Abstract
For thyroid cancer clinical trials, the inclusion of participants from diverse patient populations is uniquely important given existing racial/ethnic disparities in thyroid cancer care. Since 2011, a paradigm shift has occurred in the treatment of advanced thyroid cancer with the approval of multiple systemic therapies by the US Food and Drug Administration based on their use in the clinical trials setting. Although these clinical trials recruited patients from up to 164 sites in 25 countries, the inclusion of racial/ethnic minority patients remained low. In this mini-review, we provide an overview of barriers to accessing cancer clinical trials, framed in the context of why patients with thyroid cancer may be uniquely vulnerable. Multilevel interventions and increased funding for thyroid cancer research are necessary to increase access to and recruitment of under-represented patient populations into thyroid cancer clinical trials.
Keywords: thyroid cancer, advanced thyroid cancer, disparities, clinical trial, cancer clinical trials, barriers to clinical trial enrollment
The outcomes of cancer clinical trials help to inform our understanding of novel therapeutics and have the potential to change clinical practice guidelines on what is standard of care in the treatment of patients with cancer. Thus, the inclusion of diverse and representative patient populations in cancer clinical trials is essential to improve the generalizability of trial results, including its efficacy and safety profile, to all patient populations who may use the drugs once approved by the US Food and Drug Administration (FDA) [1]. The National Institutes of Health (NIH) Revitalization Act of 1993 mandated the appropriate inclusion of women and racial/ethnic minorities in all NIH-funded clinical research [2]. Thirty years later, the participation of racial/ethnic minority patient populations in cancer clinical trials remains low [3-8]. Among its research goals, the National Institute on Minority Health and Health Disparities (NIMHD) aims to increase the overall proportion of participants from diverse populations included in NIH-funded clinical research to 40% by 2030 [9].
For thyroid cancer clinical trials, the inclusion of participants from diverse patient populations is uniquely important given existing racial/ethnic disparities in thyroid cancer care. Hispanic, Black, and Asian/Pacific Islander patients experience a disproportionate burden of disease and mortality related to thyroid cancer, which is the second most common cancer among Hispanic and Asian/Pacific Islander women in the United States [10, 11]. Studies have consistently found that non-White patients with thyroid cancer are more likely to be diagnosed at more advanced stage of thyroid cancer, more likely to experience postoperative complications from thyroid surgery, and less likely to receive guideline-concordant thyroid cancer care [12-18]. Likely related, Hispanic and Asian/Pacific Islander women experience higher rates of mortality from thyroid cancer [19].
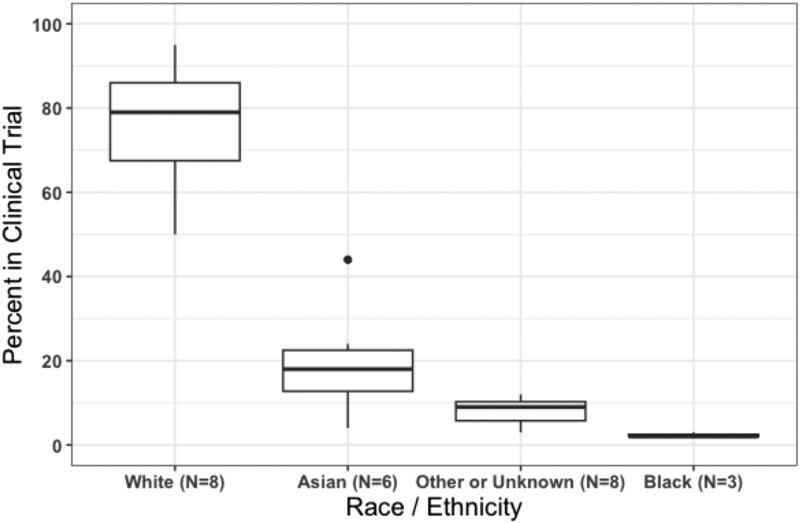
Historically, treatment options were so limited for patients with advanced thyroid cancer that access to thyroid cancer treatment often equated to access to a clinical trial. With the introduction of tyrosine kinase inhibitors for use as targeted therapy for thyroid cancer and advances in molecular genetic testing, new treatment options for advanced disease have become available. Since 2011, at least 10 systemic therapies have been approval by the FDA for treatment of advanced thyroid cancer based on its success in the clinical trial setting [20-27]. Although these clinical trials recruited patients from up to 164 sites in 25 countries, the final patient cohort was predominantly enrolled from European and North American sites. Furthermore, the non-White patient cohort ranged from 5% to 50% in each of these clinical trials, with 4% to 44% reported as Asian, 0% to 3% reported as Black, and 0% to 1% reported as Hispanic (Fig. 1) [20-31].
