Abstract
Background
Glycerol is a substrate for gluconeogenesis and fatty acid esterification in the liver, processes which are upregulated in obesity and may contribute to excess fat accumulation. Glycine and glutamate, in addition to cysteine, are components of glutathione, the major antioxidant in the liver. In principle, glycerol could be incorporated into glutathione via the TCA cycle or 3-phosphoglycerate, but it is unknown whether glycerol contributes to hepatic de novo glutathione biosynthesis.
Methods
Glycerol metabolism to hepatic metabolic products including glutathione was examined in the liver from adolescents undergoing bariatric surgery. Participants received oral [U–13C3]glycerol (50 mg/kg) prior to surgery and liver tissue (0.2–0.7g) was obtained during surgery. Glutathione, amino acids, and other water-soluble metabolites were extracted from the liver tissue and isotopomers were quantified with nuclear magnetic resonance spectroscopy.
Results
Data were collected from 8 participants (2 male, 6 female; age 17.1 years [range 14–19]; BMI 47.4 kg/m2 [range 41.3–63.3]). The concentrations of free glutamate, cysteine, and glycine were similar among participants, and so were the fractions of 13C-labeled glutamate and glycine derived from [U–13C3]glycerol. The signals from all component amino acids of glutathione - glutamate, cysteine and glycine - were strong and analyzed to obtain the relative concentrations of the antioxidant in the liver. The signals from glutathione containing [13C2]glycine or [13C2]glutamate derived from the [U–13C3]glycerol drink were readily detected, and 13C-labelling patterns in the moieties were consistent with the patterns in corresponding free amino acids from the de novo glutathione synthesis pathway. The newly synthesized glutathione with [U–13C3]glycerol trended to be lower in obese adolescents with liver pathology.
Conclusions
This is the first report of glycerol incorporation into glutathione through glycine or glutamate metabolism in human liver. This could represent a compensatory mechanism to increase glutathione in the setting of excess glycerol delivery to the liver.
Keywords: Glycerol, Glycine, Glutamate, Cysteine, Liver, Glutathione, Stable isotope, NMR
Graphical abstract
Highlights
-
•
Rates of non-alcoholic fatty liver disease and liver transplants are increasing.
-
•
Drug development requires an understanding of underlying physiology.
-
•
We traced glucose and glycerol kinetics with isotopomers in human hepatic tissue.
-
•
We describe two novel pathways of glycerol incorporation into de-novo glutathione synthesis.
-
•
These may be a protective mechanisms to prevent liver disease progression.
Nomenclature
- Ala
alanine
- ALT
alanine transaminase
- BMI
body mass index
- Cys
cysteine
- D
doublet
- DHAP
dihydroxyacetone phosphate
- DSS
4,4-dimethyl-4-silapentane-1-sulfonic acid
- GA3P
glyceraldehyde 3-phosphate
- GCL
glutamate cysteine ligase
- Glu
glutamate
- Gly
glycine
- GPx
glutathione peroxidases
- GR
glutathione reductase
- GS
glutathione synthetase
- GSH
glutathione (reduced form)
- GSSG
glutathione disulfide (oxidized form)
- α-kG
α-ketoglutarate
- Lac
lactate
- MRI
magnetic resonance imaging
- NAC
N-acetylcysteine
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NMR
nuclear magnetic resonance
- OAA
oxaloacetate
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- 3 PG
3-phosphoglycerate
- PHGDH
phosphoglycerate dehydrogenase
- PSAT
phosphoserine aminotransferase
- PSPH
phosphoserine phosphatase
- S
singlet
- SHMT
serine hydromethyl transferase
- TCA
tricarboxylic acid
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is growing in prevalence, related to increasing rates of obesity, with very concerning rates in adolescents [1,2]. Fatty liver disease is projected to be the leading cause of hepatic transplantation [3]. Of particular interest is what determines progression of simple steatosis to non-alcoholic steatohepatitis (NASH) as not all adolescents with fatty liver progress to NASH or fibrosis [4]. This process has been tied to increased inflammation in the liver, in particular reactive oxygen species and oxidative stress [5]. A critical part of the development of new pharmaceuticals is gaining a full understanding of hepatocyte substrate metabolism which may be involved in the development of NASH.
Glutathione is the major antioxidant in the body protecting cells from reactive oxygen species and free radicals [[6], [7], [8]]. It is a tripeptide composed of glutamate (Glu), cysteine (Cys), and glycine (Gly). Glutathione exists in two forms, the reduced form (GSH) and the oxidized form (GSSG, glutathione disulfide). The thiol group (-SH) of the cysteine moiety in GSH provides a reducing equivalent, and the oxidation of GSH leads to GSSG that has a disulfide bond between two molecules of GSH (Fig. 1A). GSH is dominant in vivo, but the fraction of GSSG increases under oxidative stress and other pathophysiological conditions [[9], [10], [11]]. Glutathione is maintained at a high concentration in most cells, particularly in hepatocytes [7,8,10,12], but its concentration decreases with chronic degenerative diseases or aging [13].
Fig. 1.
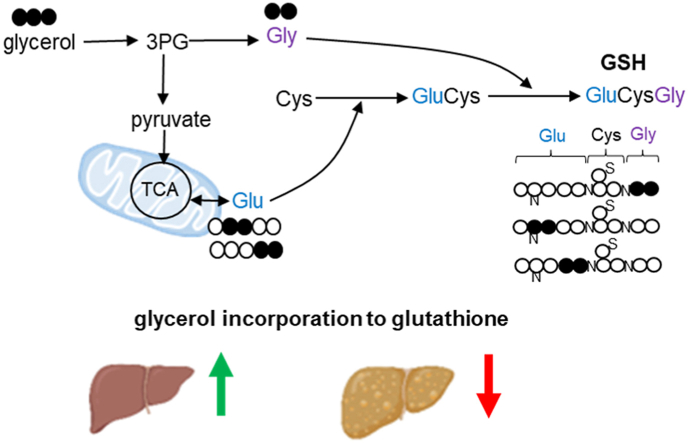
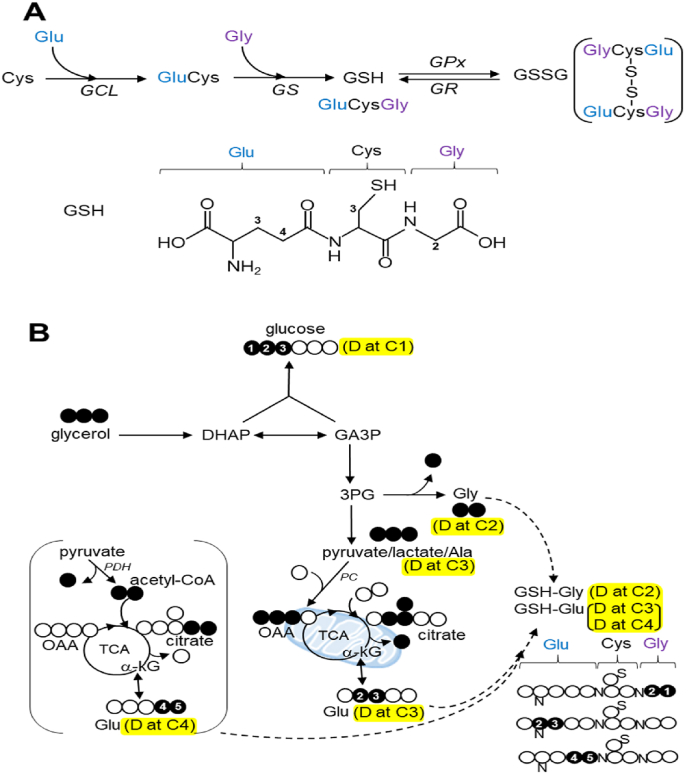
Glutathione biosynthesis and NMR detection for [U–13C3]glycerol incorporation to glutathione (A) In glutathione biosynthesis, γ-glutamylcysteine (GluCys) is formed from glutamate and cysteine via glutamate cysteine ligase (GCL) followed by the reaction between the dipeptide and glycine via glutathione synthetase (GS). The oxidation of reduced glutathione (GSH) to glutathione disulfide (GSSG) is catalyzed by glutathione peroxidase (GPx) while GSH regeneration from GSSG is catalyzed by glutathione reductase (GR). The numbers in glutathione chemical structure indicate the carbon positions of component amino acids selected for NMR analysis. (B) A simplified schematic shows glycerol metabolism in liver. Glycerol is converted to dihydroxyacetone phosphate (DHAP) or glyceraldehyde 3-phosphate (GA3P). The condensation of the two trioses leads to glucose and gluconeogenesis from [U–13C3]glycerol produces triple-labeled glucose ([13C3]glucose). GA3P may enter glycolytic pathway producing pyruvate that is in exchange with lactate or alanine (Ala). [U–13C3]glycerol metabolism through the glycolysis leads to triple-labeled ([13C3]) lactate or alanine. Pyruvate enters the TCA cycle through pyruvate carboxylase (PC) or dehydrogenase (PDH). [U–13C3]pyruvate becomes [1,2,3–13C3]oxaloacetate (OAA) through PC, and the condensation between [1,2,3–13C3]oxaloacetate and acetyl-CoA produces [2,3,6–13C3]citrate and then [2,3–13C2]α-ketoglutarate (α-kG) through the TCA cycle. Since α-ketoglutarate is in exchange with glutamate, the detection of [2,3–13C2]glutamate is evidence of PC activity. In contrast, PDH produces [1,2–13C2]acetyl-CoA from [U–13C3]pyruvate, and the reaction between [1,2–13C2]acetyl-CoA and oxaloacetate leads to [4,5–13C2]citrate, [4,5–13C2]α-ketoglutarate and consequently [4,5–13C2]glutamate. Thus, [U–13C3]glycerol incorporation to glutathione through glutamate produces [2,3–13C2]glutamate moiety or [4,5–13C2]glutamate moiety. Glycerol can be converted to glycine through 3-phosphoglycerate (3 PG), and [U–13C3]glycerol incorporation to glutathione through glycine produces glutathione-[13C2]glycine. Highlights with yellow indicate doublet (D) signals in 13C NMR spectra of metabolic products derived from [U–13C3]glycerol. open circle, 12C; black circle, 13C. