Summary
Evidence concerning PM1 exposure, maternal blood pressure (BP), and hypertensive disorders of pregnancy (HDP) is sparse. We evaluated the associations using 105,063 participants from a nationwide cohort. PM1 concentrations were evaluated using generalized additive model. BP was measured according to the American Heart Association recommendations. Generalized linear mixed models were used to assess the PM1-BP/HDP associations. Each 10 μg/m3 higher first-trimester PM1 was significantly associated with 1.696 mmHg and 1.056 mmHg higher first-trimester SBP and DBP, and with 11.4% higher odds for HDP, respectively. The above associations were stronger among older participants (> 35 years) or those educated longer than 17 years or those with higher household annual income (> 400,000 CNY). To conclude, first-trimester PM1 were positively associated with BP/HDP, which may be modified by maternal age, education level, and household annual income. Further research is warranted to provide more information for both health management of HDP and environmental policies enactment.
Subject areas: Environmental medicine, Environmental science, Environmental health
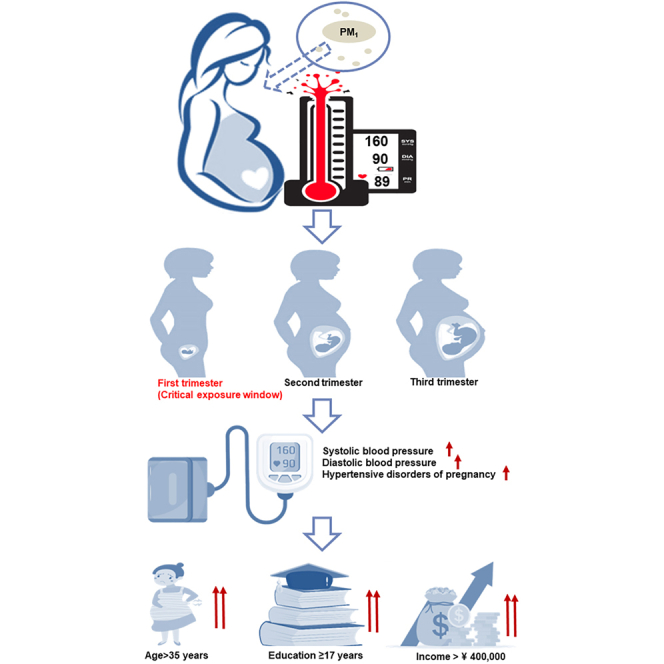
Graphical abstract
Highlights
-
•
Our study provides novel evidence on PM1- BP/HDP associations among pregnant women
-
•
First trimester was the critical exposure window for PM1-BP/HDP associations
-
•
Those > 35 years old, educated ≥ 17 years, income > 400,000 CNY were more susceptible
Environmental medicine; Environmental science; Environmental health
Introduction
Hypertensive disorders of pregnancy (HDP) are one of the most common prenatal complications, with an incidence rate of 10–20% among all pregnancies.1,2,3,4 The prevalence of HDP are rising as increased maternal age, obesity, diabetes mellitus, and greater mental stress have become more common in pregnant women.5 HDP could increase the risk of maternal and offspring adverse outcomes,6 potentially leading to placental abruption, preeclampsia, fetal growth restriction, preterm delivery, cesarean delivery, postpartum hemorrhage, low birth weight, and offspring neurodevelopmental disorders.7,8,9 Furthermore, higher maternal blood pressure (BP), which even if is lower than the diagnostic threshold for HDP, has been identified with an association with adverse maternal and fetal outcomes.10,11 It is of critical importance to identify dominant risk factors for HDP, as well as the increase in maternal BP.
Recently, epidemiological studies have explored the associations of ambient particulates with BP among pregnant women. However, the results of these studies have been inconsistent. Evidence from the United States indicated that exposure to particulate with aerodynamic diameter ≤ 2.5 μm (PM2.5) during pregnancy was positively associated with maternal BP.12 In another study of the United States, no association of PM2.5 exposure during pregnancy with elevated maternal BP was observed.13 PM2.5 was even inversely related to odds of hypertension of pregnant women in Australia.14 Mechanistic studies indicated that PM could activate sympathetic nervous system, induce endothelial dysfunction and arterial stiffness, which could further lead to elevated BP and hypertension.15 However, there are some disadvantages in existing studies: (a) sample sizes were small or conclusions remained inconsistent.12,13,14 Most of these studies addressing the effects of PM on BP were performed in developed countries with lower PM concentrations, (b) these previous analyses mainly focused on PM2.5 or larger particulates than particulate with diameter ≤ 1.0 μm (PM1). PM1 contributes to the majority of PM2.5 in China, which has smaller diameter but greater surface area to mass ratio and carries larger number of toxic compounds than PM2.5.16 Thus, PM1 tends to be more toxic than those larger particles as the small size allows them to easily reach the lung alveoli and enter the circulatory system, and eventually adversely affecting BP of pregnant women.17 Due to cardiovascular changes during pregnancy, pregnant women might be more sensitive to the toxic effects of particulates,18 especially in the first trimester.19
No research has been performed to investigate the association of PM1 exposure during pregnancy with maternal BP and HDP to our knowledge. However, these are new critical epidemiological evidences for a comprehensive understanding of the health effects of PM1. In the current study, we aimed to examine the associations of PM1 exposure with maternal BP, as well as HDP prevalence based on a nationwide population-based study (the China birth cohort study, CBCS).
Results
Descriptive statistics
As presented in Table 1, the average age of the participants was 29.59 years old, and 93.68% of them were of Han ethnicity. A total of 14,453 (13.76%) pregnant women were identified as HDP. Compared with the non-HDP, pregnant women with HDP were older, possessing higher pre-pregnancy body mass index (BMI), were more likely to be conceived in summer & autumn, were more likely to be multipara and to be exposed to secondhand smoke.
Table 1.
