Abstract
The lengthy treatment time for tuberculosis (TB) is a primary cause for the emergence of multidrug resistant tuberculosis (MDR-TB). One approach to improve TB therapy is to develop an inhalational TB therapy that when administered in combination with oral TB drugs eases and shortens treatment. Spectinamides are new semisynthetic analogues of spectinomycin with excellent activity against Mycobacterium tuberculosis (Mtb), including MDR and XDR Mtb strains. Spectinamide-1599 was chosen as a promising candidate for development of inhalational therapy. Using the murine TB model and intrapulmonary aerosol delivery of spectinamide-1599, we characterized the pharmacokinetics and efficacy of this therapy in BALB/c and C3HeB/FeJ mice infected with the Mtb Erdman strain. As expected, spectinamide1599 exhibited dose-dependent exposure in plasma, lungs, and ELF, but exposure ratios between lung and plasma were 12–40 times higher for intrapulmonary compared to intravenous or subcutaneous administration. In chronically infected BALB/c mice, low doses (10 mg/kg) of spectinamide-1599 when administered thrice weekly for two months provide efficacy similar to that of higher doses (50–100 mg/kg) after one month of therapy. In the C3HeB/FeJ TB model, intrapulmonary aerosol delivery of spectinamide-1599 (50 mg/kg) or oral pyrazinamide (150 mg/kg) had limited or no efficacy in monotherapy, but when both drugs were given in combination, a synergistic effect with superior bacterial reduction of >1.8 log10 CFU was oberved. Throughout the up to eight-week treatment period, intrapulmonary therapy was well-tolerated without any overt toxicity. Overall, these results strongly support the further development of intrapulmonary spectinamide-1599 as a combination partner for anti-TB therapy.
Keywords: aerosol, pharmacokinetics, spectinamides, tuberculosis, animal models
Graphical Abstract
Despite being a treatable disease, tuberculosis (TB) causes nearly 1.5 million deaths per year worldwide.1 Lengthy chemotherapy regimens, lack of patient compliance, and difficult management of multidrug therapies are major hurdles to cure and control morbidity and mortality of this disease. Successful treatment is further complicated by the continuous emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of Mycobacterium tuberculosis (Mtb) bacilli, the causative agent of TB. According to the World Health Organization, to control TB globally, it is essential to develop new therapies that are effective against MDR and XDR TB, simplify the current treatment regimens, and shorten the overall duration of treatment to two months.1
The first-line regimen for drug sensitive TB (DS-TB) contains four drugs, namely rifampicin, isoniazid, pyrazinamide, and ethambutol.2 For MDR-XDR-TB chemotherapy, a range of second-line injectable drugs (amikacin, kanamycin, capreomycin, and streptomycin) are used that require longer periods of treatment (approximately two years) and have less potency and higher toxicity than the first-line drugs. Forty years passed where the FDA did not approve any new drugs for TB treatment, but between 2013 and 2019, three novel oral TB drugs (bedaquiline, delamanid, and pretomanid) entered the tool kit of TB therapies and are currently used for treatment of MDR-XDR-TB.2 While several new compounds are still being evaluated for treatment of both DS-TB and MDR-TB, the focus in TB drug development is shifting to the development of new routes of administration with increased or complementary efficacy and for shorter-course regimens that range from 6 to 12 months in duration for multidrug TB therapies.2,3
To achieve effective concentrations of drug in the lungs, current TB chemotherapies require systemic administration of substantial doses of drug that often results in systemic toxicity and potentially serious side effects. This explains why one of the hardest challenges of TB chemotherapy is to reach and sustain adequate concentrations of antibiotic drugs in the lungs while avoiding toxicity and side effects if patients are to tolerate the long regimens of TB therapy. One approach to overcoming these shortcomings is to administer drugs directly into the lungs by developing inhalational TB therapies that are easy and painless to administer and facilitate targeting the lungs with high drug exposures while limiting systemic exposures and related side effects.
Inhalational therapy or “medicated vapors” for TB have been reported since the 19th century4 and in more modern times since the 1940s and 50s.5 Nebulization of liquid formulations of streptomycin5 and kanamycin6 as adjunct therapy to conventional TB therapy in patients was well-tolerated and improved therapeutic outcome.5,6 However, research on inhalational antibiotic therapy in TB has moved at a slow pace, and this therapeutic approach has not yet been incorporated as an option for treating TB disease. Importantly, during the last decades, inhaled drug therapy has become a standard of care treatment for other pulmonary diseases such as asthma, cystic fibrosis and COPD.7 During this process, we have broadened our understanding of treatment of pulmonary diseases via inhalational therapy and patient–caregiver education at the same time so that inexpensive hand-held inhaler devices with high delivery efficiencies have become commercially available.7
Spectinamides are new semisynthetic analogues of spectinomycin with excellent activity against Mtb including MDR and XDR strains. The spectinamides have a well-defined mechanism of action via protein synthesis inhibition8 with potent in vitro activity against Mtb (MIC 0.4–0.8 μg/mL) and antibiotic-resistant Mtb strains (MIC 0.4–1.6 μg/mL).8 One of the lead molecules, spectinamide-1599, is a highly soluble compound with a lengthy postantibiotic effect and good efficacy in preclinical TB models in combination regimens with other anti-TB drugs.9 However, as spectinamide-1599 is not orally bioavailable, it has to be administered as an injectable, with the risk of patients withdrawing from treatment during prolonged therapy. We previously reported that in preclinical studies, spectinamide-1599 provides high exposure and antibacterial activity when delivered directly to the lungs.8,10 Thus, we believe spectinamide-1599 has great potential to be developed as a new inhalational therapy for TB treatment. Furthermore, spectinamide-1599 is an aminocyclitol antibiotic and shares properties with other members of this family, e.g. spectinomycin, trospectomycin, tobramycin, and amikacin,11 some of which (tobramycin and amikacin) are already used as inhalational therapy.7 In this paper, we report on the pharmacokinetics (PK), tissue distribution, and efficacy of spectinamide-1599 in different mouse models of Mtb infection when administered by liquid aerosol formulation. Although all TB therapy is currently and will continue to be multidrug therapy, in the studies reported here, we tested spectinamide-1599 first as a monotherapy to determine the maximum exposure-killing activity for spectinamide-1599 in the lungs after up to eight weeks of treatment via intrapulmonary aerosol delivery. Thereafter, we demonstrated its efficacy when combined with oral pyrazinamide. To our knowledge, this systematic assessment of the in vivo pharmacology and efficacy of intrapulmonary aerosol therapy has not been performed for spectinamides or any other anti-TB drugs.
RESULTS
The assessments performed under this analysis comprise a variety of PK and efficacy studies to characterize and quantify the effect of dose, dosing regimen, and length of therapy on the disposition and the efficacy of spectinamide-1599 administered via intrapulmonary aerosol delivery.
Pharmacokinetics and Tissue Exposure after Intrapulmonary Aerosol Delivery.
The PK study program comprised single and multiple dose assessments at three different dose levels, 10, 50, and 150 mg/kg, given by intrapulmonary aerosol administration (IPA). For the multiple dose studies, dosing frequencies included once daily and every other day. For comparison, single and multiple doses (once daily) were also administered at the dose levels of 10 mg/kg by intravenous (IV) injection and 50 and 200 mg/kg by subcutaneous (SC) injection.
Exposure parameters for different dose levels after single and multiple dose administration of intrapulmonary aerosol (IPA) as well as IV and SC administration determined in plasma, lungs, and ELF are presented in Table 1, and the corresponding concentration–time profiles for multiple dosing are depicted in Figure 1. Additional exposure parameters and concentration–time profiles for spleen, liver and kidney are provided in Table S1 and Figure S1, respectively.
Table 1.
Pharmacokinetic Exposure Parameters (Geometric Mean, %CV) for Plasma, Lung, and ELF after a Single Dose or a Multiple Dose Series of Different Dose Levels (10, 50, 150, or 200 mg/kg) of Spectinamide-1599 given by IPA, SC, or IV Administration to Micea
| plasma |
lung |
ELF |
|||||
|---|---|---|---|---|---|---|---|
| dose (mg/kg) | Cmax (μg/mL) | AUClast (μg h/mL) | Cmax (μg/g) | AUClast (μg h/g) | Cmax (μg/mL) | AUClast (μg h/mL) | |
| single dose | |||||||
| IPA | 10 | 5.92 (66) | 5.51 (11) | 83.1 (15) | 162 (7.7) | 627 (44) | 747 (14) |
| IPA | 50 | 36.4 (35) | 23.2 (23) | 168 (49) | 332 (11) | 2137 (69) | 1830 (46) |
| IPA | 150 | 128 (125) | 59.5 (27) | 1620 (26) | 735 (13) | 1698 (99) | 1950 (26) |
| IV | 10 | 35.1 (16) | 7.52 (9.7) | 4.84 (25) | 3.79 (6.3) | 37.7 (13) | 41.0 (22) |
| SC | 50 | 88.5 (28) | 40.4 (13) | 18.6 (21) | 19.1 (7.5) | 113 (37) | 127 (21) |
| SCb | 200 | 191 (8.4) | 158 (6.1) | 8.82 (12) | 52.9 (23) | ND | ND |
| multiple doses: once daily (QD5) | |||||||
| IPA | 10 | 9.61 (52) | 3.56 (27) | 218 (21) | 227 (19) | 292 (144) | 307 (91) |
| IPA | 50 | 87.3 (69) | 39.8 (33) | 1043 (64) | 954 (20) | 2058 (81) | 812 (41) |
| IPA | 150 | 241 (92) | 96.9 (42) | 2409 (63) | 1910 (24) | 2537 (11) | 2100 (56) |
| IV | 10 | 31.0 (6.2) | 6.37 (5.8) | 3.97 (13) | 3.94 (16) | 61.1 (25) | 32.7 (48) |
| SC | 200 | 729 (13) | 471 (9.2) | 29.6 (3.8) | 69.1 (3.6) | 362 (23) | 297 (24) |
| multiple doses: once every other day (TIW) | |||||||
| IPA | 10 | 4.57 (54) | 5.35 (36) | 113 (41) | 150 (39) | 332 (51) | 307 (62) |
| IPA | 50 | 45.0 (44) | 24.3 (31) | 955 (72) | 789 (25) | 341 (86) | 237 (66) |
| IPA | 150 | 121 (78) | 61.7 (23) | 1330 (113) | 968 (39) | 3178 (69) | 1530 (22) |
| SC | 200 | 653 (11) | 424 (10) | 30.3 (29) | 72.0 (11) | 161 (48) | 401 (13) |
Cmax, maximum observed plasma concentration; AUClast, area under the concentration time curve until the last measured concentration. For multiple dose administrations, AUClast represents exposure after the last dose of the dosing series.
Previously reported in ref 10.
Figure 1.
