Abstract
Background and Objectives
There has been a long-standing dialog as to whether essential tremor (ET) increases the risk of developing Parkinson disease (PD). While there are relevant cross-sectional data, there are almost no longitudinal prospective data. We quantified the conversion rate from ET to ETPD in a prospective longitudinal cohort study of patients with ET. We compared the observed rate with that reported in the epidemiologic literature.
Methods
We enrolled patients with ET in a prospective, longitudinal study. A senior movement disorders neurologist evaluated standardized neurologic examinations every 18 months.
Results
One hundred ninety-three patients with ET (mean age = 78.1 ± 9.6 years, range = 55–96) had a mean follow-up duration of 4.1 years. Seven (3.6%) converted from ET to ETPD. The incidence of PD among patients with ET was 7/792.9 person-years (py; i.e., 882.8/100,000 py). A meta-analysis of the incidence (per 100,000 py) of PD in 14 studies from 13 countries across 4 continents reported an incidence of PD = 61.21 (men, 40 years or older) and 37.55 (women, 40 years or older). The incidence/100,000 py in men peaked in the 80- to 89-year-old age group (258.47) and in women in the 80- to 89-year-old age group (103.48 py). The abovementioned published values are 3.4–23.5 times lower than the value we observed for ET.
Discussion
The incidence of PD in an ET cohort is substantially higher than that reported in historical population-based control groups across numerous countries. Additional prospective longitudinal data are needed to further explore this association.
There has been a long-standing dialog as to whether essential tremor (ET) increases the risk of developing Parkinson disease (PD), with arguments made both for and against this association.1-6 There is a literature, centered around epidemiologic evidence, that ET is associated with an increased odds and risk of developing PD.1,3,7-12 One of the studies was a prospective longitudinal study in Spain, reporting that patients with ET were approximately 4–5 times more likely than age-matched individuals without ET to develop incident PD.12 There are no other data that address this risk relationship (i.e., beyond a mere cross-sectional association observed between prevalent cases), assessing whether ET at baseline is a risk factor of developing incident PD over time. We capitalized on a prospective longitudinal cohort study of patients with ET, quantifying the conversion rate from ET to ETPD during follow-up. To place this conversion rate in context, we compared the observed rate with that reported in the epidemiologic literature.
Methods
Study Design
Patients with ET were enrolled in a prospective longitudinal study of ET (Clinical Pathologic Study of Cognitive Impairment in ET [COGNET] NINDS R01 NS086736) beginning in July 2014. Nationwide enrollment in COGNET began in July 2014, with patients being geographically widespread in more than 40 US States. Inclusion criteria were (1) an ET diagnosis at baseline in the absence of other movement disorder diagnoses, (2) a minimum age of 55 years, (3) no history of brain surgery, (4) a willingness to enroll in the Essential Tremor Centralized Brain Repository, and (5) a willingness every 18 months to provide self-report clinical data and to participate in an extensive neuropsychological battery and videotaped neurologic examination. Additional details of the data collected are available elsewhere.13 We recruited patients through advertisements on the study website, along with other websites (i.e., International Essential Tremor Foundation).
Assessments were scheduled for each participant: baseline (T1), 18 months after baseline (T2), 36 months after baseline (T3), and 54 months after baseline (T4). Trained research assistants conducted these in-person evaluations in the homes of patients with ET at each study visit, many of whom lived several hours away from a tertiary medical center. These consisted of an extensive clinical evaluation, cognitive test battery, and a videotaped neurologic examination.
Standard Protocol Approvals, Registrations, and Patient Consents
All study procedures were approved by the UT Southwestern Medical Center, Yale University, and Columbia University Institutional Review Boards. All patients signed written informed consent on enrollment and at each evaluation.
Clinical Battery
At each time interval, trained personnel conducted in-person in-home evaluations; the evaluation included clinical questionnaires and a standardized videotaped neurologic examination.14 The latter included a detailed assessment of postural tremor, 5 tests for kinetic tremor, and the motor portion of the Unified Parkinson Disease Rating Scale15 excluding an assessment of rigidity.