Main characteristics of the participants (n = 105,063)
| Characteristics | Overall (n = 105,063) | HDP (n = 14,453) | Non-HDP (n = 84,589) | p value |
|---|---|---|---|---|
| Maternal age, year, mean ± SD | 29.59 ± 4.28 | 30.05 ± 4.59 | 29.50 ± 4.22 | <0.001 |
| Maternal pre-pregnancy BMI, kg/m2, mean ± SD | 21.84 ± 3.82 | 22.46 ± 4.43 | 21.74 ± 3.69 | <0.001 |
| Maternal ethnicity (%) | 0.680 | |||
| Han | 98,425 (93.68%) | 13,562 (93.84%) | 79,291 (93.74%) | |
| Minority | 6,638 (6.32%) | 891 (6.16%) | 5,298 (6.26%) | |
| Maternal education (%) | <0.001 | |||
| ≤ 12 years | 51,043 (48.58%) | 7,229 (50.02%) | 40,905 (48.36%) | |
| 13-16 years | 42,792 (40.73%) | 5,541 (38.34%) | 34,919 (41.28%) | |
| ≥ 17 years | 11,228 (10.69%) | 1,683 (11.64%) | 8,765 (10.36%) | |
| Household annual income (%) | <0.001 | |||
| < 100,000 CNY | 36,124 (34.38%) | 5,342 (36.96%) | 28,883 (34.14%) | |
| 100,000–400,000 CNY | 56,493 (53.77%) | 7,385 (51.10%) | 45,886 (54.25%) | |
| > 400,000 CNY | 12,446 (11.85%) | 1,726 (11.94%) | 9,820 (11.61%) | |
| Conception season (%) | <0.001 | |||
| Spring | 26,902 (25.61%) | 2,967 (20.53%) | 22,638 (26.76%) | |
| Summer | 20,586 (19.59%) | 3,098 (21.43%) | 15,746 (18.62%) | |
| Autumn | 28,554 (27.18%) | 4,823 (33.37%) | 22,137 (26.17%) | |
| Winter | 29,021 (27.62%) | 3,565 (24.67%) | 24,068 (28.45%) | |
| Parity (%) | <0.001 | |||
| Nullipara | 50,778 (48.33%) | 6,561 (45.40%) | 41,310 (48.84%) | |
| Multipara | 54,285 (51.67%) | 7,892 (54.60%) | 43,279 (51.16%) | |
| Maternal secondhand smoking (%) | 0.006 | |||
| No | 92,490 (88.03%) | 12,618 (87.30%) | 74,533 (88.11%) | |
| Yes | 12,573 (11.97%) | 1,835 (12.70%) | 10,056 (11.89%) | |
| Decoration (%) | 0.602 | |||
| No | 98,234 (93.50%) | 13,528 (93.60%) | 79,071 (93.48%) | |
| Yes | 6,829 (6.50%) | 925 (6.40%) | 5,518 (6.52%) | |
| Indoor chemical air pollution (%) | 0.263 | |||
| No | 9,3608 (89.10%) | 12,927 (89.44%) | 75,377 (89.11%) | |
| Yes | 11,455 (10.90%) | 1,526 (10.56%) | 9,212 (10.89%) | |
| First-trimester ambient temperature, °C; mean ± SD | 16.84 ± 7.79 | 15.57 ± 8.31 | 16.93 ± 7.74 | <0.001 |
| Second-trimester ambient temperature, °C; mean ± SD | 17.07 ± 7.32 | 15.93 ± 7.69 | 17.27 ± 7.25 | <0.001 |
| Third-trimester ambient temperature, °C; mean ± SD | 17.03 ± 7.24 | 17.47 ± 7.25 | 16.95 ± 7.27 | <0.001 |
| First-trimester SBP, mmHg; mean ± SD | 113.15 ± 12.92 | 121.92 ± 18.31 | 111.55 ± 10.93 | <0.001 |
| First-trimester DBP, mmHg; mean ± SD | 71.49 ± 11.60 | 87.03 ± 13.79 | 68.64 ± 8.49 | <0.001 |
| Second-trimester SBP, mmHg; mean ± SD | 113.57 ± 11.87 | 125.92 ± 14.46 | 111.43 ± 9.92 | <0.001 |
| Second-trimester DBP, mmHg; mean ± SD | 69.27 ± 11.64 | 86.81 ± 13.47 | 66.24 ± 8.08 | <0.001 |
| Third-trimester SBP, mmHg; mean ± SD | 114.33 ± 11.77 | 124.95 ± 15.56 | 112.50 ± 9.90 | <0.001 |
| Third-trimester DBP, mmHg; mean ± SD | 69.59 ± 10.94 | 84.50 ± 13.38 | 67.03 ± 8.04 | <0.001 |
HDP, hypertensive disorders of pregnancy; BMI, body mass index; CNY, China Yuan; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation.
Table S3 summarizes the distributions of PM1 concentrations during three trimesters in 38 hospitals from the CBCS and their pairwise correlations. The median (interquartile range) of PM1 concentration was 28.80 μg/m3 (13.00 μg/m3), 28.50 μg/m3 (12.50 μg/m3), and 27.20 μg/m3 (13.30 μg/m3) for the first, second, and third trimester, respectively. PM1 levels during three trimesters were positively correlated with each other (rspearman > 0).
Associations of PM1 during pregnancy with maternal BP and hypertensive disorders of pregnancy
Each 10 μg/m3 higher first-trimester PM1 exposure was positively associated with higher first-trimester systolic blood pressure (SBP) (β = 1.696, 95% CI: 1.537,1.855) and first-trimester diastolic blood pressure (DBP) (β = 1.056, 95% CI: 0.908,1.203). Similar effects of first-trimester PM1 were observed on second-trimester SBP/DBP but not on the third-trimester SBP/DBP. The pro-hypertensive effect of second-trimester PM1 was only observed on second- and third-trimester SBP. Furthermore, each 10 μg/m3 elevation in the first-trimester PM1 exposure was associated with 11.4% (OR = 1.114, 95% CI: 1.074,1.156) increase in the risk of HDP prevalence (Table 2). The impact of second-trimester PM1 exposure on HDP were not statistically significant (Table 2).
Table 2.
Associations of each 10 μg/m3 greater PM1 concentrations during pregnancy with maternal blood pressure and hypertensive disorders of pregnancy
| Exposure | SBP |
DBP |
HDP |
||||
|---|---|---|---|---|---|---|---|
| aβ (95% CI) | p value | aβ (95% CI) | p value | aOR (95% CI) | p value | ||
| FT PM1 | 1.313 (1.280, 1.347)a | < 0.001 | |||||
| 1.114 (1.074, 1.156)b | < 0.001 | ||||||
| FT PM1 | FT | 2.838 (2.727, 2.950)a | < 0.001 | 1.963 (1.861, 2.066)a | < 0.001 | ||
| FT | 1.696 (1.537, 1.855)b | < 0.001 | 1.056 (0.908, 1.203)b | < 0.001 | |||
| ST | 1.627 (1.529, 1.726)a | < 0.001 | 0.992 (0.896, 1.088)a | < 0.001 | |||
| ST | 0.456 (0.319, 0.592)b | < 0.001 | 0.102 (−0.032, 0.237)b | 0.136 | |||
| TT | 0.750 (0.652, 0.848)a | < 0.001 | 0.486 (0.397, 0.576)a | < 0.001 | |||
| TT | 0.093 (−0.043, 0.229)b | 0.179 | −0.137 (−0.262, −0.012)b | 0.031 | |||
| ST PM1 | 0.997 (0.970, 1.023)a | 0.813 | |||||
| 1.017 (0.981, 1.053)b | 0.350 | ||||||
| ST PM1 | ST | 0.208 (0.107, 0.309)a | < 0.001 | −0.127 (−0.226, −0.029)a | 0.011 | ||
| ST | 0.051 (−0.079, 0.181)b | 0.439 | −0.103 (−0.231, 0.025)b | 0.115 | |||
| TT | 1.045 (0.944, 1.145)a | < 0.001 | 0.359 (0.267, 0.451)a | < 0.001 | |||
| TT | 0.324 (0.195, 0.454)b | < 0.001 | −0.037 (−0.156, 0.082)b | 0.539 | |||
FT, first-trimester; ST, second-trimester; TT, third-trimester; PM1, particle with aerodynamic diameter ≤ 1.0 μm; aβ indicates adjusted estimate; CI, confidence interval; aOR, adjusted odds ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDP, hypertensive disorders of pregnancy.
Crude model: adjusted for none.
Adjusted model: adjusted for maternal age, pre-pregnancy body mass index, maternal ethnicity, maternal education, household annual income, conception season, and ambient temperature.