Figure 1.
Race/ethnicity specific percentage reported in clinical trials that led to the US Food and Drug Administration (FDA)-approval of 10 systemic therapies for the treatment of advanced thyroid cancers. Only 8 of the clinical trials included any data on race/ethnicity: the phase 3 DECISION trial (sorafenib), phase 3 SELECT trial (lenvatinib), phase 3 ZETA trial (vandetanib), phase 3 COSMIC-311 trial (cabozantinib), phase 3 EXAM trial (cabozantinib), phase 1/2 LIBRETTO-001 study (selpercatinib), phase 1/2 ARROW study (pralsetinib), and the phase 2 ROAR basket study (dabrafenib/trametinib) [20-27]. The clinical trials that led to FDA approval of Entrectinib and Larotrectinib did not include race/ethnicity data on the patients with thyroid cancer who enrolled in the studies [28-31]. The EXAM trial and ZETA trial reported race as a dichotomous variable (White vs non-White, and White vs other race, respectively) [20, 26]—we included the latter categories in “other or unknown” in this figure.
In this mini-review, we provide an overview of barriers to accessing cancer clinical trials, framed in the context of why patients with thyroid cancer may be uniquely vulnerable. In addition, we discuss potential strategies to improve access to thyroid cancer clinical trials for underrepresented patient populations in the United States.
Search Strategy
We searched PubMed using the key words “thyroid cancer,” “advanced thyroid cancer,” “disparities,” “clinical trial,” “cancer clinical trials,” and “barriers to clinical trial enrollment” to identify important themes, trends, and data described in the current mini-review. Keywords were used alone or in combination with the “AND” term. We also searched the reference lists of articles identified by this search strategy and selected those that we judged relevant. Our reference list was also modified after comments from peer reviewers.
Barriers to Accessing Cancer Clinical Trial
Financial Barriers to Participating in Clinical Trials
Patients with thyroid and nonthyroid cancer are at risk for cancer-related financial hardship due to the burden of medical and nonmedical costs, missed work, job loss, and difficulty obtaining affordable health insurance [32-37]. Such financial hardship can influence cancer patients to become nonadherent to prescribed medications (ie, take less medication than prescribed, not fill or partially fill a prescription) and to forgo needed medical care [38]. Thus, patients who are at increased risk of cancer-related financial hardship, including low-income patients who are more sensitive to marginal financial expenses, may be less able to participate in cancer clinical trials [39, 40]. Although participation in a clinical trial is free, clinical trial participants are often required to invest a substantial amount of time (ie, time off from work, time traveling) and resources (ie, transportation and lodging cost, lost wages) to complete the trial protocol [33, 40]. In a single institutional study of patients who were eligible for participation in a cancer clinical trial, 13% declined because of the distance they would need to travel to get to the trial site [41]. Furthermore, some components of the clinical trial protocol (ie, laboratory studies and diagnostic testing) may be billed to patients’ health insurance as standard of care for cancer treatment, with patients subsequently incurring routine care costs such as to copayments, coinsurance, and deductibles [33].