A senior-level movement disorders neurologist (E.D.L.) reviewed all videotaped examinations, rating the severity of postural and kinetic arm tremors on 12 examination items using a reliable scale.16 As reviewed elsewhere,14,17 ratings were 0 (absent), 0.5 (very low amplitude and almost never present), 1.0 (mild = low amplitude or intermittent tremor), 1.5 (mild-to-moderate [tremor sometimes more than mild]), 2 (moderate = clearly oscillatory and > mild amplitude), and 3 (severe), and resulted in a total tremor score (range = 0–36 [maximum]).
As described,14,17 all ET diagnoses were assigned by E.D.L. based on a review of questionnaires and videotaped neurologic examination using published diagnostic criteria.18 The criteria include gradations of possible, probable, and definite ET.18 At a minimum, possible ET required moderate or greater amplitude kinetic tremor during at least 3 activities in the absence of another known cause (e.g., medication-induced tremor and tremor from hyperthyroidism).18 These diagnostic criteria were developed for a population-based genetic study and, based on data from approximately 2,000 nondiseased controls, the criteria carefully detail the specific examination maneuvers during which tremor should be present and the severity of tremor that should be evident during these maneuvers to distinguish normal from ET.14,17 These criteria have been shown to be both reliable16 and valid19 and have been used by tremor investigators in the United States and internationally.
PD was diagnosed using published diagnostic criteria, which required the presence of at least 2 cardinal signs.20,21 As described earlier,14,21 the diagnosis of ETPD required that: (1) the ET diagnosis was present for at least 5 years before the PD diagnosis, (2) the initial ET was characterized by moderate or greater amplitude kinetic tremor in the absence of any signs of PD, and (3) the initial ET diagnosis occurred in the absence of red flags for possible emerging PD (e.g., isolated postural tremor without kinetic tremor and unilateral tremor).
Final Sample
Our initial sample included 236 patients enrolled in the COGNET project. We excluded patients (1) for whom data were available only for the initial evaluation (n = 37), (2) who received a clinical diagnosis of PD at the initial observation (n = 4), or (3) who did not receive a diagnosis of ET at baseline (2). Of the remaining 193 patients retained for analysis, 56 completed fewer than 4 evaluations because of death (n = 26) or other factors (n = 30).
Statistical Analyses
Incidence was reported per 100,000 person-years (py) and included a 95% confidence interval (CI). We used the Statistical Package for Social Sciences version 27. We tested the normality of test variables using a Kolmogorov Smirnov test and used nonparametric tests as needed. We compared baseline demographic and clinical characteristics of participants who converted to ETPD vs those who did not.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
The 193 patients with ET had a mean age of 78.1 ± 9.6 years (range = 55–96 years, 176 [91.2%] ≥65 years). Of them, 121 (62.7%) were female.
Seven (3.6%) of 193 patients with ET converted from ET to ETPD. The follow-up duration for the 193 patients with ET ranged from 1.3 to 6.4 years (mean = 4.1, median = 4.6, SD = 1.1 years); therefore, the total follow-up duration was 792.9 py. Hence, the incidence of PD among patients with ET in this sample was 7/792.9 py (i.e., 882.8/100,000 py, 95% CI = 231.72/100,00 py–1,533.94/100,000 py).
Among 72 men, 5 (6.9%) developed PD over 292.81 py (i.e., 1,707.59/100,000 py, 95% CI = 730/100,000 py–3,940/100,000 py). Among women, 2 (1.65%) developed PD over 500.06 py (i.e., 399.95/100,000 py, 95% CI = 110/100,000 py–1,450/100,000 py).
