Abstract
Vascular calcification is accelerated in patients with diabetes mellitus and increases risk of cardiovascular events and mortality. Vascular smooth muscle cells (VSMC) play a key role in regulating vascular tone and contribute significantly to the development of diabetic vasculopathy. In this study, the function of stromal interaction molecule 1 (STIM1), an important regulator for intracellular calcium homeostasis, in diabetic vascular calcification was investigated, and the underlying molecular mechanisms were uncovered.
A SMC-specific STIM1 deletion mouse model (STIM1Δ/Δ) was generated by breeding the STIM1 floxed mice (STIM1f/f) with SM22α-Cre transgenic mice. Using aortic arteries from the STIM1Δ/Δ mice and their STIM1f/f littermates, we found that SMC-specific STIM1 deletion induced calcification of aortic arteries cultured in osteogenic media ex vivo. Furthermore, STIM1 deficiency promoted osteogenic differentiation and calcification of VSMC from the STIM1Δ/Δ mice. In the low-dose streptozotocin (STZ)-induced mouse model of diabetes, SMC-specific STIM1 deletion markedly enhanced STZ-induced vascular calcification and stiffness in the STIM1Δ/Δ mice. The diabetic mice with SMC-specific STIM1 ablation also exhibited increased aortic expression of the key osteogenic transcription factor, Runx2, and protein O-GlcNAcylation, an important post-translational modulation that we have reported to promote vascular calcification and stiffness in diabetes. Consistently, elevation of O-GlcNAcylation was demonstrated in aortic arteries and VSMC from the STIM1Δ/Δ mice. Inhibition of O-GlcNAcylation with a pharmacological inhibitor abolished STIM1 deficiency-induced VSMC calcification, supporting a critical role of O-GlcNAcylation in mediating STIM1 deficiency-induced VSMC calcification. Mechanistically, we identified that STIM1 deficiency resulted in impaired calcium homeostasis, which activated calcium signaling and increased endoplasmic reticulum (ER) stress in VSMC, while inhibition of ER stress attenuated STIM1-induced elevation of protein O-GlcNAcylation.
In conclusion, the study has demonstrated a causative role of SMC-expressed STIM1 in regulating vascular calcification and stiffness in diabetes. We have further identified a novel mechanisms underlying STIM1 deficiency-induced impairment of calcium homeostasis and ER stress in upregulation of protein O-GlcNAcylation in VSMC, which promotes VSMC osteogenic differentiation and calcification in diabetes.
Keywords: STIM1, O-GlcNAcylation, Vascular calcification, Diabetes
Graphical abstract
1. Introduction
Despite of progression in the diagnosis and treatment of diabetes mellitus, developing cardiovascular disease remains a leading cause of morbidity and mortality among patients with diabetes mellitus [1,2]. Diabetic vasculopathy, including microvascular and macrovascular complications, is one of the major diabetic complications in the cardiovascular system [3]. Vascular calcification is prevalent in diabetes and leads to increased risk of major cardiovascular events and mortality [4,5]. Complex metabolic disorders, including hyperglycemia, insulin insufficiency and excessive oxidative stress, have been linked to increased vascular calcification in diabetes mellitus. Both atherosclerosis-independent medial calcification and atherosclerosis-related intimal calcification occur in diabetic vasculature, which accelerate vascular stiffness and worsens diabetic vasculopathy. Calcification of vascular smooth muscle cells (VSMC) is the common pathology in the medial and intimal lesions, contributing predominantly to the development of diabetic vasculopathy [[5], [6], [7]]. Under pathological conditions, such as hyperglycemia and oxidative stress, VSMC can undergo phenotypic switching and osteogenic differentiation, leading to the deposition of hydroxyapatite mineral and calcification in the extracellular matrix. Increased oxidative stress is a significant cause of vascular calcification [[8], [9], [10], [11], [12], [13]]. Hyperglycemia induces oxidative stress, generation of the nonenzymatic advanced glycation end products and activation of inflammation signaling in the vascularture [3,14,15], all of which promote vascular calcification in vitro and in vivo [8,9,15]. Additionally, increased glucose metabolism via the hexosamine biosynthesis pathway promotes posttranslational protein modification via O-linked β-N-acetylglucosamine modification (O-GlcNAcylation), which is associated with increased vascular calcification in human and mouse diabetic vascular lesions [5,[16], [17], [18], [19]]. Recent studies have demonstrated a causative link between increased O-GlcNAcylation and VSMC osteogenic differentiation and vascular calcification and stiffness in diabetic mice [19].
Increased oxidative stress and O-GlcNAcylation have been linked to the regulation of calcium flux and calcium-dependent signaling [20,21]. Calcium signaling plays a critical role in the development of vascular calcification, as demonstrated in both humans and animal models [22,23]. However, the role of stromal interaction molecule 1 (STIM1), a key intracellular calcium regulator located on endoplasmic reticulum membrane, in VSMC calcification remains largely unknown. STIM1 is highly conserved in eukaryotic cells and regulates intracellular calcium flux through the store-operated calcium entry [24,25]. STIM1 deficiency causes abnormal calcium influx and cellular calcium concentration, which may subsequently affect several cellular events, including cell proliferation and differentiation [[25], [26], [27]]. In humans, patients carrying a homozygous point mutation for the STIM1 gene suffer from immunodeficiency, autoimmune disease, congenital myopathy, ectodermal dysplasia, and partial iris hypoplasia [28,29]. Mice with global STIM1 deletion exhibit sudden and perinatal mortality, immunodeficiency, autoimmune and inflammatory diseases, and muscle weakness [30]. In the vascular system, STIM1 is ubiquitously expressed in all vascular cells but more abundant STIM1 mRNA levels are determined in VSMC [31]. STIM1-dependnet signaling is important for VSMC contractility, proliferation and migration [26,32,33]. Nonetheless, it is unknown how this major calcium regulators may modulate VSMC osteogenic differentiation and calcification. Using a SMC-specific STIM1 deletion animal model, the present study uncovered an important role of VSMC-expressed STIM1 in diabetic vascular calcification and elucidated how STIM1 deficiency affects calcium signals and ER stress, leading to augmented O-GlcNAcylation that mediates STMI1 deficiency-promoted VSMC osteogenic differentiation calcification.
