Abstract
Antimicrobial resistance (AMR) is a worldwide concern among infectious diseases due to increased mortality, morbidity and treatment cost. According to WHO 2019 report, among the 32 antibiotics in the clinical trials, only six were classified as innovative and containing novel moiety. The remaining antibiotics from this list contain previously known moiety (WHO AMR 2019). Therefore, the development of novel antibiotics to control resistance problems is crucial. Benzothiazole derivatives are of great interest due to their wide range of biological activities and medicinal applications. Reported data indicated that benzothiazole derivatives displayed antibacterial activity by inhibiting the dihydroorotase, DNA gyrase, uridine diphosphate-n-acetyl enol pyruvyl glucosamine reductase (MurB), peptide deformylase, aldose reductase, casdihydrofolate reductase, enoyl acyl carrier protein reductase, dialkylglycine decarboxylase, dehydrosqualene synthase, dihydropteroate synthase and tyrosine kinase. The present review analyzed the synthesis, structure-activity relationship (SAR) and mechanism of action studies of benzothiazole derivatives as antibacterial agents reported by various research groups in the last five years (2018–2022). Different patents on the antimicrobial activity of benzothiazole derivatives have also been summarized. The finding of the present review will be beneficial for the researchers in the development of novel antibacterial molecules based on benzothiazole moiety.
Graphical Abstract
Keywords: Benzothiazole derivatives, Antibacterial activity, Structure-activity relationship, Synthesis, Metal complexes, Docking
Introduction
Antimicrobial drug resistance (AMR) withholds a pressing global challenge to the healthcare system in the expression of morbidity, mortality, and economic freight [1]. Predominantly, the speedy global spreading of multidrug-resistant bacteria (superbugs) causes non-treatable infections with existing antibiotics (WHO AMR 2015), which is a frightening challenge [2]. Centers for Disease Control and Prevention asserted that in the USA 23,000 deaths occur annually due to antimicrobial drug resistance and could be enhanced by millions in the coming eternity [3]. According to WHO 2019 report, among the 32 antibiotics in the clinical trials, only six were classified as innovative and containing novel moiety. The remaining antibiotics from this list contain previously known moiety [4]. Therefore, developing new antibiotics to manage AMR problem is vital. Among the various antimicrobial resistance mechanisms, modification in the molecular targets is one of the key mechanisms. The development of effective antimicrobial drug molecules is a challenging task due to the modification in the exploited molecular targets. Therefore, the discovery of novel unexploited targets is becoming a major path for researchers to prevent antibiotic resistance [5].
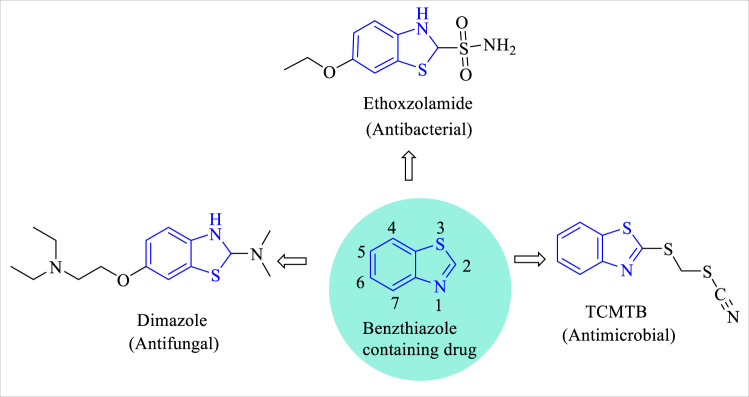
Benzothiazole is a bicyclic heterocyclic compound containing a 5-membered 1,3-thiazole ring fused with a benzene ring [6]. Their wide range of various biological activities is associated with the nature and positions of different substituents. A review of the literature concluded that different heteroaryl substitutions at the 2nd, 5th and 6th positions of benzothiazole moiety are very flexible in attaining diverse activities. Benzothiazole derivatives showed antimicrobial [7–9], anti-inflammatory [10, 11], antitumor [12, 13], anti-tubercular [14, 15], anti-HIV [16], anti-malarial [17–19], anticonvulsant [20], anti-helminthic [21, 22], anti-oxidant [23, 24] and analgesic activities [25, 26]. Ethoxzolamide, benzothiazol-2-ylthiomethyl thiocynate (TCMTB) and dimazole are the clinically approved antimicrobial drugs having benzothiazole moiety (Fig. 1) [27, 28].
Fig. 1.
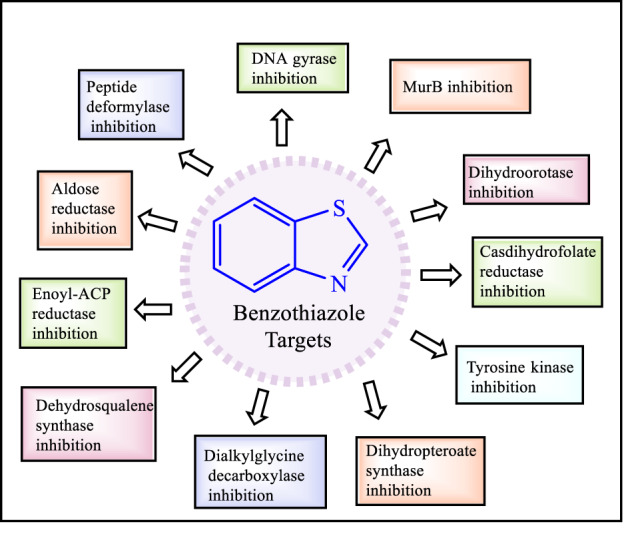
According to reported evidence, benzothiazole derivatives displayed a variety of antibacterial activities by inhibiting the dihydroorotase [29], DNA gyrase [30, 31], uridine diphosphate-n-acetyl enol pyruvyl glucosamine reductase (MurB) [32], peptide deformylase [33, 34], aldose reductase [35, 36], dihydrofolate reductase [37–39], enoyl acyl carrier protein reductase [40], casdihydrofolate reductase [41], dialkyl glycine decarboxylase [42, 43], dehydrosqualene synthase [44], dihydropteroate synthase [45] and tyrosine kinase [46, 47] (Fig. 2).
Fig. 2.
Different antibacterial targets of benzothiazole derivatives [29–47, 100]
Benzothiazole derivatives as antibacterial agents
Benzothiazole derivatives as antibacterial agents via uridine diphosphate-n-acetyl enol pyruvyl glucosamine reductase (MurB) inhibition
Maliyappa et al. (2022) synthesized antipyrine containing 6-substituted benzothiazole-based azo dyes and evaluated their antibacterial action (Scheme 1). Between the studied compounds, 4b depicted equipotent antibacterial activity in comparison to the standard drug streptomycin (MIC = 25–50 μg/ml) against Salmonella typhimurium and Klebsiella pneumonia. SAR study indicated that the presence of chloro group (4b) at the 5th location of the benzothiazole ring increased the antibacterial activity. The docking study depicted that compound 4a displayed the highest interaction (binding energy = −263.28 kcal/mol) with the target site of MurB enzyme (Fig. 2) and interaction was found to be significant as compared to standard drug streptomycin (Binding score = −289.65 kcal/mol) [32].
Scheme 1.
The chemical reaction involved in the synthesis of azo dyes based benzothiazole analogues (4a, b)
Tratrat et al. (2022) synthesized thiazolidin-4-one derivatives of benzothiazole as antibacterial agents (Scheme 2). Results showed that among the studied compounds, 8a, 8b, 8c and 8d were found to be the most active (MIC = 0.09–0.18 mg/ml) having comparable activity as standard drugs streptomycin (MIC = 0.05–0.1 mg/ml) and ampicillin (MIC = 0.2 mg/ml) against Pseudomonas aeruginosa, and Escherichia coli. SAR study indicated that the substitution of nitro (8a and 8c) and methoxy groups (8b and 8d) at the 4th position of the phenyl ring improved the antibacterial action. Docking analysis depicted that compounds 8a (binding energy = 10.21 kcal/mol) and 8d (binding energy = 10.08 kcal/mol) created the most stable complex with E. coli MurB enzyme [48].
Scheme 2.
The chemical reaction for the synthesis of thiazolidin-4-one derivatives of benzothiazole analogues (8a-d)
Haroun et al. (2021) synthesized and performed molecular docking of thiazolidinone derivatives of 2-aryl-3-(6-trifluoromethoxy) benzothiazole as an anti-bacterial agent (Scheme 3). Among the studied compounds, 11a and 11b exerted maximum antibacterial activity. Compound 11a indicated comparable activity (MIC = 0.10–0.25 mg/ml) to the standard drug streptomycin (MIC = 0.15 mg/ml) towards Listeria monocytogenes, P. aeruginosa, E. coli and Staphylococcus aureus. Compound 11b showed equivalent activity (MIC = 0.15 mg/ml) as reference compound against S. aureus and L. monocytogenes. SARs emphasized that the existence of 2,3-dichloro (11a) and 2,4-dichloro phenyl moieties (11b) linked with thiazole ring amplified the antibacterial activity. The molecular docking study depicted that the compound (11a) with a maximum in vitro activity showed the highest interaction (docking score = −10.74 kcal/mol) with the targeted enzyme E. coli MurB (Fig. 2) and formed the most stable complex among the studied derivatives. The fluoro atoms of the trifluoromethoxy moiety are involved in two hydrogen bonds with ARG A:326 and SER A:115 amino acid residues of the targeted enzyme. The keto group present on the 2nd position of the thiazole ring also formed H- bond with SER A:228 amino acid. The chloro atom on the phenyl ring (11a) interacted with PRO A:110 residue through carbon-hydrogen bond. Whereas, in compound 11b chloro group present on the para-position of the phenyl ring interacted with ALA A:123 through an alkyl bond [49].
Scheme 3.