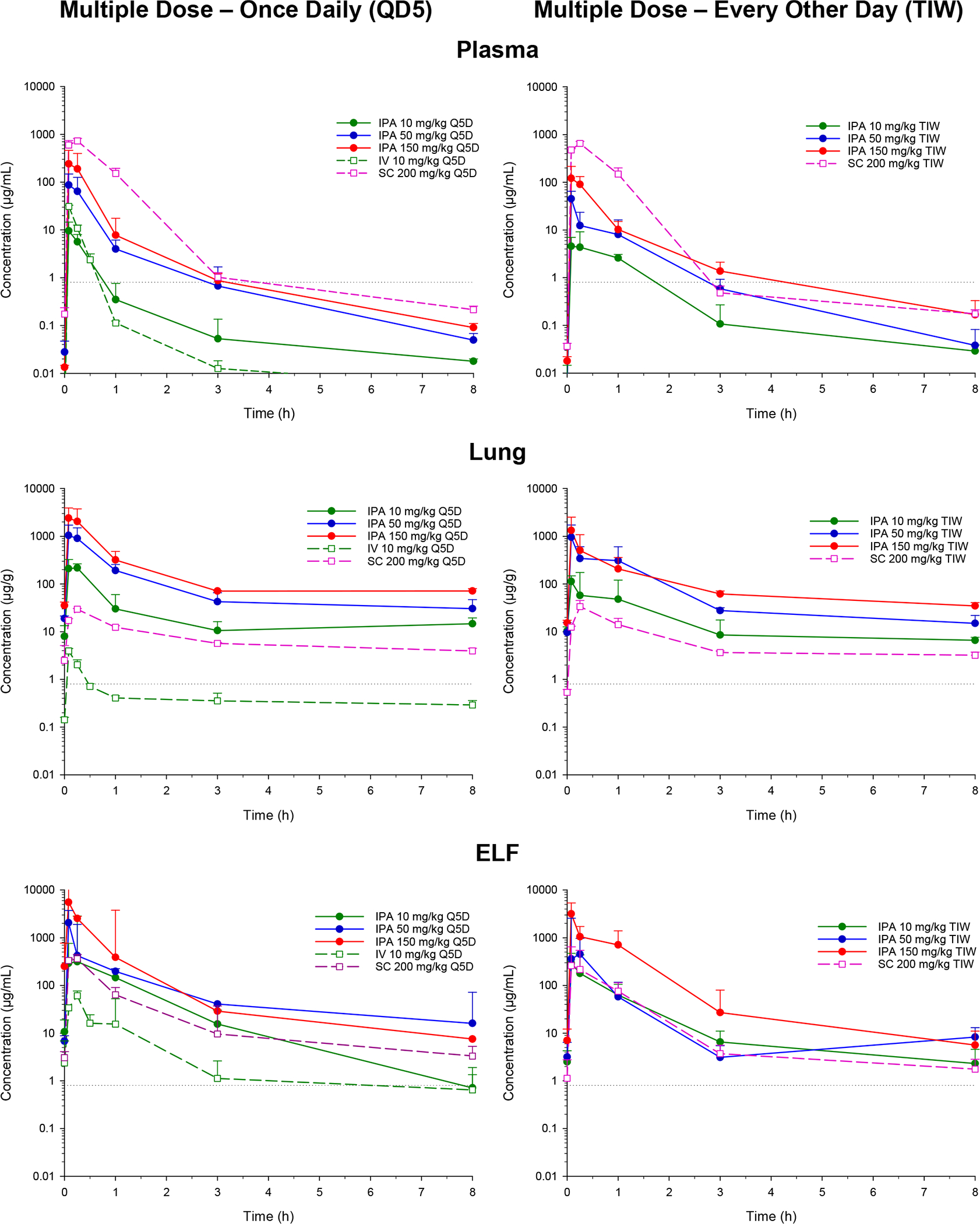
Concentration–time profiles of spectinamide-1599 in plasma, lung, and ELF of mice after the last dose of a five-day multiple dose series with administration either once daily (left column) or every other day (right column). The drug was given by IPA at dose levels of 10, 50, or 150 mg/kg and for comparison by intravenous and subcutaneous injection at doses of 10 and 200 mg/kg, respectively [geometric mean/SD]. The horizontal dotted line represents the MIC for spectinamide-1599 [0.8 μg/mL].
After single dose IPA administration, spectinamide-1599 was rapidly absorbed into the systemic circulation and plasma concentrations reached their maximum values within 5 min after administration. The peak concentration (Cmax) and the area-under-the concentration–time curve (AUC) in plasma as measures of systemic exposure increased dose dependently, with slightly less than dose proportionality for AUC. When compared with IV administration, IPA dosing reached an absolute bioavailability in plasma of 73% for the lowest dose and 53% for the highest dose, while spectinamide-1599 after SC administration was fully bioavailable (105–108%). Beyond the differences in bioavailability, the profiles showed comparable behavior independent of the route of administration with rapid decline in plasma concentrations at therapeutic concentrations with a half-life of approximately 0.37 h, and then a prolonged exposure at low concentrations levels below 1 μg/mL with a half-life of approximately 5.5 h, a pharmacokinetic behavior similar to that of spectinomycin, trospectomycin, and other aminocyclitols. Exposure ratios between lung and plasma were substantially higher after IPA administration (range 12–29) compared to the corresponding ratios after IV and SC administration (range 0.33–0.50). This indicates that although spectinamide-1599 has substantial lung penetration after SC or IV administration, dose-normalized exposures (AUC) in lung substantially increased after IPA delivery and were 12–40 times higher after IPA relative to SC or IV administration. In epithelial lining fluid (ELF) obtained via bronchoalveolar lavage (BAL), exposure differences between IPA and SC or IV administration were also pronounced: ELF-to-plasma exposure was for IPA relative to SC or IV 33–136 versus 3.1–5.5, respectively, and dose-normalized exposures in ELF were still 3.2–18 times higher than after SC or IV administration. This indicates high availability of spectinamide-1599 after IPA administration in lung and ELF relative to the systemic circulation, i.e. a targeted delivery to the pulmonary tissues relative to the systemic circulation. Figures 2A and 2B summarize the exposure ratios for lung vs plasma and ELF vs plasma, respectively.
Figure 2.
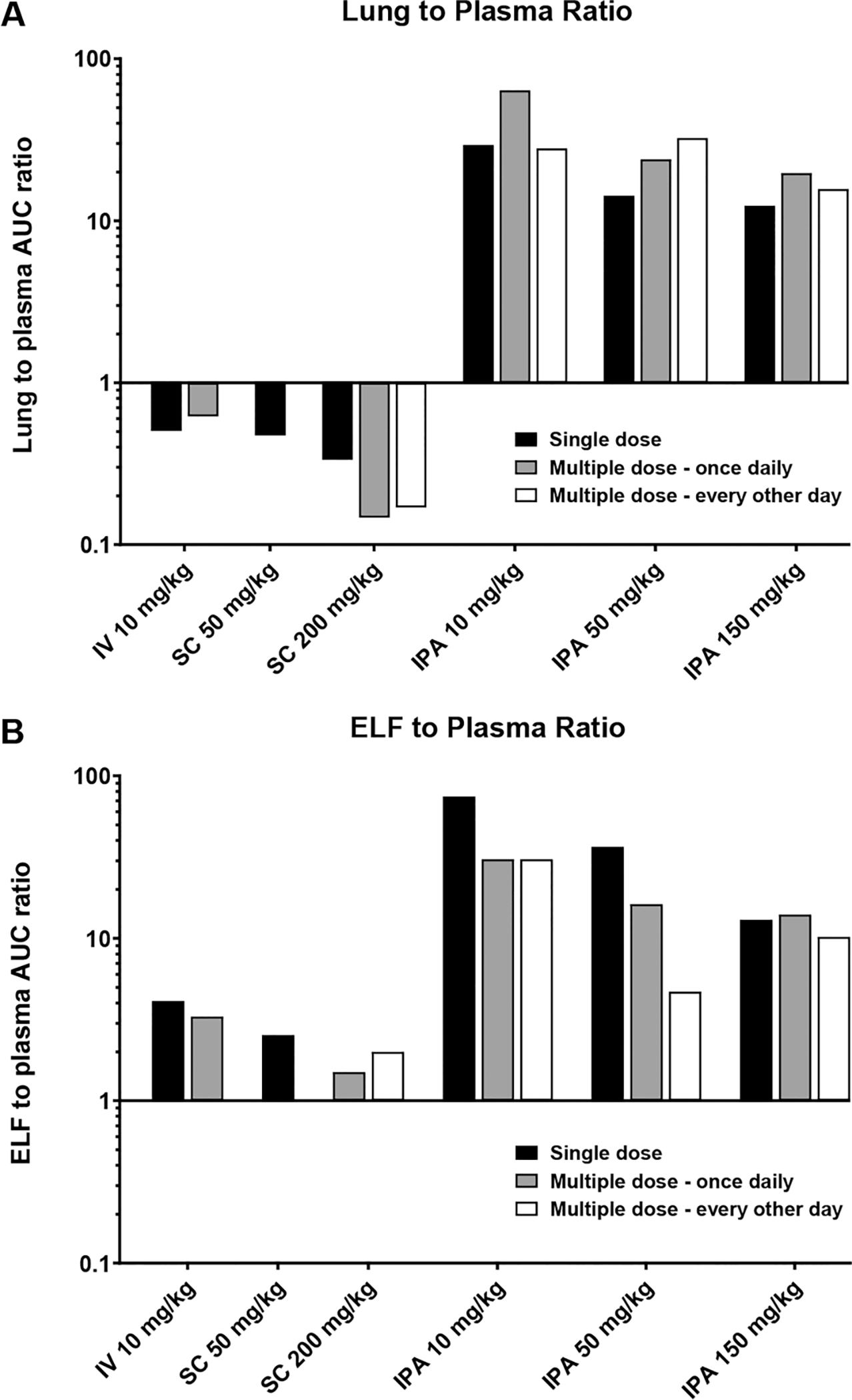
(A) Lung to plasma ratio. Exposure ratios were determined as ratios between area-under-the-concentration–time curve (AUClast) between lung homogenate and plasma. (B) ELF to plasma ratio. ELF and plasma after different doses (in mg/kg) and dosing regimens of spectinamide-1599 after IPA administration compared to IV and SC administration in mice.
Plasma and tissue distribution were also assessed after two multiple dose IPA regimens, either once daily or once every other day administration, similar to the dosing regimens used in the efficacy studies. In plasma, concentration–time profiles and pharmacokinetic exposure parameters for both regimens were similar to the single dose administration with no appreciable drug accumulation during multiple dosing. In contrast, there was a substantial increase in lung exposure during multiple dosing. This was even more pronounced for the once daily dosing regimen compared to dosing every other day. The resulting exposure ratios of lung compared to plasma were 18–61-fold, and are summarized in Figure 2A. This increase in exposure in lung was only present for IPA but not SC or IV multiple dose administration, and was not detected for ELF. This observation suggests that IPA multiple dose therapy with spectinamide-1599 selectively leads to accumulation in lung tissue that further enhances the pulmonary targeting of the IPA compared to SC or IV administration.
The effect of IPA versus SC and IV as route of administration and single versus multiple dose administration did not have any appreciable effect on tissue distribution or tissue-to-plasma ratios for the other investigated tissues, spleen, liver, and kidneys (Table S1 and Figure S1).
Dose Response of Treatment in Chronically Mtb Infected BALB/c Mice.