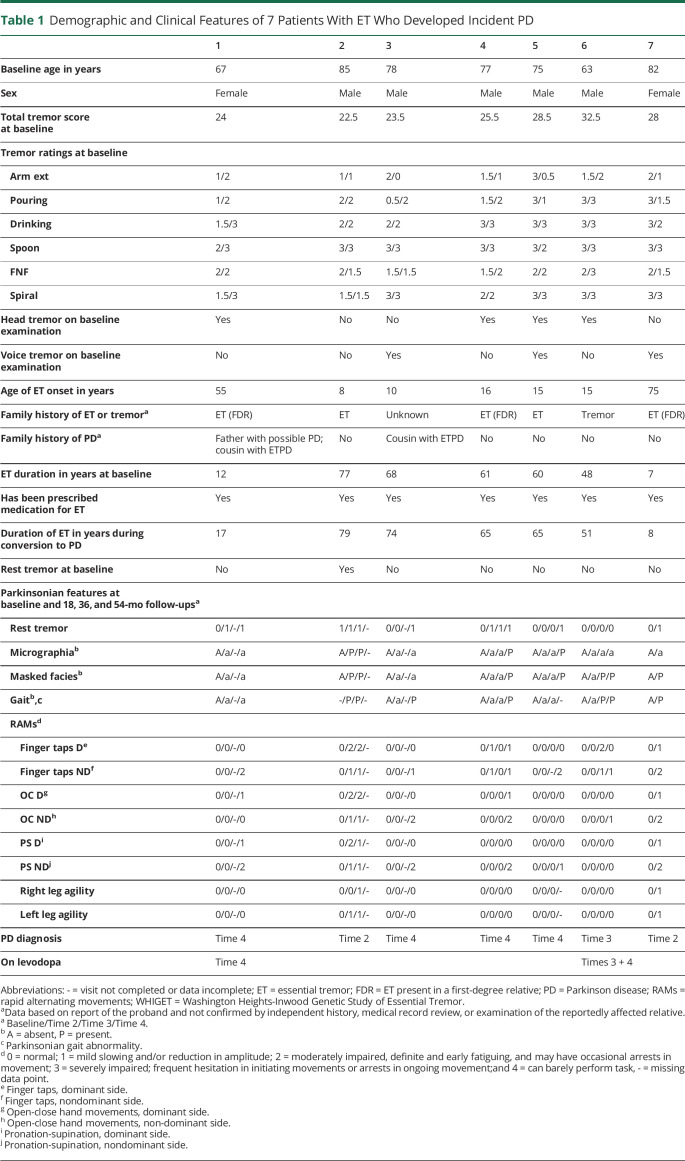
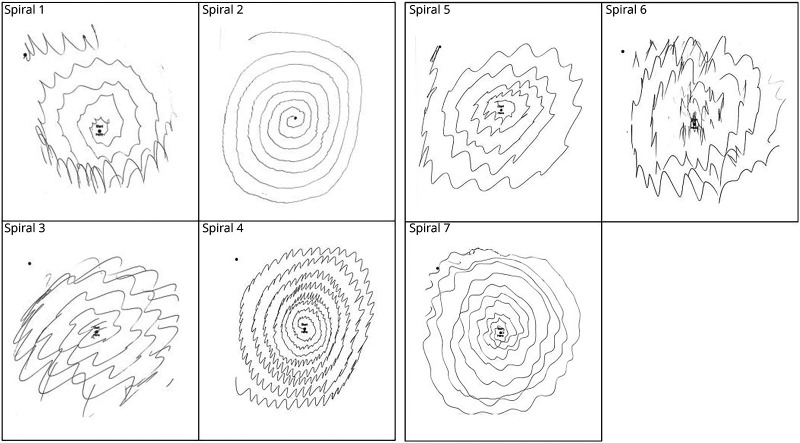
All 7 patients with ET that converted to ETPD had Washington Heights-Inwood Genetic Study of ET diagnoses of probable or definite ET, meaning that moderate (rating = 2) or severe (rating = 3) tremor was observed on at least 4 of 5 tasks that tested kinetic tremor (pouring, drinking, using spoon, finger-nose-finger, and spiral). Table 1 provides detailed demographic and clinical features on the 7 patients with ET that converted to ETPD. All 7 had been prescribed medication for their ET. Five had a family history of ET, and a sixth had a family history of nonspecific tremor. Total tremor scores ranged from 22.5 to 32.5, indicating moderate to severe ET, and all 7 patients had numerous ratings of 3 (i.e., severe tremor) on tasks that tested kinetic tremor (Table 1). Four had head tremor and 3 had voice tremor; only one had neither head tremor nor voice tremor (Table 1). The duration of ET at baseline was 7–77 years (median = 60.0). The duration of ET during conversion to PD was 8–79 years (median = 65.0). Spirals are shown on each (Figure).
Table 1.
Demographic and Clinical Features of 7 Patients With ET Who Developed Incident PD
Figure. Spirals of 7 Patients With Essential Tremor (ET) Who Converted to Those With ETPD.
ET = essential tremor; PD = Parkinson disease.