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The liver is the main source of glutathione in the body, and the antioxidant synthesized in the liver is exported to other organs through blood and bile [14]. Among the constituent amino acids in glutathione, the availability of cysteine is the rate-limiting factor in de novo glutathione synthesis, catalyzed by glutamate-cysteine ligase [15]. The supply of cysteine or its precursors stimulates glutathione synthesis [8]. N-acetylcysteine (NAC), a well-known precursor to cysteine, is used for the treatment of acetaminophen poisoning by replenishing glutathione in the liver and for the treatment of other diseases associated with oxygen radicals [[16], [17], [18]]. Under some circumstances such as diabetes or protein malnutrition, glycine may limit glutathione synthesis [[19], [20], [21]]. Low circulating glycine was reported to limit glutathione biosynthesis in fatty liver [22,23]. The administration of glycine alone, combination with NAC, or serine as a precursor also increases glutathione [[24], [25], [26], [27], [28]]. Glutamate, however, has received less attention related to its regulatory role in glutathione biosynthesis, presumably due to its abundance in most cells.
The liver receives ample nutrients through the portal vein including glycerol derived from lipolysis of triglycerides in visceral fat and from the diet. Glycerol is utilized for gluconeogenesis and fatty acid esterification producing glucose and triglycerides, respectively. Since glycerol may enter glycolytic pathways, it can be metabolized to glycine through 3-phosphoglycerate. Glycerol may also be metabolized to pyruvate and, after entry into the TCA cycle, undergo metabolism to glutamate [29]. Thus, while it is conceivable that glycerol contributes to glutathione through glycine or glutamate, glycerol incorporation into glutathione has not been reported previously in human liver. The purposes of this study are to determine the metabolic pathways involved in glycerol metabolism in the human liver and to specifically investigate the contribution of glycerol to de novo glutathione synthesis. We recruited adolescents who were undergoing bariatric surgery and administered oral [U–13C3]glycerol prior to surgery. A liver biopsy was performed during surgery and the tissue extracts were analyzed using nuclear magnetic resonance (NMR) spectroscopy to determine glycerol incorporation into glutathione and relevant metabolic processes.
2. Materials and methods
2.1. Research design
Participants: Youth aged 13–20 years old with body mass index (BMI) 41.3–63.3 kg/m2 (n = 8) were recruited from the bariatric surgery clinic at Children's Hospital Colorado (Colorado Multiple IRB 18–0479, NCT03587727). Exclusion criteria included a history of type 2 diabetes, viral hepatitis, mitochondrial disease, the use of medications known to alter insulin resistance including atypical antipsychotics, immunosuppressants, HIV medications, PPAR-γ or PPAR-α, and metformin, MRI exclusion criteria including largest circumference >200 cm and implanted metal, pregnancy, alcohol abuse, hemoglobin <10 mg/dL, and psychiatric or developmental concerns limiting informed assent and consent. The study was approved by the Colorado Institutional Review Board. All participants <18 years old provided written assent with parental consent and participants ≥18 years old provided written consent prior to enrollment and performance of any research.
Study Design: Participants underwent fasting metabolic assessments with labs and anthropomorphic measurements. Fasting laboratory values were drawn within 7 days prior to laparoscopic vertical sleeve gastrectomy. On the day of surgery, oral [U–13C3]glycerol (50 mg/kg body weight; Cambridge Isotope Laboratories, Inc. Tewksbury, MA USA) was consumed after overnight fast and 2 h prior to scheduled procedure start. Liver core and wedge biopsies were obtained for clinical and NMR analyses. Due to variation in operative schedules, biopsies occurred between 1.5 and 5.5 h after glycerol consumption (Fig. S1 and Table 1). Liver tissue was preserved immediately following biopsy by flash freezing in liquid nitrogen and stored at −80 °C prior to sample preparation for NMR spectroscopy.
Table 1.
Clinical characteristics of participants.
| Patient | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 |
|---|---|---|---|---|---|---|---|---|
| Characteristic | ||||||||
| Age (year) | 14.8 | 17.8 | 18.3 | 16.2 | 16.4 | 19.0 | 15.0 | 18.9 |
| Sex (Female/Male) | F | F | F | F | M | F | M | F |
| Race (Black/White/Other) | W | W | Other | W | Multiple | W | W | W |
| Ethnicity (Hispanic/Not Hispanic) | H | H | NH | H | H | NH | H | H |
| Body weight (kg) | 136.4 | 138.8 | 118.0 | 129.2 | 154.7 | 168.3 | 125.8 | 106.3 |
| Body mass index (kg/m2) |
43.5 |
49.8 |
42.5 |
52.1 |
44.5 |
63.3 |
41.6 |
41.3 |
| Plasma | ||||||||
| Hemoglobin A1c (%) | 6.1 | 5.6 | 5.0 | 5.8 | 5.5 | 4.9 | 5.4 | 5.4 |
| Triglycerides (<90 mg/dL) | 223 | 121 | 89 | 106 | 66 | 150 | 128 | 97 |
| ALT (11–26 U/L) | 23 | 21 | 32 | 71 | 46 | 14 | 116 | 29 |
| AST (15–40 U/L) |
26 |
23 |
25 |
47 |
35 |
23 |
51 |
31 |
| Liver | ||||||||
| Steatosis grade | 1 | 1 | 0 | 3 | 0 | 0 | 3 | 3 |
| Steatosis location | zone 1 | zone 2 | NDa | azonal | ND | azonal | zone 3 | panacinar |
| Lobular inflammation | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Liver cell injury (ballooning) | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| Fibrosis stage |
1C |
1A |
0 |
1A |
0 |
1C |
0 |
0 |
| Liver biopsy | ||||||||
| Time after13C-glycerol (hr) | 5.5 | 4.8 | 3.5 | 2.6 | 3.6 | 4.5 | 1.5 | 3.5 |
| Tissue mass for NMR (g) | 0.35 | 0.75 | 0.33 | 0.29 | 0.65 | 0.31 | 0.22 | 0.22 |
ND, not detected.