Stratified analyses of first-trimester PM1 with maternal BP and hypertensive disorders of pregnancy
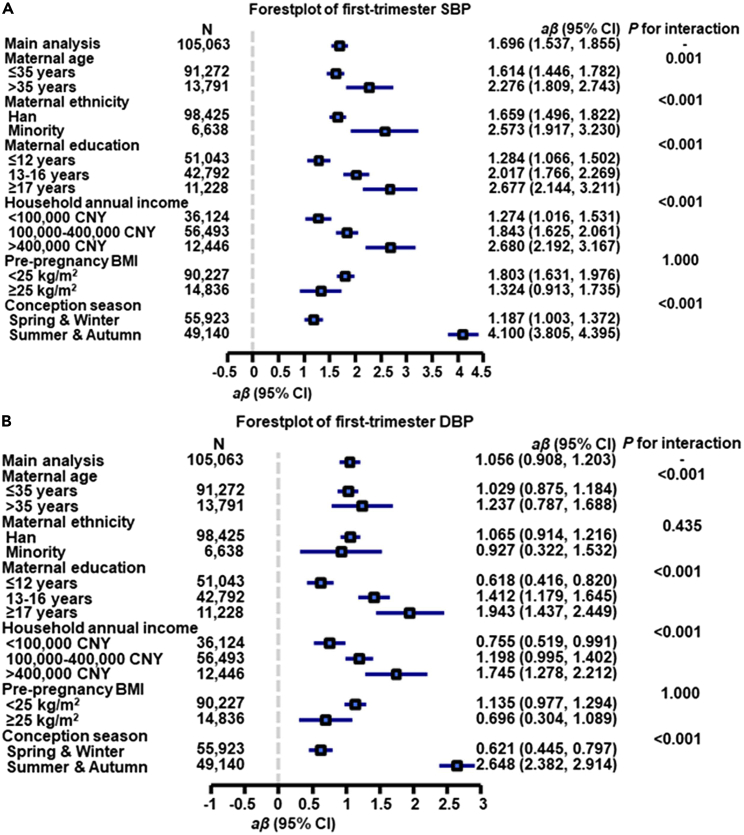
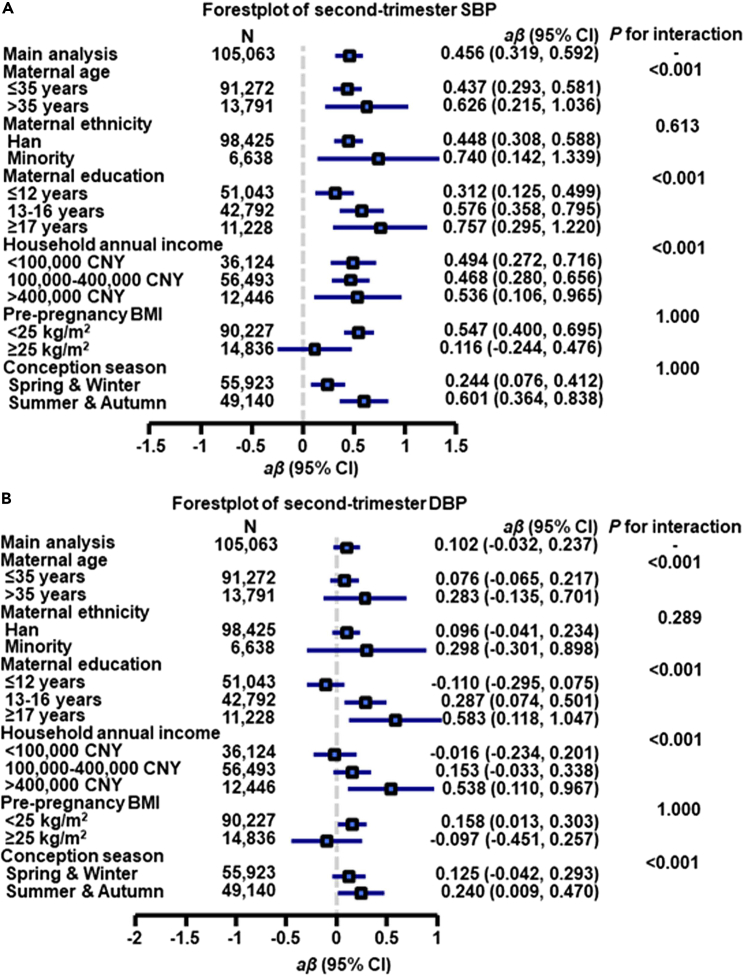
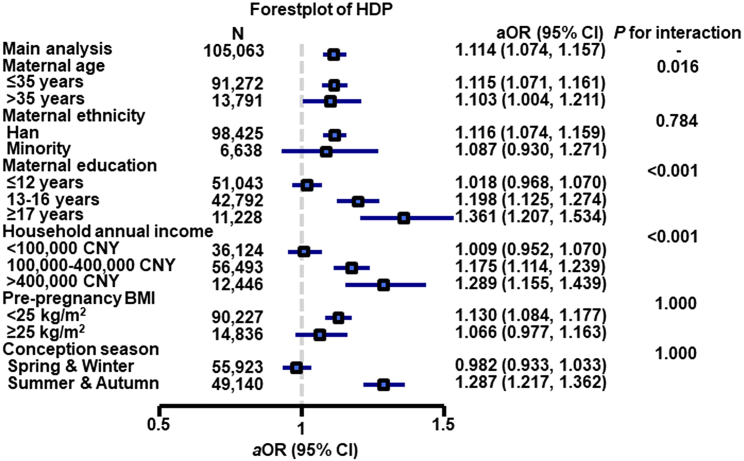
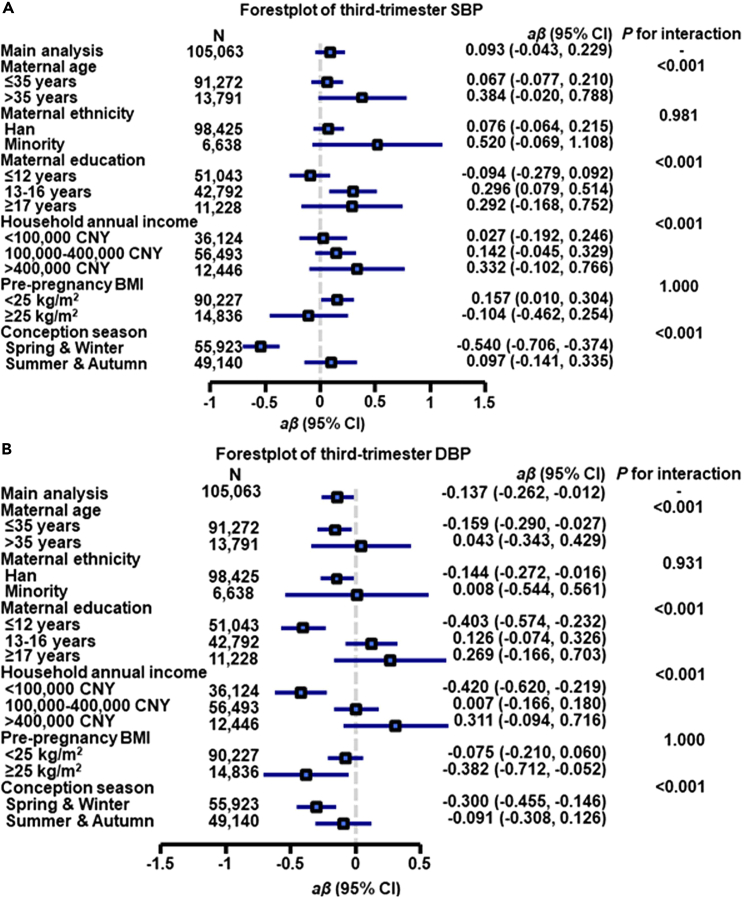
Compared with pregnant women aged less than 35 years, older pregnant women (> 35 years) had greater effect estimates (β = 2.276 for first-trimester SBP, β = 1.237 for first-trimester DBP, β = 0.626 for second-trimester SBP, β = 0.283 for second-trimester DBP, β = 0.384 for third-trimester SBP, β = 0.043 for third-trimester DBP, OR = 1.103 for HDP with all Pinteraction < 0.05) (Figures 1, 2, 3, and 4, and Tables S4–S7).
Figure 1.
Associations of each 10 μg/m3 greater first-trimester PM1 concentration with first-trimester SBP (A) and DBP (B) by potential effect modifiers∗
aβ, adjusted regression coefficient, CI, confidence interval, CNY, China yuan. SBP, systolic blood pressure, DBP, diastolic blood pressure.
∗Adjusted for maternal age, pre-pregnancy body mass index, maternal ethnicity, maternal education, household annual income, conception season, and ambient temperature.
Figure 2.
Associations of each 10 μg/m3 greater first-trimester PM1 concentration with second-trimester SBP (A) and DBP (B) by potential effect modifiers∗
aβ, adjusted regression coefficient, CI, confidence interval, CNY, China yuan. SBP, systolic blood pressure, DBP, diastolic blood pressure.
∗Adjusted for maternal age, pre-pregnancy body mass index, maternal ethnicity, maternal education, household annual income, conception season, and ambient temperature.