2. Methods
2.1. Generation of SMC-specific STIM1 deletion mice
STIM1 exon2 floxed mice (STIM1f/f) mice were bred with the SM22α-Cre transgenic mice, both were originally from The Jackson Laboratory and backcrossed to C57BL/6 genetic background, to generate SMC-specific STIM1 ablation mice (STIM1Δ/Δ). Primer sets for genotyping include: Cre: F-5′-GCGGTCTGGCAGTAAAAACTATC-3′ and R-5′-GTGAAACAGCATTGCTGTCACTT-3’; STIM1: 5′-CGATGGTCTCACGGTCTCTAGTTTC-3'; 5′-GGCTCTGCTGACCTGGAACTATAGT G-3'; and 5′-AACGTCTTGCAGTTGCTGTAGGC-3'.
2.2. Experimental animals
Eight-week old STIM1Δ/Δ mice and the STIM1f/f control littermates were intraperitoneally injected with low-dose streptozotocin (STZ, 50 mg/kg) for 5 consecutive days to induce hyperglycemia and diabetes as we previously reported [19]. Blood glucose was monitored bi-weekly for14 weeks using the AlphaTrak glucose meter and strips (Abbott, Abbott Park, IL). Both food and fluid intake were given ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
2.3. Measurement of pulse wave velocity
Aortic stiffness, indicated by pulse wave velocity (PWV), was analyzed by echocardiography using high resolution imaging system VEVO 770 (Visual Sonics, Toronto, Canada) as we previously described [19,34]. PWV was calculated as the distance divided by the time interval between the pulse wave at the aortic arch and the abdominal aorta (m/s).
2.4. Tissue harvest, processing and analysis
At the end of the experiments, mice were euthanized and aortic tissues were collected for characterization of calcium content and protein expression as we reported [9,[34], [35], [36], [37]].
Total aortic calcium was quantified by Arsenazo III calcium measurement kit (StanBio Laboratory). The amount of aortic calcium was normalized to the total protein amount in the tissues, determined by the bicinchoninic acid assay (Pierce™, PI-23225) and expressed as fold change compared to control. Aortic protein expression was determined by Western blot analysis, or immunostaining on consecutive 7-μm aortic sections using specific antibodies, including antibodies for O-GlcNAcylation (RL-2; Abcam), Runx2 (MBL, D130-3). Hematoxylin and eosin (H&E) staining was used for histology. Stained specimens were examined microscopically (leica M165 FC), and quantified using ImageJ software (NIH Bethesda, MD) as we described previously [9,[34], [35], [36], [37]].
2.5. Ex vivo aortic calcification
Calcification of aortic rings was performed as previously reported [34,36], using 3-mm mouse aortic rings cultured in osteogenic media for up to 3 weeks. Total calcium content in the aortic rings was quantified by Arsenazo III method. In parallel experiments, aortic rings were fixed in 4% paraformaldehyde, paraffin embedded and consecutive 7-μm sections were used for Alizarin Red staining and STIM1 expression was determined using specific antibody (Cell signaling) [34,36].
2.6. In vitro VSMC calcification
In vitro calcification was performed with primary VSMC at passages 3–5, as we previously reported [8,9,[34], [35], [36], [37]], in osteogenic media containing 0.25 mM l-ascorbic acid and 10 mM β-glycerophosphate and 10−8 M dexamethasone (Sigma Aldrich) for 3 weeks with media change every 3 days. Calcification was determined by Alizarin Red staining; or quantified by measuring total calcium in the cell lysates by Arsenazo III method in parallel experiments.
2.7. Inhibition of O-GlcNAcylation in VSMC
O-GlcNAcylation was inhibited in VSMC using ST49. (Sigma-Aldrich, ST045849), a pharmacological inhibitor of β-N-acetylglucosaminyltransferase. Inhibition O-GlcNAcylation was confirmed by Western blot analysis using specific antibodies for O-GlcNAcylation (RL-2, Abcam).
2.8. Intracellular calcium measurement
Intracellular calcium was measured using Fluo4 NW calcium assay kit (Molecular Probes) as we reported [34]. VSMC pre-loaded with Fluo4 NW fluorescent dye (1 μM) for 30 min were exposed to osteogenic media, and fluorescence signals were recorded for 30 min using the fluorescence microplate reader with excitation at 485 nm and emission at 525 nm (Synergy2, BioTek). Intracellular calcium was calculated by subtracting the basal fluorescence intensity from the total fluorescence intensity.
2.9. Western blot analysis
Western blot analyses were performed with the use of specific antibodies for STIM1 (Cell signaling, 5668S), α-SMA (Sigma, A5228), Col1a (Sigma, SAB2100463), Runx2 (MBL, D130-3), O-GlcNAcylation (RL-2; Abcam), p-eIF2a (Cell Signaling, 9721S), and p-CaMKII (Cell Signaling, 12716S, Phospho-CaMKII-Thr286) and detected with a Western blot chemiluminescence detection kit (Millipore). 4-Phenylbutyric Acid (4PBA, Sigma, Cat# P21005) was used to inhibit ER stress as we described [37].
2.10. Real-time polymerase chain reaction (PCR)
As we reported [8], total RNA was isolated using TRIzol (Invitrogen) and reverse transcribed into cDNA. SYBR Green–based real-time PCR was performed using specific primers for Runx2, osteocalcin (OC), α-SMA, alkaline phosphate (ALP) and collagen Ia (ColIa) with SsoFast EvaGreen Supermix (Bio-Rad) on a C1000 Thermal Cycler (Bio-Rad).
2.11. Statistical analysis
Results are presented as the mean ± SD. Differences between two groups were determined by 2-tailed Student t-tests. For multiple group comparison, one-way analysis of variance followed by a Student-Newman-Keuls test was performed. Significance was defined as p < 0.05.
