Abstract
Phagocytosis is the process whereby cells direct the spatially localized, receptor-driven engulfment of particulate materials. It proceeds via remodeling of the actin cytoskeleton and shares many of the core cytoskeletal components involved in adhesion and migration. Small GTPases of the Rho family have been widely implicated in coordinating actin dynamics in response to extracellular signals and during diverse cellular processes, including phagocytosis, yet the mechanisms controlling their recruitment and activation are not known. We show herein that in response to ligation of Fc receptors for IgG (FcγR), the guanine nucleotide exchange factor Vav translocates to nascent phagosomes and catalyzes GTP loading on Rac, but not Cdc42. The Vav-induced Rac activation proceeds independently of Cdc42 function, suggesting distinct roles for each GTPase during engulfment. Moreover, inhibition of Vav exchange activity or of Cdc42 activity does not prevent Rac recruitment to sites of particle attachment. We conclude that Rac is recruited to Fcγ membrane receptors in its inactive, GDP-bound state and that Vav regulates phagocytosis through subsequent catalysis of GDP/GTP exchange on Rac.
INTRODUCTION
Phagocytosis is an evolutionarily conserved process whereby cells coordinate the receptor driven recognition and engulfment of targets (Kwiatkowska and Sobota, 1999). It plays a central role in diverse physiological functions ranging from feeding in Dictyostelium to complex immune defense and apoptotic cell clearance mechanisms in higher eukaryotes (Aderem and Underhill, 1999). The vast repertoire of phagocytic receptors identified to date all direct engulfment via the remodeling of the actin cytoskeleton, thus strongly implicating the Rho family of small GTPases as key signaling components (Chimini and Chavrier, 2000; May and Machesky, 2001). The two best-characterized phagocytic receptors, the Fc receptor for IgG (FcγR) and the complement receptor 3 (CR3), are known to use distinct Rho GTPases to drive particle uptake. Phagocytosis through FcγR requires Cdc42 and Rac function, whereas CR3-mediated uptake requires only Rho activity (Cox et al., 1997; Caron and Hall, 1998; Massol et al., 1998). Rho GTPases also play a central role during infection by various bacterial pathogens and in the removal of apoptotic cell corpses, both in Caenorhabditis elegans (where the Rac1 ortholog Ced10 is required) and in mammalian cells (Ernst, 2000; Reddien and Horvitz, 2000; Leverrier and Ridley, 2001).
The recruitment and function of Rho GTPases specifically at the site of particle attachment is central to receptor-mediated phagocytosis. Castellano et al. (1999) showed that artificial clustering of active Cdc42 or its effector WASP beneath membrane-bound beads triggered actin polymerization and membrane protrusion around the particle. In the same system, localized recruitment of active Rac also led to actin polymerization and was sufficient to induce phagocytosis (Castellano et al., 2000). Finally, uptake through either the FcγR or the CR3 receptor is accompanied by enrichment of distinct Rho GTPases at nascent phagosomes (Caron and Hall, 1998). However, despite the overwhelming evidence detailing Rho GTPase involvement in phagocytosis, the mechanisms linking phagocytic receptors to recruitment and control of Rho GTPase function remain unclear.
Rho GTPases function as molecular switches, cycling between a GDP-bound inactive state and a GTP-bound active form. The cycle is controlled by two families of accessory proteins, guanine nucleotide exchange factors (GEFs), which catalyze GTP loading in response to upstream signals, and GTPase-activating proteins (GAPs), which promote inactivation. More than 50 RhoGEFs and 60 RhoGAPs have been identified so far in the human genome (Schultz et al., 1998), but how they participate in receptor signaling to specific GTPases is unknown. In C. elegans, genetic analysis of phagocytosis has identified two additional components involved in the upstream regulation of Rac, Ced2, and Ced5, orthologs of the mammalian adaptor proteins CrkII and DOCK180, respectively (Nolan et al., 1998; Wu and Horvitz, 1998; Reddien and Horvitz, 2000). However, neither display exchange activity for Rho GTPases in vitro and to date no GEF has been isolated using the genetic screens for Ced genes. A further complication is that Rho, Rac, and to a lesser extent Cdc42 are maintained in a cytosolic form in their resting, inactive state through interaction with Rho guanine nucleotide dissociation inhibitors (RhoGDI) (Isomura et al., 1991). Because of its spatially localized signaling, phagocytosis offers an ideal biological assay to delineate pathways linking surface receptors (such as immune receptors, growth factor receptors, and integrins) to actin remodeling via Rho GTPases and aids understanding of more complex cytoskeletal processes such as adhesion and migration.
The guanine nucleotide exchange factor Vav was first identified in hematopoietic cells and encodes a multidomain protein comprising an amino terminal calponin homology domain, an acidic region, adjacent Dbl homology (DH), and pleckstrin homology (PH) domains common to almost all Rho GEFs, a zinc finger motif, a proline-rich region, and a carboxy terminal SH3-SH2-SH3 module (Bustelo, 2000). Biochemical and structural data suggest that its GEF activity can be modulated by tyrosine phosphorylation and by phosphatidylinositol lipids (Crespo et al., 1997; Han et al., 1998) and that these two signals act synergistically to relieve the autoinhibitory amino terminus from sterically hindering the catalytic DH domain (Aghazadeh et al., 2000; Das et al., 2000). In vitro exchange assays and overexpression studies have shown that Vav can potentially activate Rho, Rac, and Cdc42 (Olson et al., 1996). It has been shown to be a critical regulator of T-cell receptor (TCR) signaling, involved in both the actin-driven clustering of TCRs and the positive selection of class I- and class II-restricted T cells, in response to antigen stimulation (Turner et al., 1997; Holsinger et al., 1998). Together, these data make Vav a good candidate for regulating phagocytosis and indeed it is reported to be one of several tyrosine phosphorylated proteins following FcγR cross-linking (Darby et al., 1994).
Herein, we show that Vav is essential for FcγR-, but not CR3-mediated phagocytosis in macrophages and COS cells. Vav is recruited to nascent FcγR phagosomes, along with filamentous actin, and is required for activation of Rac but not Cdc42. Moreover, this Vav-induced Rac activation occurs independently of Cdc42 function, suggesting that each GTPase mediates distinct stages of the phagocytosis process. Finally, Rac recruitment to nascent phagosomes occurs in the absence of Vav exchange activity, suggesting that the GTPase is recruited to activated FcγR in its inactive, GDP-bound conformation.