Patients with thyroid cancer, which is often diagnosed at a younger age than most other adult cancers [42], may be more susceptible to cancer-related financial hardship compared to patients with nonthyroid cancers. Prior studies have found that younger age at time of cancer diagnosis is associated with increased likelihood of experiencing cancer-related financial hardship [43-46]. Barrows et al [44] reported that thyroid cancer survivors were more likely to report worry about having to pay large cancer-related medical bills compared to nonthyroid cancer survivors. Furthermore, a retrospective cohort analysis of individuals diagnosed with cancer between 1995 and 2009 in the western district of Washington state by Ramsey et al [47] found that the incidence rates for bankruptcy at 1 year after diagnosis was higher for individuals with thyroid cancer compared to those with lung, colorectal, breast, and prostate cancer. Studies of cancer survivors in general have also found that racial/ethnic minority patients are at increased risk of experiencing cancer-related financial hardship [45, 46, 48]. In a survey of 273 Hispanic women with differentiated thyroid cancer reported to the Los Angeles Surveillance Epidemiology and End Results (SEER) registry, Chen et al [49] found up to 38.0% of the cohort endorsed individual material measures of financial hardship due to thyroid cancer (38.0% had to use savings, 24.6% cut down on spending for food, 17.7% could not make payments on credit cards or other bills, 5.2% had to move out of their house or apartment because they could not afford to stay there, and 2.7% had their utilities turned off because the bill was not paid). In addition to race/ethnicity, thyroid cancer-related financial hardship appears to also be influenced by level of acculturation. In their survey of Hispanic women with thyroid cancer, Chen et al [49] also reported that while financial hardship decreased with age for high-acculturated women, financial hardship for low-acculturated women remained elevated across all age groups. Thus, given the higher risk of cancer-related financial hardship among patients with thyroid cancer and among racial/ethnic minority patients, the potential financial burden associated with clinical trial participation remains a considerable barrier for racial/ethnic minority patient populations with thyroid cancer.
Structural Barriers Limits Enrollment of Diverse Patient Populations Into Clinical Trials
Despite the nearly 80% of cancer patients in general who receive their cancer care in community settings [50], most cancer clinical trials have traditionally been conducted at academic medical centers [51, 52]. In a meta-analysis of studies that examined the clinical trial decision-making pathway, Unger et al [53] found that 55.6% of cancer patients had zero cancer clinical trials available for their cancer type and stage at their treating medical center. The challenge with initiating clinical trials at nonacademic hospital sites is often related to structural barriers, including inadequate research infrastructure [53, 54]. A research infrastructure refers to the resources (ie, financial support, research staff, expertise, pool of patient participants from which to recruit, information technology systems, regulatory oversight committees, physical space, and facilities) and the coordination that is necessary to conduct a clinical trial [55]. In a Dallas safety-net hospital that cares for medically underserved patient populations, Gerber et al [56] found a substantial decrease in cancer clinical trial activation rates in the 2007 to 2014 period compared to the 1991 to 2006 period, with common reasons for nonactivation including an inability to perform the study procedure and the high startup costs. In semi-structured interviews, community oncologists noted major barriers to patient enrollment in cancer clinical trials, including insufficient infrastructure support (ie, understaffed research personnel) and increased physician burden (ie, multiple competing factors resulting in limited physician time, knowledge, and awareness of trials) [50]. With racial/ethnic minority patient populations more likely to receive their thyroid cancer care at underresourced hospitals [57-59], the lack of diversity in cancer clinical trials likely reflects the substantial impact of inadequate research infrastructures.
Related to the lack of research infrastructure, racial/ethnic minority patients with thyroid cancer are at risk of being excluded from accessing cancer clinical trials as they are less likely to receive thyroid care by medical specialists and high-volume physicians. Using pooled data from the 2015 to 2018 Medical Expenditure Panel Survey (MEPS), Cai et al [60] found that even after accounting for several social determinants of access to care, racial/ethnic minority groups in general are significantly underrepresented in the outpatient practices of many medical and surgical specialties, including oncology and otolaryngology. Furthermore, in a population-based survey of patients with differentiated thyroid cancer, Radhakrishnan et al [61] found that patient-reported involvement of primary care physicians, endocrinologists, and surgeons in their thyroid cancer care varied by race/ethnicity. Non-White patients were more likely to report discussing thyroid cancer treatment with their primary care physicians and less likely to involve their surgeons. Thus, barriers to initiation of cancer clinical trials at nonacademic medical centers ultimately act to limit access to clinical trials for racial/ethnic minority patients, many of whom obtain their cancer care in the community setting.
Language-based Barriers to Accessing Clinical Trials
In the United States, it is well documented that language barriers can lead to ineffective communication and negatively affect the quality of patients’ health care [62-65]. Cancer patients with limited English proficiency (LEP) encounter similar challenges to accessing and participating in cancer clinical trials [66]. In a retrospective cohort study of patients with gynecologic cancers, Jorge et al [67] found that even after adjusting for ethnicity and insurance status, patients with LEP had 66% lower odds of participating in clinical trials than fluent English speakers. In addition, a survey of gynecologic research staff and providers identified the most significant barriers to research participation for patients with LEP to be the lack of translated consent forms and increased time needed to enroll patients with LEP [67]. This issue disproportionately affects community-based programs and underresourced health systems. Furthermore, cancer patients with LEP may be excluded from clinical trials on the basis of their language ability. Between 2019 and 2020, nearly 20% of US interventional clinical trials registered on ClinicalTrials.gov for adult populations in general required patients to have the ability to read, speak, and/or understand English [68].
