Abstract
Background and objectives
Mitochondrial diseases present as multi-system disorders requiring a comprehensive multidisciplinary approach. The data on healthcare resource utilization associated with mitochondrial diseases and the clinical drivers of these costs are limited including for the out-patient setting where the majority of the clinical care for mitochondrial disease patients occurs. We performed a cross-sectional retrospective study of out-patient healthcare resource utilization and costs for patients with a confirmed diagnosis of mitochondrial disease.
Methods
We recruited participants from the Mitochondrial Disease Clinic in Sydney and stratified them into three groups: those with mitochondrial DNA (mtDNA) mutations (Group 1), those with nuclear DNA (nDNA) mutations and the predominant phenotype of chronic progressive external ophthalmoplegia (CPEO) or optic atrophy (Group 2) and those without a confirmed genetic diagnosis but clinical criteria and muscle biopsy findings supportive of a diagnosis of mitochondrial disease (Group 3). Data was collected through retrospective chart review and out-patient costs were calculated using the Medicare Benefits Schedule.
Results
We analyzed the data from 91 participants and found that Group 1 had the greatest average out-patient costs per person per annum ($838.02; SD 809.72). Neurological investigations were the largest driver of outpatient healthcare costs in all groups (average costs per person per annum:—Group 1: $364.11; SD 340.93, Group 2: $247.83; SD 113.86 and Group 3: $239.57; SD 145.69) consistent with the high frequency (94.5%) of neurological symptoms. Gastroenterological and cardiac-related out-patient costs were also major contributors to out-patient healthcare resource utilization in Groups 1 and 3. In Group 2, ophthalmology was the second-most resource intensive specialty ($136.85; SD 173.35). The Group 3 had the greatest average healthcare resource utilization per person over the entire duration of out-patient clinic care ($5815.86; SD 3520.40) most likely due to the lack of a molecular diagnosis and a less customized management approach.
Conclusion
The drivers of healthcare resource utilization are dependent on the phenotype–genotype characteristics. Neurological, cardiac, and gastroenterological costs were the top three drivers in the out-patient clinics unless the patient had nDNA mutations with predominant phenotype of CPEO and/or optic atrophy wherein ophthalmological-related costs were the second most resource intensive driver.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-023-02746-x.
Keywords: Mitochondrial diseases, Mitochondrial disorders, Health care costs, Health services, Health resources, Outpatients
Background
Mitochondrial diseases comprise a heterogenous group of genetic disorders resulting from mutations in either mitochondrial DNA (mtDNA) or nuclear DNA (nDNA) [1].
Clinical manifestations are protean [2–4] and the spectrum of disease severity ranges from mild or oligosymptomatic disease to severe or life-threatening multi-organ involvement. The multi-systemic manifestations may include (but are not limited to) myopathy, seizures, strokes, visual impairment, diabetes, sensorineural hearing loss, cardiomyopathy and gastrointestinal dysmotility [5–7].
Patients with mitochondrial disease require a comprehensive approach utilizing both in-patient and out-patient multidisciplinary care models with input from multiple medical specialties and allied health divisions [8–12]. Mitochondrial diseases, being representative of progressive, multisystemic chronic conditions, demonstrate the increasing challenge and impact these and similar illnesses have on population health models, not just within Australia but worldwide. As a group, chronic conditions have become “the leading cause of illness, disability and death in Australia” [13], continually adding not just to premature mortality, but also disability rates [14]. However, the persistence and practice of acute care models within public health care systems, is ineffective as costs spiral upwards without the desired improvements [15].
By better understanding healthcare resource utilisation and related costs within a chronic condition such as mitochondrial disease; future practice, skills, prevention, resources, streamlining of services, and disease monitoring systems (both within symptomatic and asymptomatic patients) to predict and prevent complications, will allow such scientific evidence to be continually integrated at all micro-, meso-, and macro-levels of health care [15], improving both health and cost-effectiveness outcomes.
There is a scarcity of data in literature on healthcare resource utilization associated with mitochondrial diseases and the clinical drivers of these healthcare costs. A retrospective analysis of healthcare utilization for patients with mitochondrial disease in USA indicated that the top three cost-intensive areas were inpatient care, surgery and prescription drugs [16]. Due to the method of data collection and patient coding to identify patients with mitochondrial disease, it was likely that many patients with mitochondrial disease were not included and that not all the coded patients had the condition. A recent Canadian study retrospectively estimated the healthcare costs incurred by the participants before and after their first (index) hospitalization with an ICD code for mitochondrial disease [17], with the authors noting similar limitations in relation to patient coding as the USA study. Notably, there is no data investigating the healthcare costs for patients with clinically or genomically confirmed mitochondrial diseases in the out-patient setting where most of the clinical care for mitochondrial disease takes place.
To address this gap, we performed a cross-sectional retrospective study of hospital out-patient resource utilization and costs associated with outpatient care of patients with a confirmed diagnosis of mitochondrial disease. We also stratified groups depending on the results of their genetic testing to assess the impact of this intervention on outpatient healthcare costs.
