Abstract
Varicella zoster virus (VZV) establishes lifelong infection after primary disease and can reactivate. Several drugs are approved to treat VZV diseases, but new antivirals with greater potency are needed. Previously, we identified β-l-5-((E)-2-bromovinyl)-1-((2S,4S)-2-(hydroxymethyl)-1,3-(dioxolane-4-yl))uracil (l-BHDU, 1), which had significant anti-VZV activity. In this communication, we report the synthesis and evaluation of numerous l-BHDU prodrugs: amino acid esters (14–26), phosphoramidates (33–34), long-chain lipids (ODE-l-BHDU-MP, 38, and HDP-l-BHDU-MP, 39), and phosphate ester prodrugs (POM-l-BHDU-MP, 41, and POC-l-BHDU-MP, 47). The amino acid ester l-BHDU prodrugs (l-phenylalanine, 16, and l-valine, 17) had a potent antiviral activity with EC50 values of 0.028 and 0.030 μM, respectively. The phosphate ester prodrugs POM-l-BHDU-MP and POC-l-BHDU-MP had a significant anti-VZV activity with EC50 values of 0.035 and 0.034 μM, respectively, and no cellular toxicity (CC50 > 100 μM) was detected. Out of these prodrugs, ODE-l-BHDU-MP (38) and POM-l-BHDU-MP (41) were selected for further evaluation in future studies.
Introduction
Varicella zoster virus (VZV), an alphaherpesvirus, causes chickenpox (varicella) on primary infection and shingles (zoster) upon reactivation from latency.1 Currently, vaccination is available with a live attenuated strain for both stages of VZV disease,2 and an adjuvant subunit vaccine is approved to prevent shingles.3 While pediatric vaccination has reduced the incidence of chickenpox, shingles remains prevalent. According to the Centers for Disease Control and Prevention,4 there are an estimated one million cases of zoster annually in the U.S. Those at highest risk are people over the age of 50, transplant recipients, people living with human immunodeficiency virus (HIV), and anyone who is immunocompromised. When VZV reactivates in the skin, it causes a painful, vesicular rash that contains abundant infectious virus. Antiviral therapy is most effective when given within three days of the appearance of the rash, and there is evidence that prompt antiviral treatment can reduce acute pain, speed healing, reduce virus shedding, and lower the incidence of herpetic neuropathic pain.5 A major complication of shingles is postherpetic neuralgia, which is a neuropathic pain that persists for months to years after the rash heals.6 There is a compelling need for improved antivirals for the treatment of VZV infections that are more effective and safe.
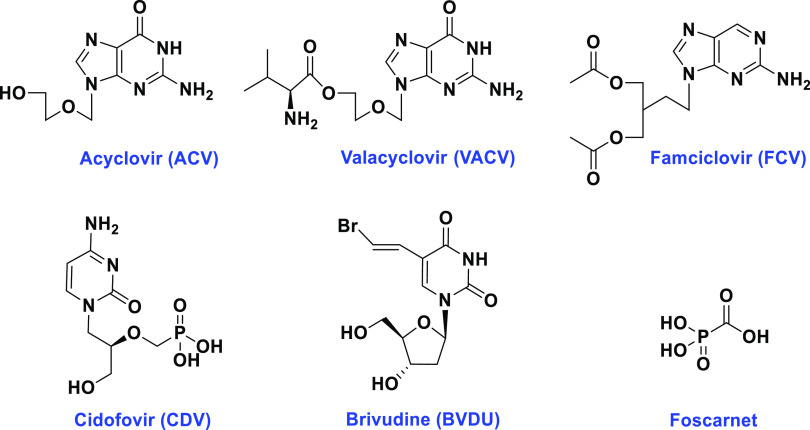
Currently, several nucleoside analogues are used to treat VZV infections, including acyclovir (ACV), valacyclovir (VACV), and famciclovir (FCV) (Figure 1).7 These nucleoside analogues act on the viral DNA polymerase to disrupt viral DNA synthesis. They are active in their triphosphate form, requiring the VZV thymidine kinase (TK) and cellular enzymes for activation.8 These drugs are not highly effective against VZV, large doses are required, and long-term use is associated with the development of drug resistance.9 Cidofovir (CDV, Figure 1), a broad-spectrum nucleotide analogue, is active against VZV. Unfortunately, nephrotoxicity and lack of oral bioavailability restrict CDV use as a first-line treatment. In selected cases, CDV may be used off-label to treat acyclovir-resistant VZV.8 Foscarnet (pyrophosphate analogue, Figure 1) is another second-line treatment; however, it is associated with many deleterious side effects.10
Figure 1.
Structure of antiviral drugs for the treatment of VZV.
Another class of compounds discovered to treat VZV infections is the cyclic derivatives of uridine. Trifluridine and idoxuridine are used primarily as topical treatments for herpes zoster ophthalmicus.11 The bromovinyl analogue, brivudine (BVDU, E-5-(2-bromovinyl)-2′-deoxy uridine, Figure 1), has been approved in Europe for the treatment of VZV infections and has potent anti-herpes activity.12 Similar to the nucleoside analogues, BVDU must be converted by the viral TK into the 5′-monophosphate and diphosphate forms. Cellular kinases then perform the final conversion step into the active 5′-triphosphate form (BVDU-TP). BVDU-TP selectively interacts with the viral DNA polymerase as a competitive inhibitor, where it is incorporated into viral DNA, leading to chain termination. BVDU has a better anti-VZV activity profile than acyclovir or its prodrug, thus requiring a smaller dose. Additionally, BVDU can be administered orally once a day, making it more appealing than other drugs used for the treatment of VZV infections. The major drawback associated with BVDU is that it is catabolized in the liver into bromovinyluracil (BVU).13 BVU inhibits dihydropyrimidine dehydrogenase (DPD), which degrades thymidine and uracil. The anticancer drug 5-fluorouracil (5-FU) is catabolized by DPD, thus causing a harmful drug interaction with brivudine.14 Due to the adverse effects associated with currently prescribed drugs to treat VZV infections, there is a critical need for new antivirals that are safe and effective against VZV and its resistant strains.
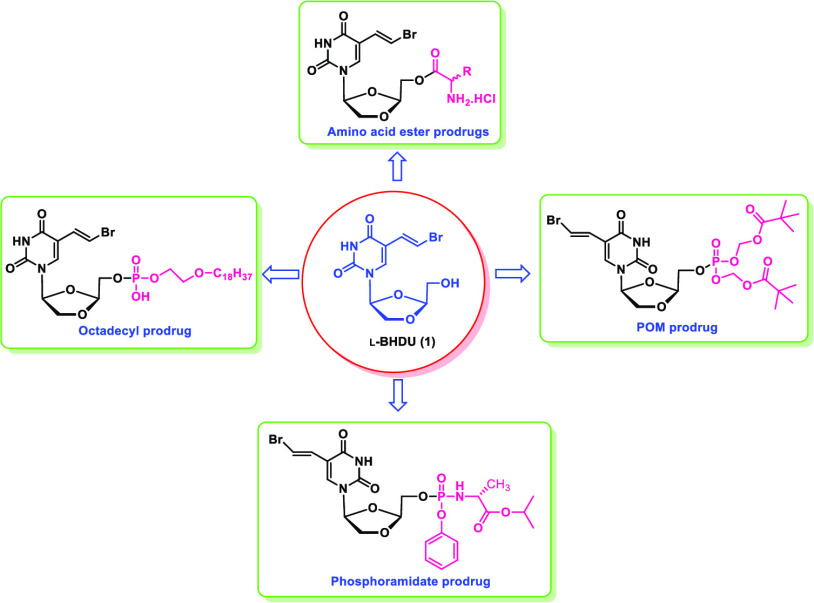
We reported a uridine derivative, β-l-5-((E)-2-bromovinyl)-1-((2S,4S)-2-(hydroxymethyl)-1,3-(dioxolane-4-yl))uracil (l-BHDU), as a potent and safe anti-VZV agent.15 In human foreskin fibroblasts (HFFs), l-BHDU was effective against VZV with an EC50 value of 0.25 μM. It was not cytotoxic in HFFs up to 200 μM, yielding a selectivity index (SI) of >909.16In vivo, l-BHDU significantly reduced VZV growth and spread compared to ACV and VACV. Metabolic studies indicated that 5-FU did not accumulate in mice treated with l-BHDU.16 It is likely that l-BHDU does not inhibit DPD and would have a better safety profile than brivudine. To increase cellular bioavailability and uptake of l-BHDU, we developed an amino acid ester prodrug, l-BHDU-l-valine, which had enhanced anti-VZV activity compared to its parent molecule. l-BHDU-l-valine had an EC50 value of 0.03 μM with a CC50 of >200 μM.16 Encouraged by these findings and to enhance the pharmacokinetic (PK) profile of l-BHDU, we explored various prodrug approaches to improve the potency of l-BHDU against VZV. Here, we describe the synthesis and antiviral evaluation of various l-BHDU prodrugs, including thirteen 5′-amino acid ester prodrugs (14–26), two phosphoramidate prodrugs (33 & 34), two long-chain phospholipid prodrugs, an octadecyloxyethyl prodrug of l-BHDU monophosphate (ODE-l-BHDU-MP, 38) and a hexadecyloxypropyl prodrug of l-BHDU monophosphate (HDP-l-BHDU-MP, 39), and two phosphate ester prodrugs (POM-l-BHDU-MP, 41, and POC-l-BHDU-MP, 47).
Results and Discussion
The development of 5′-amino acid ester prodrugs based on nucleoside analogues is well-established and known to reduce cytotoxicity.17 We previously showed that inserting an amino acid as part of the prodrug strategy enhanced bioavailability and carrier-mediated cell transport while lowering the polarity of the standard nucleoside.18 Furthermore, the phosphoramidate, phosphate esters, and long-chain phospholipid prodrugs are all known to increase the antiviral potency of nucleosides while overcoming the rate-limiting first step, monophosphorylation. Chemical and enzymatic mechanisms release nucleoside monophosphate (NMP).19 For instance, the 1-O-hexadecyloxypropyl and 1-O-octadecyloxyethyl prodrugs of CDV are more active against DNA viruses than the parent molecule.20 Due to their lipophilic nature, phospholipid prodrugs exhibit enhanced cellular uptake and oral bioavailability, and they inhibit viral replication more efficiently than standard CDV.21 However, VZV infects many human cell types and establishes latency in nerve cells. Unfortunately, l-BHDU was not lipophilic enough to penetrate nerve cells; thus, we synthesized the octadecyloxyethyl-l-BHDU-MP (ODE-l-BHDU-MP, 38) and hexadecyloxypropyl-l-BHDU-MP (HDP-l-BHDU-MP, 39) prodrugs. Esterification of l-BHDU-MP with octadecyloxyethyl (ODE) or hexadecyloxypropyl (HDP) was performed to increase its bioavailability and cell penetration, as they resemble phospholipids of the cell membrane. It was expected that the addition of these long hydrocarbon chains would improve the uptake and intracellular transport of the compounds.
To date, the FDA has approved adefovir dipivoxil [bis(pivaloyloxymethyl), POM]22,23 and tenofovir disoproxil fumarate [bis(isopropyloxymethyl carbonate), POC]24 for the treatment of hepatitis B virus (HBV) and human immunodeficiency virus (HIV) infections, respectively. The lipophilic nature of POM or POC groups may enhance the bioavailability and cellular uptake of l-BHDU.25 During the metabolism of adefovir and tenofovir prodrugs, the first POM ester group is degraded and forms an unstable hydroxymethyl alcoholate intermediate that undergoes chemical rearrangement and releases formaldehyde. Next, the second POM ester group is cleaved to generate free NMP.26 Similarly, POC prodrugs are also metabolized by enzymatic degradation. Carbonates of POC are decomposed by esterase to produce an unstable carboxylate intermediate that results in the sequential release of carbon dioxide and formaldehyde to produce a free NMP.27 Following these strategies, we synthesized POM-l-BHDU-MP (41) and POC-l-BHDU-MP (47) prodrugs for evaluation against VZV.
Chemistry
Synthesis of l-BHDU was performed as per our reported standard protocol.15 The chemical structures of the newly synthesized amino acid ester prodrugs of l-BHDU (14–25) are shown in Scheme 1. To synthesize the targeted amino acid ester prodrugs (14–25), condensation of l-BHDU was carried out with appropriate Boc-protected d- or l- amino acids. l-BHDU was stirred with the Boc-protected amino acids in the presence of catalytic 4-(dimethylamino)pyridine (DMAP) and the coupling agent 1,3-diisopropylcarbodiimide (DIC) at room temperature (rt) to produce a coupled intermediate (2–13) (60–85% yield). The Boc group was removed from compounds 2–13 by treatment with 2 M trifluoro acetic acid (TFA) in dichloromethane (DCM), followed by 1 M HCl solution in the ether, resulting in a targeted amino acid ester HCl salt (14–25) of l-BHDU (80–90% yield).
Scheme 1. Synthesis of 5′-O-Amino Acid Ester Prodrugs of l-BHDU.
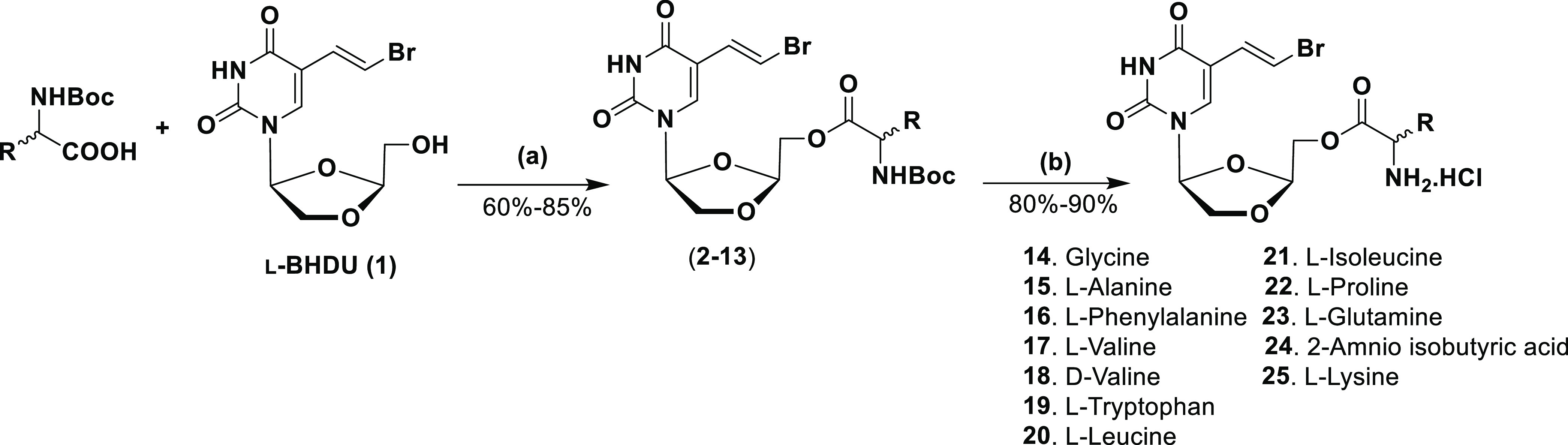
Reagents and conditions: (a) DIC, DMAP, DCM, rt, 12 h; (b) 2 M TFA in DCM, 0 °C to rt, 1 h; 1 M HCl in ether, MeOH, 0 °C to rt, 1 h.
To synthesize the tyrosine amino acid ester l-BHDU prodrug (Scheme 2), we started with the N-Boc-protected l-tyrosine amino acid (27), where the phenol of tyrosine was protected with the tert-butyldimethylsilyl chloride (TBDMSCl) in the presence of imidazole in N,N-dimethylformamide (DMF) at rt to give intermediate 28 (62% yield). Intermediate 28 was then condensed with l-BHDU via a coupling reaction in the presence of DIC at rt to afford the coupled product 29 (80% yield). Next, TBDMS deprotection of 29 was performed in a 1 M tetrabutylammonium fluoride (TBAF) solution in tetrahydrofuran (THF) to produce intermediate 30 (78% yield). Finally, deprotection of Boc was performed by treating intermediate 30 with 2 M TFA solution in DCM followed by treatment with 2 M HCl solution in ether to produce l-BHDU-l-tyrosine amino acid ester as HCl salt, 26 (85% yield).
Scheme 2. Synthesis of 5′-O-l-Amino Acid Tyrosine Ester Prodrugs of l-BHDU.