Figure 3.
Associations between first-trimester PM1 and hypertensive disorders of pregnancy by potential effect modifiers∗
aOR, adjusted odds ratio, CI, confidence intervals, CNY, China yuan.
∗Adjusted for maternal age, pre-pregnancy body mass index, maternal ethnicity, maternal education, household annual income, conception season, and ambient temperature.
Figure 4.
Associations of each 10 μg/m3 greater first-trimester PM1 concentration with third-trimester SBP (A) and DBP (B) by potential effect modifiers∗
aβ, adjusted regression coefficient, CI, confidence interval, CNY, China yuan. SBP, systolic blood pressure, DBP, diastolic blood pressure.
∗Adjusted for maternal age, pre-pregnancy body mass index, maternal ethnicity, maternal education, household annual income, conception season, and ambient temperature.
When the estimates were stratified by maternal education, we found that the association between first-trimester PM1 and first-trimester SBP was greatest among pregnant women educated longer than 17 years (β = 2.677, 95% CI: 2.144, 3.211) followed by the estimate for participants educated 13 to 16 years (β = 2.017, 95% CI: 1.766, 2.269) and participants educated less than 12 years (β = 1.284, 95% CI: 1.066, 1.502), with overall disparity across maternal education groups being significant (Pinteraction < 0.001) (Figure 1A). Similar trends were found for the first-trimester DBP, second-trimester SBP/DBP, third-trimester SBP/DBP, and HDP (all values for Pinteraction < 0.001), although the direction of effect estimates was not consistent across income groups for second-trimester DBP and third-trimester SBP/DBP (Figures 1B, 2, 4, and Tables S4–S7).
We also found that pregnant women with a household annual income above 400,000 CNY had greater effect estimates (β = 2.680 for first-trimester SBP, β = 1.745 for first-trimester DBP, β = 0.536 for second-trimester SBP, β = 0.332 for third-trimester SBP, OR = 1.289 for HDP with all Pinteraction < 0.001) than other income groups (Figures 1, 2, 3, 4, and Tables S4–S7). There were also significant modification effects found for second- and third-trimester DBP; however, the direction of effect estimates was not consistent across income groups (Figures 2 and 4), implying that further investigation is needed.
In addition, when the findings were stratified by maternal ethnicity, pre-pregnancy BMI, and conception season, interaction effects were not statistically significant for HDP (all Pinteraction > 0.05).
Sensitivity analyses
The associations were not substantially changed in sensitivity analyses when we excluded participants who lived in urban areas, when we excluded participants who lived in south China, when we excluded participants exposed to indoor decoration, when we excluded participants exposed to indoor chemical air pollution, when we excluded participants with indoor coal combustion emissions at home, when we excluded participants who kept animals during pregnancy, or when we excluded participants who were multipara (Table S8). The results were also similar to the main analyses when we repeated the analyses by adjusting additional confounding factors including ozone and PM1-2.5 (calculated by subtracting PM1 from PM2.5) (Table S9).
Discussion
Main findings
In this nationwide study of PM1-BP/HDP associations, we found positive associations of greater first-trimester PM1 exposure with first- and second-trimester BP, as well as odds of HDP. Pregnant women who were older or those with higher education level or higher household annual income may be more vulnerable to the pro-hypertensive effects of PM1 exposure. The results remained robust after a series of sensitivity analyses.
Comparison with previous studies
To date, previous studies have examined the PM1-BP association in adults and children. Wang et al.20 conducted a study involving 1.2 million couples (aged 18–45 years old) who were planning for pregnancy and reported that a 10 μg/m3 greater PM1 was associated with a 0.26 mmHg higher SBP and 0.22 mmHg higher DBP in females, as well as a 0.29 mmHg higher SBP and 0.17 mmHg higher DBP in males. Wu et al.21 found that each 10 μg/m3 greater PM1 was associated with 2.56 mmHg elevated SBP and 61% higher odds for hypertension among children. Nevertheless, no previous study has examined the associations of PM1 during pregnancy with maternal BP and HDP, and so further investigation is warranted to confirm the associations.
Although evidence on PM1 exposure and maternal BP, as well as HDP is limited, several previous studies have investigated the association between PM2.5 exposure and BP during pregnancy, among which PM2.5 levels were evaluated in different trimesters. In the current study, first-trimester PM1 was positively associated with first- and second-trimester BP, as well as odds of HDP, which are in line with some previous studies on PM. Exposure to PM2.5 during pregnancy has been found to be associated with maternal elevated BP during pregnancy in the United States,12,22 Korea,23 Poland,24 and China.8,25,26,27 However, the results of some other studies have been inconsistent. Savitz et al.28 did not find a significant association of PM2.5 exposure in the first and second trimesters with hypertension during pregnancy based on a study of 268,601 pregnant women in New York City. In another study from the United States, there was also no association between PM2.5 and HDP.13 Interestingly, in a study of Australia involving 285,594 singleton pregnancies, PM2.5 was inversely related to odds of hypertension (RR = 0.95, 95% CI: 0.93, 0.97).14 The inconsistent conclusions may result from a number of factors, including difference in exposure level, exposure estimates methods, the study populations as well as the research methods.29
Environmental factors, such as air pollution, ambient temperature, noise, and greenness may contribute to the development of hypertension,30 so the further investigations of environmental factors based on a nationwide sample are highly warranted. In our previous study, we found that exposure to cold ambient temperature in the second and third trimesters were associated with elevated BP, as well as increased HDP prevalence among most Chinese pregnant women.31 There are few reports of the health effects of PM1 worldwide due to the unavailability of PM1 data. Although no prior study has examined the effect of PM1 exposure on BP of pregnant women, the current study offers a new perspective on the association of maternal PM1 exposure with BP and risk of HDP. This finding may be used to provide information for public health interventions and environmental policies enactment.
Potential mechanisms
PM1 could be created by burning of fossil fuel, coal, and biomass.32 The mechanisms through which PM could elevate BP have not been fully clarified. One plausible pathway is that PM could stimulate receptors and nerve endings in the airways, and thereby activate sympathetic nervous system, which could further lead to elevated BP.33 The second hypothesized pathway is that PM could elicit inflammation and oxidative stress, resulting in endothelial dysfunction and further elevated BP.15,34,35,36 The third possible pathway is that sustained endocrine gland-derived vascular endothelial growth factor after the first trimester could lead to higher BP.37 Other mechanisms may via arterial stiffness and DNA hypomethylation induced by PM.15,38 The aerodynamic diameter of PM is an important factor that determining the health effects.39,40 PM1 showed smaller diameter but greater surface area to mass ratio than PM2.5, and thus has greater ability to reach the lung alveoli and enter the circulatory system, thus exerting adverse effects such as inflammatory responses on the BP.17,41,42 Additionally, PM1 absorbed more toxic substances such as organic compounds, which could induce localized oxidative and inflammatory response.43 Considering that PM1 contributes to the majority of PM2.5 in China, it is reasonable to speculate that PM1 may play an important role in the pro-hypertensive effect of PM2.5.
Susceptible population
In stratified analyses, we found pregnant women who were older or those with higher education level or higher household annual income may be more vulnerable to the pro-hypertensive effects of ambient PM1 exposure. The reason for this finding may be that advanced age-related endothelial dysfunction may amplify the endothelial dysfunction effect, which could also be caused by PM, leading to vascular damage and further development of hypertension.44 People with higher household annual income or higher education level tended to intake more total energy, meat, animal fat, protein, sugar, and sweetener, leading to an increased prevalence of overweight/obesity and dyslipidaemia, which may further increase arterial stiffness caused by PM,45,46 and these people often engaged in less physical activities, which is imperative for the maintenance of normal BP.47,48 In the current study, more participants who was educated longer than 17 years (88.28%, 9,912 of 11,228) or possessing a household annual income more than 400,000 CNY (86.06%, 10,711 of 12,446) lived in urban areas than rural areas. Urban areas were high in air pollution, noise, and heavy in traffic compared with rural areas, which could synergistically cooperate with PM to activate sympathetic nervous system, and further lead to elevated BP.49 Further study is warranted to investigate the associations of maternal age, education level, and household annual income with BP.
