnut, soybean | 50–200 | [4] | |||
| Non-legumes | Azospirillum | 5–20 | ||||
| Acetobacter | 150 | |||||
| Anabaena | rice | 600 | [41,50] | |||
| 3. | Blue green algae | Anabaena | 20–25 | [2] | ||
| Azolla | 70–100 | |||||
| Azolla | rice | 30–100 | [4] |
2.1.1.1. Symbiotic nitrogen fixation (SNF)
Symbiotic nitrogen fixation (SNF) is a mutualistic association between plants and microbes. The symbiotic nitrogen fixing microbes have the capability of fixing atmospheric nitrogen symbiotically and provided access to all types of plants. Mutualistic relationships begin once the plant starts to secrete flavonoids and iso-flavonoids in its rhizosphere, where it is identified by Rhizobium [24]. Rhizobium, Sinorhizobium, Bradyrhizobium, and Mesorhizobium are a few examples of bacteria living symbiotically with leguminous plants, Frankia with non-leguminous plants and shrubs [25]. Out of these symbiotic nitrogen fixing bacteria, Rhizobium is the leading cause of legume crops' symbiotic nitrogen fixation. Besides bacteria, some small fern is also working as symbiotic nitrogen fixers. For example, Azolla is a small, free-floating aquatic fern that collaborates with cyanobacteria (Anabaena) to fix atmospheric nitrogen. The appropriate environment, phytohormones, and nutrients are provided by Azolla to Anabaena in the interchange of fixed nitrogen. In Anabaena, the phenomenon of nitrogen fixation happens in heterocyst cell. Azolla contributes primarily to rice cultivation by fertilizing the soil with nitrogen and incorporating biomass. Actinomycetes, for example, Frankia can produce root nodules for the actinorhizal plants. Frankia can be nodulated by certain other genera, such as Allocasuarina, Myrica, Eleagnus, Coriaria and Casuarina. They are monocot plants with a promising future in agricultural and land reclamation. N is fixed by Azotobacter and Bacillus species, and they also help in the growth and development of maize plants and forest crops [26]. Inoculation of Bradyrhizobium japonicum enhanced plant growth, nodulation, and N fixation in soybean [27].
2.1.1.2. Nonsymbiotic or free living nitrogen fixation
Free-living nitrogen fixers are found in the root zone of plants and obtain food and nutrient from plants, and in favor of return fixed nitrogen under a free-living state. Non-symbiotic nitrogen fixation is also accomplished by diazotrophs that stimulate the development of non-leguminous plants such as rice and radish. Certain other rhizopheric bacteria that fall under the genus Azotobacter, Burkholderia, Azoarcus, Azospirillum, Gluconacetobacter, Diazotrophicus, Pseudomonas, Enterobacter and Cyanobacteria (Anabaena, Nostoc) that also act as non-symbiotic nitrogen fixers [28,29]. Mukherjee et al. [30] suggested that Azotobacter chroococcum can be utilized as a biofertilizer because of its capacity to fix 10 mgN/g of in-vitro-supplied carbon source. According to Galindo et al. [31] A. brasilense lowers N fertilization, enhances plant nutrition, and boosts plant biomass and wheat grain yield.
Associative nitrogen fixing bacteria are Herbaspirillum, Acetobacter, Azospirillum and diazotrophicus which are accompanied by plant root cells of the gramineae family. Azospirillum is aerobic, non-nodulating, gram-negative, associative nitrogen-fixing bacteria living with C4 plants, such as maize, bajra, sugarcane, sorghum, and cereals like rice, barley, wheat [32]. The inoculation of Azospirillum showed marked results in maize, sorghum, wheat, and other grass seedlings. According to Montanez et al. [33], bacteria can provide up to 25% of rice and corn's overall nitrogen needs.
2.1.2. Siderophores production
Siderophores are small organic molecules that carry out antibiosis by supplying iron (Fe) to crops, consequently making pathogens impoverished of iron [51]. Iron is a necessary mineral nutrient for plant development and growth and is needed as a protein cofactor used in metabolic phenomena like respiration and photosynthesis [52]. Mathiyazhagan et al. [53] reported that iron deficiency suppresses pathogen growth by obstructing main processes including sporulation and nucleic acid synthesis. PGPRs have evolved numerous iron absorption approaches to remain alive and adapted to their environment to solve this challenge and supply iron to the plant. The generation of siderophores is one of these strategies. Bacterial species such as Pseudomonas use the siderophores formed by other rhizosphere microbes to complete their iron requirements. Gouda et al. [54] reported that Pseudomonas putida has the ability to utilize heterologous siderophores made by other microbes present in the root area to increase the iron level existent in the natural environment. Sarwar et al. [55] reported that application of siderophore-producing Bacillus sp. enhances the plant growth of groundnut. The production of siderophore and antioxidant enzymes by Pseudomonas koreensis in maize plants prevented the development of plant pathogens [56]. The available literature has confirmed that fluorescent Pseudomonas sp. generates two major types of siderophores viz pseudobactins [57] and pyochellins [58]. According to Battu and Reddy [59] siderophores are considered growth promoters of plants and biocontrolling agents of fungal diseases cognate with other crops. Therefore, it is crucial to clarify the role of siderophores produced by Pseudomonas strain B324 in preventing the pathogen Pythium which causes root rot disease in wheat [60]. Table-3 summarized a few examples of siderophore producing bacteria that have been linked in various plants.
Table 3.
Instances of siderophore producing, phosphate, and potassium solubilizing bacteria in different crops.
| S. No | Mechanism /function |
Plant growth-promoting rhizobacteria (PGPRs) | Crop | Reference |
|---|---|---|---|---|
| 1. | Phosphate solubilization | Bacillus sp., Pseudomonas sp., Serratia sp | Solanum tuberosum (potato) | [99] |
|
Azospirillum, P. putida, Stenotrophomonas maltophilia, chryseobacterium |
Triticum aestivum L. (wheat) | [100] [39] |
||
| Bacillus sp., Klebsiella sp., Pseudomonas sp. | Cicer arietinum (chickpea) | [101] | ||
| Herbaspirillum spp., Bacillus spp. | Vigna unguiculata (cowpea) | [35] | ||
| B. safensis, B. simplex, Lysinibacillus fusiformis, B. pumilus | Glycine max (soybean) | [102] | ||
| S. marcescens, Pseudomonas sp. | Oryza sativa (rice) | [103] | ||
| P. brassicacerum, Acinetobacter calcoaceticus, P. marginalis | Solanum lycopersicum (tomato) | [104] | ||
| 2. | Potassium solubilization | Pseudomonas sp., Acinetobacter sp., bacillus sp. | Phaseolus vulgaris (common bean) | [105] |
| B. subtilis, K. oxycota | Zea mays (maize) | [106] | ||
| Rhizobium sp. | Vica faba (faba bean) | [107] | ||
| Bacillus, Pseudomonas sp. | Sorghum bicolr (sorghum) | [49] | ||
| B. circulans | Citrus sinensis (orange) | [108] | ||
| Pseudomonas sp., Rhizobium, Mesorhizobium, Bacillus, Azotobacter sp. | Leguminous and non-leguminous plants | [109] | ||
| 3. | Siderophore production | Bacillus sp. KB129, KB133 | Sorghum bicolr (sorghum) | [110] |
| V. paradox RAA3 | Triticum aestivum L (wheat) | [111] | ||
| Azotobacter sp. Az63, Az69 and Az70 | Zea mays (maize) | [112] | ||
| Rhizobacteria sp. | pulses | [113] | ||
| Bacillus amyloliquefaciens ROH14 | pepper | [114] | ||
| Bacillus amyloliquefaciens FZB42 | Arabidosis | [115] |
Schippers et al. [61] reported that Pseudomonads produce another form of siderophore namely pyoverdine. It was observed by Loper and Henkels [62] that mutant strains of Pseudomonads produce less amount of pyoverdine and caused less repression of the fungal pathogen as compared to their parental strains. Thus, it is proved that the production of siderophore is one of the essential process of biological control. The siderophores are categorized into 3 groups hydroxamate, phenol/catechol, and hydroxycarboxylic acid based on their chemical role that is taking part in iron chelation. Over 500 siderophores are identified till now, and out of these a chemical configuration of 270 has been identified [63]. Beneduzi et al. [64] recorded that ferric siderophore complex plays significant activity in iron absorption when plants are exposed to other metals, including cadmium and nickel. PGPRs are a major asset because of generating siderophores that provide the necessary amount of iron to plants. However, further research on the ability of PGPRs to generate siderophores is yet to be explored.
Besides, siderophore production is another mechanism of microbes to control phytopathogens. Most of the iron in the rhizospheric region is bound by siderophores, which operate as iron-chelators. This prevents bacteria and fungi from growing there by using the iron for their purposes [65]. Thus attention has been paid by researchers to develop microbial inoculants that protect plants from diseases caused by pathogens.