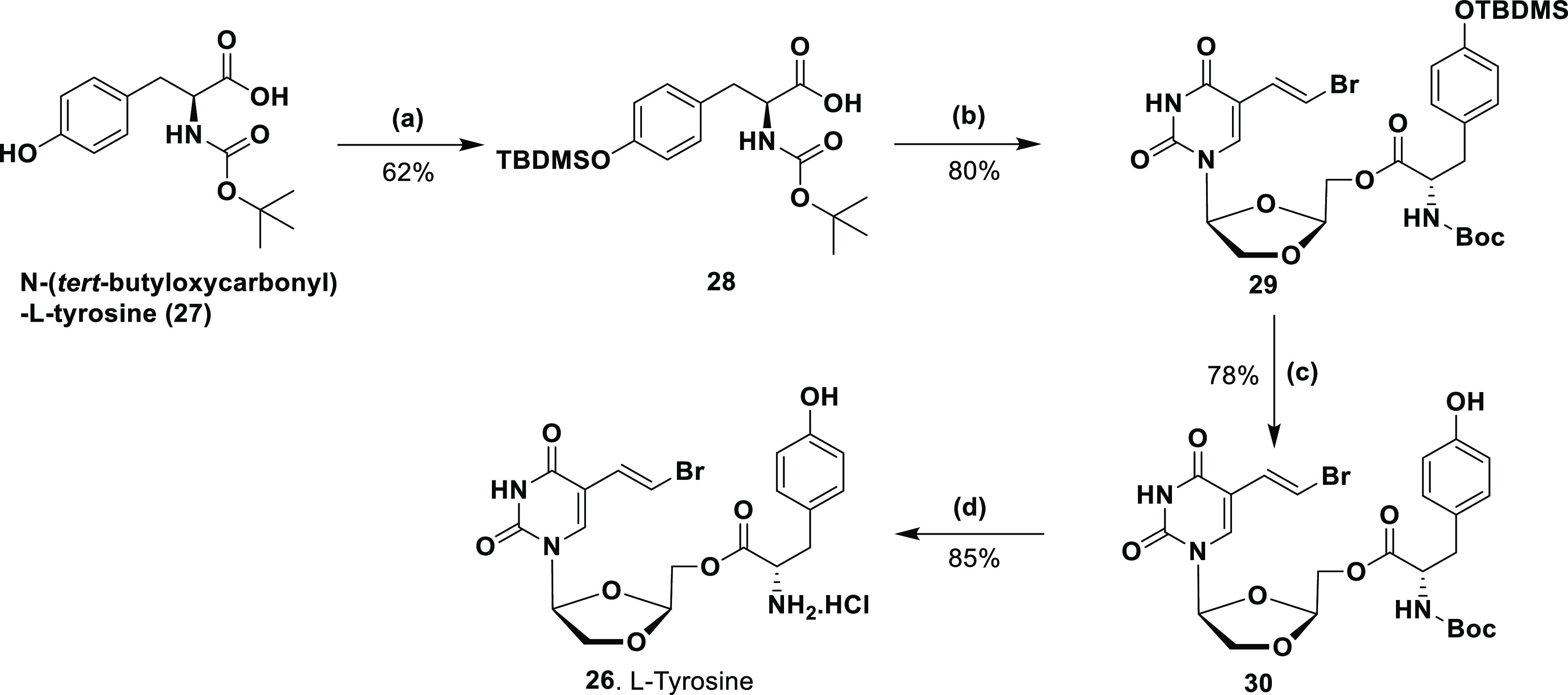
Reagents and conditions: (a) TBDMSCl, imidazole, DMF, rt, 16 h; (b) l-BHDU, DIC, DMAP, DCM, rt, 12 h; (c) 1 M TBAF solution in THF, THF, 0 °C—rt, 3 h; (d) 2 M TFA in DCM; 2 M HCl in ether, MeOH, 0 °C—rt; 1.5 h.
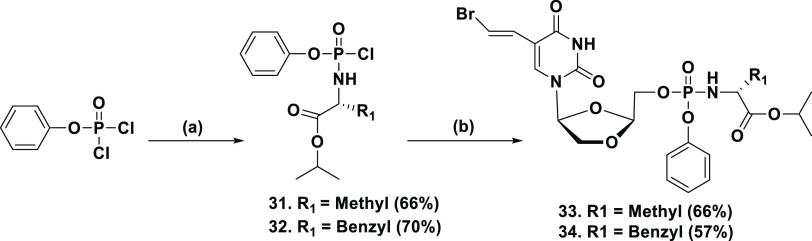
To bypass first-step rate-limiting monophosphorylation, McGuigan introduced the concept of phosphoramidate prodrugs consisting of an amino ester moiety attached via a P–N bond formation to a nucleoside aryl phosphate.28 Mechanistically, aryloxy phosphoramidate prodrugs release NMP intracellularly by chemical and enzymatic degradation. Phosphoramidate prodrugs have enhanced pharmacokinetic properties with better cellular uptake and bioavailability. Following this strategy, phosphoramidate prodrugs of l-BHDU were prepared according to our reported protocol.29 First, phosphorochloridate intermediates of the aryl alkoxy-amino ester (31 & 32) were synthesized. Phenyl dichlorophosphate was treated with l-alanine isopropyl ester hydrochloride or l-phenylalanine isopropyl ester hydrochloride in the presence of triethylamine (Et3N) in DCM at −78 °C to render intermediates 31 and 32, respectively (60–70% yield, Scheme 3). Next, condensation of phosphorochloridate intermediates (31 & 32) was carried out with l-BHDU in the presence of N-methylimidazole (NMI) in THF at 0 °C to rt to furnish phosphoramidate prodrugs 33 & 34 (50–65% yield).
Scheme 3. Synthesis of Phosphoramidate Prodrugs of l-BHDU.
Reagents and conditions: (a) l-alanine isopropyl ester hydrochloride or l-phenylalanine isopropyl ester hydrochloride, Et3N, DCM, −78 °C—rt, 3 h; (b) l-BHDU (1); NMI, THF 0 °C—rt, 12 h.
The octadecyloxyethyl prodrug of l-BHDU monophosphate (ODE-l-BHDU-MP, 38) and the hexadecyloxypropyl prodrug of l-BHDU monophosphate (HDP-l-BHDU-MP, 39) prodrugs were synthesized according to our standard protocol.30 These long-chain lipid phosphates of l-BHDU monophosphate (l-BHDU-MP) were constructed using a phosphotriester approach (Scheme 4). First, l-BHDU was condensed with 2-chlorophenyl dichlorophosphate (35) in the presence of 1,2,4-triazole and Et3N to yield the coupled intermediate. In situ, without further purification, the intermediate was treated with the appropriate long-chain lipid alcohol (3-hexadecyloxy-1-propanol or 2-octadecyloxy-1-ethanol) in the presence of NMI in THF at rt to afford a fully protected corresponding intermediate (36 & 37, 67–69% yield). The phosphotriester intermediates 36 & 37 had two closely distinguished signals in the 31P NMR, corresponding to the two diastereoisomers. This was also apparent from the 1H NMR spectroscopy. Next, the 2-chlorophenyl group of each phosphotriester 36 & 37 was removed using 0.5 N NaOH in THF at 50 °C to yield prodrugs 38 and 39 (74–85% yield).
Scheme 4. Synthesis of Long-Chain Lipid Phosphate Prodrugs of l-BHDU.
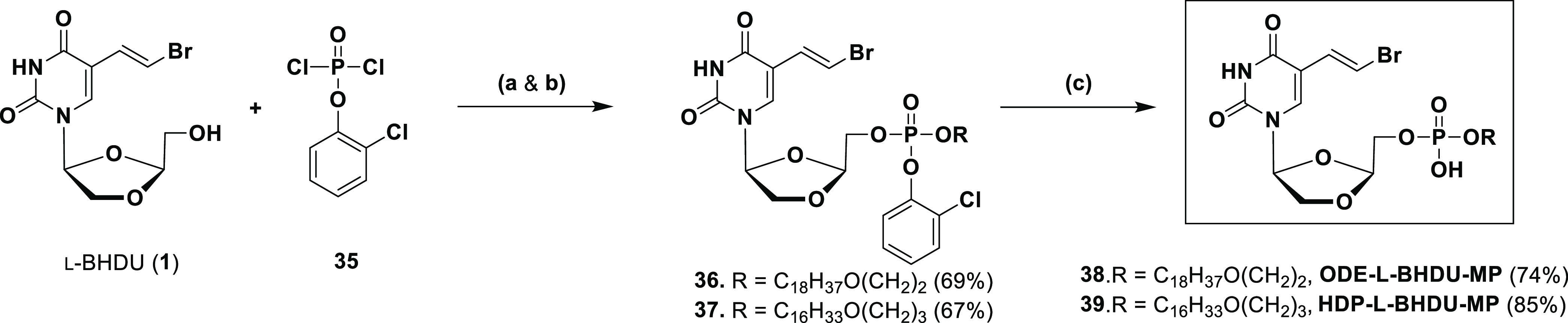
Reagents and conditions: (a) 1,2,4-triazole, Et3N, THF, rt, 1.5 h; (b) ROH, NMI, THF, rt, 12 h; (c) 0.5 N NaOH, THF/H2O, 50 °C, 2 h.
To synthesize POM-l-BHDU-MP, first, the synthesis of bis(POM)phosphorochloridate (40) was carried out according to the reported protocol by Hawang et al.31 Next, coupling of l-BHDU was performed with POM chloride (40), in the presence of NMI in THF to give POM-l-BHDU-MP (41) (47% yield, Scheme 5).
Scheme 5. Synthesis of the POM-l-BHDU-MP Prodrug.
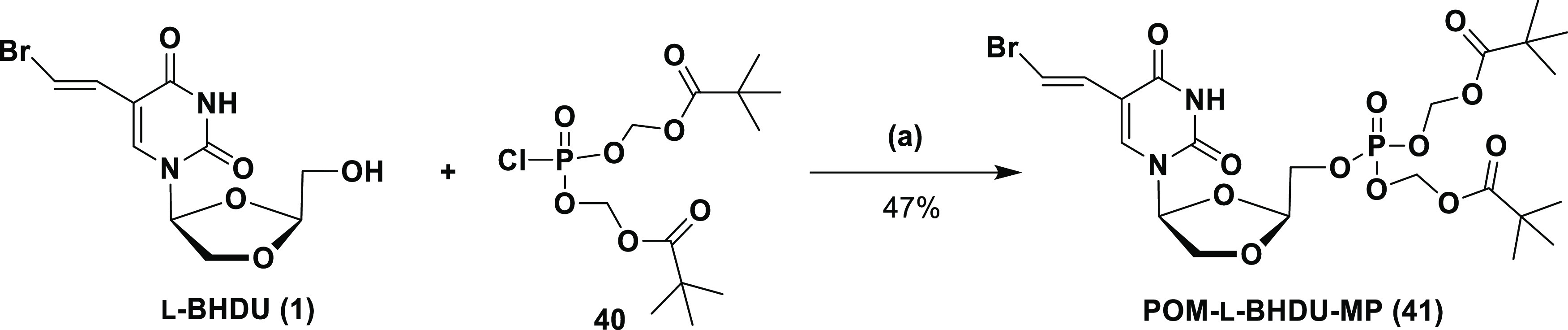
Reagents and conditions: (a) NMI, THF, 0 °C to rt, 3 h.
The synthesis of the bis-POC-l-BHDU-MP (47) prodrug was commenced with compound 42 (Scheme 6). Alkylation of 42 was carried out with the POC-I and cesium carbonate (Cs2CO3) in THF to yield POC alkylated ester 43 (82% yield). Initially, we attempted to convert 43 to 44 using the hydrogenation conditions on Pd/C. Unfortunately, selective monobenzyl deprotection was not achieved by this method, and a major didebenzylated product was obtained. Thus, selective monobenzyl deprotection was performed with LiBr, but only a small amount of compound 44 was formed (7–9% yield). Therefore, the selective monobenzyl deprotection reaction was again tried with sodium iodide (NaI) in acetonitrile, and in this case, it exclusively produced 44 in 92% yield. Repeated alkylation of 44 was performed with POC-I and Cs2CO3 in acetone to give intermediate 45 (66% yield). The final benzyl deprotection of 45 was executed on Pd/C in hydrogen at 5 psi to give the key intermediate 46 (85% yield). Finally, l-BHDU was treated with 46 in THF in the presence of N,N-diisopropylethylamine (DIPEA), bis(2-oxo-3-oxazolidinyl) phosphinic chloride (BOP-Cl), and 3-nitro-1,2,4-triazole to produce POC-l-BHDU-MP (47) (22% yield). The structures of the all-synthesized prodrugs were confirmed by 1H NMR, 13C NMR, 31P NMR, and electrospray ionization ESI high-resolution mass spectra (EI-HRMS).
Scheme 6. Synthesis of the Bis-POC-l-BHDU-MP Prodrug.
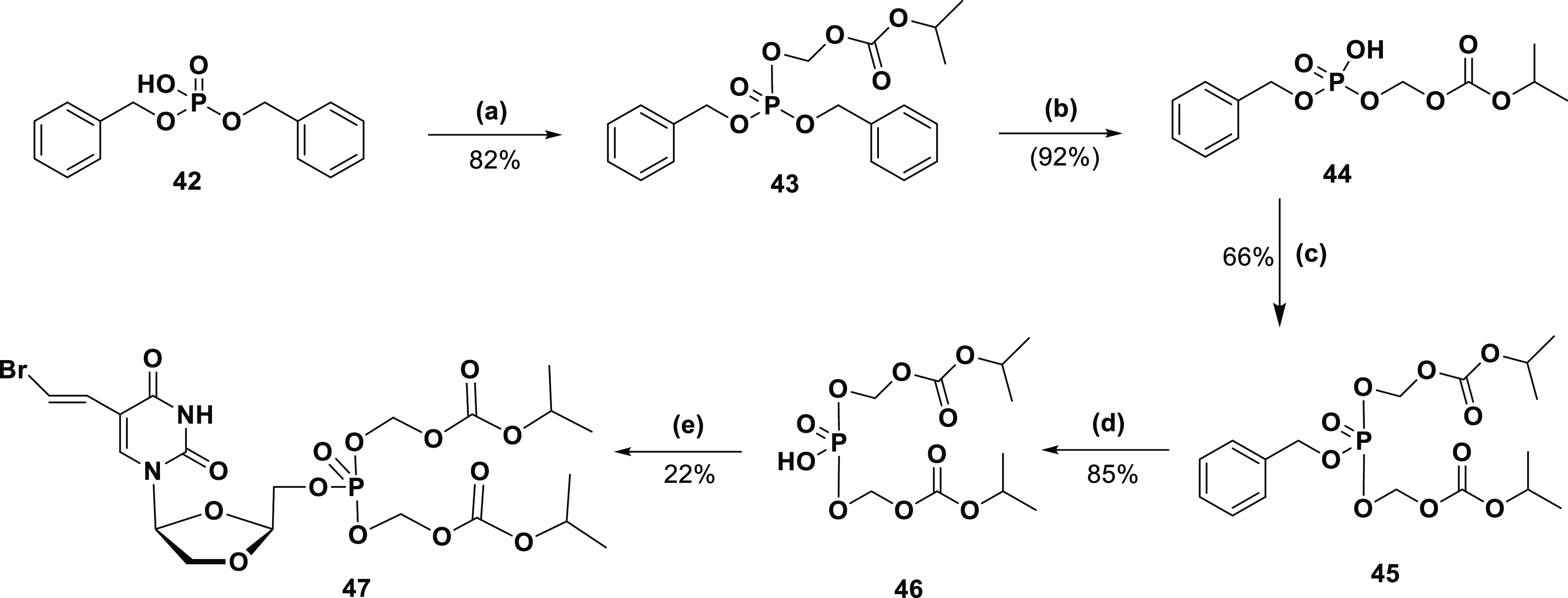
Reagents and conditions: (a) POC-I, Cs2CO3, acetone, rt, 24 h; (b) NaI, acetonitrile, rt, 24 h; (c) POC-I, Cs2CO3, acetone, rt, 24 h; (d) Pd/C, 5-10 psi, rt, 2 h; (e) l-BHDU, BOP-Cl, 3-nitro-1, 2, 4-triazole, DIPEA, THF, rt, 2–3 h.
Antiviral Activity
We previously reported that l-BHDU had good antiviral activity against VZV.16 We found that l-BHDU effectively reduced VZV replication at 15 mg/kg/day in the SCID-hu mouse with human fetal skin xenografts. It was well tolerated up to 150 mg/kg in mice and was not toxic to cells or skin.16 In the same study, ACV or VACV had no effect on VZV spread at 120 mg/kg/day. Others have corroborated these results, showing that VACV did not affect VZV replication in SCID-hu mice.32 In our previous study, we also measured l-BHDU in various mouse organs, finding that it reached high levels in most tissues but did not penetrate the brain.
The antiviral activity of l-BHDU depends on phosphorylation by VZV thymidine kinase (TK), and most antiviral resistance maps to the TK gene.33 To avoid the dependence on VZV TK for the generation of l-BHDU-MP, we utilized the phosphate ester, phosphoramidate, and long-chain phospholipid prodrug strategies. We expected these techniques to enhance the cell-bioavailability, tissue distribution, and pharmacokinetic profile of l-BHDU. The synthesized prodrugs also cap the polarity of l-BHDU, making it more lipophilic for improved cell membrane transport.