The efficacy study program consisted of dose and time response evaluations for spectinamide-1599 administered via IPA to BALB/c mice with a chronic infection with pulmonary Mtb. BALB/c mice were chosen because the course of infection and disease in these animals is well-predicted and a chronic pulmonary infection with Mtb develops into multifocal and very homogeneous type of lesions. In preclinical drug efficacy studies, this model allows to establish differences between group treatments with high statistical significance.12 In contrast to BALB/c mice, the course of pulmonary Mtb infection and disease in C3HeB/FeJ mice are unpredictable and animals develop also multifocal (as in BALB/c mice) but very heterogeneous type of lesions. According to the classification by Irwin et al. lesions are classified as necrotic (Type I), neutrophilic (type II), and cellular (Type III).13 When this animal model is used to test drug efficacy, the differences between group treatments oftentimes reach only low statistical significance.14 Thus, in separate studies, the C3HeB/FeJ mice were studied for dose response evaluation for spectinamide-1599 during chronic infection with pulmonary Mtb. Because previous studies with subcutaneous injection of spectinamide-1599 in the C3HeB/FeJ mice TB model showed low efficacy in monotherapy but substantially improved efficacy when partnered with pyrazinamide,9 we also evaluated intrapulmonary aerosol administration of spectinamide-1599 with oral administration of pyrazinamide at 150 mg/kg.
In preliminary studies, we reported that intrapulmonary aerosol delivery of spectinamide-1599 significantly reduced the bacterial burden of Mtb in chronically infected mice.8 Here, we undertook a more comprehensive assessment of intrapulmonary delivered spectinamide-1599 at three dose levels: the lowest dose level of 10 mg/kg that provides and maintains pulmonary exposure potentially sufficient for antimycobacterial activity as suggested in Figure 1, and the maximum expected human targeted dose for inhalation therapy of 50 to 100 mg/kg from a body weight extrapolation between species. It is worth noting, however, that lower human doses would likely be required if formal allometric scaling models were adopted. By changing the dose level and duration of treatment, we were aiming at improving the killing efficacy of spectinamide-1599 when administered as intrapulmonary aerosol.
We first used BALB/c mice chronically infected with Mtb. The efficacy for escalating doses of treatment with spectinamide-1599 and isoniazid (INH) in BALB/c infected with Mtb are shown in Figure 3. The drugs were delivered to chronically Mtb infected mice (n = 5–8) via intrapulmonary aerosol three times a week for 4 weeks as previously reported.8,15 In each study, one group of animals was treated with an IPA administered solution of isoniazid (INH; 25 mg/kg) and used as a positive control group for drug treatment and procedure. The results presented in Figure 3A demonstrate that spectinamide-1599 at 10, 50, or 100 mg/kg administered by IPA in BALB/c mice resulted in average CFU reduction of 0.4 (p < 0.0001), 0.7 (p < 0.0001) and 0.9 (p < 0.0001) log10 CFU, respectively, when compared to untreated or saline treated animals (thereafter referred as control groups). A similar trend was observed in lung lesions at the microscopic level in histological sections stained with H&E. The lesions in control groups consisted predominantly of histiocytic and neutrophilic aggregates at the periphery of bronchioles and small perivascular aggregates of lymphocytes and plasma cells. In treated groups, lesions were primarily composed of perivascular mononuclear cell aggregates with lesser numbers of histiocytes and rare numbers of neutrophils. There were no significant differences in reduction of bacterial burden between of the 50 and 100 mg/kg doses. In BALB/c mice treated with 25 mg/kg of INH, the bacterial burden reduction was of 1.1 (p < 0.0001) log10 CFU when compared to control groups. As expected based on our previous studies8 and the limited spectinamide-1599 exposures observed in the spleen (Table S1 and Figure S1), none of the treatments for spectinamide-1599 affected the bacterial burden in the spleen (Figure 3B). However, receiving intrapulmonary aerosols of INH, the splenic bacterial burden was reduced by 2.2 or 1.4 (p < 0.0001) log10 CFU, respectively (Figure 3B). During treatments the mice did not experience changes in body weights (Figure 3C) and did not show obvious behavioral changes. Additionally, no alveolar injury or increase in the number of alveolar macrophages was observed. We concluded that the intrapulmonary drug aerosols were well-tolerated by all groups during the 4 weeks of treatment with no apparent acute toxicity.
Figure 3.
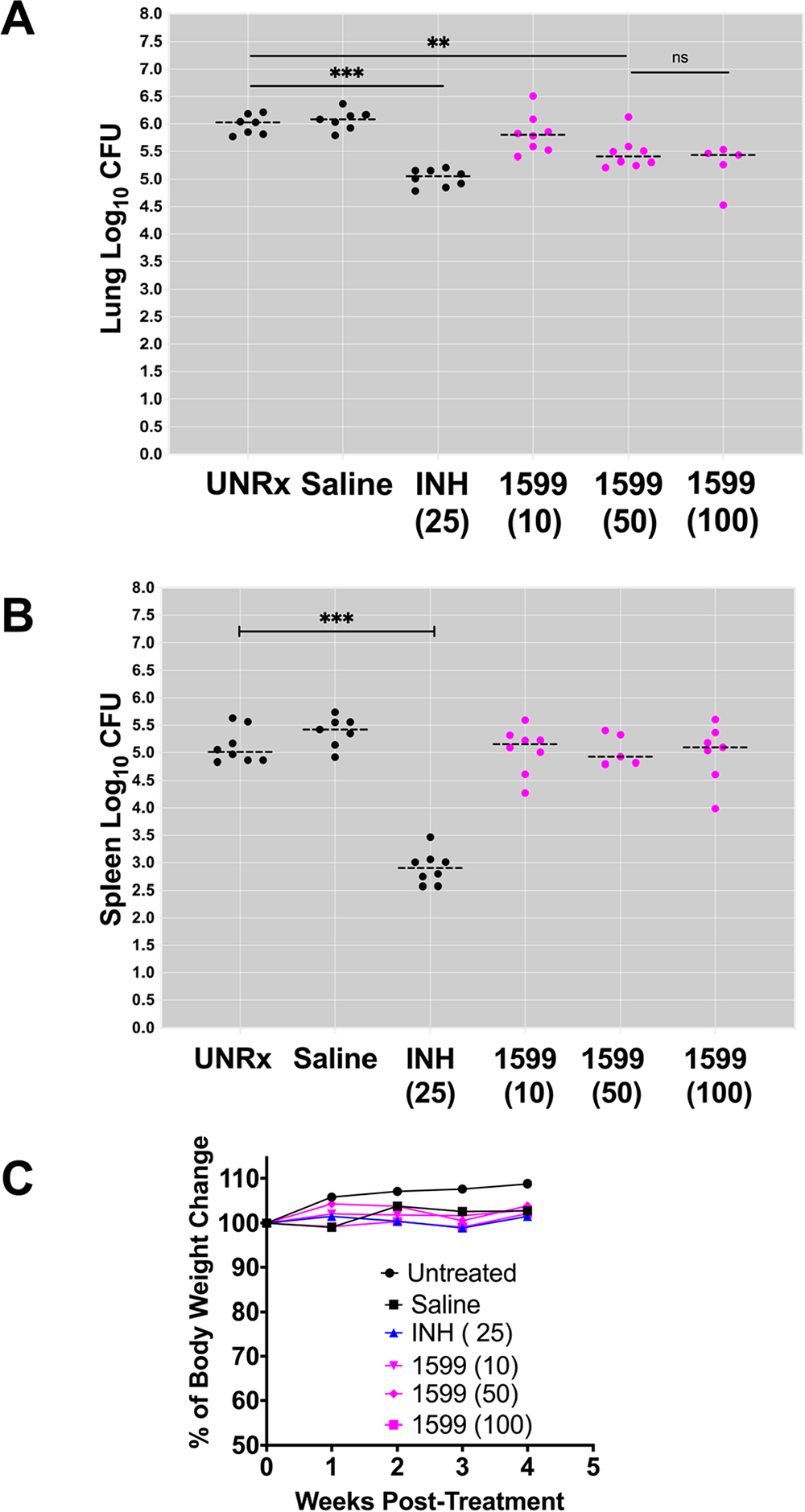
Dose response after intrapulmonary aerosol delivery of spectinamide-1599 and isoniazid to BALB/c chronically infected with Mtb. Spectinamide-1599 (1599) and INH were administered to chronically Mtb infected BALB/c mice (n = 5–8) via intrapulmonary aerosol. BALB/c female mice were infected with a low dose aerosol infection to deliver ~100 (BALB/c) Mtb bacilli per mouse. Mice were rested for four weeks until they were randomly assigned to study groups and used to test the therapy under study here. Spectinamide-1599 and INH were prepared in 0.9% low endotoxin saline (saline) at 10, 50, and 100 mg/mL [1599 (10), 1599 (50), and 1599 (100)], whereas INH was prepared at 25 mg/kg [INH (25)]. Except for mice in the untreated group (UnRx), each mouse in each group received 50 μL/dose of via intrapulmonary aerosol three times a week for 4 weeks. Three days after the last treatment, animals from all groups in the study were euthanized, and the lungs (A) and spleen (B) were prepared for bacterial load determination. The bacterial load was determined using serial dilutions of homogenized organs that were plated on 7H11 agar plates, and the CFUs in each sample were determined after 3 weeks of incubation at 37 °C. Bacterial load in each organ was expressed as the log10 of CFUs. (C) During therapy, the weights of BALB/c mice were recorded weekly as average weight of each mouse per cage (n = 5). The graph shows weekly average weight of five mice in each cage during four weeks of therapy.
Effect of Length of Treatment on Efficacy in Chronically Mtb Infected BALB/c Mice.
To evaluate the effect of extending the drug treatment period on efficacy, we repeated the experiment with groups of mice (n = 5) treated with 10 or 50 mg/kg of spectinamide-1599 for 4 or 8 weeks. Figure 4 shows the combined data from two independent studies with the same experimental design. The results in chronically infected BALB/c mice demonstrate that when mice were treated with 10, 50, and 200 mg/kg of spectinamide-1599 for 4 weeks the pulmonary bacterial load was reduced by 0.6–0.9 log 10 CFU (p < 0.0013). After 8 weeks of treatment and when compared to their corresponding control groups, doses of 10, 50, and 200 mg/kg again significantly reduced the bacterial burden (~1.0, 1.1, and 1.0 log10 CFU; p < 0.0001). Furthermore, we observed that extending the period of treatment to 8 weeks with INH provided further reduction in bacterial load beyond that provided at 4 weeks treatment. Although prolonging therapy with spectinamde-1599 from 4 to 8 weeks led to a greater reduction of the bacteria burden, the CFU difference between 4 and 8 weeks did not achieve statistical significance. The intrapulmonary drug aerosols were well-tolerated by all groups during the 4 and 8 weeks of treatment and there was no apparent acute toxicity. We concluded that shorter treatments (1 month) of spectinamide-1599 at 50 mg/kg provide similar efficacy compared to that of longer treatments (8 weeks) with lower doses of 10 mg/kg. Prolonged treatment significantly improved the statistical differences between controls and experimental groups.
Figure 4.