3. Results
3.1. STIM1 deficiency induces VSMC osteogenic differentiation and calcification
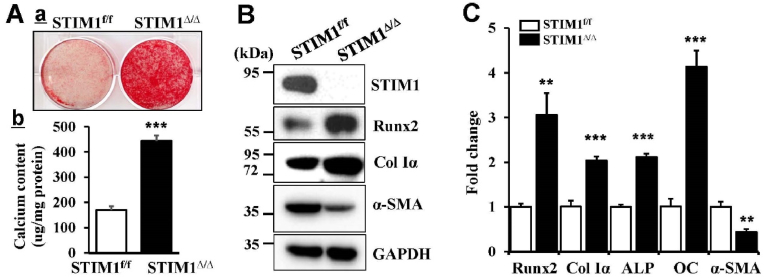
Using VSMC from the SMC-specific STIM1 deletion mice (STIM1Δ/Δ) and their control littermates (STIM1f/f), we determined the function of STIM1 in regulating VSMC osteogenic differentiation and calcification. STIM1 deletion induced calcification of VSMC culture in osteogenic media, as highlighted by both Alizarin Red staining (Fig. 1Aa) and total calcium measurements (Fig. 1Ab). Western blot analysis revealed increased protein level of the key osteogenic transcription factor, Runx2 and bone marker protein Col1α concurrently with decreased expression of the smooth muscle cell marker, α-SMA, in the STIM1Δ/Δ VSMC (Fig. 1B). Consistently, expression of genes encoding Runx2, ColIα and bone marker genes, ALP and OC, was upregulated while the α-SMA gene was downregulated in the STIM1Δ/Δ VSMC. Altogether, these results demonstrated that STIM1 deficiency induces osteogenic differentiation and calcification of STIM1Δ/Δ VSMC, supporting an important role of STIM1 in regulating VSMC function.
Fig. 1.
STIM1 deficiency induces VSMC osteogenic differentiation and calcification. Primary VSMC isolated from STIM1f/f and STIM1Δ/Δ mice were cultured in osteogenic media for 3 weeks, A) Calcification was determined by a. Alizarin Red staining. Representative images of stained dishes from 3 independent experiments are shown; and b. Total calcium content quantification by Arsenazo III assay, in parallel sets of experiments. Results shown are normalized by total protein amount. Bar values are means ± SD (n = 6, ***p < 0.001. B) Western blot analysis of the expression of STIM1, Runx2, type I collagen (Col Iα) and smooth muscle α-actin (α-SMA), using specific antibodies. The expression of GADPH was used as a loading control. C) Real-time PCR analysis of Runx2, osteogenic markers, including Col Iα, alkaline phosphatase (ALP), osteocalcin (OC) and SMA. The expression level of each gene in the STIM1f/f VSMC is normalized as 1 (n = 3, ***p < 0.001, **p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. SMC-specific STIM1 deficiency promotes ex vivo aortic calcification
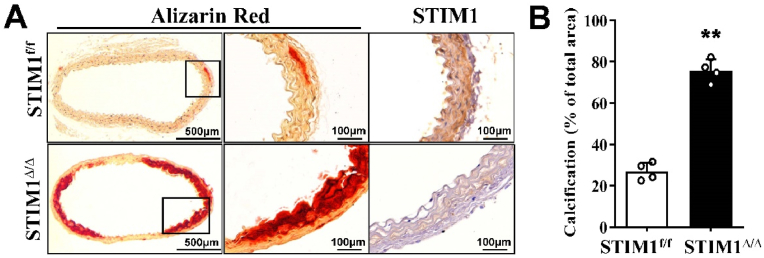
To further determine the effects of SMC-specific STIM1 deletion on calcification of VSMC in their natural milieu, we employed an ex vivo aortic ring culture system as we previously reported [36], using descending aortas from the STIM1Δ/Δ mice and their control littermates. STIM1 deficiency was evident in the aortic media of the STIM1Δ/Δ mice, as determined by immunohistochemical staining (Fig. 2A, STIM1). Consistent with the observation from the study using isolated VSMC in Fig. 1, aortas from STIM1Δ/Δ mice exhibited enhanced calcification after cultured in osteogenic media for 3 weeks, compared with that in aortas from the control STIM1f/f littermates (Fig. 2A, Alizarin Red). Quantification analysis revealed a markedly increase in vascular calcification in aortas from STIM1Δ/Δ mice (Fig. 2B), further supporting that SMC-specific STIM1 deletion promoted VSMC calcification.
Fig. 2.
SMC-specific STIM1 deletion promotes ex vivo aortic calcification. Aorta rings from STIM1f/f and STIM1Δ/Δ mice were cultured in osteogenic medium for 3 weeks. A) Consecutive sections were stained by Alizarin Red (calcification) and specific antibody for STIM1. Representative images from 4 pairs of littermates are shown. B) Quantification of vascular calcification in A with by NIH Image J software. Results presented are the percentage of positively alizarin red-stained areas in the total area (n = 4, ***p < 0.001). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. SMC-specific STIM1 deletion promotes vascular calcification and stiffness in diabetic mice
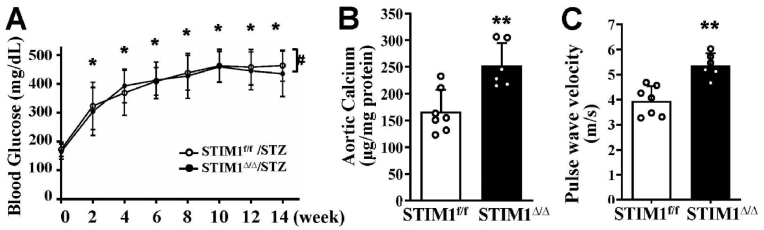
Using a low-dose STZ injection-induced diabetic mouse model, the effects of SMC-specific STIM1 deletion on vascular calcification in vivo were determined. Elevation of blood glucose levels was observed in the STZ-injected STIM1Δ/Δ mice similarly to those of the control mice (STIM1f/f/STZ) during the 14 weeks after administration of STZ (Fig. 3A). Increased calcification was determined in aortic arteries from the STZ-injected STIM1Δ/Δ mice, compared with the STZ-injected control STIM1f/f littermates (Fig. 3B). Furthermore, a significant increase in pulse wave velocity (PWV), an indicator for aortic stiffness [38], was demonstrated in the STZ-injected STIM1Δ/Δ mice (Fig. 3C). Therefore, SMC-specific STIM1 deletion in mice promoted vascular calcification and aortic stiffness, without affecting low dose STZ-induced hyperglycemia, in the diabetic mice.
