Abstract
Previously published reports support the concept that, besides promoting homotypic intercellular adhesion, cadherins may transfer intracellular signals. However, the signaling pathways triggered by cadherin clustering and their biological significance are still poorly understood. We report herein that transfection of VE-cadherin (VEC) cDNA in VEC null endothelial cells induces actin rearrangement and increases the number of vinculin positive adhesion plaques. VEC expression augments the level of active Rac but decreases active Rho. Microinjection of a dominant negative Rac mutant altered stress fiber organization, whereas inhibition of Rho was ineffective. VEC expression increased protein and mRNA levels of the Rac-specific guanosine exchange factor Tiam-1 and induced its localization at intercellular junctions. In addition, in the presence of VEC, the amounts of Tiam, Rac, and the Rac effector PAK as well as the level of PAK phosphorylation were found increased in the membrane/cytoskeletal fraction. These observations are consistent with a role of VEC in localizing Rac and its signaling partners in the same membrane compartment, facilitating their reciprocal interaction. Through this mechanism VEC may influence the constitutive organization of the actin cytoskeleton.
INTRODUCTION
Normal endothelial cells (EC) present a typical cobblestone morphology at confluence with an epithelioid phenotype. In contrast, when the cells are sparse or intercellular junctions disrupted a fibroblastoid/mesenchimal morphology predominates. All this implies that establishment of intercellular contacts may transfer intracellular signals able to mediate changes in cytoskeletal organization and cell shape.
Endothelial junctions are complex structures formed by different transmembrane adhesive proteins linked inside the cells to a network of cytoskeletal and signaling partners (Dejana et al., 1999). Cadherins are the major transmembrane adhesive proteins at adherens junctions; they present a cell-specific expression pattern (Vleminckx and Kemler, 1999) and promote homophilic type of interactions (Gumbiner, 2000, and references therein).
Cadherins establish direct molecular connections with cytoplasmic partners that bind to different and specific domains of their cytoplasmic region. Classical cytoplasmic partners of cadherins are the catenins (α-, β-, and p120) (Anastasiadis and Reynolds, 2000, and references therein), which, besides promoting anchorage to actin cytoskeleton, when released into the cytoplasm, may translocate to the nucleus and influence gene transcription (Ben-Ze'ev and Geiger, 1998). In addition, cadherins may associate to growth factor receptors (Carmeliet et al., 1999; Pece and Gutkind, 2000) and some components of their signaling cascade such as Shc (Xu et al., 1997) phosphatidylinositol 3-kinase (Carmeliet et al., 1999; Pece et al., 1999), and various protein phosphatases (Zondag et al., 2000b, and references therein). These types of interactions may play a role in controlling growth factor signaling.
Among different junctional proteins, cadherins are good candidates as mediators of the change in cytoskeletal organization observed during the mesenchymal-epithelioid shift. Loss of cadherins is a frequent phenomenon in the mesenchymal transition of tumor cells acquiring invasive properties. In many tumors cadherin expression was negatively correlated to the invasive and dedifferentiated properties of the cells (Yap, 1998). However, the molecular mechanism through which cadherins may influence cytoskeletal organization and cell shape is still unknown.
VE-cadherin (VEC) is exclusively expressed in the endothelium and regulates fundamental activities of this tissue (Dejana et al., 1999). Inactivation of VEC expression by gene targeting results in early embryonic lethality due to impairment of vascular organization and remodeling (Carmeliet et al., 1999). Antibodies blocking VEC adhesion and clustering strongly affect formation of new vascular structures in adult animals (Liao et al., 2000) and increase permeability of constitutive vessels (Corada et al., 1999).
In the present work we studied the effect of VEC in modulating EC epithelioid shape and actin cytoskeleton organization. To this end we produced EC in which the VEC gene was inactivated by gene targeting (VEC null EC). VEC cDNA was then transduced into the same lines (VEC positive EC) and the populations were compared. We found that expression of VEC alone is sufficient to determine the transition from a fibroblastoid to a well spread, cobblestone, endothelial morphology. These modifications were accompanied by reorganization of actin stress fibers and increased number of vinculin positive focal contacts. We also observed that VEC induces activation of Rac and that this small GTPase plays an important role in actin organization in EC. Furthermore, expression of VEC increases the concentration of the Rac-specific guanosine exchange factor (GEF) Tiam (Michiels et al., 1995), Rac effector p-21 activated kinase (PAK) (Aspenstrom, 1999) as well as Rac itself in the membrane/cytoskeletal compartment. In this fraction we also found a higher amount of PAK phosphorylated at positions required for its sustained activity (Manser et al., 1997; Lei et al., 2000; Sells et al., 2000). These activities were lost in VEC mutants lacking either the carboxyterminal catenin binding domain or the juxtamembrane p120 binding region. Overall, these data show that, in resting EC, VEC can modulate the activity of small GTPases, which in turn influence the cellular phenotype. This effect is important in modulating actin cytoskeleton and cell shape and, as a consequence, cell spreading and adhesion to the substrate.
MATERIALS AND METHODS
Cells and Culture Conditions
EC with homozygous null mutation of VEC gene (VEC null) were isolated from both embryonic stem cells and 9.0-d postcoitum embryos (Carmeliet et al., 1999; Balconi et al., 2000). The homogeneous endothelial nature of the cultures was proved by fluorescence-activated cell sorting (FACS), Western blot, and immunofluorescence microscopy with antibodies to endothelial markers as described in RESULTS. To express wild-type (Breviario et al., 1995) and mutant forms of the protein in VEC null EC we used retroviral vectors as described (Grignani et al., 1998). Mutant forms were as follows: Δp120 (deleted aa 621–702 of human VEC cDNA, which corresponds to p120 binding region; Lampugnani et al., 1997; Thoreson et al., 2000), Δβcat (truncated aa 703–784 of human VEC cDNA, which correspond to β-catenin binding region; Navarro et al., 1995), and IL2-VE (with VEC cytoplasmic domain, aa 621–784, linked to the extracellular and transmembrane domains of interleukin-2 [IL-2] receptor α-chain; donated by Dr. Andrew Kowalczyk, Emery University, Atlanta, GA; LaFlamme et al., 1994). The different cDNA fragments where cloned into PINCO plasmids, which were transfected in amphotrophic Phoenix packaging cells and the culture supernatant containing viral particles used to infect VEC null EC as described in detail in Introna et al. (1998). The Phoenix cell line and the PINCO plasmid were obtained through the courtesy of Dr. P.G. Pelicci (European Institute of Oncology, Milan, Italy) and their use was authorized by Dr. Nolan (Department of Molecular Pharmacology, Stanford University, Palo Alto, CA). The efficiency of cDNA transfer was tested measuring the expression of VEC protein by FACS analysis and was >60% in most infections. To avoid clonal selection heterogeneity, cells were sorted by FACS and homogeneous cell populations expressing VEC by >90% were used for the experiments.
Cells were routinely cultured in DMEM with 20% fetal calf serum (FCS), endothelial cell growth supplement, and heparin (maintenance medium; Balconi et al., 2000) on gelatin-coated tissue culture vessels. Human umbilical vein EC (HUVEC) were cultured in M199 and 20% newborn calf serum with the same supplements and coating. For the experiments, cells were put in suspension by using trypsin-EDTA (Balconi et al., 2000) seeded at the concentrations indicated in the specific sections, and cultured for 2 d in maintenance medium. Cell layers were then washed once with MCDB131 (Invitrogen, Carlsbad, CA) and cultured in MCDB131 with 1% bovine serum albumin for further total 40 h with one wash and change of medium after 24 h.