Methods
Data source
For this cross-sectional observational study, we enrolled the participants over a period of three years (from September 2018 to August 2021) from the Mitochondrial Disease Clinic at Royal North Shore Hospital (RNSH) in NSW, which provides clinical care for patients with mitochondrial disease aged sixteen years and above. RNSH is the leading Mitochondrial Disease Clinic in Australia and accepts interstate referrals and provides expert advice to clinicians in other States and Territories. Consecutive patients were recruited for enrolment in the study if they either had a genetically confirmed diagnosis or met strict diagnostic clinical criteria [18] with muscle biopsy findings supportive of a definite diagnosis of mitochondrial disease.
Standard protocol approvals, registrations, and patient consents
The research study was approved by the North Sydney Local Health District Human Research Ethics Committee (NSLHD-HREC Reference number: LNR/17/HAWKE/268) in accordance with National Health and Medical Research Council (NHMRC) National Statement [19] and NSW Health Policy Directive [20]. Written informed consent was obtained from all participants or guardians of the participants in the study.
Data collection
We accessed the Hospital and Emergency Department datasets and performed a retrospective chart review. This involved accessing the electronic medical records (eMR) as well as patients’ charts to ensure a comprehensive assessment of the multi-specialty investigations as recorded by all clinicians and allied health service providers. We confirmed data collected on demographic variables, phenotype and symptom burden and utilization of out-patient healthcare services and resources including physician consultations via face-to-face or telephone interview.
Attribution of costs
The costs of the consultations in the outpatient settings including diagnostic and surveillance investigations and day-only procedures were calculated using the listing of Government subsidized Medicare services available on Medicare Benefits Schedule (MBS) online (Table 1, Additional file 1). Costs were calculated for the baseline investigations and the follow-up investigations requested whilst providing follow-up care during the patient’s clinical course. The cost of genetic testing, muscle biopsy and blood tests such as serum lactate and pyruvate were not included in the analyses as these were standard across all study participants at baseline.
Table 1.
Demographic and socioeconomic characteristics of participants
| Total | Group 1 | Group 2 | Group 3 | |
|---|---|---|---|---|
| Mean age (years) | 53.7 | 41.4 | 54.1 | 61.5 |
| n (%) | ||||
| < 40 years | 24 (25.5) | 20 (43.5) | 5 (29.4) | 4 (13.8) |
| > 40 years | 70 (74.5) | 26 (56.5) | 12 (70.6) | 25 (86.2) |
| Gender n (%) | ||||
| Male | 34 (36.2) | 15 (32.6) | 4 (23.5) | 12 (41.4) |
| Female | 60 (63.8) | 31 (67.4) | 13 (76.5) | 17 (58.6) |
| Support level n (%) | ||||
| Have a paid carer | 22 (23.4) | 8 (17.4) | 8 (47.1) | 6 (20.7) |
| Living with parents | 10 (10.6) | 5 (10.9) | 4 (23.5) | 1 (3.4) |
| Education level n (%) | ||||
| < Year 12 | 22 (23.4) | 10 (21.7) | 7 (41.2) | 5 (17.2) |
| Year 12 | 9 (9.6) | 4 (8.7) | 0 | 5 (17.2) |
| Trade/Apprenticeship | 10 (10.6) | 5 (10.9) | 2 (11.8) | 3 (10.3) |
| Diploma/Certificate | 22 (23.4) | 11 (23.9) | 4 (23.5) | 6 (20.7) |
| Higher University Degree | 29 (30.9) | 16 (34.8) | 4 (23.5) | 9 (19.6) |
| Unknown | 2 (2.1) |
Analyses
We defined the healthcare resource utilization as the cohort’s use of out-patient healthcare services including hospital resources available through out-patient clinics and diagnostic centers and physician resources. To determine whether healthcare resource utilization was influenced by the results of genetic testing, we stratified participants into three different groups. The first group (designated as Group 1) consisted of participants with mtDNA mutations (i.e., mtDNA point mutations or deletions). The phenotypic spectrum displayed in this group included presentations such as MELAS syndrome (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes) and LHON (Leber Hereditary Optic Neuropathy), as well as other non-syndromic presentations. Participants with nDNA mutations were placed together in the second group (designated as Group 2). The predominant phenotype for these participants was autosomal dominant (AD) and autosomal recessive (AR) chronic progressive external ophthalmoplegia (CPEO) or optic atrophy. Two participants had MLASA (Myopathy, Lactic Acidosis, and Sideroblastic Anaemia) caused by YARS2 mutations. The mtDNA and nDNA mutations in groups 1 and 2 are listed in Table 2, Additional file 1. Participants with clinical features consistent with clinically definite mitochondrial disease, supportive muscle biopsy findings but no genetic cause identified were allocated to the third group (designated as Group 3).
Table 2.