MATERIALS AND METHODS
Reagents
Eukaryotic pRK5 expression vectors encoding human FcγRIIA and myc-tagged N17Cdc42, N17Rac, N19Rho, and Wasp (aa 201–310) have been previously described (Lamarche et al., 1996; Caron and Hall, 1998). Myc-tagged full-length murine Vav and Vav-C (aa 538–845) (Wu et al., 1996) were subcloned into pRK5myc by polymerase chain reaction with pSKVav as a template, a generous gift from S. Katzav (Hebrew University, Jerusalem, Israel). Green fluorescent protein (GFP)-tagged constructs were generated by subcloning full-length wild-type Rac and Cdc42 from pGEXRac and pGEXCdc42 (Self and Hall, 1995) into pEGFP-C1 (CLONTECH, Palo Alto, CA). VavΔDH (Δaa 342–348), a kind gift from C. Abrams (University of Pennsylvania, Philadelphia, PA), was subcloned into pRK5myc. Constructs were verified by DNA sequencing and prepared for microinjection by standard CsCl gradient methods. Hemagglutinin (HA)-tagged pCMVTiam1(C1199) was a kind gift from J. Collard (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Mouse monoclonal anti-Vav1 and anti-Rac (clone 23A8) were purchased from Upstate Biotechnology (Lake Placid, NY), anti-Cdc42 from Transduction Laboratories (Lexington, KY), anti-RhoA from Santa Cruz Biotechnology (Santa Cruz, CA), anti-phosphotyrosine (clone PT66) from Sigma Chemical (St. Louis, MO), rabbit polyclonal anti-Syk (clone C-20) from Santa Cruz Biotechnology, conjugated secondary antibodies from Jackson Immunoresearch Laboratories (West Grove, PA), and anti-myc monoclonal 9E10 was purified in-house. Glutathione S-transferase fused Cdc42/Rac interactive-binding domain of PAK1 (PAK-CRIB) for GTPase pulldown assays was prepared as previously described (Sander et al., 1999).
Cell Culture
The murine macrophage cell line J774.A1 and COS-7 cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% heat inactivated fetal calf serum and penicillin/streptomycin (100 U/ml and 100 μg/ml).
Microinjection and Phagocytosis Assay
J774 macrophages were used for microinjection and phagocytosis assays as previously described (Patel et al., 2000). Briefly, macrophages were seeded on glass coverslips at a density of 1 × 105 cells/ml. Immediately before injection cells were transferred to 10 mM HEPES-buffered serum-free DMEM. cDNA constructs were injected (0.1 mg/ml), together with biotin dextran as a marker, into the nucleus of 50–100 cells in a temperature- (37°C) and CO2- (10%) controlled chamber by using phase contrast microscopy. Cells were returned to the incubator for ∼3 h for optimal expression before phagocytic challenge. COS cell phagocytosis assays to investigate GTPase recruitment were performed by electroporation of cells (as described below) with cDNA encoding human FcγRIIA in combination with GFP-tagged Rac or Cdc42 and myc-tagged dominant negative Vav constructs. Cells were seeded onto glass coverslips and challenged with opsonized targets as previously described (Caron and Hall, 1998; Patel et al., 2000). Recruitment of endogenous Syk in macrophages was analyzed using IgG-opsonized latex beads (May et al., 2000) due to difficulties associated with costaining Syk and red blood cells (RBC).
Immunofluorescence
Cells seeded on coverslips were fixed in cold 4% (wt/vol) paraformaldehyde for 20 min at 4°C before permeabilization with 0.1% Triton X-100 (TX-100)/phosphate-buffered saline (PBS) for 5 min and quenching in 2.7 mg/ml NH4Cl/PBS for 10 min. For immunostaining, cells were incubated with antibodies diluted in PBS for 30 min. Where appropriate all antibody mixes contained excess human IgG (Sigma Chemical) to prevent nonspecific binding to the FcγR. Rhodamine- or Cy5-conjugated donkey anti-rabbit IgG were used to detect opsonized RBC. Myc-tagged constructs were visualized using mouse monoclonal anti-myc (9E10) followed by rhodamine- or fluorescein isothiocyanate-conjugated anti-mouse IgG. F-actin was stained using rhodamine-conjugated phalloidin (Sigma Chemical). Cells microinjected with biotin dextran were detected with aminomethylcoumarin acetate-coupled streptavidin (Molecular Probes, Eugene, OR). Coverslips were mounted in mowiol mountant (Calbiochem, San Diego, CA) containing p-phenylenediamine as an antibleaching agent and images captured using a Bio-Rad MRC 1000 confocal microscope.
Determination of Rho GTPase Activation
COS cells at 70% confluence were trypsinized, washed twice in Hebs buffer (20 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM d-glucose, pH 7.5), resuspended at ∼2 × 106 cells in 250 μl of Hebs containing 20 μg of total plasmid DNA, and electroporated with a single pulse at 250 μF, 280 V by using a Bio-Rad gene pulser. Cells were seeded onto 10-cm dishes and 16 h after transfection were serum starved for 5 h before eliciting phagocytic challenge.
For Fcγ receptor pulldown assays, 15 μl of RBC (10% suspension; ICN Biomedicals, Costa Mesa, CA), opsonized with IgG (Patel et al., 2000), was resuspended in 3 ml of cold serum-free medium and allowed to adhere to cells for 15 min at 4°C. COS cells were washed once to remove unbound particles before incubation at 37°C. At different time points, COS cells were washed twice in ice-cold 50 mM Tris, 150 mM NaCl, pH 8 and lysed by scraping on ice in radioimmunoprecipitation assay buffer (50 mM Tris, 150 mM NaCl, 1% TX-100, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM MgCl2, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin/leupeptin, pH 8). Lysates were cleared by centrifugation at 14,000 rpm for 2 min at 4°C, an aliquot saved to assess total GTPase levels, and the remaining lysates divided in two to assess levels of active Cdc42 and Rac. Lysates were incubated for 45 min at 4°C with 20 μg of a 50% slurry of PAK-CRIB coupled to glutathione agarose beads to precipitate GTPases. Beads were subsequently washed three times in cold wash buffer (50 mM Tris, 150 mM NaCl, 1% TX-100, 10 mM MgCl2, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin/leupeptin, pH 8). Equal amounts of beads and total cell lysates were analyzed by SDS-PAGE and immunoblotting, by using either mouse monoclonal anti-Cdc42 or mouse monoclonal anti-Rac clone 23A8. Fold activation of Cdc42 and Rac was assessed, relative to levels of Cdc42 and Rac in the total cell lysate, by quantification of autoradiographic exposures by using Quantity One (Bio-Rad) software.
RESULTS
Vav Is Recruited to FcγR but Not CR3 Phagosomes
The murine macrophage cell line J774.A1 was used to investigate the role of Vav during FcγR- and CR3-mediated phagocytosis. In resting cells, endogenous Vav is distributed throughout the cytosol and enriched along with F-actin in lamellipodia at the leading edge (Figure 1, A–C). No Vav2 could be detected in J774 cells by Western blotting (our unpublished data). To examine the role of Vav in phagocytosis, its distribution in response to challenge by sheep RBC was studied. The RBC were opsonized with either IgG or C3bi complement fragments, to direct phagocytosis specifically through the Fcγ or CR3 receptors, respectively (Caron and Hall, 1998).