Antibodies
Mouse monoclonal antibodies were as follows: anti-Rac-1 (clone 23A8; Upstate Biotechnology, Lake Placid, NY), anti-RhoA (clone 26C4; Santa Cruz Biotechnology, Santa Cruz, CA), anti-vinculin (clone hVIN-1; Sigma Chemical, St. Louis, MO), anti-p120 and anti-β-catenin (both from Transduction Laboratories, Lexington, KY), and anti-VEC (clone BV9, to VEC extracellular domain; Corada et al., 1999). Rat monoclonal antibodies were as follows: anti-platelet endothelial cell adhesion molecule (PECAM), anti-junctional adhesion molecule (JAM) (clone Mec 7.46 and BV12, respectively; Corada et al., 1999), and anti-endoglin (clone MJ7/18; BD PharMingen, San Diego, CA). Rabbit polyclonal antibodies anti-Tiam (C-16), Tie 2, and VEGF-R2 (C-1158) were all from Santa Cruz Biotechnology. Goat polyclonal anti-VEC (to VEC carboxyterminal region) was from Research Diagnostics (Flanders, NJ). Anti-PAK were rabbit polyclonal antibodies both from Santa Cruz Biotechnology and Cell Signaling Technology (Beverly, MA). Anti-phospho-PAK (Ser 199/204) and anti-phospho-PAK (Thr 423) were rabbit polyclonal from Cell Signaling Technology.
Immunofluorescence Microscopy
Cells (1.0 × 105/well VEC positive, VEC null, and VEC mutants or 0.7 × 105/well HUVEC) were cultured on fibronectin-coated glass coverslips set in 2-cm2 wells as described in detail in Corada et al. (1999), in 1 ml of the media indicated above. Fixation was in 3% formaldehyde from paraformaldehyde (PAF) for 15 min and was followed by permeabilization with 0.5% Triton X (TX)-100 before staining. Best junctional staining for Tiam was obtained fixing and permeabilizing cells at the same time with 0.5% TX-100 in 3% PAF for 3 min followed by 3% PAF for further 15 min. After incubation with the primary antibody for 1 h cells were double labeled with the appropriate tetramethylrhodamine B isothiocyanate (TRITC)-conjugated secondary antibody and fluorescein isothiocyanate-phalloidin for 45 min. Samples were examined under a Zeiss Axiophot or a Leica DMR fluorescence microscope and images recorded on 3200 ASA Kodak films or with a Hamamatsu 3 charge-coupled device camera before processing through Adobe Photoshop for Macintosh. In vivo treatment with blocking antibodies to VEC (80 μg/ml affinity-purified immunoglobulins; clone BV9) was for 7 h before fixation as described by Corada et al. (1999).
Microinjection
Cells were cultured on glass coverslips as described in the previous section. Production of recombinant proteins and microinjection procedure were as described in detail in Braga et al. (1997). After microinjection, samples were processed for immunofluorescence microscopy as described in the previous section.
Cell Fractionation and Western Blot
Cells (2.5 × 106) were seeded in 50-cm2 Petri dishes and cultured as described under “Cells and Culture Conditions.” Cell membrane and cytosolic fractions were obtained exactly as described by del Pozo et al. (2000). Briefly, cell layers were washed twice with ice-cold phosphate-buffered saline (PBS), scraped in ice-cold hypotonic lysis buffer (500 μl), homogenized with a Dounce homogenizer, and separated by two steps of centrifugation. Protein content was measured with the bicinchoninic acid method (Pierce Chemical, Rockford, IL). PAGE electrophoresis followed by protein transfer on nitrocellulose membrane for Western blot was used to analyze the samples. Forty micrograms of proteins was loaded per gel lane. This amount corresponded to approximately one-fiftieth of the soluble fraction and one-eighth of the particulate fraction. Total cell extracts were obtained washing twice the cell layer with PBS at room temperature and scraping the cells in boiling Laemmli sample buffer (500 μl) and immediately boiling the extract for further 5 min. To test for the presence of more than one antigen with distinct molecular weight in the same sample, the nitrocellulose membrane was either reprobed in sequence with different primary antibodies or it was cut perpendicularly to the direction of run and the separated sheets probed in parallel with different antibodies. The intensity of the bands was quantified on a G4 Macintosh computer by using the NIH Image 1.62 program developed at the National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/.
Rho and Rac Activity Assay
To recognize GTP-bound Rac and GTP-bound Rho, respectively, recombinant p21-binding domain of PAK1 (PBD) and Rho-binding domain of rhotekin (RBD) fused to glutathione S-transferase (GST) were prepared using the constructs obtained through the courtesy of Dr. M.A. Schwartz (Scripps Research Institute, La Jolla, CA) following the protocol described by Ren et al. (1999) and del Pozo et al. (2000).
Cells cultured in 50-cm2 Petri dishes, as described in the previous section, were washed once with ice-cold PBS and scraped in ice-cold lysis buffer (50 mM Tris pH 7.5, 1% TX-100, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl [500 mM NaCl for pull down with RBD-GST], 10 mM MgCl2, 1 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride). Cell extracts were centrifuged at 15,000 rpm for 5 min and the supernatants were incubated with 20 μg of recombinant PBD-GST or RBD-GST (precoupled to Sepharose-glutathione beads; Amersham Biosciences, Piscataway, NJ) for 40 min at 4°C. The beads were then washed four times with washing buffer (50 mM Tris pH 7.5, 1% TX-100, 150 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride) and eluted by boiling in Laemmli sample buffer for 5 min. Samples were then probed by Western immunoblotting for the presence of Rac-1 or Rho-A. Total cell extracts were prepared and run in parallel for normalization. To test for the specificity of the GTP-bound GTPases some samples were loaded with GDP before incubation with the resins. To this purpose, the lysates were prepared without MgCl2 and with 2 mM EDTA pH 7.5 and incubated for 10 min at room temperature, before adding 1 mM GDP, 10 mM MgCl2, and the resin.
Northern Blot
To test for Tiam transcript, mRNA was isolated, separated (3.5 μg/lane), and transferred on nitrocellulose membranes by using standard procedures. A cDNA fragment obtained by polymerase chain reaction amplification (nucleotides 953-1059) of the murine sequence (Habets et al., 1994) was used to prepare the radiolabeled probe. The transcript recognized by the probe was 7.2 kb.
RESULTS
Expression of VEC Changes EC Morphology and Actin Cytoskeletal Organization
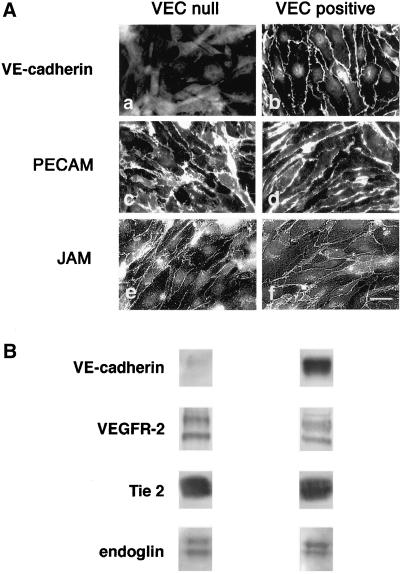
Murine EC carrying a null mutation of VEC gene (VEC null) do not express VEC but maintain control levels of endothelial specific markers such as VEGFR-2, Tie2, endoglin, and junctional proteins such as β-catenin, p120, PECAM, occludin, zonula occludens 1, and JAM (Figures 1 and 9; Carmeliet et al., 1999). When VEC cDNA was retrovirally transduced into these cells the protein was correctly expressed and homogenously distributed at cell-cell contacts in all the cell population (Figure 1; VEC positive cells). As reported in Figure 2, a and b, VEC null and positive cells show a distinct morphology. VEC positive cells have a rhomboid to polygonal shape and organize regular monolayers with no cell overlapping. VEC null cells have a triangular, fibroblastoid shape and form irregular layers with cell edge overlappings.