UDPRS scores are listed at each time (Table 1); 152 (98.7%) of 154 ratings of micrographia, masked facies, gait, and rapid alternating movements were 0 or absent before conversion to PD. There were rare (i.e., 2) ratings of 1 and none of 2 before to PD—patient 4 had 2 ratings of 1 (finger taps) at time 2, which converted to ratings of 0 at time 3.
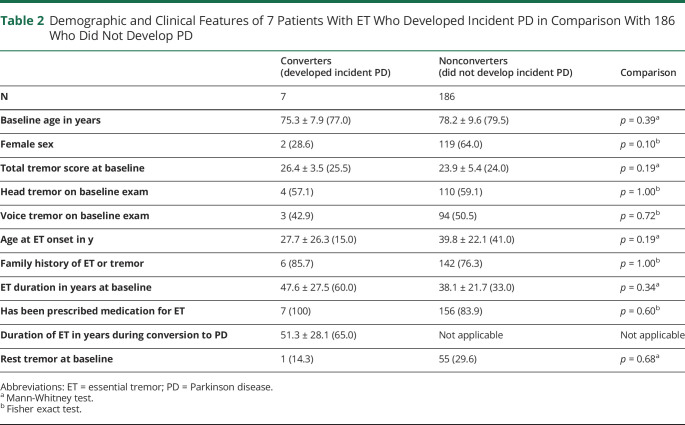
Table 2 provides detailed demographic and clinical data, comparing these 7 patients with ET with the 186 patients with ET that had not converted to ETPD. The 2 groups did not differ to a significant degree for these data, although the sample size of converters was small, limiting the power of these statistical tests. Although not significant, it is notable that few (28.6%) converters were females (vs. 64.0% for nonconverters) and the median age of ET onset in converters was 15 years (vs. 41 years in nonconverters). One (14.3%) of the converters had rest tremor at baseline (in the absence of other videotaped features of PD); that patient had the highest total tremor score, with nearly all kinetic tremor items receiving ratings of 3 (severe tremor) and had experienced ET for 51 years before converting to ETPD. Fifty-five (29.6%) nonconverters had rest tremor (in the absence of other videotaped features of PD) at baseline (Table 2); this was lower than 46.4% of patients with ET with rest tremor previously reported in a brain bank cohort.22
Table 2.
Demographic and Clinical Features of 7 Patients With ET Who Developed Incident PD in Comparison With 186 Who Did Not Develop PD
Discussion
Seven (3.6%) of 193 patients with ET converted from ET to ETPD over a mean and median follow-up duration of 4.1 and 4.6 years, respectively. In the other prospective longitudinal study of ET in Spain,12 3.0% of patients with ET developed incident PD after a median follow-up of 3.3 years, a result that is quite similar to that we reported in this study. In that study, 0.7% of controls developed incident PD during the same period.12
In our study, the incidence of PD among patients with ET was 7/792.9 py (i.e., 882.8/100,000 py, 95% CI = 231.72/100,000 py–1,533.94/100,000 py). We compared this value with that observed in historical controls further.
A meta-analysis of the incidence (per 100,000 py) of PD in 14 studies from 13 countries across 4 continents reported an incidence of PD = 61.21 (men, 40 years or older) and 37.55 (women, 40 years or older).23 The incidence/100,000 in men peaked in the 80- to 89-year-old age group (258.47 py, 95% CI = 146.46–456.16 py) and in women in the 80- to 89-year-old age group (103.48 py, 95% CI = 44.69–239.64 py).23 These values ranged from 3.4 to 23.5 times lower than the value we reported for ET. Two of these studies conducted true population-based sampling rather than relying on referrals from clinicians or data from medical record reviews. These were the Madrid and Rotterdam studies,12,24 reviewed further.
In the Madrid study,12 demographic features were similar to ours (i.e., mean age = 76.5 ± 6.5 years and 58.0% women [Spain] compared with 78.1 ± 9.6 years and 62.7% women [our study]). The incidence/100,000 of PD was 359.6 (95% CI = 216.5–561.5) in men, 147.9 (95% CI = 74.9–264.7) in women, and 235.9 (95% CI = 159.2–336.7) overall. As such, our point estimates were 4.75 times, 2.7 times, and 3.75 times higher than theirs in men, women, and overall, respectively.