Synthesis of thiazolidinone substituted of 2-aryl-3-(6-trifluoromethoxy) benzothiazole derivatives (11a-b)
Benzothiazole derivatives as antibacterial agents via DNA gyrase inhibition
Nehra et al. (2021) synthesized hybrids of 2- or 4-hydroxyphenyl benzothiazole derivatives and naphthalen-1-ol/8-hydroxyquinoline moieties linked through triazole ring and evaluated their antibacterial activity (Scheme 4). Compound 15 was found to be the most active (ZOI = 15.5–17.6 mm) out of the studied compounds, however less than reference molecule ciprofloxacin (ZOI = 22.3–24.1 mm) against all tested bacterial strains. SAR study illustrated that the substitution of the 8-hydroxyquinoline moiety at benzothiazole improved the antibacterial activity more than naphthalen-1-ol moiety. Substitution of ethyl aliphatic side chain (15) with triazole ring boosted the antibacterial activity. The docking study indicated that the compound with maximum activity also showed the highest interaction (binding affinity = −8.7 kcal mol−1) with targeted DNA gyrase B dodecamer. The oxygen and nitrogen atoms of 8-hydroxyquinoline interacted with DG2 through H-bonds, whereas the nitrogen atom of triazole formed H-bonds with DG4 [50].
Scheme 4.
Synthesis of naphthalen-1-ol and 8-hydroxyquinoline containing benzothiazole derivative (15)
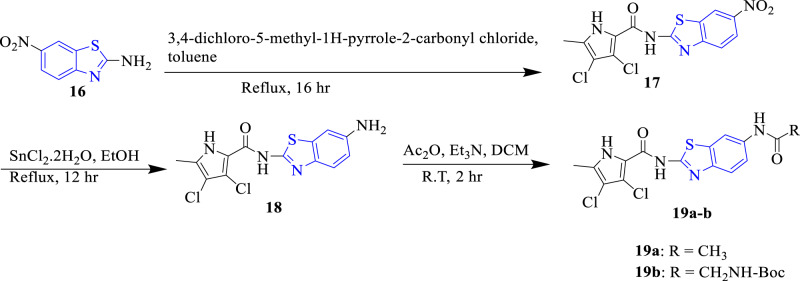
Skok et al. (2020) evaluated the antibacterial activity of benzothiazole derivatives as DNA gyraseB inhibitors (Scheme 5). Results showed that compounds 19a and 19b depicted the highest inhibition (MIC = 3.13 μM) among the screened derivatives and similar to the reference drug ciprofloxacin (MIC = 3.03 μM) against Enterococcus faecalis. DNA gyrase inhibition assay study indicated that 19a showed potent inhibition (IC50 = 9.5 nM) against DNA gyrase enzyme (Fig. 2). SARs deduced that acetamide and Boc-glycin moieties at the 5th position of the benzothiazole ring improved the antibacterial action [30].
Scheme 5.
The chemical reaction involved in the synthesis of carboxamide containing benzothiazole analogues (19a-b)
Ghannam et al. (2019) synthesized 2-arylbenzothiazole analogues and assessed their antibacterial potency (Scheme 6). Between the studied benzothiazole analogues, 25a, 25b and 25c (MIC = ~ 1 µM) showed excellent antibacterial activity in comparison with reference drug ciprofloxacin (MIC = 2.93 µM) against E. faecalis. Further, the studied compounds 25a (MIC = 2.03 µM), 25b (MIC = 1.04 µM) and 25c (MIC = 1.04 µM) showed good activity in comparison to the reference drug (MIC = 2.93 µM) against K. pneumoniae. SAR study indicated that the substitution of 4-hydroxy-3-methoxy (25a), 4-hydroxy (25b) and 3-hydroxy (25c) groups on the benzylidene moiety attached via hydrazine group increased the antibacterial activity. DNA gyrase inhibition assay demonstrated that among the studied compounds, 25a depicted the highest inhibitory action (IC50 = 4.85 μM) against the DNA gyrase enzyme (Fig. 2). However, activity was fewer than the reference drug ciprofloxacin (IC50 = 1.14 µM). Molecular docking study assessed that compound 25a has the maximum interaction (binding energy = −16.17 kcal/mol) with the targeted enzyme among the studied derivatives [51].
Scheme 6.
Synthesis of 2-arylbenzothiazole derivatives (25a-c)
Ghanavatkar et al. (2019) synthesized azo clubbed benzothiazole analogues and evaluated their antibacterial activity (Scheme 7). All the studied compounds showed very poor antibacterial activity (MIC = 312.5–1250 μg/ml) as compared to the reference drug ciprofloxacin (MIC = 50–25 μg/ml) against S. aureus and E. coli. Molecular docking study demonstrated that compound 31 formed the most stable complex (binding affinity = −7.353 kcal/mol) with DNA gyrase enzyme [52].
Scheme 7.
Synthesis of azo clubbed benzothiazole derivative (31)
Venkatesh et al. (2018) synthesized novel pyrimidine benzothiazole derivatives and studied their antibacterial activity (Scheme 8). Results showed that compounds 35d, 35e and 35 g depicted potent antibacterial action (ZOI = 17–19 mm) as compared to reference drug ciprofloxacin (ZOI = 14 mm) against S. aureus. Compound 35c indicated better inhibitory action (ZOI = 18 mm) between the evaluated derivatives and equipotent to the standard drug (ZOI = 16 mm) against Salmonella typhi. Compounds 35b, 35d and 35 h displayed equipotent activity (ZOI = 18 mm) as standard drug against E. coli. SAR study depicted that the substitution of 3-nitro (35a), 2,6-difluoro (35d), 4-hydroxy (35e) and 4-chloro (35 f) groups at the 4th position of the phenyl ring enhanced the antibacterial activity. Substitution of the halogen group at the benzothiazole moiety enhanced the activity. The most active compounds also displayed good receptor binding affinity (binding energy = −5.9 kcal/mol, −5.6 kcal/mol and −5.5 kcal/mol respectively) with target enzyme DNA gyrase in the docking study [53].
Scheme 8.
Synthesis of novel pyrimidine containing benzthiazole derivatives (35a-h)
Haroun et al. (2018) synthesized benzothiazole-based thiazolidinones compounds and assessed their antibacterial action (Scheme 9). Between the studied compounds 38a and 38b (MIC = 0.18–4.6 × 10−2 μmol/ml) showed better antibacterial action as compared to reference drug ampicillin (MIC = 24.80–74.4 × 10−2 μmol/ml) and streptomycin (MIC = 4.3–25 × 10−2 μmol/ml) against all the tested strains. SAR analysis revealed that unsubstitution (38a) and replacement of chloro group (38b) at the 3rd position of the phenyl ring enhanced the activity. Docking study showed that compounds 38a and 38b have maximum interaction (docking score = −9.0 kcal/mol) and formed a stable complex with targeted DNA gyrase B enzyme. The nitrogen atom of the intermediate chain and the hydrogen atom of the side chain formed hydrogen bond interactions with Thr165 (distance 3.01 Å). The keto group present in the thiazole ring also formed H-bond with Asn46 (distance 2.53 Å) [54].
Scheme 9.
Synthesis of benzothiazole-based thiazolidinones derivatives (38a-b)
Benzothiazole derivatives as antibacterial agents via peptide deformylase inhibition
Mishra et al. (2020) screened benzothiazole derivatives of isatin as antibacterial compounds (Scheme 10). The analysis of results displayed that benzothiazole clubbed isatin derivatives showed better antibacterial activity against Gram-negative bacterial strains rather than Gram-positive stains. Among the studied compounds, 41c indicated excellent antibacterial activity against E. coli (MIC = 3.1 μg/ml) and P. aeruginosa (MIC = 6.2 μg/ml), even more than reference drug ciprofloxacin (MIC = 12.5 μg/ml). Compound 41c also depicted equipotent activity (MIC = 12.5 μg/ml) as standard drug against Bacillus cereus and S. aureus. Compounds 41a and 41b depicted significant inhibition action (MIC = 6.2 μg/ml) in comparison to the reference drug ciprofloxacin (MIC = 12.5 μg/ml) against E. coli. SAR study assessed that the placement of the bromo group (41c) at the 5th position of the isatin ring enhanced the antibacterial activity against Gram-negative bacterial strains. Moreover, a molecular modeling study revealed that compound 41c has the highest interaction with the target enzyme peptide deformylase [55].
Scheme 10.
Synthesis of isatin containing benzothiazole derivatives (41a-c)
Benzothiazole derivatives as antibacterial agents via aldose reductase inhibitions
Kousaxidis et al. (2021) developed the non-acidic bifunctional benzothiazole-based thiazolidinones derivatives and assessed their antibacterial potency via aldose reductase inhibition assay (Fig. 2). Between the studied compounds, 42 depicted the highest aldose reductase inhibition (IC50 = 3.99 μM). SARs demonstrated that the presence of the chloro group at the 4th position of the benzylidene moiety improved the activity against tested strains and boosted the aldose reductase enzyme inhibition. Moreover, molecular docking analysis indicated that compound 42 also formed a stable complex (binding affinity = −7.34 kcal/mol) with the target site [56].
Benzothiazole derivatives as antibacterial agents via dihydroorotase inhibition
Morsy et al. (2020) performed in silico study and antimicrobial evaluation of benzothiazole derivatives. Compounds 43a and 43b displayed maximum inhibition (ZOI = 21–27 mm) against S. aureus, Bacillus subtilis and E. coli and activity was found to be better as compared to standard drug kanamycin (ZOI = 28–31 mm). SAR study demonstrated that the substitution of methyl (43a) and bromo (43b) groups at the 7th position of the benzothiazole ring enhanced the antibacterial action. Further, the substitution of hydroxy (43a) and nitro (43b) groups on phenyl moiety enhanced their antibacterial action. In silico study indicated that compound 43a has the highest interaction (docking score = −5.02 kcal/mol) with targeted dihydroorotase enzyme (Fig. 2), however, the interaction was less in comparison to reference compound HDDP (docking score = −7.37 kcal/mol) [29].
Benzothiazole derivatives as antibacterial agents via casdihydrofolate reductase inhibition
Suyambulingam et al. (2020) prepared amino-benzothiazole Schiff base analogues and evaluated their antibacterial activity (Scheme 11). Between the studied derivatives 46a and 46b were found to be the most active and exhibited equipotent antibacterial activity (MIC = 15.62 μg/ml) as compared to standard drug ciprofloxacin against E. coli and P. aeruginosa. SAR study indicated that the substitution of the hydroxyl group at the 2nd position of the benzylidene ring improved their antibacterial action. Docking study depicted that compound 46a (binding energy = −4.36 kcal/mol) and compound 46b (binding energy = −4.78 kcal/mol) formed the most stable complexes with E. coli and Lactobacillus casdihydrofolate reductase enzyme (Fig. 2). The OH group was found to be involved in the creation of hydrogen bond with the Tyr 100 residue of the targeted enzyme [41].