Reported neurological and non-neurological symptoms
| Symptoms | Group 1 [N = 46] n (%) |
Group 2 [N = 17] n (%) |
Group 3 [N = 29] n (%) |
|---|---|---|---|
| Neurological | 43 (93.5) | 16 (94.1) | 27 (93.1) |
| Cardiac | 17 (37.0) | 5 (29.4) | 14 (48.3) |
| Gastrointestinal | 34 (73.9) | 8 (47.1) | 17 (58.6) |
| Ophthalmological | 32 (69.6) | 16 (94.1) | 22 (75.9) |
| Respiratory | 4 (8.7) | 3 (17.6) | 4 (13.8) |
| Hearing impairment | 33 (71.7) | 11 (64.7) | 15 (51.7) |
| Sleep disturbance | 32 (69.6) | 15 (88.2) | 21 (72.4) |
| Diabetes mellitus | 21 (45.7) | 2 (28.6) | 7 (24.1) |
The cost figures are presented here in their original currency (Australian Dollars or $AUD). The costs are per person per annum. The Analysis of Variance (ANOVA) was performed to determine the differences in healthcare resource utilization amongst the three groups. We conducted all statistical analyses using IBM SPSS Statistics version 27.
Results
We approached one hundred and twenty-five consecutive patients for enrollment in the study. Twenty-two patients declined to participate and nine were too unwell to participate, leaving ninety-four individuals who were recruited into the study. On further review, two participants did not meet the inclusion criteria and were excluded from the final analysis (Fig. 1). Overall, the response rate was 73.6%. The demographic and socioeconomic characteristics of the participants are summarized in Table 1.
Fig. 1.
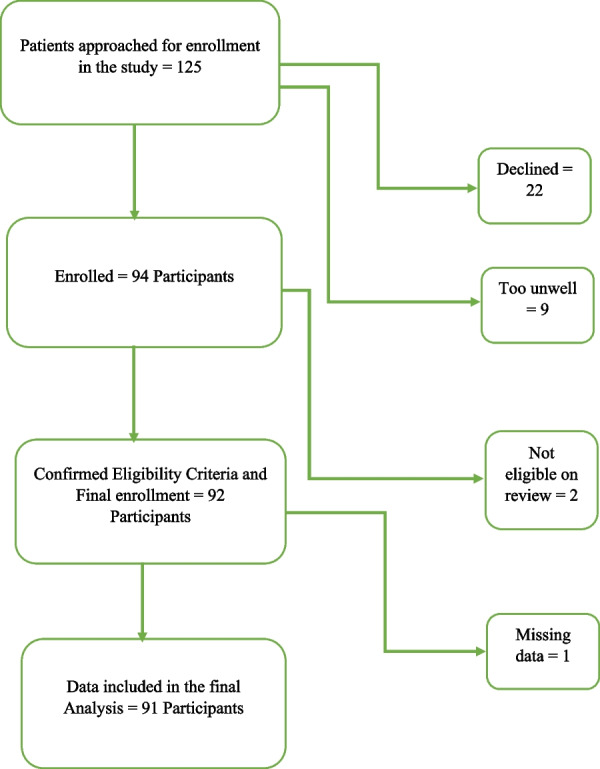
Participant enrollment and eligibility
The mean age of the participants at the time of recruitment was 53.72 years (range 16–83; SD 17.09) with 25.5% aged 16 to 40 years and 74.5% aged 41 to 83 years. Approximately two-thirds of the study participants were females (64%). The mean duration for which the participants had been attending the clinic was 9.12 years (range 1–19 years; SD 4.82).
The educational status of the study participants was variable. 30.9% had a higher university degree, 9.6% had completed higher school certificate (HSC) while 23.4% had not completed HSC. 10.6% of the participants had completed an apprenticeship after HSC and another 23.4% had a graduate diploma or certificate.
Neurological symptoms were the most commonly reported problem in our cohort as shown in Table 2. Table 2 also summarizes the frequency of the non-neurological symptoms reported in the three groups.
The most frequently reported non-neurological symptoms in Group 1 included gastrointestinal dysmotility (73.9%), hearing impairment (71.7%), ophthalmological problems (69.6%) and sleep disturbance and/or irregular sleep pattern (69.6%). In Group 2, ophthalmological problems were most prevalent (94.1%). These included reduced visual acuity (legal blindness in some), ptosis and problems associated with poor ocular motility secondary to CPEO. Sleep disturbance was frequently reported (88.2%) followed by hearing impairment (64.7%) and gastrointestinal dysmotility (47.1%). Ophthalmological problems were frequent in Group 3 (75.9%) followed by sleep disturbance (72.4%), gastrointestinal dysmotility (58.6%) and hearing impairment (51.7%). Cardiac symptoms were most prevalent in Group 3 (48.3%) and Diabetes Mellitus in Group 1 (45.7%). Non-sleep related respiratory symptoms were reported most frequently in Group 2 (17.6%) followed by Group 3 (13.8%) and then Group 1 (8.7%).
Figure 2 illustrates the composite of the average out-patient costs per person per annum. We found that patients with mtDNA encoded mitochondrial disease (Group 1) had the highest average out-patient costs per person per annum ($838.02; SD 809.72) followed by the patients without a confirmed genetic diagnosis (Group 3) which had the second highest average out-patient costs per person per annum ($669.12; SD 406.31). The annual average out-patient costs for nDNA encoded mitochondrial disease (Group 2) were $71 less ($598.12; SD 370.28) than that in Group 3. There was no significant difference in the average out-patient costs per person per annum (p = 0.34) between the three groups.