Liver biopsies were evaluated by author MAL in the clinical pathology service at Children's Hospital Colorado using standard liver pathology techniques [30]. The liver biopsy was preserved in 10% neutral buffered formalin for fixation, embedded in paraffin, and sectioned on a microtome at 4 μm followed by staining with routinely performed hematoxylin and eosin, trichrome, and periodic acid-Schiff with and without diastase.
2.2. Sample preparation and NMR spectroscopy
Metabolites were extracted from liver tissues using a method reported previously with modifications [31]. Frozen liver tissue (0.2–0.7 g) was powdered using a mortar and pestle chilled with liquid nitrogen, and ground tissue was transferred into a 15-mL conical tube containing a mixture of acetonitrile-isopropanol-water (3:3:2; 7 mL) on ice, vortexed for 1 min, and centrifuged at 25,000 g and 4 °C for 10 min. The supernatant was transferred to a 50-mL conical tube, and the remaining pellet was treated with the additional mixture of acetonitrile-isopropanol-water (5 mL). The pellet was disrupted with a spatula, vortexed for 1 min and centrifuged, and the supernatant was transferred to the same 50-mL tube. The extraction was repeated one more time by adding the mixture of solvents (3 mL) to the pellet. Combined supernatant was centrifuged at 25,000 g and 4 °C for 10 min and it was transferred to a 20-mL glass vial to dry under vacuum. Dichloromethane (1 mL x 3) was slowly added to dried extracts to dissolve lipids, and dichloromethane solution was transferred to a new 15-mL conical tube. After centrifuging, the dichloromethane solution was discarded, and the residue was dissolved with the mixture of acetonitrile-isopropanol-water (100 μL x 3) to combine with the original extracts in the 20-mL glass vial. After drying under vacuum, the extracts were dissolved in heavy water (2H2O, 200 μL) containing 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS; 5 mM; NMR reference), centrifuged at 20,000 g for 5 min and the supernatant was transferred to a 3-mm NMR tube.
NMR spectra were collected using a 14.1 T Bruker Avance III HD equipped with a 5-mm cryoprobe (Bruker, MA, USA) with the observe coil tuned to 13C (150 MHz). Proton-decoupled 13C NMR was acquired using Bruker standard zgpg30 sequence (a 30° pulse, a 36k‐Hz sweep width, and a 2‐s acquisition time with 1.5‐s interpulse delay) at 25 °C. Proton decoupling was performed using a standard WALTZ-16 pulse sequence. Spectra were averaged with 8000–20,000 scans and a line broadening of 0.5 Hz was applied prior to Fourier transformation. Spectra were analyzed using ACD/Labs NMR spectral analysis program (Advanced Chemistry Development, Inc., Toronto, Canada).
2.3. NMR analysis of metabolites
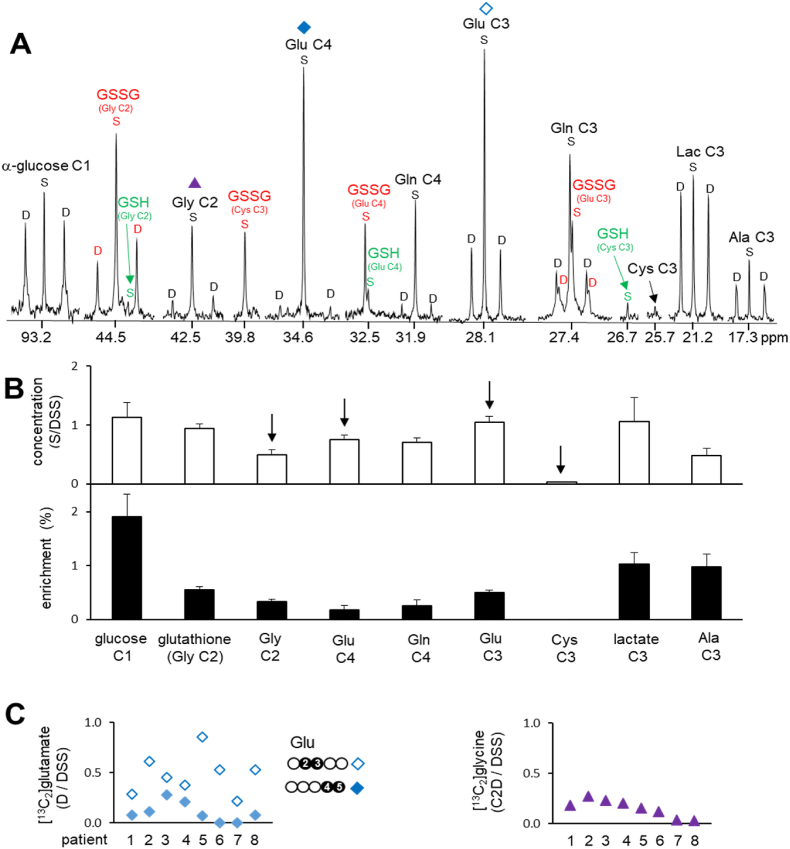
In the liver, [U–13C3]glycerol is phosphorylated by glycerol kinase and can be further converted to another triose such as dihydroxyacetone phosphate (DHAP) or glyceraldehyde 3-phosphate (GA3P). Since the condensation of these two trioses leads to glucose, the appearance of triple-labeled (i.e., [1,2,3–13C3] or [4,5,6–13C3]) glucose is evidence of gluconeogenesis from [U–13C3]glycerol (Fig. 1B). In this study, [1,2,3–13C3]glucose was quantified using the peak areas of doublets (D12) at α-glucose carbon 1 (C1; 93.2 ppm; Fig. 2A) and β-glucose C1 (97.0 ppm). Instead of entering gluconeogenesis, [U–13C3]GA3P may experience the terminal steps of glycolysis to produce pyruvate that is in exchange with lactate or alanine (Ala). Thus, the appearance of [U–13C3]lactate or [U–13C3]alanine is evidence of [U–13C3]glycerol metabolism through glycolysis. These products were detected using the doublet (D23) at lactate C3 (21.2 ppm) or alanine C3 (17.3 ppm), respectively. 3-phosphoglycerate (3 PG) is an intermediate of glycolysis and it can be metabolized to serine through the multiple enzymatic activities of phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase (PSAT), and phosphoserine phosphatase (PSPH), and then glycine after decarboxylation by serine hydromethyl transferase (SHMT). [U–13C3]glycerol metabolism to glycine was detected by the doublet at glycine C2 (42.5 ppm) in 13C NMR, a signal from [U–13C2]glycine.
Fig. 2.
NMR analysis of metabolites in liver extracts (A) In 13C NMR, a singlet (S) reflects the pool size of a metabolite with natural 13C abundance and a double (D) is the signal from 13C–13C spin-spin coupling with two adjacent carbons originated from [U–13C3]glycerol. A doublet at glucose C1 is from [1,2,3–13C3]glucose demonstrating gluconeogenesis from [U–13C3]glycerol while a doublet at lactate C3 is from [U–13C3]lactate demonstrating the glycerol metabolism through glycolytic pathway. Doublet signals from other metabolites also inform [U–13C3]glycerol metabolism to glycine, glutamate, glutamine, glutathione and so on. (B) Graphs show relative concentrations of metabolites and their enrichments based on NMR analysis. Arrows indicate free glycine, glutamate and cysteine. Cysteine was not enriched by 13C and its concentration was low. Data are average ± SE (n = 8). (C) Graphs show relative concentrations of [2,3–13C2]glutamate, [4,5–13C2]glutamate and [1,2–13C2]glycine based on doublet signals from the amino acids.