Figure 1.
Endothelial markers and junctional molecules in VEC null and positive EC. (A) When examined by immunofluorescence microscopy the neo-expressed VEC was concentrated to intercellular contacts in VEC positive cells (b). In both VEC null and positive cells the endothelial junctional markers PECAM (c and d) and JAM (e and f) were concentrated at cell-cell contacts. Bar, 20 μm. (B) VEC null cells were found positive in Western blot for the endothelial-selective VEGF and angiopoietin 1 receptors VEGFR-2 and Tie 2, respectively, as well as for the transforming growth factor-β coreceptor endoglin. The level of endothelial markers was comparable in VEC null and positive EC.
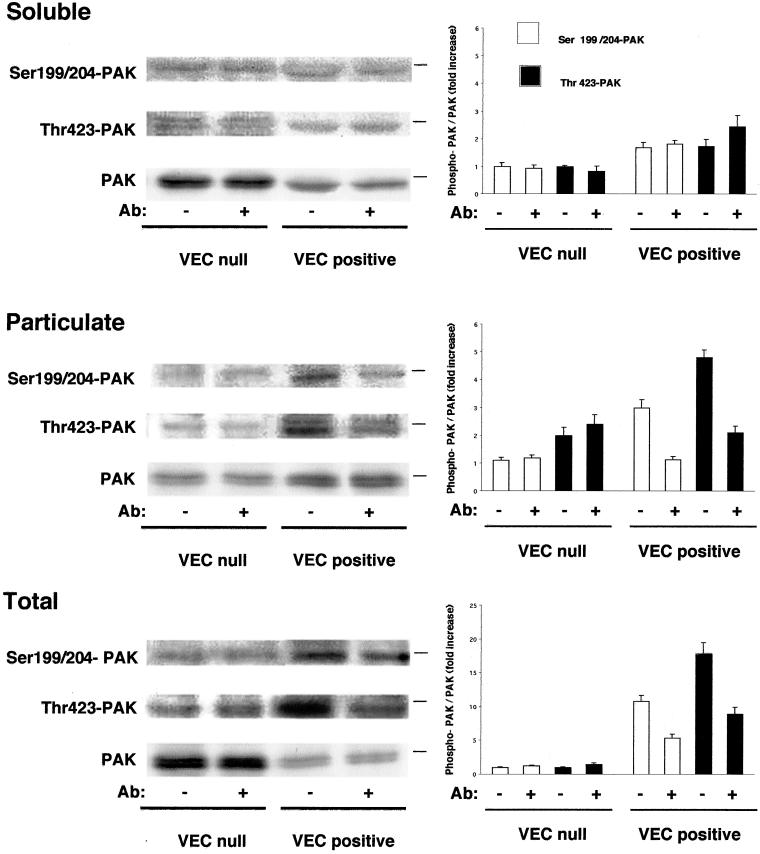
Figure 9.
PAK phosphorylation and distribution between soluble and particulate compartment. PAK was 1.6 ± 0.1-fold (mean ± SD of five experiments) concentrated in the particulate fraction of VEC positive cells (lower lane in middle gel, PAK). The lanes marked Ser 199–204 and Thr 423 are Western blot performed using two antibodies that recognize PAK only when phosphorylated in these respective positions. Particulate PAK was 2.5- (Ser 199–204) and 2-fold (Thr 423) more phosphorylated in VEC positive than in VEC null cells. Treatment with VEC antibodies for 7 h reduced phosphorylation by 50–60% in both positions. The graphics on the right report the ratio between the respective phospho-PAK and PAK in the same fraction. For soluble and particulate fractions the reference value is the ratio phospho-PAK/PAK of soluble control VEC null EC for the respective amino acid. For the total the reference value is the ratio phospho-PAK/PAK of control VEC null. They report the mean ± SD of three experiments. The dash on the right of each gel panel indicates the position of the 66-kDa marker.
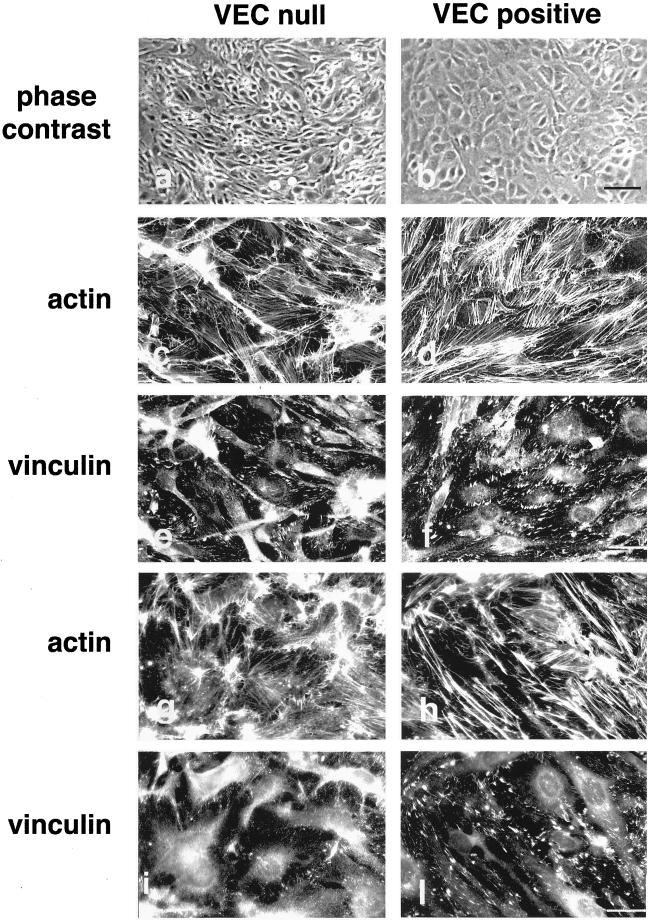
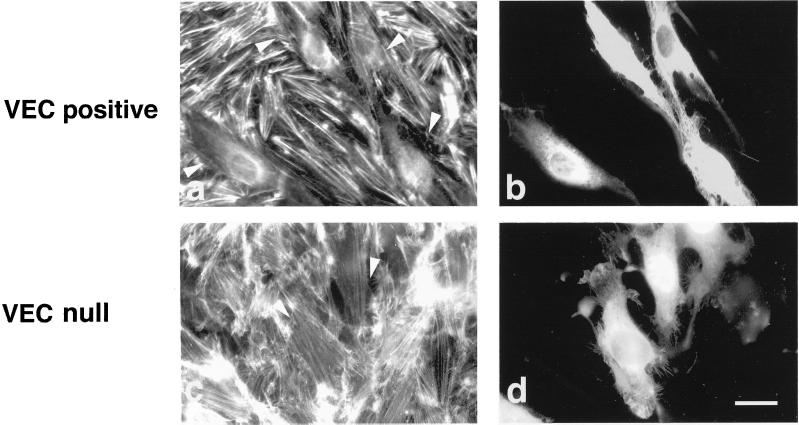
Figure 2.