2.1.3. Nutrient solubilization
2.1.3.1. Potassium solubilization
The third most important macronutrient that plants need is potassium (K). More than 90% of potassium is found in the form of silicate minerals and insoluble rock. It is particularly involved in the control of stomatal opening and closure, protein synthesis, nutrient uptake, increasing the quality of products, and providing resistance to stressful environmental conditions [66]. It is a crucial component of protein synthesis, enzyme activation, and photosynthesis, making it the third necessary nutrient for plants. The deficiency of potassium causes many major problems in the development of plants [67] and bore underdeveloped roots, slow growth, and lower seed and yield production due to lack of proper potassium content. Kumar and Dubey [68] suggested that it is necessary to discover the source of potassium to conserve potassium eminence and plant absorption in the soil to support crop production. Comprehensive research has been done on potassium rock's solubilization through PGPRs by generating and releasing organic acids [[69], [70], [71]]. Liu et al. [72] reported that PGPRs such as B. edaphicus, Acidothiobacillus sp, Burkholderia sp, Ferrooxidans sp., and Paenibacillus sp can solubilize and release potassium elements from potassium containing minerals in the soil so that plants can easily assess that. The application of potassium solubilizing PGPRs as biofertilizers to boost agricultural nutrients can therefore inhibit the utilization of chemical fertilizers and encourage sustainable agricultural production [40,73,74]. According to Macik et al. [75], biofertilizers are substances containing living microorganisms or their inocula and dormant spores that have positive effects on plants, particularly on the seed, root, or soil [76]. Inoculation of seeds of various plants with potassium solubilizing bacteria (KSB) generally exhibited a pronounced increase in seedling vigor, germination rate, plant growth, productivity, and plant potassium uptaking under both greenhouse as well as field conditions [69,77]. Production of organic acids is a significant phenomenon that either directly increases K dissolution through proton or ligand-mediated mechanism or indirectly does so by forming complexes in solution with reaction products. This is one of the mechanisms used by KSB to make K available to plants. As a result, using KSB as a biofertilizer can help promote environmentally sustainable agricultural production by reducing the usage of chemical fertilizers while also increasing plant growth and output [78]. These technologies are becoming essential in the farming practices of today. In the coming years, the changing plan of agricultural practices and environmental threats linked with chemical fertilizers demands a greater position for biofertilizers. Some examples of potassium solubilizing rhizobacteria and improved K uptake in various crops have been presented in Table-3.
2.1.3.2. Phosphate solubilization
The second-most important plant element is phosphorus [79] which can only be absorbed as monobasic or dibasic ions [54,80]. 95–99% of soil P exists in insoluble, frozen, or precipitated forms that are not available for the plant. As a result, only a small amount of the total soil P is useable by crops and is rarely enough [81,82]. Numerous microorganisms have been demonstrated to contribute to the biogeochemical cycling of inorganic and organic P in the plant rhizosphere, and inoculants based on P-solubilizing microorganisms are anticipated to expand quickly on the commercial market in the future [83,84]. Due to their capacity to solubilize P, many PGPRs have caught the interest of researchers for use as plant inoculants [54,85]. Due to the intrinsic P deficiency in many agricultural soils, these organisms are frequently suggested as potential P biofertilizers [38]. P-solubilizing bacteria (PSB) are referred heterotrophic bacteria chosen for their ability to solubilize phosphate compounds that are only weakly soluble in manufactured media by secreting low molecular weight organic ions, which acidify the medium [86].
According to reports, PSB can increase the solubility of precipitated inorganic P ions by acidifying the rhizosphere, which helps desorb inorganic P and complex organic P compounds from clay particles in soil. The solubilization of P is widely advanced to occur by acidification, despite the fact that PSB secrete a variety of enzymes and metabolites that do so [40,84]. It has been possible to extract PSB strains from a variety of genera, including Pseudomonas, Bacillus, and Burkholderia [87]. Although many strains have been demonstrated to solubilize P in numerous in vitro tests and pot trials [88], only a small number of PSB have been made available for purchase commercially to date [89]. For instance, recent research by Zeng et al. [90] effectively showed a positive correlation between the production of organic acids and Pseudomonas frederiksbergensis's P solubilizing activities. According to Zhang et al. [91] Bacillus subtilis shields plants from environmental stress and improves safflower growth. Under field circumstances, NanoPhos with phosphate-solubilizing bacteria increased the population of soil enzymes and bacteria, which improved maize production [92]. The commonly known P-solubilizers include Pseudomonas, 18 microbial inoculants as biofertilizer Azotobacter [93], 314 Bacillus, Rhodococcus, Arthrobacter, Serratia, Gordonia, Phyllobacterium, Delftia sp. [94], Pantoea, Klebsiella, Enterobacter [95], Xanthomonas, Chryseobacterium [96], Rhizobium leguminosarum bv. Trifolii [97], Pseodomonas sp. [98].
2.1.4. Phytohormone production
Plant hormones are important and secreted by both plants and microorganisms that play a significant role in plant growth and development [116]. Plant hormone production is the beneficial phenomenon of valuable microbes which is producing indole-3-acetic acids, cytokinin, gibberellins, ethylene, and abscisic acid [117]. Our earth supports abundant microbes capable of employing useful effects on plant growth and development. Microbes produce and transport plant hormones are the organic constituents having the capability of inducing physiological, morphological, and biochemical activities of plants even at minute concentrations. These hormones act as signaling molecules and show the direct impact, as they induce root growth, enhance nutrient uptake, and increase nodulation [118]. These are mainly five different types of plant hormones viz; auxin, gibberellins, abscisic acid, cytokinins, and 1-Aminocyclopropane-1-carboxylase (ACC). Some plants also produce brassinosteroids and polyamines in their young tissues. Phytohormones are endogenous in the origin of plants. Numerous reports confirmed that soil microbes can produce phytohormones and can improve the development and growth of plants [119].
2.1.4.1. Auxin production
Auxins are naturally forming growth hormones. There are various types of auxins, and the most prevalent auxin that occurs naturally is indole-3-acetic acid. Involving in the regulation of plant growth. Indole acetic acid (IAA) induced lateral root formation, cell elongation and differentiation, apical dominance, and also induced flowering, fruit setting as well as ripening [120,121]. Plants themselves synthesize IAA from tryptophan via involving the oxidative deamination mechanism or decarboxylation mechanism [122]. However, some microbes such as Azospirillum, Rhizobium, Bradyrhizobium, Enterobacter, Agrobacterium, Pseudomonas, Xanthomonas, Bacillus, and Klebsiella are also capable of synthesizing phytohormones via indole-3-pyruvic acid, indole-3-acetic acid aldehyde pathway and through indole-3-acetamide formation [[123], [124], [125]]. The ability of IAA production was found in cyanobacteria such as Anabeana, Nostoc, Calothrix, Gloeothece, Chlorogloeopsis, Plectonema, and Cylindrospermum.
2.1.4.2. Gibberellins production
Gibberellins are tetracyclic diterpenoid compounds, involved in various physiological as well developmental processes in plants [126]. There are more than 136 known gibberellins that are widely spread in nature [127], but GA3 is the most frequently used while GA1 is the most active among all. Gibberellins are synthesized from geranyl diphosphate via several pathways. GAs induces maximum biological activities such as seed germination as it breaks the dormancy of seeds, stem elongation, activation, and synthesis of amylolytic enzymes, floral induction and fruit growth in plants [128]. Stem growth is highly dependent upon the production of gibberellins and its absence or low concentration results in the minimum height of plants. The gibberellins are produced by plants themselves as well as by the fungal strain Gibberella fujikuroi. However, several reports showed that PGPRs like Azospirillum, Rhizobium Bacillus, Micrococcus, Agrobacterium, Clostridium, Xanthomonas, and Pseudomonas also produce gibberellins [[129], [130], [131], [132]].
2.1.4.3. Cytokinin production
Cytokinins are adenine derivatives that regulate cytokinesis in plant tissues [133]. Numerous microorganisms, including Azospirillum, Bacillus, Pseudomonas fluorescens, Pseudomonas putida, Bradyrhizobium, and Paenibacillus polymyxa, have produced cytokinin, primarily zeatin [132,[134], [135], [136]] and by certain streptomycetes. Cytokinins induce cell division, root hair proliferation, inhibit lateral root elongation, and control root meristem differentiation in plants [137]. In addition, cytokinins have a significant impact on plants, encouraging mitotic cell division in roots and shoots and delaying the aging of leaves [138]. Arkhipova et al. [139] described that cytokinin producing bacterial inoculation in plants rouses shoot growth in plants and reduced root to shoot ratio. A maize plant inoculated with cytokinins producing bacteria A. chroococcum which enhanced its growth conditions [140].
2.1.4.4. Abscisic acid production
Abscisic acid (ABA) is also known as stress hormone which is primarily involved in plant development and environmental stresses like drought, temperature, and high salinity [141]. ABA production provides drought as well as water tolerance in plants. Bacteria such as A. brasilense are capable to enhance the production of ABA in plants under drought or water stress by causing the closure of stomata, consequently preventing water loss [142]. Additionally, lateral roots are also developed as a result of this.
2.1.4.5. Aminocyclopropane-1- carboxylate (ACC) deaminase production
Ethylene is a crucial growth hormone involved in the regulation of the usual growth and development of plants at very low concentrations [143]. It is also called stress hormone due to its production during biotic and abiotic stress conditions [29]. However, it is beneficial for plant growth at low levels but proved to be harmful at higher levels. Ethylene inhibits auxin transportation and stops root elongation, promoting fruit ripening, senescence, and abscission of different plant parts [144,145]. ACC is the direct precursor of ethylene and some PGPRs such as Azospirillum brasilense, Enterobacter, Pseudomonas, Achromobacter, Azospirillum, Agrobacterium, Alcaligenes, Ralstonia, Serratia, Burkholderia spp., Rhizobium, etc. have ACC deaminase activity and support the plant development by decreasing the levels of ethylene and provide tolerance to plants under stress conditions [[146], [147], [148]]. ACC deaminase hydrolyses are the immediate precursor of ethylene produced ammonia and α-ketobutyrate which is utilized by microbes as carbon and nitrogen source for their growth [149]. Microbes with ACC deaminase activity are accredited to enhanced growth and productivity and therefore are considered potential agents for biofertilizer origination [150]. The various kind of phytohormones produced by PGPRs in several crops is listed in Table 4.