In the Rotterdam study,24 the mean age of the PD cohort was 68.8 years, a value that was 9.3 years younger than ours. If we were to remove their younger participants (i.e., those in the age range of 55–65 years), then their mean age would be approximately 75 years, which is similar to ours. Their sample comprised 59.0% women, which is similar to ours (62.7%). In their sample age 65 years and older, the incidence/100,000 of PD was 261.14 (95% CI = 180–370) in men, 206.11 (95% CI = 150–290) in women, and 227.87 (95% CI = 180–290) overall. As such, our point estimates were 6.5 times, 2 times, and 4 times higher than theirs in men, women, and overall.
In summary, the incidence of PD in our ET cohort seems to be substantially higher than that reported in historical, population-based control groups across numerous countries. It is also similar to that reported in the other prospective longitudinal study of ET.12 These data suggest that ET is a robust risk factor of incident PD. Along these lines, there is a sizable literature, which although controversial,5,6 presents epidemiologic evidence that ET seems to be associated with an increased odds and risk of developing PD1,3,7-12; as noted earlier, one of these studies is a longitudinal prospective study, reporting that patients with ET were 4–5 times more likely than age-matched individuals without ET to develop PD.12 The magnitude of that increased risk of incident PD in ET (i.e., 4–5 times)12 is very similar to the magnitude of the increased incidence we reported earlier for ET relative to historical controls (i.e., 3.75–4 times higher overall).
The potential mechanisms for an increased incidence of PD among patients with ET is not clear, although there is a literature, again with mixed findings, reporting in several studies an increase in the prevalence of Lewy pathology in patients with ET.25-27 Adding to that literature, a large clinical pathological cohort study of 231 patients with ET recently reported that the proportion of patients with ET with Lewy pathology was 25.1% (i.e., one in four patients with ET), a proportion that in the context of other studies among control populations seemed to be 50%–75% higher than expected (i.e., 25.1% observed vs 14.5%–16.4% expected).28
All 7 patients with ET that converted to ETPD had moderate-to-severe ET and all had been prescribed medication for their ET. Most of them had a family history of ET or tremor and had either head or voice tremor. The duration of ET during conversion to PD was 8–79 years (8, 17, 51, 65, 65, 74, 79, median = 65.0). Although not significant, it is notable that most of the converters were male, consistent with the male preponderance of PD.
This study was not without limitations. First, we did not enroll a control group without ET; hence, we were not able to directly compare the incidence of PD in ET with that of a control group. Thus, we were not able to report a relative risk. As an alternative, we used data on historical controls, which are both abundant and from many different source populations worldwide. These provide the needed comparison of base rates. In several of these analyses, we were careful to compare our data with those from studies featuring the most robust designs and including participants with demographic features similar to our own. It is important to note that the absence of an enrolled control group does not invalidate our primary observation, which is a point estimate and CI for the conversion of ET to ETPD in a large cohort of patients with ET. Second, whereas many PD incidence studies evaluate samples from the general population or samples derived from door-to-door screening, in our study, the prevalence of PD was determined in a sample containing expertly selected patients with a specific diagnosis of ET. Individuals ascertained from the community have been shown to have fewer concurrent medical conditions than those derived from treatment settings,29 raising the theoretic concern that the former might be less likely to exhibit both ET and PD. It is important to note, however, that our patients with ET were not derived from treatment settings, which lessens the possibility of such bias. In addition, in this study, we did not assess the concurrent prevalence of PD in an ET sample, but rather, the incidence of PD (i.e., the subsequent conversion to ETPD) in that sample. Thus, the study focused on the evolution of a diagnosis rather than simple co-occurrence of 2 diagnostic entities. Third, the absolute number of cases that converted from ET to ETPD was modest, resulting in a wide CI. Fourth, we used videotaped neurologic examinations for the diagnosis of PD rather than in-person assessment by a neurologist. This design, however, has been used in numerous other studies as well.30-33 Furthermore, a movement disorders neurologist rather than a general neurologist viewed all videotaped examinations. One major advantage of videotaped rather than in-person assessments is that the examination may be replayed as many times as needed to review ambiguous phenomenology. Furthermore, it