Pyruvate enters the TCA cycle through pyruvate dehydrogenase (PDH) or pyruvate carboxylase (PC). The entry of [U–13C3]pyruvate through PDH produces [1,2–13C2]acetyl-CoA after decarboxylation, and the condensation between [1,2–13C2]acetyl-CoA and oxaloacetate (OAA) generates [4,5–13C2]citrate and then [4,5–13C2]α-ketoglutarate (αKG) through the TCA cycle. Since α-ketoglutarate is in exchange with glutamate, glycerol metabolism through PDH produces [4,5–13C2]glutamate that can be detected by a doublet (D45, JCC ∼ 52 Hz) at glutamate C4 (34.6 ppm) in 13C NMR (Fig. 2A). The entry of [U–13C3]pyruvate through PC produces [1,2,3–13C3]oxaloacetate after carboxylation, and the reaction between [1,2,3–13C3]oxaloacetate and acetyl-CoA produces [2,3,6–13C3]citrate. The citrate becomes [2,3–13C2]α-ketoglutarate through the TCA cycle and then [2,3–13C2]glutamate that can be quantified using a doublet (D23, JCC ∼ 34 Hz) at glutamate C3 (28.1 ppm; Fig. 2A).
The relative concentration of a metabolite was calculated based on the peak area of a singlet (S) from each metabolite because the singlet represents natural 13C abundance. The level of a13C-labeled metabolite derived from [U–13C3]glycerol was measured using a doublet (D), a signal from 13C–13C spin-spin coupling (Fig. 2A). The peak area of a singlet or a doublet was normalized by the peak area of DSS, the NMR reference.
2.4. NMR analysis of glutathione
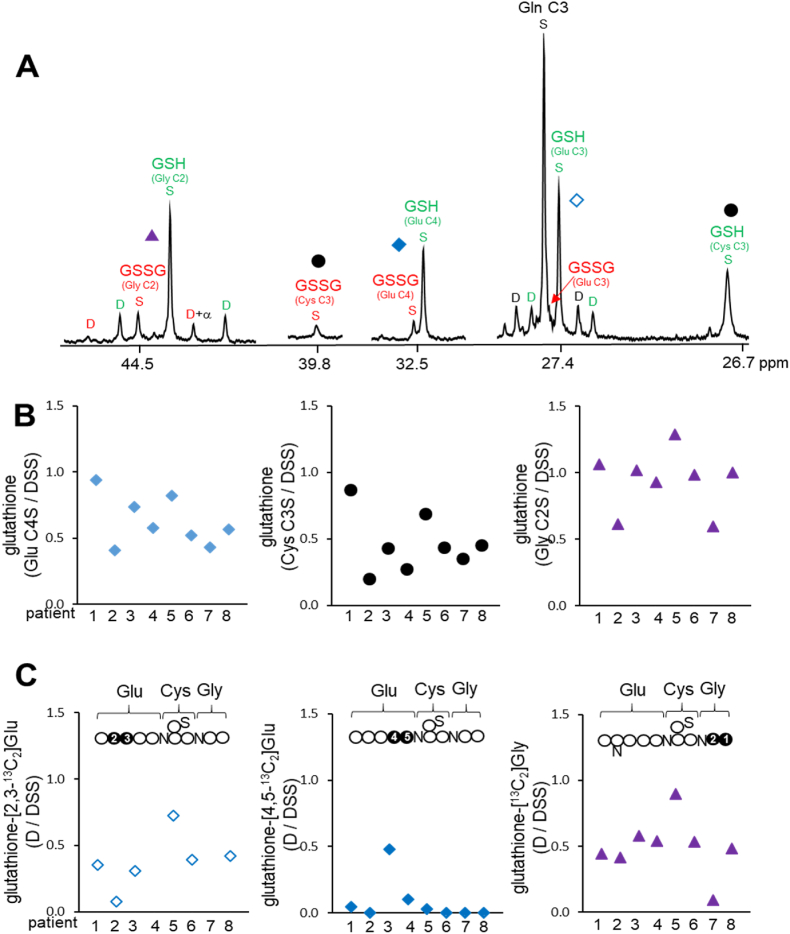
Signals from GSH and GSSG were resolved in 13C NMR spectra (Fig. 2, Fig. 3A). Among many carbons in glutathione, signals from glycine moiety C2, cysteine moiety C3, and glutamate moiety C3 and C4 were selected for analysis because these signals were stronger and better resolved compared to signals from other carbons. Chemical shifts and NMR signals from the selected carbons in GSH and GSSG are as follow:
Fig. 3.
Quantitation of glutathione and newly synthesized glutathione with [U–13C3]glycerol (A) 13C NMR of liver extracts shows signals from component amino acids in glutathione. Singlet signals represent the pool size of glutathione and doublets represent [U–13C3]glycerol incorporation to glutathione. (B) Graphs show relative glutathione contents (sum of GSH and GSSG) based on singlet signals from glutamate moiety C4, cysteine moiety C3 and glycine moiety C2 in 13C NMR spectra. (C) Graphs show the relative level of glutathione containing [2,3–13C2]glutamate, [4,5–13C2]glutamate or [U–13C2]glycine. Newly synthesized glutathione with [U–13C3]glycerol should be cautiously compared among participants due to varied timing for a liver biopsy. The low level of newly synthesized glutathione in patient #7, for example, could be in part due to the short duration from [U–13C3]glycerol administration to liver biopsy (1.5 h).
Glutamate C3 in GSH and GSSG at 27.4 ppm: S ([3–13C1]Glu) and D23 ([2,3–13C2]Glu).
Glutamate C4 in GSH and GSSG at 32.5 ppm: S ([4–13C1]Glu) and D45 ([4,5–13C2]Glu).
Cysteine C3 in GSH at 26.7 ppm and GSSG at 39.8 ppm: S ([3–13C1]Cys).
Glycine C2 in GSH and GSSG at 44.5 ppm: S ([2–13C1]Gly) and D ([U–13C2]Gly).
A singlet (S) reflects the pool size of glutathione with natural 13C abundance and a doublet (D) is evidence of [U–13C3]glycerol metabolism to glutathione. Doublets were detected in glutamate and glycine moieties, but not in the cysteine moiety.
2.5. Statistical analysis
NMR Comparisons between two groups were made using a Mann Whitney U test for non-parametric data and ANOVA was used for analysis of greater than 2 groups, where p < 0.05 was considered significant. Data are expressed as mean ± standard error.
3. Theory
As previously described, the 13C label from an oral load of [U–13C3]glycerol can be traced to specific locations within a metabolite, as detected by isotopomer NMR analysis. Label locations and signal abundance can be used to measure hepatic relative contributions of the TCA cycle, the pentose phosphate pathway and indirect metabolism, in both the glucose and triglyceride products that are released from the liver [29]. In this study, we performed this isotopomer NMR analysis on liver tissue, rather than blood, and assessed for the location of the tracer in a broader range of metabolites, to better understand intrahepatic energy metabolism. By performing the analysis in the liver tissue, the hypothesis was that we would be able to find the 13C label in a broader range of metabolites where either the enrichment was too low or there is not direct secretion to the blood stream.
4. Results
4.1. Participant characteristics
Clinical characteristics of participants are noted in Table 1. The average age of participants was 17.0 years with a range 14.8–19.0 years. Participants were predominantly female (75%) and identified as Hispanic (75%). Average BMI was 47.4 kg/m2 with a range 41.3–63.3 kg/m2. By design, no participants had diabetes (Hemoglobin A1c > 6.5%) and two had prediabetes (defined as hemoglobin A1c 5.7–6.4%). Five participants had elevations in ALT, two were >2x upper limit normal. Five of eight had hepatic steatosis, three had inflammation consistent with NASH, and four had some degree of fibrosis.
4.2. Assessment of metabolites and glycerol metabolism in the liver
Gluconeogenesis from [U–13C3]glycerol was evidenced by doublet (D12) signals at α-glucose C1 (93.2 ppm) and β-glucose C1 (97.0 ppm). [U–13C3]glycerol incorporation into glycolytic pathway was also evidenced by doublet (D23) signals at lactate C3 (21.2 ppm) and alanine C3 (17.3 ppm; Fig. 2A). The amplitude of the doublet relative to the natural abundance singlet in glucose, alanine and lactate indicates that ∼1–3% of the metabolite pools were enriched by [U–13C3]glycerol (Fig. 2B). A doublet at glycine C2 (42.5 ppm), the signal from [U–13C2]glycine, was also detected demonstrating [U–13C3]glycerol metabolism to glycine. A small singlet only, without a doublet, was detectable at cysteine C3 (25.7 ppm). The relative concentrations and 13C enrichments of these metabolites in the liver from each participant are listed in Table 2.