Table 4.
Phytohormones produced by PGPRs and their role in plants.
| S. No. | PGPR | Phytohormones | Plant | References |
|---|---|---|---|---|
| 1. | Pseudomanas putida | ACC deaminase | Tomato | [151] |
| 2. | Bacillus circulans | ACC deaminase | Mustard | [152] |
| 3. | Achromobacter xylosoxidans, Enterobacter Cloacae | ACC deaminase, IAA | Maize | [153] |
| 4. | Bacillus spp. | ACC deaminase, IAA, EPS | Rice | [154] |
| 5. | Pseudomonas putida | IAA | Canola | [155] |
| 6. | Bacillus subtilis | IAA | Edible tubercle | [124] |
| 7. | Herbaspirillum seropedicae | IAA | Ocimum sanctum | [17] |
| 8. | Bacillus amyloliquefaciens QST713 | IAA, EPS | Alfalfa (Medicago sativa L.) | [156] |
| 9. | Azospirillum sp | Cytokinin production | Mimosa pudica | [157] |
| 10. | Bacillus | Cytokinin production | Cucumber | [158] |
| 11. | Pseudomonas BA-8 | Cytokinin production | Strawberry | [159] |
| 12. | 25Acinetobactersp. ALEB16 | Abscisic acid | Atractylodes lancea | [160] |
| 13. | Bacillus sp. | Gibberellin production | Alder | [161] |
| 14. | Sphingomonas | Gibberellin production | Tomato | [162] |
| 15. | Bacillus | Gibberellin production | Pepper | [163] |
2.2. Indirect mechanism of PGPRs
2.2.1. Induced system resistance
Plants possess a wide range of active defense systems that are expressed in response to phytopathogens. These pathogens affect plant health and create a chronic threat to food production and ecosystem sustainability. Plants possess an induced system resistance (ISR) which protects the plant from biotic challenges and is effective against numerous pathogens [164]. In plants, ISR is instigated by microbes mostly by Pseudomonas sp via pathways regulated by ethylene and jasmonic acid [[165], [166], [167], [168]]. It was observed that P. fluorescens triggered ISR in various plants such as Arabidopsis, tobacco, and radish via jasmonic acid/ethylene (JA/ET) signaling pathways and substantially reduced the pathogenicity caused by phytopathogens like viruses, fungi as well as bacteria [169]. It was reported that microbes derived some compounds called elicitors which have been proposed to be responsible for the induction of ISR is mediated by a variety of plant hormones [170,171]. Microbial elicitors include cell wall components such as flagellin, lipopolysaccharides, and chitin [172], volatile organic compounds (VOC) like alkanes, terpenoids, sulfides, alcohols, phenolic compounds and ketones [171] or metabolites including antibiotics, siderophores [173]. These elicitors act synergistically to induce ISR against different pathogens, and control plant diseases as well. Sometimes the elicitors generate ISR by affecting phytohormones that are crucial to the plant signaling process and leading to the initiation of defense response [174]. Besides, various strains of Pseudomonas, different Bacillus sp. such as B. pumilus, B. subtilis, B. amyloliquefaciens, B. cereus, and B. mycoides were reported to induce in opposition to a variety of diseases [175]. It was observed that B. cereus synthesize dimethyl disulfide (DMDS) and showed ISR-eliciting action toward various pathogenic fungi [176]. Arabidopsis plants inoculated with P. simiae release elicitor coumarin scopoletin, which is a phenolic compound and inhibits the soil borne pathogens [177]. It was also observed that PGPRs can change the morphology or physiology of plant roots upon pathogen attack by secreting the phytohormones such as JA, auxin, NO and cytokinins, thus protecting the plant from pathogen attack [[178], [179], [180]]. Sometimes, microbes amplified the ISR in plants via structural barrier, production of molecules such as chitinase, β-1,3-glucanase, peroxidases, and phenylalanine ammonia-lyase [181].
2.2.2. Biocontrol of phytopathogen
Pathogen attack and disease development in agricultural plants are the dominating factor in reducing crop productivity and contamination of food products. Therefore, to protect crop productivity from pathogens different chemical constituents like pesticides are used [182]. However, prolonged utilization of these chemical compounds has advanced resistance to pathogens and creates a threat to the environment. Therefore, biological control is intended as a substitute for chemicals to manage the pathogen attack. Rhizobacteria encourage plant development, are potential biological agents for phytopathogens, and are used as biofertilizers due to their substantial influence on plant health, suppression of pathogens and diseases. The use of beneficial microbes in crop fields is non-toxic and ecofriendly thus preventing pathogen attacks through different mechanisms. PGPRs belonging to genera Acetobacter, Burkholderia Arthrobacter, Klebsiella, Azoarcus, Azotobacter, Azospirillum, pseudomonas, Enterobacter, Beijerinckia, Alcaligenes, Bacillus, Derxia, Gluconacetobacter, Rhodococcus, Acinetobacter and Stenotrophomonas, etc. have the property of biocontrol agents [183]. The production of antibiotics is the well-known mechanism of PGPRs to counter the toxic effects of pathogens in plants. Antibiotics such as amphisin, pyrrolnitrin, phenazines, hydrogen cyanide (HCN), oomycin A, tropolone, kanosamine, etc. produced from Streptomyces, Stenotrophomonas, Pseudomonas, and Bacillus that have antifungal, antibacterial, antiviral properties and protects the plant from diseases and pathogens [184].
2.2.3. Chitinase
The production of enzymes by PGPRs is another mechanism to control phytopathogens. PGPR's such as S. plymuthica, P. stutzeri, S. marcescens, and Paenibacillus sp. secrete enzymes including chitinase, protease, lipase, and various other enzymes that hydrolyze the chitin, proteins, cellulose, and hemicellulose as well as other cell wall components of fungal pathogens [185,186].
2.2.4. Hydrogen cyanide (HCN)
Microbes such as Rhizobium, Bacillus, and Pseudomonas secrete hydrogen cyanide that degrades pathogens and provides the plant defense against pathogenic diseases [187]. El-Rahman et al. [188] recorded that rhizobacteria produce HCN, and inhibit the growth of Agrobacterium tumefacience and Meloidogyne incognita.
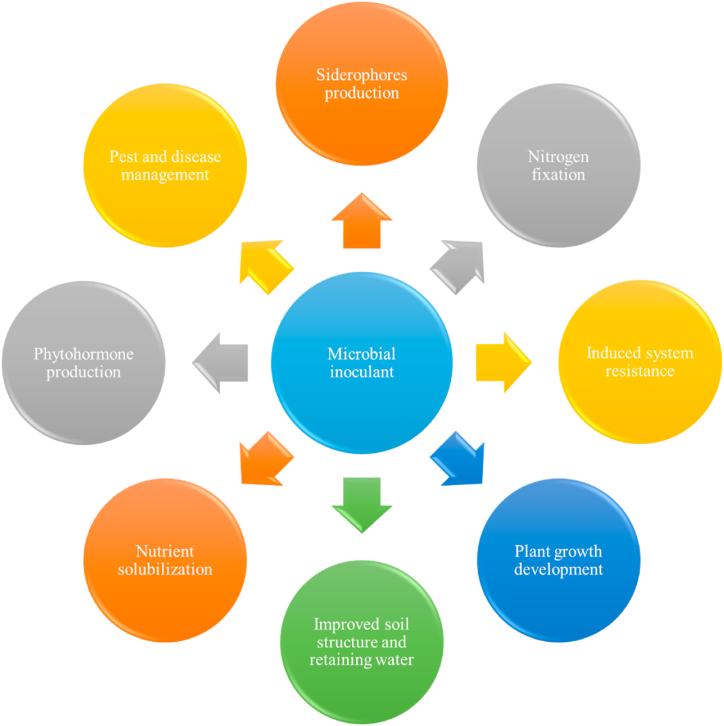
So, the PGPRs are vital microorganisms and played significant role in plant growth development and enhancing crop improvement by performing several activities like nitrogen fixation, potassium solubilization, phosphate solubilization phytohormone production, pest and disease management, siderophore production, improved soil structure. The aforementioned substantial contribution of microbial inoculants in improving crop yield and productivity is documented in Fig. 2.
Fig. 2.
Diagrammatic representation of role of microbial inoculants/PGPRs.
3. Future perspective and challenges
The utilization of biofertilizers as an integral aspect of agricultural techniques is rapidly becoming widespread worldwide. To ensure food security on a global scale, it is imperative to enhance crop yields while adapting to varying climatic conditions. The potential use of PGPRs as biofertilizers and biopesticides provides hope for doing this. However, additional investigation is required to understand the contradictory results regarding the advantages of PGPRs in field settings [189]. The future prospects of microbial inoculants as biofertilizers are promising as they offer several advantages over traditional chemical fertilizers. With the growing concerns about the environmental impacts of traditional farming practices, there is a need to increase sustainable agricultural practices. In addition, microbial inoculants as biofertilizers can contribute to sustainable agriculture by reducing the dependence on chemical fertilizers and promoting soil health. The ability of PGPR with the potential to be converted into inoculants for a variety of crops is being evaluated in an increasing number of studies [190,191]. Therefore, it is reasonable to anticipate that the widespread use of biofertilizers will give a variety of strategies for the overall growth of sustainable crop production systems in near future [192].