Fig. 3.
SMC-specific STIM1 deletion promotes vascular calcification and stiffness in diabetic mice. STIM1f/f and STIM1Δ/Δ mice were injected with streptozotocin (STZ) for 5 consecutive days. A) Blood glucose levels were monitored using the AlphaTrak glucose meter up to 14 weeks after the STZ injection. (*p < 0.05 compared with week 0; # indicating not significant between two groups). B) Calcium contents were determined in descending aortas, the calcium content in each sample was normalized to its protein level (n = 7 and 6 respectively, ***p < 0.001). C) Echocardiography was performed in mice 14 week after STZ injection, to determine pulse wave velocity, an indicator for aortic stiffness (n = 7 and 6 respectively, ***p < 0.001).
3.4. Increased O-GlcNAcylation and Runx2 are associated with diabetic vascular calcification in the SMC-specific STIM1 deletion mice
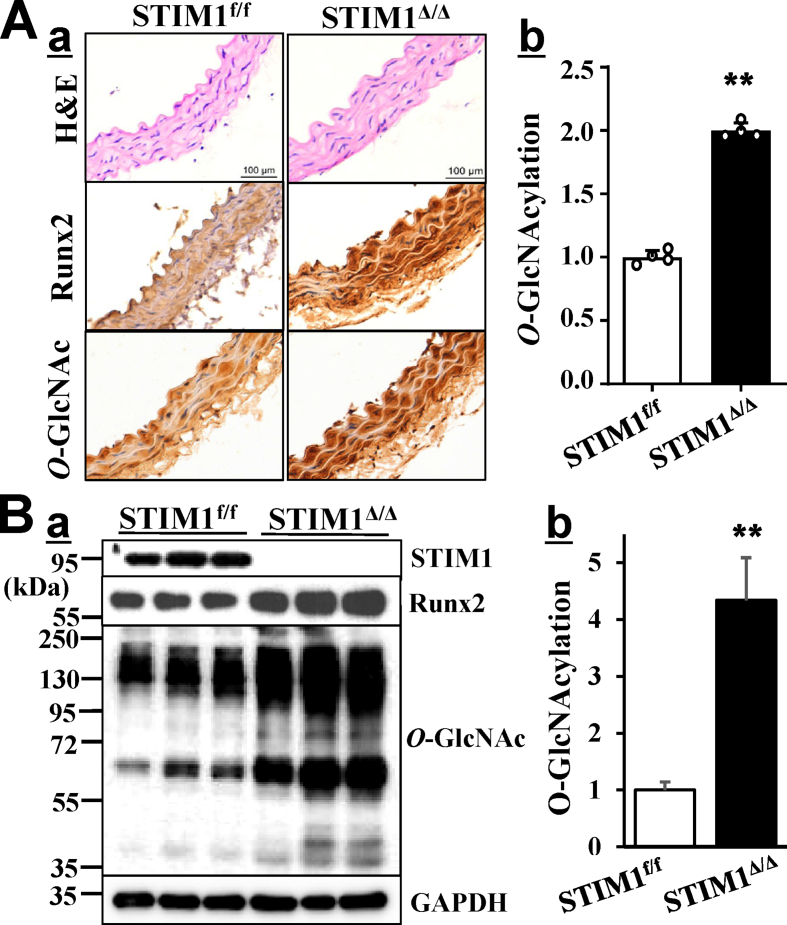
We have previously reported that increased protein O-GlcNAcylation promotes VSMC calcification in vitro and vascular calcification in diabetic mice [19]. To evaluate whether SMC-specific STIM1 deletion-increased vascular calcification in the diabetic mice was associated with O-GlcNAcylation, we determined protein O-GlcNAcylation in arteries from the STZ-injected STIM1Δ/Δ mice and the control STIM1f/f littermates. Immunohistochemical staining revealed markedly increased vascular O-GlcNAcylation in the media of arteries from STZ-injected STIM1Δ/Δ mice compared with those from the STIM1f/f mice (Fig. 4Aa, O-GlcNAc) and quantified in Fig. 4Ab. Western blotting analysis further confirmed significant increases in protein O-GlcNAcylation in aortic arteries from the STIM1Δ/Δ mice (Fig. 4B). Increased aortic O-GlcNAcylation in the STIM1Δ/Δ mice was accompanied by increased Runx2 (Fig. 3Aa & Ba, Runx2), the key osteogenic transcription factor that determines vascular calcification355,86. Accordingly, increased vascular O-GlcNAcylation in the STIM1Δ/Δ mice may be responsible for STIM1 deletion-promoted vascular calcification in the diabetic mice.
Fig. 4.
Increased O-GlcNAcylation and Runx2 are associated with diabetic vascular calcification in the SMC-specific STIM1 deletion mice. The expression of Runx2 and protein O-GlcNAcylation in aortas from the STZ-injected STIM1f/f and STIM1Δ/Δ mice as described in Fig. 3 were determined by A) Immunostaining in aortic sections, using specific antibodies for Runx2 and O-GlcNAc. H&E staining was used for histology. b. Vascular O-GlcNAcylation in a was quantified by NIH ImageJ and compared with that in STZ-injected STIM1f/f, which was defined as 1, (n = 4/group, **p < 0.001). B) Protein extracts from descending aortas (n = 3/group) were used for Western blot with specific antibodies. The expression of GADPH was used as a loading control. b. Total protein O-GlcNAcylation was quantified by the intensity of the bands in a (O-GlcNAc), normalized to the intensity of GAPDH. Results are shown as fold changes compared to GlcNAcylation in protein extracts from STIM1f/f mice which was defined as 1 (n = 3, **p = 0.002).
