Abstract
Pharmacokinetic/pharmacodynamic studies of anti-tuberculosis agents in animal models of tuberculosis are hampered by the frequent necessity to perform sample bioanalysis outside the biosafety level-3 environment. Thus, each specimen has to undergo tedious and time-consuming sample sterilization procedures that may also affect drug stability. Here, we tested treatment of Mycobacterium tuberculosis (Mtb) infected samples with methanol to sterilize samples while preserving drug integrity for further pharmacokinetic/pharmacodynamic evaluations. Tissue samples harvested from Mtb infected mice were homogenized, incubated in methanol, and tested for sterility. Once sterility was confirmed, the samples were used to determine concentrations of the anti-tuberculosis drug spectinamide-1599 in lung homogenates using liquid chromatography coupled with mass spectrometry. The results demonstrate that methanol sterilizes tissue samples harvested from Mtb infected mice without altering the integrity of the drug in the tissue.
Keywords: Mycobacterium-tuberculosis, Sample sterilization, Methanol, Pharmacokinetics, PK
1. Introduction
Mycobacterium tuberculosis (Mtb) bacilli are the causative agent of tuberculosis disease (TB) and are transmitted as aerosols via the pulmonary route with only few bacilli needed to establish infection. After infection, Mtb can survive for long periods of time within the granuloma formed in the lungs [1]. Chemotherapy is available to treat TB but in the best-case scenario, a regimen of at least six-months of multidrug treatment is required to successfully treat the infection. The protracted treatment in many cases leads to patient non-adherence and contributes to the emergence of multi-drug resistant Mtb (MDR) and extensively drug resistant Mtb (XDR) strains [2]. In 2017, the World Health Organization reported 558,000 new cases of MDR (4.1% of all newly TB reported cases) [3,4]. There is an urgent need for shorter, safer, and more effective treatment options against Mtb infections. Multiple new chemical entities are currently in the early drug discovery and development stage. Understanding the pharmacokinetic and pharmacodynamic (PK/PD) properties of novel anti-TB drug candidates is essential to the development of more effective therapies for TB. While Mtb is classified as a biosafety level III (BSL-3) pathogen [5] and studies in animal models of Mtb infection are performed under Animal Biosafety Level 3 (ABSL-3), bioanalytical equipment used in pharmacology studies to quantify drug exposures is often outside of the BSL-3 environment. Thus, each specimen subjected to drug content analysis must undergo a sterilization procedure that inactivates Mtb while preserving the integrity of the drug present in each sample. The inability to sterilize samples poses a major impediment to performing PK/PD studies in animal models of TB.
Spectinamides are a new class of semi-synthetic analogues derived from spectinomycin with anti-tuberculosis activity [6]. In preclinical studies, Spectinamide-1599 has shown excellent killing activity against Mtb, including MDR and XDR Mtb strains [7]. The antimycobacterial activity of spectinamide-1599 is attributed to its ability to inhibit RNA translation in Mtb and avoid the mycobacterial Rv1258 efflux pump [8]. PK/PD studies are essential to evaluate the relationship between drug exposure and Mtb killing of these new semisynthetic drugs. In previous studies, liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) assays were used to determine spectinamide-1599 concentrations and resulting PK/PD and exposure-response parameters in vitro [9], and in non-infected, naïve animals [7]. However, studies in Mtb infected animals have been limited by the required sample sterilization process prior to drug analysis. Here, we demonstrate that treatment of Mtb infected tissues with methanol, a solvent used in mass spectrometry, sterilizes samples and allows for further analysis using LC-MS/MS assay methodology.
2. Materials and methods
2.1. Animal model of infection
This study used frozen lung tissue samples previously harvested from Mtb infected mice. In brief, pathogen-free 8–10 week old female Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the BSL-3 facility at Colorado State University. Studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved for use of female Balb/c mice by the Colorado State University Institutional Animal Care and Use Committee under protocol #16–6592A. Mice were infected with a low dose aerosol of Mtb. The inoculum (2 × 106 CFU/5 ml) was administered using the Glas-Col inhalation exposure system (Glas-Col, Terre Haute, IN) for a targeted infection of 50–100 bacilli in the lungs of each mouse. At day 1 and at 8 weeks post-infection, necropsies were performed to harvest the lungs and determine bacterial burden. At the time of necropsy the right and middle right lobes of the lungs were frozen at −80 °C.