Furthermore, advanced technology, such as precision agriculture, can help optimize the use of microbial inoculants by providing information on soil conditions and plant nutrient requirements leading to the precise and targeted application of biofertilizers, improving their efficacy and reducing waste. The impact of climate change on agricultural productivity can be mitigated by the use of microbial inoculants, which can assist in adapting to changing conditions. Certain strains of beneficial microorganisms have shown promise in assisting plants in coping with various stresses associated with climate change, such as drought and salinity. Besides, advances in microbiology and genetic engineering can lead to the development of new microbial strains with improved capabilities, for instance, researchers are working on developing microbial strains that can fix nitrogen in non-leguminous plants, which could greatly reduce the need for nitrogen fertilizers. Despite being the third most important macronutrient for plant growth and development, research on K solubilization by plant growth-promoting rhizobacteria (PGPRs) has not progressed as rapidly as studies on nitrogen fixation and phosphorus solubilization [193]. The outcome of this research will serve to promote and instill confidence in the application of bioinoculants. Additionally, future studies should concentrate on the optimization of growth conditions that are economical to maintain, can withstand unfavorable environmental conditions, and increase productivity [194]. Overall, microbial inoculants as biofertilizers are promising, and their use is likely to increase as more farmers adopt sustainable and environmentally friendly farming practices.
As we know that microbial inoculants are a promising alternative to chemical fertilizers but, there are several limitations and challenges associated with the use of microbial inoculants as biofertilizers. They possess a restricted shelf life and may lose their effectiveness if not stored properly or used within a certain period. Further investigations are required in this area to advance knowledge, enable broad-scale application and commercialization, and enhance the ability to harness and manipulate plant microbiomes in situ for agricultural purposes [195]. The inoculant industry faces several difficulties when creating formulations with extended shelf lives. The creation of formulations with longer shelf lives, broader therapeutic spectra, and constant field performance may help this technology to become quicker and commercially viable [196]. To create formulations with longer shelf lives, new biotechnological methods should be assessed. Besides, there are also certain limitations to the use of microbial inoculants including control of quality and consistency of microbial inoculants, application methods, compatibility of microbial inoculants with chemical fertilizers, and environmental factors such as temperature, moisture, and pH. To overcome these challenges, researchers and industry professionals should explore several strategies, including the selection of improved microbial strains, the development of new formulations, standardized quality control, and educating farmers and agricultural professionals regarding the benefits and proper use of microbial inoculants.
4. Conclusion
Pesticides and chemical fertilizers are effective for the production and disease control of plants but, their continuous application is a threat to the soil ecosystem, plants as well as human beings. Thus to overcome this problem, use of beneficial microbes as biofertilizers and biocontrol agents is an ecofriendly and cheap method for sustainable agriculture. Biofertilizers have the potential to replace chemical fertilizers as well as pesticides and exert a positive impact on crop productivity and encouragement should be given to its implementation in agriculture. Farmers should be made aware of the benefits of using PGPRs as biofertilizers, and the commercialization of PGPRs should be emphasized. Thus in general we concluded that PGPRs have countless benefits in agriculture. Consequently, we can say that the use of biofertilizers in agricultural fields is the best alternative to chemical fertilizers which influence hazardous effects on flora as well fauna and soil health.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
Authors do not have any conflict of interest.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research in King Saud University.
References
- 1.Suyal D.C., Soni R., Sai S., Goel R. Microbial inoculants as biofertilizer. Microbial Inoculants in Sustainable Agricultural Productivity. 2016;1:311–318. Research Perspectives. [Google Scholar]
- 2.Pathak D.V., Kumar M. Microbial Inoculants in Sustainable Agricultural Productivity. Springer; New Delhi: 2016. Microbial inoculants as biofertilizers and biopesticides; pp. 197–209. [Google Scholar]
- 3.Kaminsky L.M., Trexler R.V., Malik R.J., Hockett K.L., Bell T.H. The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol. 2019;37(2):140–151. doi: 10.1016/j.tibtech.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary R.S., Yadav N.K. Biofertilizers (microbial inoculants) JUST AGRICULTURE. 2021;1:1–10. [Google Scholar]
- 5.Mordor Intelligence . 2022. Global Biofertilisers Market-Growth, Trends and Forecast; pp. 2022–2027. [Google Scholar]
- 6.Dunham W.C., DunhamTrimmer L.L.C. vol. 20. 2015, October. Evolution and future of biocontrol. (10th Annual Biocontrol Industry Meeting (ABIM)). Basel, Switzerland. [Google Scholar]
- 7.Gupta A., Meyer J.M., Goel R. Development of heavy metal-resistant mutants of phosphate solubilizing Pseudomonas sp. NBRI 4014 and their characterization. Curr. Microbiol. 2002;45(5):323–327. doi: 10.1007/s00284-002-3762-1. [DOI] [PubMed] [Google Scholar]
- 8.Hayat R., Ali S., Amara U., Khalid R., Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann. Microbiol. 2010;60(4):579–598. [Google Scholar]
- 9.Ahemad M., Malik A. Bioaccumulation of heavy metals by zinc resistant bacteria from agricultural soils irrigated with wastewater. Bacteriol. J. 2011;2:12. 2. [Google Scholar]
- 10.Ahemad M. Implications of bacterial resistance against heavy metals in bioremediation: a review. J. Instit. Integr. Omics Appl. Biotechnol. (IIOAB) 2012;3(3):39–46. [Google Scholar]
- 11.Saharan B.S., Nehra V. Plant growth promoting rhizobacteria: a critical review. Int. J. Life Sci. Med. Res. 2011;21(1):30. [Google Scholar]
- 12.Werner D. In: The Rhizosphere: Biochemistry and Organic Substances at the Soil Plant Interface. Pinton R., Varanini Z., Nannipieri P., editors. Marcel Dekker; New York, USA: 2000. Organic signals between plants and microorganisms; pp. 197–222. [Google Scholar]
- 13.Kumar A., Singh R., Yadav A., Giri D.D., Singh P.K., Pandey K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016;6:60. doi: 10.1007/s13205-016-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A., Singh V., Singh M., Singh P.P., Singh S.K., Singh P.K., et al. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocatal. Agric. Biotechnol. 2016;8:1–7. [Google Scholar]
- 15.Babalola O.O., Sanni A.I., Odhiambo G.D., Torto B. Plant growth-promoting rhizobacteria do not pose any deleterious effect on cowpea and detectable amounts of ethylene are produced. World J. Microbiol. Biotechnol. 2007;23(6):747–752. [Google Scholar]
- 16.Kumar K.V., Srivastava S., Singh N., Behl H.M. Role of metal resistant plant growth promoting bacteria in ameliorating fly ash to the growth of Brassica juncea. J. Hazard Mater. 2009;170(1):51–57. doi: 10.1016/j.jhazmat.2009.04.132. [DOI] [PubMed] [Google Scholar]
- 17.Barnawal D., Bharti N., Maji D., Chanotiya C.S., Kalra A. 1- Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 2012;58:227–235. doi: 10.1016/j.plaphy.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Kaymak H.C., Guvenc I., Yarali F., Donmez M.F. The effects of bio-priming with PGPR on germination of radish (Raphanus sativus L.) seeds under saline conditions. Turk. J. Agric. For. 2009;33(2):173–179. [Google Scholar]
- 19.Kang S.M., Khan A.L., Waqas M., You Y.H., Kim J.H., Kim J.G.…Lee I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014;9(1):673–682. [Google Scholar]
- 20.Zahir Z.A., Munir A., Asghar H.N., Shaharoona B., Arshad M. Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J. Microbiol. Biotechnol. 2008;18(5):958–963. [PubMed] [Google Scholar]
- 21.Marschner H. Academic Press; London: 1995. Mineral Nutrition of Higher Plants. [Google Scholar]
- 22.Damam M., Kaloori K., Gaddam B., Kausar R. Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. Int. J. Pharmaceut. Sci. Rev. Res. 2016;37(1):130–136. [Google Scholar]
- 23.Schlesinger W.H. 1991. The Global Carbon Cycle. Biogeochemistry, an Analysis of Global Change. [Google Scholar]