Fig. 2.
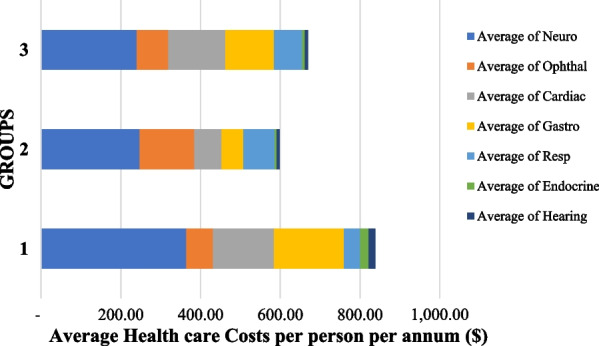
Total Average health care resource utilization and costs per person per annum
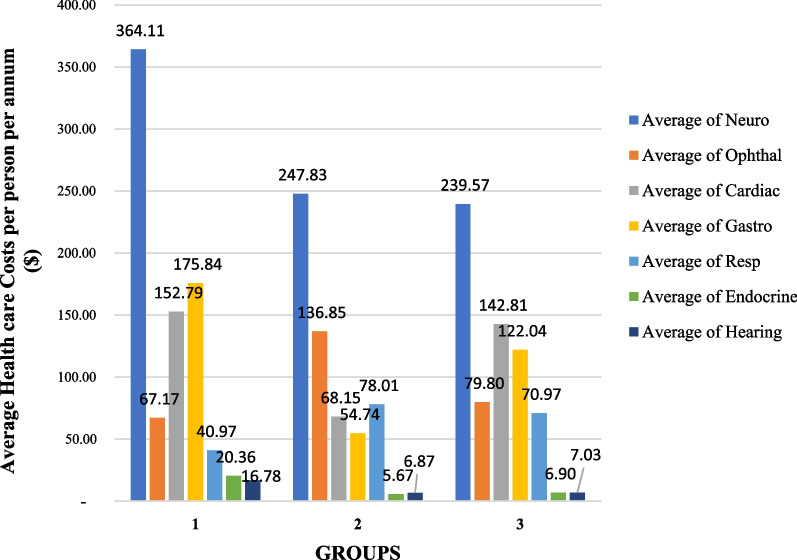
We found that the neurological investigations were the largest driver of outpatient healthcare costs in all groups when compared to other investigations (Fig. 3). In Group 1, the average neurological cost per person per annum amounts to $364.11 (SD 340.93), in Group 2, to $247.83 (SD 113.86) and in Group 3, to $239.57 (SD 145.69). The difference in the neurological out-patient costs per person per annum amongst the three groups was not statistically significant (p = 0.09). Gastroenterological and cardiac related investigations and consultations were also major contributors to outpatient health care costs in Groups 1 and 3. In Group 1, the average gastroenterology-related costs per person per annum amounted to $175.84 (SD 300.06) and in Group 3, to $122.04 (SD 139.20) with no statistically significance difference between the two (p = 0.17). For cardiac investigations, cost contributions were $152.79 (SD 229.55) and $142.81 (SD 128.41) (p = 0.25) in Groups 1 and 3 respectively.
Fig. 3.
Average health care costs per person per annum by Specialty
For patients with nDNA encoded mitochondrial disease (Group 2), following neurological investigations, ophthalmological ($136.85; SD 173.35), and then respiratory and cardiac investigations were the main drivers of out-patient costs for this group.