VEC expression modifies actin and vinculin organization in EC. VEC null and VEC positive cells formed morphologically different monolayers as observed at phase contrast (a and b). The organization of actin stress fibers was deeply modified by the expression of VEC (c and d). Actin stress fibers were thick and short in VEC positive cells (d) in comparison with the thin and elongated fibers in VEC null cells (c). Distribution of vinculin positive adhesion plaques (e and f) at the end of the actin stress fibers reflected the different setting of actin microfilaments. Vinculin positive plaques were numerous and marked the cell margins in VEC positive cells (f). They were few, thin, and mostly polar in VEC null cells (e). EC isolated from 9.0-d-postcoitum embyros with wild-type (h and l) or homozygous null mutation of VEC gene (g and i) showed differences in thickness and organization of actin stress fibers and vinculin positive adhesion plaques comparable with EC differentiated from embryonic stem (ES) cells. Cells in c–l were double stained for vinculin and actin. Black bar, 100 μm; white bar, 20 μm.
When the organization of actin stress fibers was examined, VEC positive cells presented thick actin stress fibers mostly disposed in a zig-zag pattern in part connecting the two longer sides of the cell and in part ending in the middle of the cell body. At the ends of actin stress fibers vinculin positive adhesion plaques were present (Figure 2, d and f). VEC null cells have thin actin stress fibers arranged in a parallel pattern along the major axis of the cell and ending in thin, elongated vinculin positive adhesion plaques (Figure 2, c and e). The density of adhesion plaque was of 44 ± 6.8 and 20 ± 3.5/500 μm2 for VEC positive and VE null cells, respectively.
When the morphology of EC obtained from embryos carrying a null mutation for VEC (Figure 2, g and i) was compared with that of EC from control animals (Figure 2, h and l) we found that, also in this case, VEC positive cells presented better organized stress fibers (Figure 2, g and h) and a higher number of focal contacts (Figure 2, i and l). The experiments reported in the following sections were performed using EC derived from embryonic stem cells. The same experiments were repeated at least once using EC from early embryos (Figure 2) with comparable results.
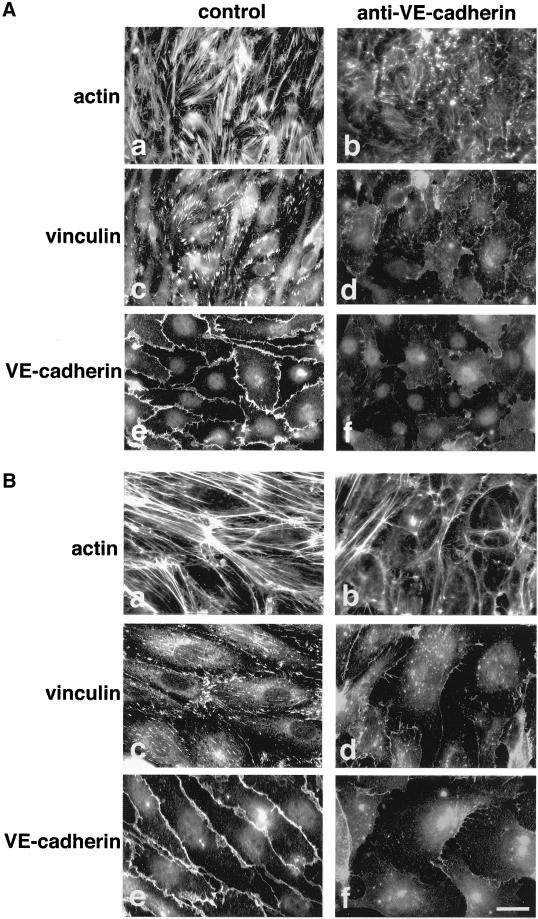
To test whether VEC needs to be engaged at intercellular junctions to maintain its effects, we exposed VEC positive cells (Figure 3A) and HUVEC (Figure 3B) to blocking anti-VEC monoclonal antibodies (mAbs) able to disrupt VEC junctional clusters (Figure 3, A and B, e and f) without affecting the distribution of other junctional molecules (Corada et al., 1999, 2001). As reported in Figure 3, A and B, a–d, these mAbs induced rearrangement of actin filaments and pulverization of vinculin positive adhesion plaques in both cell types.
Figure 3.
VEC antibodies disorganize actin and vinculin in VEC positive murine EC and in HUVEC. VEC positive murine EC (A) responded to monoclonal antibodies in a way comparable to HUVEC (B). As reported previously in detail (Corada et al., 1999) VEC antibodies induced release of VEC from cell-cell junctions (f in A and B in comparison with control antibodies in e in A and B). This effect paralleled a strong reduction of actin stress fibers (compare b with control a both in A and B) and loss of the vinculin positive adhesion plaques (compare d with control c both in A and B). The cells shown in the figure were treated with the antibodies for 7 h. For analysis in immunofluorescence microscopy they were double labeled for vinculin (c and d) and actin (a and b) or labeled for VEC (e and f). Bar, 20 μm.
VEC Expression Activates Rac
General cell morphology, actin stress fibers, and cell shape are regulated by the Rho family of small GTPases (Hall, 1998). Therefore, the observations reported above prompted us to examine the state of activation of Rho and Rac in the presence or absence of VEC (Figure 4).
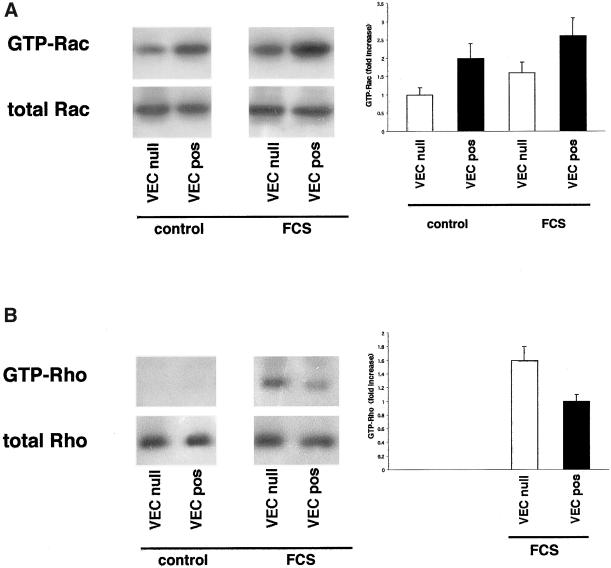
Figure 4.