3.5. Increased O-GlcNAcylation mediates STIM1 deletion-promoted VSMC calcification
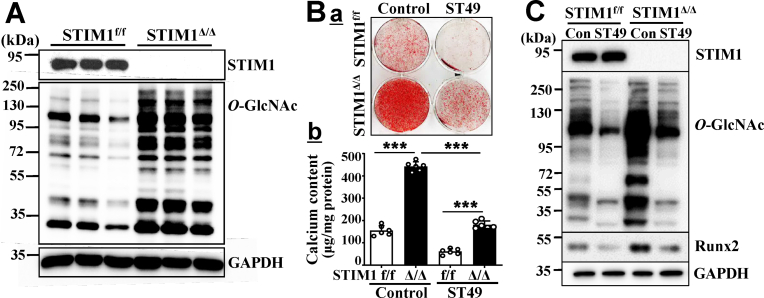
The effect of STIM1 deficiency on O-GlcNAcylation was further characterized using primary VSMC from the STIM1Δ/Δ mice and the control STIM1f/f mice. STIM1 deficiency led to significant increases in O-GlcNAcylation in VSMC grown in culture media (Fig. 5A), suggesting a direct contribution of STIM1 deficiency to increased O-GlcNAcylation in VSMC. The role of increased O-GlcNAcylation in mediating STIM1 deficiency-promoted VSMC calcification was further determined using ST49, a selective pharmacological inhibitor for O-GlcNActransferase (OGT), which is the essential enzyme for protein O-GlcNAcylation. Inhibition of O-GlcNAcylation by ST49 not only abolished calcification of the control STIM1f/f VSMC cultured in osteogenic media, but also attenuated STIM1 deficiency-promoted calcification of the STIM1Δ/Δ VSMC (Fig. 5Ba). Quantitative measurements of calcium in parallel sets of experiments further confirmed that ST49 potently inhibited STIM1 deficiency-promoted VSMC (Fig. 5Bb). Consistently, ST49-inhibited O-GlcNAcylation in VSMC was associated with inhibition of STIM1 deficiency-induced Runx2 in the STIM1Δ/Δ VSMC. Collectively, these results support a critical role of increased O-GlcNAcylation in mediating STIM1 deficiency-induced Runx2 upregulation and calcification of VSMC.
Fig. 5.
STIM1 deletion promotes VSMC calcification via increased O-GlcNAcylation. A) Western blot analysis of protein O-GlcNAcylation in VSMC isolated from STIM1f/f and STIM1Δ/Δ mice. The expression of GAPDH was used as a loading control. (n = 3/group). B) STIM1f/f and STIM1Δ/Δ VSMC were cultured in osteogenic medium with or without inhibitor for O-GlcNAcylation, ST49 (10 μM) for 3 weeks. Calcification was determined by a. Alizarin red staining; or b. quantified by Arsenazo III assay in separate dishes (n = 3, ***p<0.001). C) Western blot analysis of Runx2 expression and O-GlcNAcylation in protein lyses isolated from B with specific antibodies. Representative blots from 3 independent experiments are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. STIM1 deficiency-induced calcium signaling and endoplasmic reticulum (ER) stress increase O-GlcNAcylation in VSMC
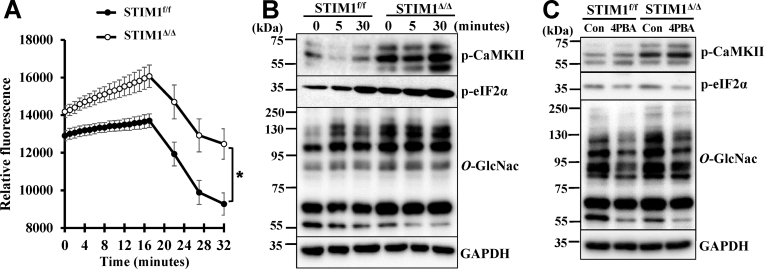
STIM1 is known to regulate intracellular calcium flux, therefore, we initially compared calcium flux in the STIM1f/f and STIM1Δ/Δ VSMC. Elevation of intracellular calcium was observed in VSMC within minutes after exposure to osteogenic medium, followed by slow decreases after 16 min. STIM1 deficiency in VSMC led to a significant increase in intracellular calcium flux in the STIM1Δ/Δ VSMC. Such signal was sustained at a higher level during the experimental period (Fig. 6A). Consistently, increased phosphorylation and activation of calcium/calmodulin-dependent kinase II (CaMKII), a calcium signal mediator, was observed in the STIM1Δ/Δ VSMC (Fig. 6B, p-CaMKII). As increased intracellular calcium flux has been linked to ER stress, we further evaluated ER stress, as highlighted by increased phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) determined in the STIM1Δ/Δ VSMC. Accordingly, increased calcium flux, activation of calcium signaling and elevated ER stress in the STIM1Δ/Δ VSMC may contribute to STIM1 deficiency-induced O-GlcNAcylation and VSMC calcification.
Fig. 6.
STIM1 deficiency-induced calcium signaling and ER stress increase O-GlcNAcylation in VSMC. A) VSMC from STIM1f/f and STIM1Δ/Δ were exposed to osteogenic media and calcium flux was monitored by Fluo-4 for up to 32 min. Results from 8 independent experiments are shown. (*p < 0.05). B) VSMC from STIM1f/f and STIM1Δ/Δ were serum starved and exposed to osteogenic media for 0, 5 and 30 min. Western blots were performed to determine phosphorylation of CaMKII (p-CaMKII, Ca2+/calmodulin-dependent protein kinase), phosphorylation of eIF2a (p-eIF2a, eukaryotic translation initiation factor 2A, an ER stress signal), and protein O-GlcNAcylation (O-GlcNac). Representative blots from 3 independent experiments are shown. C) VSMC were serum starved and then exposed to osteogenic media with or without ER stress inhibitor, 4PBA (10 mM), for 24 h. Western blots were performed to determine p-CaMKII, p-eIF2a and O-GlcNAc using specific antibodies. Representative blots from 3 independent experiments are shown.
Using 4PBA, an ER stress inhibitor that inhibits VSMC calcification [37], we further determined whether increased ER stress might mediate STIM1 deficiency-induced O-GlcNAcylation. 4PBA inhibited p-eIF2α, but did not affect activation of pCaMKII in the STIM1Δ/Δ VSMC (Fig. 6C), suggesting that activation of calcium signaling may precede STIM1 deficiency-induced ER stress. Importantly, inhibition of ER stress also decreased O-GlcNAcylation (Fig. 6C, O-GlcNAc), supporting that increased ER stress mediates STIM1 deficiency-induced O-GlcNAcylation in VSMC. Taken together, these results suggest that STIM1 deficiency in VSMC leads to increased intracellular calcium flux, activation of CaMKII, and ER stress, which mediates upregulation of O-GlcNAcylation that promotes VSMC calcification.