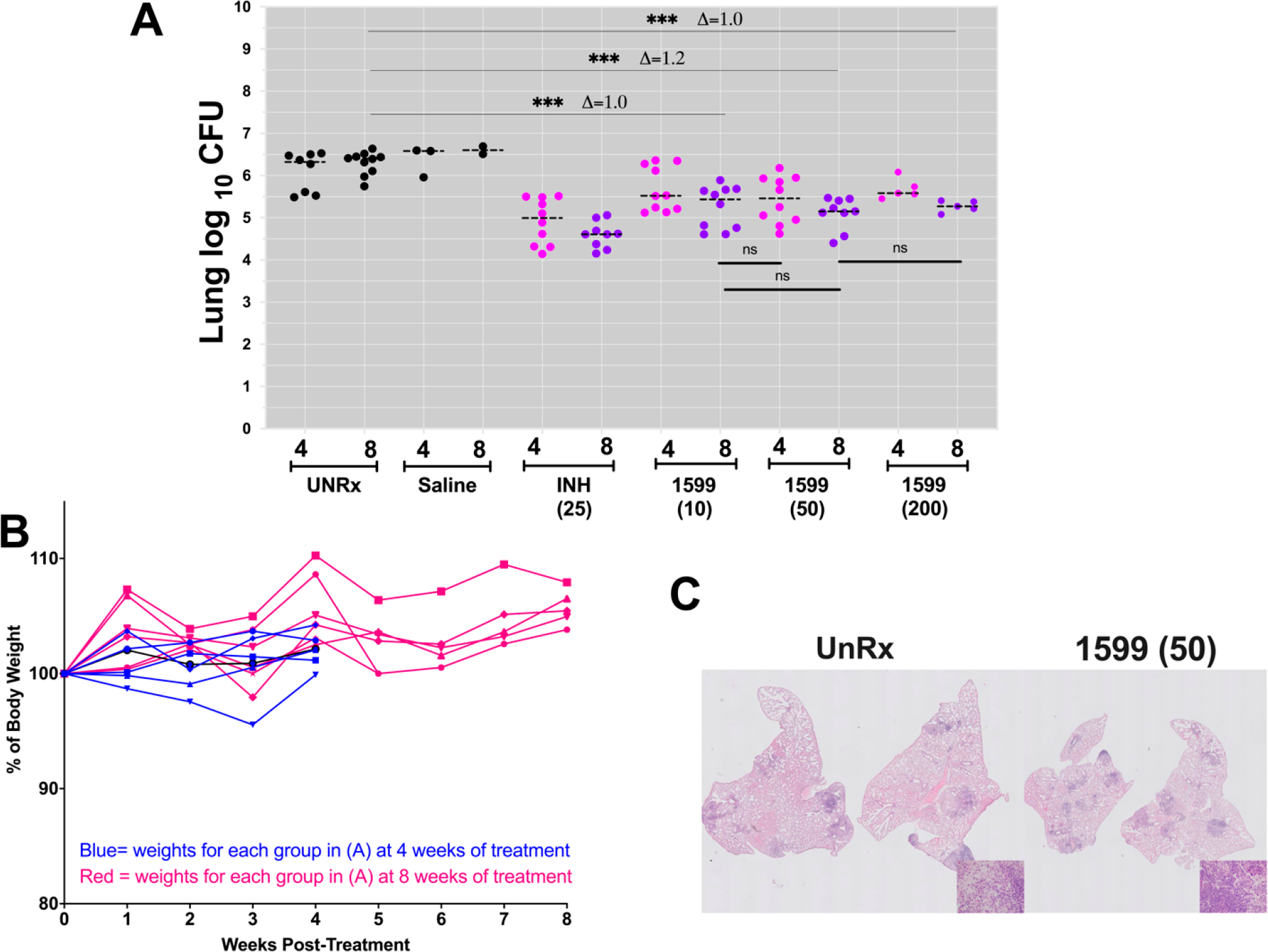
Efficacy for length of treatment after intrapulmonary aerosol delivery of spectinamide-1599 and isoniazid to BALB/c chronically infected with Mtb. As in Figure 3, spectinamide-1599 (1599) and INH were administered to chronically Mtb infected BALB/c (A and B) mice (n = 5) via intrapulmonary aerosol. Spectinamide-1599 and INH were prepared in 0.9% low endotoxin saline (saline) at 10, 50, and 200 mg/kg [1599 (10); 1599 (50) and 1599 (200)], whereas INH was prepared at 25 mg/kg [INH (25)]. Except for mice in the untreated group (UnRx), each mouse in each group received 50 μL/dose via intrapulmonary aerosol 3 times a week during 4 (magenta dots) or 8 (purple dots) weeks. Three days after the last treatment, animals from all groups in the study were euthanized, and the lungs were prepared for bacterial load determination as in Figure 3. Bacterial load in each organ was expressed as the log10 of CFUs. The graph shows combined data from two independent studies, and only one of the studies included the test group (n = 5) of 200 mg/kg [1599 (200)]. Each dot represents lung CFU per animal in each group. During therapy, the weights of mice in each group were recorded weekly (B). ns (p < 0.05); * (p < 0.01); ** (p < 0.001), and *** (p < 0.001); **** p < 0.0001. (B) During therapy, the weights of each mouse were recorded weekly as average weight of each mouse per cage (n = 5). The graph shows weekly average of weights of 5 mice in each cage during 4 (blue lines) and 8 weeks (red lines) of therapy. (C) Representative photomicrographs of H&E-stained lung tissue sections from an infected but untreated control mouse (left) and from an infected mouse receiving spectinamide-1599 at 50 mg/kg IPA per day for 4 weeks (right). Histopathology revealed perivascular and peribronchiolar, histiocyte- and neutrophil-rich aggregates along with small perivascular lymphocytic and plasmacytic aggregates in the control animals. In treated mice, perivascular lymphocytic and plasmacytic inflammation predominated, and histiocytic and neutrophilic aggregates were significantly decreased. Background photomicrograph, 10×; inset picture, 40×.
Lung Exposure to Intrapulmonary Spectinamide-1599 during Prolonged Therapy Chronically Mtb Infected BALB/c Mice.
To confirm that the spectinamide-1599 exposures observed in healthy BALB/c mice during multiple dose therapy are also observed in the infected mice, we also measured plasma and lung tissue homogenate concentrations in chronically Mtb infected BALB/c mice treated for 4 or 8 weeks with IPA spectinamide-1599. As shown in Figure 5, plasma concentrations assessed 0.5 h after the last dose of a 4-week dosing series showed dose-dependent exposures that agreed with the 95% confidence interval for the expected exposure based on the multiple dose pharmacokinetic assessments in healthy mice. Similarly, plasma concentrations determined 72 h after the last dose after 4 or 8 weeks of dosing declined to concentrations below 0.1 μg/mL for all dose levels, as expected based on the observed plasma pharmacokinetic behavior in healthy mice. In contrast, spectinamide-1599 concentrations in lung homogenate remained at high concentrations between 27 to 70 μg/g tissue even 72 h after the last dose of the multiple dose series, which is similar to the exposures observed at the last sampling time point in the pharmacokinetic studies in healthy mice (Figure 1). While lung concentrations increased from 10 to 50 mg/kg exposures, there was no further increase observed with 200 mg/kg dosing. Furthermore, there was no significant difference between the concentrations observed after 4 vs 8 weeks of therapy. These data indicate that the PK of IPA administered spectinamide-1599 is not impacted by chronic Mtb infection. As was seen in healthy mice, there was substantial tissue accumulation and retention of spectinamide-1599 in lung tissue after prolonged IPA therapy in Mtb infected mice that is dose-dependent but reaches a plateau at 50 mg/kg and is fully achieved with 4 weeks of treatment.
Figure 5.
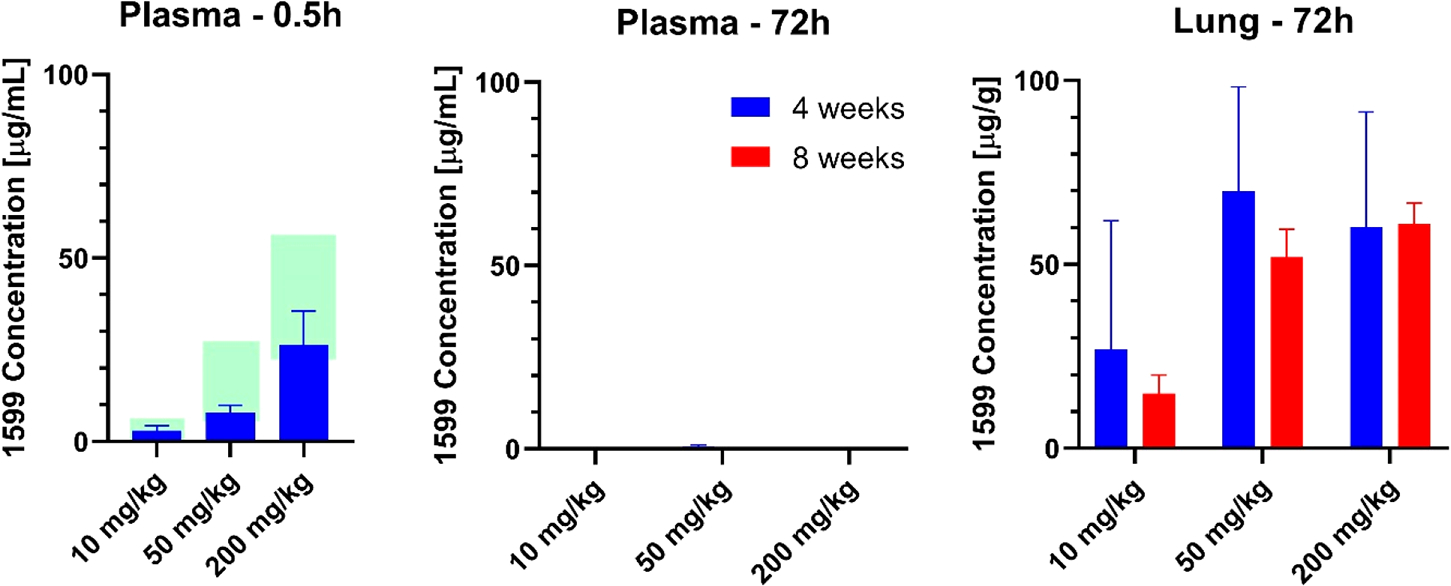
Spectinamide-1599 concentrations in plasma and lung after 4 or 8 weeks of treatment in Mtb infected mice. Spectinamide-1599 concentrations (mean ± SD) were measured in chronically Mtb infected BALB/c mice (n = 3–5) in plasma 0.5 or 72 h and in lung homogenate 72 h after the last dose of either 4 or 8 weeks of intrapulmonary aerosol administration with 10, 50, or 200 mg/kg 3 times weekly. The shaded areas for the 0.5 h plasma concentrations are the 95% confidence intervals for the expected concentrations based on the multiple dose pharmacokinetic studies in healthy mice.
Intrapulmonary Aerosol Treatment of Chronically Mtb Infected C3HeB/FeJ Mice.