To evaluate these new prodrugs, we tested them in ARPE-19 cells infected with VZV-ORF57-Luc.34 VZV-ORF57-Luc expresses firefly luciferase; thus, virus spread was measured by bioluminescence imaging (total flux [photons/s/cm2/steradian]) in the IVIS 50 instrument (Caliper Life Sciences/Xenogen, Hopkinton, MA). ARPE-19 cells were seeded in 96-well tissue culture plates 3 d prior to infection and grown to confluence. Cells were infected with cell-free VZV-ORF57-Luc at an MOI of 0.01, as previously described.16,34−36 At 2 h post-infection, the virus inoculum was removed, and the medium was replaced with controls or test compounds in 6 replicate wells. CDV or ACV (positive controls), or l-BHDU and its prodrugs, were incubated with infected cells for 72 h. Virus yield was determined by dividing the average total flux at each concentration by the average total flux of the untreated wells.
Cellular toxicity was measured using a neutral red dye uptake assay, as previously described.16,36,37 Briefly, ARPE-19 cells were seeded in tissue culture plates for 3 d. Cells were then treated for 72 h with a range of concentrations of the control and experimental compounds. Staurosporine, which causes apoptosis, served as the control for cell death. For both efficacy and cytotoxicity assays, the 50% reduction in efficacy (EC50) and cell viability (CC50) was determined using GraphPad software (San Diego, California, www.graphpad.com).
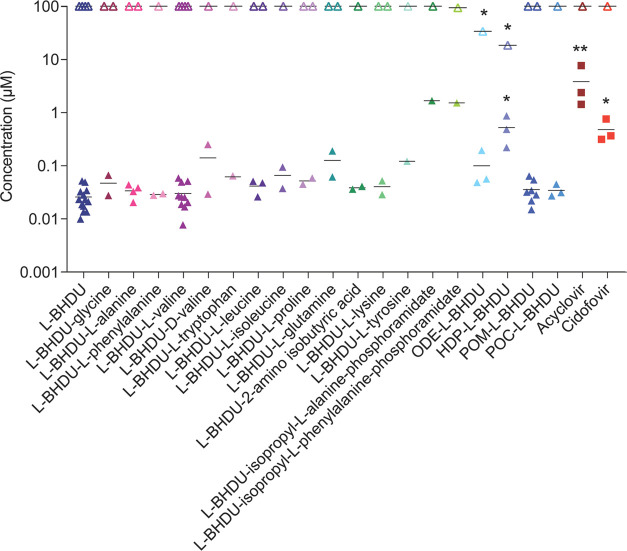
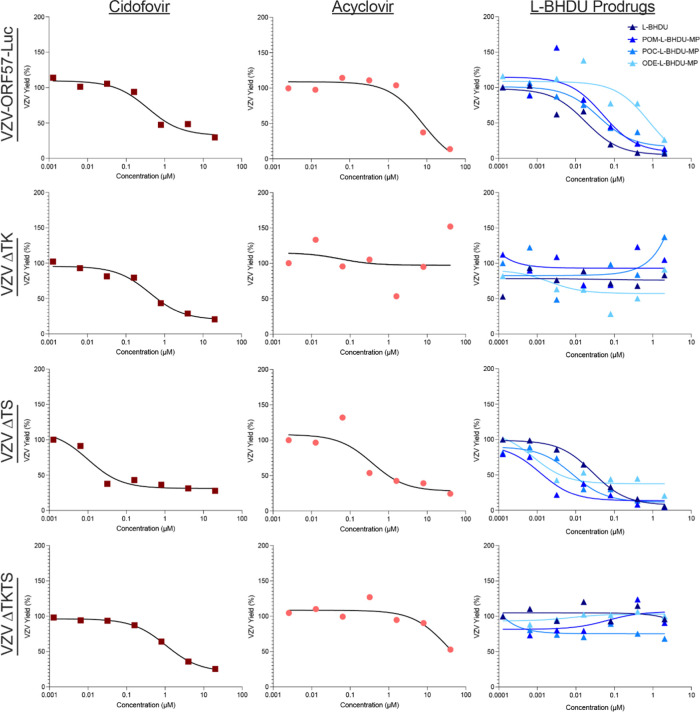
In this study, l-BHDU prevented VZV-ORF57-Luc spread in APRE-19 cells better than when it was evaluated against another reporter virus, VZV-BAC-Luc, in human foreskin fibroblasts (HFFs) (Figure 2).16 This suggests that the virus strain and/or cell type may slightly alter the effects of l-BHDU. Figure 2 shows the EC50 and CC50 values of synthesized prodrugs; all results are summarized in Table 1.
Figure 2.
Antiviral activity and cytotoxicity of l-BHDU and its prodrugs against VZV. l-BHDU and its prodrugs were evaluated against VZV-ORF57-Luc in ARPE-19 cells. Closed symbols represent EC50 values; open symbols represent CC50 values. N = 1–13 individual experiments. Each symbol represents the average of 6 replicates per experiment. Asterisks indicate significance [*p < 0.05,**p < 0.01, one-way analysis of variance (ANOVA) with Dunnett’s post hoc test].
Table 1. Antiviral Activity of l-BHDU and its Prodrugs against Cell-Free VZV in ARPE-19 Cells.
| s.no. | compounds | EC50 (μM)a | CC50 (μM)b | SI (CC50/EC50)c |
|---|---|---|---|---|
| 1. | l-BHDU (1) | 0.0258 | >100 | >3878 |
| 2. | l-BHDU-glycine (14) | 0.0469 | >100 | >2132 |
| 3. | l-BHDU-l-alanine (15) | 0.0338 | >100 | >2959 |
| 4. | l-BHDU-l-phenylalanine (16) | 0.0287 | >100 | >3484 |
| 5. | l-BHDU-l-valine (17) | 0.0301 | >100 | >3322 |
| 6. | l-BHDU-d-valine (18) | 0.141 | >100 | >709 |
| 7. | l-BHDU-l-tryptophan (19) | 0.0645 | >100 | >1550 |
| 8. | l-BHDU-l-leucine (20) | 0.0414 | >100 | >2415 |
| 9. | l-BHDU-l-isoleucine (21) | 0.0658 | >100 | >1520 |
| 10. | l-BHDU-l-proline (22) | 0.0517 | >100 | >1934 |
| 11. | l-BHDU-l-glutamine (23) | 0.126 | >100 | >794 |
| 12. | l-BHDU-2-aminoisobutyric acid (24) | 0.0385 | >100 | >2597 |
| 13. | l-BHDU-l-lysine (25) | 0.0403 | >100 | >2481 |
| 14. | l-BHDU-l-tyrosine (26) | 0.121 | >100 | >826 |
| 15. | l-BHDU-isopropyl-l-alanine-phosphoramidate (33) | 1.689 | >100 | >206 |
| 16. | l-BHDU-isopropyl-l-phenylalanine-phosphoramidate (34) | 1.530 | 93.72 | 61 |
| 17. | ODE-l-BHDU-MP (38) | 0.100 | 33.93 | 339 |
| 18. | HDP-l-BHDU-MP (39) | 0.526 | 18.47 | 35 |
| 19. | POM-l-BHDU-MP (41) | 0.0356 | >100 | >2809 |
| 20. | POC-l-BHDU-MP (47) | 0.0343 | >100 | >2915 |
| 21. | acyclovir (ACV) | 3.866 | >100 | >26 |
| 22. | cidofovir (CDV) | 0.482 | >100 | >208 |
50% inhibitory concentration 72 hpi determined by bioluminescence imaging, mean from at least three experiments.
50% cytotoxic concentration at 72 h determined by neutral red assay from 3 experiments.
Selectivity index = CC50/EC50.
Most l-BHDU prodrugs had good anti-VZV activity without cytotoxicity, similar to the parent molecule. Among the synthesized amino acid ester prodrugs (14–26), l-phenylalanine (16) and l-valine (17) prodrugs were not cytotoxic and had the lowest EC50 values at 0.028 μM (SI > 3484) and 0.030 μM (SI > 3322), respectively. Interestingly, we observed a significant drop in antiviral activity when the d-valine configuration was used for the prodrug (18, EC50 = 0.14 μM). Based on this result, we did not synthesize additional d-amino acid analogues of l-BHDU. The other l-amino acid prodrugs also exhibited moderate to good anti-VZV activity. There was no cytotoxicity associated with l-BHDU-amino acid ester prodrugs (CC50 > 100 μM). Comparably, phosphoramidate prodrugs (33, 34) had less antiviral activity. l-BHDU-isopropyl-l-alanine-phosphoramidate (33) had a weak antiviral activity with an EC50 of 1.689 μM but was not cytotoxic (CC50 > 100 μM). l-BHDU-isopropyl-l-phenylalanine-phosphoramidate (34) had a weak antiviral activity with an EC50 of 1.53 μM and was mildly cytotoxic (CC50 = 93.72 μM).
We also evaluated the long-chain lipid phosphate prodrugs octadecyloxyethyl-l-BHDU-MP (ODE-l-BHDU-MP, 38) and hexadecyloxypropyl-l-BHDU-MP (HDP-l-BHDU-MP, 39). ODE-l-BHDU-MP (38) had moderate anti-VZV activity with an EC50 of 0.10 μM, but was also moderately cytotoxic with a CC50 of 33.93 μM. HDP-l-BHDU-MP (39) had reduced antiviral activity with an EC50 of 0.53 μM, and was cytotoxic (CC50 = 18.47 μM). We believe that the slower metabolic rate of the long hydrocarbon in the cell causes higher cytotoxicity.
Finally, we tested the phosphate ester prodrugs, POM-l-BHDU-MP (41) and POC-l-BHDU-MP (47), against VZV in ARPE-19 cells. Both POM-l-BHDU-MP and POC-l-BHDU-MP had good antiviral efficacy and were not cytotoxic. POM-l-BHDU-MP and POC-l-BHDU-MP had EC50 values of 0.036 μM (SI > 2809) and 0.034 μM (SI > 2915), respectively, similar to its parent compound, l-BHDU. Other benefits of the long-chain phospholipid and phosphonate ester prodrugs of l-BHDU-MP are that these prodrugs resemble lysophosphatidylcholine20 and enhance cellular uptake.
Since l-BHDU (1), ODE-l-BHDU-MP (38), POM-l-BHDU-MP (41), and POC-l-BHDU-MP (47) had some of the best antiviral profiles against VZV-ORF57-Luc, we then screened them against VZV TK and TS (thymidylate synthase) mutants.34 These studies were performed in the same manner as the efficacy studies described above, except cell-associated VZV-ORF57-Luc, VZV-ORF57-ΔTK, VZV-ORF57-ΔTS, and VZV-ORF57-ΔTKTS were used to infect ARPE-19 cells. As before, CDV and ACV were used as positive controls.
The EC50 for each compound against cell-associated VZV was similar to or a little higher than those obtained with cell-free VZV (Table 2). In most cases, l-BHDU and its prodrugs were more potent against VZV-ORF57-Luc than CDV or ACV (Table 2, Figure 3). The exception is ODE-l-BHDU-MP (38), which was less potent compared to CDV but 9-fold more potent compared to ACV. As expected, CDV was effective against VZV-ORF57-ΔTK and VZV-ORF57-ΔTKTS, while ACV was not due to its requirement for phosphorylation by viral TK. Unfortunately, l-BHDU and its prodrugs were not active against VZV-ORF57-ΔTK or VZV-ORF57-ΔTKTS.
Table 2. Antiviral Activity of l-BHDU and Several Prodrugs against Cell-Associated Wild-Type and Mutant VZV Viruses in ARPE-19 Cells.
| EC50 (μM) |
||||||
|---|---|---|---|---|---|---|
| viral strains | CDV | ACV | l-BHDU | POM-l-BHDU-MP (41) | POC-l-BHDU-MP (47) | ODE-l-BHDU-MP (38) |
| VZV-ORF57-Luc | 0.37 | 7.75 | 0.18 | 0.051 | 0.040 | 0.82 |
| VZV-ORF57-ΔTK | 0.42 | >20 | >2.0 | >2.0 | >2.0 | >2.0 |
| VZV-ORF57-ΔTS | 0.01 | 0.37 | 0.028 | 0.0012 | 0.0084 | 0.0008 |
| VZV-ORF57-ΔTKTS | 1.05 | >20 | >2.0 | >2.0 | >2.0 | >2.0 |
Figure 3.
Antiviral screening results for l-BHDU and its C18 (ODE-l-BHDU-MP, 38) POM (41), and POC-l-BHDU-MP (47) against cell-associated VZV-ORF57-Luc, VZV TK-, VZV TS-, and VZV TKTS-. Cidofovir and acyclovir are positive controls. Each symbol represents the average of 6 replicate wells; the line is the best-fit curve (error bars are omitted for clarity).
Interestingly, l-BHDU and the prodrugs POM-l-BHDU-MP, POC-l-BHDU-MP, and ODE-l-BHDU-MP were more potent against the VZV-ORF57-ΔTS mutant virus than the wild type. This phenomenon was also observed for CDV and ACV. We previously noted this phenomenon with other antivirals against VZV-ORF57-ΔTS, including the thymidine analogue brivudine (BVDU).34 We suspect that the loss of TS reduces the dTMP pools in the cell, resulting in less competition with l-BHDU or its prodrugs for incorporation during viral DNA synthesis. Thus, while these prodrugs do not work against VZV TK mutants, l-BHDU and its prodrugs are still highly effective at reducing VZV spread and should be investigated further. In the event of viral thymidine kinase (TK) mutation, the nucleoside class of antivirals does not proceed to the first monophosphorylation step.
The unphosphorylated nucleoside remains in its inactive form and does not interfere with viral replication. Therefore, it was thought to select the prodrugs that bypass the viral TK-driven monophosphorylation or do not require the often rate-limiting initial phosphorylation step for activation. Considering these points, POM-l-BHDU-MP and ODE-l-BHDU-MP were selected for the elaborated in vivo studies via subcutaneous and oral routes. Studies to evaluate these prodrugs in human skin organ culture and human skin xenografts in athymic nude mice (NuSkin mouse model) are forthcoming.
Pharmacokinetic Studies
The antiviral efficacy of POM-l-BHDU-MP prompted us to perform pharmacokinetic studies on POM-l-BHDU-MP in vitro and in vivo. POM-l-BHDU-MP demonstrated good stability in gastric and intestinal juice (data not shown). In liver homogenates, POM-l-BHDU-MP converted to l-BHDU and l-BHDU-MP (Figure 4). POM-l-BHDU-MP incubated with homogenized mouse liver was unstable and converted to l-BHDU and l-BHDU-MP. The concentration of l-BHDU increased steadily, reaching 1.9 μg/mL after 60 min. l-BHDU-MP appeared rapidly and reached maximal concentration (4.6 μg/mL) after 4 min, which is 32-fold higher than the corresponding l-BHDU concentration. Thus, POM-l-BHDU-MP likely converts rapidly into l-BHDU-MP and more slowly into l-BHDU in the liver.
Figure 4.
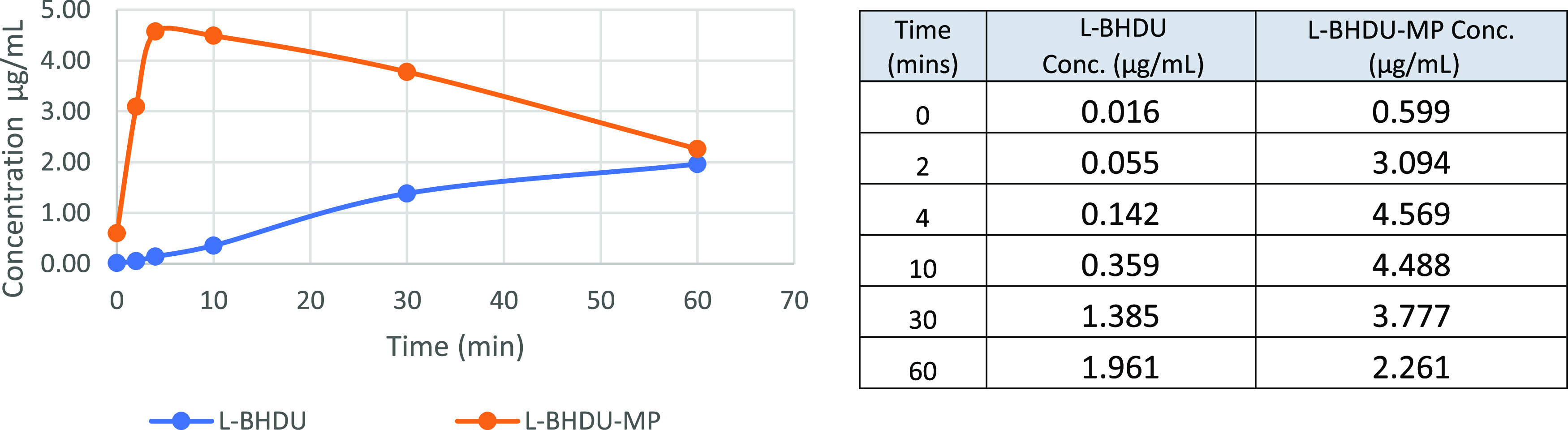
In vitro stability study of POM-l-BHUD-MP in mouse liver homogenates.