Table 2.
Relative concentrations and13C enrichments of metabolites.
| Metabolite | Chemical shift | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 |
|---|---|---|---|---|---|---|---|---|---|
| Glucose (α & β; C1) | 92.9 & 97.0 ppm | 2.67 | 1.82 | 0.87 | 0.87 | 0.77 | 0.77 | 0.65 | 0.59 |
| Glucose enrichment (%) | 0.23 | 0.80 | 1.03 | 1.99 | 2.97 | 3.03 | 3.50 | 1.72 | |
| Glycerol 3-phosphate (C1) | 63.3 ppm | 0.32 | 0.64 | 0.39 | 0.12 | 0.28 | NDa | ND | 0.08 |
| G3P enrichment (%) | 0.14 | 0.88 | 0.95 | 0.40 | 1.91 | ND | ND | ND | |
| Serine (C2) | 57.4 ppm | 0.19 | 0.16 | 0.09 | 0.01 | 0.04 | 0.09 | ND | 0.08 |
| Serine enrichment (%) | 0.21 | 0.12 | 0.24 | ND | ND | ND | ND | ND | |
| Glycine (C2) | 42.6 ppm | 0.85 | 0.79 | 0.56 | 0.45 | 0.50 | 0.32 | 0.19 | 0.30 |
| Glycine enrichment (%) | 0.24 | 0.38 | 0.45 | 0.50 | 0.34 | 0.40 | 0.21 | 0.11 | |
| Phosphoethanolamine (N) | 41.8 ppm | 0.18 | 0.20 | 0.16 | 0.08 | 0.19 | 0.20 | 0.09 | 0.47 |
| Succinate (C2&C3) | 35.0 ppm | 0.44 | 1.14 | 0.51 | 0.24 | 0.41 | 0.16 | 0.15 | 0.34 |
| Succinate enrichment (%) | 0.07 | 0.17 | 0.16 | 0.24 | 0.30 | ND | ND | 0.15 | |
| Glutamate (C4) | 34.0 ppm | 1.07 | 1.06 | 0.57 | 0.47 | 0.96 | 0.74 | 0.52 | 0.59 |
| Glutamate enrichment (%) | 0.08 | 0.11 | 0.53 | 0.49 | 0.09 | ND | ND | 0.15 | |
| Glutamine (C4) | 31.8 ppm | 1.11 | 0.65 | 0.82 | 0.64 | 0.66 | 0.68 | 0.44 | 0.68 |
| Glutamine enrichment (%) | 0.10 | 0.21 | 0.86 | 0.51 | 0.14 | 0.14 | ND | 0.10 | |
| Glutamate (C3) | 27.9 ppm | 1.31 | 1.42 | 0.91 | 0.78 | 1.37 | 0.99 | 0.77 | 0.82 |
| Glutamate enrichment (%) | 0.24 | 0.48 | 0.54 | 0.53 | 0.69 | 0.59 | 0.32 | 0.58 | |
| Cysteine (C3) | 25.7 ppm | 0.03 | 0.05 | 0.01 | 0.03 | 0.06 | 0.04 | 0.02 | 0.03 |
| Lactate (C3) | 21.0 ppm | 1.81 | 3.48 | 1.28 | 0.66 | 0.65 | 0.09 | 0.08 | 0.45 |
| Lactate enrichment (%) | 0.23 | 0.75 | 0.87 | 0.90 | 2.12 | 1.72 | 0.97 | 0.65 | |
| Alanine C3 | 17.3 ppm | 0.92 | 0.93 | 0.73 | 0.63 | 0.27 | 0.10 | ND | 0.25 |
| Alanine enrichment (%) | 0.28 | 0.69 | 0.95 | 1.10 | 1.86 | 2.02 | ND | 0.89 |
Common metabolites were analyzed using 13C NMR of liver extracts. The relative concentration of a metabolite was measured using a singlet with natural 13C abundance normalized by DSS, a NMR reference. Excess 13C enrichment in each metabolite was calculated using a doublet by assuming a singlet from the metabolite as natural abundance (1.1%). Since the amount of tissue collected through a biopsy varied among participants, the concentrations of metabolites were calculated for 1 g. 13C enrichments in metabolites cannot be directly compared among participants because the interval from [U–13C3]glycerol administration to a liver biopsy varied.
ND, not detected.
As noted, 13C-labeling patterns in glutamate provide information about the path of pyruvate entry to the TCA cycle through PC or PDH. Both [2,3–13C2]glutamate (doublet at glutamate C3; 28.1 ppm) and [2,3–13C2]glutamine (doublet at glutamine C3; 27.4 ppm) were detected demonstrating [U–13C3]glycerol metabolism through PC. In addition, the presence of [4,5–13C2]glutamate (doublet at glutamate C4; 34.6 ppm) and [4,5–13C2]glutamine (doublet at glutamine C4; 31.9 ppm) demonstrated [U–13C3]glycerol metabolism through PDH. The signal from [2.3–13C2]glutamate was stronger than that from [4,5–13C2]glutamate in all participants demonstrating pyruvate entry to the TCA cycle mainly through PC rather than PDH.
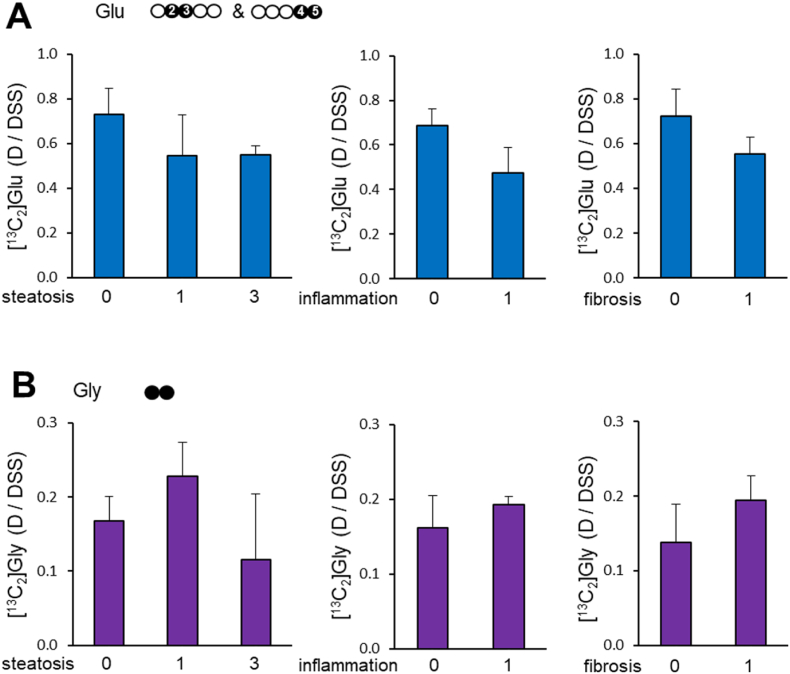
Glutamate and glycine were abundant in the liver of all participants, but the concentration of cysteine was low (Fig. 2A–B). The levels of these free amino acids among participants were similar. The presence of 13C-labeled glutamate and glycine showed active [U–13C3]glycerol metabolism to these amino acids in all participants. The levels of [13C2]glycine were not noticeably different among patients, but the levels of [13C2]glutamate varied (Fig. 2C).
4.3. Assessment of glutathione
The relative concentration and 13C enrichment in hepatic glutathione were based on the signals from glycine moiety C2, cysteine moiety C3, glutamate moiety C3 and C4 (Fig. 3A). Chemical shifts for the carbons of component amino acids between GSH and GSSG were very close, except the cysteine moiety C3 (26.7 ppm in GSH; 39.8 ppm in GSSG). The different chemical shifts in the cysteine moiety were due to dissimilar chemical environments between a thiol (-SH) group in GSH and a disulfide bond (-S-S-) in GSSG. The relative concentration of total glutathione (GSH and GSSG) was measured using singlets from glycine moiety C2, cysteine moiety C3, and glutamate moiety C4. The results based on the signals from different component amino acids in glutathione were generally consistent in each patient (Fig. 3B). The concentrations of glutathione were high and comparable with common metabolites such as glucose, glutamate, and lactate (Fig. 2B), and they somewhat varied among patients (Fig. 3B and Table 3).