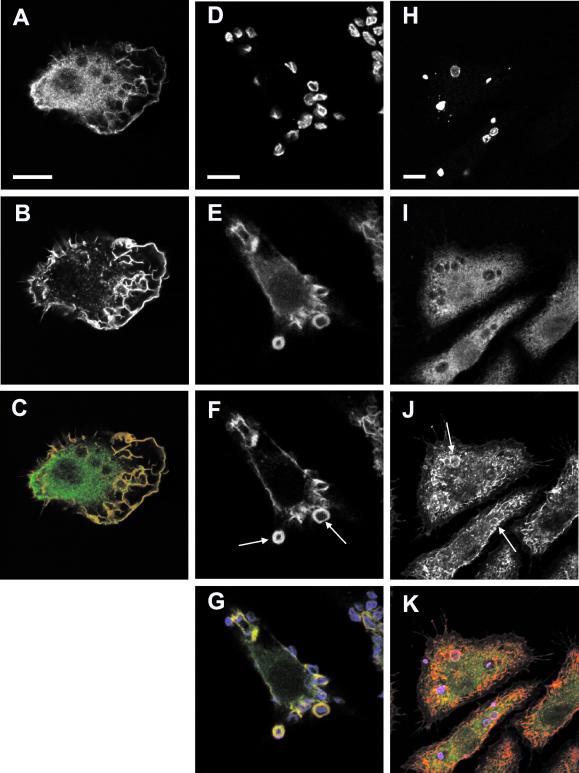
Figure 1.
Endogenous Vav is recruited to phagosomes during FcγR- but not CR3-mediated phagocytosis in J774.A1 macrophages. (A–C) In resting cells Vav (A) and F-actin (B) colocalize mainly at lamellipodia and dorsal ruffles. (C) Merged image showing Vav in green and F-actin in red, colocalization is seen in yellow. (D–G) FcγR-mediated phagocytosis of IgG-opsonized RBC (D) triggers a localized recruitment of Vav (E) to nascent phagosomes where it colocalizes with F-actin (F). In contrast, during CR3 phagocytosis (H–K) C3bi-opsonized RBC (H) fail to accumulate Vav (I) at the sites of attachment, despite inducing F-actin (J) reorganization. (G and K) Merged images showing RBC in blue, Vav in green, and F-actin in red. Arrows indicate sites of localized actin remodeling. Images are representative of cells from at least five independent experiments. Bar, 10 μm.
During FcγR-mediated phagocytosis, endogenous Vav was recruited to nascent phagosomes, where it colocalized with F-actin (Figure 1, D–G). This local enrichment of Vav was transient and coincided with RBC engulfment because macrophages incubated at 4°C, and thus unable to signal downstream of the FcγR (Griffin et al., 1975), failed to recruit Vav to sites of particle attachment (our unpublished data). Similar studies were done using C3bi-coated RBC to investigate CR3-mediated uptake. As before, engulfment was accompanied by local polymerization of F-actin beneath bound targets (Figure 1, H–K). However, no specific enrichment of Vav around bound targets could be seen at any stage of phagocytosis. This suggested a possible role for Vav during FcγR, but not CR3-mediated phagocytosis in macrophage cells.
Vav Is Required for FcγR-mediated Phagocytosis
To determine whether Vav activity was necessary for FcγR-mediated phagocytosis, we examined the effects of inhibiting its function in macrophages. Two previously described dominant negative mutants of Vav were used (Wu et al., 1996; Gringhuis et al., 1998; Ma et al., 1998): Vav-C comprising just the carboxy-terminal SH3-SH2-SH3 domains and VavΔDH containing a deletion in the catalytic DH domain (see MATERIALS AND METHODS). Macrophages microinjected with Vav DNA constructs were challenged with IgG- or C3bi-opsonized RBC and scored for their ability to bind and internalize targets.
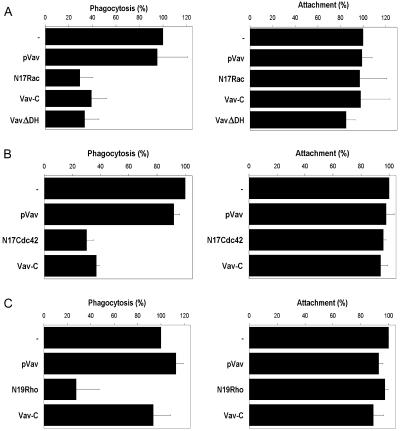
Cells expressing full-length, wild-type Vav were as competent as control cells in binding and internalizing IgG- (Figure 2, A and B) or C3bi- (Figure 2C) coated RBC. In contrast, macrophages expressing Vav-C or VavΔDH exhibited a marked reduction in their FcγR-dependent phagocytic potential (Figure 2A). The degree of inhibition was comparable to that seen when inhibiting endogenous Cdc42 (our unpublished data) or Rac function (Figure 2A), each of which have previously been shown to be essential for FcγR-directed uptake (Cox et al., 1997; Caron and Hall, 1998; Massol et al., 1998). Despite the phagocytic defect, the target binding capacity of macrophages was unaffected, suggesting that Vav function is not necessary for efficient clustering or recycling of the FcγR. Similar results were observed in COS cells, which have been extensively used as an alternative model for studying phagocytosis after transfection with the FcγR (Downey et al., 1999) (Figure 2B). Although the hematopoietic-specific form of Vav (Vav1) is not expressed in COS cells (Bustelo, 2000; our unpublished data), it has been proposed that VavΔDH can interfere with the activity of the very closely related GEFs Vav2 and Vav3 (Ma et al., 1998). To assess the specificity of the dominant negative Vav constructs, we examined their effects on CR3-dependent phagocytosis in macrophages (Figure 2C). Consistent with a lack of Vav recruitment, expression of the dominant negative Vav-C mutant had no effect on CR3-mediated phagocytosis. In contrast, blocking Rho function effectively abolished engulfment as previously reported (Caron and Hall, 1998).
Figure 2.
Vav exchange activity is required for FcγR- but not CR3-mediated phagocytosis. The effect of expressing different Vav or GTPase constructs on the attachment (right) and phagocytosis (left) of IgG- or C3bi-opsonized RBC was analyzed. Results are shown relative to expression of a control vector (−). Dominant negative Vav constructs (Vav-C and VavΔDH) but not full-length Vav (pVav) inhibited FcγR-mediated phagocytosis in J774.A1 macrophages (A) and receptor-transfected COS cells (B) to levels comparable to blocking Rac (N17Rac) or Cdc42 (N17Cdc42) function. In contrast, dominant negative Vav had no effect on CR3 phagocytosis in J774 macrophages (C). Data shown are the mean ± SEM of at least three independent experiments.