Conclusion
Exposure to first-trimester PM1 might be positively associated with elevated first- and second-trimester BP, as well as HDP prevalence, particularly among pregnant women who were older or those with higher education level or higher household annual income. Further studies are warranted to investigate the mechanisms of increased vulnerability to PM1 and validate our findings. Corresponding work should also be conducted for government to protect pregnant women from adverse effects associated with PM1 exposure.
Limitations of the study
The current study has several limitations. First, the temporal association between first-trimester PM1 exposure and BP of pregnant women could not be confirmed due to the cross-sectional nature of the current study. Second, PM1 concentrations during pregnancy were predicted based on ground monitoring data, satellite remote sensing, meteorologic data, and land use information. Although the satellite-based prediction of PM1 has been widely used by many epidemiological studies, predictive error of PM1 still exists. The accuracy and spatial resolution of estimated PM1 could be improved in future by including more detailed environmental data with novel models. More advanced devices and technologies are needed to measure exposure level more accurately in future research. Third, maternal BP could be affected by humidity, diet, exercise, and the technical proficiency of the tester. However, maternal BP was measured by trained nurses with same digital BP monitors according to the American Heart association recommendations for BP measurements. Forth, although we adjusted for the important potential confounders in this study, like most previous studies, we cannot rule out the possibility of the bias related to residual confounders, such as maternal stress, family history of hypertension, heart, and kidney disease, diabetes, other metabolic disorders, pregnancy via in vitro fertilization, physical activity, traffic noise exposure, and other pollutants, which might affect the BP but were not included in this study.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| R Version 4.1.1 | R Foundation | https://www.r-project.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wentao Yue (yuewt@ccmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Method details
Study population
Data was collected from the CBCS, a nationwide cohort study, that investigating the risk factors underlying maternal-fetal health. Details of the CBCS have been presented elsewhere.50 Briefly, the CBCS covering 38 research centers in 17 provinces in China from November 2017 through December 2021 (Table S1 and Figure S1). In CBCS, pregnant women were enrolled in the first trimester (6-12 weeks’ gestation). At this time, each participant was asked to complete a self-filled questionnaire, including social and demographic characteristics, previous medical status, health lifestyle behaviors, residential environments as well as housing information. The first and second follow-up visits were conducted in the second (20-24 weeks’ gestation) and third trimesters (28-34 weeks’ gestation), respectively. Medical examination records during pregnancy were collected by trained researchers, doctors, or nurses. If a participant experienced a pregnancy loss, all clinical information would also be recorded. The third follow-up visit was conducted after delivery to collect the birth records of newborns for further analysis.
In the current study, pregnant women (n = 106,087) were initially included, their information (i.e., maternal age, ethnicity, pre-pregnancy weight, height, education, household annual income, secondhand smoke exposure, alcohol consumption, conception season, parity, blood pressure, residential addresses, etc.) was collected using the unique identification numbers. Exclusion criteria was that participants who failed to complete the survey (n = 957), participants with outliers or missing data for maternal age (n = 13) or pre-pregnancy BMI (n = 54). Thus, 105,063 pregnancies were eligible for final analyses (Figure S2).
The current study was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (number: 2018-KY-003-01). Written informed consents were signed by each participant before data collection.
BP measurement and HDP definition
Maternal BP was measured by trained nurses at 6-12 weeks, 20-24 weeks, and 28-34 weeks of gestation with digital blood pressure monitors (A&D Medical Life Source TM-2655) according to the American Heart Association recommendations for BP measurements. Before the measurement, pregnant women should stop smoking, eating, drinking alcohol/caffeinated drinks, or physical exercising for more than 30 minutes and have a break for more than five minutes in a comfortable room.21,51 SBP and DBP on the upper right arm were measured with pregnant women in a sitting position, and the elbow was at the same level with heart when measuring. The measurements were repeated in three successive pairs after an interval of at least two minutes, and mean values of the three measurements were recorded in the electronic data capturing system.
HDP were divided into four categories as follows: (a) gestational hypertension, (b) preeclampsia or eclampsia, (c) chronic hypertension, and (d) superimposed preeclampsia.52 Gestational hypertension was defined as new-onset hypertension (mean SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) after 20 weeks of gestation.53 Preeclampsia was defined as hypertension (SBP/DBP ≥140/90 mmHg) and proteinuria of 1+ or more on a dipstick test or the protein level in the urine ≥ 300 mg/24-hour after 20 weeks of gestation, or hypertension plus the involvement of one organ or system in women with previously normal blood pressure.54 Eclampsia was defined as new-onset grand mal seizures in women with pre-eclampsia.55 Chronic hypertension refers to hypertension (≥140/90 mmHg) predating the pregnancy or before 20 weeks of gestation.56 Superimposed preeclampsia refers to chronic hypertension associated with preeclampsia.55
PM1 exposure assessment
Daily PM1 concentrations in 17 provinces of CBCS were predicted at a spatial resolution of 0.1 ° × 0.1 ° from 2017 to 2021, based on a combination of ground monitoring data, satellite remote sensing, meteorologic data, and land use information, which were previously reported.32,57 Briefly, we combined two Moderate Resolution Imaging Spectroradiometer (MODIS) aerosol optical depth (AOD) product, Dark Target and Deep Blue data, using an inverse variance weighting method, after filling their gaps. Daily meteorological data (i.e., temperature, barometric pressure, relative humidity, and wind speed) were obtained from the China meteorological data sharing service system (http://data.cma.cn/). We obtained annual land cover data (i.e., urban cover, forest cover, and water cover) at a spatial resolution of 500 m from Global Mosaics of the standard MODIS land cover type data Collection 5.1 product of Global Land Cover Facility (http://glcf.umd.edu/). We downloaded monthly average Normalized Difference Vegetation Index data (MODIS Level 3) from the NASA Earth Observatory (http://neo.sci.gsfc.nasa.gov). Aqua and Terra active fires during the study period were downloaded from NASA Fire Information for Resource Management System (https://earthdata.nasa.gov/data/near-real-time-data/firms). In addition, elevation (http://srtm.csi.cgiar.org/) were also collected.
We developed a generalized additive model (GAM) to link the ground monitored PM1 concentrations with information of AOD, meteorology, land cover, vegetation, active fires, and other spatial predictors.16 During the process of developing GAM, AOD was included firstly, and then other predictors were included sequentially to achieve an optimal model that maximized the explained variability in air pollutant concentrations. In addition, we have considered the variability of PM1 level in different areas and over time by using a range of spatial and temporal predictors, including region (province), month, and day of the week.16 Results of 10-fold cross-validation method showed the adjusted coefficient of determination (R2) and root mean squared error (RMSE) were 59% and 22.5 μg/m3 for daily predicted PM1, respectively.
Collected residential addresses of participants were geocoded into longitude and latitude using the Application Programming Interface (API) provided by Auto Navi Map (https://www.autonavi.com). We then assigned trimester (i.e., first, second, and third trimesters) average PM1 concentrations to each study participant based on their coordinates.
Confounders
According to the suggestion of Textor et al.,58 potential confounders were identified based on three primary criteria as follows: (a) it could lead to elevated BP or hypertension, (b) it should be a “cause” of air pollution, and (c) it should not be the “effect” of exposure (air pollution), nor be an intermediate factor in the causal chain of outcome (hypertension). A directed acyclic graph (DAG, Figure S3) presenting the published studies (Table S2) was constructed with DAGitty v3.0 software (http://www.dagitty.net/development/dags.html) to identify a minimally sufficient set of potential confounders,59 leaving maternal age, pre-pregnancy BMI, maternal ethnicity, maternal education, household annual income, conception season, and ambient temperature as confounders in the adjusted models.