Table 3.
Relative concentrations and13C enrichments of glutathione.
| moiety | Chemical shift | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 |
|---|---|---|---|---|---|---|---|---|---|
| GSSG-Gly C2 | 44.5 ppm | 0.23 | 0.44 | 0.97 | 0.55 | 1.20 | 0.74 | 0.52 | 0.86 |
| enrichment (%) | 0.45 | 0.82 | 0.63 | 0.60 | 0.78 | 0.60 | 0.17 | 0.58 | |
| GSH-Gly C2 | 44.4 ppm | 0.84 | 0.18 | 0.04 | 0.38 | 0.09 | 0.25 | 0.08 | 0.17 |
| enrichment (%) | 0.47 | 0.56 | 0.60 | 0.70 | 0.57 | 0.60 | NDa | 0.55 | |
| Sum (Gly moiety C2) |
1.07 |
0.62 |
1.01 |
0.93 |
1.29 |
0.99 |
0.60 |
1.03 |
|
| GSSG-Cys C3 | 39.8 ppm | 0.12 | 0.12 | 0.39 | 0.24 | 0.60 | 0.39 | 0.34 | 0.35 |
| GSH-Cys C3 | 26.7 ppm | 0.75 | 0.09 | 0.04 | 0.04 | 0.08 | 0.04 | 0.02 | 0.11 |
| Sum (Cys moiety C3) |
0.87 |
0.21 |
0.43 |
0.28 |
0.68 |
0.43 |
0.36 |
0.46 |
|
| GSSG-Glu C4 | 32.5 ppm | 0.14 | 0.23 | 0.52 | 0.26 | 0.59 | 0.32 | 0.43 | 0.38 |
| enrichment (%) | ND | ND | 0.77 | 0.20 | 0.06 | ND | ND | ND | |
| GSH-Glu C4 | 32.5 ppm | 0.80 | 0.18 | 0.22 | 0.32 | 0.24 | 0.20 | ND | 0.19 |
| enrichment (%) | 0.07 | ND | 0.59 | 0.20 | ND | ND | ND | ND | |
| Sum (Glu moiety C4) |
0.94 |
0.41 |
0.74 |
0.58 |
0.83 |
0.52 |
0.43 |
0.57 |
|
| GSSG-Glu C3⸹ | 27.4 ppm | 0.06 | 0.06 | 0.75 | – | 0.83 | 0.47 | – | 0.42 |
| enrichment (%) | ND | ND | 0.45 | – | 0.96 | 0.92 | – | 1.09 | |
| GSH-Glu C3 | 27.4 ppm | 0.87 | 0.15 | 0.07 | – | 0.07 | 0.30 | ND | 0.14 |
| enrichment (%) | 0.45 | 0.58 | ND | – | ND | ND | ND | ND | |
| Sum (Glu moiety C3) | 0.93 | 0.21 | 0.82 | – | 0.90 | 0.77 | – | 0.56 |
The relative concentration of glutathione was based on singlet signals from the carbons of component amino acids, glycine (Gly), cysteine (Cys) and glutamate (Glu), normalized by the signal of DSS. Excess 13C enrichment was calculated using a doublet from glycine moiety C2, glutamate moiety C3 and C4 in glutathione by assuming a singlet from the corresponding carbon as natural abundance. Since the amount of tissue collected through a biopsy varied among participants, the concentration of glutathione was calculated for 1 g. 13C enrichments in glutathione cannot be directly compared among participants because the interval from [U–13C3]glycerol administration to a liver biopsy varied.
ND, not detected; ⸹Due to overlap with glutamine C3, the quantitation of glutamate moiety C3 could be not-precise.
4.4. Glutathione derived from [U–13C3]glycerol through glycine or glutamate
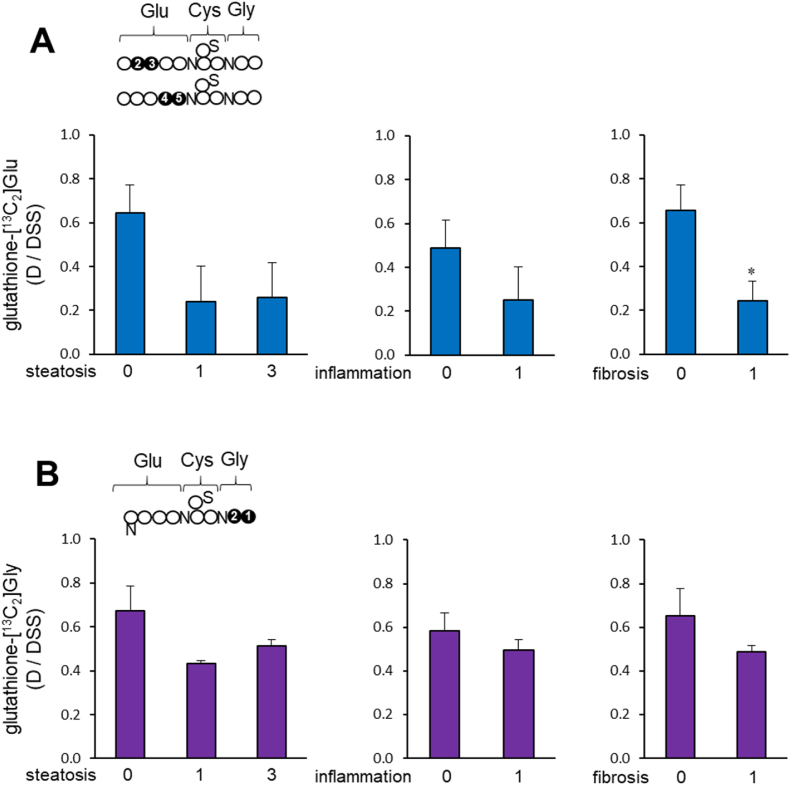
[U–13C3]glycerol incorporation into glutathione was demonstrated by the detection of [13C2]glycine and [13C2]glutamate moieties in glutathione (Fig. 3C and Table 3). Glutathione-[13C2]glycine was readily observed in all participants, but its concentrations were somewhat varied among them. Notably, patient # 7 had trivial 13C-labeled glycine moiety, possibly due to the shortest interval (1.5 h) between the glycerol load and the biopsy (Table 1). As seen in free glutamate, the glutamate moiety in glutathione had 13Cs at carbons 2 & 3 or carbons 4 & 5 depending on pyruvate entry to the TCA cycle. Glutathione-[2,3–13C2]glutamate was detectable in most participants, except two patients (#4 and #7) having unresolved signals due to overlap with glutamine C3. Glutathione-[4,5–13C2]glutamate was also detectable, but only in a few volunteers. Also, the doublet from glutathione-[2,3–13C2]glutamate was generally larger than that from glutathione-[4,5–13C2]glutamate in each participant. The sum of [2,3–13C2]- and [4,5–13C2]glutamate moieties reflects glutathione synthesized from [U–13C3]glycerol regardless of pyruvate entry to the TCA cycle, and the sum was comparable among most participants, excepting patients #2 and #7 with minimal levels (Fig. 3C).
4.5. Liver pathology and [U–13C3]glycerol incorporation to glutathione
Among 8 participants, hepatic steatosis was detected in 5 patients, inflammation in 3 patients and fibrosis in 4 patients (Table 1). The levels of free 13C-glutamate and 13C-glycine were not different in these patients with different liver conditions (Fig. 4). Overall hepatic glutathione levels were not noticeably different depending on liver pathology, either (Fig. S2), but the levels of newly synthesized glutathione with [U–13C3]glycerol differed, p = 0.05. Glutathione synthesis through glutamate tended to decrease in the presence of steatosis or inflammation, and in the presence of fibrosis (Fig. 5A). In addition, glutathione synthesized through glycine tended to decrease with steatosis, inflammation or fibrosis without a statistical significance (Fig. 5B). Patient #7 was excluded in these analyses due to the exceptionally short duration (1.5 h) from [U–13C3]glycerol administration to the biopsy.