- 24.Hawkins J.P., Oresnik I.J. The Rhizobium-legume symbiosis: co-opting successful stress management. Front. Plant Sci. 2022;3 doi: 10.3389/fpls.2021.796045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahran H.H. Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol. 2001;91(2):143–153. doi: 10.1016/s0168-1656(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 26.Azeem M., Haider M.Z., Javed S., Saleem M.H., Alatawi A. Drought stress amelioration in maize (Zea mays L.) by inoculation of Bacillus spp. Strains under sterile soil conditions. Agriculture. 2022;12:50. [Google Scholar]
- 27.Htwe A.Z., Moh S.M., Soe K.M., Moe K., Yamakawa T. Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of mung bean, cowpea, and soybean. Agronomy. 2019;9(2):77. [Google Scholar]
- 28.Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255(2):571–586. [Google Scholar]
- 29.Bhattacharyya P.N., Jha D.K. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 2012;28(4):1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee A., Gaurav A.K., Singh S., Yadav S., Bhowmick S., Abeysinghe S., et al. The bioactive potential of phytohormones: a review. Biotechnol. Rep. 2022;8 doi: 10.1016/j.btre.2022.e00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galindo F.S., Pagliari P.H., Fernandes G.C., Rodrigues W.L., Boleta E.H.M., Jalal A., et al. Improving sustainable field-grown wheat production with Azospirillum brasilense under tropical conditions: a potential tool for improving nitrogen management. Front. Environ. Sci. 2022;10 [Google Scholar]
- 32.Yasuda M., Dastogeer K.M.G., Sarkodee-Addo E., Tokiwa C., Isawa T., Shinozaki S., et al. Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions. Agronomy. 2022;12:1367. [Google Scholar]
- 33.Montanez A., Rodriguez Blanco A., Barlocco C., Beracochea M., Sicardi M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl. Soil Ecol. 2012;58:21–28. [Google Scholar]
- 34.Abdel-Salam M.A., Shams A.S. Feldspar-K fertilization of potato (Solanum tuberosum L.) augmented by biofertilizer. J. Agric. Environ. Sci. 2012;12(6):694–699. [Google Scholar]
- 35.Manoel da Silva J., Carvalho dos Santos T.M., Santos de Albuquerque L., Coentro Montaldo Y., Ubaldo Lima de Oliveira J., Mesquita da Silva S.G.…da Rocha Oliveira Teixeira R. Potential of the endophytic bacteria ('Herbaspirillum'spp. and'Bacillus' spp.) to promote sugarcane growth. Aust. J. Crop. Sci. 2015;9(8):754–760. [Google Scholar]
- 36.Crespo J.M., Boiardi J.L., Luna M.F. Mineral phosphate solubilization activity of Gluconacetobacter diazotrophicus under P-limitation and plant root environment. Agric. Sci. 2011;2 [Google Scholar]
- 37.Karimi N., Zarea M.J., Mehnaz S. Endophytic Azospirillum for enhancement of growth and yield of wheat. Environ. Sustain. 2018;1:149–158. [Google Scholar]
- 38.Emami S., Alikhani H.A., Pourbabaei A.A., Etesami H., Motashare Zadeh B., Sarmadian F. Improved growth and nutrient acquisition of wheat genotypes in phosphorus deficient soils by plant growth-promoting rhizospheric and endophytic bacteria. Soil Sci. Plant Nutr. 2018;64(6):719–727. [Google Scholar]
- 39.Youseif S.H. Genetic diversity of plant growth promoting rhizobacteria and their effects on the growth of maize plants under greenhouse conditions. Ann. Agric. Sci. (Cairo) 2018;63(1):25–35. [Google Scholar]
- 40.Bakhshandeh E., Pirdashti H., Lendeh K.S. Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol. Eng. 2017;103:164–169. [Google Scholar]
- 41.Fattah Q. Third International Botanical Conference 2005. Bangladesh Botanical Society; Dhaka, Bangladesh: 2005. “A Plant Resources for Human Development”. [Google Scholar]
- 42.Choudhary M., Patel B.A., Meena V.S., Yadav R.P., Ghasal P.C. Seed bio-priming of green gram with Rhizobium and levels of nitrogen and sulphur fertilization under sustainable agriculture. Legume Res.-An Int. J. 2019;42(2):205–210. [Google Scholar]
- 43.Yousaf S., Zohaib A., Anjum S.A., Tabassum T., Abbas T., Irshad S.…Farooq N. Effect of seed inoculation with plant growth promoting rhizobacteria on yield and quality of soybean. Pakistan J. Agric. Res. 2018;32(1):177–184. [Google Scholar]
- 44.Htwe A.Z., Moh S.M., Moe K., Yamakawa T. Biofertilizer production for agronomic application and evaluation of its symbiotic effectiveness in soybeans. Agronomy. 2019;9:162. [Google Scholar]
- 45.Saini R., Dudeja S.S., Giri R., Kumar V. Isolation, characterization, and evaluation of bacterial root and nodule endophytes from chickpea cultivated in Northern India. J. Basic Microbiol. 2015;55(1):74–81. doi: 10.1002/jobm.201300173. [DOI] [PubMed] [Google Scholar]
- 46.Maheshwari R., Bhutani N., Suneja P. Screening and characterization of siderophore producing endophytic bacteria from Cicer arietinum and Pisum sativum plants. J. Appl. Biol. Biotechnol. 2019;7(5):7–14. [Google Scholar]
- 47.Sandhya V., Shrivastava M., Ali S.Z., Sai Shiva Krishna Prasad V. Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ. Agric. Sci. 2017;43(1):22–34. [Google Scholar]
- 48.Akintokun A.K., Ezaka E., Akintokun P.O., Shittu O.B., Taiwo L.B. Isolation, screening and response of maize to plant growth promoting Rhizobacteria inoculants. Sci. Agric. Bohem. 2019;50(3):181–190. [Google Scholar]
- 49.Archana D.S., Nandish M.S., Savalagi V.P., Alagawadi A.R. Characterization of potassium solubilizing bacteria (KSB) from rhizosphere soil. Bioinfolet-A Q. J. Life Sci. 2013;10(1b):248–257. [Google Scholar]
- 50.Postgate J.R. Cambridge University Press; 1982. The Fundamentals of Nitrogen Fixation. [Google Scholar]
- 51.Maksimov I.V., Abizgil'Dina R.R., Pusenkova L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens. Appl. Biochem. Microbiol. 2011;47(4):333–345. [PubMed] [Google Scholar]
- 52.Gao D., Ran C., Zhang Y., Wang X., Lu S., Geng Y., et al. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022;185:112–122. doi: 10.1016/j.plaphy.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Mathiyazhagan S., Kavitha K., Nakkeeran S., Chandrasekar G., Manian K., Renukadevi P., et al. PGPR mediated management of stem blight of Phyllanthus amarus (Schum and Thonn) caused by Corynespora cassiicola (Berk and Curt) Wei. Arch. Phytopathol. Plant Protect. 2004;37(3):183–199. [Google Scholar]
- 54.Gouda S., Kerry R.G., Das G., Paramithiotis S., Shin H.S., Patra J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Sarwar S., Khaliq A., Yousra M., Sultan T., Ahmad N., Khan M.Z. Screening of siderophore-producing PGPRs isolated from groundnut (Arachis hypogaea L.) rhizosphere and their influence on iron release in soil. Commun. Soil Sci. Plant Anal. 2020;51:1680–1692. [Google Scholar]
- 56.Ghazy N., El-Nahrawy S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 2021;203:1195–1209. doi: 10.1007/s00203-020-02113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemanceau P., Bakker P.A.H.M., De Kogel W.J., Alabouvette C., Schippers B. Antagonistic effect of nonpathogenic Fusarium oxysporum Fo47 and pseudobactin 358 upon pathogenic Fusarium oxysporum f. sp. Dianthi. Appl. Environ. Microbiol. 1993;59(1):74–82. doi: 10.1128/aem.59.1.74-82.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leeman M., den Ouden F.M., van Pelt J.A., Dirkx F.P.M., Steiji H., Bakker P.A.H.M., Schippers B. Iron availability affects induction of systemic resistance to Fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology. 1996;86:149–155. [Google Scholar]
- 59.Battu P.R., Reddy M.S. Siderophore-mediated antibiosis of rhizobacterial fluorescent pseudomonads against rice fungal pathogens. Int. J. Pharm. Tech. Res. 2009;1(2):227–229. [Google Scholar]
- 60.Becker J.O., Cook R.J. Role of siderophore in suppression of Pythium species and production of increased growth response of wheat by fluorescent Pseudomonas. Phytopathology. 1988;78:778–782. [Google Scholar]
- 61.Schippers B., Bakker A.W., Bakker P.A.H. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annu. Rev. Phytopathol. 1987;25:339–358. [Google Scholar]
- 62.Loper J.E., Henkels M.D. Utilization of heterologous siderophores enhances level of iron available to Pseudomonas putida in the rhizosphere. Appl. Environ. Microbiol. 1999;65(12):5357–5363. doi: 10.1128/aem.65.12.5357-5363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hider R.C., Kong X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010;27(5):637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 64.Beneduzi A., Ambrosini A., Passaglia L.M. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012;35(4):1044–1051. doi: 10.1590/s1415-47572012000600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33(11):197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santosh S., Velmourougane K., Idapuganti R.G., Manikandan A., Blaise D. Potassium solubilizing potential of native bacterial isolates from cotton rhizosphere of rainfed vertisols. Natl. Acad. Sci. Lett. 2022;45(3):209–212. [Google Scholar]
- 67.Nath D., Maurya B.R., Meena V.S. Documentation of five potassium-and phosphorus- solubilizing bacteria for their K and P-solubilization ability from various minerals. Biocatal. Agric. Biotechnol. 2017;10:174–181. [Google Scholar]
- 68.Kumar P., Dubey R.C. Plant growth promoting rhizobacteria for biocontrol of phytopathogens and yield enhancement of Phaseolus vulgaris L. J. Curr. Perspect. Appl. Microbiol. 2012;1:6–38. [Google Scholar]