Scheme 11.
Synthesis of substituted 2-(imino) phenol clubbed benzothiazole Schiff analogues (46a-b)
Benzothiazole derivatives as antibacterial agents via enoyl acyl carrier protein reductase inhibition
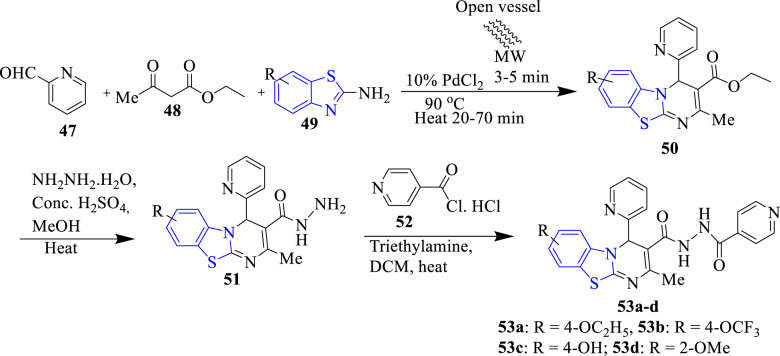
Bhoi et al. (2019) synthesized isoniazid containing 4H-pyrimido[2,1-b] benzothiazoles derivatives as antibacterial agents (Scheme 12). Among the studied compounds, 53a (ZOI = 25 ± 1.115 mm) and 53d (ZOI = 23 ± 1.112 mm) displayed excellent antibacterial activity and similar to reference drug streptomycin (ZOI = 24 mm) against Enterobacter aerogenes. Compound 53c (ZOI = 24 mm) indicated equipotent activity as compared to the standard drug against E. coli and B. cereus. SARs demonstrated that the substitution of 4-ethoxy (53a), 4-trifluoro methoxy (53b), 4-hydroxy (53c) and 2-methoxy (53d) groups at the benzothiazole ring improved the activity. Docking study indicated that compound 53b formed the most stable complex (binding energy = −9.077 kcal/mol) with targeted enoyl acyl carrier protein reductase enzyme (Fig. 2), among the studied compounds. Compound 53b formed four hydrogen bond interactions with Thr164, Thr164, His208, and Thr304 through -NH atoms of side chain of pyrimidine and pyridine rings [57].
Scheme 12.
Synthesis of isoniazid containing 4H-pyrimido[2,1-b] benzothiazoles derivatives (53a-d)
Benzothiazole derivatives as antibacterial agents via dialkylglycine decarboxylase inhibition
Mishra et al. (2019) synthesized Schiff base containing benzothiazole derivatives and evaluated their antibacterial activity (Scheme 13). Studied compounds, 56 and 59a-d (MIC = 0.4–0.8 μg/ml) showed good activity as compared to the reference drug ciprofloxacin (MIC = 1 μg/ml) against K. pneumonia. SAR analysis depicted that the presence of diethylamino group (59b) at the 4th location of the benzylidene ring augmented the antibacterial activity. Further, the substitution of nitro (59c) and bromo (59d) groups at the 3rd position of benzylidene moiety improved the antibacterial activity. The docking study indicated that compound 59c showed the highest inhibition against DNA gyrase of E. coli (docking score = −6.793 kcal/mol) and α-amylase of B. subtilis (docking score = −6.577 kcal/mol). Compound 59d also showed excellent interaction (docking score = −4.218 kcal/mol) with dialkyl glycine decarboxylase enzyme [42].
Scheme 13.
Synthesis of substituted 2-(((3-hydroxyphenyl) imino) methyl) phenol benzothiazole analogues (59a-d)
Benzothiazole derivatives as antibacterial agents via dehydrosqualene synthase inhibition
Gondru et al. (2018) synthesized pyrazole-thiazole hybrids of benzothiazole derivatives and assessed their antibacterial activity (Scheme 14). Antibacterial study indicated that compound 63a exhibited maximum activity (MIC = 1.9 μg/ml) and was found to be comparable to the reference drug ciprofloxacin (MIC = 0.9 μg/ml) against B. subtilis. SAR study assessed that substitution of a CH3 group (63a) at the 4th position of the phenyl ring increased the antibacterial activity. Molecular docking results depicted that compound 63b exhibited maximum interaction (binding affinity = −9.92 kcal/mol) with the targeted enzyme dehydrosqualene synthase (Fig. 2). Compound 63b showed two hydrogen bond interactions with Lys20 [43].
Scheme 14.
Synthesis of pyrazole-thiazole hybrids of benzothiazole derivatives (63a-b)
Benzothiazole derivatives as antibacterial agents via dihydropteroate synthase inhibition
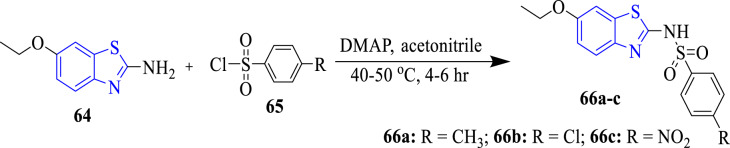
Naaz et al. (2018) synthesized sulfonamide analogues of benzothiazole and performed their antibacterial activity (Scheme 15). Compound 66c (MIC = 3.1–6.2 μg/ml) showed equipotent antibacterial activity as compared to reference drugs chloramphenicol and sulphamethoxazole against P. aeruginosa, S. aureus and E. coli. Further studied compounds 66b and 66c (MIC = 12.5 μg/ml) showed excellent antibacterial activity as compared to standard drug (MIC = 50 μg/ml) against mutant type E. coli. SARs depicted that the presence of chloro (66b) and nitro (66c) groups at the 4th position of the phenyl ring attached to sulfonamide group improved the antibacterial activity. Moreover, a molecular in silico study displayed that compounds 66a (binding energy = −9.17 kcal/mol) and 66c (binding affinity = −8.60 kcal/mol) depicted improved interactions with dihydropteroate synthase enzyme (Fig. 2). The NO2 group (66c) was found to be involved in the H-bonding with the target site [58].
Scheme 15.
Synthesis of benzothiazole sulfonamide derivatives (66a-c)
Benzothiazole derivatives as antibacterial agents via DNA gyrase and tyrosine kinase inhibition
Kumari et al. (2018) prepared conjugates of benzothiazole and N-acetyl-glucosamine as antibacterial agents (Scheme 16). Studied compounds, 72b and 72c showed equipotent antibacterial activity (MIC = 6.25 μg/ml) as reference drug ampicillin against S. aureus and E. coli. SAR study emphasized that the substitution of 4-chloro (72a), 4-methoxy (72b) and 6-nitro (72c) groups on the benzothiazole moiety enhanced their antibacterial activity. Docking study revealed that all the studied compounds formed complexes with targeted enzyme DNA gyrase and tyrosine kinase. Compound 72c (docking score = −9.59 kcal/mol) and 72a (docking score = −9.61 kcal/mol) showed maximum interactions with DNA gyrase and tyrosine kinase enzyme, respectively [59].
Scheme 16.
Preparation of bioconjugates of benzothiazole derivatives (72a-c)
Other miscellaneous benzothiazole derivatives as antibacterial agents
Mutairi et al. (2022) synthesized pyrimidine and pyrrole analogues of benzothiazole analogues and evaluated their activity against bacterial strains (Scheme 17). Among the studied compounds, 78a, 78b, and 80b were found to be most active. Compound 78a depicted better inhibition against Gram-positive bacterial strains (MIC = 8 µmol L−1 against S. aureus and MIC = 4 µmol L–1 against Streptococcus pneumonia) than Gram-negative bacterial strains and activity was higher than standard drug cefotaxime (MIC = 6–10 µmol L–1). Compound 78a also showed good activity (MIC = 5 µmol L–1) in contrast to the standard drug (MIC = 6 µmol L–1) against E. coli. Compound 78b indicated better antibacterial activity in contrast to the standard drug (MIC = 6–10 µmol L–1) towards Gram-positive bacterial strains (MIC = 6 µmol L–1 against S. aureus and MIC = 5 µmol L–1 against S. pneumonia) as well as Gram-negative bacterial strains (MIC = 10 µmol L–1 against Chlamydia pneumoniae and MIC = 5 µmol L–1 against E. coli). Compound 80b also deduced the potent antibacterial action against studied Gram-positive bacterial strains (MIC = 4–10 µmol L–1) and activity was found to be similar to the reference drug. SAR analysis proved that the inclusion of thiophene-2-yl (78a) and para-fluoro phenyl (78b and 80b) at the 4th position of pyridopyrimidine and pyrrole moieties enhanced the antibacterial activity [60].
Scheme 17.
Synthesis of pyrimidine and pyrrole containing benzothiazole derivatives (78a-b and 80a-b)
Catalano et al. (2021) synthesized benzothiazole analogues of phenyl urea and performed their antibacterial activity (Scheme 18). Out of the studied compounds, 83a demonstrated excellent activity (MIC = 8 μg/ml), which was 8 times higher than the reference compound TCC (MIC = 64 μg/ml) against E. faecalis. Compounds 83b and 83c exhibited two times more activity (MIC = 8 μg/ml) than reference compound triclocarban (MIC = 16 μg/ml) against S. aureus [61].
Scheme 18.
Chemical reaction involved in the preparation of phenyl urea clubbed benzothiazole analogues (83a-c)
Geesi et al. (2021) synthesised benzothiazole derivatives of pyrrolidine-2-ones using metal-doped TiO2 nano catalysts in an MX2/urea mixture and investigated their antibacterial activity (Scheme 19). The combination of pyrrolidine-2-ones with benzothiazole in MX2 mixture improved catalytic performance and antimicrobial activity. Among the studied compounds 88a, 88b, 88c, and 88d were found to be most active and exhibited equipotent inhibition as standard drug ciprofloxacin (MIC = 12.5 μg/ml) towards P. aeruginosa and E. coli. Compound 88c (MIC = 12.5 μg/ml) exerted excellent antibacterial action in contrast to reference drug (MIC = 18.75 μg/ml) against MRSA. SARs demonstrated that unsubstitution (88a) and substitution of chloro (88b), methyl (88c) and sulfonamide (88d) moieties at the 4thposition of phenyl ring (connected to benzothiazole via pyrrolidine-2-one) improved the antibacterial activity [62].