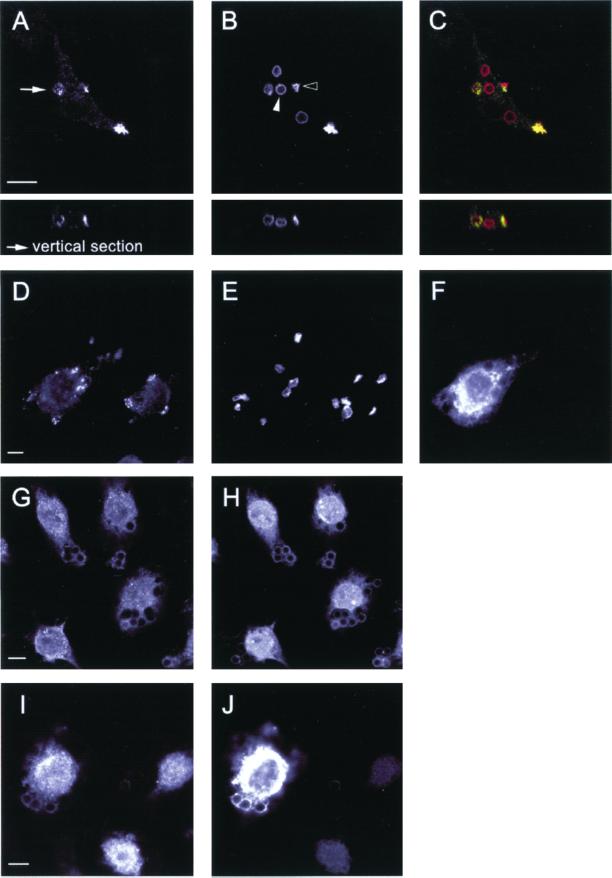
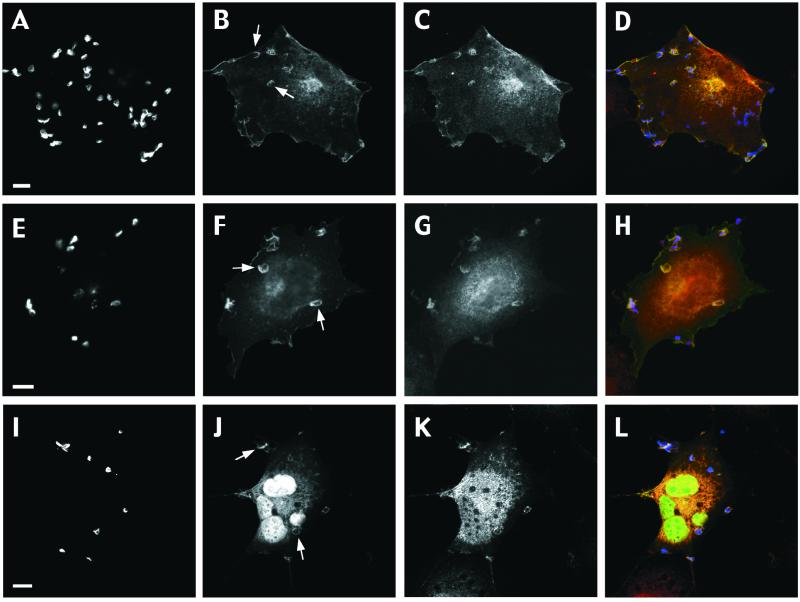
The polymerization and remodeling of F-actin necessary for phagocytosis is absolutely dependent on tyrosine phosphorylation (Greenberg et al., 1993). After target binding and receptor clustering, several tyrosine-phosphorylated proteins, including the FcγR itself, transiently accumulate at the forming phagosome (Greenberg et al., 1993; Strzelecka et al., 1997). Hence, we investigated whether these receptor proximal signaling events were affected by expression of dominant negative Vav in macrophages. As previously shown, bound RBC (evident by their crenated morphology; Figure 3B, open arrowhead; Greenberg et al., 1993) undergoing FcγR-mediated phagocytosis were accompanied by the underlying punctate accumulation of tyrosine phosphoproteins (Figure 3, A–C). In contrast, fully internalized RBC, which appear as swollen particles within vacuoles (Figure 3B, closed arrowhead; Greenberg et al., 1993), no longer displayed the focal enrichment of tyrosine-phosphorylated proteins. Expression of Vav-C failed to alter the enhanced localized staining for phosphotyrosine proteins despite blocking engulfment (Figure 3, D–F). Moreover, the tyrosine kinase p72Syk, which becomes enriched at nascent phagosomes in response to FcγR ligation (Figure 3, G and H; Strzelecka et al., 1997), exhibited identical recruitment kinetics in macrophages coexpressing Vav-C (Figure 3, I and J). Thus, we conclude that the exchange activity of Vav is necessary for efficient FcγR-mediated phagocytosis in both J774 macrophages and COS cells but is not required for CR3-directed uptake. Moreover, its inhibitory effects appear to lie downstream of initial FcγR-mediated signaling events, given that enrichment of phosphotyrosine-containing proteins and Syk proceeds normally in macrophages expressing dominant negative Vav.
Figure 3.
Early FcγR-mediated signaling and recruitment events proceed normally in macrophages expressing dominant negative Vav. (A–F) FcγR-mediated phagocytosis of RBC is accompanied by the underlying accumulation of phosphotyrosine proteins in control (A–C) and in Vav-C expressing (D–F) macrophages. Endogenous phosphotyrosine proteins (A), RBC (B), and merged image (C) showing RBC in red and endogenous tyrosine-phosphorylated proteins in green. Note the transient nature of the local enrichment in phosphotyrosine staining; in B, open arrowhead indicates bound RBC (phosphotyrosine positive), whereas closed arrowhead shows fully internalized RBC (negative for phosphotyrosine proteins). Expression of Vav-C (F) fails to alter the enrichment of phosphotyrosine proteins (D) beneath bound RBC (E). (G–J) Recruitment of the protein tyrosine kinase Syk, which accompanies FcγR-mediated engulfment in macrophages, proceeds normally in control (G–H) and Vav-C–expressing cells (I–J). Endogenous Syk (G and I), IgG-opsonized latex beads (H), and Vav-C and IgG-opsonized latex beads (both detected in J, due to cross-reactivity of the secondary antibody). Latex beads were used to overcome difficulties in staining for Syk. Representative images from four independent experiments are shown. Bar, 10 μm.
Vav Regulates Rac but Not Cdc42 Activation during Phagocytosis
It has previously been shown that FcγR-mediated phagocytosis requires both Cdc42 and Rac function, although their individual contributions to the process are unknown (Cox et al., 1997; Caron and Hall, 1998; Massol et al., 1998). Because when overexpressed Vav is capable of activating both Cdc42 and Rac (Olson et al., 1996) and Cdc42 is capable of activating Rac in some cell types (Nobes and Hall, 1995; Wojciak-Stothard et al., 1998), the link between Vav, Cdc42, and Rac was examined. To study the kinetics of Cdc42 and Rac activation, cells were challenged with IgG-opsonized RBC for different periods of time. Cell lysates were divided in two and GTP-loaded Cdc42 and Rac were precipitated using the Cdc42/Rac binding domain of p21 activated kinase (PAK-CRIB) fused to glutathione S-transferase (Sander et al., 1999). Initial studies in J774 macrophages revealed high basal levels of GTP-bound Cdc42 and Rac before stimulation with RBC, thus making GTPase activation upon phagocytosis difficult to assess (our unpublished data). Also, the inability to transfect these macrophages made it impossible to biochemically address the role of Vav in GTPase activation in this system, by using the dominant negative Vav mutants. As a result, experiments were performed in COS cells transiently transfected with the FcγR. To synchronize the onset of phagocytosis, targets were allowed to bind to transfected COS cells at 4°C, before inducing engulfment by transferring cells to 37°C.