A pharmacokinetic absorption study was performed in BALB/c mice (Charles River). A single equimolar dose of 22.5 mg/kg POM-l-BHDU-MP or 11.4 mg/kg l-BHDU was dissolved in Cremophor–dimethyl sulfoxide (DMSO)–saline (1:1:8) and administered by oral gavage to two groups of mice with equal numbers of male and female mice (N = 15 each male and female).
Mouse blood was collected by cardiac puncture at the times presented in Figure 5 (see table). Plasma was separated from red blood cells and immediately frozen at −80 °C. l-BHDU absorption profiles are shown in Figure 5. The results indicate that l-BHDU was significantly more bioavailable when administered as the prodrug form of POM-l-BHDU-MP.
Figure 5.
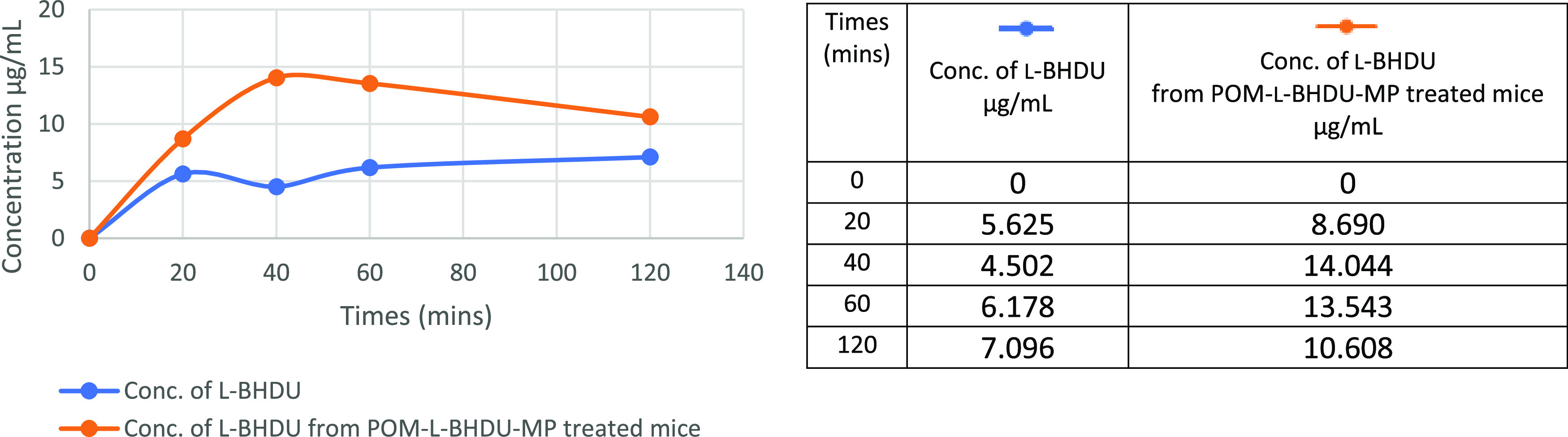
l-BHDU plasma concentration–time profiles after oral administration of l-BHDU or POM-l-BHDU-MP.
The AUC value for l-BHDU in POM-l-BHDU-MP treated mice is approximately 2.2-fold higher than for l-BHDU treated mice (Table 3). In mice given POM-l-BHDU-MP, the maximal plasma concentration of l-BHDU was 14 μg/mL at 40 min (Figure 5), whereas a lower l-BHDU plasma concentration, 4.5 to 7.0 μg/mL, was observed in mice given l-BHDU. Cmax for l-BHDU was significantly higher after administration of prodrug POM-l-BHDU-MP than the parent molecule (l-BHDU, Table 3). Thus, the POM prodrug had better oral absorption/availability than l-BHDU. We found that in mice administered POM-l-BHDU-MP, only the l-BHDU metabolite was detected via liquid chromatography/mass spectrometry (LC/MS) in plasma, and the trace amount of l-BHDU-MP detected was below the limit of quantitation. This suggests that POM-l-BHDU-MP is absorbed and rapidly cleaved by esterases at the (P–O) bond of l-BHDU-MP to release the parental nucleoside, l-BHDU.
Table 3. Pharmacokinetic Parameters after Oral Application of 22.5 mg/kg POM-l-BHDU-MP and 11.4 mg/kg l-BHDU to BALB/c Mice.a.
| l-BHDU derived from POM-l-BHDU-MP | l-BHDU | |
|---|---|---|
| AUC (μg·min/mL) | 1680 | 764 |
| Tmax (min) | 40 | 120 |
| Cmax (μg/mL) | 10.6 | 7.1 |
LC-MS quantification of l-BHDU from POM-l-BHDU-MP- and l-BHDU-treated mice. AUC: area under the curve from 0 to 120 min; Tmax: time taken to reach maximum conc.; and Cmax: maximum concentration.
Conclusions
Here, we described the synthesis and antiviral evaluation of l-BHDU and its prodrugs, including 5′-amino acid esters, phosphoramidates, long-chain phospholipids, and phosphate esters. In vitro, most of these prodrugs exhibited significant anti-VZV activity. Of particular interest were POM-l-BHDU-MP, POC-l-BHDU-MP, and ODE-l-BHDU-MP. POM-l-BHDU-MP (41) and POC-l-BHDU-MP (42) had similar antiviral activity compared to l-BHDU, whereas ODE-l-BHDU-MP (38) was less active than the parental molecule. The pharmacokinetic study revealed that POM-l-BHDU-MP prodrugs have 2.2 times better oral absorption/availability in comparison to l-BHDU. Based on these data, we conclude that ODE-l-BHDU-MP (38), POM-l-BHDU-MP (41), and POC-l-BHDU-MP (42) have potent antiviral profiles and warrant continued development as novel antiviral treatments for VZV infections. Additional studies are planned to evaluate these compounds in more clinically relevant systems, including a human skin organ culture system and in the NuSkin mouse model,38 as well as the elaborated pharmacokinetics of the prodrugs to select them as potential clinical candidates.
Experimental Section
General Analytical Methods
Reagents and anhydrous solvents were purchased from commercial sources and used without further purification. Moisture-sensitive reactions were performed using oven-dried glassware under a nitrogen or argon atmosphere. Reactions were monitored by thin-layer chromatography plates (TLC silica gel GF 250 μm) that were visualized using a Spectroline UV lamp (254 nm) and developed with a 15% solution of sulfuric acid in methanol. Column chromatography was performed on silica gel 60 Å, 40–63 μM (230 × 400 mesh, Sorbent Technologies). Preparative normal phase chromatography was performed on a CombiFlash Rf 150 (Teledyne Isco) with prepacked RediSep Rf silica gel cartridges or on RediSep gold C18 reverse phase columns. Melting points were recorded on a Mel-temp II laboratory device and were uncorrected. Nuclear magnetic spectra were recorded on a Varian Inova 500 spectrometer at 500 MHz for 1H NMR, 202 MHz for 31P NMR, and 125 MHz for 13C NMR with tetramethylsilane as an internal standard. Chemical shifts (δ) are quoted as s (singlet), bs (broad singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (double doublet), and dt (double triplet). Optical rotations were measured on a JASCO DIP-370 digital polarimeter. High-resolution mass spectroscopy (HRMS) spectra were measured on a Bruker Ultrahigh-resolution QTOF MS Impact II spectrometer. Samples were infused at 3 μL/min, and spectra were obtained in the positive or negative ionization mode with a typical resolution of 20,000 or greater. The purity of all tested compounds is ≥95%, as determined by their elemental analysis (Table S1) or by high-performance liquid chromatography/ultraviolet (HPLC/UV). Elemental analyses were performed by Atlantic Microlab Inc. Norcross, GA. HPLC/UV was determined with a Waters HPLC coupled to a photodiode array. Five microliters of sample 0.5 mg/mL in methanol, acetonitrile, or a mixture of DMSO/MeOH (0.5:10) was injected using an XBridge C18, 3.5 μm (4.6 × 150 mm) column at 25 °C with a flow rate of 0.8 mL/min or with UPLC BEH C18, 1.7 μm (100 × 2.1) mm at 50 °C with a flow rate of 0.55 mL/min. The mobile phases were a mixture of A = 10 mM ammonium acetate in water and B = acetonitrile (ACN), and A = 0.05% formic acid (FA) in water and B = 0.05% in acetonitrile (ACN). Purity is given as % of absorbance at the Max plot.
General Procedure for the Synthesis of Boc-N-protected-5′-amino Acid Ester of l-BHDU (2–13)
A solution of l-BHDU (1 mmol), Boc-protected amino acid (2.5 mmol), DMAP (2.5 mmol), and 1,3-diisopropylcarbodiimide (DIC, 2.5 mmol) in DCM (10 mL) was stirred at rt from 12 h. The mixture was diluted with DCM (10 mL) and washed with aqueous sodium bicarbonate (2 × 10 mL) and finally with water (2 × 10 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography to provide 5′-O-Boc-protected amino acid ester of l-BHDU in 60–85% yield.
l-BHDU-5′-O-l-Boc-glycine Ester (2)
Yield: 78%; 1H NMR (500 MHz, CDCl3) δ 9.59 (bs, 1H), 7.54 (s, 1H), 7.41 (d, J = 15.0 Hz, 1H), 6.73 (d, J = 13.6 Hz, 1H), 6.31 (d, J = 4.4 Hz, 1H), 5.18 (t, J = 3.2 Hz, 1H), 5.11 (s, 1H), 4.51 (dd, J = 12.4 & 3.3 Hz, 1H), 4.38 (dd, J = 12.4 & 3.0 Hz, 1H), 4.27 (d, J = 10.1 Hz, 1H), 4.19–4.16 (m, 1H), 3.98–3.88 (m, 2H), 1.44 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 170.1, 161.4, 155.8, 149.6, 136.9, 128.1, 112.2, 110.9, 102.9, 81.5, 80.4, 71.3, 62.8, 42.3, 28.4; HRMS (EI) calcd for (C17H22BrN3O8 + H)+ 476.0669, found 476.0663.
l-BHDU-5′-O-l-Boc-alanine Ester (3)
Yield: 71%; 1H NMR (500 MHz, CDCl3) δ 9.53 (bs, 1H), 7.58 (s, 1H), 7.44 (d, J = 13.6 Hz, 1H), 6.76 (d, J = 13.6 Hz, 1H), 6.34 (dd, J = 5.7 & 1.8 Hz, 1H), 5.18 (t, J = 3.3 Hz, 1H), 5.03 (d, J = 6.4 Hz, 1H), 4.57 (d, J = 10.2 Hz, 1H), 4.39–4.23 (m, 3H), 4.18 (dd, J = 10.4 & 5.7 Hz, 1H), 1.43 (s, 9H), 1.38 (d, J = 7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 173.2, 161.2, 155.2, 150.0, 137.0, 128.1, 112.3, 110.9, 102.9, 81.4, 71.8, 62.8, 49.3, 42.3, 28.3, 23.5, 18.1; HRMS (EI) calcd for (C18H24BrN3O8 – H)− 488.0669, found 488.0670.
l-BHDU-5′-O-l-Boc-phenylalanine Ester (4)
Yield: 82%; 1H NMR (500 MHz, CDCl3) δ 9.69 (bs, 1H), 7.54 (s, 1H), 7.47 (d, J = 13.6 Hz, 1H), 7.32–7.25 (m, 3H), 7.13 (d, J = 13.6 Hz, 2H), 6.77 (d, J = 13.6 Hz, 1H), 6.33 (dd, J = 5.7 & 1.7 Hz, 1H), 5.12 (t, J = 3.4 Hz, 1H), 5.02 (d, J = 7.7 Hz, 1H), 4.58–4.52 (m, 2H), 4.28–4.23 (m, 2H), 4.20–4.14 (m, 1H), 3.13–3.0 (m, 1H), 3.03 (dd, J = 13.8 & 6.9 Hz, 1H), 1.41 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 171.9, 161.5, 152.2, 149.7, 137.0, 135.6, 129.2, 128.8, 128.2, 127.3, 112.3, 111.0, 102.9, 81.4, 80.3, 71.1, 62.9, 54.6, 53.5, 38.1, 28.3; HRMS (EI) calcd for (C24H28BrN3O8 – H)− 564.0982, found 564.0985.
l-BHDU-5′-O-l-Boc-valine Ester (5)
Yield: 83%; 1H NMR (500 MHz, CDCl3) δ 8.84 (bs, 1H), 7.60 (s, 1H), 7.48 (d, J = 13.6 Hz, 1H), 6.78 (d, J = 13.6 Hz, 1H), 6.33 (d, J = 7.1 Hz, 1H), 5.19 (t, J = 3.2 Hz, 1H), 4.96 (d, J = 8.8 Hz, 1H), 4.61 (dd, J = 12.3 & 4.0 Hz, 1H), 4.30–4.23 (m, 2H), 4.20–4.13 (m, 2H), 2.14–2.05 (m, 1H), 1.43 (s, 9H), 0.97 (d, J = 6.8 Hz, 3H), 0.90 (d, J = 6.8 Hz, 3H).13C NMR (125 MHz, CDCl3) δ 172.3, 161.1, 155.8, 149.5, 137.1, 128.2, 112.3, 110.0, 103.0, 81.5, 80.2, 71.2, 62.6, 58.9, 30.9, 28.4, 19.2, 17.8; HRMS (EI) calcd for (C20H28BrN3O8 + H)+ 518.1138, found 518.1132.
l-BHDU-5′-O-d-Boc-valine Ester (6)
Yield: 73%; 1H NMR (500 MHz, CDCl3) δ 9.39 (bs, 1H), 7.56 (s, 1H), 7.46 (d, J = 13.6 Hz, 1H), 6.75 (d, J = 13.6 Hz, 1H), 6.28 (d, J = 7.1 Hz, 1H), 5.19 (t, J = 3.7 Hz, 1H), 4.99 (d, J = 8.7 Hz, 1H), 4.51 (dd, J = 12.3 & 4.0 Hz, 1H), 4.37 (dd, J = 12.3 & 3.2 Hz, 1H), 4.25 (dd, J = 10.4 & 1.5 Hz, 1H), 4.22–4.15 (m, 2H), 2.15–2.08 (m, 1H), 1.43 (s, 9H), 0.97 (d, J = 6.8 Hz, 3H), 0.90 (d, J = 6.8 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 172.2, 161.2, 155.7, 149.4, 136.7, 128.1, 112.1, 111.0, 103.1, 81.9, 80.2, 71.7, 62.9, 58.8, 31.0, 28.4, 19.2, 17.7; HRMS (EI) calcd for (C20H28BrN3O8 + H)+ 518.1138, found 518.1135.
l-BHDU-5′-O-l-Boc-tryptophan Ester (7)
Yield: 71%; 1H NMR (500 MHz, CDCl3) δ 9.42 (bs, 1H), 8.34 (bs, 1H), 7.52 (d, J = 7.9 Hz, 1H), 7.49–7.38 (m, 2H), 7.33 (d, J = 8.1 Hz, 1H), 7.17 (t, J = 7.4 Hz, 1H), 7.09 (t, J = 7.2 Hz, 1H), 7.03 (s, 1H), 6.73 (d, J = 13.6 Hz, 1H), 6.23 (d, J = 5.6 Hz, 1H), 5.14 (d, J = 7.8 Hz, 1H), 4.96 (s, 1H), 4.61 (d, J = 6.9 Hz, 1H), 4.44 (d, J = 9.5 Hz, 1H), 4.22–4.14 (m, 2H), 4.04 (dd, J = 10.4 & 5.7 Hz, 1H), 3.24 (d, J = 6.0 Hz, 2H), 1.43 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 172.3, 161.3, 155.3, 149.6, 137.0, 136.2, 128.2, 127.6, 123.0, 122.2, 119.7, 118.6, 112.3, 111.4, 111.0, 109.6, 103.0, 81.3, 80.3, 71.0, 62.8, 54.3, 53.5, 28.4; HRMS (EI) calcd for (C26H29BrN4O8 – H)− 603.1091, found 603.1096.
l-BHDU-5′-O-l-Boc-leucine Ester (8)
Yield: 67%; 1H NMR (500 MHz, CDCl3) δ 9.48 (bs, 1H), 7.61 (s, 1H), 7.45 (d, J = 13.6 Hz, 1H), 6.78 (d, J = 13.6 Hz, 1H), 6.33 (dd, J = 5.7 & 1.9 Hz, 1H), 5.18 (t, J = 3.0 Hz, 1H), 4.95 (d, J = 8.1 Hz, 1H), 4.61 (d, J = 12.2 Hz, 1H), 4.28–4.21 (m, 3H), 4.17 (dd, J = 10.3 & 5.8 Hz, 1H), 1.74–1.69 (m, 1H), 1.60–1.48 (m, 2H), 1.41 (s, 9H), 0.92 (dd, J = 6.6 & 3.6 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.4, 161.5, 155.5, 150.0, 137.2, 128.3, 112.3, 111.0, 103.0, 81.5, 80.2, 71.2, 63.0, 52.4, 50.8, 41.1, 28.4, 25.0, 23.5, 23.0, 21.9; HRMS (EI) calcd for (C21H30BrN3O8 – H)− 530.1138, found 530.1141.