Scheme 19.
Preparation of substituted 1-phenylpyrolidinone containing benzothiazole derivatives (88a-e)
Zhang et al. (2021) prepared the 5-methylphenanthridium benzothiazoles analogues as antibacterial agents (Scheme 20). Compounds 98a and 98b (MIC = 1–4 μg/ml) showed maximum inhibition and better than reference drug ciprofloxacin (MIC = 4–16 μg/ml) against B. subtilis, Bacillus pumilus, S. aureus and Streptococcus pyogenes. SARs showed that existence of methyl group at the 9th (98a) and 10th (98b) positions of phenanthridium ring enhanced the antibacterial activity [63].
Scheme 20.
Synthesis of 5-methylphenanthridium benzothiazoles derivatives (98a-b)
Poonia et al. (2021) synthesized urea benzothiazole-triazole derivatives and evaluated their antibacterial activity (Scheme 21). Compound 101 showed maximum inhibition (MIC = 0.0071 µmol/ml) and also better than standard drug ciprofloxacin (MIC = 0.0094 µmol/mL) against Pseudomonas fluorescence. SAR study depicted that the existence of the p-OCH3 group at the phenyl urea moiety improved the antibacterial activity [64].
Scheme 21.
Preparation of urea benzothiazole-triazole derivative (101)
Durcik et al. (2021) synthesized dichloropyrazole-based benzothiazole analogues and assessed their antibacterial activity (Scheme 22). Studied compound, 104 depicted better inhibitory activity against Gram-positive strains (MIC = 0.0156–0.25 µg/mL) and Gram-negative strains (MIC = 1–4 µg/ml) as compared to standard drug novobiocin (MIC = 0.125–8 µg/ml). SARs deduced that the presence of methoxy thiophene-3-yl moiety at the 7th position of benzothiazole improved the antibacterial activity [65].
Scheme 22.
The chemical reaction involved in the preparation of 2-(3,4-dichloro-5-methyl-1H-pyrrole-2-carboxamido)-7-(methoxythiophen-3-yl)benzothiazole-5-carboxylic acid derivative (104)
Racane et al. (2021) synthesized and performed the antibacterial activity of 2,5-disubstituted furane benzimidazole and benzothiazole derivatives (Scheme 23). The results showed that benzothiazole compounds were found to be more active in comparison with benzimidazole derivatives. The antibacterial study demonstrated that compounds 107a (MIC = 12.5 μM), 107b (MIC = 1.6 μM), 107c (MIC = 6.25 μM) and 107d (MIC = 3.13 μM) showed potent antibacterial activity as compared to standard drug ampicillin (MIC = > 64 μM) against S. cerevisiae. SAR study indicated that the placement of isopropylamide (107a and 107c) and 4,5-dihydro-1H-imidazol-2-yl (107b and 107d) moieties at the 5th position of benzothiazole ring improved the antibacterial activity. The existence of nitro (107a and 107b) and chloro (107c and 107d) groups at the para-position of the phenyl ring improved the bacterial inhibition [66].
Scheme 23.
The chemical reaction involved in the preparation of 2,5-disubstituted furan benzothiazole derivatives (107a-d)
Basha et al. (2021) synthesized pyrimidinylbenzazolyl urea containing benzothiazole, benzoxazole and benzimidazole derivatives as antibacterial agents (Scheme 24). Results showed that benzothiazole derivatives depicted better antibacterial activity as compared to benzoxazole and benzimidazole derivatives. Compound 111 demonstrated better activity (ZOI = 40 ± 1.3 mm) in contrast to the reference drug chloramphenicol (ZOI = 34 mm) against B. subtilis. SARs demonstrated that the substitution of para-nitrophenyl moiety at the 3rd and 5th positions of the pyrimidine ring enhanced the bacterial inhibition [67].
Scheme 24.
Preparation of urea containing benzothiazole derivative (111)
Khaled et al. (2021) synthesized and assessed antibacterial activity of heterocycles comprising benzothiazole moiety (Schemes 25 and 26). Out of the screened compounds, 115 and 118 showed similar activity as the reference drug ampicillin (ZOI = 23–24 mm). Compound 115 demonstrated ZOI = 19 mm (S. aureus) and ZOI = 20 mm (E. coli), whereas compound 118 displayed ZOI = 10 mm (S. aureus) and ZOI = 9 mm (E. coli). SARs illustrated the that substitution of pyrano-2,3-pyrazole (115) and ethylene nitrile-amino benzoxazole (118) moieties at 2nd position of the benzothiazole ring improved the bacterial inhibition [68].
Scheme 25.
Preparation of 5-(benzothiazol-2-yl)-3-methyl-4-phenyl-1,4-dihydropyrano-2,3-pyrazol-6-amine derivative (115)
Scheme 26.
Synthesis of (E)-3-(4-amino-2-oxo-2H-chromen-3-yl)-2-(benzothiazol-2-yl) but-2-enenitrile analogue (118)
Daisy et al. (2020) synthesized 2-mercaptobenzothiazole derivatives from 2-bromomethyl mesitylene and 1,4-bis(bromomethyl) durene moieties (Scheme 27). Compounds 124 and 125 (ZOI = 9–11 mm) showed good antibacterial activity and similar to the reference compound gentamicin (ZOI = 11.5 mm) against B. subtilis and S. aureus. SAR study indicated the that substitution of 2,4,6-trimethyl (124) and 2,3,5,6-tetramethyl (125) moieties on the phenyl ring improved the antibacterial action [69].
Scheme 27.
The chemical reaction involved in the synthesis of 2-benzylthiobenzothiazole derivatives (124 and 125)
Racane et al. (2020) synthesized 2,6-disubstituted benzothiazole derivative and assessed their antibacterial activity (Scheme 28). Results showed that compounds, 130a, 130b and 130c were found to be the most active (MIC = 4 µg/ml) and showed the highest activity against Moraxella catarrhalis among the studied compounds. However, antibacterial effect was lower than the standard drug azithromycin (MIC = 0.06 µg/ml) [70].
Scheme 28.
Synthesis of 6-substituted-2- phenylbenzothiazole derivatives (130a-c)
Mishra et al. (2019) synthesized benzothiazole derivatives as antibacterial agents (Scheme 29). Compound 133 showed the highest antibacterial activity (MIC = 78.125 µg/ml) against S. aureus and E. coli among the studied derivatives. However, activity was found to be less than the reference drug ciprofloxacin (MIC = 25–50 µg/ml) [71].
Scheme 29.
Preparation of azo dyes containing benzothiazole derivative (133)
Evren et al. (2020) synthesized N-naphthalen-1-yl-propenamide benzothiazole derivatives and screened their antibacterial action. Studied compound 134 (MIC = 187.5 μg/ml) was found to be the most active out of the studied compounds against Yersinia enterocolitica, however, antibacterial activity was very less than standard drug chloramphenicol (MIC = 93.75 μg/ml) [72].
Wang et al. (2019) synthesized chalcone-based benzothiazole derivatives against resistant bacterial strains (Scheme 30). The antibacterial study indicated that compounds 138a, 138b and 138c showed excellent antibacterial activity (33–72% inhibition at 50 μg/cm3) as compared to standard drug bismerthiazole (28–49% inhibition at 50 μg/cm3) against Xoo, Xac, and Rs. SAR study indicated the hat substitution of heteroaryl moieties viz. pyridine-4-yl (138a) and furan-2-yl (138c) improved the antibacterial activity [73].
Scheme 30.
The chemical reaction involved in the preparation of chalcone-based benzothiazole analogues (138a-c)
Rao et al. (2018) synthesized benzothiazole-piperazine sulfonamide derivatives as antibacterial agents (Scheme 31). Among the studied compounds, 144a, 144b and 144c exhibited maximum antibacterial activity (MIC = 2.34–18.75 μg/ml) against all tested bacterial strains. However, effect was lower than the standard drug ciprofloxacin (MIC = 0.58 μg/ml) [74].
Scheme 31.
Synthesis of benzothiazole-piperazine sulfonamide derivatives (144a-c)
Cindric et al. (2018) synthesized 2-benzimidazolyl and 2-benzothiazolyl substituted benzothieno-2-carboxamide as antibacterial agents (Scheme 32). Compound 149 showed twice antibacterial activity (MIC = 8 μg/ml) as compared to the standard drug azithromycin (MIC = 16 μg/ml) against E. faecalis. SAR study indicated that the presence of an amino group and its conversion to salt form at the 5th position of the benzothiazole ring enhanced the activity [75].
Scheme 32.
Preparation of 2-benzothiazolyl substituted benzothieno-2-carboxamide salt analogues (149)
Al-Harthy et al. (2018) synthesized piperazine containing 2-arylbenzothiazole derivatives as antibacterial agents (Scheme 33). Out of the studied antibacterial compounds, 154a and 154b (MIC = 32 μg/ml) exhibited the highest bacterial growth inhibition against S. aureus. However, the affect was lower than the reference drug tamoxifen (MIC = 10 μg/ml) [76].
Scheme 33.
Chemical reaction for synthesis of piperazine containing benzothiazole derivatives (154a-b)
Saraswat et al. (2018) estimated the antibacterial effect of benzothiazole-thiophene derivatives (Scheme 34). The antibacterial study demonstrated that compound 159 was found to be the most active and depicted equipotent inhibition (MIC = 6.25 ± 0.27 μg/ml) as standard drug ciprofloxacin (MIC = 6.25 ± 0.60 μg/ml) against S. aureus. SARs demonstrated that the presence of electronegative chloro group at the 5th position of benzothiazole moiety enhanced the antibacterial activity [77].
Scheme 34.
Synthesis of benzothiazole-thiophene derivative (159)
Metal complexes of benzothiazole derivatives as antibacterial agents
Adeleke et al. (2021) synthesized silver (I) containing quinoline Schiff base as antibacterial agent (Scheme 35). Complex 161 (MIC = 1.6 µg/ml) exhibited maximum inhibition among screened compounds and showed eight times higher inhibition than reference drug ciprofloxacin (MIC = 25 µg/ml) against S. aureus. The inclusion of perchlorate anion with Ag atom improved the antibacterial activity [78].
Scheme 35.