For thyroid cancer, which has the highest mortality among Hispanic women and men and Asian/Pacific Islander women in the United States [19], language-based barriers to clinical trial participation may be especially relevant. In the United States, 35% of the Hispanic and Asian population have LEP [69]. Furthermore, about 1 in 5 Hispanic and Asian households are linguistically isolated, which the US Census Bureau defines as a household in which all adults speak English less than “very well” [69]. For some patients with LEP, the barriers of clinical trial enrollment due to language discordance is magnified by literacy-related challenges during the informed consent process. Although it is generally recommended that consent forms be written at or below an eighth-grade reading level, most research-related documents are written at much higher reading levels [70-72]. However, 41% of the Hispanic population and 13% of the Asian population have below basic health literacy, which corresponds to being able to identify short straight-forward information in a text [73]. Thus, for Hispanic and Asian patients with thyroid cancer, language-based barriers may substantially limit their access to thyroid cancer clinical trials.
Strategies to Improve Access to Thyroid Cancer Clinical Trials
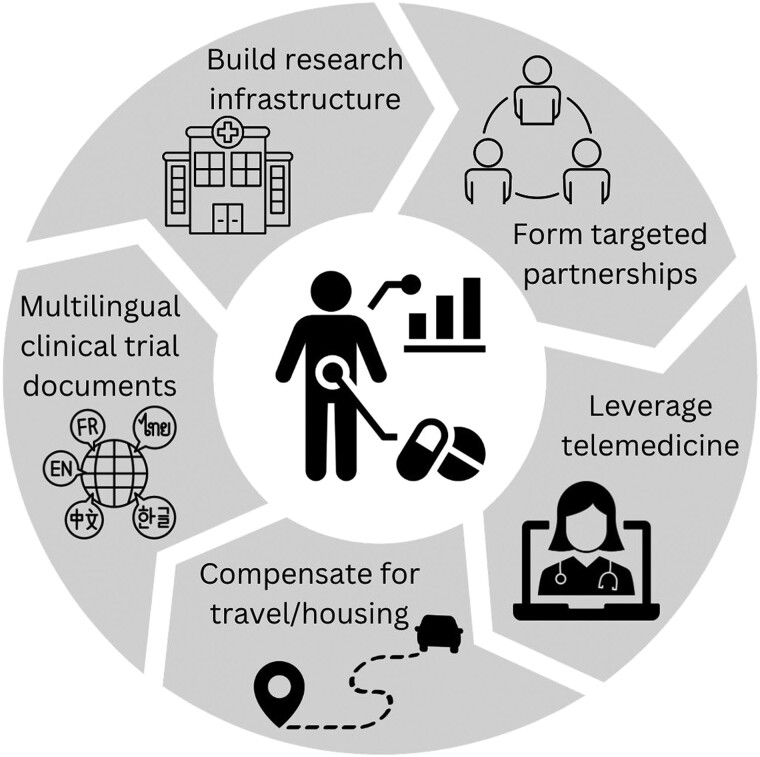
The lack of diversity and representation in cancer clinical trials is a decades-old issue. As such, nonthyroid cancer research has increasingly focused on multilevel strategies to overcome identified barriers to enhance the recruitment and retention of diverse patient populations. We next discuss potential strategies to increase access to thyroid cancer clinical trials for underrepresented patient populations (Fig. 2).
Figure 2.
Strategies to increase access to thyroid cancer clinical trials. To increase access to thyroid cancer clinical trials, interventions that address multilevel factors are necessary. At the level of the institution, health care executives and administrators need to prioritize the reduction of structural barriers to clinical trial participation. In addition, when designing clinical trial protocols, researchers should leverage existing technological advances in telemedicine to increase access to thyroid cancer clinical trials for underrepresented patient populations. Furthermore, for thyroid cancer clinical trials to be accessible to all patients regardless of socioeconomic status and English-language proficiency, funding agencies such as the National Institutes of Health and industry sponsors need to preemptively address financial and logistical barriers that may be relevant to patients with lower socioeconomic status and to racial/ethnic minority patient populations.