Fig. 4.
Liver pathology vs. [U–13C3]glycerol metabolism to glutamate or glycine (A) [U–13C3]glycerol metabolism to glutamate was not significantly changed in the liver with steatosis, inflammation or fibrosis. [13C2]Glu at the x-axis is the sum of [2,3–13C2]glutamate and 4,5–13C2]glutamate. (B) According to the appearance of [U–13C2]glycine, [U–13C3]glycerol metabolism to glycine remained unchanged in the liver with steatosis, inflammation or fibrosis. The data from patient 7 was excluded due to a short duration from [U–13C3]glycerol administration to a liver biopsy.
Fig. 5.
Liver pathology vs. [U–13C3]glycerol incorporation to glutathione (A) The newly synthesized glutathione through glutamate after the administration of [U–13C3]glycerol significantly decreased in the liver with fibrosis, and tended to decrease in the presence of steatosis or inflammation without a statistical significance. Glutathione-[13C2]Glu at the x-axis is the sum of glutathione (GSH + GSSG) containing [2,3–13C2]glutamate and [4,5–13C2]glutamate. (B) Based on the amount of glutathione-[13C2]glycine, the newly synthesized glutathione through glycine tended to decrease in the liver with steatosis, inflammation or fibrosis without a statistical significance. The data from patient 7 was excluded due to a short duration from [U–13C3]glycerol administration to a liver biopsy.
5. Discussion
We demonstrated metabolism of [U–13C3]glycerol to amino acids and incorporation into glutathione in the liver of adolescents undergoing bariatric surgery. Glycerol was metabolized to glycine or glutamate and both amino acids were further incorporated into de novo glutathione biosynthesis. To our knowledge, this is the first mammalian report of glycerol participation in hepatic glutathione synthesis. The levels of free glutamate, cysteine, glycine, 13C-labeled glutamate, and 13C-labeled glycine were generally similar among participants. Overall the levels of hepatic glutathione, glutathione-[13C2]glycine, and glutathione-[13C2]glutamate were high in most patients. However, there were obvious trends of lower newly synthesized glutathione with [U–13C3]glycerol in patients with liver pathology, and glutathione-[13C2]glutamate trended to be lower in the liver with fibrosis. It is difficult to draw definitive conclusions about some results because of varied timing for liver biopsy relative to glycerol administration, slightly different metabolic conditions for each patient, and intrahepatic heterogeneity among volunteers. Nevertheless, the current study clearly demonstrated that glycerol was a substrate for hepatic glutathione through glutamate or glycine metabolism, and glutathione synthesized with glycerol was reduced in obese youth with liver pathology.
Glycerol is well-known to serve as a substrate for gluconeogenesis and as the backbone for fatty acid esterification. This study proves a third role of glycerol in liver metabolism, incorporation into glutathione. The utilization of glycine or glutamate derived from [U–13C3]glycerol for glutathione biosynthesis was supported by consistent 13C-labeling patterns between free amino acids and corresponding units in glutathione. The substrate incorporation to glutathione was not trivial because the amount of 13C-labeled glutathione was comparable to those of 13C-labeled common metabolites such as glucose and lactate, produced through gluconeogenesis and glycolytic pathway, respectively. In retrospect, glycerol metabolism to glutathione is not unexpected because metabolic pathways from glycerol to glycine or glutamate are established, and the incorporation of these amino acids into glutathione is also known. Glycerol from lipolysis of triglycerides may protect the liver from oxidative stress by contributing to glutathione synthesis. This new finding is interesting because the well-known roles of glycerol for glucose and triglyceride production are associated with poor glycemic control, excess lipid burden, and potentially oxidative stress [32,33]. Glycerol incorporation to glutathione must contribute to counteracting these adverse processes. Partitioning of glycerol released from lipolysis for the biosynthesis of glucose, triglycerides, or glutathione could play a role in the progression of fatty liver disease since it can be advantageous or disadvantageous for liver health depending on its metabolism. Asides component amino acids of glutathione and their precursors, contributions of other substrates to glutathione production are mostly unknown, but glucose was reported to produce the antioxidant in erythrocytes and pancreatic islets [34,35]. The earlier study with the islets specified PC activity needed for glutathione synthesis from glucose [34]. It was consistent with the current result about glycerol incorporation to glutathione through the series of processes including glycolytic pathway, PC, the TCA cycle and glutamate.
Though the two forms of glutathione (GSH and GSSG) were distinguished in 13C NMR and their relativity in vivo could differ depending on liver condition [36], the levels of GSH and GSSG were not separately reported in this study. This was because the relative concentrations of GSH and GSSG ex vivo may not reflect relative concentrations in the liver in vivo. GSH is dominant in vivo, but GSH exposure to air causes time-dependent oxidation [37,38]. We also noticed that the lag of NMR acquisition from tissue preparation tended to increase the ratio of GSSG/GSH, suggesting GSH oxidation during sample preparation and storage. In the quantitation of total glutathione, the signal from each amino acid subunit was analyzed focusing on glycine moiety C2, glutamate moiety C4, and cysteine moiety C3, and the results based on different parts of glutathione were consistent. In theory, singlets from all the carbons in glutathione reflect their pool size, but many other carbons, except the several carbons selected in this study, were not suitable for precise analysis. The signals from other carbons in glutathione were hard to discern due to close proximity or overlap with signals from other metabolites. In our previous study with hamsters, the singlet signal from glutamate moiety C4 was quantified to report a glutathione pool size in the liver altered by an acetaminophen overdose [39]. Though [U–13C3]glycerol was injected intraperitoneally to the rodents, liver glutathione was not labeled by 13C. It was not a surprise because the experimental conditions with the hamsters were quite different from the current work. In our understanding, this study is the first report regarding glycerol incorporation to hepatic glutathione in mammals.
The use of [U–13C3]glycerol provided some insight about the biosynthesis of glutathione in the liver of obese young volunteers though it should be taken with caution because the duration from the glycerol administration to liver biopsy varied among patients. Nevertheless, the trends of impaired glutathione synthesis was obvious in the presence of steatosis or inflammation, and the newly synthesized glutathione through glutamate was significantly decreased in the fibrotic liver. There were controversial reports about hepatic glutathione contents in rodent models of hepatic steatosis. A choline deprived diet initially increased liver glutathione up to 3 days but decreased it afterward [40] while mitochondrial glutathione was reported to increase in the fatty liver of ob/ob mice [41]. It has been proposed that glutathione is consumed as a compensatory protection against further hepatic pathology. This idea is supported by several recent clinical investigations of glutathione for treatment of NAFLD. Specifically, previous reports have shown that four months of 300 mg/day of glutathione treatment improves ALT and lipid profiles in NAFLD patients [36] and decreases oxidative stress measured by serum 8-hydroxy-2-deoxyguanosine in a second cohort of individuals with NASH [42]. Taken together with our data, this suggests that the demand and utilization of glutathione is closely related with liver pathophysiology. Given that our study included young volunteers with normal to mild liver pathology, we cannot make any definitive conclusions about how glutathione metabolism may differ across the NAFLD spectrum. Therefore, future work with larger sample sizes should investigate the interactions among glutathione synthesis, utilization, and detoxification in individuals with advanced or chronic fatty liver disease and further, explore how these processes may relate to disease progression.
6. Conclusions
This study demonstrates glycerol incorporation into glutathione through glycine and glutamate metabolism in human liver. Glycerol, a substrate traditionally associated with gluconeogenesis, was utilized for glutathione synthesis. Since the majority of participants studied had little evidence of significant liver disease, the roles of glycerol in liver pathophysiology require further investigation in larger sample sizes and individuals across the entire spectrum of fatty liver disease. It would be interesting to investigate whether glycerol incorporation into glutathione is related to fatty liver disease progression. Further work should also investigate the partitioning of glycerol for gluconeogenesis, fatty acid esterification, or glutathione synthesis in relation to other chronic liver diseases.