Preparation of metal complex of silver (I) and quinoline clubbed benzothiazole derivative (161)
Shadap et al. (2021) performed an antibacterial evaluation of metal complexes of benzamide-benzothiazole derivatives (Scheme 36). Compound 163 (ZOI = 25 mm) showed most potent activity among studied compounds and equivalent to the reference drug kanamycin (ZOI = 24 mm) against Bacillus thuringiensis [79].
Scheme 36.
The chemical reaction for the synthesis of metal complexes of benzamide-benzothiazole derivative (163)
Noreen et al. (2021) assessed antibacterial activity of prepared amino benzothiazole-linked metal complexes (Scheme 37). The antibacterial study emphasized that activity increased on the complexation of the ligand molecules (167). However, all the screened compounds displayed lower activity (ZOI = 12–21 mm) in comparison to reference drugs azithromycin and cefixime (ZOI range = 30.5–32 mm) against tested bacterial strains [80].
Scheme 37.
The chemical reaction involved in the synthesis of amino benzothiazole-linked metal complexes (167)
Chakraborty et al. (2019) synthesised Cu (II) and Ag(I) complexes of 2,6-bis(benzothiazole)-pyridine derivatives as antibacterial agents. Complex 168 (ZOI = 27 mm) indicated good bacterial inhibitory action in contrast to standard compound AgNO3 (ZOI = 25 mm) against Staphylococcus epidermidis [81].The antibacterial results indicated that Ag-complexes showed improved bacterial inhibition as compared to Cu-complexes.
Daravath et al. (2019) synthesized and screened antibacterial activity of Cu (II) and Co (II) complexes of benzothiazole-based Schiff bases (Scheme 38). Compound 172 depicted the highest activity against B. amyloliquefaciens (MIC = 10 µg/ml), S. aureus (MIC = 9 µg/ml) and E. coli (MIC = 8 µg/ml) and activity was found to be better than reference drug streptomycin (MIC = 18 µg/ml). SARs deduced that the substitution of the iodine group at the 3rd position of benzylidene moiety improved the antibacterial activity [82].
Scheme 38.
The chemical reaction involved in the synthesis of amino benzothiazole containing complexes (172)
Mishra et al. (2019) synthesized benzothiazole-imino-benzoic acid metal complexes and examined their bacterial inhibition efficacy (Scheme 39). The outcomes revealed that metal complexes 175a and 175b exhibited excellent antibacterial efficacy (ZOI = 12–18 mm) as compared to standard drug streptomycin (ZOI = 8 mm) against L. monocytogenes [83].
Scheme 39.
Preparation of amino benzothiazole-imino-benzoic acid complexes (175a-b)
Gulab et al. (2018) assessed the antimicrobial activity of calcium complexes of 2-mercaptobenzothiazole and 1,10-phenanthroline (Scheme 40). The study showed that complex 179 exhibited a better antibacterial effect (ZOI = 25 mm) as compared to the reference compound levofloxacin (ZOI = 22 mm) towards P. aeruginosa [84]. Structure activity relationship of above-mentioned active benzothiazole derivatives have been presented in Fig. 3.
Scheme 40.
Synthesis of calcium complex of 2-mercaptobenzothiazole and 1,10-phenanthroline (179)
Fig. 3.
SAR of different substituted benzothiazole derivatives as antibacterial agent
Conclusion
Benzothiazole derivatives are promising antibacterial agents that can act against various bacterial targets viz. dihydroorotase, DNA gyrase, uridine diphosphate-n-acetyl enol pyruvyl glucosamine reductase (MurB), peptide deformylase, aldose reductase, casdihydrofolate reductase, enoyl acyl carrier protein reductase, dialkylglycine decarboxylase, dehydrosqualene synthase, dihydropteroate synthase, and tyrosinekinase etc. (Fig. 2). The present review summarized antibacterial effect of various substituted benzothiazole derivatives based on their mechanism of actions reported by different research groups in last 5 years. The results indicated that different aryl and heteroaryl substitutions at the 2nd position of benzothiazole moiety enhanced the antibacterial action against different strains such as, E. coli, E. faecalis, S. aureus, P. aeruginosa, S. pneumonia, P. fluorescence, L. monocytogenes etc (Fig. 3). The electron-withdrawing and electron releasing groups at the 5th and 6th positions of benzothiazole ring enhanced the antibacterial activity. In general, substitution of halogen groups viz, F, Cl and Br at the 5th position of benzothiazole ring significantly enhanced the antibacterial activity. Analysis of various mechanism of action studies showed that different aryl and heteroaryl substitutions at the 2nd position of benzothiazole ring formed most stable complexes with their target proteins/enzymes. Various types of antibacterial targets and their interactions with benzothiazole derivatives have been presented in Table 1. Benzothiazole metal complexes were also discussed and displayed good results in the improvement of bacterial inhibition. Silver (against S. aureus and S. epidermis) and Cadmium (against L. monocytogenes) metal complexes of benzothiazole derivatives showed potent antibacterial activity. Different patents on antimicrobial activity of benzothiazole derivatives were also summarized (Table 2).
Table 1.
Protein targets and interactions of benzothiazole derivatives as antibacterial agents
| S. No. | Best-docked compounds | Enzyme/Protein name | PDB id | H-bond interaction | References |
|---|---|---|---|---|---|
| 1 | 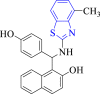 |
E. coli dihydroorotase | 2eg7 | H-bond formed between the OH group of naphthalene moiety and ASN44, OH group of phenyls with LEU222. | [85] |
| 2 | 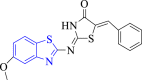 |
E. coli Mur B | 2Q85 | H-bond formed between NH and O group of thiazolidine ring and GLY123A, N atom of thiazolidine and benzothiazole ring and ARG214A, O atom of -OCH3 group and TYR190A. | [54] |
| 3 |  |
Biotin carboxylase | 3JZI | – | [86] |
| 4 | 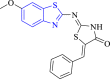 |
Aldose reductase | 4JIR | N atom of benzothiazole moiety formed H-bond with LEU 301 A. | [56] |
| 5 | 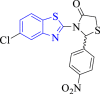 |
E. coli Mur B | 2Q85 | O atom of NO2 interacted with GLNA:287, S atom of benzothiazole interacted with ARGA:158 and O atom of thiazolidine ring formed H- bond with SERA:158 | [48] |
| 6 | 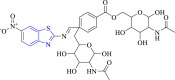 |
Dialkylglycine decarboxylase and S. aureus Gyrase | 2XCT and 1D7U | OH of pyrrole ring with DA H:13, TYR 20 and O atom of benzene ring interacted with GLN52, ARG 406 and LYS 272 via H-bond. | [52] |
| 7 | 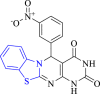 |
DNA gyrase | 2XCT | Nitro and both carboxyl groups formed H-bond with Asp437, Arg458, Gly459, Ser1085, Phe1123 | [53] |
| 8 | 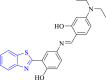 |
E. coli 24 kDa α-amylase | 1KZN and 1BAG | – | [87] |
| 9 | 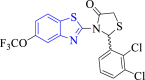 |
E. coli Mur B | 2Q85 | O-atom of OCF3 interacted with ARGA:326, F atom of OCF3 interacted with SERA:115, N atom of benzothiazole with ARGA:158 and O-atom of thiazolidine ring with SERA:228 through H-bonds. | [49] |
| 10 | 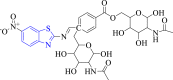 |
S. aureus DNA gyrase | 3TTZ | – | [59] |
| 11 | 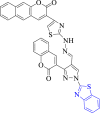 |
Dehydrosqualene synthase | 2ZCS | Both ketooxygens formed two H-bond with LYS 20 | [43] |
Table 2.
Patents on benzothiazole derivatives as antimicrobial agents
| Sr. No. | Title | Patent No. | Publication date | References |
|---|---|---|---|---|
| 1 | Novel benzothiazole derivatives with enhanced biological activity | WO2017025980 A1 | 2017 | [88] |
| 2 | Antibacterial benzothiazole derivatives | WO2016079688A | 2015 | [89] |
| 3 | Antibacterial composition | US20120004221A1 | 2012 | [90] |
| 4 | Substituted benzothiazoles as antifungal agents and their preparation, pharmaceutical compositions and use in the treatment of fungal infections. | CN103193770A | 2013 | [91] |
| 5 | Cycloalkylamidobenzoxazoles and benzothiazoles as fungicides and their preparation and use for fighting harmful microorganisms in plants. | EP2277869A1 | 2011 | [92] |
| 6 | Substituted benzoxazoles and benzothiazoles as fungicides and their preparation and use for fighting harmful microorganisms. | EP2277870A1 | 2011 | [93] |
| 7 | Synthesis of new benzothiazole derivatives as potential antitubercular agents | US20120095021 A1 | 2011 | [94] |
| 8 | Antibacterial composition | WO2009074810 A1 | 2009 | [95] |
| 9 | Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid | US005719172A | 1998 | [96] |
| 10 | Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid | US005494904A | 1996 | [97] |
| 11 | Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid. | WO1995008267A1 | 1995 | [98] |
| 12 | Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid | WO9508267A1 | 1995 | [99] |
Acknowledgements
We gratefully acknowledge Prof. Kiran Singh, Director, Institute of Pharmaceutical Sciences, Kurukshetra University, Kurukshetra and Honorable Vice-Chancellor, Prof. Som Nath Sachdeva, Kurukshetra University, Kurukshetra-136119, India, for providing their unconditional support and necessary facilities.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moutafchieva R, Mladenov D. Antimicrobial resistance: review. Trakia J Sci. 2020;18:01–404. doi: 10.15547/tjs.2020.04.015. [DOI] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Cli Microbio Inf. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SG, Adithan C, Harish BN, Sujatha S, Roy G, Malini A. Antimicrobial resistance in India: a review. J Nat Sci Biol Med. 2013;4:286–91. doi: 10.4103/0976-9668.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medelson M, Matsotso MP. Antimicrobial resistance national strategy framework, 2014–2024 in AMR CONTROL. 2015;1–136. www.globalhealthdynamics.co.uk.