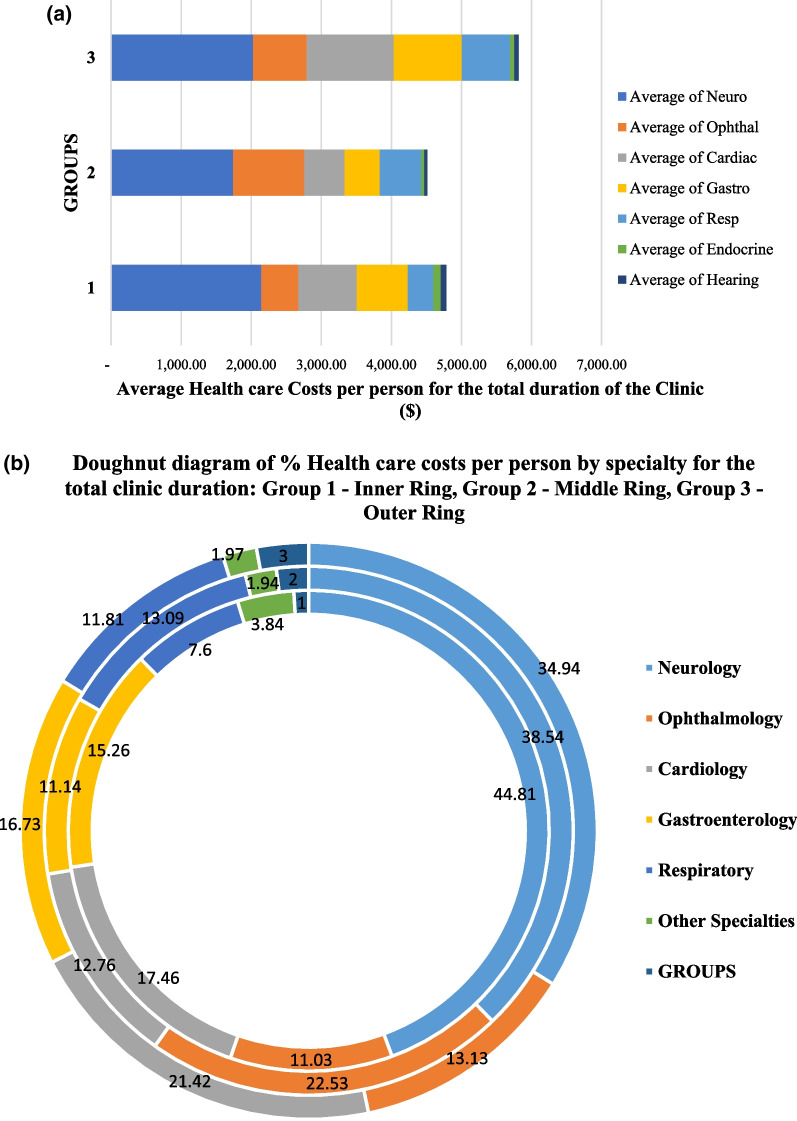
When assessed over the entire duration of the patients’ medical care in the mitochondrial out-patient clinic, our study demonstrates that patients without a confirmed genetic diagnosis (Group 3) had the highest average healthcare resource utilization and costs per person. The average out-patient costs per person are illustrated in Fig. 4a. For Group 3, the out-patient costs per person amount to $5815.86 (SD 3520.40). This is followed by Group 1 at $4783.56 (SD 2859.11) and then Group 2 at $4514.84 (SD 2457.35).
Fig. 4.
a Average health care resource utilization and costs per person for total duration of clinic attendance. b Percentage health resource utilization by specialty for the total duration of clinic attendance
For the total duration of medical out-patient care in the mitochondrial clinic, healthcare costs related to neurological investigations and consultations ranked the highest, contributing 44.81%, 38.54% and 34.94% respectively for groups 1, 2 and 3 (Fig. 4b). In group 2, ophthalmological investigations contributed 22.53%, ranking second highest. This was followed by respiratory and cardiac-related healthcare costs which contributed 13.09% and 12.76% of the out-patient costs respectively. In Groups 1 and 3, cardiac-related costs ranked second highest at 17.46% and 21.42% respectively. The gastroenterological investigations ranked third in both Groups 1 and 3 at 15.26% and 16.73% respectively.
Discussion
Our study examines the clinical drivers of healthcare resource utilization in adult patients with mitochondrial disease in Australia in the out-patient setting.
Our results show that neurological consultations and investigations are the leading driver of healthcare costs in the out-patient clinics in all the three groups of participants: those with mtDNA mutations, those with nDNA mutations, and those with no genetic cause identified. This is consistent with the high frequency (94.5%) of neurological symptoms in our study population necessitating a higher frequency of neurological investigations and consultations. Patient symptoms requiring outpatient care included muscle fatigue and weakness, exercise intolerance, migraines, seizures, stroke-like episodes, cognitive deterioration, gait impairment, paraesthesia and sensory deficits and incoordination.
Those patients with mtDNA point mutations (Group 1) accrued the highest per person per annum neurological-driven out-patient costs compared to Groups 2 and 3 (Fig. 2). This is not surprising, given that neurological symptoms and signs contribute significantly to the clinical manifestations in the patients with mtDNA mutations [2–4]. In these patients, neurological investigations such as MRI brain with MR spectroscopy and electroencephalogram (EEG) were carried out more frequently due to the higher prevalence of certain neurological symptoms such as stroke-like episodes and seizures.
Despite the clinical heterogeneity and variability in their disease course, neurological complaints constituted a high symptom burden in the other two groups as well (Group 2: 94.1%; Group 3: 93.1%). Similarly, neurological investigation was the most resource intensive field in Groups 2 and 3 as well (Fig. 3). Neurological symptoms due to myopathy and peripheral neuropathy were frequently investigated and treated in patients with OPA1 and TWNK mutations despite their features of optic atrophy, ptosis and ophthalmoplegia constituting the predominant phenotype [3, 21]. This higher frequency of neurological symptoms is similarly reflected in our study Group 3 which comprises participants with a clinical definite diagnosis but no confirmed genetic diagnosis to date, and is consistent with the study by Grier et al., where weakness, fatigue, difficulty in walking, incoordination, numbness and seizures were amongst the top ten self-reported symptoms in a cohort of patients with either a clinical diagnosis or biochemical deficiency or both [22].