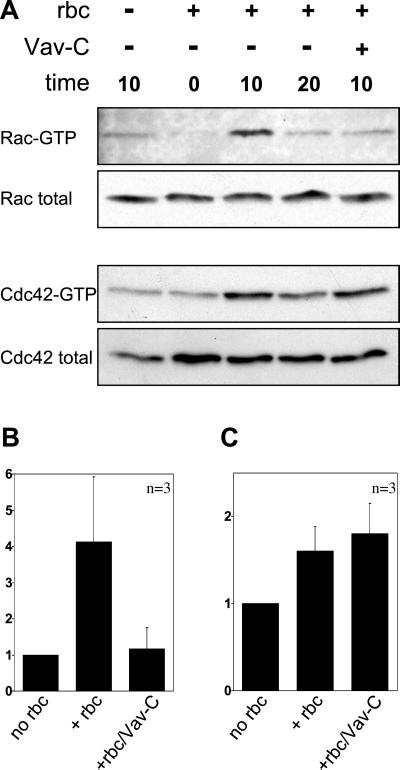
As shown in Figure 4A, progression of FcγR-directed phagocytosis correlated with a transient increase in GTP loading of endogenous Rac and Cdc42. The level of Rac activation peaked after 10 min at 37°C, resulting in a fourfold increase, before returning to basal levels by 20 min (Figure 4, A and B). Endogenous Cdc42 also showed a transient activation after addition of IgG-opsonized RBC (Figure 4A), with an indistinguishable time course from that of Rac, although the fold activation was lower (Figure 4C). The rise in the levels of GTP-bound GTPases was not a consequence of the temperature shift during the experiment, because cells warmed in the absence of targets failed to elicit GTPase activation (Figure 4A, lane 1).
Figure 4.
Vav activates Rac but not Cdc42 in response to FcγR-mediated phagocytosis. (A) Kinetics of GTP loading on Rac and Cdc42 in COS cells after induction of FcγR-directed phagocytosis. Coexpression of Vav-C blocks activation of endogenous Rac but not Cdc42. Fold activation of Rac (B) and Cdc42 (C) based on the 10-min time point is shown relative to levels of Rac and Cdc42 in the total cell lysate. Data shown are the mean ± SEM of at least three independent experiments.
To determine the role of Vav in FcγR phagocytosis, the receptor was coexpressed with dominant negative Vav in COS cells. As shown in Figure 4, A and B, coexpression of dominant negative Vav (Vav-C) completely abolished the FcγR-induced Rac activation seen at the 10-min time point. This inhibition was not a result of reduced surface expression of the Fcγ receptor, as shown by the similar RBC attachment index (Figure 2, A and B). However, expression of dominant negative Vav did not prevent the Cdc42 activation seen upon phagocytic challenge (Figure 4, A and C). We conclude that Vav functions as a Rac-specific GEF during FcγR-mediated phagocytosis.
VavΔDH Does Not Function by Titrating Endogenous Rac
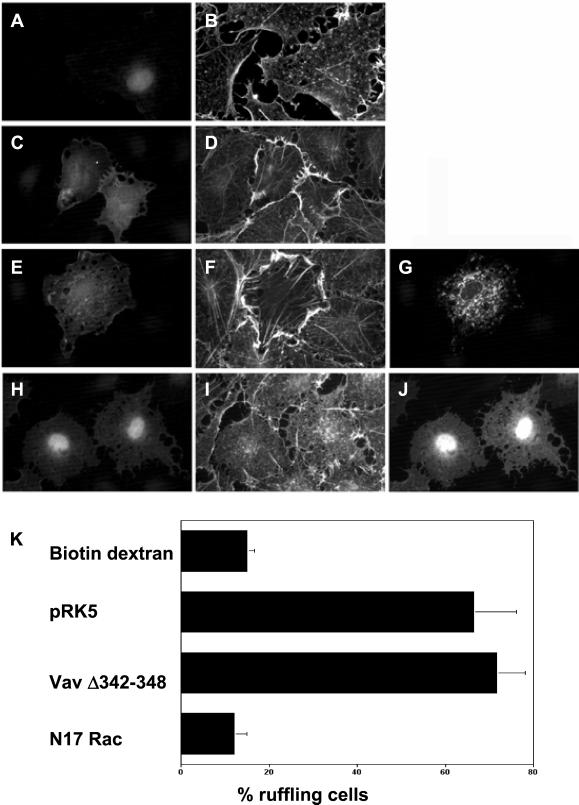
Guanine nucleotide exchange factors are believed to interact with their target Rho GTPases via their DH domains (Aghazadeh et al., 1998; Soisson et al., 1998). To ensure that the phagocytic defects apparent when using the dominant negative VavΔDH mutant were due to a block in Vav exchange activity and not attributable to sequestration of endogenous Rac or a nonspecific effect on Rac-induced actin polymerization, we tested whether VavΔDH was capable of blocking Rac activation in response to a Vav-independent Rac stimulus (Figure 5). We used a constitutively active mutant of Tiam1 (Tiam1C1199), an alternative exchange factor known to function as a Rac-specific GEF and induce lamellipodia when expressed in fibroblasts (Michiels et al., 1995). Hence, Tiam1 and VavΔDH were coinjected (1:1 ratio) into quiescent serum-starved Swiss 3T3 fibroblasts, which lack organized actin filaments. Cells were seen to elicit cytoskeletal changes indicative of Rac activation, namely, peripheral ruffling (Figure 5F) (Ridley et al., 1992). Indeed, the phenotype was indistinguishable from control cells expressing Tiam1 and empty vector (Figure 5D). In contrast, coexpression of a dominant negative Rac mutant (N17Rac) completely inhibited, as expected, the Tiam1-induced lamellipodia (Figure 5I) with cells appearing identical to control biotin dextran-injected fibroblasts (Figure 5B). Thus, the effects of the VavΔDH construct on phagocytosis are not attributable to titration of endogenous Rac protein or nonspecific effects on actin assembly.
Figure 5.