- 5.Undale VR, Gupta S, Lakhadive K. Novel targets for antimicrobials. Turk J Pharm Sci. 2020;17:565–75. doi: 10.4274/tjps.galenos.2020.90197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booysen IN, Ismail MB, Akerman MP. N-[(E)-Thio-phen-2-yl-methyl-idene]-1,3-benzothia-zol-2-amine. Acta Crystallogr Sect E Struct Rep Online. 2012;68:1–6. 10.1107/S1600536812030498. [DOI] [PMC free article] [PubMed]
- 7.Prajapat P, Rathore KK, Gandhi D, Agarwal S, Hussain N, Talesara GL. A facile synthesis of biologically significant 2-(1,3-benzothiazol-2-ylimino)-1,3-thiazolidin-4-one / 3-(1,3-benzothiazol-2-yl)-2-thioxoimidazolidin-4-one Analogues from 1-(1,3-benzothiazol-2-yl)thiourea and their Alpha-hydroxylamine derivatives. Iran J Org Chem. 2016;8:1795–801. [Google Scholar]
- 8.Cano NH, Ballari MS, López AG, Santiago AN. New Synthesis and biological evaluation of benzothiazole derivates as antifungal agents. J Agric Food Chem. 2015;63:3681–6. doi: 10.1021/acs.jafc.5b00150. [DOI] [PubMed] [Google Scholar]
- 9.Patel RV, Park SW. Catalytic N-formylation for synthesis of 6-substituted-2-benzothiazolylimino-5-piperazinyl-4-thiazolidinone antimicrobial agents. Res Chem Intermed. 2015;41:5599–609. doi: 10.1007/s11164-014-1684-8. [DOI] [Google Scholar]
- 10.Jyothi M, Ranganatha VL, Khamees HA, Khadri MJN, Khanum SA. Design, synthesis, characterization and analysis of anti-inflammatory properties of novel N-(benzo[d]thiazol-2-yl)-2-[phenyl(2-(piperidin-1-yl) ethylamino] benzamides and N-(benzo[d]thiazol-2-yl)-2-[phenyl (2-morpholino) ethylamino] benzamides derivatives through in vitro and in silico approach. J Iran Chem Soc. 2022;1–13. 10.1007/s13738-022-02719-0.
- 11.Kumbhare RM, Kosurkar UB, Ramaiah MJ, Dadmal TL, Pushpavalli SNCLV, Pal-Bhadra M. Synthesis and biological evaluation of novel triazoles and isoxazoles linked 2-phenyl benzothiazole as potential anticancer agents. Bioorg Med Chem Lett. 2012;22:5424–7. doi: 10.1016/j.bmcl.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Hiyoshi H, Goto N, Tsuchiya M, Iida K, Nakajima Y, Hirata N, et al. 2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole suppresses tumor progression and metastatic potential of breast cancer cells by inducing ubiquitin ligase CHIP. Sci Rep. 2014;4:1–10. doi: 10.1038/srep07095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khedr MA, Zaghary WA, Elsherif GE, Azzam RA, Elgemeie GH. Purine analogs: synthesis, evaluation and molecular dynamics of pyrazolopyrimidines based benzothiazole as anticancer and antimicrobial CDK inhibitors. Nucleosides Nucleotides Nucleic Acids. 2022:1–29. 10.1080/15257770.2022.2109169. [DOI] [PubMed]
- 14.Hazra K, Nargund LVG, Rashmi P, Chandra JNNS, Nandha B, Harish MS. Synthesis and comparative study of anti-mycobacterium activity of a novel series of fluoronitrobenzothiazolopyrazolineregio isomers. Arch Pharm. 2012;345:137–46. doi: 10.1002/ardp.201100072. [DOI] [Google Scholar]
- 15.Mir F, Shafi S, Zaman MS, Kalia NP, Rajput VS, Mulakayala C, et al. Sulfur rich 2-mercaptobenzothiazole and 1,2,3-triazole conjugates as novel antitubercular agents. Eur J Med Chem. 2014;76:274–83. doi: 10.1016/j.ejmech.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Xu YS, Zeng CC, Jiao ZG, Hu LM, Zhong RG. Design, synthesis and anti-HIV integrase evaluation of 4-oxo-4H- quinolizine-3-carboxylic acid derivatives. Molecules. 2009;14:868–83. doi: 10.3390/molecules14020868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takasu K, Inoue H, Kim HS, Suzuki M, Shishido T, Wataya Y, et al. Rhodacyanine dyes as antimalarials. Prelim Eval Act Toxic J Med Chem. 2002;45:995–8. doi: 10.1021/jm0155704. [DOI] [PubMed] [Google Scholar]
- 18.Burger A, Sawhney SN. Antimalarials. III. Benzothiazole amino alcohols. J Med Chem. 1968;11:270–3. [DOI] [PubMed]
- 19.Aggarwal S, Paliwal D, Kaushik D, Gupta GK, Kumar A. Pyrazole schiffbase hybrids as anti-malarial agents: synthesis, in vitro screening and computational study. Comb Chem High Scr. 2018;21:194–203. doi: 10.2174/1386207321666180213092911. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui N, Rana A, Khan SA, Haque SE, Alam MS, Ahsan W, et al. Synthesis of 8-substituted-4-(2/4-substituted phenyl)-2H-[1,3,5]triazino[2, 1-b][1,3]benzothiazole-2-thiones and their anticonvulsant, anti-nociceptive, and toxicity evaluation in mice. J Enzym Inhib Med Chem. 2009;24:1344–50. doi: 10.3109/14756360902888176. [DOI] [PubMed] [Google Scholar]
- 21.Munirajasekhar D, Himaja M, Mali SV. A facile and efficient synthesis of 2-(5-(4-substitutedphenyl)-4, 5-dihydro-3-phenylpyrazol-1-yl)-6-substitutedbenzothiazoles and their biological studies. J Heterocycl Chem. 2014;51:459–65. doi: 10.1002/jhet.1618. [DOI] [Google Scholar]
- 22.Sarkar S, Dwivedi J, Chauhan R. Synthesis of 1-[2(substituted phenyl)-4-oxothiazolidin-3-yl]-3-(6-fluro-7-chloro-1,3-benzothiazol-2-yl)-ureas as anthelmintic agent. J Pharm Res. 2013;7:439–42. 10.1016/j.jopr.2013.05.008.
- 23.Gouda MA, Eldien HF, Girges MM, Berghot MA. Synthesis and antioxidant activity of novel series of naphthoquinone derivatives attached to benzothiophene moiety. Med Chem. 2013;3:2228–30. doi: 10.4172/2161-0444.1000143. [DOI] [Google Scholar]
- 24.Suresh C, Rao JV, Jayaveera KN, Reddy GJ. Synthesis of 2-hydrazinobenzothiazole-2-amino-(-4-substituted)-acetanilides for antioxidant activity. Int J Pharm Biomed Sci. 2011;1:2230–7605. [Google Scholar]
- 25.Praveen C, Nandakumar A, DheenkumarP, Muralidharan D, Perumal PT. Microwave-assisted one-pot synthesis of benzothiazole and benzoxazole libraries as analgesic agents. J Chem Sci. 2012;124:609–24. doi: 10.1007/s12039-012-0251-3. [DOI] [Google Scholar]
- 26.Kumar V, Sharma S, Husain A. Synthesis and in vivo anti-inflammatory and analgesic activities of Oxadiazoles clubbed with Benzothiazole nucleus. Int Curr Pharm Sci. 2015;12:457–61. doi: 10.3329/icpj.v4i12.25597. [DOI] [Google Scholar]
- 27.Rahman MM, Tikhomirova A, Modak JK, Hutton ML, Supuran CT, Roujeinikova A. Antibacterial activity of ethoxzolamide against Helicobacter pylori strains SS1 and 26695. Gut Pathog. 2020;12:1–7. doi: 10.1186/s13099-020-00358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salem ME, Darweesh AF, Shaaban MR, Elwahy AHM Synthesis of novel bis- and poly(hydrazinylthiazole) linked to benzofuran or benzothiazole as new hybrid molecules. Arkivoc. 2019: 73–88. 10.24820/ark.5550190.p010.810.
- 29.Morsy MA, Ali EM, Kandeel M, Venugopala KN, Nair AB, Greish K, et al. Screening and molecular docking of novel benzothiazole derivatives as potential antimicrobial agents. Antibiotics. 2020;9:1–15. doi: 10.3390/antibiotics9050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skok Z, Barančoková M, Benek O, Cruz CD, Tammela P, Tomašič T, et al. Exploring the chemical space of benzothiazole-based DNA Gyrase B inhibitors. ACS Med Chem Lett. 2020;11:2433–40. doi: 10.1021/acsmedchemlett.0c00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axford LC, Agarwal PK, Anderson KH, Andrau LN, Atherall J, Barker S, et al. Design, synthesis and biological evaluation of α-substituted isonipecotic acid benzothiazole analogues as potent bacterial type II topoisomerase inhibitors. Bioorg Med Chem Lett. 2013;23:6598–603. doi: 10.1016/j.bmcl.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 32.Maliyappa MR, Keshavayya J, Nazrulla MA, Sudhanva MS, Rangappa S. Six-substituted benzothiazole based dispersed azo dyes having antipyrine moiety: synthesis, characterization, DFT, antimicrobial, anticancer and molecular docking studies. J Iran Chem Soc. 2022;1–21. 10.1007/s13738-022-02569-w.