4. Discussion
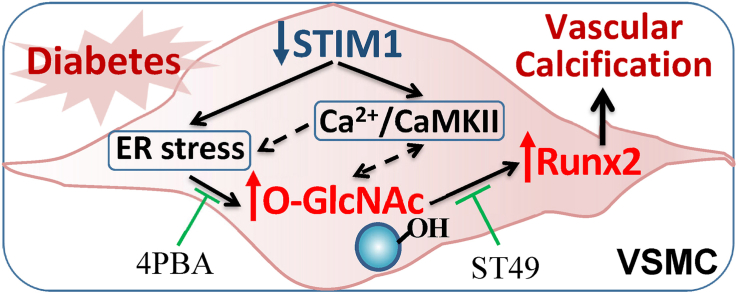
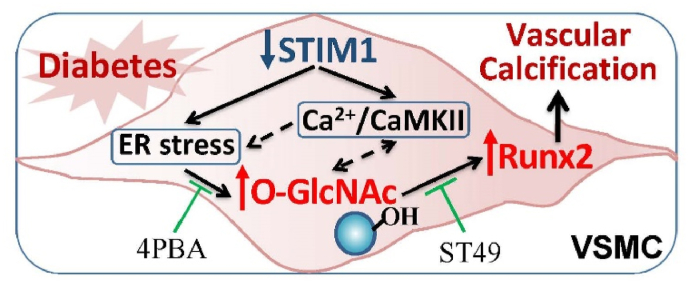
Vascular calcification is prevalent in diabetes mellitus and is correlated with adverse cardiovascular outcome and mortality in diabetic subjects. Emerging studies have improved our understanding of the active cell-driven process of vascular calcification and highlighted the major contributions of the osteogenic differentiation of VSMC in the development of vascular calcification in atherosclerosis, diabetes and chronic renal failure. In diabetic vascular calcification, we and others have demonstrated the major role of increased oxidative stress and upregulation of protein O-GlcNAcylation in promoting VSMC calcification. The present studies further elucidated a novel function of STIM1, a calcium sensor and key regulator of the intracellular calcium signals, in the regulation of VSMC calcification via ER stress-mediated upregulation of O-GlcNAcylation (Fig. 7).
Fig. 7.
STIM1 deficiency promotes VSMC calcification in diabetes via ER stress-induced O-GlcNAcylation. Diabetic conditions enhance STIM1 deficiency-induced intracellular calcium/CaMKII activation and ER stress in VSMC, leading to increased protein O-GlcNAcylation that upregulates Runx2 and promotes VSMC calcification.
STIM1 mutations have been linked to human diseases, including immunodeficiency and autoimmunity and defects in platelets, fibroblasts, skeletal muscle [28,29]. However, the role of STIM1 in diabetic vascular calcification in human has not been reported. STIM1 expression was reduced in islets from human donor with type II diabetes and STZ-induced diabetic mice [39]. By generating SMC-specific STIM1 deletion mouse model, we have provided the first evidence linking a causative regulation of STIM1 in VSMC calcification in vitro and diabetic vascular calcification in vivo. Similar to our previous reports, minimal calcification was determined in the control VSMC cultured in the osteogenic media without additional stimuli in vitro or ex vivo, while STIM1 deficiency was sufficient to increase calcification of the STIM1Δ/Δ VSMC cultured in osteogenic media. Furthermore, decreased SMC marker gene and increased osteogenic transcription factor Runx2 and bone markers were determined in the STIM1Δ/Δ VSMC, supporting the notion that STIM1 deficiency in VSMC led to dedifferentiation and osteogenic differentiation of VSMC. Consistently, SMC-specific STIM1 deletion markedly increased vascular calcification of the STIM1Δ/Δ mice in vivo in the low dose STZ-induced diabetes model, which we have previously reported to induce vascular calcification [19]. Similar to our previous observations in diabetic mice [19], we found that increased vascular calcification in the diabetic STIM1Δ/Δ mice was associated with increased aortic stiffness.
Of note, SMC-specific STIM1 deletion did not affect STZ-induced development of hyperglycemia per se, suggesting that intrinsic intracellular signaling in the STIM1Δ/Δ VSMC promotes VSMC osteogenic differentiation and calcification in diabetic conditions. Hyperglycemia has been linked to increased oxidative stress, upregulation of O-GlcNAcylation and activation of calcium signaling [19,20,40], all of which promote VSMC calcification. The observation of an increase in vascular O-GlcNAcylation in the STIM1Δ/Δ mice prompted us to further determine the role of O-GlcNAcylation in VSMC from the STIM1Δ/Δ mice. Intriguingly, deletion of STIM1 in VSMC resulted in elevation of O-GlcNAcylation, a key inducing factor that promotes VSMC calcification in vitro and in vivo [19]. Importantly, inhibition of O-GlcNAcylation attenuated STIM1-decifiency induced VSMC calcification in the STIM1Δ/Δ VSMC, supporting that STIM1 deficiency induces VSMC calcification via a mechanism mediated by increased O-GlcNAcylation.
Consistent with the known function of STIM1 in regulating intracellular calcium flux, we found that STIM1 deficiency led to increased calcium flux in the STIM1Δ/Δ VSMC. The findings of a sustained elevation of calcium flux in the STIM1Δ/Δ VSMC under basal condition that was further induced by the osteogenic media suggests that STIM1 deficiency may prime VSMC to a status that is prone to osteogenic differentiation. Consistent with this notion, STIM1 deficiency was sufficient to promote VSMC calcification in osteogenic media without additional stimuli.