VavΔDH does not act by sequestering endogenous Rac protein or inhibiting Rac-induced actin polymerization. Quiescent serum-starved S3T3 fibroblasts were microinjected with biotin dextran (A and B), or cDNAs encoding HA-tagged Tiam1 and either empty vector (C and D), myc-tagged VavΔDH (E–G), or myc-tagged N17Rac (H–J). Cells were stained for F-actin (B, D, F, and I), HA-tagged constructs (C, E, and H), and myc-tagged constructs (G and J). Representative images from three independent experiments are shown. (K) Quantitation of cells showing a ruffling phenotype after expression of the indicated constructs. Data shown are the mean ± SEM of at least three independent experiments. Bar, 10 μm.
Inactive Rac Is Recruited to Phagosomes
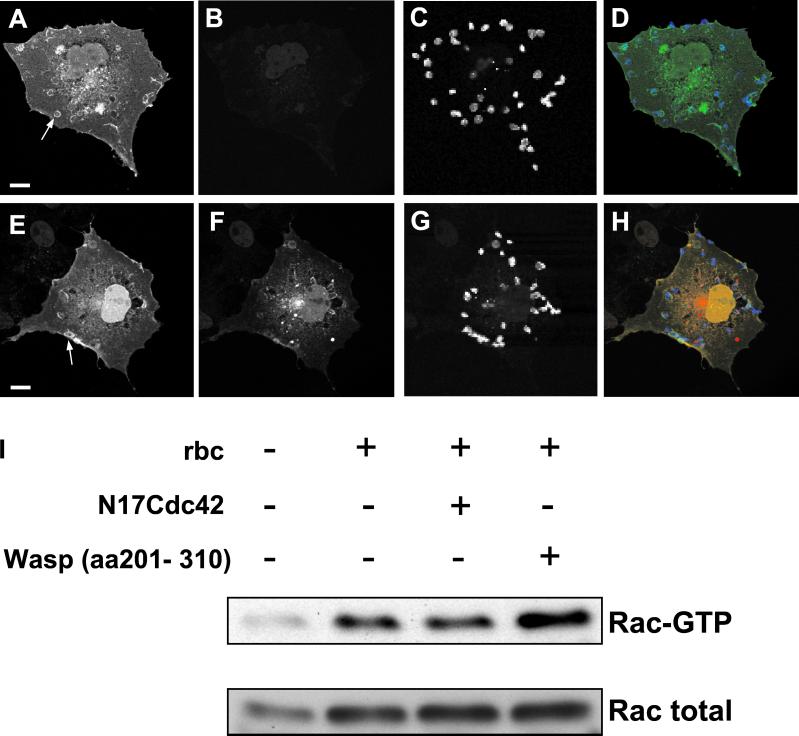
The spatially localized remodeling of the actin cytoskeleton that accompanies phagocytosis provides an ideal opportunity for investigating the recruitment of signaling components. Although the accumulation of Rho GTPases at nascent phagosomes has been previously shown, the mechanisms of recruitment remain unclear (Caron and Hall, 1998). To examine whether membrane targeting of Cdc42 or Rac is dependent on Vav exchange activity, we studied the recruitment of GFP-tagged GTPases to nascent phagosomes in COS cells coexpressing FcγR and dominant negative Vav. Recruitment of both wild-type Cdc42 (Figure 6, A–D) and Rac (Figure 6, E–H) to forming phagosomes was unaffected by either Vav-C or VavΔDH (our unpublished data). Moreover, GFP-tagged N17Rac, which mimics the inactive form of the GTPase, was recruited beneath bound RBC in Vav-C–expressing cells (Figure 6, I–L). Both Vav mutants colocalized with Rac and Cdc42 at sites of RBC attachment, suggesting that the SH2 domain, which has been proposed to play a key role in receptor complex association (Arudchandran et al., 2000), may direct the accumulation of the Vav mutants at sites of RBC attachment. Because dominant negative Vav prevents GTP loading on Rac, we conclude that the Rac recruitment seen in Figure 6F must be the inactive GDP-bound form. The enrichment of GFP-tagged N17Rac to nascent phagosomes in cells expressing dominant negative Vav strengthens this conclusion.
Figure 6.
Rac recruitment to phagosomes occurs independently of activation by Vav. Recruitment of GFP-Cdc42 (A–D), GFP-Rac (E–H), or GFP-N17Rac (I–L) to sites of RBC attachment in COS cells cotransfected with FcγR and dominant negative Vav. RBC (A, E, and I), GFP-Cdc42 (B), GFP-Rac (F), GFP-N17Rac (J), and Vav-C (C, G, and K). (D, H, and L) Merged images showing GFP-Cdc42, GFP-Rac, or GFP-N17Rac in green; RBC in blue; and Vav-C in red. Arrows indicate sites of GTPase recruitment. Images are representative of cells from at least three independent experiments. Bar, 10 μm.
FcγR-induced Rac Recruitment and Activation Are Cdc42 Independent
Cdc42 has been shown to be a potent activator of Rac in several cell types (Nobes and Hall, 1995; Wojciak-Stothard et al., 1998). To determine whether activation of Cdc42 leads to a sequential activation and/or recruitment of Rac during phagocytosis, we investigated FcγR-induced recruitment and GTP loading of Rac in the presence of a dominant negative Cdc42 mutant. As shown in Figure 7, A–D, Rac localized to discrete foci beneath FcγR-attached particles in COS cells. Recruitment was transient and maximal at 10 min coincident with GTP loading (our unpublished data). Coexpression of a dominant negative Cdc42 mutant (N17Cdc42), which also accumulated at sites of RBC attachment, did not prevent recruitment of Rac (Figure 7, E–H) or of full-length Vav (our unpublished data). Finally, inhibition of Cdc42 function, by using either the N17Cdc42 mutant or a fragment of the Cdc42 effector Wasp (aa 201–310), failed to block Rac GTP loading in response to phagocytic stimuli (Figure 7I). Thus, ligation of the Fcγ receptor triggers the independent recruitment and activation of Cdc42 and Rac.
Figure 7.
FcγR-induced recruitment and activation of Rac is independent of Cdc42 function. Recruitment of GFP-Rac to nascent phagosomes in COS cells coexpressing FcγR and either empty vector (A–D) or myc-tagged N17Cdc42 (E–H). GFP-Rac (A and E), myc (B and F), and RBC (C and G) were stained and appear green, red, and blue, respectively, on the merged images (D and H). Arrows indicate sites of GFP-Rac recruitment. Images are representative of cells from at least three independent experiments. Bars, 10 μm. (I) GTP-loading on Rac in response to FcγR-induced phagocytosis is not blocked by inhibiting Cdc42 function with N17Cdc42 or the Cdc42 binding domain of Wasp (aa 201–310).
DISCUSSION
The engulfment of targets by phagocytic cells involves a complex signaling cascade that drives localized actin remodeling in a manner controlled by Rho GTPases. In previous work we and others have reported that both Cdc42 and Rac are essential for FcγR-induced phagocytosis (Cox et al., 1997; Caron and Hall, 1998; Massol et al., 1998). We have now identified a unique role for the guanine nucleotide exchange factor Vav during FcγR-mediated phagocytosis. In response to FcγR ligation, Vav translocates to sites of particle attachment, where it regulates the activation of Rac to induce engulfment. Although Vav has been reported to be capable of activating other Rho GTPases, it does not regulate Cdc42 during FcγR-mediated phagocytosis (this study), nor does it regulate Rho in CR3-directed uptake (our unpublished data).