- 33.Takayama W, Shirasaki Y, Sakai Y, Nakajima E, Fujita S, Sakamoto-Mizutani K, et al. Synthesis and PDF inhibitory activities of novel benzothiazolylidenehydroxamic acid derivatives. Bioorg Med Chem Lett. 2003;13:3273–6. doi: 10.1016/S0960-894X(03)00675-9. [DOI] [PubMed] [Google Scholar]
- 34.Singh A, Srivastava R, Singh RK. Design, synthesis, and antibacterial activities of novel heterocyclic arylsulphonamidederivatives, Interdiscip. Science. 2018;10:748–61. doi: 10.1007/s12539-016-0207-2. [DOI] [PubMed] [Google Scholar]
- 35.Aotsuka T, Abe N, Fukushima K, Ashizawa N, Yoshida M. Benzothaizol-2-ylcarboxylic acid with diverse spacers: a noval class of potent, orally active aldose reductase inhibitors. Bioorg Med Chem Let. 1997;7:1677–82. doi: 10.1016/S0960-894X(97)00287-4. [DOI] [Google Scholar]
- 36.Zaher N, Nicolaou I, Demopoulos VJ. Pyrrolylbenzothiazole derivatives as aldose reductase inhibitors. J Enzym Inhib Med Chem. 2002;17:131–5. doi: 10.1080/1475636029002658. [DOI] [PubMed] [Google Scholar]
- 37.Tariq S, Kamboj P, Amir M. Therapeutic advancement of benzothiazole derivatives in the last decennial period. Arch Pharm. 2019;352:1–17. doi: 10.1002/ardp.201800170. [DOI] [PubMed] [Google Scholar]
- 38.Khilya OV, Milokhov DS, Kononets LA, Kobzar OL, Vovk AI, Volovenko YM. Synthesis and evaluation of new 2,6-diamino-5-hetarylpyrimidines as inhibitors of dihydrofolate reductase. Monatsh Chem. 2018;149:813–22. doi: 10.1007/s00706-017-2032-7. [DOI] [Google Scholar]
- 39.Priyadarsini R, Tharani CB, Suganya S, Kavitha S. Pharmacophore modeling and 3D-QSAR studies on substituted benzothiazole/benzimidazole analogues as DHFR inhibitors with antimycobacterial activity. Int J Pharm Sci. 2012;3:441–50. [Google Scholar]
- 40.Ozawa T, Kitagawa H, Yamamoto Y, Takahata S, Iida M, Osaki Y, et al. Phenylimidazole derivatives as specific inhibitors of bacterial enoyl-acyl carrier protein reductase FabK. Bioorg Med Chem. 2007;15:7325–36. doi: 10.1016/j.bmc.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Suyambulingam JK, Karvembu R, Bhuvanesh NSP, Enoch IVMV, Selvakumar PM, Premnath D, et al. Synthesis, structure, biological/chemosensor evaluation and molecular docking studies of aminobenzothiazole Schiff bases. J Adhes Sci Technol. 2020;34:2590–612. doi: 10.1080/01694243.2020.1775032. [DOI] [Google Scholar]
- 42.Mishra VR, Ghanavatkar CW, Mali SN, Chaudhari HK, Sekar N. Schiff base clubbed benzothiazole: synthesis, potent antimicrobial and MCF-7 anticancer activity, DNA cleavage and computational study. J Biomol Struct Dyn. 2020;38:1772–85. doi: 10.1080/07391102.2019.1621213. [DOI] [PubMed] [Google Scholar]
- 43.Gondru R, Sirisha K, Raj S, Gunda SK, Kumar CG, Pasupuleti M, et al. Design, synthesis, in vitro evaluation and docking studies of pyrazole-thiazole hybrids as antimicrobial and antibiofilm agents. Chem Sel. 2018;3:8270–6. doi: 10.1002/slct.201801391. [DOI] [Google Scholar]
- 44.Ekennia AC, Onwudiwe DC, Osowole AA, Okpareke OC, Olubiyi OO, Lane JR. Coordination compounds of heterocyclic bases: synthesis, characterization, computational and biological studies. Res Chem Intermed. 2019;45:1169–205. doi: 10.1007/s11164-018-3664-x. [DOI] [Google Scholar]
- 45.Azzam RA, Elboshi HA, Elgemeie GH. Synthesis, physicochemical properties and molecular docking of new benzothiazole derivatives as antimicrobial agents targeting DHPS enzyme. Antibiotics. 2022;11:1–21. doi: 10.3390/antibiotics11121799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahare HV, Talele GS. Designing of benzothiazole derivatives as promising EGFR tyrosine kinase inhibitors: a pharmacoinformatics study. J Biomol Struct Dyn. 2020;38:1365–74. doi: 10.1080/07391102.2019.1604264. [DOI] [PubMed] [Google Scholar]
- 47.Mokhtar AM, El-Messery SM, Ghaly MA, Hassan GS. Targeting EGFR tyrosine kinase: synthesis, in vitro antitumor evaluation, and molecular modeling studies of benzothiazole-based derivatives. Bioorg Chem. 2020;104:1–12. doi: 10.1016/j.bioorg.2020.104259. [DOI] [PubMed] [Google Scholar]
- 48.Tratrat C, Petrou A, Geronikaki A, Ivanov M, Kostić M, Soković M, et al. Thiazolidin-4-ones as potential antimicrobial agents: experimental and in silico evaluation. Molecules. 2022;27:1–25. doi: 10.3390/molecules27061930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haroun M, Tratrat C, Petrou A, Geronikaki A, Ivanov M, Ciric A, et al. 2-Aryl-3-(6-trifluoromethoxy)benzo[d]thiazole-based thiazolidinone hybrids as potential anti-infective agents: Synthesis, biological evaluation and molecular docking studies. Bioorg Med Chem Lett. 2021;32:1–12. doi: 10.1016/j.bmcl.2020.127718. [DOI] [PubMed] [Google Scholar]
- 50.Nehra N, Tittal RK, Ghule VD. 1,2,3-Triazoles of 8-Hydroxyquinoline and HBT: synthesis and studies (DNA binding, antimicrobial, molecular docking, ADME, and DFT. ACS Omega. 2021;6:27089–100. 10.1021/acsomega.1c03668. [DOI] [PMC free article] [PubMed]
- 51.Ghannam IAY, El-Meguid EAA, Ali HI, Sheir DH, Kerdawy AM. Novel 2-arylbenzothiazole DNA gyrase inhibitors: synthesis, antimicrobial evaluation, QSAR and molecular docking studies. Bioorg Chem. 2019;93:1–44. doi: 10.1016/j.bioorg.2019.103373. [DOI] [PubMed] [Google Scholar]
- 52.Ghanavatkar CW, Mishra VR, Mali SN, Chaudhari HK, Sekar N. Synthesis, bioactivities, DFT and in-silico appraisal of azo clubbed benzothiazole derivatives. J Mol Struct. 2019;1192:162–71. doi: 10.1016/j.molstruc.2019.04.123. [DOI] [Google Scholar]
- 53.Venkatesh T, Bodke YD, KuruniN, Kumar S. One-pot synthesis of novel substituted phenyl-1,5-dihydro-2Hbenzo[4,5]thiazolo[3,2-a]pyrimido[4,5-d]pyrimidine derivatives as potent antimicrobial agents. Med Chem. 2018;8:1–8. doi: 10.4172/2161-0444.1000488. [DOI] [Google Scholar]
- 54.Haroun M, Tratrat C, Kositzi K, Tsolaki E, Petrou A, Aldhubiab B, et al. New benzothiazole-based thiazolidinones as potent antimicrobial agents. Design, synthesis and biological evaluation. Curr Top Med Chem. 2018;18:75–87. doi: 10.2174/1568026618666180206101814. [DOI] [PubMed] [Google Scholar]
- 55.Mishra R, Chaurasia H, Singh VK, Naaz F, Singh RK. Molecular modeling, QSAR analysis and antimicrobial properties of Schiff base derivatives of isatin. J Mol Struct. 2021;1243:1–13. doi: 10.1016/j.molstruc.2021.130763. [DOI] [Google Scholar]
- 56.Kousaxidis A, Kovacikova L, Nicolaou I, Stefek M, Geronikaki A. Non-acidic bifunctional benzothiazole-based thiazolidinones with antimicrobial and aldose reductase inhibitory activity as a promising therapeutic strategy for sepsis. Med Chem Res. 2021;30:1837–48. doi: 10.1007/s00044-021-02778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhoi MN, Borad MA, Jethava DJ, Acharya PT, Pithawala EA, Patel CN, et al. Synthesis, biological evaluation and computational study of novel isoniazid containing 4H-Pyrimido[2,1-b]benzothiazoles derivatives. Eur J Med Chem. 2019;177:12–31. doi: 10.1016/j.ejmech.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 58.Naaz F, Srivastava R, Singh A, Singh N, Verma R, Singh VK, et al. Molecular modeling, synthesis, antibacterial and cytotoxicity evaluation of sulfonamide derivatives of benzimidazole, indazole, benzothiazole and thiazole. Bioorg Med Chem. 2018;26:3414–28. doi: 10.1016/j.bmc.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 59.Kumari B, Chauhan K, Trivedi J, Jaiswal V, Kanwar SS, Pokharel YR. Benzothiazole-based-bioconjugates with improved antimicrobial, anticancer and antioxidant potential. Chem Sel. 2018;3:11326–32. doi: 10.1002/slct.201801936. [DOI] [Google Scholar]
- 60.Al-Mutairi A, Hafez HN, El-Gazzar ARBA, Mohamed MYA. Synthesis and antimicrobial, anticancer and anti-oxidant activities of novel 2,3-dihydropyrido[2,3-d]pyrimidine-4-one and pyrrolo[2,1-b][1,3]benzothiazole derivatives via microwave-assisted synthesis. Molecules. 2022;27:1–18. doi: 10.3390/molecules27041246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catalano A, Rosato A, Salvagno L, Iacopetta D, Ceramella J, Fracchiolla G, et al. Benzothiazole-containing analogues of triclocarban with potent antibacterial activity. Antibiotics. 2021;10:1–12. doi: 10.3390/antibiotics10070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geesi MH, Ouerghi O, Dehbi O, Riadi Y. Metal-doped TiO2nanocatalysts in an MX2/urea mixture for the synthesis of benzothiazoles bearing substituted pyrrolidin-2-ones: Enhanced catalytic performance and antibacterial activity. J Environ Chem Eng. 2021;9:1–10. doi: 10.1016/j.jece.2021.105344. [DOI] [Google Scholar]
- 63.Zhang N, Song D, Chen W, Zhang S, Zhang P, Zhang N, et al. Modification of 5-methylphenanthridium from benzothiazoles to indoles as potent FtsZ inhibitors: Broadening the antibacterial spectrum toward vancomycin-resistant enterococci. Eur J Med Chem. 2021;224:1–17. doi: 10.1016/j.ejmech.2021.113723. [DOI] [PubMed] [Google Scholar]
- 64.Poonia N, Lal K, Kumar A, Kumar A, Sahu S, Baidya ATK, et al. Urea-thiazole/benzothiazole hybrids with a triazole linker: synthesis, antimicrobial potential, pharmacokinetic profile and in silico mechanistic studies. Mol Divers. 2022;26:2375–91. doi: 10.3390/molecules27061930. [DOI] [PubMed] [Google Scholar]
- 65.Durcik M, Nyerges A, Skok Z, Skledar DG, Trontelj J, Zidar N, et al. New dual ATP-competitive inhibitors of bacterial DNA gyrase and topoisomerase IV active against ESKAPE pathogens. Eur J Med Chem. 2021;213:1–22. doi: 10.1016/j.ejmech.2021.113200. [DOI] [PubMed] [Google Scholar]
- 66.Racané L, Zlatar I, Perin N, Cindrić M, Radovanović V, Banjanac M, et al. Biological activity of newly synthesized benzimidazole and benzothizole 2,5‐disubstituted furane derivatives. Molecules. 2021;26:1–21. doi: 10.3390/molecules26164935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basha NH, Babu NK, Shanker PS, Reddy GD, Padmaja A, Padmavathi V. Synthesis of pyrimidinylbenzazolyl urea derivatives as antimicrobial and antioxidant agents. Polycycl Aromat Compd. 2021;1–16. 10.1080/10406638.2021.1998153.
