Abstract
BACKGROUND:
Metformin is the only oral therapy for youth with type 2 diabetes, but up to 50% require additional agents within 2 years of diagnosis. Extended-release (XR) metformin formulations may improve adherence and tolerability-- important mediators of treatment response-- but data in youth is lacking.
OBJECTIVES:
To evaluate rates of gastrointestinal (GI) symptoms in patients treated with metformin (SR and XR) and the change in GI symptoms after changes in metformin therapy.
METHODS:
Retrospective chart review of youth with Type 2 or prediabetes seen in a multidisciplinary clinic during 2016–2019.
RESULTS:
Of 488 eligible patients, 41.4% and 21.1% were taking metformin SR and XR respectively, with most (58%, n=178/305) taking a total daily dose of ≥ 1500mg/day. Those not on metformin tended to be younger, leaner, and had lower HbA1cs than those taking metformin, P<0.05. 30% of patients described GI symptoms, most commonly, abdominal pain and diarrhea. There was no difference in GI symptoms in those on SR vs XR (18.3% vs. 14.6%, P=0.41). Among patients who initiated metformin, rates of GI symptoms increased (13% to 33%, P=0.001, n=99), while rates tended to decrease when metformin was discontinued (28% to 12%, P=0.076, n=50). Rates of GI symptoms were unchanged among those that switched from SR to XR metformin (17% vs 14%, P=0.6, n=58).
CONCLUSIONS:
GI symptoms are common in youth with type 2 diabetes taking metformin XR and SR. Adjuncts to mitigate GI symptoms in youth on metformin therapy are needed to improve quality of life and medication adherence.
Keywords: type 2 diabetes, metformin, pediatrics, adverse effects, gastrointestinal
BACKGROUND
Metformin is the only first-line oral medication approved by the Federal Drug Administration for treatment of type 2 diabetes (T2DM) in children [1, 2]. However, the clinical response to metformin monotherapy in youth, defined as a reduction in glycemia, is highly variable both in randomized controlled trials and in real-world clinical centers. At diagnosis, up to half of youth require intensive insulin therapy [3], and a similar percentage who are treated with metformin monotherapy require additional anti-hyperglycemic agents within 2 years of diagnosis [4]. The reduced long-term efficacy of metformin in youth starkly contrasts data in adults. On average, ~80% of adults maintained glycemic control on metformin monotherapy over a 5-year period [5], suggesting that pediatric-specific factors may play an important role.
Though there are likely many contributions to this disparity, including both psychosocial and physiologic factors, medication adherence is a key determinant of treatment responsiveness in the pediatric population, as higher HbA1c and poorer glycemic control has been linked with reduced metformin adherence in adolescence [6]. Specifically, suboptimal metformin adherence in youth may be the result of 2 key factors: metformin is associated with high rates of gastrointestinal (GI) side effects, and when using the standard release (SR) formulation, the twice daily dosing may be associated with significant medication burden [7]. One popular clinical approach to minimize the burden is to prescribe the once daily extended release (XR) formulation, which has similar efficacy and safety in adult trials. However, some [8, 9], but not all [10–13], trials demonstrate an improvement in side effects when using the XR formulation in adults with diabetes. Further, a head-to-head comparator trial of metformin SR and XR formulations in youth is lacking, and whether XR formulations are associated with a meaningful reduction in GI symptomatology in youth is unclear. Critical evaluation of the clinical response and acceptability of metformin XR formulation in youth is needed since some XR formulations are significantly more expensive than SR and improving medication adherence is important for reducing the growing burden of type 2 diabetes in youth.
In this retrospective analysis we determined the prevalence of GI symptoms in youth followed in a tertiary care prediabetes and diabetes clinic. In a cross-sectional analysis, we compared the rates of symptoms in patients treated with metformin (SR and XR) to those not on metformin treatment at their most recent visit. In a subset of youth, we evaluated the change in GI symptomatology and its association with changes in metformin therapy over time.
METHODS
The protocol was approved by the Institutional Review Board of the Children’s National Hospital (CNH). A retrospective chart review was performed for all patients with T2DM or pre-diabetes who were evaluated in the outpatient diabetes multi-disciplinary clinic at CNH and had at least 1 visit between January 2016 to May 2019. This date range was selected because it coincided with the transition to ICD-10 billing and coding system and the formalization of the multidisciplinary clinic practice management guideline modeled after the American Diabetes Association Standard of Care guidelines. Practitioners followed the guideline that recommended metformin as first-line therapy for treatment in youth with type 2 diabetes who have modest hyperglycemia, i.e. hemoglobin A1c <9%, starting with 500mg and gradually increasing the dose over the course of 2–4 weeks [14]. Patients were identified according to the following ICD-10 diagnosis codes: E11.X (type 2 diabetes) and E73.03 (pre-diabetes) during the study period, and data were extracted into and managed using REDCap® electronic data capture tools hosted at CNH [15]. A total of 2827 encounters were identified for 829 patients. After exclusion of clinic visits unrelated to prediabetes or T2DM, as well as those with no documentation of the absence or presence of GI symptoms in the history or in the review of systems sections, complete data was available for 488 patients at the most recent clinic visit (Figure 1). Electronic charts were reviewed and the following data collected for each clinic visit: age, race, ethnicity, weight, blood pressure, labs including Hba1c, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), diagnosis of diabetes or prediabetes, metformin formulation and dose, use of other diabetes and anti-hypertensive medications. In youth (n=9) with HbA1c >14% (the upper limit of the clinical assay, DCA Vantage Analyzer, Siemens, Malvern, PA), a value of 14.1% was used for analysis. Data on GI symptoms were extracted from the charts if current history or review of systems sections were documented. A standard clinical review of systems questionnaire was given to all clinic patients and a provider template was used to systematically ask about the presence of GI side effects and adherence in patients who were taking metformin. The GI symptoms that were designated a priori and recorded were: diarrhea, abdominal pain/cramps, constipation, nausea, retching/vomiting, bloating, decreased appetite, and “other”. The “other” category included the following complaints recorded in the medical record: “stomach upset”, “GI upset”, “GI symptoms - does not tolerate well”, “abdominal discomfort”, as well as “decreased appetite”. Metformin adherence was categorized by reported missed doses per week – 0–1 doses, 2–4 doses, or 5 or more doses. Adherence was not documented in 81/305 youth taking metformin.