l-BHDU-5′-O-l-Boc-isoleucine Ester (9)
Yield: 72%; 1H NMR (500 MHz, CDCl3) δ 9.62 (bs, 1H), 7.58 (s, 1H), 7.46 (d, J = 13.8 Hz, 1H), 6.77 (d, J = 13.6 Hz, 1H), 6.33 (dd, J = 5.7 & 1.7 Hz, 1H), 5.18 (t, J = 3.4 Hz, 1H), 5.02 (d, J = 8.7 Hz, 1H), 4.59 (dd, J = 12.3 & 2.9 Hz, 1H), 4.26 (d, J = 11.3 Hz, 2H), 4.21–4.15 (m, 2H), 1.85–1.77 (m, 1H), 1.42 (s, 10H), 1.20–1.12 (m, 1H), 0.93–0.87 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 172.3, 161.5, 155.7, 149.7, 137.0, 128.2, 112.3, 111.0, 103.0, 81.5, 80.2, 71.1, 62.6, 58.3, 37.6, 28.5, 25.0, 15.7, 11.5; HRMS (EI) calcd for (C21H30BrN3O8 + H)+ 532.1295, found 532.1284.
l-BHDU-5′-O-l-Boc-proline Ester (10)
Yield: 75%; 1H NMR (500 MHz, CDCl3) δ 9.80 (bs, 1H), 7.7–7.58 (m, 1H), 7.43 (d, J = 11.0 Hz, 1H), 6.74 (dd, J = 28.4 & 13.6 Hz, 1H), 6.36 (t, J = 4.0 Hz, 1H), 5.19–5.15 (m, 1H), 4.57 (ddd, J = 38.3, 12.5 & 3.0 Hz, 1H), 4.30–4.14 (m, 4H), 3.59–3.36 (m, 2H), 2.26–2.11 (m 1H), 1.98–1.94 (m, 3H), 1.43–1.40 (m, 9H); 13C NMR (125 MHz, CDCl3) δ 172.7, 161.5, 154.5, 149.9, 137.7, 128.5, 112.4, 110.9, 103.0, 81.4, 80.2, 71.4, 62.4, 58.9, 53.6, 46.6, 29.9, 23.7; HRMS (EI) calcd for (C20H26BrN3O8 – H)− 514.0825, found 514.0826.
l-BHDU-5′-O-l-Boc-glutamine Boc Amide Ester (11)
Yield: 79%; 1H NMR (500 MHz, CDCl3) δ 9.99 (s, 1H), 7.57 (s, 1H), 7.44 (d, J = 13.6 Hz, 1H), 6.78 (d, J = 13.6 Hz, 1H), 6.33 (d, J = 5.6 Hz, 1H), 6.08 (d, J = 36.8 Hz, 2H), 5.48 (d, J = 7.6 Hz, 1H), 5.20 (t, J = 3.1 Hz, 1H), 4.52 (d, J = 11.9 Hz, 1H), 4.36–4.15 (m, 4H), 2.33 (t, J = 7.1 Hz, 2H), 2.14–2.0 (m, 1H), 1.98–1.87 (m, 1H), 1.43 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 175.1, 172.2, 161.9, 155.8, 150.0, 137.5, 128.5, 112.2, 110.5, 102.7, 81.3, 80.3, 70.8, 63.0, 53.4, 31.8, 28.4; HRMS (EI) calcd for (C20H27BrN4O9+Na)+ 569.0859, found 569.0851.
l-BHDU-5′-O-Boc-2-aminoisobutyric Ester (12)
Yield: 66%; 1H NMR (500 MHz, CDCl3) δ 9.57 (bs, 1H), 7.52 (s, 1H), 7.44 (d, J = 13.6 Hz, 1H), 6.73 (d, J = 13.6 Hz, 1H), 6.30 (d, J = 5.3 Hz, 1H), 5.17 (t, J = 3.8 Hz, 1H), 5.02 (bs, 1H), 4.46 (dd, J = 12.1 & 3.7 Hz, 1H), 4.33 (dd, J = 12.2 & 3.6 Hz, 1H), 4.23 (d, J = 10.4 Hz, 1H), 4.16 (dd, J = 10.4 & 5.6 Hz, 1H), 1.49 (s, 6H), 1.41 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 174.2, 161.4, 154.6, 149.6, 136.9, 128.2, 112.3, 110.9, 103.2, 81.5, 71.4, 63.4, 56.1, 28.3, 25.6; HRMS (EI) calcd for (C19H26BrN3O8 – H)− 502.0825, found 502.0830.
l-BHDU-5′-O-l-Boc-lysine Amide Ester (13)
Yield: 85%; 1H NMR (500 MHz, CDCl3) δ 9.67 (bs, 1H), 7.58 (s, 1H), 7.48–7.41 (m, 1H), 6.77 (d, J = 13.8 Hz, 1H), 6.33 (d, J = 5.4 Hz, 1H), 5.17 (t, J = 3.3 Hz, 2H), 4.67 (s, 1H), 4.56 (d, J = 12.4 Hz, 1H), 4.27 (d, J = 11.2 Hz, 2H), 4.22–4.13 (m, 2H), 3.11–3.04 (m, 2H), 1.80–1.73 (m, 1H), 1.67–1.58 (m, 1H), 1.47–1.40 (m, 22H); 13C NMR (125 MHz, CDCl3) δ 172.7, 161.4, 156.2, 155.6, 149.7, 137.1, 128.3, 112.3, 110.8, 102.9, 81.4, 80.2, 79.4, 71.0, 62.7, 53.6, 53.5, 39.9, 31.8, 29.7, 28.6, 28.4, 22.6; HRMS (EI) calcd for (C26H39BrN4O10 + H)+ 647.1928, found 647.1926.
General Procedure for the Synthesis of 5′-Amino Acid Ester of l-BHDU (14–25)
The Boc-protected amino acid prodrug (150 mg) was dissolved in DCM (10 mL), and the solution was cooled to 0 °C, then TFA (2 mL) was added to the solution with vigorous stirring. The mixture was warmed to rt and stirred for 1 h. The excess volatiles were removed under pressure. The obtained crude was dissolved in methanol (10 mL) and cooled to 0 °C, then 2 M HCl solution was added in ether and continued stirring for 20 min. The solvents were removed under reduced pressure, and the crude solid obtained was further washed with anhydrous ether to give l-BHDU-5′-O-amino acid ester as a hydrochloride salt in approximately 80–90% yield.
l-BHDU-5′-O-glycyl Ester Hydrochloride (14)
Yield: 82%; 1H NMR (500 MHz, CD3OD) δ 7.70 (s, 1H), 7.39 (d, J = 15.0 Hz, 1H), 6.86 (d, J = 15.0 Hz, 1H), 6.30 (dd, J = 5.0 & 2.5 Hz, 1H), 5.24 (t, J = 5.0 Hz, 1H), 4.56–4.49 (m, 2H), 4.38 (dd, J = 10.0 & 5.0 Hz, 1H), 4.15–4.13 (m, 1H), 3.87–3.77 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 167.0, 162.1, 150.1, 138.3, 129.0, 111.3, 108.4, 102.4, 81.7, 70.2, 63.5, 39.6; anal. calcd for C12H15BrClN3O6·(H2O)1.5: C, 32.78; H, 4.13; N, 9.56. Found: C, 32.39, H, 3.90; N, 9.23; HRMS (EI) calcd for (C12H14BrN3O6 + H)+ 376.0144, found 376.0141.
l-BHDU-5′-O-l-alanyl Ester Hydrochloride (15)
Yield: 89%; [α]D24 = +4.27 (c 0.5, MeOH); 1H NMR (500 MHz, DMSO-d6) δ 11.6 (s, 1H, NH), 8.42 (bs, 3H), 7.65 (s, 1H), 7.30 (d, J = 15.0 Hz, 1H), 6.99 (d, J = 10.0 Hz, 1H), 6.23 (dd, J = 5.0 & 2.5 Hz, 1H), 5.16 (t, J = 2.5 Hz, 1H), 4.46–4.40 (m, 2H), 4.32 (dd, J = 10.0 & 5.0 Hz, 1H), 4.14–4.06 (m, 2H), 1.33 (d, J = 10.0 Hz, 3H); 13C NMR (125 MHz, DMSO-d6) δ 169.5, 162.1, 150.0, 138.2, 129.0, 111.4, 108.4, 102.3, 81.7, 70.1, 63.8, 14.8; anal. calcd for C13H17BrClN3O6·(H2O)1.0: C, 35.11; H, 4.31; N, 9.45. Found: C, 35.05, H, 4.32; N, 9.28; HRMS (EI) calcd for (C13H16BrN3O6 + H)+ 390.0301, found 390.0296.
l-BHDU-5′-O-l-phenylalanyl Ester Hydrochloride (16)
Yield: 85%; [α]D24 = −11.26 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.63 (s, 1H), 7.38–7.28 (m, 4H), 7.18 (d, J = 8.5 Hz, 2H), 6.82 (d, J = 14.0 Hz, 1H), 6.30 (dd, J = 5.5 & 1.5 Hz, 1H), 5.17 (t, J = 3.5 Hz, 1H), 4.52 (dd, J = 12.5 & 3.5 Hz, 1H), 4.44 (dd, J = 12.5 & 3.0 Hz, 1H), 4.38 (dd, J = 10.5 & 1.5 Hz, 1H), 4.27 (t, J = 6.5 Hz, 1H), 4.19–4.15 (m, 1H), 3.21–3.10 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 168.6, 162.0, 150.0, 138.1, 133.4, 129.1, 127.8, 111.5, 108.5, 102.1, 100.0, 81.6, 69.9, 63.7, 53.8, 36.1; anal. calcd for C19H21BrClN3O6·(H2O)1.0: C, 43.82; H, 4.45; N, 8.07. Found: C, 43.75, H, 4.51; N, 7.98; HRMS (EI) calcd for (C19H20BrN3O6 + H)+ 466.0614, found 466.0615.
l-BHDU-5′-O-l-valyl Ester Hydrochloride (17)
Yield: 84%; [α]D24 = −7.4 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.74 (s, 1H), 7.47 (d, J = 13.5 Hz, 1H), 6.91 (d, J = 14.0 Hz, 1H), 6.37 (d, J = 4.5 Hz, 1H), 5.25 (t, J = 3.0 Hz, 1H), 4.63–4.54 (m, 2H), 4.45 (d, J = 10.5 Hz, 1H), 4.26–4.23 (m, 1H), 3.95 (d, J = 4.5 Hz, 1H), 2.31–2.28 (m, 1H), 1.09 (d, J = 7.0 Hz, 3H), 1.05 (d, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CD3OD) δ 168.6, 162.1, 150.1, 138.3, 129.0, 111.4, 108.5, 102.2, 81.8, 70.0, 63.8, 58.1, 29.7, 17.2, 16.6; anal. calcd for C15H21BrClN3O6·(H2O)0.5: C, 38.85; H, 4.78; N, 9.06. Found: C, 38.82, H, 4.85; N, 8.96; HRMS (EI) calcd for (C15H20BrN3O6 + H)+ 418.0614, found 418.0612.
l-BHDU-5′-O-d-valyl Ester Hydrochloride (18)
Yield: 86%; [α]D24 = −15.85 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.70 (s, 1H), 7.39 (dd, J = 13.5 & 3.5 Hz, 1H), 6.84 (d, J = 13.0 Hz, 1H), 6.27 (dd, J = 6.0 & 2.0 Hz, 1H), 5.25 (t, J = 3.5 Hz, 1H), 4.60–4.57 (m, 2H), 4.38 (dd, J = 5.5 & 1.5 Hz, 1H), 4.20–4.17 (m, 1H), 3.85 (d, J = 4.5 Hz, 1H), 2.31–2.24 (m, 1H), 1.04 (d, J = 7.0 Hz, 6H); 13C NMR (125 MHz, CD3OD) δ 168.5, 162.1, 150.0, 138.1, 129.0, 111.2, 108.4, 102.3, 82.0, 70.3, 63.5, 58.0, 29.7, 17.2, 16.5; anal. calcd for C15H21BrClN3O6·(H2O)1.2: C, 37.82; H, 4.95; N, 8.82. Found: C, 37.69, H, 5.12; N, 8.65; HRMS (EI) calcd for (C15H20BrN3O6 + H)+ 418.0614, found 418.0611.
l-BHDU-5′-O-l-tryptophan Ester Hydrochloride (19)
Yield: 90%; [α]D24 = −39.12 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.69 (s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.37–7.32 (m, 2H), 7.12–7.07 (m, 2H), 7.01 (t, J = 7.5 Hz, 1H), 6.84 (d, J = 13.6 Hz, 1H), 6.29 (d, J = 4.2 Hz, 1H), 5.11 (t, J = 3.0 Hz, 1H), 4.45 (dd, J = 12.5 & 3.1 Hz, 1H), 4.35 (d, J = 11.9 Hz, 1H), 4.25 (dd, J = 12.5 & 3.0 Hz, 1H), 4.16 (dd, J = 10.4 & 5.8 Hz, 1H), 3.81 (t, J = 6.0 Hz, 1H), 3.17 (dd, J = 6.0, 2.8 Hz, 2H); 13C NMR (125 MHz, CD3OD) δ 168.8, 162.0, 150.0, 138.0, 136.9, 128.9, 126.9, 124.3, 121.7, 119.1, 117.4, 111.3, 108.5, 105.6, 102.1, 81.6, 70.1, 63.9, 53.3, 26.1; anal. calcd for C15H21BrClN3O6·(H2O)1.0: C, 45.06; H, 4.32; N, 10.01. Found: C, 44.75, H, 4.46; N, 9.78; HRMS (EI) calcd for (C21H21BrN4O6 + H)+ 505.0723, found 505.0717.
l-BHDU-5′-O-l-leucine Ester Hydrochloride (20)
Yield: 89%; [α]D24 = −4.9 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.70 (s, 1H), 7.45 (d, J = 13.6 Hz, 1H), 6.90 (d, J = 13.6 Hz, 1H), 6.36 (dd, J = 5.8 & 1.8 Hz, 1H), 5.30 (t, J = 3.7 Hz, 1H), 4.62 (dd, J = 12.2 & 3.8 Hz, 1H), 4.50 (dd, J = 12.2 & 3.7 Hz, 1H), 4.43 (dd, J = 10.4 & 1.7 Hz, 1H), 4.24 (dd, J = 10.4 & 5.9 Hz, 1H), 4.06 (dd, J = 7.5 & 6.3 Hz, 1H), 1.87–1.70 (m, 3H), 1.00 (dd, J = 6.2 & 3.3 Hz, 6H); 13C NMR (125 MHz, CD3OD) δ 169.6, 162.0, 138.1, 129.0, 111.5, 108.5, 102.1, 81.7, 70.0, 64.1, 51.1, 39.2, 24.1, 21.2, 21.0; anal. calcd for C16H23BrClN3O6·(H2O)1.0: C, 39.48; H, 5.18; N, 8.63. Found: C, 39.29, H, 5.31; N, 8.41; HRMS (EI) calcd for (C16H22BrN3O6 + H)+ 432.0770, found 432.0774.
l-BHDU-5′-O-l-isoleucine Ester Hydrochloride (21)
Yield: 86%; [α]D26 = −1.9 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.73 (s, 1H), 7.46 (d, J = 13.6 Hz, 1H), 6.91 (d, J = 13.6 Hz, 1H), 6.37 (dd, J = 5.8 & 1.6 Hz, 1H), 5.30 (t, J = 3.4 Hz, 1H), 4.61 (dd, J = 12.3 & 3.3 Hz, 1H), 4.53 (dd, J = 12.3 & 3.5 Hz, 1H), 4.45 (dd, J = 10.4 & 1.5 Hz, 1H), 4.25 (dd, J = 10.4 & 5.9 Hz, 1H), 4.01 (d, J = 4.1 Hz, 1H), 2.03–1.96 (m, 1H), 1.56–1.46 (m, 1H), 1.39–1.31 (m, 1H), 1.06 (d, J = 7.0 Hz, 3H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CD3OD) δ 168.5, 162.1, 150.1, 138.3, 129.0, 111.5, 108.5, 102.2, 81.7, 70.0, 63.8, 57.3, 36.4, 24.9, 13.8, 10.6; anal. calcd for C16H23BrClN3O6: C, 41.00; H, 4.95; N, 8.96. Found: C, 40.82, H, 4.93; N, 8.74; HRMS (EI) calcd for (C16H22BrN3O6 + H)+ 432.0770, found 432.0767.