- 68.Mohamed KS, Elbialy EE, Fadda AA. Synthesis of novel heterocycles comprising benzothiazole moiety and their antimicrobial evaluations. Polycycl Aromat Compd. 2021;1–15. 10.1080/10406638.2021.1947332.
- 69.Daisy C, Asha RN, Kumar GSS, Vadivel E, Bhuvanesh N, Nayagam BRD. Experimental and theoretical studies of 2-Mercaptobenzothiazole with 2-Bromomethylmesitylene and 1,4-Bis(bromomethyl)durene. J Mol Struct. 2020;1222:1–13. doi: 10.1016/j.molstruc.2020.128894. [DOI] [Google Scholar]
- 70.Racané L, Ptiček L, Fajdetić G, Tralić-Kulenović V, Klobučar M, KraljevićPavelić S, et al. Green synthesis and biological evaluation of 6-substituted-2-(2-hydroxy/methoxy phenyl)benzothiazole derivatives as potential antioxidant, antibacterial and antitumor agents. Bioorg Chem. 2020;95:1–10. doi: 10.1016/j.bioorg.2019.103537. [DOI] [PubMed] [Google Scholar]
- 71.Mishra VR, Ghanavatkar CW, Mali SN, Qureshi SI, Chaudhari HK, Sekar N. Design, synthesis, antimicrobial activity and computational studies of novel azo linked substituted benzimidazole, benzoxazole and benzothiazole derivatives. Comput Biol Chem. 2019;78:330–7. doi: 10.1016/j.compbiolchem.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Evren AE, Yurttas L. Yılmaz-CankilicM.Synthesis of novel N-(naphthalen-1-yl)propanamide derivatives and evaluation their antimicrobial activity. Phosphorus Sulfur Silicon Relat Elem. 2020;195:158–64. doi: 10.1080/10426507.2019.1657428. [DOI] [Google Scholar]
- 73.Wang Y, Li P, Jiang S, Chen Y, Su S, He J, et al. Synthesis and antibacterial evaluation of novel chalcone derivatives containing a benzothiazole scaffold. Monatsh Chem. 2019:1–8. 10.1007/s00706-019-02399-2.
- 74.Rao BR, Katiki MR, Dileep K, Kumar CG, Reddy GN, Nanubolu JB, et al. Synthesis and biological evaluation of benzothiazole-piperazine sulfonamide conjugates and their antibacterial and antiacetylcholinesterase activity. Lett Org Chem. 2018;16:723–34. doi: 10.2174/1570178615666181113094539. [DOI] [Google Scholar]
- 75.Cindrić M, Perić M, Kralj M, Martin-Kleiner I, David-Cordonnier MH, Paljetak HC, et al. Antibacterial and antiproliferative activity of novel 2-benzimidazolyl- and 2-benzothiazolyl-substituted benzo[b]thieno-2-carboxamides. Mol Divers. 2018;22:637–46. doi: 10.1007/s11030-018-9822-7. [DOI] [PubMed] [Google Scholar]
- 76.Al-Harthy T, Zoghaib WM, Stoll R, Abdel-Jalil R. Design, synthesis, and antimicrobial evaluation of novel 2-arylbenzothiazole analogs bearing fluorine and piperazine moieties. Monatsh Chem. 2018;149:645–51. doi: 10.1007/s00706-017-2088-4. [DOI] [Google Scholar]
- 77.Saraswat P, Jeyabalan G, Hassan MZ, Ahsan MJ. Design, synthesis and biological evaluation of benzothiazole-thiophene hybrids: a new class of potent antimicrobial agents. Antiinfect Agents. 2018;16:57–63. doi: 10.2174/2211352515666171124155327. [DOI] [Google Scholar]
- 78.Adeleke AA, Zamisa SJ, Islam MS, Olofinsan K, Salau VF, Mocktar C, et al. Quinoline functionalized schiff base silver (I) complexes: Interactions with biomolecules and in vitro cytotoxicity, antioxidant and antimicrobial activities. Molecules. 2021;26:1–34. doi: 10.3390/molecules26051205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shadap L, Agarwal N, Chetry V, Poluri KM, Kaminsky W, Kollipara MR. Arene ruthenium, rhodium and iridium complexes containing benzamide derivative ligands: Study of interesting bonding modes, antibacterial, antioxidant and DNA binding studies. J Organomet Chem. 2021;937:1–13. doi: 10.1016/j.jorganchem.2021.121731. [DOI] [Google Scholar]
- 80.Noreen S, Sumrra SH. Aminothiazole-linked metal chelates: synthesis, density functional theory, and antimicrobial studies with antioxidant correlations. ACS Omega. 2021;6:33085–99. doi: 10.1021/acsomega.1c05290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakraborty I, Pinto M, Stenger-Smith J, Martinez-Gonzalez J, Mascharak PK. Synthesis, structures and antibacterial properties of Cu(II) and Ag(I) complexes derived from 2,6-bis(benzothiazole)-pyridine. Polyhedron. 2019;172:1–7. doi: 10.1016/j.poly.2019.02.001. [DOI] [Google Scholar]
- 82.Daravath S, Rambabu A, Vamsikrishna N, Ganji N, Raj S. Synthesis, structural characterization, antioxidant, antimicrobial, DNA incision evaluation and binding investigation studies on copper(II) and cobalt(II) complexes of benzothiazole cored Schiff bases. J Coord Chem. 2019;72:1973–93. doi: 10.1080/00958972.2019.1634263. [DOI] [Google Scholar]
- 83.Mishra N, Gound SS, Mondal R, Yadav R, Pandey R. Synthesis, characterization and antimicrobial activities of benzothiazole-imino-benzoic acid ligands and their Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes. Results Chem. 2019;1:1–21. doi: 10.1016/j.rechem.2019.100006. [DOI] [Google Scholar]
- 84.Gulab H, Shah Z, Mahmood M, Shah SR, Ali S, Iqbal M, et al. Synthesis, characterization and antibacterial activity of a new calcium complex using sodium 2-mercaptobenzothiazole and 1, 10-phenanthroline as ligands. J Mol Struct. 2018;1154:140–4. doi: 10.1016/j.molstruc.2017.10.045. [DOI] [Google Scholar]
- 85.Morsy MA, Ali EM, Kandeel M, Venugopala KN, Nair AB, Greish K, et al. Screening and molecular docking of novel benzothiazole derivatives as potential antimicrobial agents. Antibiotics. 2020;9:1–15. doi: 10.3390/antibiotics9050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ojaswi G, Divya N, Digna P, Mahajan SS. Design, synthesis and antimicrobial evaluation of N-phenylactylamido derivatives of heterocyclic compounds. Eur J Pharm Med Res. 2018;5:291–8. [Google Scholar]
- 87.Mishra VR, Ghanavatkar CW, Mali SN, Chaudhari HK, Sekar N. Schiff base clubbed benzothiazole: synthesis, potent antimicrobial and MCF-7 anticancer activity, DNA cleavage and computational study. J Biomol Struct Dyn. 2020;38:1772–85. doi: 10.1080/07391102.2019.1621213. [DOI] [PubMed] [Google Scholar]
- 88.Chauhan K, Kumari B. Novel benzothiazole derivatives with enhanced biological activity. WO 2017025980 A2, 2017.
- 89.Schmitt C, Survet JP, Chapoux G, Mirre A, Specklin JL. Antibacterial benzothiazole derivatives. WO2016079688A1, 2016.
- 90.Haydon D, Czaplewski LG, Palmer NJ, Mitchell DR, Atherall JF, Steele CR, Ladduwahetty T. Antibacterial composition. US20120004221A1, 2012.
- 91.Sheng C, Zhang W, Liu Y. Substituted benzothiazoles as antifungal agents and their preparation, pharmaceutical compositions and use in the treatment of fungal infections. CN103193770A, 2013.
- 92.Vor Die ELNN. Cycloalkylamidobenzoxazoles and benzothiazoles as fungizides. EP2277869A1, 2011.
- 93.Vor Die ELNN. Substituted benzoxa(thia)zole. EP2277870A1, 2011.
- 94.Ahmad K, Shetti R VCRNC, Swapna P, Azeeza S, Reddy AM, Khan IA, Abdullah ST, Sharma S, Kalia NP. Synthesis of new benzothiazole derivatives as potential anti-tubercular agents. US 2012O095021A1, 2012.
- 95.Haydon DJ, Czaplewski LG. Antibacterial composition. WO2009074810A1, 2009.
- 96.Oppong D, Hollis CG. Synergistic antimicrobial composition containing (thiocyanomethylthio)benzothiazole and an organic acid. US005719172A, 1998.
- 97.Oppong D, Hollis CG. Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid. US005494904A, 1996.
- 98.Oppong D, Hollis CG. Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid. WO1995008267A1, 1995.
- 99.Oppong D, Hollis CG. Synergistic antimicrobial composition containing 2-(thiocyanomethylthio)benzothiazole and an organic acid. WO9508267A1, 1995.
- 100.Rakowitz D, Hennig B, Nagano M, Steger S, Costantino L, Matuszczak B. Synthesis of novel benzoic acid derivatives with benzothiazolyl subunit and evaluation as aldose reductase inhibitors. Arch Pharm. 2005;338:411–8. doi: 10.1002/ardp.200500101. [DOI] [PubMed] [Google Scholar]