Unlike BALB/c mice which exhibit homogeneous granulomatous lesions with Mtb infection, C3HeB/FeJ mice provide an animal TB model associated with high heterogeneity of lesion types that are more similar to those found in tuberculosis patients.13 The course of pulmonary Mtb infection and disease in C3HeB/FeJ mice is unpredictable, whereas ~30% of animals may succumb to infection within 4–6 weeks and develop neutrophil-rich-type granulomas (type II), other animals survive to a longer chronic state of infection and develop multifocal but very heterogeneous type of lesions classified as necrotic (Type I) and cellular granulomas (Type III).13 When this animal model is used to test drug efficacy, the differences between group treatments oftentimes reach only low statistical significance.14 Thus, using a similar experimental design as with BALB/c mice, we used C3HeB/FeJ mice to evaluate IPA treatment efficacy of spectinamide-1599. Figures 6A and B show representative studies on changes in pulmonary bacterial burden after monotherapy treatment with three doses (10, 50, and 100 mg/kg) of 1599 (Figure 6A) and efficacy of therapy when 1599 is administered as combination therapy with pyrazinamide (Figure 6B). The bacterial burden in C3HeB/FeJ mice chronically infected with Mtb ranged between >6.5–8 log10 CFU by 56 days postinfection when the model is reported to develop highest heterogenicity of lesions.13 IPA treatment for 4 weeks with spectinamide-1599 at 10, 50, or 100 mg/kg did not provide significant CFU reduction when compared to controls groups (Figure 6A). Similarly, no significant histopathologic changes were observed between control and treated groups (data not shown). During histological assessment of tissue sections stained by H&E, tissues from both control and treated mice contained necrotic (Type I), and cellular (Type III) lesions and necrotic lesions appeared larger in control mice compared to treated mice (data not shown). Importantly, in Figure 6B is shown that while there were no statistically significant differences between animal controls and animal receiving monotherapy with either spectinamide-1599 at 50 mg/kg or PZA (150 mg/kg), the combined administration of spectinamide-1599 (50 mg/kg) via IPA with oral administration of PZA (150 mg/kg) synergized well and provided a significant reduction of >1.8 log10 CFU (p < 0.03) in lung bacterial burden. In C3HeB/FeJ mice, IPA administration of 25 mg/kg of INH, resulted in a bacterial burden reduction of 0.6–1.3 (p < 0.05) log10 CFU when compared to control groups. Also, no necrotic lesions were observed in mice treated with 25 mg/kg of INH (data not shown). When using the C3HeB/FeJ mouse TB model, we did not attempt administration of therapy for 8 weeks as it was previously reported by our group13 that in these models after 12 weeks of infection there is rapid progression in severity of TB disease, and mice would not tolerate drug treatment beyond 4 weeks.
Figure 6.
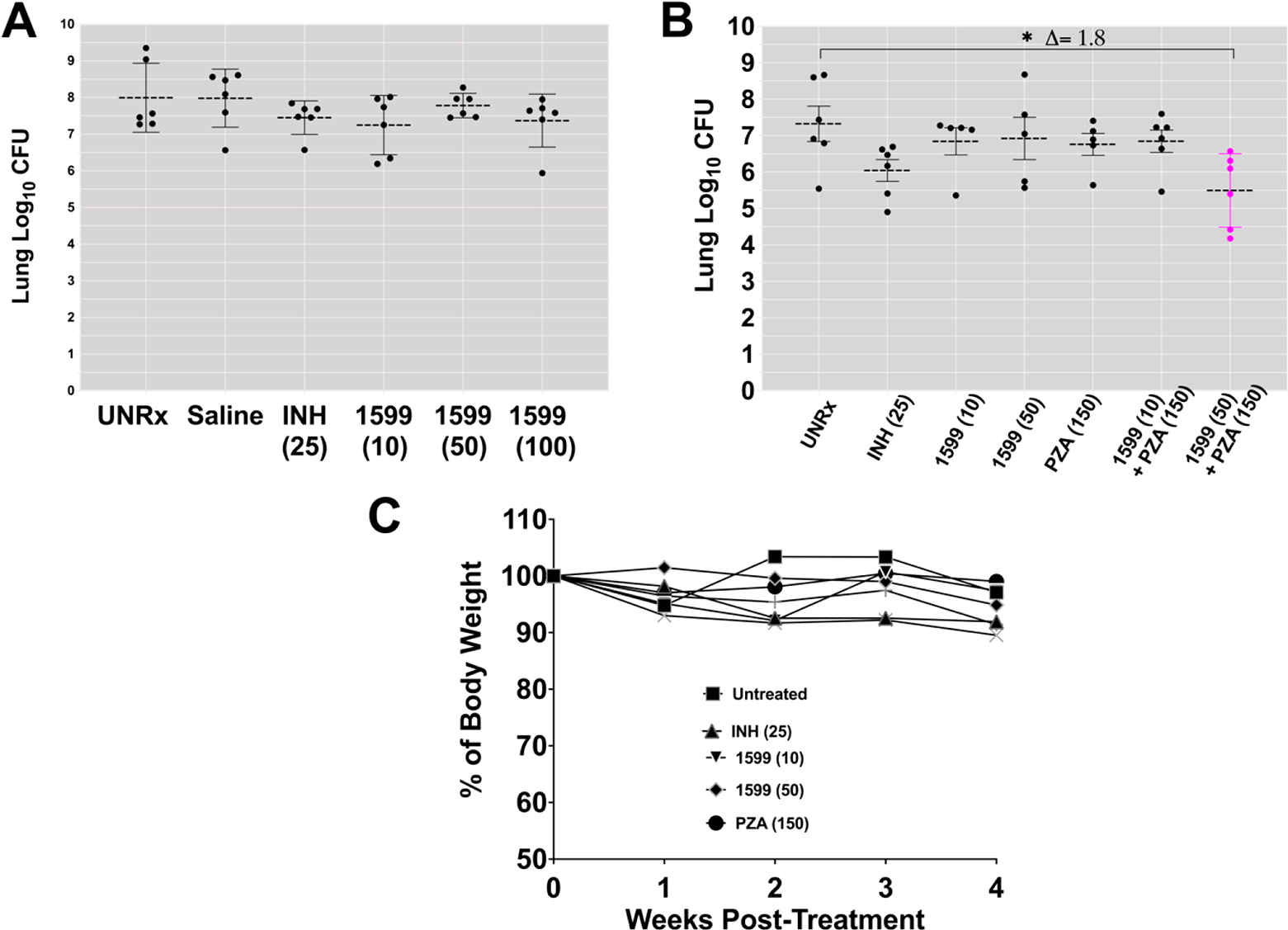
Dose response after intrapulmonary aerosol delivery of spectinamide-1599 and pyrazinamide to C3HeB/FeJ chronically infected with Mtb. (A) Spectinamide-1599 (1599) and INH were administered to chronically Mtb infected C3HeB/FeJ mice (n = 5–6) via intrapulmonary aerosol. C3HeB/FeJ female mice were infected with a low dose aerosol infection to deliver ~75 CFU Mtb bacilli per mouse. Mice were rested during 8 weeks until they were randomly assigned to study groups and used to test the therapy under study here. Spectinamide-1599 and INH were prepared in 0.9% low endotoxin saline (saline) at 10, 50, and 100 mg/mL [1599 (10); 1599 (50), and 1599 (100)], whereas INH was prepared at 25 mg/kg [INH (25)]. Except for mice in the untreated group (UnRx), each mouse in each group received 50 μL/dose via intrapulmonary aerosol 3 times a week for 4 weeks. Three days after the last treatment, animals from all groups in the study were euthanized, and the whole lungs were prepared for bacterial load determination. The bacterial load was determined using serial dilutions of homogenized organs that were plated on 7H11 agar plates, and the CFU in each sample were determined after 3–4 weeks of incubation at 37 °C. Bacterial load in each organ was expressed as the log10 of CFUs. The graph shows representative data from two separate studies. (B) Spectinamide-1599 (1599) and PZA were administered to chronically Mtb infected C3HeB/FeJ mice (n = 5–6) via intrapulmonary aerosol. As in panel A, spectinamide-1599, INH, and PZA were prepared in 0.9% low endotoxin saline at 10 and 50 mg/kg, respectively [1599 (10); 1599 (50)] INH at 25 mg/kg [INH (25)] and PZA at 150 mg/kg [PZA (150)]. Except for mice in the untreated group (UnRx), each mouse in each group received 50 μL/dose of via intrapulmonary aerosol. Some groups received PZA administered orally via gavage daily for 4 weeks. Three days after the last treatment, animals from all groups in the study were euthanized, and the whole lungs were prepared for bacterial load determination as explained in panel A. The graph shows representative data from two separate studies. (C) During therapy, the weights of each mouse in panel B were recorded weekly as average weight of each mouse per cage (n = 5). The graph shows weekly average of weights of 5 mice in each cage during the 4 weeks of therapy.
DISCUSSION
Targeted delivery of antibiotics directly to the site of infection through pulmonary delivery is gaining increasing interest in numerous infective conditions involving the lungs.16 Especially in situations where (i) the antibiotic has limited penetration of the lung tissues, (ii) the antibiotic is not orally bioavailable and has to be administered by intravenous infusion or injection, (iii) the maximum tolerated systemic exposure is insufficient to support efficacious concentrations in the lungs, or (iv) when there is systemic toxicity of the drug.18–20
Spectinamide-1599 has been shown to be well-tolerated and efficacious in treating acute and chronic infections in mouse models of tuberculosis.8,9 While spectinamides, including spectinamide-1599, have limited plasma protein binding (45%), good penetration into lung tissue and are metabolically stable with renal excretion in unchanged form as the major elimination pathway,8 they are not orally bioavailable and thus, have to be administered parenterally.21 Therefore, pulmonary delivery directly to the primary site of Mtb infection emerged as a potential strategy to administer efficacious doses of spectinamide-1599 without the need for an invasive administration procedure.10 The current study investigates the disposition and distribution behavior as well as efficacy and tolerability of spectinamide-1599 after IPA in mouse models of chronic Mtb infection.
As a first step, we investigated the plasma and tissue pharmacokinetics of spectinamide-1599 after single and multiple escalating doses given by IPA relative to IV or SC administration. After single dose administration, spectinamide-1599 exposures in plasma, lung, and ELF increased dose-dependently, with a systemic bioavailability in plasma of approximate 60–75% relative to SC and IV administration. This comports with our previous observations of linear and reproducible pharmacokinetics of spectinamides in rats and mice, with modest interindividual coefficients of variation of <40%.8,10
The advantage of local administration of the drug to the lungs became apparent when lung-to-plasma exposure ratios were compared among the different routes of administration. While exposure ratios after IV and SC administration confirmed earlier reports of good tissue penetration into the lungs with exposure relative to plasma of approximately 33–50%,21 this ratio dramatically increased with IPA administration to 1200–2900%. Even more importantly, during multiple dosing, lung homogenate exposures after IPA administration exhibited selective and substantial accumulation: While plasma concentrations remained nearly identical between single and multiple dose administration as expected based on the relative short plasma half-life at therapeutic concentrations, lung exposures showed substantial accumulation, which further increased the relative exposure of lung tissue relative to plasma. This accumulation in lung tissue was particularly pronounced after daily dosing, with a much lesser degree after thrice weekly dosing, and seemed to be specific for IPA administration whereas it was absent after IV or SC multiple dosing.