Ophthalmological problems constituted the most frequent non-neurological symptoms (94.1%) and are consistent with outpatient monitoring of the ocular symptoms of participants stratified to Group2. Ophthalmological investigations and consultations are the second greatest driver of healthcare resource utilization. Ophthalmological costs were also attributed to day-only interventional procedures, of which surgery for correction of ptosis was most common. Surgical correction of ptosis in CPEO has shown safety and efficacy [23]. Eshaghi et al. have reported significant improvement in Margin-to-Reflex Distance 1 (MRD1) and chin-up posture at 1-year post-surgical follow up [24] and Doherty et al. demonstrated low complication rate and objective benefit following surgery in patients with ocular myopathy and ptosis [25]. In our cohort, 7 participants in Group 2 had ptosis correction surgery (41.2%) of which 3 patients required more than one operation. One participant in Group 1 and three participants in Group 3 also required ptosis correction surgery.
Gastroenterological and cardiac-related out-patient costs were also amongst the stronger drivers of healthcare resource utilization. Gastroenterological-related costs were the second most resource intensive field in Group 1 and third-most in Group 3. Almost three-quarters of the participants in Group 1 (73.9%) reported a high prevalence of gastrointestinal dysmotility and other symptoms associated with alimentary disorders. More than half of the participants in Group 3 (58.6%) also reported gastrointestinal symptoms. Similar to other studies [26–29], gastroenterological symptoms such as dysphagia, gastroesophageal reflux disease and feeding difficulties manifesting as delayed gastric emptying, constipation and intestinal pseudo-obstruction were observed and investigated with gastric motility and colonic transit studies and were main contributors to the higher out-patient costs. Gastroscopies and colonoscopies were performed at a lesser frequency. Consultations with gastroenterologists also contributed to the costs in this category especially for patients with severe or progressive symptoms.
Cardiac investigations and surveillance cardiac testing also contributed significantly to the healthcare resource utilization. The out-patient costs related to cardiac category are driven by surveillance investigations such as 12-lead electrocardiograms (ECGs), 24-h Holter monitoring and transthoracic echocardiograms (TTEs). Patients with mitochondrial disease are at an increased risk of cardiomyopathy and arrythmia as cardiac muscle is a high-energy-dependent tissue [30]. The progression to a high-grade AV block can be unpredictable in patients with mtDNA mutations, hence there is a need for regular monitoring despite minimal to no reported symptoms [31, 32]. Additionally, cardiac day procedures and investigations such as stress echocardiograms, electrophysiology studies (EPS) and myocardial perfusion scans (MIBI scans) are often more expensive compared to investigations in other specialties. Additional file 1: Table 1 lists the costs of specialty-based investigations and day-only procedures. Of the three groups, participants in Group 3 reported the highest prevalence of cardiac symptoms (48.3%), and investigations were more frequent because without a confirmed genetic diagnosis, the risks for cardiac complications are more difficult to ascertain.
When the health care costs were analyzed for the participants’ entire clinic duration, Group 3 showed the greatest healthcare resource utilization (Fig. 4). This observed difference is most likely due to the lack of a molecular diagnosis which can otherwise enable a more customized approach through utilization of streamlined standards of care and investigations [8, 9, 33] and undertaking further ancillary investigations to determine a potential molecular diagnosis.
Strengths and limitations
The main strength of this study is that it is based on a sample of patients identified as having mitochondrial disease by a genetically confirmed diagnosis or clinically definite (with muscle biopsy positive) diagnosis of mitochondrial disease in a hospital out-patient setting when compared to other studies where there was uncertainty about whether the patients had a confirmed mitochondrial disorder [13].
One limitation of the study is underrepresentation of those patients with mitochondrial disease who were severely affected and deemed too unwell for recruitment. This may lead to under-estimation of health care resource utilization. Such patients require in-patient care more frequently. However, useful insights into the healthcare resource utilization and trends can be gained by analyzing the data from out-patient clinics prior to these patients requiring in-patient hospital care. A future study to estimate health care resource utilization for mitochondrial patients admitted to the hospital may help understand this gap further.
Children have a different disease pattern to adults with earlier age of onset, often greater disease severity and shorter disease duration. Our study focused on assessing the healthcare costs in the adult population, rather than a mixed adult and paediatric population given these differences. Hence, presentation and management in chronic condition outpatient departments is less. Studies investigating out-patient health care resource utilization in children with mitochondrial diseases may provide further insight into the breadth of the problem.
Reasons given by individuals who declined to enroll in the study included that they were either clinically stable or too busy managing daily routine and healthcare appointments and/or caring for additional family members. Individuals aged over 40 years who were not working full time were more amenable to recruitment. Similarly, there was greater participation by females compared to working male participants and this was independent of disease severity.
Conclusion
Mitochondrial disease has protean clinical manifestations and individuals affected by this genetic disorder experience a complex multi-specialty clinic journey during their lifespan. The drivers of healthcare resource utilization and costs are dependent on the phenotype-genotype characteristics of the patients as well as the extent of the multisystem involvement, disease evolution and the rate of progression. The top three drivers for health care resource utilization and costs in the out-patient clinics are neurology, cardiology, and gastroenterology specialties unless the patient has nDNA mutations with predominant phenotype of CPEO and/or optic atrophy wherein ophthalmology is the second most resource intensive specialty after neurology.