It is currently thought that upon ligand binding, FcγR is phosphorylated on tyrosine residues at immunoreceptor tyrosine-based activation motifs by Src family kinases. This allows docking and activation of the tyrosine kinase p72Syk, which subsequently triggers signaling events leading to engulfment (Darby et al., 1994; Greenberg et al., 1996). Vav is a complex 95-kDa, multidomain molecule that can interact, through its SH2 domain, directly with Syk (Deckert et al., 1996). Vav can be phosphorylated by Syk or the Src kinase Lck, leading to increased exchange activity in vitro and following overexpression in vivo (Crespo et al., 1997). A similar pathway has been reported in T-cell receptor signaling where the Syk homolog Zap-70 targets Vav to clustered receptors at the membrane (Salojin et al., 2000). However, Vav1, Syk, and Zap70 are hematopoietic-specific (Law et al., 1994; Bustelo, 2000), yet FcγR will promote efficient tyrosine kinase-dependent phagocytosis when expressed in COS cells (Indik et al., 1995). Furthermore, FcγR clustering still induces tyrosine phosphorylation of Vav in macrophages derived from Syk-deficient mice (Crowley et al., 1997). These results suggest that signaling pathways used by FcγR to activate Rac and Cdc42 are essentially conserved in nonhematopoietic cells and that there is redundancy in some of the components of the pathways. Our results show that Vav is recruited to nascent phagosomes and that its activity is essential for FcγR phagocytosis in macrophages and in COS cells expressing FcγR. We can conclude from this that in COS cells one or both of the close relatives, Vav2 or Vav3, fulfill the same function as Vav in coupling FcγR to Rac. These two additional Vav family members both have high sequence homology to the hematopoietic-specific Vav, but they exhibit a broader expression profile (Bustelo, 2000). Furthermore, recent work with Vav knockout mice reveals functional compensatory effects by Vav2 during B-cell receptor signaling in B cells (Tedford et al., 2001).
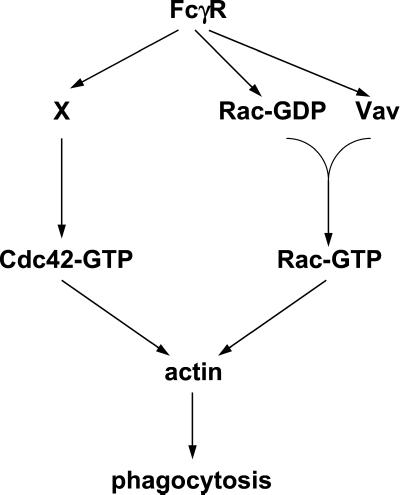
The exchange activity of Vav for Rho GTPases has been well documented, although its specificity in vivo remains unresolved. Expression of truncated versions of Vav in Swiss 3T3 fibroblasts leads to activation of Cdc42, Rac, and Rho (Olson et al., 1996), whereas in vitro GEF assays suggest a preference for Rac and Rho (Crespo et al., 1997). In T cells Vav is essential for TCR-dependent activation of Rac, although it is unknown whether it also activates Cdc42 and/or Rho in this context (Salojin et al., 1999). We have now shown that Vav acts as a Rac-specific exchange factor downstream of FcγR. Although FcγR also activates Cdc42, this is not mediated by Vav and the exchange factor involved is unknown. Furthermore, we have found that Cdc42 is not required for FcγR-induced Rac activation, or Rac translocation. This rules out the possibility that the only role of Cdc42 is to activate Rac and leads us to the conclusion that Rac and Cdc42 control distinct biochemical steps in the phagocytic process as postulated by Massol et al. (1998) (Figure 8). Accordingly, inhibition of both Cdc42 and Rac results in a greater inhibition of FcγR-mediated phagocytosis than blocking the function of either GTPase alone (Caron and Hall, 1998).
Figure 8.
Model for the role of Vav in FcγR-mediated phagocytosis. Activation and recruitment of Cdc42 and Rac during FcγR-mediated phagocytosis occur via independent mechanisms. Rac is recruited to nascent phagosomes in its GDP-bound form where it is locally activated by Vav. Rac recruitment may occur independently of Vav (as shown) or via an indirect association with the SH3/SH2 domains of Vav. Once activated, both Rac and Cdc42 direct the actin remodeling required to complete phagocytosis.
The experiments we report herein also begin to address another poorly understood aspect of Rho GTPase activation. We have found that Rac is recruited to sites of particle attachment before activation by Vav, suggesting that there is a receptor-mediated mechanism for recruiting the inactive, GDP-bound form of Rac. How this occurs is unknown. Two gene products acting upstream of Rac, Ced2/CrkII and Ced-5/DOCK180, have been identified through the genetic analysis of apoptotic cell uptake in C. elegans (Reddien and Horvitz, 2000). Neither is capable of activating Rac directly and so far, no GEF has been isolated using the genetic screens. However, DOCK180 has been reported to couple the integrin αvβ5 to Rac activation during uptake of apoptotic cells in mammalian cells (Albert et al., 2000). What is very interesting is that DOCK180 can interact directly with Rac (Nolan et al., 1998). Therefore, one possibility is that DOCK180 mediates recruitment of Rac.GDP to FcγR where it is activated by Vav. Alternatively, the SH3-SH2-SH3 domains of Vav (i.e., Vav-C) may recruit Rac.GDP indirectly, perhaps via DOCK180 or another protein interaction. Several groups have shown that the inactive form of Rac exists in a cytosolic complex with RhoGDI and that GEFs are unable to promote nucleotide exchange while Rac is in this complex (Hancock and Hall, 1993; Longenecker et al., 1999). Whether RhoGDI remains bound, or is induced to dissociate, for example, by the interaction with ezrin/radixin/moesin family proteins (Takahashi et al., 1997), which are found at phagosomes (Defacque et al., 2000), is unknown. Moreover, it has recently been reported that the amino terminus of Vav can interact directly with RhoGDI (Groysman et al., 2000), although because Rac.GDP is still recruited to nascent phagosomes in the presence of Vav-C, this is unlikely to be the mechanism involved herein. We are currently investigating the mechanisms of Rac recruitment to phagosomes.
In conclusion, we have shown that Vav specifically regulates the activation of Rac, but not Cdc42, during FcγR-mediated phagocytosis and plays no role in the Rho-dependent CR3-mediated engulfment. Cdc42 and Rac are recruited and activated at FcγR-dependent phagosomes through distinct pathways, suggesting that they regulate different aspects of the phagocytic process. Finally, Rac is recruited to forming phagosomes in its GDP-bound form before activation by Vav.