l-BHDU-5′-O-l-proline Ester Hydrochloride (22)
Yield: 85%; [α]D25 = −15.78 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.74 (s, 1H), 7.46 (d, J = 13.6 Hz, 1H), 6.92 (d, J = 14.1 Hz, 1H), 6.36 (dd, J = 5.8 & 1.7 Hz, 1H), 5.30 (t, J = 3.1 Hz, 1H), 4.62 (dd, J = 12.4 & 3.2 Hz, 1H), 4.55 (dd, J = 12.4 & 3.1 Hz, 1H), 4.47–4.42 (m, 2H), 4.24 (dd, J = 10.4 & 5.9 Hz, 1H), 3.48–3.42 (m, 1H), 3.40–3.36 (m, 1H), 2.44–2.36 (m, 1H), 2.19–2.07 (m, 3H); 13C NMR (125 MHz, CD3OD) δ 168.5, 162.1, 150.0, 138.3, 129.1, 111.4, 108.4, 102.2, 100.0, 81.7, 70.1, 64.0, 59.3, 28.1, 23.2; anal. calcd for C15H19BrClN3O6·(H2O)1.0: C, 38.28; H, 4.50; N, 8.93. Found: C, 38.30, H, 4.50; N, 8.82; HRMS (EI) calcd for (C15H18BrN3O6 + H)+ 416.0457, found 416.0457.
l-BHDU-5′-O-l-glutamine Ester Hydrochloride (23)
Yield: 87%; [α]D25 = +1.9 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.74 (s, 1H), 7.45 (d, J = 13.5 Hz, 1H), 6.91 (d, J = 13.6 Hz, 1H), 6.35 (dd, J = 5.8 & 1.8 Hz, 1H), 5.30 (t, J = 3.8 Hz, 1H), 4.61 (dd, J = 12.1 & 4.0 Hz, 1H), 4.52 (dd, J = 12.1 & 3.7 Hz, 1H), 4.43 (dd, J = 10.3 & 1.7 Hz, 1H), 4.24 (dd, J = 10.4 & 5.9 Hz, 1H), 4.16 (t, J = 6.3 Hz, 1H), 2.54–2.50 (m, 2H), 2.26–2.13 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 168.7, 162.1, 150.0, 138.1, 129.0, 111.5, 108.5, 102.1, 100.0, 81.9, 70.1, 64.3, 52.3, 30.3, 25.5; anal. calcd for C15H20BrClN4O7·(H2O)1.5: C, 35.28; H, 4.54; N, 10.97. Found: C, 35.43, H, 4.63; N, 10.63; HRMS (EI) calcd for (C15H19BrN4O7 + H)+ 447.0515, found 447.0508.
l-BHDU-5′-O-2-aminoisobutyric Acid Ester Hydrochloride (24)
Yield: 82%; 1H NMR (500 MHz, CD3OD) δ 7.71 (s, 1H), 7.44 (d, J = 13.6 Hz, 1H), 6.90 (d, J = 13.6 Hz, 1H), 6.35 (dd, J = 5.9 & 1.8 Hz, 1H), 5.29 (t, J = 3.8 Hz, 1H), 4.60 (dd, J = 12.2 & 4.0 Hz, 1H), 4.54 (dd, J = 12.2 & 3.6 Hz, 1H), 4.44 (dd, J = 10.4 & 1.8 Hz, 1H), 4.25 (dd, J = 10.4 & 5.9 Hz, 1H), 1.63 (s, 3H), 1.61 (s, 3H); 13C NMR (125 MHz, CD3OD) δ 171.23, 162.1, 150.0, 138.0, 129.0, 111.5, 108.4, 102.2, 81.9, 70.1, 64.3, 56.6, 22.7; anal. calcd for C14H19BrClN3O6·(H2O)1.0: C, 36.66; H, 4.61; N, 9.16. Found: C, 36.94, H, 4.64; N, 8.77; HRMS (EI) calcd for (C14H18BrN3O6 + H)+ 404.0457, found 404.0451.
l-BHDU-5′-O-l-lysine Ester Hydrochloride (25)
Yield: 85%; [α]D25 = −1.6 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.74 (s, 1H), 7.46 (d, J = 13.5 Hz, 1H), 6.92 (d, J = 13.5 Hz, 1H), 6.37 (dd, J = 5.8 & 1.6 Hz, 1H), 5.32 (t, J = 3.6 Hz, 1H), 4.61 (dd, J = 12.2 & 3.7 Hz, 1H), 4.54 (dd, J = 12.2 & 3.4 Hz, 1H), 4.46 (dd, J = 10.4 & 1.6 Hz, 1H), 4.25 (dd, J = 10.4 & 5.9 Hz, 1H), 4.11 (t, J = 6.5 Hz, 1H), 3.02–2.95 (m, 2H), 2.06–1.89 (m, 2H), 1.78–1.72 (m, 2H), 1.62–1.48 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 168.9, 162.2, 150.1, 138.4, 129.0, 111.4, 108.5, 102.2, 100.0, 70.0, 64.2, 52.4, 39.0, 29.7, 26.7, 21.7; anal. calcd for C16H25BrCl2N4O6·(H2O)1.5: C, 35.12; H, 5.16; N, 10.24. Found: C, 35.26, H, 5.28; N, 9.87; HRMS (EI) calcd for (C16H23BrN4O6 + H)+ 447.0879, found 447.0869.
(S)-2-((tert-Butoxycarbonyl)amino)-3-(4-((tert-butyldimethylsilyl)oxy)phenyl)propanoic Acid (28)
To a solution of (tert-butoxycarbonyl)-L-tyrosine (27, 0.50 g, 1.77 mmol) in DMF (10 mL) were added TBDMSCl (0.4 g, 2.65 mmol) and imidazole (0.24 g, 3.54 mmol) at 0 °C, then the reaction mixture was stirred at rt for 16 h. It was quenched with water (15 mL), and the aqueous layer was extracted with diethyl ether (25 mL × 2); the combined organic layer was washed with brine (25 mL), dried over Na2SO4, and concentrated under reduced pressure. The crude residue was purified by silica gel chromatography (10–25% EtOAc/hexane) to give compound 28 as a viscous solid. Yield (0.44 g, 62%). 1H NMR (500 MHz, CDCl3) δ 7.04 (d, J = 8.0 Hz, 2H), 6.78 (d, J = 7.9 Hz, 2H), 4.89 (d, J = 8.0 Hz, 1H), 4.54 (s, 1H), 3.12 (d, J = 12.3 Hz, 1H), 3.00 (d, J = 14.6 Hz, 1H), 1.41 (s, 9H), 0.97 (s, 9H), 0.18 (s, 6H); HRMS (EI) calcd For (C20H33NO5Si+Na)+ 418.2026, found m/z 418.2033.
((2S,4S)-4-(5-((E)-2-Bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methyl (S)-2-((tert-Butoxycarbonyl)amino)-3-(4-((tert-butyldimethylsilyl)oxy)phenyl) Propanoate (29)
Compound 28 was coupled with l-BHDU by following the above-described coupling procedure for compounds 2–13. Yield: 80%; 1H NMR (500 MHz, CDCl3) δ 8.54 (bs, 1H), 7.55 (s, 1H), 7.48 (d, J = 13.6 Hz, 1H), 6.99 (d, J = 9.3 Hz, 3H), 6.76 (d, J = 8.2 Hz, 1H), 6.31 (d, J = 5.6 Hz, 1H), 5.12 (t, J = 3.3 Hz, 1H), 4.90 (d, J = 7.4 Hz, 1H), 4.61–4.53 (m, 1H), 4.47 (d, J = 8.0 Hz, 1H), 4.28–4.09 (m, 3H), 3.05–2.91 (m, 2H), 1.41 (s, 9H), 0.97 (s, 9H), 0.10 (s, 6H); HRMS (EI) calcd for (C30H42BrN3O9Si + H)+ 696.1952, found m/z 696.1957.
((2S,4S)-4-(5-((E)-2-Bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methyl (tert-Butoxycarbonyl)-l-tyrosinate (30)
To a stirred solution of compound 29 (100 mg, 0.143 mmol) in THF (5 mL) was added 1 M TBAF in THF (0.21 mL) at 0 °C. The mixture was warmed to rt and stirred for 3 h. Then, the mixture was quenched with water (10 mL), and the aqueous layer was extracted with EtOAc (15 mL × 2); the combined organic layer was washed with brine (15 mL), dried over Na2SO4, and concentrated under reduced pressure. The obtained crude compound was purified by silica gel chromatography (45–50% EtOAc/hexane) to give 30 as a sticky solid. Yield (65 mg, 78%). 1H NMR (500 MHz, CDCl3) δ 9.95 (bs, 1H), 7.50–7.37 (m, 2H), 6.92 (d, J = 7.7 Hz, 2H), 6.72 (d, J = 9.9 Hz, 3H), 6.28 (s, 1H), 5.11 (d, J = 7.5 Hz, 1H), 5.03 (s, 1H), 4.44 (d, J = 12.5 Hz, 2H), 4.32–4.18 (m, 2H), 4.15–4.07 (m, 1H), 2.99–2.89 (m, 2H), 1.42 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 174.3, 161.7, 155.4, 149.6, 137.3, 130.3, 128.2, 126.9, 115.6, 112.3, 110.9, 102.6, 81.3, 80.5, 70.9, 62.9, 54.9, 53.5, 37.3, 28.5; HRMS (EI) calcd for (C24H28BrN3O9 – H)− 580.0931, found 580.0936.
l-BHDU-5′-O-l-tyrosine Ester Hydrochloride (26)
To a stirred solution of compound 30 (100 mg, 0.171 mmol) in DCM (5 mL) at 0 °C was added TFA (1 mL), and the mixture was stirred at rt for 1 h. After checking TLC, the volatiles were removed under reduced pressure. The obtained residue was dissolved in methanol (3 mL), cooled to 0 °C, and a solution of 2 M HCl was added in ether and stirred for 30 min. The volatiles were distilled off under reduced pressure, and the crude solid was washed with anhydrous ether to obtain 26 as the HCl salt as a white solid. Yield (75 mg, 85%). [α]D25 = −2.4 (c 0.5, MeOH); 1H NMR (500 MHz, CD3OD) δ 7.68 (s, 1H), 7.40 (d, J = 13.5 Hz, 1H), 7.03 (d, J = 5.9 Hz, 2H), 6.87 (d, J = 13.1 Hz, 1H), 6.79–6.74 (m, 2H), 6.34 (dd, J = 5.9 & 1.7 Hz, 1H), 5.22 (t, J = 3.3 Hz, 1H), 4.56 (dd, J = 12.4 & 3.4 Hz, 1H), 4.49 (dd, J = 12.4 & 3.2 Hz, 1H), 4.42 (dd, J = 10.4 & 1.6 Hz, 1H), 4.28–4.19 (m, 2H), 3.17–3.04 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 168.7, 162.1, 157.1, 150.1, 138.2, 130.3, 129.0, 123.7, 115.7, 111.5, 108.5, 102.2, 81.7, 70.0, 63.7, 54.0, 35.2; anal. calcd for C19H21BrClN3O7·(H2O)1.0: C, 42.52; H, 4.32; N, 7.83. Found: C, 42.54, H, 4.42; N, 7.68; HRMS (EI) calcd for (C19H20BrN3O7 – H)− 480.0406, found 480.0410.
Isopropyl (Chloro(phenoxy)phosphoryl)-l-alaninate (31)
To a solution of phenyl dichlorophosphate (1.0 mol) and l-alanine isopropyl ester hydrochloride (1.0 mol) in anhydrous DCM at −78 °C, triethylamine (2.0 mol) was added dropwise, and the mixture was stirred at the same temperature for 1 h. The reaction mixture was slowly warmed to rt and stirred for 2 h. The solvent was removed under reduced pressure, and the crude residue was resuspended in anhydrous ether and filtered through a Celite bed under nitrogen. The filtrate was concentrated in vacuo to give compound 31 as an oily liquid, which was used as such for the next step. 1H NMR (500 MHz, CDCl3) δ 7.38–7.24 (m, 2H), 7.23–7.21 (m, 3H), 5.11–5.04 (m, 1H), 4.33–4.07 (m, 2H), 1.49 (d, J = 10.0 Hz, 3H), 1.26 (dd, J = 15.0 & 5.0 Hz, 6H); 31P NMR (CDCl3, 202 MHz): δ 8.69, 8.31.
Isopropyl (Chloro(phenoxy)phosphoryl)-l-phenylalaninate (32)
To a solution of phenyl dichlorophosphate (1.0 mol) and the l-phenylalanine isopropyl ester hydrochloride (1.0 mol) in anhydrous DCM at −78 °C, triethylamine (2.0 mol) was added dropwise, and the mixture was stirred at the same temperature for 1 h. The reaction mixture was slowly warmed to rt and stirred for 2 h. The solvent was removed under reduced pressure, and the crude residue was resuspended in anhydrous ether and filtered through a Celite bed under nitrogen. The filtrate was concentrated in vacuo to give compound 32 as an oily liquid, which was used as such for the next step. 1H NMR (500 MHz, CDCl3) δ 7.37–7.14 (m, 10H), 5.04–4.95 (m, 1H), 4.42–4.30 (m, 1H), 4.17–4.03 (m, 1H), 3.18–3.06 (m, 1H), 1.19 (dd, J = 10.0 & 5.0 Hz, 6H); 31P NMR (CDCl3, 202 MHz): δ 8.63, 8.60.
Isopropyl ((((2S,4S)-4-(5-((E)-2-Bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)-l-alaninate (33)
To a stirred suspension of l-BHDU, 1 (0.1 g, 0.31 mmol) in anhydrous THF (8 mL) and N-methylimidazole (0.15 mL, 1.86 mmol) were added at 0 °C. Phosphorochloridate 31 (0.37 g, 1.24 mmol) in THF (10 mL) was added dropwise, and the mixture was stirred for 12 h at ambient temperature. The volatiles were removed under reduced pressure, and the residue was purified by silica gel column chromatography (2% methanol/DCM) to give compound 33 as an off-white solid. Yield (0.12 g, 66%). Mp 63–65 °C; 1H NMR (500 MHz, CDCl3) δ 7.71–7.65 (m, 1H), 7.45–7.41 (m, 1H), 7.31–7.28 (m, 2H), 7.25–7.18 (m, 1H), 7.13 (t, J = 15.0 & 5.0 Hz, 1H), 6.87 (s, 1H), 6.70 (dd, J = 10.0 & 5.0 Hz, 1H), 6.30 (dd, J = 5.0 & 2.5 Hz, 1H), 5.17–5.15 (m, 2H), 5.01–4.95 (m, 1H), 4.46–4.21 (m, 2H), 4.18–4.14 (m, 2H), 4.02–3.67 (m, 2H), 1.32 (d, J = 5.0 Hz, 3H), 1.19 (dd, J = 10.0 & 5.0 Hz, 6H); 31P NMR (CDCl3, 202 MHz): δ 4.07, 3.73; 13C NMR (125 MHz, CDCl3) δ 173.0, 161.4, 150.4, 149.6, 137.3, 129.9, 129.5, 128.4, 125.2, 120.3, 112.1, 110.6, 103.3, 81.4, 71.9, 69.5, 64.2, 50.5, 33.4, 21.8, 21.0; anal. calcd for C22H27BrN3O9P: C, 44.91; H, 4.63; N, 7.14. Found: C, 45.23, H, 4.92; N, 6.85; HRMS (EI) calcd for (C22H27BrN3O9P + H)+ 588.0747; found 588.0743.