Supplementary Information
Additional file 1: Table 1 Cost of investigations per specialty. Table 2 Mutation details in groups 1 and 2.
Acknowledgements
Not applicable.
Abbreviations
- AD
Autosomal dominant
- ANOVA
Analysis of variance
- AR
Autosomal recessive
- $AUD
Australian dollars
- AV block
Atrioventricular block
- CPEO
Chronic progressive external ophthalmoplegia
- ECG
Electrocardiogram
- EEG
Electroencephalogram
- eMR
Electronic medical records
- EPS
Electrophysiology study
- HSC
Higher school certificate
- ICD
International classification of diseases
- LHON
Leber hereditary optic neuropathy
- MBS
Medicare benefits schedule
- MELAS
Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes
- MIBI scan
Myocardial perfusion scan/sestamibi scan
- MLASA
Myopathy, lactic acidosis, and sideroblastic anaemia
- MRD1
Margin-to-reflex distance 1
- MRI
Magnetic resonance imaging
- mtDNA
Mitochondrial DNA
- nDNA
Nuclear DNA
- NHMRC
National Health and Medical Research Council
- NSLHD-HREC
North Sydney Local Health District Human Research Ethics Committee
- NSW
New South Wales
- OPA1
Optic atrophy 1
- RNSH
Royal North Shore Hospital
- SD
Standard deviation
- TTE
Transthoracic echocardiogram
- TWNK
Twinkle mtDNA helicase
- USA
United States of America
- YARS2
Tyrosyl-tRNA synthetase 2
Author contributions
SH: Major role in the acquisition of data; Study concept and design; Analysis and interpretation of data; Drafting of the manuscript for content, including medical writing for content. KC: Major role in the acquisition of data; Study concept and design. RS: Study concept and design; Analysis and interpretation of data. DS: Study concept and design; Analysis and interpretation of data. CMS: Study concept and design; Interpretation of data; Drafting of the manuscript for content, including medical writing for content. All authors read and approved the final manuscript.
Funding
This is not a sponsored study.
Availability of data and materials
The authors have access to all the data in this study. The authors take full responsibility for the integrity of the data, the accuracy of the data analysis and interpretation and the conduct of the research. Due to data protection in accordance with approved ethical standards, the data cannot be made available publicly.
Declarations
Ethics approval and consent to participate
The Methods section in the manuscript includes North Sydney Local Health District Human Research Ethics Committee (NSLHD-HREC) approval reference number (LNR/17/HAWKE/268) in accordance with National Health and Medical Research Council (NHMRC) National Statement and NSW Health Policy Directive. The principal author has received consent forms from the participants in this study and has them on file.
Consent for publication
As indicated above in the section: Ethics approval and Consent to participate.
Competing interests
The authors report no disclosures relative to the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davis RL, Raj R, Kumar K, Puttick C, Liang C, Ahmad KE, Edema-Hildebrand F, Park JS, Minoche AE, Gayevskiy V, Mallawaarachchi AC, Christodoulou J, Schofield D, Dinger ME, Cowley MJ, Sue CM. Use of whole-genome sequencing for mitochondrial disease diagnosis. Neurology. 2022;99(7):e730–e742. doi: 10.1212/WNL.0000000000200745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng YS, Bindoff LA, Gorman GS, et al. Mitochondrial disease in adults: recent advances and future promise. Lancet neurol. 2021;20(7):573–584. doi: 10.1016/S1474-4422(21)00098-3. [DOI] [PubMed] [Google Scholar]
- 3.La Morgia C, Maresca A, Caporali L, Valentino ML, Carelli V. Mitochondrial diseases in adults. J Intern Med. 2020;287(6):592–608. doi: 10.1111/joim.13064. [DOI] [PubMed] [Google Scholar]
- 4.Klopstock T, Priglinger C, Yilmaz A, Kornblum C, Distelmaier F, Prokisch H. Mitochondrial disorders. Dtsch Arztebl int. 2021;118(44):741–748. doi: 10.3238/arztebl.m2021.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis RL, Liang C, Sue CM. Mitochondrial diseases. Handb Clin Neurol. 2018;147:125–141. doi: 10.1016/B978-0-444-63233-3.00010-5. [DOI] [PubMed] [Google Scholar]
- 6.Liang C, Ahmad K, Sue CM. The broadening spectrum of mitochondrial disease: shifts in the diagnostic paradigm. Biochim Biophys Acta. 2014;1840(4):1360–1367. doi: 10.1016/j.bbagen.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Prim. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 8.Sue CM, Balasubramaniam S, Bratkovic D, et al. Patient care standards for primary mitochondrial disease in Australia: an Australian adaptation of the Mitochondrial Medicine Society recommendations. Intern Med J. 2022;52(1):110–120. doi: 10.1111/imj.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh S, Goldstein A, Karaa A, et al. Patient care standards for primary mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med. 2017;19(12):1380. doi: 10.1038/gim.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet neurol. 2010;9(8):829–840. doi: 10.1016/S1474-4422(10)70116-2. [DOI] [PubMed] [Google Scholar]
- 11.Meyers DE, Basha HI, Koenig MK. Mitochondrial cardiomyopathy: pathophysiology, diagnosis, and management. Tex Heart Inst J. 2013;40(4):385–394. [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Hong SQ, Jiang L. An interpretation of the expert consensus on standards for the management of patients with primary mitochondrial disease from the Mitochondrial Medicine Society. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20(11):887–892. doi: 10.7499/j.issn.1008-8830.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.aihw.gov.au/getmedia/9844cefb-7745-4dd8-9ee2-f4d1c3d6a727/19787-AH16.pdf.aspx.