At the level of public policy, more funding for thyroid cancer research is needed to better understand barriers and facilitators to enrollment of underrepresented patient populations in thyroid cancer clinical trials. Although thyroid cancer is the seventh most common cancer in women [74], the second most common cancer in Hispanic and Asian/Pacific Islander women [10, 11], and was one of the most rapidly increasing cancers in the United States until recently [19], thyroid cancer research at the NIH continues to be underfunded relative to other cancer types. Despite the substantial clinical and economic burden associated with thyroid cancer [75], the National Cancer Institute allocated only $13.5 million (ranked 28th) to thyroid cancer research in 2018 [76].
At the level of the institution, health care executives and administrators need to prioritize the reduction of structural barriers to clinical trial participation for vulnerable patient populations. This includes the development and strengthening of well-resourced research infrastructure in the community setting where racial/ethnic minority patient populations are more likely to obtain their cancer care [50, 53, 77]. In addition, the formation of targeted partnerships between community hospitals, including hospitals that are embedded in underserved communities, and academic medical centers is important to increase access to cancer clinical trials for underrepresented patient populations [78].
When designing clinical trial protocols, researchers can leverage existing technological advances to increase access to thyroid cancer clinical trials for underrepresented patient populations [79]. With the rapid adoption of telemedicine in cancer care delivery in recent years [80, 81], the incorporation of telehealth visits in cancer clinical trials may make it more accessible for patients by avoiding the inconvenience and expense of travel and time off from work [82, 83]. In addition, researchers may be able to leverage patient portals, which are associated with institutional electronic health records, to increase engagement with and recruitment of underrepresented patient populations.
For thyroid cancer clinical trials to be accessible to all patients regardless of socioeconomic status and English-language proficiency, funding agencies such as the NIH and industry sponsors need to preemptively address financial and logistical barriers that may be relevant to patients with lower socioeconomic status and to racial/ethnic minority patient populations. Allocation of funds for financial reimbursement of trial-related expenditures (ie, for travel and/or lodging) would help to mitigate the financial burden of out-of-pocket expenses associated with clinical trial participation. Pilot studies of financial reimbursement programs for nonthyroid cancer clinical trials have yielded promising results [84-86]. Furthermore, since Hispanic and Asian/Pacific Islander patients with thyroid cancer are more likely to experience worse outcomes [10, 11], and both of these racial/ethnic groups have high rates of LEP [69], it may be beneficial to invest in the translation of clinical trial documents into non-English languages. In addition, the availability of patient information, both in English and non-English text, about thyroid cancer clinical trials in the ClinicalTrials.gov database would improve access through increased patient awareness of existing thyroid cancer clinical trials.
Conclusion
Since 2011, a paradigm shift has occurred in the treatment of advanced thyroid cancer with the recent FDA approval of multiple systemic therapies based on their use in the clinical trials setting. Thus, the lack of diversity in thyroid cancer clinical trials not only limits the generalizability of trial results, but also represents a disparity in access to high-quality thyroid cancer care. Multilevel interventions and increased funding for thyroid cancer research are necessary to increase access to and recruitment of underrepresented patient populations into thyroid cancer clinical trials.
Disclosures
The authors have nothing to disclose.
Abbreviations
- FDA
US Food and Drug Administration
- LEP
limited English proficiency
- NIH
National Institutes of Health
Contributor Information
Debbie W Chen, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, MI 48106, USA.
Francis P Worden, Division of Hematology and Medical Oncology, University of Michigan, Ann Arbor, MI 48106, USA.