Isopropyl ((((2S,4S)-4-(5-((E)-2-Bromovinyl)-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-1,3-dioxolan-2-yl)methoxy)(phenoxy)phosphoryl)-l-phenylalaninate (34)
To a stirring suspension of l-BHDU, 1 (0.1 g, 0.31 mmol) in anhydrous THF and N-methylimidazole, (NMI, 0.15 mL, 1.86 mmol), were added at 0 °C. Phosphorochloridate 32 (0.47 g, 1.24 mmol) in THF (10 mL) was added dropwise, and the mixture was stirred for 12 h at ambient temperature. The volatiles were removed under reduced pressure, and the residue was purified by silica gel column chromatography (1.5% methanol/DCM) to give compound 34 as a white solid. Yield (0.12g, 57%). Mp 71–73 °C; 1H NMR (500 MHz, CDCl3) δ 8.27 (m, 1H), 7.64 (d, J = 5.0 Hz, 1H), 7.45–7.39 (m, 1H), 7.31–7.29 (m, 2H), 7.28–7.02 (m, 8H), 6.70 (d, J = 15.0 Hz, 1H), 6.29–6.26 (m, 1H), 5.08–5.07 (m, 1H), 4.97–4.91 (m, 1H), 4.30–4.05 (m, 5H), 3.52–3.34 (m, 1H), 3.02–2.91 (m, 2H), 1.17 (dd, J = 10.0 & 5.0 Hz, 6H); 31P NMR (CDCl3, 202 MHz): δ 3.91, 3.82; 13C NMR (125 MHz, CDCl3) δ 171.9, 161.0, 150.4, 149.3, 137.3, 135.5, 129.8, 129.7, 129.6, 128.6, 128.3, 127.2, 125.3, 125.2, 120.2, 112.1, 110.6, 103.4, 81.6, 71.6, 69.6, 63.8, 55.6, 40.4, 21.8; anal. calcd for C28H31BrN3O9P: C, 50.61; H, 4.70; N, 6.32. Found: C, 50.92, H, 4.95; N, 6.11; HRMS (EI) calcd for (C28H31BrN3O9P + H)+ 664.1060; found 664.1056.
l-BHDU-5′-[(2-octadecyloxyethyl)phosphate] (ODE-l-BHDU-MP, 38)
A solution of 1,2,4-triazole (0.28 g, 4.1 mmol) and triethylamine (0.57 mL, 4.1 mmol) in anhydrous THF (10 mL) was added to a solution of 2-chlorophenyl dichlorophosphate (35, 0.5 g, 2.0 mmol) in THF (10 mL). The reaction mixture was stirred at rt for 30 min and filtered. In another flask, to a prestirred (20 min.) solution of l-BHDU (1, 0.49 g, 1.5 mmol) in dry THF (20 mL), NMI (0.17 mL, 2.0 mmol) was added above the filtrate intermediate at 0 °C and the mixture was stirred at rt for 1 h. Then, 2-(octadecyloxy)ethanol (0.48 g, 1.5 mmol) was added to the mixture and stirred overnight at rt. The solvent was evaporated under reduced pressure, and the obtained crude was purified by silica gel column chromatography (3% MeOH/DCM) to produce l-BHDU 5′-[(2-chlorophenyl 2-octadecyloxyethyl) phosphate] 36 as a white solid. Yield (0.85 g, 69%). 1H NMR (500 MHz, CDCl3) δ 9.13 (bs, 1H, NH), 7.71 (d, J = 2.5 Hz, 1H), 7.48–7.38 (m, 3H), 7.20 (t, J = 16.0 Hz, 1H), 7.10 (t, J = 15.5 Hz, 1H), 6.72 (dd, J = 13.5 & 4.5 Hz, 1H); 6.33 (dd, J = 16.5 & 7.5 Hz, 1H), 5.17–5.16 (m, 1H), 4.55–4.43 (m, 2H), 4.37–4.32 (m, 2H), 4.22–4.16 (m, 2H), 3.66–3.64 (m, 2H), 3.42 (t, J = 13.5 & 8.0 Hz, 2H), 1.52–1.48 (m, 2H), 1.29–1.23 (m, 30H), 0.86 (t, J = 7.0 Hz, 3H); 31P NMR (202 MHz, CDCl3): δ −6.10, 6.36; 13C NMR (125 MHz, CDCl3) δ 160.9, 149.3, 146.3, 130.9, 130.5, 128.2, 127.6, 126.6, 126.0, 125.5, 112.1, 110.8, 110.4, 81.5, 81.4, 81.2, 71.5, 71.4, 68.3, 31.9, 29.7, 29.5, 29.4, 26.0, 22.7, 14.2. The obtained intermediate 36 (0.45 g, 0.55 mmol) was dissolved in THF (5 mL), and 0.5 N NaOH solution (1.5 mL) was added at 0 °C. The mixture was stirred at 50 °C for 2 h and neutralized with 1 N HCl at 0 °C. The volatiles were removed under reduced pressure, and the residue was purified by silica gel column chromatography (10% MeOH/DCM) to give 38 as a white solid. Yield (0.29 g, 74%). Mp 115–117 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.59 (s, 1H), 8.20 (s, 1H), 7.39 (d, J = 13.5 Hz, 1H), 7.26 (d, J = 14.0 Hz, 1H), 6.18 (d, J = 4.5 Hz, 1H), 5.04–5.03 (m, 1H), 4.23 (d, J = 9.5 Hz, 1H), 4.08 (t, J = 4.5 Hz, 1H), 3.91–3.93 (m, 2H), 3.72–3.69 (m, 2H), 3.43 (t, J = 4.5 Hz, 2H), 3.35–3.34 (m, 2H), 1.46–1.43 (m, 2H), 1.28–1.23 (m, 30H), 0.85 (t, J = 8.6 Hz, 3H); 31P NMR (202 MHz, DMSO-d6): δ −0.7; 13C NMR (125 MHz, DMSO-d6) δ 162.3, 150.0, 139.4, 130.6, 110.9, 107.7, 103.2, 81.3, 70.8, 69.6, 66.0, 64.6, 31.9, 29.7, 29.6, 29.4, 29.2, 26.1, 22.6, 14.5; anal. calcd for C30H52BrN2O9P·(H2O)0.5: C, 51.14; H, 7.58; N, 3.98. Found: C, 51.20, H, 7.55; N, 3.92; HRMS (EI) calcd for (C30H52BrN2O9P + Na)+ 717.2492, found 717.2485.
l-BHDU-5′-[(2-hexadecyloxyethyl)phosphate] (HDP-l-BHDU-MP, 39)
Compound 39 (50 mg) was synthesized in qualitative yield by following the same procedure explained for compound 38. Yield 85%; mp 122–123 °C; 1H NMR (500 MHz, CD3OD) δ 8.02 (s, 1H), 7.46 (d, J = 14.0 Hz, 1H), 7.04 (d, J = 13.5 Hz, 1H), 6.32 (dd, J = 7.5 & 1.5 Hz, 1H), 5.19 (s, 1H), 4.29 (dd, J = 7.0 & 2.0 Hz, 1H), 4.22–4.19 (m, 1H), 4.15–4.14 (m, 2H), 3.99 (d, J = 6.5 & 2.0 Hz, 2H), 3.55 (t, J = 7.0 Hz, 2H), 3.43 (t, J = 6.5 Hz, 2H), 1.92–1.89 (m, 2H), 1.56–1.52 (m, 2H), 1.37–1.31 (m, 26H), 0.93 (t, J = 7.0 Hz, 3H); 31P NMR (202 MHz, CD3OD): δ 0.58; 13C NMR (125 MHz, CD3OD) δ 162.3, 150.0, 138.6, 129.6, 129.1, 111.2, 108.3, 107.8, 104.5, 104.0, 81.7, 71.1, 70.7, 67.0, 63.6, 62.4, 31.7, 30.8, 30.7, 30.6, 29.4, 29.3, 29.1, 25.9, 22.4, 13.0; anal. calcd for C29H50BrN2O9P·(H2O)0.9: C, 49.92; H, 7.48; N, 4.01. Found: C, 49.65, H, 7.62; N, 3.69; HRMS (EI) calcd for (C29H50BrN2O9P + H)+ 681.2516, found 681.2507.
Bis(POM) Prodrug of l-BHDU-MP (POM-l-BHDU-MP, 41)
To a stirred solution of l-BHDU (1, 80 mg, 0.25 mmol) and N-methylimidazole (0.16 mL, 2.0 mmol) in dry THF (3 mL), bis(POM)phosphorochloridate3140 (500 mg, 1.23 mmol) at 0 °C was added by dissolving in dry THF (3 mL) and stirred for 15 min. Then, the reaction was warmed to rt and stirred for 3 h. The mixture was quenched with methanol, and the solvent was removed under reduced pressure. The crude was purified by silica gel column chromatography (0.5% MeOH/DCM) to give 41 as a colorless sticky oil, which was crystallized in DCM-pentane to render an off-white solid. Yield: (75 mg, 47%). Mp: 80–85 °C; 1H NMR (500 MHz, CDCl3) δ 8.97 (bs, 1H), 7.71 (s, 1H), 7.45 (d, J = 13.6 Hz, 1H), 6.79 (d, J = 13.6 Hz, 1H), 6.35 (d, J = 4.5 Hz, 1H), 5.72–5.65 (m, 4H), 5.15 (s, 1H), 4.43–4.39 (m, 1H), 4.35–4.31 (m, 1H), 4.26–4.21 (m, 1H), 4.20–4.17 (m, 1H), 1.23 (s, 18H); 31P NMR (202 MHz, CDCl3) δ −3.02; 13C NMR (125 MHz, CDCl3) δ 176.9, 161.1, 149.5, 137.3, 128.4, 112.2, 110.6, 102.9, 83.2, 81.4, 71.6, 65.1, 38.9, 26.9; anal. calcd for C22H32BrN2O12P: C, 42.12; H, 5.14; N, 4.47. Found: C, 42.34, H, 5.23; N, 4.26; HRMS (EI) calcd for (C22H32BrN2O12P + H)+ 627.0954, found m/z 627.0953.
((Bis(benzyloxy)phosphoryl)oxy)methyl Isopropyl Carbonate (43)
To a stirred mixture of compound 42 (560 mg, 2.0 mmol) and cesium carbonate (1.6 g, 4.97 mmol) in acetone (10 mL), POC-I (610 mg, 2.38 mmol) was added dropwise at rt and stirred overnight. The reaction mixture was filtered through a Buchner funnel, the obtained filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (20% EtOAc/hexane) to produce compound 43 as a colorless oil. Yield (650 mg; 82%). 1H NMR (500 MHz, CDCl3) δ 7.35–7.30 (m, 10H), 5.61–5.58 (d, J = 15.0 Hz, 2H), 5.07–5.05 (d, J = 10.0 Hz, 4H), 4.90–4.85 (m, 1H), 1.29–1.28 (d, J = 5.0 Hz, 6H); 31P NMR (202 MHz, CDCl3) δ −2.02; HRMS (EI) calcd for (C19H23O7P + Na)+ 417.1079, found m/z 417.1085.
(((Benzyloxy)(hydroxy)phosphoryl)oxy)methyl Isopropyl Carbonate (44)
To a stirred solution of compound 43 (1.0 g, 2.54 mmol) in acetonitrile (20 mL), NaI (0.76 g, 5.07 mmol) was added and stirred at 45 °C for 12 h. The reaction mixture was concentrated under reduced pressure, and the obtained crude was washed with dry ether and dried under high vacuum. The obtained residue was used as such for the next step without further purification.
((Benzyloxyphosphoryl)bis(oxy))bis(methylene) Diisopropyl Bis(carbonate) (45)
To a stirred mixture of compound 44 (231 mg, 0.75 mmol) and cesium carbonate (371 mg, 1.13 mmol) in acetone (10 mL), POC-I (240 mg, 0.98 mmol) was added dropwise at rt and continued stirring overnight. The reaction mixture was filtered through a Buchner funnel, the obtained filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (15% EtOAc/hexane) to produce 45 as a colorless oil. Yield (210 mg, 66%) 1H NMR (500 MHz, CDCl3) δ 7.38–7.33 (m, 5H), 5.64–5.62 (d, J = 10.0 Hz, 4H), 5.13–5.12 (d, J = 5.0 Hz, 2H), 4.94–4.86 (s, 2H), 1.30–1.28 (dd, J = 10.0 Hz, 12H); 31P NMR (202 MHz, CDCl3) δ −3.77; HRMS (EI) calcd for (C17H25O10P + H)+ 420.1185, found m/z 420.1192.
((Hydroxyphosphoryl)bis(oxy))bis(methylene) Diisopropyl Bis(carbonate) (46)
A suspension of compound 45 (300 mg, mmol) and 10% Pd/C (30 mg) in methanol at ambient temperature was treated with H2 at 5 psi for 2 h. The mixture was passed through a Celite bed and concentrated under reduced pressure to give 46 as a colorless sticky liquid. Yield (200 mg, 85%). Compound 46 was used as such for the next step reaction without further purification. 1H NMR (500 MHz, CDCl3) δ 7.99 (bs, 1H), 5.63–5.60 (d, J = 15.0 Hz, 4H), 4.95–4.88 (s, 2H), 1.31–1.30 (d, J = 5.0 & 2.0 Hz, 12H); 13C NMR (125 MHz, CDCl3) δ 153.1, 85.4, 73.4, 21.7; 31P NMR (202 MHz, CDCl3) δ −3.36; HRMS (EI) calcd for (C10H19O10P + Na)+ 353.0614, found m/z 353.0621.
Bis(POC) Prodrug of l-BHDU-MP (POC-l-BHDU-MP, 47)
Compound 46 (92 mg, 0.282 mmol) was taken in NEt3 (1 mL) and pyridine (0.5 mL), stirred at rt for 10 min, and then the contents were concentrated under reduced pressure followed by co-evaporation with toluene (3 mL). The residue was dissolved in dry THF (3 mL) and cooled to 0 °C. After that, l-BHDU (30 mg, 0.094) was added, followed by the addition of DIPEA (0.05 mL, 0.282 mmol), BOP-Cl (48.0 mg, 0.189 mmol), and 3-nitro-1,2,4-triazole sequentially (21 mg, 0.189 mmol). The mixture was stirred at the same temperature for 2 h and diluted with EtOAc (50 mL). The organic layer was washed with saturated NaHCO3 solution (20 mL × 2), followed by brine solution (10 mL), and dried over Na2SO4. The solvent was removed under reduced pressure, and the obtained crude was purified by silica gel column chromatography (0.8% Methanol/DCM) to give compound 47 as a colorless sticky solid. Yield (13 mg, 22%). 1H NMR (500 MHz, CDCl3) δ 8.92 (s, 1H), 7.70 (s, 1H), 7.44 (d, J = 13.6 Hz, 1H), 6.78 (d, J = 13.6 Hz, 1H), 6.36 (dd, J = 5.6 & 2.0 Hz, 1H), 5.73–5.64 (m, 4H), 5.16 (d, J = 2.0 Hz, 1H), 4.92 (dq, J = 12.4 & 6.2 Hz, 2H), 4.46–4.33 (m, 2H), 4.28–4.17 (m, 2H), 1.33–1.30 (m, 12H); 31P NMR (202 MHz, CDCl3) −3.2; 13C NMR (125 MHz, CDCl3) δ 161.0, 153.0, 149.4, 137.3, 128.3, 112.2, 110.5, 102.9, 85.8, 81.4, 77.4, 73.7, 71.5, 65.2, 21.7; HRMS (EI) calcd for (C20H28BrN2O14P + H)+ 631.0540, found 631.0538; HPLC purity ≥ 97%.
Cells and Viruses
Adherent retinal pigmented epithelial cells (ARPE-19; CRL-2302; ATCC) were used for all cell culture-based assays and can be used for at least 30 passages. Cells were grown in Dulbecco’s Modified Eagle Medium with 4.5 g/l-glucose, l-glutamine, and sodium pyruvate (DMEM, 1X, Corning, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (Benchmark FBS; Gemini Bio Products, West Sacramento, CA), penicillin–streptomycin (5000 IU/mL), and amphotericin B (250 μg/mL). The VZV strains used for the cell-based assays included VZV-ORF57-Luc, along with the mutant strains VZV-ORF57-ΔTK, VZV-ORF57-ΔTS, and VZV-ORF57-ΔTKTS.34 All viruses were passaged no more than 10 times in ARPE-19 cells.
Compounds and Formulations
All compounds were prepared as 10 mM stocks in DMSO and stored at −80 °C. The control compounds, cidofovir (CDV; BEI Resources, Manassas, VA) and acyclovir (ACV; Millipore Sigma, Burlington, MA), are commercially available. Stock compounds were diluted in DMSO and/or complete tissue culture media prior to being added to cells.
Efficacy and Cytotoxicity Assays in ARPE-19 Cells
Antiviral activity against the VZV strains used here was evaluated in ARPE-19 cells as previously described.16,36 Briefly, confluent ARPE-19 cells in a 96-well plate were infected with VZV at an approximate MOI of 0.01 for 2 h. Virus inoculum was removed, then the infected cells were treated with l-BHDU, its prodrugs, or the control compounds, CDV or ACV, at a range of concentrations. Virus spread was measured by bioluminescence (Total Flux) after 72 h. VZV yield was calculated as the average total flux for each concentration divided by the average Total Flux of the untreated wells. Cytotoxicity studies were performed using a neutral red assay as previously described.16,37 Cytotoxicity was measured after 72 h of co-culture with the test compounds under the same conditions as the antiviral evaluations at higher concentration ranges.
Bioluminescence Imaging
Imaging was performed using the IVIS 50 instrument (Caliper Life Sciences/Xenogen, Hopkinton, Massachusetts) as previously described.37 Infected 96-well plates were imaged for 30 s–1 min, and VZV spread was measured as Total Flux (photons/s/cm2/steradian) in each well or region of interest (ROI).
Statistical Analysis
Calculations for the 50% effective concentrations (EC50) and 50% cytotoxic concentrations (CC50) were performed using GraphPad software (San Diego, California, www.graphpad.com). Other calculations and graphs were also performed using GraphPad. A p-value of ≤0.05 was considered statistically significant.
Procedure for Liver Homogenate Samples
Untreated BALB/c mice were euthanized, and their livers were immediately removed. Liver tissue was homogenized using a Qiagen TissueLyser LT (Qiagen LLC, Germantown MD). Exactly 0.95 mL of liver homogenate was combined with POM-l-BHDU-MP (0.3 μg/mL) and spiked with the internal standard azidothymidine (a final concentration of 20 ng/mL, Cayman Chemical, Ann Arbor, MI). The samples were incubated at 37 °C, and the enzymatic reactions were stopped by adding 2× volumes of methanol at 0, 2, 4, 10, 30, and 60 min. The samples were vortexed and centrifuged to remove the precipitated protein. Supernatants were dried using an N-EVAP 116 analytical nitrogen evaporator and reconstituted with 50% methanol in water. The concentration of POM-l-BHDU-MP, l-BHDU, and l-BHDU-MP were determined using LC-MS/MS.