Differences in the duration of intracellular calcium flux may lead to diverse downstream molecular signals that differentially regulate VSMC physiology and pathology. Unlike physiologic intracellular calcium influx that is associated with excitation-contraction of SMC, sustained increase in intracellular calcium may lead to VSMC pathology, such as calcification [41]. We found the prolonged increases in intracellular calcium in the STIM1Δ/Δ VSMC were associated with the activation of the calcium signaling mediator CaMKII, a multifunctional serine/threonine protein kinase that is expressed abundantly in vascular tissue [42,43]. CaMKII activation has been linked to pathophysiological changes in Ca2+ handling and regulating gene expression in cardiomyocytes [44], however, the role of CaMKII signaling in regulating vascular calcification is not clear. Autophosphorylation of CaMKII on Threonine (Thr) 286/287 during sustained calcium transients is associated with a constitutively Ca2+/CaM-independent activity even at lower intracellular calcium concentration [45]. Accordingly, sustained increased intracellular calcium led to autophosphorylation and activation of CaMKII in the STIM1 deficiency VSMC. Given that ER stress inhibitor did not affect activation of CaMKII in the control or STIM1 deletion VSMC in the present study, it is possible that increased phosphorylation of CaMKII may precede STIM1 deficiency-induced ER stress. Furthermore, hyperglycemia can promote direct O-GlcNAc modification of CaMKII in diabetes mellitus, which activates CaMKII autonomously [44]. In addition, hyperglycemia-induced O-GlcNAcylation and CaMKII activation increases cytosolic reactive oxygen species (ROS) in mouse myocytes [20]; while ROS can induce CaMKII activation via oxidation of methionine 281/282 in the regulatory domain of CaMKII, similar to it Thr287 autophosphorylation [46]. Therefore, elevated O-GlcNAcylation in the STIM1Δ/Δ VSMC may further promote CaMKII activation. Our observation of increased CaMKII phosphorylation and activation in the STIM1Δ/Δ VSMC has provided new insights into the activation of CaMKII in VSMC.
Our studies revealed that increased ER stress contributed to the upregulation of O-GlcNAcylation in the STIM1Δ/Δ VSMC. STIM1-deficiency in VSMC increased phosphorylation of eIF2α, an ER stress signal transducer that regulates protein translation. In hearts from the SMC-specific STIM1 deletion mice with myocardial infarct, ER stress markers GRP78 and CHOP was reduced while ATF6 was increased [47]. Accordingly, STIM1-deficiency in different cells, VSMC or cardiomyocytes, may differently affect ER stress responsive signals, such as eIF2α for protein translation, CHOP for apoptosis, or ATF6 for protein degradation. In cardiomyocytes, increased intracellular calcium induces ER stress markers, including GRP78 and CHOP, which is associated with O-GlcNAcylation elevation [48]. Increased O-GlcNAcylation impairs calcium cycling and cardiomyocyte function, while inhibition of O-GlcNAcylation improves calcium handling and contractile function of the diabetic heart [49,50], supporting the role of O-GlcNAcylation in regulating calcium flux and cardiomyocyte function in the development of diabetic cardiomyopathy. Furthermore, O-GlcNAcylation of eIF2α was found to regulate eIF2α-mediated ER stress response in the HepG2 cells [51]. Together with our finding of the role of ER stress in mediating STIM1 deficiency-induced O-GlcNAcylation, these studies suggest a dynamic interplay of intracellular calcium, ER stress and O-GlcNAcylation, which contribute to STIM1-regulated VSMC pathophysiology.
As STIM1 mutations have been linked to human diseases but the function of STIM1 in vascular disease are not fully understood, our funding of a causative effect of STIM1 deficiency on VSMC calcification has shed lights on the novel function of STIM1 in the pathogenesis of vascular disease. Of note, reduction of STIM1 expression has been demonstrated in islets from human donor with type II diabetes [39]. In addition, decreased STIM1 expression has been determined in aged arteries, such as rat mesenteric and mouse cerebral arteries [52,53], which may contribute to increased vascular calcification and stiffness with aging. Further investigations are warrant to uncover the regulation and function of STIM1 in different vascular beds in aging and age-related vascular diseases. In addition, the novel mechanisms uncovered in our studies have highlighted STIM1 deficiency-increased upregulation of O-GlcNAcylation in promoting Runx2 upregulation and VSMC calcification. O-GlcNAcylation is emerging recognized as an “integrated sensor” for many diseases, including cardiovascular disease [54], uncovering the STIM1-dependent and independent O-GlcNAcylation signaling network in VSMC may provide important molecular insights into the development of new strategies or targets for detection, prevention and therapy of vascular diseases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Ming He, Department of Pathology at the University of Alabama at Birmingham, for helpful discussion; and grant support from the National Institutes of Health (NIH, HL136165, HL146103 and HL158097) as well as United States Department of Veterans Affairs Basic Sciences R&D Service (BX005800and BX004426) to YC.
Data availability
No data was used for the research described in the article.