is reassuring to note that data from numerous studies indicate that when assessed remotely, 95–100% of individuals with self-reported PD are judged to experience PD and 95–97% of those who did not report PD were judged to be without the disease.31-33 While it is conceivable that 1 or more of our patients with ET had tremor-dominant PD that was mislabeled as ET, we think this is highly unlikely. None had been diagnosed by their treating neurologist as PD and none were on dopamine replacement therapy. As part of our evaluation, each had a detailed neurologic examination, which included assessments of hypomimia, hypophonia, rapid alternating movements in all limbs, axial bradykinesia, changes in arm swing, gait, posture, and rest tremor, and only rest tremor was present in 1 patient. Furthermore, at baseline, all but 1 had had action tremor for 10 or more years, and the phenotype of the tremor differed from that reported in tremor-dominant PD, which is reportedly characterized by unilateral or asymmetric postural tremor rather than bilateral kinetic tremor, as seen in our patients34,35—in 1 study of 439 patients with tremor-dominant PD, only 10% had kinetic tremor at the initial visit and 6% at the final visit.35 Fifth, our assessment of participants did not include an assessment of rigidity. Nonetheless, there are substantial data showing that the motor features of bradykinesia and rigidity in ET are part of the same factor,36,37 suggesting that evaluation of both is somewhat redundant. Hence, it is unlikely that we failed to detect patients with PD whose sole clinical manifestation was rigidity in the absence of bradykinesia. Finally, it would have been interesting to have dopamine transporter imaging in these cases as an objective measure of dopamine deficiency.
This study had several strengths: First, it is 1 of only 2 prospective longitudinal studies of the incidence of PD in ET; the other study was reviewed earlier.12 Second, all participants underwent an extensive videotaped neurologic examination, which allowed for a detailed and repeated review of subtle motor phenomenology. Third, all videotaped examinations were reviewed by and diagnoses assigned by a senior movement disorders neurologist.
In summary, 7 (3.6%) of 193 patients with ET converted from ET to ETPD over a mean and median follow-up duration of 4.1 and 4.6 years, respectively. The incidence of PD in this ET cohort seems to be substantially higher than that reported in historical, population-based control groups across numerous countries. Several mechanisms are discussed. Additional prospective longitudinal data are needed to further study this association.
Appendix. Authors
Study Funding
NINDS R01 NS086736.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Thenganatt MA, Jankovic J. The relationship between essential tremor and Parkinson's disease. Parkinsonism Relat Disord. 2016;22(suppl 1):S162-S165. doi: 10.1016/j.parkreldis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Tarakad A, Jankovic J. Essential tremor and Parkinson's disease: exploring the relationship. Tremor Other Hyperkinet Mov (N Y). 2019;8:589. doi: 10.5334/tohm.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaRoia H, Louis ED. Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology. 2011;37:1-10. doi: 10.1159/000328866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Ottman R. Is there a one-way street from essential tremor to Parkinson's disease? Possible biological ramifications. Eur J Neurol. 2013;20(11):1440-1444. doi: 10.1111/ene.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler CH, Shill HA, Beach TG. Essential tremor and Parkinson's disease: lack of a link. Mov Disord. 2011;26(3):372-377. doi: 10.1002/mds.23509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Algarni M, Fasano A. The overlap between Essential tremor and Parkinson disease. Parkinsonism Relat Disord. 2018;46(suppl 1):S101-S104. doi: 10.1016/j.parkreldis.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Tan EK, Lee SS, Fook-Chong S, Lum SY. Evidence of increased odds of essential tremor in Parkinson's disease. Mov Disord. 2008;23(7):993-997. doi: 10.1002/mds.22005. [DOI] [PubMed] [Google Scholar]
- 8.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2008;80(4):423-425. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Bower JH, Ahlskog JE, et al. Increased risk of essential tremor in first-degree relatives of patients with Parkinson's disease. Mov Disord. 2007;22(11):1607-1614. doi: 10.1002/mds.21584. [DOI] [PubMed] [Google Scholar]
- 10.Costello S, Bordelon Y, Bronstein J, Ritz B. Familial associations of Alzheimer disease and essential tremor with Parkinson disease. Eur J Neurol. 2010;17(6):871-878. doi: 10.1111/j.1468-1331.2010.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spanaki C, Plaitakis A. Essential tremor in Parkinson's disease kindreds from a population of similar genetic background. Mov Disord. 2009;24(11):1662-1668. doi: 10.1002/mds.22655. [DOI] [PubMed] [Google Scholar]