Megan R Haymart, Email: meganhay@med.umich.edu, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, MI 48106, USA; Division of Hematology and Medical Oncology, University of Michigan, Ann Arbor, MI 48106, USA.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. National Institute on Minority Health and Health Disparities . Diversity and Inclusion in Clinical Trials. Accessed January 15, 2023. https://www.nimhd.nih.gov/resources/understanding-health-disparities/diversity-and-inclusion-in-clinical-trials.html
- 2. National Institutes of Health . NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. Accessed February 1, 2023. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm
- 3. Tanne JH. US must urgently correct ethnic and racial disparities in clinical trials, says report. BMJ. 2022;377:o1292. [DOI] [PubMed] [Google Scholar]
- 4. Aldrighetti CM, Niemierko A, Van Allen E, Willers H, Kamran SC. Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open. 2021;4(11):e2133205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunlop H, Fitzpatrick E, Kurti K, et al. Participation of patients from racial and ethnic minority groups in phase 1 early cancer drug development trials in the US, 2000-2018. JAMA Netw Open. 2022;5(11):e2239884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720‐2726. [DOI] [PubMed] [Google Scholar]
- 7. Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: have we improved? Cancer. 2013;119(16):2956‐2963. [DOI] [PubMed] [Google Scholar]
- 8. Nazha B, Mishra M, Pentz R, Owonikoko TK. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019;39(39):3‐10. [DOI] [PubMed] [Google Scholar]
- 9. National Institute on Minority Health and Health Disparities . Leap Forward Research Challenge. Accessed January 10, 2023. https://www.nimhd.nih.gov/about/strategic-plan/nih-strategic-plan-leap-forward-research-challenge.html
- 10. Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68(6):425‐445. [DOI] [PubMed] [Google Scholar]
- 11. Torre LA, Sauer AM, Chen MS Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: converging incidence in males and females. CA Cancer J Clin. 2016;66(3):182‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weeks KS, Kahl AR, Lynch CF, Charlton ME. Racial/ethnic differences in thyroid cancer incidence in the United States, 2007-2014. Cancer. 2018;124(7):1483‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg. 2007;246(6):1083‐1091. [DOI] [PubMed] [Google Scholar]
- 14. Hauch A, Al-Qurayshi Z, Friedlander P, Kandil E. Association of socioeconomic status, race, and ethnicity with outcomes of patients undergoing thyroid surgery. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1173‐1183. [DOI] [PubMed] [Google Scholar]
- 15. Harari A, Li N, Yeh MW. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2014;99(1):133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen DW, Haymart MR. Disparities research in thyroid cancer: challenges and strategies for improvement. Thyroid. 2020;30(9):1231‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wenaas AE, Nagy CZ, Yiu Y, Xu L, Horter K, Zevallos JP. Demographic and socioeconomic factors predictive of compliance with American Thyroid Association guidelines for the treatment for advanced papillary thyroid carcinoma. Head Neck. 2015;37(12):1776‐1780. [DOI] [PubMed] [Google Scholar]
- 18. Kovatch KJ, Reyes-Gastelum D, Hughes DT, Hamilton AS, Ward KC, Haymart MR. Assessment of voice outcomes following surgery for thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2019;145(9):823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Surveillance, Epidemiology, and End Results Program . Cancer Stat Facts: Thyroid Cancer. Accessed March 20, 2023. https://seer.cancer.gov/statfacts/html/thyro.html
- 20. Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brose MS, Nutting CM, Jarzab B, et al. ; DECISION Investigators . Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491‐501. [DOI] [PubMed] [Google Scholar]
- 23. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621‐630. [DOI] [PubMed] [Google Scholar]
- 24. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol. 2022;33(4):406‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639‐3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brose MS, Robinson B, Sherman SI, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(8):1126‐1138. [DOI] [PubMed] [Google Scholar]
- 28. US Food and Drug Administration . FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. Accessed February 28, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc
- 29. Doebele RC, Drilon A, Paz-Ares L, et al. ; Trial Investigators . Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Food and Drug Administration . FDA approves larotrectinib for solid tumors with NTRK gene fusions. Accessed February 28, 2023. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions
- 31. Waguespack SG, Drilon A, Lin JJ, et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol. 2022;186(6):631‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Regenbogen SE, Veenstra CM, Hawley ST, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. 2014;120(19):3074‐3081. [DOI] [PubMed] [Google Scholar]
- 33. Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39(39):105‐114. [DOI] [PubMed] [Google Scholar]
- 34. Zafar SY. Financial toxicity of cancer care: it's time to intervene. J Natl Cancer Inst. 2016;108(5):djv370. [DOI] [PubMed] [Google Scholar]
- 35. Rotter J, Spencer JC, Wheeler SB. Financial toxicity in advanced and metastatic cancer: overburdened and underprepared. J Oncol Pract. 2019;15(4):e300‐e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenup RA, Rushing C, Fish L, et al. Financial costs and burden related to decisions for breast cancer surgery. J Oncol Pract. 2019;15(8):e666‐e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veenstra CM, Braun TM, Abrahamse PH, Wittmann D, Hawley ST. Employment outcomes in family supporters of patients with early stage breast cancer and their association with patients’ health-related quality of life and financial burden. Cancer Med. 2022;11(5):1324‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zullig LL, Peppercorn JM, Schrag D, et al. Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer. J Oncol Pract. 2013;9(6S):60s‐63s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huey RW, George GC, Phillips P, et al. Patient-reported out-of-pocket costs and financial toxicity during early-phase oncology clinical trials. Oncologist. 2021;26(7):588‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728‐1733. [DOI] [PubMed] [Google Scholar]
- 42. American Cancer Society . Key Statistics for Thyroid Cancer. Accessed February 28, 2023. https://www.cancer.org/cancer/thyroid-cancer/about/key-statistics.html
- 43. Banegas MP, Guy GP Jr, de Moor JS, et al. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff (Millwood). 2016;35(1):54‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barrows CE, Belle JM, Fleishman A, Lubitz CC, James BC. Financial burden of thyroid cancer in the United States: an estimate of economic and psychological hardship among thyroid cancer survivors. Surgery. 2020;167(2):378‐384. [DOI] [PubMed] [Google Scholar]
- 45. Han X, Zhao J, Zheng Z, de Moor JS, Virgo KS, Yabroff KR. Medical financial hardship intensity and financial sacrifice associated with cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(2):308‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710‐3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen DW, Reyes-Gastelum D, Veenstra CM, Hamilton AS, Banerjee M, Haymart MR. Financial hardship among Hispanic women with thyroid cancer. Thyroid. 2021;31(5):752‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wong AR, Sun V, George K, et al. Barriers to participation in therapeutic clinical trials as perceived by community oncologists. JCO Oncol Pract. 2020;16(9):e849‐e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Copur MS. Inadequate Awareness of and Participation in Cancer Clinical Trials in the Community Oncology Setting. Accessed February 10, 2023. https://www.cancernetwork.com/view/inadequate-awareness-and-participation-cancer-clinical-trials-community-oncology-setting [PubMed]
- 52. McAlearney AS, Reiter KL, Weiner BJ, Minasian L, Song PH. Challenges and facilitators of community clinical oncology program participation: a qualitative study. J Healthc Manag. 2013;58(1):29‐44; discussion 45-26. [PMC free article] [PubMed] [Google Scholar]
- 53. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minasian LM, Unger JM. What keeps patients out of clinical trials? JCO Oncol Pract. 2020;16(3):125‐127. [DOI] [PubMed] [Google Scholar]
- 55. Institute of Medicine . Building an Infrastructure to Support Clinical Trials. Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020 (Workshop Summary). National Academies Press; 2012:53‐62. [PubMed] [Google Scholar]
- 56. Gerber DE, Lakoduk AM, Priddy LL, Yan J, Xie XJ. Temporal trends and predictors for cancer clinical trial availability for medically underserved populations. Oncologist. 2015;20(6):674‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Megwalu UC, Ma Y. Racial/ethnic disparities in use of high-quality hospitals among thyroid cancer patients. Cancer Invest. 2021;39(6-7):482‐488. [DOI] [PubMed] [Google Scholar]
- 59. Zagzag J, Kenigsberg A, Patel KN, Heller KS, Ogilvie JB. Thyroid cancer is more likely to be detected incidentally on imaging in private hospital patients. J Surg Res. 2017;215:239‐244. [DOI] [PubMed] [Google Scholar]
- 60. Cai C, Gaffney A, McGregor A, et al. Racial and ethnic disparities in outpatient visit rates across 29 specialties. JAMA Intern Med. 2021;181(11):1525‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Radhakrishnan A, Reyes-Gastelum D, Abrahamse P, et al. Physician specialties involved in thyroid cancer diagnosis and treatment: implications for improving health care disparities. J Clin Endocrinol Metab. 2022;107(3):e1096‐e1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim EJ, Kim T, Paasche-Orlow MK, Rose AJ, Hanchate AD. Disparities in hypertension associated with limited English proficiency. J Gen Intern Med. 2017;32(6):632‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Divi C, Koss RG, Schmaltz SP, Loeb JM. Language proficiency and adverse events in US hospitals: a pilot study. Int J Qual Health Care. 2007;19(2):60‐67. [DOI] [PubMed] [Google Scholar]