- 69.Meena V.S., Maurya B.R., Verma J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014;169:337–347. doi: 10.1016/j.micres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Sindhu S.S., Parmar P., Phour M., Sehrawat A. Springer; India: 2016. Potassium-solubilizing Microorganisms (KSMs) and its Effect on Plant Growth Improvement. Potassium Solubilizing Microorganisms for Sustainable Agriculture; pp. 171–185. [Google Scholar]
- 71.Bahadur I., Maurya B.R., Meena V.S., Saha M., Kumar A., Aeron A. Mineral release dynamics of tricalcium phosphate and waste muscovite by mineral-solubilizing Rhizobacteria isolated from Indo-Gangetic Plain of India. Geomicrobiol. J. 2017;34(5):454–466. [Google Scholar]
- 72.Liu D., Lian B., Dong H. Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol. J. 2012;29(5):413–421. [Google Scholar]
- 73.Setiawati T.C., Mutmainnah L. Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agric. Agric. Sci. Procedia. 2016;9:108–117. [Google Scholar]
- 74.Wei Y., Zhao Y., Fan Y., Lu Q., Li M., Wei Q., et al. Impact of phosphate solubilizing bacteria inoculation methods on phosphorus transformation and long term utilization in composting. Bioresour. Technol. 2017;241:134–141. doi: 10.1016/j.biortech.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 75.Mącik M., Gryta A., Frąc M. Biofertilizers in agriculture: an overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020;162:31–87. [Google Scholar]
- 76.Hamid B., Bashir Z. Potassium solubilizing microorganisms: an alternative technology to chemical fertilizers. J. Res. Dev. 2019;19:79–84. [Google Scholar]
- 77.Anjanadevi I.P., John N.S., John K.S., Jeeva M.L., Misra R.S. Rock inhabiting potassium solubilizing bacteria from Kerala, India: characterization and possibility in chemical K fertilizer substitution. J. Basic Microbiol. 2016;56:67–77. doi: 10.1002/jobm.201500139. [DOI] [PubMed] [Google Scholar]
- 78.Etesami H., Emami S., Alikhani H.A. Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017;17(4):897–911. [Google Scholar]
- 79.Goswami D., Thakker J.N., Dhandhukia P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. 2016;2(1) [Google Scholar]
- 80.Verma M., Mishra J., Arora N.K. Plant growth-promoting rhizobacteria: diversity and applications. Environ. Biotechnol.: for sustainable future. 2019:129–173. [Google Scholar]
- 81.Alori E.T., Glick B.R., Babalola O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malhotra H., Sharma S., Pandey R. In: Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. Plant nutrients and Abiotic Stress Tolerance. Hasanuzzaman M., Fujita M., Oku H., Nahar K., Hawrylak-Nowak B., editors. Springer; Singapore: 2018. pp. 171–190. [Google Scholar]
- 83.Parnell J.J., Berka R., Young H.A., Sturino J.M., Kang Y., Barnhart D.M., DiLeo M.V. From the lab to the farm: an industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016;7:1110. doi: 10.3389/fpls.2016.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rafi M.M., Krishnaveni M.S., Charyulu P.B.B.N. Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. Recent Dev. Appl. Microbiol. Biochem. 2019:223–233. [Google Scholar]
- 85.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J., Dowling D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barrow N.J., Lambers H. Phosphate-solubilising microorganisms mainly increase plant phosphate uptake by effects of pH on root physiology. Plant Soil. 2022:1–6. [Google Scholar]
- 87.Hsu P.C.L., Condron L., O'Callaghan M., Hurst M.R. hemX is required for production of 2‐ketogluconate, the predominant organic anion required for inorganic phosphate solubilization by B urkholderia sp. H a185. Environ. Microbiol. Rep. 2015;7(6):918–928. doi: 10.1111/1758-2229.12326. [DOI] [PubMed] [Google Scholar]
- 88.Hsu P.C.L., O'Callaghan M., Condron L., Hurst M.R. Use of a gnotobiotic plant assay for assessing root colonization and mineral phosphate solubilization by Paraburkholderia bryophila Ha185 in association with perennial ryegrass (Lolium perenne L.) Plant Soil. 2018;425:43–55. [Google Scholar]
- 89.Owen D., Williams A.P., Griffith G.W., Withers P.J. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015;86:41–54. [Google Scholar]
- 90.Zeng Q., Wu X., Wen X. Identification and characterization of the rhizosphere phosphate-solubilizing bacterium Pseudomonas frederiksbergensis JW-SD2, and its plant growth-promoting effects on poplar seedlings. Ann. Microbiol. 2016;66(4):1343–1354. [Google Scholar]
- 91.Zhang T., Hu F., Ma L. Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth. Open Life Sci. 2019;14(1):246–254. doi: 10.1515/biol-2019-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaudhary A., Parveen H., Chaudhary P., Khatoon H., Bhatt P. Microbial technology for sustainable environment; 2021. Rhizospheric Microbes and Their Mechanism; pp. 79–93. [Google Scholar]
- 93.Kumar V., Behl R.K., Narula N. Establishment of phosphate-solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under green house conditions. Microbiol. Res. 2001;156(1):87–93. doi: 10.1078/0944-5013-00081. [DOI] [PubMed] [Google Scholar]
- 94.Wani P.A., Zaidi A., Khan A.A., Khan M.S. Effect of phorate on phosphate solubilization and indole acetic acid releasing potentials of rhizospheric microorganisms. Ann. Plant Protect. Sci. 2005;13(1):139–144. [Google Scholar]
- 95.Chung H., Park M., Madhaiyan M., Seshadri S., Song J., Cho H., Sa T. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005;37(10):1970–1974. [Google Scholar]
- 96.Singh A.V., Chandra R., Goel R. Phosphate solubilization by Chryseobacterium sp. and their combined effect with N and P fertilizers on plant growth promotion. Arch. Agron Soil Sci. 2013;59(5):641–651. [Google Scholar]
- 97.Abril A., Zurdo-Pineiro J.L., Peix A., Rivas R., Velázquez E. First International Meeting on Microbial Phosphate Solubilization. Springer Netherlands; 2007. Solubilization of phosphate by a strain of Rhizobium leguminosarum bv. trifolii isolated from Phaseolus vulgaris in El Chaco Arido soil (Argentina) pp. 135–138. [Google Scholar]
- 98.Rani A., Souche Y., Goel R. Comparative in situ remediation potential of Pseudomonas putida 710A and Commamonas aquatica 710B using plant (Vigna radiata (L.) wilczek) assay. Ann. Microbiol. 2013;63:923–928. [Google Scholar]
- 99.Abd El-Moaty N.M., Khalil H.M., Gomaa H.H., Ismail M.A., El-Dougdoug K.A. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting. Middle East J. Appl. Sci. 2018;8:554–566. [Google Scholar]
- 100.Zabihi H.R., Savaghebi G.R., Khavazi K., Ganjali A., Miransari M. Pseudomonas bacteria and phosphorous fertilization, affecting wheat (Triticum aestivum L.) yield and P uptake under greenhouse and field conditions. Acta Physiol. Plant. 2011;33:145–152. [Google Scholar]
- 101.Chhabra D., Sharma P. Non rhizobial endophytic bacteria from Chickpea (Cicer arietinum L.) tissues and their antagonistic traits. J. Appl. Nat. Sci. 2019;11(2):346–351. [Google Scholar]
- 102.Kadmiri I.M., Chaouqui L., Azaroual S.E., Sijilmassi B., Yaakoubi K., Wahby I. Phosphate-solubilizing and auxin-producing rhizobacteria promote plant growth under saline conditions. Arabian J. Sci. Eng. 2018;43:3403–3415. [Google Scholar]
- 103.Kolekar S.S., Desai P.D., Pancahal H.K., Shah K.B. Study of phosphate solubilizing microorganisms with biofertilizer potential. Int. J. Pharma Bio Sci. 2017;8(2):751–757. [Google Scholar]
- 104.Castillo A.R., Gerding M., Oyarzúa P., Zagal E., Gerding J., Fischer S. Plant growth-promoting rhizobacteria able to improve NPK availability: selection, identification and effects on tomato growth. Chil. J. Agric. Res. 2019;79(3):473–485. [Google Scholar]
- 105.Kumar A., Kumar A., Devi S., Patil S., Payal C., Negi S. Isolation, screening and characterization of bacteria from Rhizospheric soils for different plant growth promotion (PGP) activities: an in vitro study. Recent Res. Sci. Technol. 2012;4(1):1–5. [Google Scholar]
- 106.Imran M., Shahzad S.M., Arif M.S., Yasmeen T., Ali B., Tanveer A. Inoculation of potassium solubilizing bacteria with different potassium fertilization sources mediates maize growth and productivity. Pakistan J. Agric. Sci. 2020;57:1045–1055. [Google Scholar]
- 107.Shravanthi G.V., Panchatcharam P., AS S.R., Ambikapathy V. Screening of potassium solubilizing bacteria and their growth promoters. J. Pharmacogn. Phytochem. 2019;8(2):661–664. [Google Scholar]
- 108.Shaaban E.A., El-Shamma M.S., El-Shazly S., El-Gazzar A., Abdel-Hak R.E. Efficiency of rock-feldspar combined with silicate dissolving bacteria on yield and fruit quality of Valencia orange fruits in reclaimed soils. J. Appl. Sci. Res. 2012;(August):4504–4510. [Google Scholar]
- 109.Verma T., Pal P. Isolation and Screening of Rhizobacteria for various plant growth promoting attributes. J. Pharmacogn. Phytochem. 2020;9(1):1514–1517. [Google Scholar]
- 110.Grover M., Bodhankar S., Sharma A., Sharma P., Singh J., Nain L. PGPR mediated alterations in root traits: way toward sustainable crop production. Front. Sustain. Food Syst. 2021;4 [Google Scholar]