References
- 1.Matheus A.S., et al. Impact of diabetes on cardiovascular disease: an update. Int. J. Hypertens. 2013 doi: 10.1155/2013/653789. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon B.M., Maddox T.M. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes. 2015;6:1246–1258. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt A.M. Diabetes mellitus and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2019;39:558–568. doi: 10.1161/ATVBAHA.119.310961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiha M., Njeim M., Chedrawy E.G. Diabetes and coronary heart disease: a risk factor for the global epidemic. Int. J. Hypertens. 2012 doi: 10.1155/2012/697240. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr J.J., et al. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: the diabetes heart study. Bone. 2008;42:43–52. doi: 10.1016/j.bone.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raggi P., Shaw L.J., Berman D.S., Callister T.Q. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J. Am. Coll. Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 7.Hoff J.A., et al. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J. Am. Coll. Cardiol. 2003;41:1008–1012. doi: 10.1016/s0735-1097(02)02975-3. [DOI] [PubMed] [Google Scholar]
- 8.Byon C., Javed A., Dai Q., Kappes J., Clemens T., Darley-Usmar V., McDonald J., Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008;30:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byon C.H., et al. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler. Thromb. Vasc. Biol. 2011;31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mody N., Parhami F., Sarafian T.A., Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 11.Liberman M., et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 12.Miller J.D., et al. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byon C.H., Heath J.M., Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa T., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 15.Wei Q., et al. Advanced glycation end products accelerate rat vascular calcification through RAGE/oxidative stress. BMC Cardiovasc. Disord. 2013;13:13. doi: 10.1186/1471-2261-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federici M., et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 17.Bostrom K.I., Jumabay M., Matveyenko A., Nicholas S.B., Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ. Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stabley J.N., Towler D.A. Arterial calcification in diabetes mellitus: preclinical models and translational implications. Arterioscler. Thromb. Vasc. Biol. 2017;37:205–217. doi: 10.1161/ATVBAHA.116.306258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath J.M., et al. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ. Res. 2014;114:1094–1102. doi: 10.1161/CIRCRESAHA.114.302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu S., et al. Hyperglycemia acutely increases cytosolic reactive oxygen species via O-linked GlcNAcylation and CaMKII activation in mouse ventricular myocytes. Circ. Res. 2020;126:e80–e96. doi: 10.1161/CIRCRESAHA.119.316288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngoh G.A., Watson L.J., Facundo H.T., Jones S.P. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byon C.H., Chen Y. Molecular mechanisms of vascular calcification in chronic kidney disease: the link between bone and the vasculature. Curr. Osteoporos. Rep. 2015;13:206–215. doi: 10.1007/s11914-015-0270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Zhao X., Wu H. Arterial stiffness: a focus on vascular calcification and its link to bone mineralization. Arterioscler. Thromb. Vasc. Biol. 2020;40:1078–1093. doi: 10.1161/ATVBAHA.120.313131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins H.E., Zhu-Mauldin X., Marchase R.B., Chatham J.C. STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H446–H458. doi: 10.1152/ajpheart.00104.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou J., et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisaillon J.M., et al. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am. J. Physiol. Cell Physiol. 2010;298:C993–C1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong J., et al. The deregulation of STIM1 and store operative calcium entry impaired aortic smooth muscle cells contractility in aortic medial degeneration. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard C., et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacruz R.S., Feske S. Diseases caused by mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci. 2015;1356:45–79. doi: 10.1111/nyas.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol. Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson M., et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan V., et al. STIM1-dependent peripheral coupling governs the contractility of vascular smooth muscle cells. Elife. 2022;11 doi: 10.7554/eLife.70278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi Y., et al. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2007;361:934–940. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., et al. Dietary potassium regulates vascular calcification and arterial stiffness. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y., Byon C.H., Yuan K., Chen J., Mao X., Heath J.M., Javed A., Zhang K., Anderson P.G., Chen Y. Smooth muscle cell-specific Runx2 deficiency inhibits vascular calcification. Circ. Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng L., et al. Inhibition of FOXO1/3 promotes vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2015;35:175–183. doi: 10.1161/ATVBAHA.114.304786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y., et al. AKT-independent activation of p38 MAP kinase promotes vascular calcification. Redox Biol. 2018;16:97–103. doi: 10.1016/j.redox.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drueke T.B., Massy Z.A. Role of vitamin D in vascular calcification: bad guy or good guy? Nephrol. Dial. Transplant. 2012;27:1704–1707. doi: 10.1093/ndt/gfs046. [DOI] [PubMed] [Google Scholar]
- 39.Kono T., et al. Impaired store-operated calcium entry and STIM1 loss lead to reduced insulin secretion and increased endoplasmic reticulum stress in the diabetic beta-cell. Diabetes. 2018;67:2293–2304. doi: 10.2337/db17-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Zhao X., Wu H. Transcriptional programming in arteriosclerotic disease: a multifaceted function of the Runx2 (Runt-Related transcription factor 2) Arterioscler. Thromb. Vasc. Biol. 2021;41:20–34. doi: 10.1161/ATVBAHA.120.313791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen N.T., et al. Oxidative stress by Ca(2+) overload is critical for phosphate-induced vascular calcification. Am. J. Physiol. Heart Circ. Physiol. 2020;319:H1302–H1312. doi: 10.1152/ajpheart.00305.2020. [DOI] [PubMed] [Google Scholar]
- 42.Ebenebe O.V., Heather A., Erickson J.R. CaMKII in vascular signalling: friend or foe. Heart Lung Circ. 2018;27:560–567. doi: 10.1016/j.hlc.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Saddouk F.Z., Ginnan R., Singer H.A. Ca(2+)/Calmodulin-Dependent protein kinase II in vascular smooth muscle. Adv. Pharmacol. 2017;78:171–202. doi: 10.1016/bs.apha.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Erickson J.R., et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier L.S. Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) in the heart. Adv. Exp. Med. Biol. 2012;740:685–702. doi: 10.1007/978-94-007-2888-2_30. [DOI] [PubMed] [Google Scholar]
- 46.Anderson M.E. Oxidant stress promotes disease by activating CaMKII. J. Mol. Cell. Cardiol. 2015;89:160–167. doi: 10.1016/j.yjmcc.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mali V., Haddox S., Belmadani S., Matrougui K. Essential role for smooth muscle cell stromal interaction molecule-1 in myocardial infarction. J. Hypertens. 2018;36:377–386. doi: 10.1097/HJH.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou L., et al. Glucose deprivation-induced increase in protein O-GlcNAcylation in cardiomyocytes is calcium-dependent. J. Biol. Chem. 2012;287:34419–34431. doi: 10.1074/jbc.M112.393207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark R.J., et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y., et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 51.Jang I., et al. O-GlcNAcylation of eIF2alpha regulates the phospho-eIF2alpha-mediated ER stress response. Biochim. Biophys. Acta. 2015;1853:1860–1869. doi: 10.1016/j.bbamcr.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y., et al. Contrasting patterns of agonist-induced store-operated Ca2+ entry and vasoconstriction in mesenteric arteries and aorta with aging. J. Cardiovasc. Pharmacol. 2015;65:571–578. doi: 10.1097/FJC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgeon-Chartier C., Menguy C., Prevot A., Morel J.L. Effect of aging on calcium signaling in C57Bl6J mouse cerebral arteries. Pflügers Archiv. 2013;465:829–838. doi: 10.1007/s00424-012-1195-7. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y., Zhao X., Wu H. Metabolic stress and cardiovascular disease in diabetes mellitus: the role of protein O-GlcNAc modification. Arterioscler. Thromb. Vasc. Biol. 2019;39:1911–1924. doi: 10.1161/ATVBAHA.119.312192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.