- 64. Cheng EM, Chen A, Cunningham W. Primary language and receipt of recommended health care among Hispanics in the United States. J Gen Intern Med. 2007;22(S2):283‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. [Google Scholar]
- 66. Unger JM. Lost in translation: participation in cancer clinical trials for patients with limited English proficiency. J Natl Compr Canc Netw. 2023;21(1):99‐100. [DOI] [PubMed] [Google Scholar]
- 67. Jorge S, Masshoor S, Gray HJ, Swisher EM, Doll KM. Participation of patients with limited English proficiency in gynecologic oncology clinical trials. J Natl Compr Canc Netw. 2023;21(1):27‐32.e22. [DOI] [PubMed] [Google Scholar]
- 68. Muthukumar AV, Morrell W, Bierer BE. Evaluating the frequency of English language requirements in clinical trial eligibility criteria: a systematic analysis using ClinicalTrials.gov. PLoS Med. 2021;18(9):e1003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ramakrishnan K, Ahmad FZ. State of Asian Americans and Pacific Islanders Series: A Multifaceted Portrait of a Growing Population. Center for American Progress; 2014. [Google Scholar]
- 70. Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348(8):721‐726. [DOI] [PubMed] [Google Scholar]
- 71. Hochhauser M. Consent forms: no easy read. Accessed April 1, 2023. https://www.appliedclinicaltrialsonline.com/view/consent-forms-no-easy-read [Google Scholar]
- 72. Friedman DB, Kim SH, Tanner A, Bergeron CD, Foster C, General K. How are we communicating about clinical trials? An assessment of the content and readability of recruitment resources. Contemp Clin Trials. 2014;38(2):275‐283. [DOI] [PubMed] [Google Scholar]
- 73. Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America's Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006-483). U.S. Department of Education. National Center for Education Statistics; 2006. [Google Scholar]
- 74. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 75. Aschebrook-Kilfoy B, Schechter RB, Shih YC, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1252‐1259. [DOI] [PubMed] [Google Scholar]
- 76. National Cancer Institute . FY 2018 Research Funding by Cancer Type. Accessed February 26, 2023. https://fundedresearch.cancer.gov/nciportfolio/search/funded;jsessionid=3CE3C77AAED25AC4A998F3BBD2A43374?action=full&fy=PUB2018&type=site
- 77. Somkin CP, Altschuler A, Ackerson L, et al. Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care. 2005;11(7):413‐421. [PubMed] [Google Scholar]
- 78. Ledesma Vicioso N, Lin D, Gomez DR, et al. Implementation strategies to increase clinical trial enrollment in a community-academic partnership and impact on Hispanic representation: an interrupted time series analysis. JCO Oncol Pract. 2022;18(5):e780‐e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. National Institutes of Health . Request for Information (RFI): Advancing Clinical and Translational Science through Accelerating the Decentralization of Clinical Trials. Accessed March 3, 2023. https://grants.nih.gov/grants/guide/notice-files/NOT-TR-23-006.html
- 80. American Medical Association . AMA survey shows widespread enthusiasm for telehealth. Accessed February 25, 2023. https://www.ama-assn.org/press-center/press-releases/ama-survey-shows-widespread-enthusiasm-telehealth
- 81. Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book. 2018;38:540‐545. [DOI] [PubMed] [Google Scholar]
- 82. Meghiref Y, Parnot C, Duverger C, et al. The use of telemedicine in cancer clinical trials: connect-patient-to-doctor prospective study. JMIR Cancer. 2022;8(1):e31255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chiang A, Herbst RS. How Telemedicine Can Transform Clinical Research and Practice. Accessed February 10, 2023. https://ascopost.com/issues/december-25-2022/how-telemedicine-can-transform-clinical-research-and-practice/
- 84. Borno HT, Zhang L, Zhang S, et al. Implementation of a multisite financial reimbursement program in cancer clinical trials integrated with patient navigation: a pilot randomized clinical trial. JCO Oncol Pract. 2022;18(6):e915‐e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Medina SP, Zhang S, Nieves E, et al. Experiences of a multiethnic cohort of patients enrolled in a financial reimbursement program for cancer clinical trials. JCO Oncol Pract. 2023;19(5):e801‐e810. [DOI] [PubMed] [Google Scholar]
- 86. Nipp RD, Lee H, Powell E, et al. Financial burden of cancer clinical trial participation and the impact of a cancer care equity program. Oncologist. 2016;21(4):467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.