- 111.Chandra D., Srivastava R., Gupta V.V.S.R., Franco C.M.M., Sharma A.K. Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can. J. Microbiol. 2019;65:387–403. doi: 10.1139/cjm-2018-0636. [DOI] [PubMed] [Google Scholar]
- 112.Shirinbayan S., Khosravi H., Malakouti M.J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 2019;133:138–145. [Google Scholar]
- 113.Andy A.K., Masih S.A., Gour V.S. Isolation, screening and characterization of plant growth promoting rhizobacteria from rhizospheric soils of selected pulses. Biocatal. Agric. Biotechnol. 2020;27 [Google Scholar]
- 114.Gupta S., Pandey S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019;10:1506. doi: 10.3389/fmicb.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu X., Liu S.-F., Yue L., Zhao X., Zhang Y.-B., Xie Z.-K., et al. Epsc involved in the encoding of exopolysaccharides produced by Bacillus amyloliquefaciens FZB42 Act to boost the drought tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018;19:3795. doi: 10.3390/ijms19123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Usman M., Balsalobre-Lorente D., Jahanger A., Ahmad P. Pollution concern during globalization mode in financially resource-rich countries: do financial development, natural resources, and renewable energy consumption matter? Renew. Energy. 2022;183:90–102. [Google Scholar]
- 117.Eichmann R., Richards L., Schafer P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021;105:518–541. doi: 10.1111/tpj.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garcìa J.A.L., Probanza A., Ramos A.B., Barriuso J., Mañero F.J.G. Effect of inoculation with plant growth promoting rhizobacteria (PGPRs) and Sinorhizobium fredii on biological nitrogen fixation, nodulation and growth of Glycine max cv. Osumi. Plant Soil. 2004;267:143–153. [Google Scholar]
- 119.Spaepen S., Dobbelaere S., Croonenborghs A., Vanderleyden J. Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil. 2008;312:15–23. [Google Scholar]
- 120.Calvo P., Nelson L., Kloepper J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014;383:3–41. [Google Scholar]
- 121.Numan M., Bashir S., Khan Y., Mumtaz R., Shinwari Z.K., Khan A.L.…Ahmed A.H. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol. Res. 2018;209:21–32. doi: 10.1016/j.micres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Ahemad M., Khan M.S. Toxicological assessment of selective pesticides towards plant growth promoting activities of phosphate solubilizing Pseudomonas aeruginosa. Acta Microbiol. Immunol. Hung. 2011;58:169–187. doi: 10.1556/AMicr.58.2011.3.1. [DOI] [PubMed] [Google Scholar]
- 123.Hariprasad P., Niranjana S.R. Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil. 2009;316:13–24. [Google Scholar]
- 124.Swain M.R., Naskar S.K., Ray R.C. Indole-3-acetic acid production and effect on sprouting of Yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol. J. Microbiol. 2007;56:103–110. [PubMed] [Google Scholar]
- 125.Bharucha U., Kamlesh P., Ujjval B., Trivedi Optimization of indole acetic acid production by Pseudomonas putida UB1 and its effect as plant growth-promoting rhizobacteria on mustard (Brassica nigra) Agric. Res. 2013;2(3):215–221. [Google Scholar]
- 126.Crozier A., Kamiya Y., Bishop G., Yokota T. In: Biochemistry and Molecular Biology of Plants. Buchanan B.B., Grussem W., Jones R.L., editors. American Society of Plant Biologists; Rockville: 2001. Biosynthesis of hormones and elicitors molecules; pp. 850–900. [Google Scholar]
- 127.Kozaki A., Aoyanagi T. Molecular aspects of seed development controlled by gibberellins and abscisic acids. Int. J. Mol. Sci. 2022;23(3):1876. doi: 10.3390/ijms23031876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sponsel V.M. The deoxyxylulose phosphate pathway for the biosynthesis of plastidic isoprenoids: early days in our under-standing of the early stages of gibberellin biosynthesis. J. Plant Growth Regul. 2002;20:332–345. doi: 10.1007/s003440010032. [DOI] [PubMed] [Google Scholar]
- 129.Janzen R., Rood S., Dormar J., McGill W. Azospirillum brasilense produces gibberellins in pure culture and chemi-cally-medium and in co-culture on straw. Soil Biol. Biochem. 1992;24:1061–1064. [Google Scholar]
- 130.Cassán F., Bottini R., Schneider G., Piccoli P. Azospirillum brasilense and Azospirillum lipoferum hydrolyze conjugates of GA20 and metabolize the resultant a glycones to GA1 in seedlings of rice dwarf mutants. PlantPhysiol. 2001;125:2053. doi: 10.1104/pp.125.4.2053. –2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacMillan J. Occurrence of gibberellins in vascular plants, fungi and bacteria. J. Plant Growth Regul. 2002;20:387–442. doi: 10.1007/s003440010038. [DOI] [PubMed] [Google Scholar]
- 132.AlAli H.A., Khalifa A., Al-Malki M. Plant growthpromoting rhizobacteria from Ocimum basilicum improve growth of Phaseolus vulgaris and Abelmoschus esculentus. South Afr. J. Bot. 2021;139:200–209. [Google Scholar]
- 133.Skoog F., Strong F.N., Miller C.O. Cytokinins Sci. 1965;148:532–533. doi: 10.1126/science.148.3669.532-a. [DOI] [PubMed] [Google Scholar]
- 134.Perrig D., Boiero M.L., Masciarelli O.A., Penna C., Ruiz O.A., Cassán F.D., et al. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007;75:1143–1150. doi: 10.1007/s00253-007-0909-9. [DOI] [PubMed] [Google Scholar]
- 135.de García Salamone I.E., Hynes R.K., Nelson L.M. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can. J. Microbiol. 2001;47:404–411. doi: 10.1139/w01-029. [DOI] [PubMed] [Google Scholar]
- 136.Hussain A., Hasnain S. Cytokinin production by some bacteria: its impact on cell division in cucumber cotyledons. Afr. J. Microbiol. Res. 2009;3:704–712. [Google Scholar]
- 137.Riefler M., Novak O., Strnad M., Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.De Pascale S., Rouphael Y., Colla G. Plant biostimulants: innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017;82(6):277–285. [Google Scholar]
- 139.Arkhipova T.N., Prinsen E., Veselov S.U., Martinenko E.V., Melentiev A.I., Kudoyarova G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil. 2007;292:305–315. [Google Scholar]
- 140.Nieto K.F., Frankenberger W.T., Jr. Influence of adenine, isopentyl alcohol and Azotobacter chroococcum on the vegetative growth of Zea mays. Plant Soil. 1991;135:213–221. [Google Scholar]
- 141.Miyakawa T., Fujita Y., Yamaguchi-Shinozaki K., Tanokura M. Structure and function of abscisic acid receptors. Trends Plant Sci. 2013;18(5):259–266. doi: 10.1016/j.tplants.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 142.Bauer H., Ache P., Lautner S., Fromm J., Hartung W., Al-Rasheid Khaled A.S., et al. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 2013;1:53–57. doi: 10.1016/j.cub.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 143.Ahemad M., Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: curent perspective. J. King Saud Univ. Sci. 2014;26(1):1–20. [Google Scholar]
- 144.Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase producing soil bacteria (review) Eur. J. Plant Pathol. 2007;119(3):329–339. [Google Scholar]
- 145.Yuan Y., Zu M., Sun L., Zuo J., Tao J. Isolation and Screening of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing PGPR from Paeonia lactiflora rhizosphere and enhancement of plant growth. Sci. Hortic. 2022;297 [Google Scholar]
- 146.Pandey P., Kang S.C., Maheshwari D.K. Isolation of endophytic plant growth promoting Burkhoderia sp. MSSP from root nodules of Mimosa pudica. Curr. Sci. 2005;89:177–180. [Google Scholar]
- 147.Shaharoona B., Arshad M., Khalid A. Differential response of etiolated pea seedling to 1-aminocyclopropane-1-carboxylate and/or L-methionine utilizing rhizobacteria. J. Microbiol. 2007;45:15–20. [PubMed] [Google Scholar]
- 148.Kang B.G., Kim W.T., Yun H.S., Chang S.C. Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol. Rep. 2010;4:179–183. [Google Scholar]
- 149.Prigent-Combaret C., Blaha D., Pothier J.F., Vial L., Poirier M.A., Wisniewski-Dyé F., et al. Physical organization and phylogenetic analysis of acdR as leucine-responsive regulator of the 1-aminocyclopropane-1-carboxylate deaminase gene acdS in phytobeneficial Azospirillum lipoferum 4B and other proteobacteria. FEMS Microbiol. Ecol. 2008;65:202–219. doi: 10.1111/j.1574-6941.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 150.Shaharoona B., Arshad M., Zahir Z.A. Effect of plant growth promoting rhizobacteria containing ACC deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.) Lett App Microbiol. 2006;42:155. doi: 10.1111/j.1472-765X.2005.01827.x. [DOI] [PubMed] [Google Scholar]
- 151.Grichko V.P., Glick B.R. Amelioration of flooding stress by ACC deaminase containing plant growth-promoting bacteria. Plant Physiol. Biochem. 2001;39:11–17. [Google Scholar]
- 152.Ghosh S., Penterman J.N., Little R.D., Chavez R., Glick B.R. Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of canola, Brassica campestris. Plant Physiol. Biochem. 2003;41:277–281. [Google Scholar]
- 153.Danish S., Zafar-ul-Hye M., Fahad S., Saud S., Brtnicky M., Hammerschmiedt T., Datta R. Drought stress alleviation by ACC deaminase producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without timber waste biochar in maize. Sustainability. 2020;12(15):6286. [Google Scholar]