We obtained the individual information of pregnant women using self-filled questionnaires: maternal age (years), maternal ethnicity (Han versus Minority), maternal pre-pregnancy BMI (kg/m2), maternal education (≤12 years versus 13-16 years versus ≥17 years), household annual income(<100,000 CNY versus 100,000-400,000 CNY versus >400,000 CNY), maternal secondhand smoke exposure (yes versus no), maternal alcohol consumption (yes versus no), conception season (spring versus summer versus autumn versus winter), parity (nullipara versus multipara). Pre-pregnancy BMI was obtained as the weight in kilograms divided by the square of the height in meters before conception. Secondhand smoke exposure was identified as non-smokers being exposed to cigarette smoke for more than 15 minutes daily for more than one day per week.60 Maternal alcohol consumption was defined as pregnant women who drinking once a week for more than six months.61 Ambient temperature from 2017 to 2021 (period of data collection) were collected from the ERA5-land reanalysis dataset of the European Centre for Medium-Range Weather Forecasts (ECMWF) (https://www.ecmwf.int/).
Quantification and statistical analysis
Descriptive statistics
Continuous and categorical variables were presented as mean ± standard deviation (SD) or as frequency with percentage, respectively.
Main analyses
Generalized linear mixed models with a random intercept for hospital were used to evaluate the associations of PM1 with SBP/DBP levels of three trimesters or HDP prevalence, respectively. The effect estimates were presented as regression coefficient (β for continuous outcomes) and odds ratio (OR for dichotomous outcomes), respectively per 10 μg/m3 higher PM1. The unadjusted models and adjusted models were developed. The variance inflation factors (VIFs) of all the variables in the models were calculated to ensure the absence of collinearity for the adjusted models (all VIFs < 5).
Stratified analyses
Furthermore, the PM1-BP/HDP associations may be different among subgroups of participants. To explore potential effect modification, we performed stratified analyses by maternal age of the participants (≤ 35 years versus > 35 years), maternal ethnicity (Han versus Minority), maternal education (≤12 years versus 13-16 years versus ≥ 17 years), household annual income (<100,000 CNY versus 100,000-400,000 CNY versus >400,000 CNY), pre-pregnancy BMI (<25 kg/m2 versus ≥ 25 kg/m2), and conception season (spring & winter versus summer & autumn).
Sensitivity analyses
To assess the robustness of our findings, several sensitivity analyses were performed. The PM1-BP/HDP associations were estimated by individually excluding participants who lived in urban areas, participants who lived in south China, participants exposed to indoor decoration, participants exposed to indoor chemical air pollution, participants with indoor coal combustion emissions at home, participants who kept animals during pregnancy, or multipara. In addition, we reran our models by adjusting additional confounding factors including ozone and PM1-2.5.
Statistical analyses were performed using R 4.1.1. Statistical significance of main effects and interactions were assumed at P < 0.05 for a 2-tailed test.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (grant number 2016YFC1000101 and 2019YFC1005100), National Natural Science Foundation of China (grant number 82202182), China Postdoctoral Science Foundation (grant number 2020TQ0207), Postdoctoral Foundation provide by Beijing Obstetrics and Gynecology Hospital, Capital Medical University.
Author contributions
Conceptualization, M.Z., Y.Q.S., W.T.Y., C.H.Y., G.H.D., and W.J.Z., methodology, M.Z., B.Y.Y., Y.Q.S., Y.Q.Z., G.B.C., and W.J.Z., formal analysis, M.Z., B.Y.Y., Y.Q.Z., and Y.Q.S., investigation, R.X.L., Y.Z., S.F.S., E.J.Z., X.T.Z., Q.Z.W., L.X.H., Y.T.Z., L.B.W., Y.N.L., X.X.L., J.X.L., S.H.W., and X.M., resources, C.H.Y., W.T.Y., R.X.L., Y.Z., S.F.S., E.J.Z., and X.T.Z., data curation, R.X.L., Y.Z., S.F.S., E.J.Z., X.T.Z., Q.Z.W., L.X.H., Y.T.Z., L.B.W., Y.N.L., X.X.L., J.X.L., S.H.W., and X.M., writing—original draft, M.Z. and Y.Q.S., writing—review & editing, B.Y.Y., W.T.Y., C.H.Y., G.H.D., and W.J.Z., visualization, M.Z., Y.Q.S., and Y.Q.Z., funding acquisition, W.T.Y., C.H.Y., Y.Q.S., and M.Z., supervision, W.T.Y., C.H.Y., G.H.D., and W.J.Z.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: May 12, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106863.
Contributor Information
Wangjian Zhang, Email: Zhangwj227@mail.sysu.edu.cn.
Guanghui Dong, Email: donggh5@mail.sysu.edu.cn.
Chenghong Yin, Email: yinchh@ccmu.edu.cn.
Wentao Yue, Email: yuewt@ccmu.edu.cn.
Supplemental information
Data and code availability
-
•
Data reported in this study cannot be deposited in a public repository due to confidentiality reasons, which are mandatory according to the Ethical Committee. However, they might be available upon request to the lead contact. To request access, contact Wentao Yue (yuewt@ccmu.edu.cn).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Timpka S., Markovitz A., Schyman T., Mogren I., Fraser A., Franks P.W., Rich-Edwards J.W. Midlife development of type 2 diabetes and hypertension in women by history of hypertensive disorders of pregnancy. Cardiovasc. Diabetol. 2018;17:124. doi: 10.1186/s12933-018-0764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand J.S., Lawlor D.A., Larsson H., Montgomery S. Association between hypertensive disorders of pregnancy and neurodevelopmental outcomes among offspring. JAMA Pediatr. 2021;175:577–585. doi: 10.1001/jamapediatrics.2020.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett H.L., Callaway L.K. Hypertensive disorders of pregnancy. BMJ. 2017;358:j3245. doi: 10.1136/bmj.j3245. [DOI] [PubMed] [Google Scholar]
- 4.Webster K., Fishburn S., Maresh M., Findlay S.C., Chappell L.C., Guideline Committee Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:l5119. doi: 10.1136/bmj.l5119. [DOI] [PubMed] [Google Scholar]
- 5.Ramlakhan K.P., Johnson M.R., Roos-Hesselink J.W. Pregnancy and cardiovascular disease. Nat. Rev. Cardiol. 2020;17:718–731. doi: 10.1038/s41569-020-0390-z. [DOI] [PubMed] [Google Scholar]
- 6.Vonck S., Staelens A.S., Lanssens D., Tomsin K., Oben J., Bruckers L., Gyselaers W. Development of a biophysical screening model for gestational hypertensive diseases. J. Biomed. Sci. 2019;26:38. doi: 10.1186/s12929-019-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher G.M., O'Keeffe G.W., Kearney P.M., Kenny L.C., Dinan T.G., Mattsson M., Khashan A.S. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatr. 2018;75:809–819. doi: 10.1001/jamapsychiatry.2018.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Li J., Liao J., Hu C., Cao Z., Xia W., Xu S., Li Y. Impacts of ambient fine particulate matter on blood pressure pattern and hypertensive disorders of pregnancy: evidence from the Wuhan cohort study. Hypertension. 2021;77:1133–1140. doi: 10.1161/HYPERTENSIONAHA.120.15608. [DOI] [PubMed] [Google Scholar]
- 9.Ankumah N.A., Cantu J., Jauk V., Biggio J., Hauth J., Andrews W., Tita A.T.N. Risk of adverse pregnancy outcomes in women with mild chronic hypertension before 20 weeks of gestation. Obstet. Gynecol. 2014;123:966–972. doi: 10.1097/AOG.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 10.Dunietz G.L., Strutz K.L., Holzman C., Tian Y., Todem D., Bullen B.L., Catov J.M. Moderately elevated blood pressure during pregnancy and odds of hypertension later in life: the POUCHmoms longitudinal study. BJOG. 2017;124:1606–1613. doi: 10.1111/1471-0528.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D.D., Gao L., Huang O., Ullah K., Guo M.X., Liu Y., Zhang J., Chen L., Fan J.X., Sheng J.Z., et al. Increased adverse pregnancy outcomes associated with stage 1 hypertension in a low-risk cohort: evidence from 47 874 cases. Hypertension. 2020;75:772–780. doi: 10.1161/HYPERTENSIONAHA.119.14252. [DOI] [PubMed] [Google Scholar]