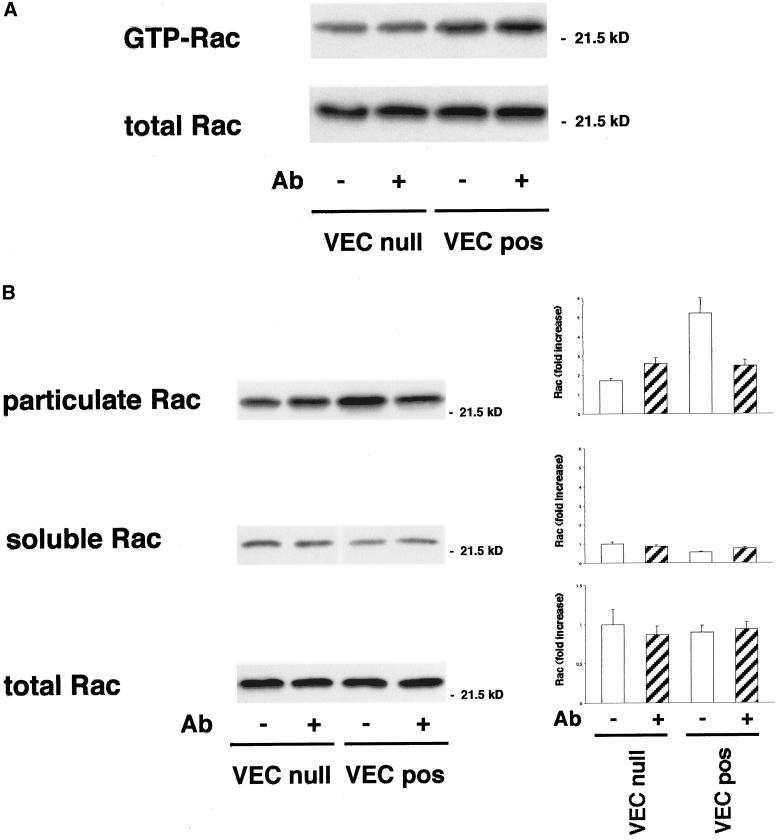
Activity of Rac-1 and Rho-A in VEC null and VEC positive EC. (A) GTP-bound Rac recovered in pulldown experiments with recombinant PBD-GST was twice as much in VEC positive in comparison with VEC null EC. The mean fold increase over control VEC null ± SD of five experiments is reported in the right panel. This difference was slightly reduced (to 1.6 increase), but still significant, after stimulation with FCS for 9 min. (B) GTP-bound Rho could not be recovered in pulldown experiments with recombinant RBD-GST from unstimulated VEC null or VEC positive EC. After stimulation with FCS for 3 min GTP-Rho became measurable and it was 1.6-fold more in VEC null than in VEC positive cells. The mean fold increase over FCS-stimulated VEC positive ± SD of five experiments is shown in the right panel.
The active GTP-bound forms of Rac and Rho GTPases were isolated using the recombinant proteins PBD-GST and RBD-GST, respectively. GTP-Rac could be detected in unstimulated cells and was twofold more in VEC positive than in VEC null cells (Figure 4A). After acute, short-lasting stimulation with serum for 9 min GTP-Rac increased 1.3- and 1.6-fold in VEC positive and VEC null cells, respectively, reducing the difference to 1.6-fold (Figure 4A). GTP-bound Rho was undetectable in control cells (Figure 4B) and could be measured only after stimulation with serum for 3 min. At variance with GTP-Rac, GTP-Rho was 1.6-fold more abundant in VEC null than in VEC positive cells (Figure 4B).
Activation of Rac Is Required for Actin Stress Fiber Organization
The relative contribution of Rac and Rho to actin organization in VEC null and positive cells was further studied by microinjecting the cells with dominant negative Rac (N17Rac). In VEC positive cells N17Rac disassembled actin stress fibers in 80% of microinjected cells within 1 h (Figure 5a). In VEC null cells N17 Rac had virtually no effect (Figure 5c; mean number of stress fibers ± SD was 15 ± 5 and 12 ± 5 for each of 20 control or N17-injected cells).
Figure 5.
Actin reorganization in response to N17Rac microinjection in VEC null and VEC positive EC. VEC positive EC responded to microinjected N17Rac with a strong reduction of actin stress fibers (a). After the same treatment VEC null did not show appreciable modification of actin microfilaments (c). Microinjected cells were TRITC-dextran labeled as shown in b and d. They are indicated by arrowheads in a and c. The effects presented in the figure were observed 1 h after microinjection, but similar responses were already present 30 min after injection (our unpublished data). Bar, 20 μm.
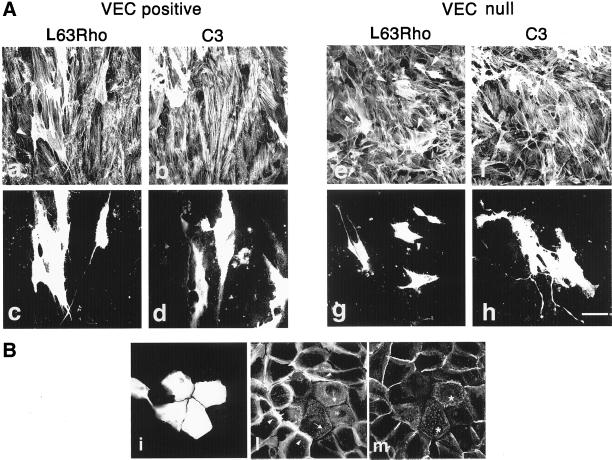
The microinjection of a constitutively active form of Rac (L61Rac) in VEC negative cells induced a phenotype similar to that of VEC positive cells (our unpublished data). These observations suggest that Rac activation is required for stress fibers organization induced by VEC expression. This hypothesis was further challenged using a constitutively active form of Rho (L63Rho). The reasoning was that if the more active Rac is present the better it could counterbalance the effect of active Rho as suggested by various reports (Sander et al., 1999; Zondag et al., 2000a). In VEC positive cells L63Rho increased the number of actin stress fibers and modified their shape and distribution within the cells, inducing deposition of bundles of thick actin stress fibers along the longest axis of the cell (Figure 6a). VEC null cells were more susceptible to the action of L63Rho, and not only presented a strong increase in number and dimension of actin microfilaments but also appeared strongly contracted (Figure 6, e and g). The Rho inhibitor C3 did not change actin organization either in VEC positive or null cells (Figure 6, b and f). Similarly, in resting, confluent HUVEC, N17Rac disassembled actin stress fibers in >70% of microinjected cells, whereas C3 induced disappearance of actin stress fibers only in 20% of microinjected cells (our unpublished data). Different preparations of C3, effective in disassembling actin stress fibers in epithelial cells, gave results comparable to the ones reported above when tested in VEC positive cells, VEC null cells, and HUVEC. The same preparation of C3 used on the same day on keratinocytes disorganized actin stress fibers and junctional E-cadherin (Figure 6B).
Figure 6.
Actin reorganization in response to L63Rho and C3 microinjection in VEC null and VEC positive EC. (A) Microinjection of the constitutively active L63Rho induced formation of thick cables of actin stress fibers both in VEC positive and null EC (a and e). In null cells this effect was followed by contraction (e, arrowheads, and g). In contrast, microinjected VEC positive cells remained mostly flat (a, arrowheads, and c). Microinjection of C3 transferase did not modify actin stress fibers either in VEC null (f) or VEC positive EC (b). Microinjected cells were TRITC-dextran labeled as shown in c, d, g, and h. The figure reports the effects observed 1 h after microinjection. Bar, 30 μm. (B) In keratinocytes, the same preparation of C3 transferase as in A (0.1 mg/ml for 1 h) microjniected on the same day as for the experiments in A induced dissolution (arrows, in microinjected cells, l) of the peripheral ring of actin stress fibers (arrowhead, in control cells, l) and diffusion of E-cadherin from cell-cell contacts (asterisks, m). i shows microjniected cells labeled with TRITC-dextran.
VEC Concentrates Rac, PAK, and Tiam in Particulate Compartment
The association of Rac (del Pozo et al., 2000) and its partners (Stam et al., 1997) to the membrane/cytoskeletal compartment appears crucial for local and selective activation of the related signaling pathways. VEC positive and null EC were fractionated into soluble and particulate fractions corresponding to cytosol and membranes/cytoskeleton, respectively (del Pozo et al., 2000). We then analyzed expression and distribution of Rac, the selective Rac activator Tiam-1, and the Rac effector PAK (Aspenstrom, 1999).