2.2. Sterilization of lung samples with methanol
Sample sterilization was investigated with two different methodologies, one for whole lung tissue (Method A), and one for lung tissue homogenates (Method B). For Method A, frozen lung lobes from 10 mice were weighed and cut to have 500 mg tissue in each sample. The tissue samples were placed in safe-lock tubes (Article #22363204, Fisher Scientific, Waltham, MA) and thawed, then mixed with methanol (Article #179957, Honeywell). Triplicate samples were placed in 0, 500 or 1000 μL of methanol and were incubated at 4 °C for 24 h. After the incubation period each tube received three sterile beads (Article #NC9084634, Fisher Scientific, Waltham, MA) and was homogenized in the Next Advance Bullet Blender (Averill Park, NY) during 4 min at 800 rpm. For Method B, samples were treated with methanol after homogenization of the lung tissue (in PBS at a ratio of 1:4). For this purpose, triplicate samples of lung homogenates were mixed with methanol at (v/v) ratios of 1:0, 1:1, 1:2, 1:4, 1:8 and incubated at 4 °C for 24 or 48 h. For both methods, after methanol treatment the samples were centrifuged at 800×g for 10 min and supernatant was removed. The tissue pellet was resuspended in PBS. Thereafter, 100 μL of ten-fold dilutions in sterile PBS of each lung homogenate was plated on 7H11 agar and incubated at 37 °C for 6 weeks and checked for bacterial colony formation.
2.3. Sterility testing with mycobacteria growth indicator tube (MGIT)
Sterility of samples after methanol treatment was further verified using the BACTEC MGIT 320 system (Becton-Dickinson Microbiology Systems, Sparks, MD). Briefly, 200 μL of the lung homogenate from each sample in triplicates (untreated or treated with methanol as in Method B above) was added to the BACTEC MGIT. The BD BACTEC MGIT barcoded 7 mL tubes (Becton-Dickinson, article #245122) containing 4 mL of 7H9 broth with 0.25% glycerol were supplemented with 0.1 mL per vial of an antimicrobial mixture containing polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA). Additionally, 0.5 mL of BD BACTEC MGIT 960 supplement kit (Becton-Dickinson, article #245124) with oleic acid, bovine albumin, dextrose, and catalase was added to each vial. Bacterial growth was determined via Time to Positivity (TTP in days) measured using the BACTEC/MGIT system.
2.4. Integrity of drug concentrations in methanol sterilized samples
Spectinamide-1599 was synthesized and provided by Dr. Richard Lee (St. Jude Children’s Research Hospital, Memphis TN) as previously described [8]. Lung homogenates (200 μL) of untreated Balb/c mice were spiked with spectinamide-1599 for final concentrations of 0, 0.5, 5, or 50 μg/mL. Samples for each concentration were prepared in triplicate. Lung homogenates containing spectinamide-1599 and their respective controls were then mixed with 800 μL of 99.6% methanol (containing 10 ng/mL spectinamide 1329 [(3′-Dihydro-3′-deoxy-3′(R)-(pyridin-2yl) acetylamino spectinomycin] as internal standard) and incubated for 24 h at 4 °C according to Method B. To confirm sterilization of the samples after methanol treatment, one tenth of each sample was plated on 7H11 agar plates and incubated at 37 °C during 6 weeks as described above. The remaining volume in each sample was frozen at −80 °C until the results from the sterility test were available.