The combined phenomena, increased tissue exposure relative to plasma and drug accumulation during multiple dosing, led to substantially increased lung exposure to spectinamide-1599 after IPA compared to SC or IV administration. We confirmed that this high exposure and retention in the lungs is not only limited to healthy animals, but is also observed after 4 and 8 weeks of therapy in Mtb infected mice. This observation is not unique for spectinamides. A similar behavior with regard to the lack of plasma accumulation during multiple dosing but substantial lung exposures has for example also been described after pulmonary administration of tobramycin, another aminocyclitol antibiotic.22
It has been suggested that pulmonary delivery is especially favorable for those antibiotics that exhibit concentration-dependent killing as the high local exposures would be favorable to further improve their killing efficiency.17 Examples of which include tobramycin and amikacin. In chemostat-based in vitro experiments, spectinamide-1599 also exhibited concentration-dependent mycobacterial killing,23,24 and in vivo studies in mouse models of chronic Mtb infection indicate the same PK/PD behavior for spectinamides in vivo.25 ELF exposures were similar or in some cases even slightly higher than lung homogenate exposures, suggesting that spectinamide-1599 does not remain sequestered in substructures of lung tissue but is able to maintain high ELF concentrations. These concentrations were at least 60-fold higher than the MIC of 0.8 μg/mL for spectinamide-1599.8 As ELF is assumed to be constantly recycled and ELF concentrations for most systemically administered antibiotics are thought to be identical to free, unbound plasma concentrations,26,27 lung tissue may serve as a reservoir to supply and maintain the observed high ELF concentrations for spectinamide-1599. A high antibiotic exposure in ELF after inhaled administration despite low serum concentrations has also been described for inhaled amikacin.28 The pharmacokinetic behavior of spectinamides in general and spectinamide-1599 in particular are similar to other aminocyclitol antibiotics such as spectinomycin, trospectomycin, and classic aminoglycoside antimicrobials such as tobramycin and amikacin.29,30 As these compounds are highly hydrophilic, their disposition is characterized by a rapid elimination from plasma with short half-lives at therapeutically relevant concentrations, largely by renal excretion in unchanged form, followed by a prolonged terminal phase at very low concentrations. While the latter does not contribute substantially to the overall systemic exposure (<2% of AUC), it is assumed to be caused by slow redistribution of the drug from tissue storage sites and is a typical feature of aminoglycosides and many other amines.31
Aminoglycosides and other aminocyclitols such as streptomycin, gentamicin, tobramycin, amikacin, and trospectomycin are well-known to affect intracellular vesicular processes.32 Similar to many other cationic amphiphilic drugs, they are known to associate with membrane phospholipids and interfere with lysosomal storage, may enrich in lysosomes, and may cause phospholipidosis.33–35 As the cellular components of the lungs have a high abundance of lysosomes relative to most other tissues,34 one explanation for the observed enrichment of spectinamide-1599 in lung tissue after IPA administration is the potential for similar mechanisms to be involved. Further studies are currently ongoing to identify the cellular and subcellular locations of spectinamide-1599 storage. It remains to be seen whether the pulmonary retention leads to higher local exposures of free, pharmacologically active drug, and ultimately increased local efficacy.
In addition, we investigated the efficacy and relationship between dose, dosing frequency and duration of therapy via intrapulmonary delivery of spectinamide-1599 in two preclinical murine TB models. This choice was based on the facts that the progression of human TB is both a complex and dynamic process resulting in a wide spectrum of disease forms. Pulmonary TB in humans can develop with a wide range in number of pulmonary lesions (1–2 or many lesions) and with multiple histologically distinct lesion types.36 Within the spectrum of lesion types, one particular lesion type is characterized by a rim of fibrosis and a central region of caseum containing a high burden of free bacilli while another type of lesion is characterized by cellular aggregations containing intracellular bacilli. Thus, bacilli within lesions in human TB are found in different physicochemical and biological environments leading to multiple distinct bacterial subpopulations within different environments in the same patient. Despite this complex scenario, TB is a treatable disease but requires long regimens of multidrug therapy with variable treatment outcomes and a wide range of adverse effects.2 In an attempt to cover the wide spectrum of human TB forms, our preclinical studies in support of the development of inhaled spectinamide-1599 used the BALB/c and C3HeB/FeJ TB mouse models. BALB/c mice were chosen because the course of infection and disease develops into multifocal and very homogeneous cellular types of lesions12 and most bacilli are found in the intracellular compartments.13 In contrast to BALB/c mice, the course of pulmonary Mtb infection and disease in C3HeB/FeJ mice develops multifocal but very heterogeneous types of lesions including small and large necrotic lesions.13 Bacilli in these lesions can be found free within the caseum of necrotic lesions or intracellularly in cellular lesions (type III lesions as in ref 13). Of important consideration is that in this TB model the response in drug efficacy studies is often bimodal and referred to as responders and nonresponders.9
Altogether our results here are in line with the characteristics described above for the two TB preclinical models. In BALB/c mice there is a dose-dependent efficacy where 50 and 100 mg/kg of spectinamide-1599 given as intrapulmonary aerosol three times a week provide highest efficacy, and prolonged duration of treatment up to 8 weeks resulted in significant improvement in reduction of bacterial burden with differences reaching high statistical significance. Lower concentrations (10 mg/kg) achieved similar pathogen killing as larger 50–100 mg/kg doses if given for prolonged periods of time (8 weeks).
All doses and regimens tested during these studies were very well-tolerated by the animals. Moreover, a preliminary histopathological evaluation of lung sections in the treated animals did not show evidence for drug effects on host tissue. No evidence of inflammatory responses, ELF accumulation in alveoli or pathological changes were observed in animals treated by IPA spectinamide-1599.
Considering the results from the PK studies discussed above indicate high concentrations of spectinamide-1599 in ELF and lung tissue (>40–80 times the MIC) during prolonged therapy, accumulation of drug in the lungs appears to reach a ceiling of benefit for reduction of bacterial burden. If we consider that during the chronic state of infection in BALB/c mice most of the bacilli in the lungs remain within granulomas and within intracellular compartments, we can speculate that the potential storage sites in the lung tissue are likely located within intracellular compartments and away from bacilli, but provide a storage compartment to maintain effective free drug concentrations in the lung microenvironment for a prolonged period of time. Ongoing studies are investigating this essential question.
IPA treatment for 4 weeks with spectinamide-1599 at 10, 50, or 100 mg/kg in C3HeB/FeJ mice with advanced chronic Mtb infection, when animals are known to present with multifocal and necrotic type I lesions, did not provide statistical significance in CFU reduction when compared to controls groups and no histopathologic changes were observed between control and treated groups. However, it was previously reported9 that when given by the SC route, one of the most attractive properties of spectinamide-1599 is its synergistic effect and capacity to partner well with the antimicrobial activity of other TB drugs, including in animal models with advanced and severe pathology as in C3HeB/FeJ mice.9 Based on these results, C3HeB/FeJ mice infected with Mtb with advanced pathology were used in our studies to test the synergistic effect of intrapulmonary aerosols of spectinamide-1599 and oral administration of pyrazinamide (PZA). As expected, monotherapy with intrapulmonary aerosol of spectinamide-1599 (50 mg/kg) or oral pyrazinamide (150 mg/kg) had limited or no efficacy in the C3HeB/FeJ mice TB model, but when both drugs were given in combination, a superior reduction >1.8 log10 CFU was observed. This result is interesting as it was reported previously in in vitro checkerboard assays with spectinamide-1599 and PZA revealed no synergistic effect on free Mtb.9 Thus, we speculate that the synergistic effect between both drugs observed in vivo during the chronic state of Mtb infection in C3HeB/FeJ mice may be associated with an interaction of both drugs at the host and not bacterial cell level, and ongoing studies are intended to address this question.
In summary these studies demonstrated that spectinamide-1599 administered as a liquid formulation via intrapulmonary aerosol is very well-tolerated and effective in the treatment of pulmonary tuberculosis. It is worth noting that our studies provide preliminary information for preclinical efficacy of the drug when administered directly to the lungs, and we reason that if formal allometric scaling models between the mouse and humans’ lungs were adopted, the actual dose in human will be lower. It is also important to mention that our results using direct administration of the drug into the lungs can serve only to guide the development of inhalational therapy for humans which will require passive inhalation and will likely result in substantial changes of the amount of drug needed for inhalation depending on the efficiency of the delivery method adopted.
Direct administration to the lungs provided a dose-dependent, rapid exposure in plasma and accumulation in the lungs during extended regimens. However, we encountered the intriguing observation that at higher doses (50–100 mg/kg) there is no increased efficacy with prolonged therapy despite drug being accumulated in the lungs in large quantities. This is not a unique property to spectinamide-1599 as similar properties have been reported for other aminocyclitol drugs (tobramycin and amikacin) when used as inhalational therapy. In addition, the maximum efficacy of spectinamide-1599 after SC administration as 4-week monotherapy in the chronic infection model in BALB/c mice has consistently been reported as a ~1.2–1.5 log10 CFU reduction in lung (with dosing performed in 5 of 7 days per week): 1.2 for 150 mg/kg once daily,45 1.2 for 200 mg/kg once daily,9 and 1.5 for 200 mg/kg twice daily.8 A similar plateau effect for efficacy under monotherapy has recently also been described for structurally closely related spectinamide-1810.25 Similar efficacy has been observed after IPA administration of substantially lower doses: 50 and 100 mg/kg given 3 times per week. These observations suggest that the benefit of the spectinamide accumulation in the lungs under IPA therapy is not a higher efficacy, but that substantially lower IPA doses seem to be necessary for the same maximum efficacy as observed under SC monotherapy. The overall observation of a ceiling effect of spectinamide monotherapy can be explained by the coexistence of multiple bacterial subpopulations with different metabolic states in infected animals in vivo, and a preferential killing of some of these populations, but not all of them by the spectinamide.25
Moreover, we believe that the therapeutic window of inhaled spectinamide-1599 is substantially improved when administered with pyrazinamide. Pyrazinamide is a critical component in TB chemotherapy and is a drug that has played an essential role in shortening the standard treatment regimen from nine to six months,37 although its mechanism of action is still not well-understood. Thus, future studies should address mechanisms of synergy for inhalational spectinamide-1599 and PZA as well as with additional TB drug combinations; most importantly, these should determine the potential of inhaled spectinamide-1599 for replacing currently used antimycobacterial agents that exhibit substantial toxicity in long-term therapy (e.g., linezolid).
CONCLUSION
These results strongly support the further development of intrapulmonary administration of spectinamide-1599 as a combination partner for anti-TB therapy.
METHODS
Compliance.
All animal experiments were conducted in accordance with the Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Prior to initiation, all animal protocols were approved by the Institutional Animal Care and Use Committees of Colorado State University or the University of Tennessee Health Science Center, respectively.
Drug Compounds.
Spectinamide-1599 (dihydrochloride) was provided by Dr. Lee at St. Jude Children’s Research Hospital and was synthesized as reported.8 Pharmaceutical grade INH was purchased from Fluka Analytical (Charlotte, NC), whereas PZA was purchased from Acros Organics (Fair Lawn, NJ). All drugs were prepared in filtered endotoxin-free RNase/DNase-free saline and sterile 0.9% saline (TEKnova, Hollister, CA). Mice were weighed each week to calculate the amount of drug required. Spectinamide-1599 was prepared at 10, 50, and 100 mg/kg; INH was prepared at 25 mg/kg, and PZA was prepared at 150 mg/kg. For SC and IV administration, spectinamide-1599 was formulated in variable mixtures of PlasmaLyte A Injection (Multiple Electrolytes Injection type I USP, Baxter Healthcare, Deerfiled, IL) and sterile water.