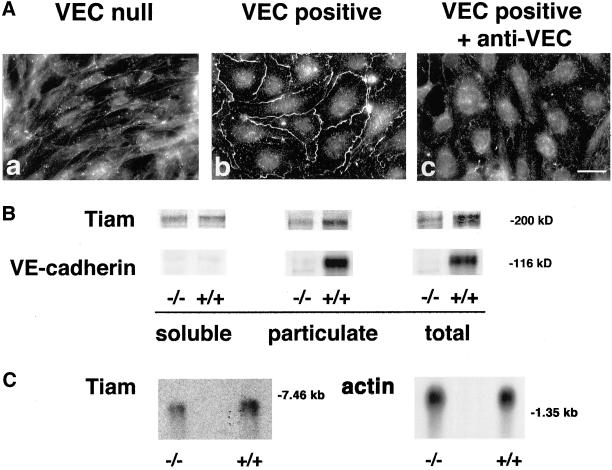
As shown in Figure 7B when antibodies against Tiam were used, we observed more (2 ± 0.28-fold; mean ± SD of five experiments) Tiam in the particulate fraction of VEC positive compared with null cells. The amount of the antigen detected in total extract of VEC positive cells was 1.8 ± 0.3-fold (mean ± SD of five experiments) higher than in VEC null. The reactivity of the antibody for Tiam was specific and was blocked by the corresponding immunopeptide (our unpublished data). These data indicate that expression of VEC increases Tiam protein levels and distribution in the particulate fraction. These observations were further confirmed by Northern blot analysis of Tiam mRNA. As reported in Figure 7C expression of VEC induced a twofold increase in Tiam mRNA in comparison with null cells. In addition, Tiam colocalized with VEC at intercellular junctions only in VEC positive but not in VEC null cells (Figure 7A, a and b) and the antibody that induces diffusion of VEC out of junctions (Figure 3A) also caused loss of Tiam from cell-cell contacts of VEC positive EC (Figure 7A, c). As reported in Figures 8B and 9, Rac and its effector PAK were concentrated at higher levels in the membrane pellet of VEC positive cells 2 ± 0.4- and 1.6 ± 0.1-fold (mean of five experiments ± SD, respectively, vs. VEC null cells). When PAK phosphorylation at positions Ser 199–204 and Thr 423, an index of PAK activation (Manser et al., 1997; Lei et al., 2000; Sells et al., 2000), was tested using specific antibodies, 2.5- and 2-fold increase in the level of Ser 199–204 and Thr 423, respectively, was observed in the particulate fraction of VEC positive in comparison with VEC null cells (Figure 9, middle). VEC was almost exclusively present in the particulate fraction (Figure 7B).
Figure 7.
(A) Junctional localization of Tiam requires VEC expression and clustering. VEC positive (b) but not negative EC (a) localized Tiam to intercellular junctions. Antibodies to VEC that disrupted VEC clusters at junctions (Figure 3) also induced disappearance of Tiam from cell-cell contacts (c). Bar, 20 μm. (B) Distribution of Rac activators and effectors between soluble and particulate fractions in VEC null and positive EC. When tested by Western blot, the Rac GEF Tiam was twofold concentrated in the particulate fraction of VEC positive (+/+) than in VEC null (−/−) EC. VEC was also selectively distributed in this fraction. The position of mw markers is reported on the right. (C) Northern blot analysis at Tiam mRNA. Expression of VEC (+/+) was accompanied by a twofold increase of Tiam mRNA in comparison to null (−/−) cells. The position of molecular weight markers is indicated on the right.
Figure 8.
Effect of VEC antibodies on Rac-1 activity and distribution between soluble and particulate fraction. (A) Incubation of EC with VEC antibodies for 7 h did not modify the amount of GTP-Rac recovered in pulldown experiments with PBD-GST in both VEC positive and negative EC. (B) Rac is 2 ± 0.4-fold (mean ± SD from five experiments) more concentrated in the pellet of VEC positive than VEC null EC. Incubation of the cells with a VEC mAb reduced the particulate Rac by ∼50% in VEC positive EC, leading to values similar to VEC null cells. Graphics on the right report Rac fold increase in the respective fractions. For soluble and particulate fractions the reference level is soluble Rac in control VEC null. For the total, the reference value is Rac in control VEC null. The mean ± SD of three experiments is reported.
VEC Antibodies Reduce Rac Level and PAK Phosphorylation in Particulate Compartment
VEC antibody that affects actin and vinculin organization (Figure 3) and VEC and Tiam distribution (Figures 3 and 7, respectively) reduced Rac in the particulate fraction of VEC positive cells by 40–50% (Figure 8B). The antibody had no effect on the level of active Rac (Figure 8A). It also did not reduce particulate fraction-associated Tiam and VEC (our unpublished data) as well as PAK (Figure 9, middle). It reduced PAK phosphorylation in the particulate fraction by 50–60% both at the level of Ser 199–204 and Thr 423 (Figure 9, middle).
Wild-Type VEC Is Necessary for Rac Activation and for Reshaping Actin Stress Fibers
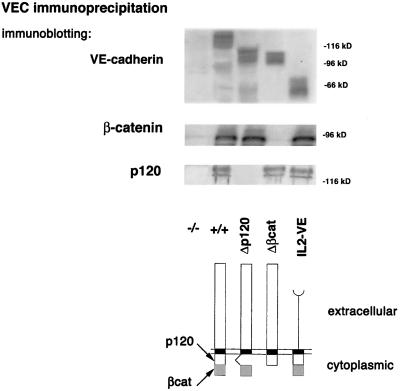
To the aim of defining the region of VEC responsible for the effects described above, various endothelial mutants expressing different truncated forms of VEC have been produced. The mutant cDNAs were transduced into VEC null EC as described above. The mutants were as follows: 1) Δβcat, truncated in the carboxyterminal cytoplasmic domain responsible for VEC binding to β- and γ-catenins (Navarro et al., 1995); 2) Δp120, truncated in the juxtamembrane half of the cytoplasmic domain responsible for binding to p120 (Lampugnani et al., 1997; Thoreson et al., 2000); and 3) IL2-VE, where the cadherin extracellular domain was substituted with the IL-2 receptor sequence linked to the cadherin cytoplasmic tail (Figure 10). The mutants were expressed at levels comparable to the wild type (Figure 10) and were correctly distributed to the plasma membrane; indeed, a similar amount of labeled VEC molecules was recovered from surface biotinylated wild-type and mutant cells (our unpublished data). Δβcat and Δp120 could be found at cell-cell junctions (Figure 11, d and g), whereas IL2-VE remained diffused on the cell membrane (Figure 11, l). The mutants coimmunoprecipitated (Figure 10) with the expected catenins: Δβcat only with p120, Δp120 only with β-catenin, and IL2-VE with both p120 and β-catenin.
Figure 10.
Association of catenins to VEC mutants. Mutant cDNAs with deletion of the cytoplasmic domain required for p120 (Δp120), or β-catenin (Δβcat) binding, or with substitution of the extracellular domain with the extracellular domain of the IL-2 receptor (IL2-VE) (bottom) were transduced into VEC null cells. By immunoprecipitation and Western blot analysis VEC mutants were expressed at the expected molecular weight of ∼110 kDa for Δp120 and Δβcat and 50 kDa for IL2-VE, in comparison to 120 kDa of the full-length VEC (top). Δp120 mutant coimmunoprecipitated only with β-catenin, Δβcat mutant only with p120, and IL2-VE chimera with both β-catenin and p120. The total amount of both β-catenin and p120 in the different cell types was not significantly modified (our unpublished data). The position of molecular weight markers is shown on the right.