2.5. LC-MS/MS quantitation of spectinamide-1599
Samples with demonstrated absence of colony forming units in the sterility test were moved outside of the BSL-3 environment for LC-MS/MS analysis. After thawing, vortexing and centrifugation at 10,000×g and 4 °C for 10 min, the supernatant was chromatographically separated on a HILIC 3.5 μm, 100 mm × 4.6 mm column (Phenomenex, Torrance, CA) using a Shimadzu Nexera XR liquid chromatograph (Shimadzu Corporation, Columbia, MD) consisting of two pumps, online degasser, system controller and an auto sampler. A mobile phase consisting of (a) water with 5 mM ammonium formate buffer and (b) methanol with 5 mM ammonium formate buffer was used at a flow rate of 0.4 mL/min in gradient mode. Detection of the analyte and internal standard was achieved by an API 4500 triple quadruple mass spectrometer (AB-Sciex, Foster City, CA) equipped with a turbospray ion source operated in positive ion mode. The characteristic mass transfers for spectinamide-1599 (487.2 → 207.1) and internal standard (453.0 → 247.1) were monitored in multiple reaction monitoring mode with a declustering potential of 45 V and collision energy of 25 eV. Data were acquired and processed with Analyst software version 1.6.2 (AB-Sciex, Foster City, CA). Spectinamide-1599 concentrations were determined by comparing peak area ratios between spectinamide-1599 and internal standard for unknown samples to a previously established calibration curve. The accuracy was on average ± 3.8% over the entire range of the calibration curve, and the precision (coefficient of variation) of the assay was 3.2%, with a lower limit of quantitation of 1 ng/mL.
3. Results and discussion
Since the lungs are the major site of Mtb infection in tuberculosis disease, our study focused on sterilization of lung tissues from mouse models of Mtb infection. Sterility of Mtb infected lungs after methanol treatment was demonstrated by culturing lung homogenates on 7H11 agar plates followed by incubation at 37 °C for 6 weeks. Fig. 1 (A–C) shows that whole lung (Fig. 1A) or lung homogenates (Fig. 1B) if left untreated when plated on 7H11 agar plates and cultured at 37 °C during 4–6 weeks yielded 5.27 or 4.6 log10 CFU ( ± SEM 0.01) respectively. When samples were incubated with methanol at 4 °C for 24 h as explained in the material and methods section and then cultured onto 7H11 agar plates as above, no CFU were observed after incubation at 4 or 6 weeks (Fig. 1A–C). These studies were repeated for two additional times with the same results.
Fig. 1. CFU growth of lung specimens after methanol sterilization.