- 14.Nolte E, McKee M. Integration and chronic care: a review. In: Nolte E, McKee M, editors. Caring for people with chronic conditions: a health system perspective. Maidenhead: Open University Press; 2008. pp. 64–91. [Google Scholar]
- 15.https://apps.who.int/iris/handle/10665/42500.
- 16.Cohen B, Balcells C, Hotchkiss B, Aggarwal K, Karaa A. A retrospective analysis of health care utilization for patients with mitochondrial disease in the United States: 2008–2015. Orphanet J Rare Dis. 2018;13(1):210. doi: 10.1186/s13023-018-0949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buajitti E, Rosella LC, Zabzuni E, Young LT, Andreazza AC. Prevalence and health care costs of mitochondrial disease in Ontario, Canada: a population-based cohort study. PLoS ONE. 2022;17(4):e0265744. doi: 10.1371/journal.pone.0265744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morava E, van den Heuvel L, Hol F, et al. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67(10):1823–1826. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- 19.https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018.
- 20.https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2010_055.pdf.
- 21.Viscomi C, Zeviani M. MtDNA-maintenance defects: syndromes and genes. J Inherit Metab Dis. 2017;40(4):587–599. doi: 10.1007/s10545-017-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grier J, Hirano M, Karaa A, Shepard E, Thompson JLP. Diagnostic odyssey of patients with mitochondrial disease: results of a survey. Neurol Genet. 2018;4(2):e230. doi: 10.1212/NXG.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn J, Kim NJ, Choung HK, et al. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. Br J Ophthalmol. 2008;92(12):1685–1688. doi: 10.1136/bjo.2008.144816. [DOI] [PubMed] [Google Scholar]
- 24.Eshaghi M, Arabi A, Eshaghi S. Surgical management of ptosis in chronic progressive external ophthalmoplegia. Eur J Ophthalmol. 2021;31(4):2064–2068. doi: 10.1177/1120672120952344. [DOI] [PubMed] [Google Scholar]
- 25.Doherty M, Winterton R, Griffiths PG. Eyelid surgery in ocular myopathies. Orbit. 2013;32(1):12–15. doi: 10.3109/01676830.2012.736599. [DOI] [PubMed] [Google Scholar]
- 26.Chinnery PF, Turnbull DM. Mitochondrial medicine. QJM. 1997;90(11):657–667. doi: 10.1093/qjmed/90.11.657. [DOI] [PubMed] [Google Scholar]
- 27.Parsons T, Weimer L, Engelstad K, et al. Autonomic symptoms in carriers of the m3243A>G mitochondrial DNA mutation. Arch Neurol. 2010;67(8):976–979. doi: 10.1001/archneurol.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amiot A, Tchikviladze M, Joly F, et al. Frequency of mitochondrial defects in patients with chronic intestinal pseudo-obstruction. Gastroenterology. 2009;137(1):101–109. doi: 10.1053/j.gastro.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 29.Hom XB, Lavine JE. Gastrointestinal complications of mitochondrial disease. Mitochondrion. 2004;4(5–6):601–607. doi: 10.1016/j.mito.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Wahbi K, Bougouin W, Behin A, et al. Long-term cardiac prognosis and risk stratification in 260 adults presenting with mitochondrial diseases. Eur Heart J. 2015;36(42):2886–2893. doi: 10.1093/eurheartj/ehv307. [DOI] [PubMed] [Google Scholar]
- 31.Kabunga P, Lau AK, Phan K, et al. Systematic review of cardiac electrical disease in Kearns–Sayre syndrome and mitochondrial cytopathy. Int J Cardiol. 2015;181:303–310. doi: 10.1016/j.ijcard.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 32.Bates MG, Bourke JP, Giordano C, d'Amati G, Turnbull DM, Taylor RW. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur Heart J. 2012;33(24):3023–3033. doi: 10.1093/eurheartj/ehs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boggan RM, Lim A, Taylor RW, McFarland R, Pickett SJ. Resolving complexity in mitochondrial disease: towards precision medicine. Mol Genet Metab. 2019;128(1–2):19–29. doi: 10.1016/j.ymgme.2019.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1 Cost of investigations per specialty. Table 2 Mutation details in groups 1 and 2.
Data Availability Statement
The authors have access to all the data in this study. The authors take full responsibility for the integrity of the data, the accuracy of the data analysis and interpretation and the conduct of the research. Due to data protection in accordance with approved ethical standards, the data cannot be made available publicly.