- 12.Nobles C.J., Williams A., Ouidir M., Sherman S., Mendola P. Differential effect of ambient air pollution exposure on risk of gestational hypertension and preeclampsia. Hypertension. 2019;74:384–390. doi: 10.1161/HYPERTENSIONAHA.119.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assibey-Mensah V., Glantz J.C., Hopke P.K., Jusko T.A., Thevenet-Morrison K., Chalupa D., Rich D.Q. Ambient wintertime particulate air pollution and hypertensive disorders of pregnancy in Monroe County, New York. Environ. Res. 2019;168:25–31. doi: 10.1016/j.envres.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melody S.M., Wills K., Knibbs L.D., Ford J., Venn A., Johnston F. Maternal exposure to ambient air pollution and pregnancy complications in victoria, Australia. Int. J. Environ. Res. Publ. Health. 2020;17:2572. doi: 10.3390/ijerph17072572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bont J., Jaganathan S., Dahlquist M., Persson Å., Stafoggia M., Ljungman P. Ambient air pollution and cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022;291:779–800. doi: 10.1111/joim.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G., Knibbs L.D., Zhang W., Li S., Cao W., Guo J., Ren H., Wang B., Wang H., Williams G., et al. Estimating spatiotemporal distribution of PM1 concentrations in China with satellite remote sensing, meteorology, and land use information. Environ. Pollut. 2018;233:1086–1094. doi: 10.1016/j.envpol.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.Y., Li Q., Guo Y., Zhou H., Wang X., Wang Q., Shen H., Zhang Y., Yan D., Zhang Y., et al. Association of long-term exposure to airborne particulate matter of 1 mum or less with preterm birth in China. JAMA Pediatr. 2018;172:e174872. doi: 10.1001/jamapediatrics.2017.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su X., Zhao Y., Yang Y., Hua J. Correlation between exposure to fine particulate matter and hypertensive disorders of pregnancy in Shanghai, China. Environ. Health. 2020;19:101. doi: 10.1186/s12940-020-00655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grazuleviciene R., Dedele A., Danileviciute A., Vencloviene J., Grazulevicius T., Andrusaityte S., Uzdanaviciute I., Nieuwenhuijsen M.J. The influence of proximity to city parks on blood pressure in early pregnancy. Int. J. Environ. Res. Publ. Health. 2014;11:2958–2972. doi: 10.3390/ijerph110302958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y.Y., Li Q., Guo Y., Zhou H., Wang Q.M., Shen H.P., Zhang Y.P., Yan D.H., Li S., Chen G., et al. Long-term exposure to airborne particulate matter of 1 mum or less and blood pressure in healthy young adults: a national study with 1.2 million pregnancy planners. Environ. Res. 2020;184:109113. doi: 10.1016/j.envres.2020.109113. [DOI] [PubMed] [Google Scholar]
- 21.Wu Q.Z., Li S., Yang B.Y., Bloom M., Shi Z., Knibbs L., Dharmage S., Leskinen A., Jalaludin B., Jalava P., et al. Ambient airborne particulates of diameter </=1 mum, a leading contributor to the association between ambient airborne particulates of diameter </=2.5 mum and children's blood pressure. Hypertension. 2020;75:347–355. doi: 10.1161/HYPERTENSIONAHA.119.13504. [DOI] [PubMed] [Google Scholar]
- 22.Mobasher Z., Salam M.T., Goodwin T.M., Lurmann F., Ingles S.A., Wilson M.L. Associations between ambient air pollution and hypertensive disorders of pregnancy. Environ. Res. 2013;123:9–16. doi: 10.1016/j.envres.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe S.A., Jun Y.B., Kim S.Y. Exposure to air pollution during preconceptional and prenatal periods and risk of hypertensive disorders of pregnancy: a retrospective cohort study in Seoul, Korea. BMC Pregnancy Childbirth. 2018;18:340. doi: 10.1186/s12884-018-1982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jedrychowski W.A., Perera F.P., Maugeri U., Spengler J., Mroz E., Flak E., Stigter L., Majewska R., Kaim I., Sowa A., Jacek R. Prohypertensive effect of gestational personal exposure to fine particulate matter. Prospective cohort study in non-smoking and non-obese pregnant women. Cardiovasc. Toxicol. 2012;12:216–225. doi: 10.1007/s12012-012-9157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai W., Li Y., Niu Y., Ding Y., Yu X., Zhu B., Duan R., Duan H., Kou C., Li Y., Sun Z. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ. Res. 2020;185:109471. doi: 10.1016/j.envres.2020.109471. [DOI] [PubMed] [Google Scholar]
- 26.Sun M., Yan W., Fang K., Chen D., Liu J., Chen Y., Duan J., Chen R., Sun Z., Wang X., Xia Y. The correlation between PM2.5 exposure and hypertensive disorders in pregnancy: a Meta-analysis. Sci. Total Environ. 2020;703:134985. doi: 10.1016/j.scitotenv.2019.134985. [DOI] [PubMed] [Google Scholar]
- 27.Xia B., Zhou Y., Zhu Q., Zhao Y., Wang Y., Ge W., Yang Q., Zhao Y., Wang P., Si J., et al. Personal exposure to PM2.5 constituents associated with gestational blood pressure and endothelial dysfunction. Environ. Pollut. 2019;250:346–356. doi: 10.1016/j.envpol.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Savitz D.A., Elston B., Bobb J.F., Clougherty J.E., Dominici F., Ito K., Johnson S., McAlexander T., Ross Z., Shmool J.L.C., et al. Ambient fine particulate matter, nitrogen dioxide, and hypertensive disorders of pregnancy in New York city. Epidemiology. 2015;26:748–757. doi: 10.1097/EDE.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., Guo C., Lau A.K.H., Chan T.C., Chuang Y.C., Lin C., Jiang W.K., Yeoh E.K., Tam T., Woo K.S., et al. Long-term exposure to fine particulate matter, blood pressure, and incident hypertension in Taiwanese adults. Environ. Health Perspect. 2018;126:017008. doi: 10.1289/EHP2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Münzel T., Sørensen M. Noise pollution and arterial hypertension. Eur. Cardiol. 2017;12:26–29. doi: 10.15420/ecr.2016:31:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y., Zhang M., Chen S., Zhang W., Zhang Y., Su S., Zhang E., Sun L., Yang K., Wang J., et al. Potential impact of ambient temperature on maternal blood pressure and hypertensive disorders of pregnancy: a nationwide multicenter study based on the China birth cohort. Environ. Res. 2023;227:115733. doi: 10.1016/j.envres.2023.115733. [DOI] [PubMed] [Google Scholar]
- 32.He P., Liu L., Salas J.M.I., Guo C., Cheng Y., Chen G., Zheng X., Wang H., Knibbs L.D., Williams G., et al. Spatiotemporal variation of PM1 pollution in China. Br. J. Nutr. 2018;120:198–203. [Google Scholar]
- 33.Brook R.D., Rajagopalan S., Pope C.A., 3rd, Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Cai J., Chen R., Zhao Z., Ying Z., Wang L., Chen J., Hao K., Kinney P.L., Chen H., Kan H. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. 2017;136:618–627. doi: 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
- 35.Chin M.T. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart. 2015;101:253–256. doi: 10.1136/heartjnl-2014-306379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao X., Zhong J., Brook R.D., Rajagopalan S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxidants Redox Signal. 2018;28:797–818. doi: 10.1089/ars.2017.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sergent F., Hoffmann P., Brouillet S., Garnier V., Salomon A., Murthi P., Benharouga M., Feige J.J., Alfaidy N. Sustained endocrine gland-derived vascular endothelial growth factor levels beyond the first trimester of pregnancy display phenotypic and functional changes associated with the pathogenesis of pregnancy-induced hypertension. Hypertension. 2016;68:148–156. doi: 10.1161/HYPERTENSIONAHA.116.07442. [DOI] [PubMed] [Google Scholar]