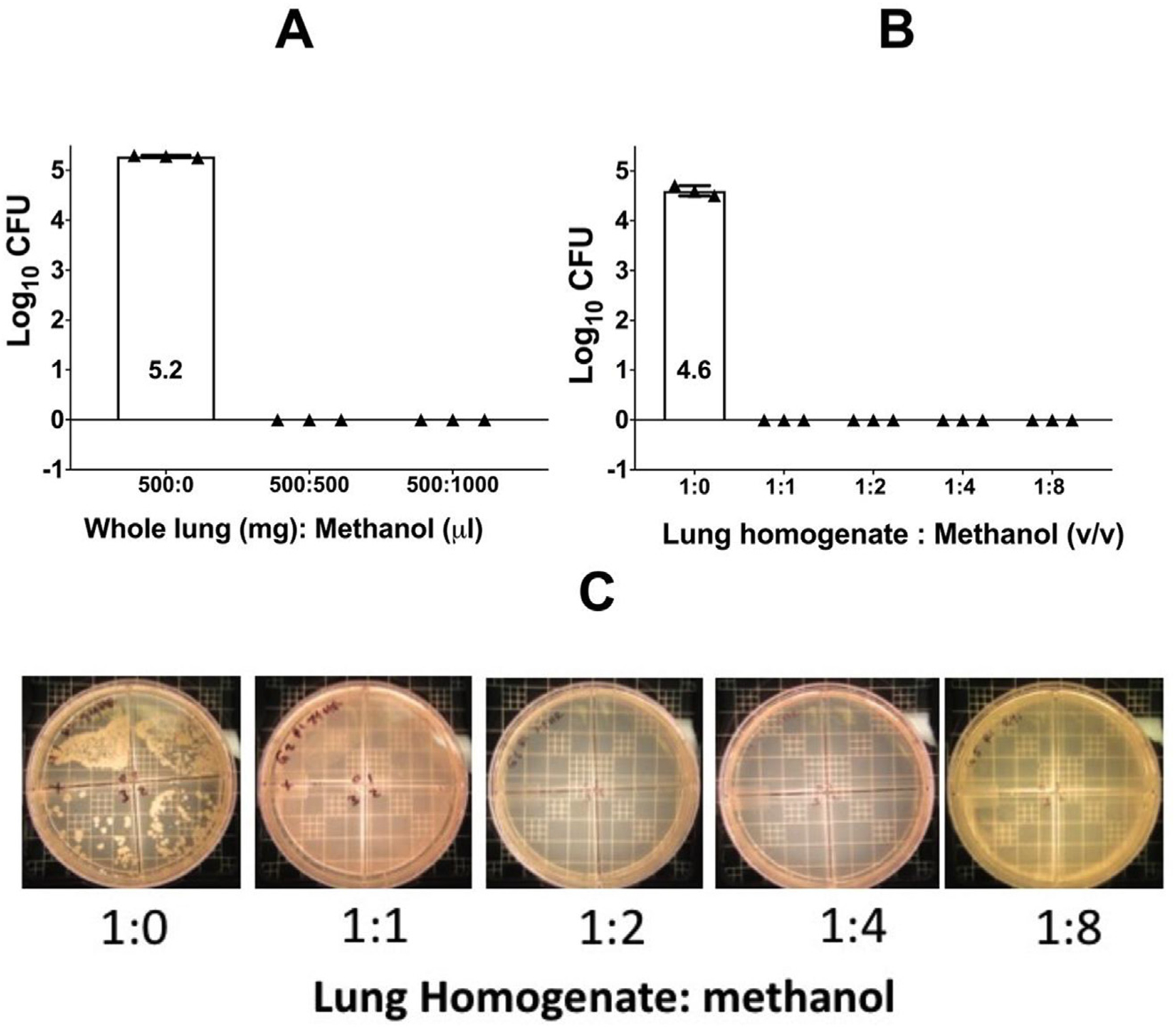
Panel A shows CFU growth of untreated whole lung compared to 1:1 and 1:2 tissue to methanol (mg/ul). Panel B shows CFU growth of untreated lung homogenate compared to 1:1 and 1:2 tissue to methanol (v/v). Panel C shows CFU growth of lung homogenate on 7H11 agar after 6 weeks incubation at 37 °C and diluted ten-fold. Homogenate was treated with 0 μl of methanol or 1:1, 1:2, 1:4, or 1:8 (v/v) of methanol as shown.
We further tested sterility in lung specimens after methanol treatment using a BACTEC-MGIT system. Lung homogenates were diluted in 7H9 media at 1:1, 1:5, 1:8, and 1:10 ratios. Samples had previously been incubated in 1:1 or 1:2 ratios of methanol, spun down, and resuspended in PBS as previously described. Samples without methanol treatment were also included as controls. Thereafter, 200 μL of each sample or their respective controls was added to a BACTEC barcoded vial and placed in the BACTEC-MGIT system for 42 days. Positive growth in this system was determined as Time to Positivity (TTP in days). As expected, positive control samples demonstrated TTP at 3, 7 and 17 days whereas none of the samples treated with methanol had TTP positivity during 42 days of culture in the BACTEC-MGIT system. We concluded that the methanol treatment of lung tissues infected with Mtb sterilizes these samples.
To ensure that the sterilization procedure of lung tissue homogenates with methanol does not affect the stability of spectinamide-1599, we spiked lung homogenate specimens with three different concentrations of spectinamide-1599, performed the methanol sterilization step, verified the sterility of each sample, and quantified the drug concentration in each specimen. Since none of the samples showed colony forming units after 6 weeks of incubation, they were moved out of the BSL-3 facility and were analyzed for drug concentrations by LC-MS/MS. A comparison of the actually measured concentrations with the nominal concentrations (Fig. 2A) indicates good agreement within the limits of the accuracy of the applied LC-MS/MS assay. These results suggest that integrity of the spectinamide-1599 was maintained during the methanol sterilization step. This observation was expected since sample treatment with methanol for protein denaturation in preparation of LC-MS/MS analyses has been used routinely for spectinamide-1599 without detectable impact on estimated concentrations [7,10]. In addition, spectinamide-1599 has shown good chemical stability in aqueous solution over 96 h at 37 °C [9].
Fig. 2. Integrity of spectinamide-1599 in lung specimens after methanol sterilization.