ACKNOWLEDGMENTS
We thank A. Schmidt and A. Jaffe for discussions and M. Shipman for technical assistance. J.P is supported by a Medical Research Council Ph.D. fellowship. E.C and A.H are supported by a Wellcome Trust project grant.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–01-0002. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–01–0002.
REFERENCES
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Aghazadeh B, Zhu K, Kubiseski TJ, Liu GA, Pawson T, Zheng Y, Rosen MK. Structure and mutagenesis of the Dbl homology domain. Nat Struct Biol. 1998;5:1098–1107. doi: 10.1038/4209. [DOI] [PubMed] [Google Scholar]
- Albert ML, Kim JI, Birge RB. αvβ5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- Arudchandran R, Brown MJ, Peirce MJ, Song JS, Zhang J, Siraganian RP, Blank U, Rivera J. The Src homology 2 domain of Vav is required for its compartmentation to the plasma membrane, and activation of c-Jun NH(2)-terminal kinase 1. J Exp Med. 2000;191:47–60. doi: 10.1084/jem.191.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Castellano F, Montcourrier P, Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. 2000;113:2955–2961. doi: 10.1242/jcs.113.17.2955. [DOI] [PubMed] [Google Scholar]
- Castellano F, Montcourrier P, Guillemot JC, Gouin E, Machesky L, Cossart P, Chavrier P. Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr Biol. 1999;9:351–360. doi: 10.1016/s0960-9822(99)80161-4. [DOI] [PubMed] [Google Scholar]
- Chimini G, Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat Cell Biol. 2000;2:E191–E196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby C, Geahlen RL, Schreiber AD. Stimulation of macrophage Fc gamma RIIIA activates the receptor-associated protein tyrosine kinase Syk and induces phosphorylation of multiple proteins including p95Vav and p62/GAP-associated protein. J Immunol. 1994;152:5429–5437. [PubMed] [Google Scholar]
- Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, Broek D. Control of intramolecular interactions between the pleckstrin homology, and Dbl homology domains of Vav, and Sos1 regulates Rac binding. J Biol Chem. 2000;275:15074–15081. doi: 10.1074/jbc.M907269199. [DOI] [PubMed] [Google Scholar]
- Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- Defacque H, et al. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey GP, Botelho RJ, Butler JR, Moltyaner Y, Chien P, Schreiber AD, Grinstein S. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcγRIIA receptors. J Biol Chem. 1999;274:28436–28444. doi: 10.1074/jbc.274.40.28436. [DOI] [PubMed] [Google Scholar]
- Ernst JD. Bacterial inhibition of phagocytosis. Cell Microbiol. 2000;2:379–386. doi: 10.1046/j.1462-5822.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S, Chang P, Wang DC, Xavier R, Seed B. Clustered syk tyrosine kinase domains trigger phagocytosis. Proc Natl Acad Sci USA. 1996;93:1103–1107. doi: 10.1073/pnas.93.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin FM, Jr, Griffin JA, Leider JE, Silverstein SC. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, de Leij LF, Coffer PJ, Vellenga E. Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in human mature T lymphocytes. Mol Cell Biol. 1998;18:1725–1735. doi: 10.1128/mcb.18.3.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groysman M, Russek CS, Katzav S. Vav, a GDP/GTP nucleotide exchange factor, interacts with GDIs, proteins that inhibit GDP/GTP dissociation. FEBS Lett. 2000;467:75–80. doi: 10.1016/s0014-5793(00)01121-2. [DOI] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Hall A. A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 1993;12:1915–1921. doi: 10.1002/j.1460-2075.1993.tb05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–4399. [PubMed] [Google Scholar]
- Isomura M, Kikuchi A, Ohga N, Takai Y. Regulation of binding of rhoB p20 to membranes by its specific regulatory protein, GDP dissociation inhibitor. Oncogene. 1991;6:119–124. [PubMed] [Google Scholar]
- Kwiatkowska K, Sobota A. Signaling pathways in phagocytosis. Bioessays. 1999;21:422–431. doi: 10.1002/(SICI)1521-1878(199905)21:5<422::AID-BIES9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Law CL, Sidorenko SP, Chandran KA, Draves KE, Chan AC, Weiss A, Edelhoff S, Disteche CM, Clark EA. Molecular cloning of human Syk. A B cell protein-tyrosine kinase associated with the surface immunoglobulin M-B cell receptor complex. J Biol Chem. 1994;269:12310–12319. [PubMed] [Google Scholar]
- Leverrier Y, Ridley AJ. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol. 2001;11:195–199. doi: 10.1016/s0960-9822(01)00047-1. [DOI] [PubMed] [Google Scholar]
- Longenecker K, et al. How RhoGDI binds Rho. Acta Crystallogr D Biol Crystallogr. 1999;55:1503–1515. doi: 10.1107/s090744499900801x. [DOI] [PubMed] [Google Scholar]
- Ma AD, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase gamma, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol P, Montcourrier P, Guillemot JC, Chavrier P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 1998;17:6219–6229. doi: 10.1093/emboj/17.21.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- May RC, Machesky LM. Phagocytosis, and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–40. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nolan KM, Barrett K, Lu Y, Hu KQ, Vincent S, Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MF, Pasteris NG, Gorski JL, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- Patel JC, Hall A, Caron E. Rho GTPases, and macrophage phagocytosis. Methods Enzymol. 2000;325:462–473. doi: 10.1016/s0076-6879(00)25466-9. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Horvitz HR. CED-2/CrkII, and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Salojin KV, Zhang J, Delovitch TL. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J Immunol. 1999;163:844–853. [PubMed] [Google Scholar]
- Salojin KV, Zhang J, Meagher C, Delovitch TL. ZAP-70 is essential for the T cell antigen receptor-induced plasma membrane targeting of SOS, and Vav in T cells. J Biol Chem. 2000;275:5966–5975. doi: 10.1074/jbc.275.8.5966. [DOI] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self AJ, Hall A. Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 1995;256:3–10. doi: 10.1016/0076-6879(95)56003-3. [DOI] [PubMed] [Google Scholar]
- Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J. Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell. 1998;95:259–268. doi: 10.1016/s0092-8674(00)81756-0. [DOI] [PubMed] [Google Scholar]
- Strzelecka A, Pyrzynska B, Kwiatkowska K, Sobota A. Syk kinase, tyrosine-phosphorylated proteins and actin filaments accumulate at forming phagosomes during Fcγ receptor-mediated phagocytosis. Cell Motil Cytoskeleton. 1997;38:287–296. doi: 10.1002/(SICI)1097-0169(1997)38:3<287::AID-CM7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- Tedford K, Nitschke L, Girkontaite I, Charlesworth A, Chan G, Sakk V, Barbacid M, Fischer KD. Compensation between Vav-1, and Vav-2 in B cell development, and antigen receptor signaling. Nat Immunol. 2001;2:548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- Turner M, Mee PJ, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VL. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wu J, Motto DG, Koretzky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]