- 38.Bellavia A., Urch B., Speck M., Brook R.D., Scott J.A., Albetti B., Behbod B., North M., Valeri L., Bertazzi P.A., et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J. Am. Heart Assoc. 2013;2:e000212. doi: 10.1161/JAHA.113.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G., Li S., Zhang Y., Zhang W., Li D., Wei X., He Y., Bell M.L., Williams G., Marks G.B., et al. Effects of ambient PM1 air pollution on daily emergency hospital visits in China: an epidemiological study. Lancet Planet. Health. 2017;1:e221–e229. doi: 10.1016/S2542-5196(17)30100-6. [DOI] [PubMed] [Google Scholar]
- 40.Huang L., Mao F., Zang L., Zhang Y., Zhang Y., Zhang T. Estimation of hourly PM1 concentration in China and its application in population exposure analysis. Environ. Pollut. 2020;273:115720. doi: 10.1016/j.envpol.2020.115720. [DOI] [PubMed] [Google Scholar]
- 41.Xiao X., Yao T., Du S., Zhang J., Huang T., Lei Y., Cao L., Shen Z., Cao Y. Age differences in the pulmonary and vascular pathophysiologic processes after long-term real-time exposure to particulate matter in rats. Chemosphere. 2020;261:127710. doi: 10.1016/j.chemosphere.2020.127710. [DOI] [PubMed] [Google Scholar]
- 42.Wang F., Liu J., Zeng H. Interactions of particulate matter and pulmonary surfactant: implications for human health. Adv. Colloid Interface Sci. 2020;284:102244. doi: 10.1016/j.cis.2020.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin L., Xie J., Wong C.K.C., Chan S.K.Y., Abbaszade G., Schnelle-Kreis J., Zimmermann R., Li J., Zhang G., Fu P., Li X. Contributions of city-specific fine particulate matter (PM2.5) to differential in vitro oxidative stress and toxicity implications between beijing and Guangzhou of China. Environ. Sci. Technol. 2019;53:2881–2891. doi: 10.1021/acs.est.9b00449. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z., Li H., Luo P., Yan D., Yang N., Zhang Y., Huang Y., Liu Y., Zhang L., Yan J., Zhang C. UNC5B promotes vascular endothelial cell senescence via the ROS-mediated P53 pathway. Oxid. Med. Cell. Longev. 2021;2021:5546711. doi: 10.1155/2021/5546711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang S., Kang M., Lim H. Global and regional patterns in noncommunicable diseases and dietary factors across national income levels. Nutrients. 2021;13:3595. doi: 10.3390/nu13103595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo C., Yang C., Wang X., Chen Y., Liu X., Deng H. Nicotinamide reprograms adipose cellular metabolism and increases mitochondrial biogenesis to ameliorate obesity. J. Nutr. Biochem. 2022;107:109056. doi: 10.1016/j.jnutbio.2022.109056. [DOI] [PubMed] [Google Scholar]
- 47.Brown S.C., Lombard J., Wang K., Byrne M.M., Toro M., Plater-Zyberk E., Feaster D.J., Kardys J., Nardi M.I., Perez-Gomez G., et al. Neighborhood greenness and chronic health conditions in medicare beneficiaries. Am. J. Prev. Med. 2016;51:78–89. doi: 10.1016/j.amepre.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Dipietro L., Evenson K.R., Bloodgood B., Sprow K., Troiano R.P., Piercy K.L., Vaux-Bjerke A., Powell K.E., 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE∗ Benefits of physical activity during pregnancy and postpartum: an umbrella review. Med. Sci. Sports Exerc. 2019;51:1292–1302. doi: 10.1249/MSS.0000000000001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warembourg C., Nieuwenhuijsen M., Ballester F., de Castro M., Chatzi L., Esplugues A., Heude B., Maitre L., McEachan R., Robinson O., et al. Urban environment during early-life and blood pressure in young children. Environ. Int. 2021;146:106174. doi: 10.1016/j.envint.2020.106174. [DOI] [PubMed] [Google Scholar]
- 50.Yue W., Zhang E., Liu R., Zhang Y., Wang C., Gao S., Su S., Gao X., Wu Q., Yang X., et al. The China birth cohort study (CBCS) Eur. J. Epidemiol. 2022;37:295–304. doi: 10.1007/s10654-021-00831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morawski K., Ghazinouri R., Krumme A., Lauffenburger J.C., Lu Z., Durfee E., Oley L., Lee J., Mohta N., Haff N., et al. Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Intern. Med. 2018;178:802–809. doi: 10.1001/jamainternmed.2018.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts J.M., August P.A., Bakris G., Barton J.R., Bernstein I.M., Druzin M., Gaiser R.R., Granger J.P., Jeyabalan A., Johnson D.D., et al. Hypertension in pregnancy, report of the American college of obstetricians and gynecologists' task force on hypertension in pregnancy. Obstet. Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 53.Steinthorsdottir V., McGinnis R., Williams N.O., Stefansdottir L., Thorleifsson G., Shooter S., Fadista J., Sigurdsson J.K., Auro K.M., Berezina G., et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat. Commun. 2020;11:5976. doi: 10.1038/s41467-020-19733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters T.P., Dyer A.R., Scholtens D.M., Dooley S.L., Herer E., Lowe L.P., Oats J.J.N., Persson B., Sacks D.A., Metzger B.E., et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care. 2016;39:2204–2210. doi: 10.2337/dc16-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong T., Chen P., Mu Y., Li X., Di B., Li J., Qu Y., Tang J., Liang J., Mu D. Association between ambient temperature and hypertensive disorders in pregnancy in China. Nat. Commun. 2020;11:2925. doi: 10.1038/s41467-020-16775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P., Saito S., Hall D.R., Warren C.E., Adoyi G., Ishaku S., International Society for the Study of Hypertension in Pregnancy ISSHP Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 57.Yang B.Y., Qian Z.M., Li S., Chen G., Bloom M.S., Elliott M., Syberg K.W., Heinrich J., Markevych I., Wang S.Q., et al. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in China: a cross-sectional study with findings from the 33 Communities Chinese Health Study. Lancet Planet. Health. 2018;2:e64–e73. doi: 10.1016/S2542-5196(18)30001-9. [DOI] [PubMed] [Google Scholar]
- 58.Textor J., van der Zander B., Gilthorpe M.S., Liskiewicz M., Ellison G.T. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int. J. Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 59.Leopold S.J., Watson J.A., Jeeyapant A., Simpson J.A., Phu N.H., Hien T.T., Day N.P.J., Dondorp A.M., White N.J. Investigating causal pathways in severe falciparum malaria: a pooled retrospective analysis of clinical studies. PLoS Med. 2019;16:e1002858. doi: 10.1371/journal.pmed.1002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carroll X., Liang X., Zhang W., Zhang W., Liu G., Turner N., Leeper-Woodford S. Socioeconomic, environmental and lifestyle factors associated with gestational diabetes mellitus: a matched case-control study in Beijing, China. Sci. Rep. 2018;8:8103. doi: 10.1038/s41598-018-26412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Zhu Y., Jia W., Sun D., Zhao L., Zhang C., Wang C., Chen G., Fu S., Bo Y., Xing Y. Association between lipid profiles and presence of carotid plaque. Sci. Rep. 2019;9:18011. doi: 10.1038/s41598-019-54285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data reported in this study cannot be deposited in a public repository due to confidentiality reasons, which are mandatory according to the Ethical Committee. However, they might be available upon request to the lead contact. To request access, contact Wentao Yue (yuewt@ccmu.edu.cn).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.