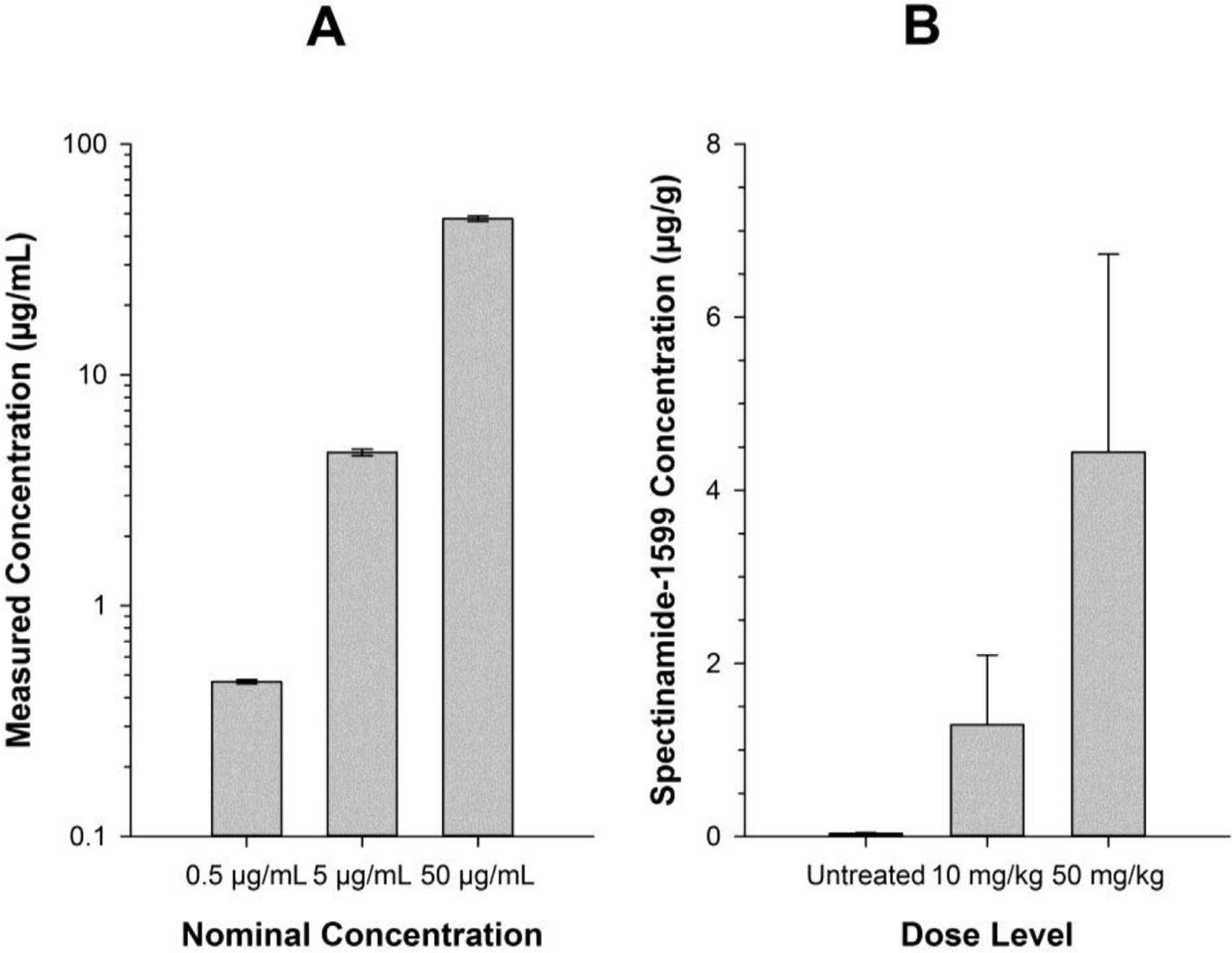
Panel A shows nominal versus actually measured concentrations for lung homogenates spiked with three different known concentrations of spectinamides-1599. The homogenates have undergone the methanol sterilization methodology as described (n = 3 per concentration). Panel B shows measured spectinamide-1599 concentrations in lung homogenates obtained from Balb/c mice infected with Mtb and treated with doses of either 0, 10 or 50 mg/kg three times weekly for four week by intrapulmonary aerosol delivery. The specimens were obtained 72 h after administration of the last dose (n = 3 per dose level).