Funding
This study was supported by the National Institutes of Health [EB015908; DK058398; multi-center nutrition and obesity center pilot from P30DK056336; NIH/NCATS Colorado CTSA Grant Number UL1 TR002535] and the Childrens Hospital Colorado Department of Pediatric Surgery.
Author declarations
1. Eunsook S. Jin: Conceptualization; Data curation; Formal analysis; Methodology; Roles/Writing - original draft;
2. Craig R. Malloy: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Visualization; Roles/Writing - original draft;
3. Gaurav Sharma: Data curation; Formal analysis; Methodology; Validation; Visualization; Writing - review & editing.
4. Erin Finn Data curation; Formal analysis; Investigation; Roles/Writing - original draft;
5. Kelly N. Z. Fuller Data curation; Formal analysis; Investigation; Roles/Writing - original draft;
6. Yesenia Garcia Reyes Data curation; Investigation; Methodology; Project administration; Supervision; Writing - review & editing.
7. Mark A. Lovell Data curation; Investigation; Writing - review & editing.
8. Sarkis C. Derderian Data curation; Investigation; Writing - review & editing.
9. Jonathan A. Schoen Data curation; Investigation; Writing - review & editing.
10. Thomas H. Inge Conceptualization; Data curation; Funding acquisition; Investigation; Supervision; Writing - review & editing.
11. Melanie G. Cree Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Roles/Writing - original draft; .
Declaration of competing interest
None.
Acknowledgements
The authors would like to thank the patients and their family members for participating, and the Children's Hospital Colorado operating room staff for their assistance in performing the research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102749.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Schwimmer J.B., et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Anderson E.L., et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhouri N., et al. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl. Int. 2016;29(4):418–424. doi: 10.1111/tri.12694. [DOI] [PubMed] [Google Scholar]
- 4.Xanthakos S.A., et al. Progression of fatty liver disease in children receiving standard of care lifestyle advice. Gastroenterology. 2020;159(5):1731–1751 e10. doi: 10.1053/j.gastro.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandato C., et al. Metabolic, hormonal, oxidative, and inflammatory factors in pediatric obesity-related liver disease. J. Pediatr. 2005;147(1):62–66. doi: 10.1016/j.jpeds.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Ribas V., Garcia-Ruiz C., Fernandez-Checa J.C. Glutathione and mitochondria. Front. Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzorno J. Glutathione! Integr. Med. 2014;13(1):8–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu G., et al. Glutathione metabolism and its implications for health. J. Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 9.Pastore A., et al. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin. Chem. 2001;47(8):1467–1469. [PubMed] [Google Scholar]
- 10.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830(5):3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halprin K.M., Ohkawara A. The measurement of glutathione in human epidermis using glutathione reductase. J. Invest. Dermatol. 1967;48(2):149–152. doi: 10.1038/jid.1967.24. [DOI] [PubMed] [Google Scholar]
- 12.Kretzschmar M. Regulation of hepatic glutathione metabolism and its role in hepatotoxicity. Exp. Toxicol. Pathol. 1996;48(5):439–446. doi: 10.1016/S0940-2993(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 13.E P., K J.J. Glutathione: physiological and clinical relevance. Journal of Restorative Medicine. 2012;1(1) [Google Scholar]
- 14.Ookhtens M., Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin. Liver Dis. 1998;18(4):313–329. doi: 10.1055/s-2007-1007167. [DOI] [PubMed] [Google Scholar]
- 15.Lu S.C. Regulation of glutathione synthesis. Mol. Aspect. Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira P.I., et al. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007;12(2):195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- 17.Lee W.M. Acetaminophen and the U.S. Acute liver failure study group: lowering the risks of hepatic failure. Hepatology. 2004;40(1):6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 18.Lee W.M., et al. Acute liver failure: summary of a workshop. Hepatology. 2008;47(4):1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persaud C., Forrester T., Jackson A.A. Urinary excretion of 5-L-oxoproline (pyroglutamic acid) is increased during recovery from severe childhood malnutrition and responds to supplemental glycine. J. Nutr. 1996;126(11):2823–2830. doi: 10.1093/jn/126.11.2823. [DOI] [PubMed] [Google Scholar]
- 20.Grimble R.F., et al. Cysteine and glycine supplementation modulate the metabolic response to tumor necrosis factor alpha in rats fed a low protein diet. J. Nutr. 1992;122(11):2066–2073. doi: 10.1093/jn/122.11.2066. [DOI] [PubMed] [Google Scholar]
- 21.Mabrouk G.M., Jois M., Brosnan J.T. Cell signalling and the hormonal stimulation of the hepatic glycine cleavage enzyme system by glucagon. Biochem. J. 1998;330(Pt 2):759–763. doi: 10.1042/bj3300759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mardinoglu A., et al. Personal model-assisted identification of NAD(+) and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 2017;13(3):916. doi: 10.15252/msb.20167422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rom O., et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci. Transl. Med. 2020;12(572) doi: 10.1126/scitranslmed.aaz2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekhar R.V., et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34(1):162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauriz J.L., et al. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic. Biol. Med. 2001;31(10):1236–1244. doi: 10.1016/s0891-5849(01)00716-x. [DOI] [PubMed] [Google Scholar]
- 26.Cieslik K.A., et al. Improved cardiovascular function in old mice after N-acetyl cysteine and Glycine supplemented diet: inflammation and mitochondrial factors. J Gerontol A Biol Sci Med Sci. 2018;73(9):1167–1177. doi: 10.1093/gerona/gly034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen D., et al. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014;99(1):169–177. doi: 10.1210/jc.2013-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Ramirez A., et al. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. (Lond.) 2014;126(1):19–29. doi: 10.1042/CS20130164. [DOI] [PubMed] [Google Scholar]
- 29.Jin E.S., Sherry A.D., Malloy C.R. An oral load of [13C3]Glycerol and blood NMR analysis detect fatty acid esterification, pentose phosphate pathway, and glycerol metabolism through the tricarboxylic acid cycle in human liver. J. Biol. Chem. 2016;291(36):19031–19041. doi: 10.1074/jbc.M116.742262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiner D.E., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Fiehn O. Metabolomics by gas chromatography-mass spectrometry: combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016;114:30 4 1–30 4 32. doi: 10.1002/0471142727.mb3004s114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelkonen R., Nikkila E.A., Kekki M. Metabolism of glycerol in diabetes mellitus. Diabetologia. 1967;3(1):1–8. doi: 10.1007/BF01269904. [DOI] [PubMed] [Google Scholar]
- 33.Puhakainen I., Koivisto V.A., Yki-Jarvinen H. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1992;75(3):789–794. doi: 10.1210/jcem.75.3.1517368. [DOI] [PubMed] [Google Scholar]
- 34.Fu A., et al. Glucose metabolism and pyruvate carboxylase enhance glutathione synthesis and restrict oxidative stress in pancreatic islets. Cell Rep. 2021;37(8) doi: 10.1016/j.celrep.2021.110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki M., et al. Substrates for glutathione regeneration in mammalian erythrocytes. Comparative Haematology International. 1997;7:70–73. [Google Scholar]
- 36.Honda Y., et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: an open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017;17(1):96. doi: 10.1186/s12876-017-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomin T., Schittmayer M., Birner-Gruenberger R. Addressing glutathione redox status in clinical samples by two-step alkylation with N-ethylmaleimide isotopologues. Metabolites. 2020;10(2) doi: 10.3390/metabo10020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003;57(3–4):145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin E.S., Lee M.H., Malloy C.R. Divergent effects of glutathione depletion on isocitrate dehydrogenase 1 and the pentose phosphate pathway in hamster liver. Phys. Rep. 2020;8(16) doi: 10.14814/phy2.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grattagliano I., et al. Severe liver steatosis correlates with nitrosative and oxidative stress in rats. Eur. J. Clin. Invest. 2008;38(7):523–530. doi: 10.1111/j.1365-2362.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 41.Yang S., et al. Mitochondrial adaptations to obesity-related oxidant stress. Arch. Biochem. Biophys. 2000;378(2):259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 42.Irie M., et al. Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease. Euroasian J. Hepato-Gastroenterol. 2016;6(1):13–18. doi: 10.5005/jp-journals-10018-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.