Drug Administration.
IPA delivery was performed under isoflurane anesthesia using a MicroSprayer device (MicroSprayer, model IA-C; Penn Century, Philadelphia, PA) attached to an FMJ-250 high pressure-syringe device (Penn Century) as described previously.8,15,38,39 Each dose administered using the Penn Century device delivers 50 μL of solution. Mice received one dose per day of treatment. During the procedure the animals were monitored for regular breathing and clinical symptoms. SC and IV administration were performed using a fixed volume of 50 μL, given by injection into the loose skin over the shoulders or by retroorbital injection under isoflurane anesthesia, respectively. Gavage was administered in 200 μL volumes as previously reported.12
Bacteria.
Mycobacterium tuberculosis (Erdman strain, TMC107; ATCC 35801, American Type Culture Collection, Manassas, VA) was used to infect the animals. Frozen stocks of the bacilli were used to prepare the inoculum in double distilled water for infection as previously described.15
Establishment of Chronic Infection of BALB/c Mice.
Six- to eight-week-old BALB/c female mice were purchased from Jackson Lab (Bar Harbor, ME). The mice were kept in BSL3 facilities and rested for a week prior to infection. The mice were infected with a low dose aerosol infection using the Glass-Col System to deliver ~100 Mtb bacilli per mouse. To verify bacilli deposition in the lungs, on day 1 post infection, three mice were sacrificed, the lungs were harvested and prepared for bacilli burden determination as explained below. The rest of the mice were rested for 4 weeks until they were randomly assigned to study groups and used to test the therapy under study here. To “washout” the drugs before determination of bacterial burden, the mice were rested for three days after the last treatment when they mice were euthanized and the lungs and spleen harvested and prepared for bacteria load determination and histology.
Establishment of Chronic Infection in C3HeB/FeJ Mice.
Six- to eight-week-old C3HeB/FeJ female mice were purchased from Jackson Lab (Bar Harbor, ME). The mice were infected with a Mtb inoculum concentration adjusted to deliver ~50–75 bacilli in the lungs of each mouse and at day 1 post infection, three mice were euthanized to quantify bacilli deposition in the lungs.13,40 During the first 21 days post infection, mice were monitored daily for activity and weight loss. Mice with ~20% weight loss were euthanized (20–30% of mice in study), the remaining mice were rested until 8 weeks post infection when treatment was initiated. Three days after the last treatment, animals were euthanized and the lungs prepared for bacterial load determination as explained below.
Bacterial Load Determination.
Following euthanasia, mouse tissues (lung and spleen) were homogenized using the Next Advance Bullet Blender (Averill Park, NY). Briefly, the left lobe for BALB/c or whole lung for C3HeB/FeJ mice or spleen was placed in 1.5 mL sterile, safe lock Eppendorf tubes containing 0.5 mL of sterile PBS and 3 × 3.2 mm, sterile stainless-steel beads, thereafter the tubes were placed in the Bullet Blender and homogenized during 4 min and 8000 rpm. The bacterial load was determined using serial dilutions of homogenized organs that were plated on 7H11 agar plates, and the colony forming units (CFU) in each sample were determined after 3 weeks of incubation at 37 °C. Bacterial load in each organ was expressed as the log10 of CFUs.
Histopathology Analysis.
The middle right lobe of the lungs of each mouse was placed into a histology cassette and fixed in 4% paraformaldehyde. Samples were inactivated in 4% paraformaldehyde solution for 48 h and then processed using standard histological protocols for sectioning and staining with Haematoxylin-Eosin (H&E). Histopathological assessments of the H&E stained specimens were performed by a pathologist blinded to the study groups.
Pharmacokinetic Studies.
Naïve female BALB/c mice (~20 g body weight; Charles River, Wilmington, MA) were acclimatized for 1 week prior to drug administration with access to food and water ad libitum. For single dose studies, groups of mice (n = 3 of 27 per study) were sacrificed predose and 0.083, 0.25, 0.5, 1, 2, 3, 8, and 24 h after drug administration. For multiple dose studies, groups of mice (n = 3 of 15 per study) were sacrificed immediately prior to and 0.25, 1, 3, and 8 h after the last drug administration in the dosing series. An additional 0.5 h sample was collected in the multiple dose intravenous study.
Each mouse was terminally anesthetized, and blood was collected via retroorbital bleed into a heparinized container. ELF was sampled by bronchoalveolar lavage: making a fine incision into the trachea to allow a catheter tubing (BD Insyte Autoguard, 22 gauge, 0.9 × 25 mm, Becton Dickinson, Franklin Lakes, NJ) to pass through, a tuberculin syringe prefilled with PBS (200 μL) was inserted into the catheter and infused. After infusion, the same fluid was aspirated back and collected into a clean tube. This process of infusion and aspiration was repeated thrice for each mouse. Subsequently, lung, liver, kidney and spleen tissues were collected. Plasma was immediately separated by centrifugation (6000g for 10 min at 4 °C) and stored at −70 °C until analysis. Tissues and bronchoalveolar lavage fluid (BALF) were immediately frozen and stored at −70 °C until analysis.
Determination of Spectinamide-1599 Concentrations in Plasma, ELF, and Tissue Sample Preparation.
Prior to analysis, frozen tissues were thawed at room temperature, weighed and homogenized in PBS (4 volumes) using an UltraTurrax tissue homogenizer (IKA, Wilmington, NC). Sample preparation was conducted by protein precipitation using methanol. Proteins were precipitated by the addition of 4 volumes (100 μL) of internal standard (3′-dihydro-3′-deoxy-3′(R)-isopropylacetylamino spectinomycin,41 10 ng/mL) in methanol to a sample (25 μL) of plasma, BALF or tissue homogenate. Samples were vortexed for 0.5 min and centrifuged at 10 000g for 10 min at 4 °C, and the supernatants were collected for further analysis. Samples obtained from Mtb infected animal under BSL3 underwent methanol-based sterilization as previously described by our group.42 To further confirm sterility of the samples after methanol treatment, one tenth of each sample was plated on 7H11 agar plates and incubated at 37 °C for 6 weeks and checked for bacterial growth prior to removal from the BSL3 environment.
Correction for ELF Dilution during Bronchoalveolar Lavage.
ELF concentrations of spectinamide-1599 were calculated from BALF concentrations using the urea method where the concentration of drug in ELF is the concentration of drug in BALF corrected by the ratio between the urea concentrations in plasma and BALF.43 Urea concentrations in both plasma and BALF were determined with a Quantichrom Urea Assay Kit (BioAssay Systems, Hayward, CA, United States) per the manufacturer’s instructions using a Cytation 5 Multi Mode Reader (BioTek, Winnoski, VT) at 420 nm.
Bioanalytical Spectinamide-1599 Assay.
Concentrations of spectinamide-1599 in plasma, BALF, and tissue homogenates were determined by a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay as previously described in.8 In brief, chromatographic separation of the supernatant was carried out on a Luna HILIC 3 μm, 100 × 4.6 mm column (Phenomenex, Torrance, CA) using a gradient mobile phase of methanol and 5 mM ammonium formate buffer, pH 2.75, at a flow rate of 0.4 mL/min. Detection was performed with an API 4500 triple quadruple mass spectrometer (AB Sciex, Foster City, CA) with electrospray ionization in multiple reaction monitoring mode using the mass transfers of m/z 487.2 → 207.1 for spectinamide-1599 and m/z 453.0 → 247.1 for the internal standard. A calibration curves with 11 calibrants measured in duplicate was constructed and validated in each analytical run with spiked samples of mouse plasma or respective homogenized tissue at four different concentration levels that were scattered between unknown samples throughout an analytical run. The lower limit of quantification was 1 ng/mL, with acceptable accuracy and precision.
Pharmacokinetic Analysis.
The plasma, ELF, and tissue concentrations were analyzed by standard noncompartmental pharmacokinetic (PK) approaches using a pooled sample approach and the software package Phoenix WinNonlin 8.0 (Certara, Princeton, NJ). Penetration of drug in tissues was estimated from the ratios of the area under the concentration–time curve from time 0 to the last measured data point (AUC0–last) for tissues and ELF compared to the AUC0–last in plasma. Variability measures (standard deviation) of AUC as aggregate PK parameter were estimated by bootstrap analysis as previously suggested for sparse sampling situations in preclinical PK.44 Absolute bioavailability for the IPA and SC administration were determined by calculating the dose normalized ratios of AUC after IPA or SC administration relative to IV administration.
Statistical Analysis.
For the mouse TB model experiments, the bacterial burden was expressed as CFU, and data were analyzed using Graph Pad Prism version 8.1.1. The statistical analysis was performed using a Dunnett’s multiple comparisons test as part of a one-way ANOVA test.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grant R01AI120670 by the National Institute of Allergy and Infectious Diseases and by grant S10OD016226 by the Office of the Director of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge the staff of the Laboratory Animal Resources at Colorado State University for their animal care.
ABBREVIATIONS
- AUC
area-under-the concentration–time curve
- CFU
colony forming units
- DS-TB
drug-sensitive TB
- ELF
epithelial lining fluid
- Cmax
peak concentration
- H&E
hematoxylin and eosin
- IPA
intrapulmonary aerosol
- INH
isoniazid
- IV
intravenous
- MDR-TB
multidrug-resistant tuberculosis
- Mtb
Mycobacterium tuberculosis
- MIC
minimum inhibitory concentration
- TB
tuberculosis
- PD
pharmacokinetics
- PK
pharmacokinetics
- PZA
pyrazinamide
- SC
subcutaneous
- XDR-TB
extensively drug-resistant TB
- 1599
spectinamide-1599
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.1c00213.
Table S1, pharmacokinetic exposure parameters; Figure S1, concentration–time profiles of spectinamide-1599 in liver, spleen, and kidney of mice (PDF)
Contributor Information
Mercedes Gonzalez-Juarrero, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States.
Pradeep B. Lukka, Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, Tennessee 38163, United States; Present Address: AstraZeneca, Gaithersburg, Maryland 20878, United States.
Santosh Wagh, Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, Tennessee 38163, United States; Present Address: Pfizer, Cambridge, MA 02139, United States.
Amanda Walz, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States.
Jennifer Arab, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States; Present Address: Vitrolife Inc., Englewood, Colorado 80110, United States.
Camron Pearce, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States.
Zohaib Ali, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States.
Josiah T. Ryman, Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, Tennessee 38163, United States; Present Address: Merck & Co, Kenilworth, NJ 07033, United States
Keyur Parmar, Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, Tennessee 38163, United States.
Zaid Temrikar, Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, Tennessee 38163, United States.
Juan Munoz-Gutierrez, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States.
Gregory T. Robertson, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States
Jiuyu Liu, Department of Chemical Biology, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, United States.
Anne J. Lenaerts, Mycobacteria Research Laboratories, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523, United States
Charles Daley, Division of Mycobacterial and Respiratory Infections, National Jewish Health, Denver, Colorado 80206, United States.