We further demonstrated the feasibility of the sterilization of Mtb-infected lung homogenates by determining the spectinamide concentrations in lung samples (n = 3 per dose group) taken 72 h after termination of a 4 week-long treatment of Mtb-infected mice with trice weekly administration of 0, 10, or 50 mg/kg spectinamide-1599 as intrapulmonary aerosol delivery as previously described [10]. The ob served lung concentrations [Fig. 2B] increased in a dose-dependent manner and were within the range of expected exposure after therapy with such a dosing regimen based on the extensively assessed lung exposure profiles of spectinamide-1599 in healthy, uninfected Balb/c mice after intrapulmonary aerosol administration [unpublished data].
In summary, we have presented a simple sterilization methodology for lung homogenates of Mtb infected mice that is amenable to subsequent specimen analysis by LC-MS/MS and thus facilitates the performance of PK/PD studies for anti-tubercular drug candidates without the need to have bioanalytical equipment in the BSL-3 environment. Each laboratory should establish its own protocol for sterilization of samples. Our recommended guidelines for sterilization of samples is mixing of Mtb infected samples with methanol at 1:1 ratio and incubation at 4 °C during 24hr. To confirm sterility, one tenth of each sample should be plated on 7H11 agar plates and incubated 4–6 weeks prior removal from BSL-3 and PK analysis.
Funding
This research was supported by the National Institute of Allergy and Infectious Diseases and the Office of the Director of the National Institutes of Health (grant numbers R01AI120670, S10OD016226). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Singh R, Manjunatha U, Boshoff HI, Ha YH, Nyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 2008;322(5906):1392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003;167(11):1472–7. [DOI] [PubMed] [Google Scholar]
- [3].WHO. Global tuberculosis report 2018:40–53.
- [4].Zumla A, Chakaya J, Centis R, D’Ambrosio L, Mwaba P, Bates M, Kapata N, Nyirenda T, Chanda D, Mfinanga S, Hoelscher M, Maeurer M, Migliori GB. Tuberculosis treatment and management–an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir. Med 2015;3(3):220–34. [DOI] [PubMed] [Google Scholar]
- [5].Herman P, Fauville-Dufaux M, Breyer D, Van Vaerenbergh B, Pauwels K, Do Thi CD, Sneyers M, Wanlin M, Snacken R, Moens W. Biosafety recommendations for the contained use of Mycobacterium tuberculosis complex isolates in industrialized countries. M. tuberculosis and Biosafety; 2006.
- [6].Borovinskaya MA, Shoji S, Holton JM, Fredrick K, Cate JHD. A steric block in translation caused by the antibiotic spectinomycin. ASC Chem. Biol 2007;2(8):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman MS, Vaddady PK, Zheng Z, Qi J, Akbergenov R, Das S, Madhura DB, Rathi C, Trivedi A, Villellas C, Lee RB, Rakesh, Waidyarachchi SL, Sun D, McNeil MR, Ainsa JA, Boshoff HI, Gonzalez-Juarrero M, Meibohm B, Bottger EC, Lanaerts AJ, Spectinamides. A new class of semisynthetic anti-tuberculosis agents that overcome native drug efflux. Nat Med 2014;20(2):152–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu J, Bruhn DF, Lee RB, Zheng Z, Janusic T, Scherbakov D, Scherman MS, Boshoff HI, Das S, Rakesh Waidyarachchi SL, Brewer TA, Gracia B, Yang L, Bollinger J, Robertson GT, Meibohm B, Lanaerts AJ, Ainsa J, Bottger EC, Lee RE. Structure-activity relationships of spectinamide antituberculosis agents: a dissection of ribosomal inhibition and native efflux avoidance contributions. ACS Infect Dis 2016;3(1):72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vaddady PK, Trivedi A, Rathi C, Madhura DB, Liu J, Lee RE, Meibohm B. Dynamic time-kill curve characterization of spectinamide antibiotics 1445 and 1599 for the treatment of tuberculosis. Eur J Pharm Sci 2019;127:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rathi C, Lukka PB, Wagh S, Lee RE, Lenaerts AJ, Braunstein M, Hickey A, Gonzalez-Juarrero M, and Meibohm B. Comparative pharmacokinetics of spectinamide 1599 after subcutaneous and intrapulmonary aerosol administration in mice. Tuberculosis, 114, 119–122. 10.1016/j.tube.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