Collection of Mouse Plasma Samples for PK Analysis
This animal protocol #282 was approved by the IACUC at SUNY Upstate Medical University, which has been fully accredited by AAALAC since 7/31/1999. This method was previously used to evaluate l-BHDU effects on 5-fluorouracil metabolism.16 Briefly, a single equimolar dose of 22.5 mg/kg POM-l-BHDU-MP or 11.4 mg/kg l-BHDU was dissolved in Cremophor–DMSO–saline (1:1:8) and administered by oral gavage to two groups of BALB/c mice (Charles River) with equal numbers of male and female mice (N = 15 each male and female). Six untreated mice were used for blank controls and were labeled time 0. At 20, 40, 60, and 120 min, three mice from the POM-l-BHDU-MP group and three mice from the l-BHDU group were anesthetized with inhaled isoflurane and then exsanguinated by cardiac puncture. Blood samples were collected into a tube containing heparin. Plasma was separated from red blood cells by centrifugation at 10,000 rpm for 5 min. Plasma was transferred to a new tube and immediately frozen at −80 °C.
Plasma Preparation for LC-MS/MS
Plasma samples were thawed, and exactly 0.95 mL was removed for analysis. The internal standard azidothymidine was added to mouse plasma samples at a final concentration of 20 ng/mL. Proteins were precipitated from the plasma by adding 2× volumes of methanol, then the samples were clarified by centrifugation. The supernatants were dried using an N-EVAP 116 analytical nitrogen evaporator and then reconstituted with 50% methanol in water. LC-MS/MS was used to analyze the concentrations of POM-l-BHDU-MP, l-BHDU, and l-BHDU-MP. Calibration curves were made using BALB/c mouse plasma (Innovative Research, Inc. Novi MI) prior to analyzing the experimental samples.
Protocol of LC/MS Analysis
The primary stock solutions for the calibration were prepared at 0.5 mg/mL for l-BHDU, 1 mg/mL for POM-l-BHDU-MP, and 1 mg/mL for l-BHDU-MP. The primary stock solutions were diluted to working solutions that were in turn used to spike the biological matrix of interest. l-BHDU calibration points were 100.0, 400.0, 800.0, 1000.0, 4000.0, 6000.0, 8000.0, 10,000.0, and 12,000.0 ng/mL. l-BHDU-MP calibration points were 100.0, 200.0, 400.0, 800.0, 1000.0, 2000.0, 4000.0, 6000.0, and 8000.0 ng/mL. The ISTD working solution of AZT was prepared at 20 ng/mL. The standard stock solutions were stored in −80 °C freezer until used. A Waters Xevo Micro TQS UPLC Mass spectrometer with an ESI (−) source was operated for the LC/MS analysis. Waters MassLynx 4.2 software (Milford, MA) was used for instrumentation and obtaining data. An Acquity Premier Oligonucleotide BEH C18, 130 Å, 1.7 μm 2.1 × 150 mm column coupled with an Acquity UPLC BEH C18 1.7 μm (2.1 × 5 mm) VanGuard Pre-Column was used to separate the analytes. The column temperature was set at 30 °C, and the autosampler was set at 5 °C. The mobile phase A was 5 mM ammonium formate in water, and the mobile phase B was 5 mM ammonium formate in methanol. The injection volume was 5 μL. The analytes were separated using a gradient method, with a 0.15 mL/min flow rate (time/minute, % mobile phase b): (0/10), (2/10), (20/95), (20.1/10), and (30,10). The samples were analyzed in a negative ESI mode. The capillary voltage was 1.50 kV, and the cone voltage was 25 V. The desolvation gas was nitrogen and was used at a flow rate of 650 L/h. The desolvation temperature was 350 °C, and the source temperature was 150 °C. The collision gas was argon with a collision cell pressure of 3.18 × 10–3 mbar, and the collision energy was 30 V. A multiple reaction monitoring (MRM) function was used to quantify the analytes using the following ion transitions: 266.1 → 223.2 for AZT (ISTD), 318.7 → 80.7 for l-BHDU, 398.7 → 180.9 for l-BHDU-MP, and 626.6 → 325 for POM-l-BHDU-MP.
Acknowledgments
The authors express their gratitude to Dr. Dennis Philip, in charge of the proteomics and mass spectrometry facility (PAMS), who helped to provide all high-resolution mass spectroscopy (HRMS) spectra of intermediates and final compounds.
Glossary
Abbreviations
- ACV
acyclovir
- BVU
bromovinyluracil
- CDV
cidofovir
- DPD
dihydropyrimidine dehydrogenase
- DIC
1,3-diisopropylcarbodiimide
- DIPEA
N,N-diisopropylethylamine
- DMAP
4-(dimethylamino)pyridine
- Et3N
triethylamine
- FCV
famciclovir
- 5-FU
5-fluorouracil
- HFFs
human foreskin fibroblasts
- HBV
hepatitis B virus
- HDP
hexadecyloxypropyl
- HIV
human immunodeficiency virus
- NMP
nucleoside monophosphate
- NMI
N-methylimidazole
- ODE
octadecyloxyethyl
- POM
bis(pivaloyloxymethyl)
- POC
bis(isopropyloxymethyl carbonate)
- TK
thymidine kinase
- TBDMSCl
tert-butyldimethylsilyl chloride
- TBAF
tetrabutylammonium fluoride
- TFA
trifluoro acetic acid
- VZV
varicella zoster virus
- VACV
valacyclovir
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c00545.
Author Contributions
All authors have contributed to the write-up and preparation of the manuscript. All authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
Special Issue
Published as part of the Journal of Medicinal Chemistry virtual special issue “New Drug Modalities in Medicinal Chemistry, Pharmacology, and Translational Science”.
Supplementary Material
References
- Gershon A. A.; Breuer J.; Cohen J. I.; Cohrs R. J.; Gershon M. D.; Gilden D.; Grose C.; Hambleton S.; Kennedy P. G. E.; Oxman M. N.; Seward J. F.; Yamanishi K. Varicella Zoster Virus Infection. Nat. Rev. Dis. Primers 2015, 1, 15016 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M.; Leung J.; Gershon A. A. Transmission of Vaccine-Strain Varicella-Zoster Virus: A Systematic Review. Pediatrics 2019, 144, e20191305 10.1542/peds.2019-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha T.; Ming D.; Breuer J. A Critical Appraisal of ″Shingrix’, A Novel Herpes Zoster Subunit Vaccine (HZ/Su or GSK1437173A) for Varicella Zoster Virus. Hum. Vaccines Immunother. 2017, 13, 1789–1797. 10.1080/21645515.2017.1317410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cdc.gov/shingles/index.html.
- Bruxelle J.; Pinchinat S. Effectiveness of Antiviral Treatment on Acute Phase of Herpes Zoster and Development of Post Herpetic Neuralgia: Review of International Publications. Med. Mal. Infect. 2012, 42, 53–58. 10.1016/j.medmal.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Sampathkumar P.; Drage L. A.; Martin D. P. Herpes Zoster (Shingles) and Postherpetic Neuralgia. Mayo Clin. Proc. 2009, 84, 274–280. 10.4065/84.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J.; Hodge R. A. V. Recent Developments in Anti-herpesvirus Drugs. Br. Med. Bull. 2013, 106, 213–249. 10.1093/bmb/ldt011. [DOI] [PubMed] [Google Scholar]
- Andrei G.; Snoeck R. Advances and Perspectives in the Management of Varicella-Zoster Virus Infections. Molecules 2021, 26, 1132. 10.3390/molecules26041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil A.; Goldust M.; Wollina U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. 10.3390/v14020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. Y.; Kim K. S.; Lee W. K. Intravitreal Foscarnet for the Treatment of Acyclovir-resistant Acute Retinal Necrosis Caused by Varicella Zoster Virus. Ocul. Immunol. Inflammation 2011, 19, 212–213. 10.3109/09273948.2010.544857. [DOI] [PubMed] [Google Scholar]
- Hoffman J. Overview of Antiviral Medications Used in Ophthalmology. Community Eye Health 2020, 33, 85–88. [PMC free article] [PubMed] [Google Scholar]
- Clercq E. D. (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU). Med. Res. Rev. 2005, 25, 1–20. 10.1002/med.20011. [DOI] [PubMed] [Google Scholar]
- Clercq E. D. Discovery and Development of BVDU (brivudin) as a Therapeutic for the Treatment of Herpes zoster. Biochem. Pharmacol. 2004, 68, 2301–2315. 10.1016/j.bcp.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Keizer H. J.; Debruijn E. A.; Tjaden U. R.; Declercq E. Inhibition of Fluorouracil Catabolism in Cancer-Patients by the Antiviral Agent (E)-5-(2-Bromovinyl)-2′-Deoxyuridine. J. Cancer Res. Clin. Oncol. 1994, 120, 545–549. 10.1007/BF01221032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.; Li L.; Grill S.; Gullen E.; Lee C. S.; Gumina G.; Tsujii E.; Cheng Y. C.; Chu C. K. Structure-Activity Relationships of (E)-5-(2-bromovinyl) Uracil and Related Pyrimidine Nucleosides as Antiviral Agents for Herpes Viruses. J. Med. Chem. 2000, 43, 2538–2546. 10.1021/jm990543n. [DOI] [PubMed] [Google Scholar]
- De C.; Liu D. M.; Zheng B.; Singh U. S.; Chavre S.; White C.; Arnold R. D.; Hagen F. K.; Chu C. K.; Moffat J. F. beta-L-1-[5-(E-2-bromovinyl)-2-(hydroxymethyl)-1,3-(dioxolan-4-yl)] uracil (L-BHDU) Prevents Varicella-Zoster Virus Replication in a SCID-Hu Mouse Model and Does not Interfere with 5-Fluorouracil Catabolism. Antiviral Res. 2014, 110, 10–19. 10.1016/j.antiviral.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krečmerová M. Amino Acid Ester Prodrugs of Nucleoside and Nucleotide Antivirals. Mini. Rev. Med. Chem. 2017, 17, 818–833. 10.2174/1389557517666170216151601. [DOI] [PubMed] [Google Scholar]
- Coen N.; Singh U.; Vuyyuru V.; Van den Oord J. J.; Balzarini J.; Duraffour S.; Snoeck R.; Cheng Y. C.; Chu C. K.; Andrei G. Activity and Mechanism of Action of HDVD, a Novel Pyrimidine Nucleoside Derivative with High Levels of Selectivity and Potency Against Gammaherpesviruses. J. Virol. 2013, 87, 3839–3851. 10.1128/JVI.03338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W.; McKenna C. E. Prodrug Approaches to Improving the Oral Absorption of Antiviral Nucleotide Analogues. Expert Opin. Drug Delivery 2009, 6, 405–420. 10.1517/17425240902824808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y. Alkoxyalkyl Prodrugs of Acyclic Nucleoside Phosphonates Enhance Oral Antiviral Activity and Reduce Toxicity: Current State of the Art. Antiviral Res. 2009, 82, A84–A98. 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle J. R.; Hartline C.; Aldern K. A.; Rodriguez N.; Harden E.; Kern E. R.; Hostetler K. Y. Alkoxyalkyl Esters of Cidofovir and Cyclic Cidofovir Exhibit Multiple-log Enhancement of Antiviral Activity Against Cytomegalovirus and Herpesvirus Replication in vitro. Antimicrob. Agents Chemother. 2002, 46, 2381–2386. 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naesens L.; Neyts J.; Balzarini J.; Bischofberger N.; Declercq E. In-Vivo Antiretroviral Efficacy of Oral Bis(Pom)-Pmea, the Bis(Pivaloyloxymethyl)Prodrug of 9-(2-Phosphonylmethoxyethyl)Adenine (Pmea). Nucleosides, Nucleotides Nucleic Acids 1995, 14, 767–770. 10.1080/15257779508012468. [DOI] [Google Scholar]
- Marcellin P.; Chang T.; Lim S. G.; et al. Adefovir dipivoxil for the Treatment of Hepatitis B e Antigen-Positive Chronic Hepatitis B. N. Engl. J. Med. 2003, 348, 808–816. 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- Fung H. B.; Stone E. A.; Piacenti F. J. Tenofovir Disoproxil Fumarate: A Nucleotide Reverse Transcriptase Inhibitor for the Treatment of HIV Infection. Clin. Ther. 2002, 24, 1515–1548. 10.1016/S0149-2918(02)80058-3. [DOI] [PubMed] [Google Scholar]
- Pradere U.; Garnier-Amblard E. C.; Coats S. J.; Amblard F.; Schinazi R. F. Synthesis of Nucleoside Phosphate and Phosphonate Prodrugs. Chem. Rev. 2014, 114, 9154–9218. 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer A. J.; Wiemer D. F.. Prodrugs of Phosphonates and Phosphates: Crossing the Membrane Barrier. In Topics in Current Chemistry; Springer International Publishing, 2015; Vol. 360, pp 115–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel K. M.; Dowd C. S. Phosphonate Prodrugs: An Overview and Recent Advances. Future Med. Chem. 2019, 11, 1625–1643. 10.4155/fmc-2018-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan C.; Tsang H. W.; Sutton P. W.; De Clercq E.; Balzarini J. Synthesis and Anti-HIV Activity of Some Novel Chain-Extended Phosphoramidate Derivatives of d4T (Stavudine): Esterase Hydrolysis as a Rapid Predictive Test for Antiviral Potency. Antiviral Chem. Chemother. 1998, 9, 109–115. 10.1177/095632029800900202. [DOI] [PubMed] [Google Scholar]
- Singh U. S.; Mulamoottil V. A.; Chu C. K. Synthesis of an Anti-hepatitis B Agent, 2′-Fluoro-6′-methylene-carbocyclic Adenosine (FMCA) and Its Phosphoramidate (FMCAP). J. Org. Chem. 2019, 84, 752–759. 10.1021/acs.joc.8b02599. [DOI] [PubMed] [Google Scholar]
- Liang Y.; Narayanasamy J.; Schinazi R. F.; Chu C. K. Phosphoramidate and Phosphate Prodrugs of (-)-beta-D-(2R,4R)-dioxolane-thymine: Synthesis, Anti-HIV Activity and Stability Studies. Bioorg. Med. Chem. 2006, 14, 2178–2189. 10.1016/j.bmc.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Hwang Y. S.; Cole P. A. Efficient Synthesis of Phosphorylated Prodrugs with Bis(POM)-phosphoryl Chloride. Org. Lett. 2004, 6, 1555–1556. 10.1021/ol049714v. [DOI] [PubMed] [Google Scholar]
- Oliver S. L.; Zerboni L.; Sommer M.; Rajamani J.; Arvin A. M. Development of Recombinant Varicella-Zoster Viruses Expressing Luciferase Fusion Proteins for Live in vivo Imaging in Human Skin and Dorsal Root Ganglia Xenografts. J. Virol. Methods 2008, 154, 182–193. 10.1016/j.jviromet.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De C.; Liu D.; Singh U. S.; Chu C. K.; Moffat J. F. β-L-1-[5-(E-2-Bromovinyl)-2-(Hydroxymethyl)-1,3 Dioxolan-4-yl)] Uracil (L-BHDU) Inhibits Varicella Zoster Virus Replication by Depleting the Cellular dTTP Pool. bioRxiv 2020, 10.1101/2020.02.13.948216. [DOI] [Google Scholar]
- Lloyd M. G.; Yee M. B.; Flot J. S.; Liu D. M.; Geiler B. W.; Kinchington P. R.; Moffat J. F. Development of Robust Varicella Zoster Virus Luciferase Reporter Viruses for In Vivo Monitoring of Virus Growth and Its Antiviral Inhibition in Culture, Skin, and Humanized Mice. Viruses 2022, 14, 826. 10.3390/v14040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M. G.; Liu D.; Lyu J.; Fan J.; Overhulse J. M.; Kashemirov B. A.; Prichard M. N.; McKenna C. E.; Moffat J. F. An Acyclic Phosphonate Prodrug of HPMPC is Effective Against VZV in Skin Organ Culture and Mice. Antiviral Res. 2022, 199, 105275 10.1016/j.antiviral.2022.105275. [DOI] [PubMed] [Google Scholar]
- Lloyd M. G.; Liu D.; Legendre M.; Markovitz D. M.; Moffat J. F. H84T BanLec has Broad Spectrum Antiviral Activity Against Human Herpesviruses in Cells, Skin, and Mice. Sci. Rep. 2022, 12, 1641 10.1038/s41598-022-05580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J.; Greenblatt R. J.; Liu D. M.; Moffat J. F. Compounds that Target Host Cell Proteins Prevent Varicella-Zoster Virus Replication in Culture, Ex vivo, and in SCID-Hu Mice. Antiviral Res. 2010, 86, 276–285. 10.1016/j.antiviral.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M. G.; Smith N. A.; Tighe M.; Travis K. L.; Liu D.; Upadhyaya P. K.; Kinchington P. R.; Chan G. C.; Moffat J. F. A Novel Human Skin Tissue Model To Study Varicella-Zoster Virus and Human Cytomegalovirus. J. Virol. 2020, 94, e01082-20 10.1128/JVI.01082-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.