- 12.Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62(5):734-741. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 13.Cersonsky TEK, Kellner S, Chapman S, Huey ED, Louis ED, Cosentino S. Profiles of normal cognition in essential tremor. J Int Neuropsychol Soc. 2020;26(2):197-209. doi: 10.1017/s1355617719001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis ED, Hernandez NC, Ottman R, Clark LN. Mixed motor disorder: essential tremor families with heterogeneous motor phenomenology. Neurol Clin Pract. 2021;11(6):e817-e825. doi: 10.1212/CPJ.0000000000001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41-47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Ford B, Bismuth B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord. 1998;13(2):287-293. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Hernandez N, Sebastian AA, Clark LN, Ottman R. Validity of probands' reports and self-reports of essential tremor: data from a large family study in North America. J Neurol Sci. 2018;393:45-50. doi: 10.1016/j.jns.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood genetic study of essential tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124-133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Pullman SL. Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Mov Disord. 2001;16(4):668-673. doi: 10.1002/mds.1144. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42(6):1142-1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 21.Louis ED, Wise A, Alcalay RN, Rao AK, Factor-Litvak P. Essential tremor-Parkinson's disease: a double whammy. J Neurol Sci. 2016;366:47-51. doi: 10.1016/j.jns.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis ED, Hernandez N, Michalec M. Prevalence and correlates of rest tremor in essential tremor: cross-sectional survey of 831 patients across four distinct cohorts. Eur J Neurol. 2015;22(6):927-932. doi: 10.1111/ene.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The incidence of Parkinson's disease: a systematic review and meta-analysis. Neuroepidemiology. 2016;46(4):292-300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- 24.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63(7):1240-1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 25.Louis ED, Honig LS, Vonsattel JP, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Arch Neurol. 2005;62(6):1004-1007. doi: 10.1001/archneur.62.6.1004. [DOI] [PubMed] [Google Scholar]
- 26.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(12):3297-3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 27.Shill HA, Adler CH, Beach TG. Lewy bodies in essential tremor are no different than in controls. Parkinsonism Relat Disord. 2016;23:106-107. doi: 10.1016/j.parkreldis.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis ED, Iglesias-Hernandez D, Hernandez NC, et al. Characterizing Lewy pathology in 231 essential tremor brains from the essential tremor centralized brain repository. J Neuropathol Exp Neurol. 2022;81(10):796-806. doi: 10.1093/jnen/nlac068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis ED, Barnes LF, Ford B, Ottman R. Family history information on essential tremor: potential biases related to the source of the cases. Mov Disord. 2001;16(2):320-324. doi: 10.1002/mds.1040. [DOI] [PubMed] [Google Scholar]
- 30.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18):1434-1440. doi: 10.1212/wnl.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen-Roberts S, Myers TL, Auinger P, et al. A remote longitudinal observational study of individuals at genetic risk for Parkinson disease: baseline results. Neurol Genet. 2022;8(5):e200008. doi: 10.1212/nxg.0000000000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorsey ER, Darwin KC, Mohammed S, et al. Virtual research visits and direct-to-consumer genetic testing in Parkinson's disease. Digit Health. 2015;1:205520761559299. doi: 10.1177/2055207615592998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers TL, Tarolli CG, Adams JL, et al. Video-based Parkinson's disease assessments in a nationwide cohort of Fox Insight participants. Clin Park Relat Disord. 2021;4:100094. doi: 10.1016/j.prdoa.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long duration asymmetrical postural tremor is likely to predict development of Parkinson's disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005;76(1):115-117. doi: 10.1136/jnnp.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konno T, Deutschlander A, Heckman MG, et al. Comparison of clinical features among Parkinson's disease subtypes: a large retrospective study in a single center. J Neurol Sci. 2018;386:39-45. doi: 10.1016/j.jns.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 36.van Rooden SM, Visser M, Verbaan D, Marinus J, van Hilten JJ. Motor patterns in Parkinson's disease: a data-driven approach. Mov Disord. 2009;24(7):1042-1047. doi: 10.1002/mds.22512. [DOI] [PubMed] [Google Scholar]
- 37.van Rooden SM, Visser M, Verbaan D, Marinus J, van Hilten JJ. Patterns of motor and non-motor features in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2009;80(8):846-850. doi: 10.1136/jnnp.2008.166629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.