Richard E. Lee, Department of Chemical Biology, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, United States
Miriam Braunstein, Department of Microbiology and Immunology, University of North Carolina-Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Anthony J. Hickey, Discovery Science and Technology, RTI International, RTP, Durham, North Carolina 27709, United States
Bernd Meibohm, Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, Tennessee 38163, United States.
REFERENCES
- (1).WHO. Global tuberculosis report 2020. Euro Surveill; World Health Organization: Geneva, 2020, 18. [Google Scholar]
- (2).Tiberi S; du Plessis N; Walzl G; Vjecha MJ; Rao M; Ntoumi F; Mfinanga S; Kapata N; Mwaba P; McHugh TD; Ippolito G; Migliori GB; Maeurer MJ; Zumla A Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, e183–e198. [DOI] [PubMed] [Google Scholar]
- (3).WHO. Global Tuberculosis Report 2018. World Health Organization: Geneva, 2018. [Google Scholar]
- (4).Stein SW; Thiel CG The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Delivery 2017, 30, 20–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Miller JB; Abramson HA; Ratner B Aerosol streptomycin treatment of advanced pulmonary tuberculosis in children. Am. J. Dis Child 1950, 80, 207–237. [DOI] [PubMed] [Google Scholar]
- (6).Hickey AJ; Misra A; Fourie PB Dry powder antibiotic aerosol product development: inhaled therapy for tuberculosis. J. Pharm. Sci. 2013, 102, 3900–3907. [DOI] [PubMed] [Google Scholar]
- (7).Hickey AJ Emerging trends in inhaled drug delivery. Adv. Drug Delivery Rev. 2020, 157, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lee RE; Hurdle JG; Liu J; Bruhn DF; Matt T; Scherman MS; Vaddady PK; Zheng Z; Qi J; Akbergenov R; Das S; Madhura DB; Rathi C; Trivedi A; Villellas C; Lee RB; Rakesh; Waidyarachchi SL; Sun D; McNeil MR; Ainsa JA; Boshoff HI; Gonzalez-Juarrero M; Meibohm B; Bottger EC; Lenaerts AJ Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat. Med. 2014, 20, 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Robertson GT; Scherman MS; Bruhn DF; Liu J; Hastings C; McNeil MR; Butler MM; Bowlin TL; Lee RB; Lee RE; Lenaerts AJ Spectinamides are effective partner agents for the treatment of tuberculosis in multiple mouse infection models. J. Antimicrob. Chemother. 2016, 72, 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Rathi C; Lukka PB; Wagh S; Lee RE; Lenaerts AJ; Braunstein M; Hickey A; Gonzalez-Juarrero M; Meibohm B Comparative pharmacokinetics of spectinamide-1599 after subcutaneous and intrapulmonary aerosol administration in mice. Tuberculosis (Oxford, U. K.) 2019, 114, 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ho YI; Chan CY; Cheng AF In-vitro activities of aminoglycoside-aminocyclitols against mycobacteria. Journal of antimicrobial chemotherapy 1997, 40, 27–32. [DOI] [PubMed] [Google Scholar]
- (12).De Groote MA; Gilliland JC; Wells CL; Brooks EJ; Woolhiser LK; Gruppo V; Peloquin CA; Orme IM; Lenaerts AJ Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Irwin SM; Driver E; Lyon E; Schrupp C; Ryan G; Gonzalez-Juarrero M; Basaraba RJ; Nuermberger EL; Lenaerts AJ Presence of multiple lesion types with vastly different microenvironments in C3HeB/FeJ mice following aerosol infection with Mycobacterium tuberculosis. Dis. Models & Mech . 2015, 8, 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Irwin SM; Prideaux B; Lyon ER; Zimmerman MD; Brooks EJ; Schrupp CA; Chen C; Reichlen MJ; Asay BC; Voskuil MI; Nuermberger EL; Andries K; Lyons MA; Dartois V; Lenaerts AJ Bedaquiline and Pyrazinamide Treatment Responses Are Affected by Pulmonary Lesion Heterogeneity in Mycobacterium tuberculosis Infected C3HeB/FeJ Mice. ACS Infect. Dis. 2016, 2, 251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gonzalez-Juarrero M; Woolhiser LK; Brooks E; DeGroote MA; Lenaerts AJ Mouse model for efficacy testing of antituberculosis agents via intrapulmonary delivery. Antimicrob. Agents Chemother. 2012, 56, 3957–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hickey AJ; Mansour HM, Eds. Inhalation Aerosols: Physical and Biological Basis for Therapy, 3rd ed.; CRC Press: Park City, Utah, 2019. [Google Scholar]
- (17).Flume P; Klepser ME The rationale for aerosolized antibiotics. Pharmacotherapy 2002, 22, 71S–79S. [DOI] [PubMed] [Google Scholar]
- (18).Braunstein M; Hickey AJ; Ekins S Why Wait? The Case for Treating Tuberculosis with Inhaled Drugs. Pharm. Res. 2019, 36, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ho DK; Nichols BLB; Edgar KJ; Murgia X; Loretz B; Lehr CM Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [DOI] [PubMed] [Google Scholar]
- (20).Maselli DJ; Keyt H; Restrepo MI Inhaled Antibiotic Therapy in Chronic Respiratory Diseases. Int. J. Mol. Sci. 2017, 18, 1062–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Madhura DB; Trivedi A; Liu J; Boyd VA; Jeffries C; Loveless V; Lee RE; Meibohm B Tissue Penetration of a Novel Spectinamide Antibiotic for the Treatment of Tuberculosis. AAPS J. 2016, 18, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Cheer SM; Waugh J; Noble S Inhaled tobramycin (TOBI): a review of its use in the management of Pseudomonas aeruginosa infections in patients with cystic fibrosis. Drugs 2003, 63, 2501–2520. [DOI] [PubMed] [Google Scholar]
- (23).Vaddady PK; Lee RE; Meibohm B In vitro pharmacokinetic/pharmacodynamic models in anti-infective drug development: focus on TB. Future Med. Chem. 2010, 2, 1355–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Vaddady PK; Trivedi A; Rathi C; Madhura DB; Liu J; Lee RE; Meibohm B Dynamic time-kill curve characterization of spectinamide antibiotics 1445 and 1599 for the treatment of tuberculosis. Eur. J. Pharm. Sci. 2019, 127, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wagh S; Rathi C; Lukka PB; Parmar K; Temrikar Z; Liu J; Scherman MS; Lee RE; Robertson GT; Lenaerts AJ; Meibohm B Model-based exposure-response assessment for spectinamide 1810 in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 2021. DOI: 10.1128/AAC.01744-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kiem S; Schentag JJ Interpretation of Epithelial Lining Fluid Concentrations of Antibiotics against Methicillin Resistant Staphylococcus aureus. Infect. Chemother. 2014, 46, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zeitlinger MA; Traunmuller F; Abrahim A; Muller MR; Erdogan Z; Muller M; Joukhadar C A pilot study testing whether concentrations of levofloxacin in interstitial space fluid of soft tissues may serve as a surrogate for predicting its pharmacokinetics in lung. Int. J. Antimicrob. Agents 2007, 29, 44–50. [DOI] [PubMed] [Google Scholar]
- (28).Luyt CE; Clavel M; Guntupalli K; Johannigman J; Kennedy JI; Wood C; Corkery K; Gribben D; Chastre J Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Crit Care 2009, 13, R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Madhura DB; Lee R; Meibohm B Pharmacokinetic profile of spectinomycin in rats. Pharmazie 2013, 68, 675–676. [PMC free article] [PubMed] [Google Scholar]
- (30).Nichols DJ; Bye A; Novak E Pharmacokinetics of trospectomycin sulphate in healthy subjects after single intravenous and intramuscular doses. Br. J. Clin. Pharmacol. 1991, 32, 255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mingeot-Leclercq MP; Piret J; Brasseur R; Tulkens PM Effect of acidic phospholipids on the activity of lysosomal phospholipases and on their inhibition by aminoglycoside antibiotics–I. Biochemical analysis. Biochem. Pharmacol. 1990, 40, 489–497. [DOI] [PubMed] [Google Scholar]
- (32).Muro S Alterations in Cellular Processes Involving Vesicular Trafficking and Implications in Drug Delivery. Biomimetics (Basel) 2018, 3, 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hruban Z Pulmonary and generalized lysosomal storage induced by amphiphilic drugs. Environ. Health Perspect. 1984, 55, 53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kaufmann AM; Krise JP Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J. Pharm. Sci. 2007, 96, 729–746. [DOI] [PubMed] [Google Scholar]
- (35).Nonoyama T; Fukuda R Drug-induced Phospholipidosis - Pathological Aspects and Its Prediction. J. Toxicol. Pathol. 2008, 21, 9–24. [Google Scholar]
- (36).Hunter RL Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 2016, 97, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Controlled trial of four thrice-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Lancet 1981, 1, 171–174. [PubMed] [Google Scholar]
- (38).Higgins DM; Sanchez-Campillo J; Rosas-Taraco AG; Higgins JR; Lee EJ; Orme IM; Gonzalez-Juarrero M Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J. Immunol. 2008, 180, 4892–4900. [DOI] [PubMed] [Google Scholar]
- (39).Rosas-Taraco AG; Higgins DM; Sanchez-Campillo J; Lee EJ; Orme IM; Gonzalez-Juarrero M Local pulmonary immunotherapy with siRNA targeting TGFbeta1 enhances antimicrobial capacity in Mycobacterium tuberculosis infected mice. Tuberculosis 2011, 91, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Driver ER; Ryan GJ; Hoff DR; Irwin SM; Basaraba RJ; Kramnik I; Lenaerts AJ Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 3181–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Liu J; Bruhn DF; Lee RB; Zheng Z; Janusic T; Scherbakov D; Scherman MS; Boshoff HI; Das S; Rakesh; Waidyarachchi SL; Brewer TA; Gracia B; Yang L; Bollinger J; Robertson GT; Meibohm B; Lenaerts AJ; Ainsa J; Bottger EC; Lee RE Structure-Activity Relationships of Spectinamide Antituberculosis Agents: A Dissection of Ribosomal Inhibition and Native Efflux Avoidance Contributions. ACS Infect. Dis. 2017, 3, 72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Walz A; Lukka PB; Pearce C; Creissen E; Braunstein M; Hickey AJ; Meibohm B; Gonzalez-Juarrero M Sterilization of Mycobacterium tuberculosis infected samples using methanol preserves anti-tuberculosis drugs for subsequent pharmacological testing studies. Tuberculosis 2019, 117, 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).He J; Gao S; Hu M; Chow DS; Tam VH A validated ultra-performance liquid chromatography-tandem mass spectrometry method for the quantification of polymyxin B in mouse serum and epithelial lining fluid: application to pharmacokinetic studies. J. Antimicrob. Chemother. 2013, 68, 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Mager H; Goller G Resampling methods in sparse sampling situations in preclinical pharmacokinetic studies. J. Pharm. Sci. 1998, 87, 372–378. [DOI] [PubMed] [Google Scholar]
- (45).Bruhn DF; Scherman MS; Liu J; Scherbakov D; Meibohm B; Böttger EC; Lenaerts AJ; Lee RE In vitro and in vivo Evaluation of Synergism between Anti-Tubercular Spectinamides and Non-Classical Tuberculosis Antibiotics. Sci. Rep. 2015. Sep 14, 5, 13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