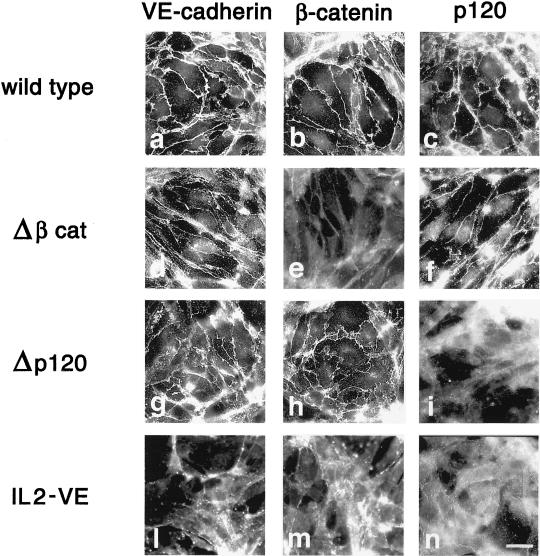
Figure 11.
Junctional distribution of VEC mutants and associated catenins. Δβcat and Δp120 distributed to intercellular junctions (d and g, respectively) as wild-type VEC (a). Δβcat codistributed with p120 (f), but not βcat (e), whereas Δp120 codistributed with β-catenin (h), but not p120 (i). IL2-VE and associated β-catenin and p120 did not show preferential junctional distribution (l–n). Bar, 20 μm.
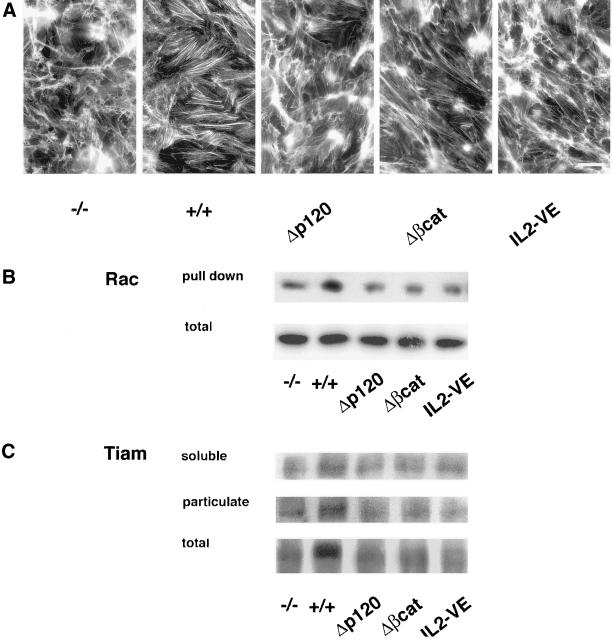
Δβcat and Δp120 codistributed at junctions only with p120 (Figure 11, f) and β-catenin (Figure 11, h), respectively. In transfectants with IL2-VE, which does not cluster at junctions, no significant junctional staining of catenins was observed (Figure 11, l–n). The general morphology of the cell layer as well as the organization of actin stress fibers in all the mutants was similar to that of VEC null cells (Figure 12). The level of GTP-Rac was low in the three mutants (2–3-fold lower than in VEC positive cells) and comparable to VEC null cells (Figure 12). Furthermore, the mutants did not show a detectable increase of Tiam in the particulate fractions compared with wild-type VEC cells (Figure 12). Rac and PAK showed a pattern of distribution between soluble and particulate fraction superimposable on that found in VEC null cells (our unpublished data).
Figure 12.
Actin organization, Rac activity, and Tiam distribution in soluble and particulate fractions in VEC mutants. (A) Compared with wild-type VEC none of the mutants induced actin reorganization. (B) In parallel, VEC mutants did not increase the level of activated Rac measured in pulldown experiments with PBD-GST (GTP-Rac was 2–3-fold less than in wild-type VEC). (C) Tiam was not concentrated in the particulate compartment of any VEC mutants in contrast to wild-type VEC. Tiam associated to particulate fraction was 1.7–2.3-fold more in wild-type than in mutant cells, whereas the reactivity in the total extract of VEC positive cells was 1.8–2-fold more than in the other transfectants. Bar, 20 μm (A).
DISCUSSION
In this study we show that VEC expression influences EC shape and that this effect is accompanied by reorganization of actin stress fibers and associated vinculin positive adhesion plaques. The specific role of VEC in the reorganization of actin and vinculin was demonstrated both by adding specific blocking antibodies to confluent EC and by comparing VEC positive and null EC. Within few hours, disruption of VEC clusters at junctions by blocking antibodies (Corada et al., 1999, 2001) paralleled the disorganization of actin and vinculin clusters, indicating that the continuous presence of VEC at junctions is required for a correct signaling and assembly of actin microfilaments. VEC effect seems specific and not due to a more general disorganization of intercellular junctions because in the presence of the antibodies or in VEC null cells all the other junctional proteins we could test, including occludin, zonula occludens 1, JAM, and PECAM (Carmeliet et al., 1999; this study), were correctly clustered at intercellular contacts.
We found that activation of Rac GTPase plays a role in these VEC-mediated activities. VEC expression augmented the amount of GTP-bound Rac in comparison with null cells, and microinjection of a Rac dominant negative form induced a strong disorganization of actin stress fibers in VEC positive cells. It has been recently observed, using a Ca2+ switch assay or blocking antibodies that clustering of E-cadherin activates Cdc42 (Kim et al., 2000) and Rac (Nakagawa et al., 2001; Noren et al., 2001). These data are consistent with the present report. However, in our system we could measure Rac activation in resting endothelial monolayers, indicating that VEC can exert a steady-state control on Rac GTPase. Because in physiological situations in vivo the endothelium is virtually quiescent, the mechanism we are describing should reflect the common functional state of the endothelium.
Activation of Rac signaling may down-regulate Rho GTPase (Sander et al., 1999; Zondag et al., 2000a), and it has been reported for epithelial cells and 3T3 fibroblasts that this effect accompanies the shift from a mesenchymal to an epithelial phenotype with increase in cell spreading and inhibition of motility. Decreased RhoA and elevated Rac activity are also found in confluent epithelial cells in comparison with subconfluent cultures (Noren et al., 2001). In our cells, irrespective of VEC expression, activated Rho could not be measured in resting conditions. Only upon stimulation with serum, GTP-bound Rho was detectable and its levels were lower in VEC positive compared with VEC null EC.
Other studies showed that C3 could disrupt stress fibers in HUVEC activated by different stimuli (Wojciak-Stothard et al., 1998, 1999) and also in unstimulated confluent EC from bovine pulmonary arteries (Carbajal and Schaeffer, 1999). It is likely that Rho may promote actin reshaping upon acute cell activation by inflammatory cytokines and permeability-increasing agents, whereas its role in the maintenance of cytoskeletal organization in resting and confluent EC may depend on the specific endothelial origin. An important issue is the mechanism through which VEC maintains Rac activation. We found that VEC expression increases the total amount of the Rac-specific GEF Tiam and its association to the particulate fraction. In addition, VEC induces Tiam redistribution at junctions as shown by immunofluorescence staining of confluent EC. The effect was specific because no junctional Tiam could be observed in VEC null cells or after treatment with a VEC blocking antibody.