- 154.Joshi B., Chaudhary A., Singh H., Kumar P.A. Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.) Plant Soil. 2020;457:225–240. [Google Scholar]
- 155.Ahmad F., Ahmad I., Khan M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005;29:29–34. [Google Scholar]
- 156.Han L., Zhang M., Du L., Zhang L., Li B. Effects of Bacillus amyloliquefaciens QST713 on photosynthesis and antioxidant characteristics of Alfalfa (Medicago sativa L.) under drought stress. Agronomy. 2022;12(9):2177. [Google Scholar]
- 157.Sabat S., Murthy V.K., Shantha S.L., Kushnoor D., Agarwal G., Thomas J., et al. Comparative study of cytokinin production isolated from bacteria and shoot induction. Indian J. Biotechnol. 2014;13(4):544–546. [Google Scholar]
- 158.Sokolova M.G., Akimova G.P., Vaishlya O.B. Effect of phytohormones synthesized by rhizosphere bacteria on plants. Appl. Biochem. Microbiol. 2011;47:274–278. [PubMed] [Google Scholar]
- 159.Aslantas R., Cakmakci R., Sahin F. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci. Hortic. 2007;111:371–377. [Google Scholar]
- 160.Wang X.M., Yang B., Ren C.G., Wang H.W., Wang J.Y., Dai C.C. Involvement of abscisic acid and salicylic acid in signal cascade regulating bacteria. Plant. 2015;153:30–42. doi: 10.1111/ppl.12236. [DOI] [PubMed] [Google Scholar]
- 161.Gutierrez-Manero F.J., Ramos-Solano B., Probanza A., Mehouachi J., Tadeo F.R., Talon M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant. 2001;111(2):206–211. [Google Scholar]
- 162.Khan A.L., Waqas M., Kang S.M., Al-Harrasi A., Hussain J., Al-Rawahi A.…Lee I.J. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014;52:689–695. doi: 10.1007/s12275-014-4002-7. [DOI] [PubMed] [Google Scholar]
- 163.Joo G.J., Kim Y.M., Kim J.T., Rhee I.K., Kim J.H., Lee I.J. Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. J. Microbiol. 2005;43(6):510–515. [PubMed] [Google Scholar]
- 164.van Loon L.C. Mechanisms of Resistance to Plant Diseases (Slusarenko AJ, Fraser RSS & Van Loon LC. Kluwer; Dordrechet: 2000. Systemic induced resistance; pp. 521–574. [Google Scholar]
- 165.Pieterse C.M.J., Ton J., van Loon L.C. Cross-talk between plant defence signaling pathways: boost or burden? Agric. Biotech. Net. 2001;3:1–18. [Google Scholar]
- 166.Yan Z., Reddy M.S., Ryu C.M., Mc Inroy JA Wilson M., Kloepper J.W. Induced systemic protection against tomato late blight elicited by PGPR. Phytopathology. 2002;92:1329–1333. doi: 10.1094/PHYTO.2002.92.12.1329. [DOI] [PubMed] [Google Scholar]
- 167.Verbon E.H., Trapet P.L., Stringlis I.A., Kruijs S., Bakker P.A.H.M., Pieterse C.M.J. Iron and immunity. Annu. Rev. Phytopathol. 2017;55:355–375. doi: 10.1146/annurev-phyto-080516-035537. [DOI] [PubMed] [Google Scholar]
- 168.Martínez-Medina A., Van Wees S.C.M., Pieterse C.M.J. Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant Cell Environ. 2017;40:2691–2705. doi: 10.1111/pce.13016. [DOI] [PubMed] [Google Scholar]
- 169.Van Loon L.C., Bakker P.A.H.M., Pieterse C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998;36(1):453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 170.Sharifi R., Ryu C.M. Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 2018;44:88–97. doi: 10.1016/j.pbi.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 171.Tyagi S., Mulla S.I., Lee K.J., Chae J.C., Shukla P. VOCs-mediated hormonal signaling and cross talk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018;38:1277–1296. doi: 10.1080/07388551.2018.1472551. [DOI] [PubMed] [Google Scholar]
- 172.Pieterse C.M.J., Zamioudis C., Berendsen R.L., Weller D.M., Van Wees S.C.M., Bakker P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 173.Iavicoli A., Boutet E., Buchala A., Metraux J.P. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 2003;16(10):851–858. doi: 10.1094/MPMI.2003.16.10.851. [DOI] [PubMed] [Google Scholar]
- 174.Pieterse C.M.J., VanderDoes D., Zamioudis C., Leon-Reyes A., VanWees S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 175.Wu G., Liu Y., Xu Y., Zhang G., Shen Q., Zhang R. Exploring elicitors of the beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 to induce plant systemic resistance and their interactions with plant signaling pathways. Mol. Plant Microbe Interact. 2018;31:560–567. doi: 10.1094/MPMI-11-17-0273-R. [DOI] [PubMed] [Google Scholar]
- 176.Huang C.J., Tsay J.F., Chang S.Y., Yang H.P., Wu W.S., Chen C.Y. Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag. Sci. 2012;68(9):1306–1310. doi: 10.1002/ps.3301. [DOI] [PubMed] [Google Scholar]
- 177.Stringlis I.A., Proietti S., Hickman R., Van Verk M.C., Zamioudis C., Pieterse C.M.J. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J. 2018;93:166–180. doi: 10.1111/tpj.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shoresh M., Yedidia I., Chet I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology. 2005;95:76–84. doi: 10.1094/PHYTO-95-0076. [DOI] [PubMed] [Google Scholar]
- 179.Zhang H., Kim M.S., Krishnamachari V., Payton P., Sun Y., Grimson M., et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 2007;226:839–851. doi: 10.1007/s00425-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 180.GradyEN, MacDonald J., Liu L., Richman A., Yuan Z.C. Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Fact. 2016;15:203. doi: 10.1186/s12934-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Harish S., Kavino M., Kumar N., Balasubramanian P., Samiyappan R. Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against banana Bunchy top virus. Biol. Control. 2009;5:16–25. [Google Scholar]
- 182.Guo R.F., Yuan G.F., Wang Q.M. Effect of NaCl treatment on glucosinolate metabolism in broccoli sprouts. J. Zhejiang Univ. - Sci. B. 2013;14(2):124. doi: 10.1631/jzus.B1200096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kumar A., Verma H., Singh V.K., Singh P.P., Singh S.K., Ansari W.A., et al. In: Agriculturally Important Microbes for Sustainable Agriculture. Meena V., Mishra P., Bisht J., Pattanayak A., editors. Springer; Singapore: 2017. Role of Pseudomonas sp. in sustainable agriculture and disease management; pp. 195–215. [Google Scholar]
- 184.Compant S., Reiter B., Sessitsch A., Nowak J., Clement C., Barka E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005;71(4):1685–1693. doi: 10.1128/AEM.71.4.1685-1693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Frankowski J., Lorito M., Scala F., Schmidt R., Berg G., Bahl H. Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch. Microbiol. 2001;176:421–426. doi: 10.1007/s002030100347. [DOI] [PubMed] [Google Scholar]
- 186.Kamensky M., Ovadis M., Chet I., Chernin L. Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biol. Biochem. 2003;35:323–331. [Google Scholar]
- 187.Xu Z., Zhang R., Wang D., Qiu M., Feng H., Zhang N., et al. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of its DegU phosphorylation. Appl. Environ. Microbiol. 2014;80(9):2941–2950. doi: 10.1128/AEM.03943-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.El-Rahman A., Shaheen H.A., El-Aziz A., Rabab M., Ibrahim D.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control. 2019;29(1):1–11. [Google Scholar]
- 189.Kelbessa B.G., Dubey M., Catara V., Ghadamgahi F., Ortiz R., Vetukuri R.R. CABI Reviews; 2023. Potential of Plant Growth-Promoting Rhizobacteria to Improve Crop Productivity and Adaptation to a Changing Climate. 2023. [Google Scholar]
- 190.Yanni Y., Zidan M., Dazzo F., Rizk R., Mehesen A., Abdelfattah F., Elsadany A. Enhanced symbiotic performance and productivity of drought stressed common bean after inoculation with tolerant native rhizobia in extensive fields. Agric. Ecosyst. Environ. 2016;232:119–128. [Google Scholar]
- 191.Manasa K., Reddy R.S., Triveni S., Kumar B.K., Priya N.G. Characterization of Rhizobium isolates and their potential PGPR characteristics of different rhizosphere soils of Telangana region, India. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(5):2808–2813. [Google Scholar]
- 192.Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P.N., Das B., Gosh A., Tribedi P. Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017;24:3315–3335. doi: 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- 193.Ijaz M., Ali Q., Ashraf S., Kamran M., Rehman A. 2019. Development of Future Bioformulations for Sustainable Agriculture. Microbiome in Plant Health and Disease: Challenges and Opportunities; pp. 421–446. [Google Scholar]
- 194.Gupta G., Parihar S.S., Ahirwar N.K., Snehi S.K., Singh V. Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015;7(2):96–102. [Google Scholar]
- 195.Qiu Z., Egidi E., Liu H., Kaur S., Singh B.K. New frontiers in agriculture productivity: optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019;37(6) doi: 10.1016/j.biotechadv.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 196.Nakkeeran S., Fernando W.D., Siddiqui Z.A. Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. PGPR: biocontrol and biofertilization. 2006:257–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.