Taking all these data into account it appears that VEC expression increases and stabilizes Tiam at the membrane. Tiam may then activate Rac and indirectly PAK, which are concurrently concentrated in the particulate compartment of VEC positive cells, starting in this way a chain of molecular events responsible for the observed functional effects. Other studies (del Pozo et al., 2000; Kraynov et al., 2000; Sells et al., 2000) demonstrated that Rho family GTPases regulate signaling components that are localized in specific domains at the cell membrane. It has been proposed (Sander et al., 1999; Zondag et al., 2000a) that Tiam can control the transition from mesenchymal to epithelial phenotype and that a critical element for this effect is its relocalization at junctions with consequent sustained activation of Rac. It was shown that, when cells are confluent, Tiam (Sander et al., 1999) and Rac (Kraynov et al., 2000) would be mostly concentrated at cell-to-cell junctions, whereas in motile cells these effectors would relocalize to membrane ruffles. Herein, we add a new element to the picture that is the direct role of cadherin in inducing the junctional distribution of Tiam and in maintaining the Tiam/Rac system constitutively activated. Supporting this issue we also observed that PAK recovered in the membrane/cytoskeletal compartment of VEC positive EC is more phosphorylated at positions that positively modulate its activity (Manser et al., 1997; Lei et al., 2000; Sells et al., 2000) than in VEC null EC.
The involvement of the Rac-PAK pathway in the activity of VEC on actin stress fibers and vinculin organization is further illustrated by the effect of VEC antibodies. These antibodies do not decrease the amount of active Rac. However, they reduce by ∼50% Rac level and ∼60% PAK phosphorylation, specifically in the particulate fraction of VEC positive cells. Treatment with VEC antibodies does not reduce PAK protein, as well as Tiam and VE-cadherin, in the particulate compartment. These results would fit in a picture in which de-/mislocalized activation of Rac, as a consequence of VEC and Tiam diffusion out of intercellular junctions, would result in functional failure even if the activation of Rac itself is not impaired. A similar regulatory mechanism operating in adherent versus suspended cells has been reported by del Pozo et al. (2000).
Even if PAK seems to be the most likely effector of the VEC-Rac pathway we cannot exclude at this stage that other partners could play a relevant role in actin and vinculin distribution. For instance, we studied the effect of a Rho-associated coiled-coil kinase (ROCK) inhibitor, Y-27632, but at concentrations able to disrupt focal contacts and adhesion plaques in other cell types (keratinocytes and fibroblasts), it was ineffective in VEC positive cells (our unpublished data).
A further question is whether the known partners of VEC may play a role in the processes described. The cytoplasmic domain of VEC binds β- and γ-catenins and p120. These proteins when released into the cytoplasm exert important biological activities. p120 may act as an inhibitor of Rho GTPase (Anastasiadis et al., 2000; Noren et al., 2000) and as β- and γ-catenins may translocate to the nucleus and modulate gene transcription by binding specific transcription factors. The binding of these proteins to cadherins would therefore prevent their release into the cytosol and inhibit their activity. p120 binds to a juxtamembrane region of VEC tail (Lampugnani et al., 1997), whereas β- and γ-catenins bind to the carboxyterminal domain (Navarro et al., 1995). Deletion mutants of VEC lacking either one of these domains are unable to concentrate Tiam in the particulate compartment, to activate Rac, and to induce actin reorganization, although they retain the ability to link catenins in the respective residual cytoplasmic region and to localize at cell-cell contacts. This would suggest that the concomitant binding of all the partners is necessary for a correct activity of VEC. However, the chimeric mutant IL2-VE, containing a full-length VEC cytoplasmic tail, was also ineffective despite its ability to correctly link β-catenin and p120. This mutant, although correctly expressed on the cell surface, is unable to localize at junctions, suggesting that VEC clustering at intercellular junctions is also a requirement for its activity.
In conclusion, although in previous reports Rac was able to modulate cadherin activity by an “inside out” mechanism, our observations support the idea of an “outside in” signaling pathway from cadherins to Tiam, Rac, and PAK. Therefore, the cross-talk between regulators of cell-cell recognition and adhesion, such as the cadherins, and regulators of cytoskeletal organization and substrate adhesion, such as Rho-like GTPases, can operate in both directions.
We have previously shown that embryos lacking VEC show a lethal phenotype due to defects in the normal development of the vasculature (Carmeliet et al., 1999). The data reported herein add further information about the mechanism leading to the null phenotype. The lack of a correct actin organization may affect several cellular responses, including growth, apoptosis, and formation of vascular structures. Therefore, modulation of Rac by VEC may have a more general impact on EC functional behavior and be required for the normal organization of the vasculature in vivo.
ACKNOWLEDGMENTS
This work was supported in part by Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale delle Ricerche (grant 97.01299.PF49), The European Community (BMH4 C983380, QLG1-CT-1999-01036 and QLK3-CT-1999-00020), Ministero Universita' Ricerche Scientifiche e Tecnologiche (9906317157-003), and Telethon-Italy (grant E.1254).
Footnotes
DOI: 10.1091/mbc.01–07–0368.
REFERENCES
- Anastasiadis PZ, Reynolds AB. The p120 catenin family. complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113:1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- Balconi G, Spagnuolo R, Dejana E. Development of endothelial cell lines from embryonic stem cells. A tool for studying genetically manipulated endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2000;20:1443–1451. doi: 10.1161/01.atv.20.6.1443. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5) an endothelial specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15:1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Schaeffer RC. RhoA inactivation enhances endothelial barrier function. Am J Physiol. 1999;77:C955–C964. doi: 10.1152/ajpcell.1999.277.5.C955. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion, and clustering of the protein, and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Corada M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Grignani F, Lanfrancone L, Peschle C, Nolan GP, Pelicci PG. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Introna M, et al. Rapid retroviral infection of human hemopoietic cells of different lineages: efficient transfer in fresh T cells. Br J Hematol. 1998;103:449–461. doi: 10.1046/j.1365-2141.1998.01020.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Li Z, Sacks DB. E-cadherin-mediated cell-cell attachment activates Cdc42. J Biol Chem. 2000;275:36999–37005. doi: 10.1074/jbc.M003430200. [DOI] [PubMed] [Google Scholar]
- Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110:2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini M, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Liao F, et al. Monoclonal antibody to vascular endothelial (VE)-cadherin is a potent inhibitor of angiogenesis, tumor growth, and metastasis. Cancer Res. 2000;60:6805–6810. [PubMed] [Google Scholar]
- Manser E, Huang H, Loo T, Chen X, Dong J, Leung T, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Habets GGM, Stam JC, Van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment, and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- Navarro P, Caveda L, Breviario F, Mandoteanu I, Lampugnani MG, Dejana E. Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem. 1995;270:30965–30972. doi: 10.1074/jbc.270.52.30965. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind J. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment, and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Pfaff A, Chernoff J. Temporal, and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol. 2000;151:1449–1458. doi: 10.1083/jcb.151.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam JC, Sander EE, Michiels F, van Leeuwen FN, Kain HE, van der Kammen RA, Collard JG. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272:28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Guo DF, Davidson M, Inagami T, Carpenter G. Interaction of the adaptor protein Shc and the adhesion molecule cadherin. J Biol Chem. 1997;272:13463–13466. doi: 10.1074/jbc.272.21.13463. [DOI] [PubMed] [Google Scholar]
- Yap AS. The morphogenetic role of cadherin cell adhesion molecules in human cancer: a thematic review. Cancer Invest. 1998;16:252–261. doi: 10.3109/07357909809039774. [DOI] [PubMed] [Google Scholar]
- Zondag GC, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity, and epithelial-mesenchymal transition. J Cell Biol. 2000a;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag GC, Reynolds AB, Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPmu binds to, and dephosphorylates the catenin p120(ctn) J Biol Chem. 2000b;275:11264–11269. doi: 10.1074/jbc.275.15.11264. [DOI] [PubMed] [Google Scholar]