Figure 1:
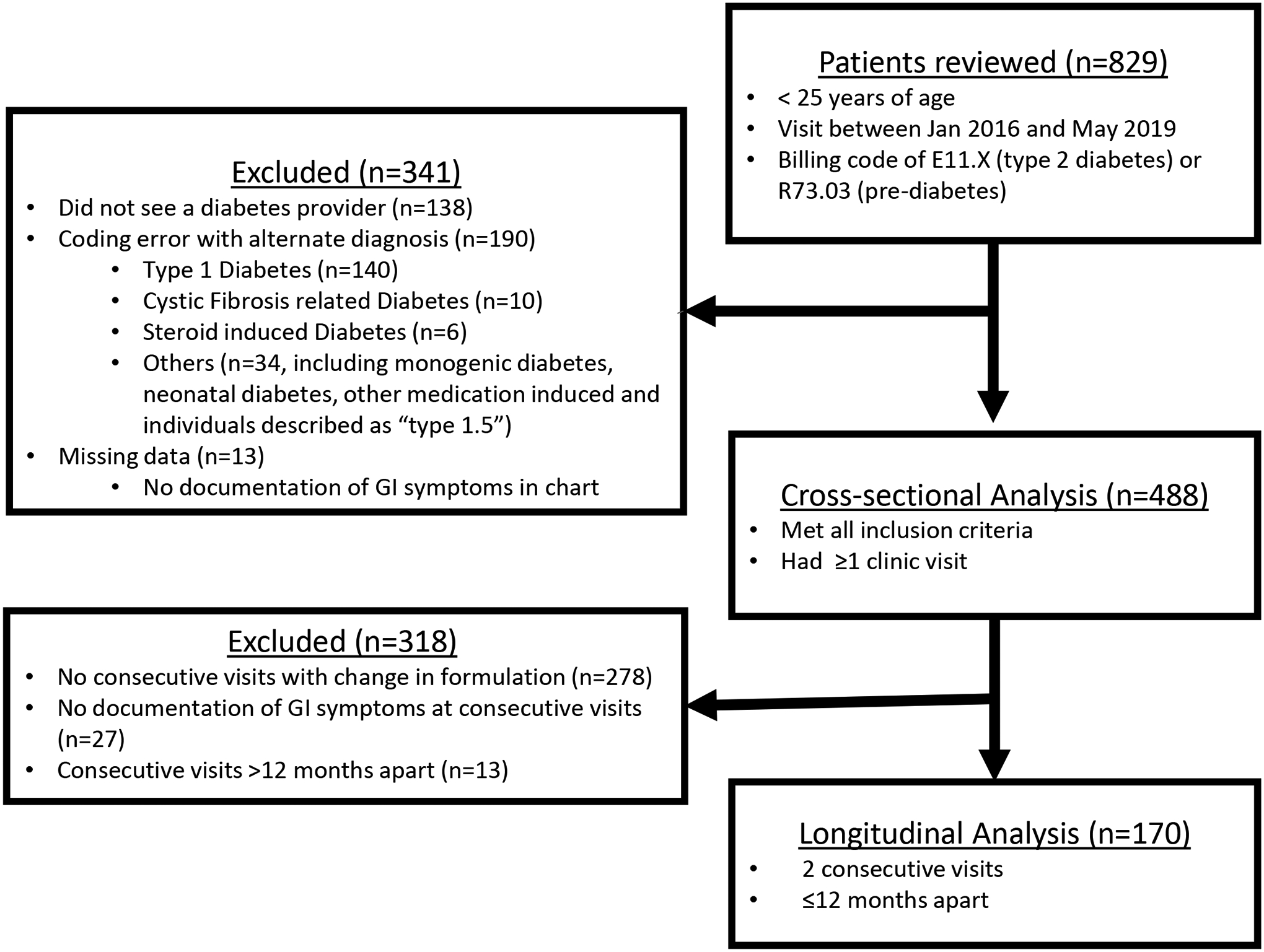
Patient flow diagram.
In a subset of 170 youth who had 2 consecutive visits within ≤12 months and longitudinal data on metformin change, we determined the change in GI symptoms when metformin was initiated (n=99), switched from metformin SR to metformin XR (n=58), and discontinued (n=50), Figure 1. Some of these 170 individuals experienced 2 or more of these changes in medication throughout the study period (see supplemental figure 1). Patients were excluded from the longitudinal analysis if there was no formulation change or if visits were not consecutive, had missing data, or were >12 months apart (Figure 1). Metformin adherence was documented at the visit immediately following initiation of metformin, at the visit where metformin was discontinued, and at the visits before and after a switch from standard release (SR) to extended release (XR). Visits were within 12 months of the medication change (4.2 ± 2.4 months, mean±SD). Adherence data was not available for n=22/99 in the initiation group and n=8/58 in the SR to XR group).
Statistics
Data are expressed as mean±SD, median (25th-75th percentile) for continuous variables and frequency (percent) for categorical variables. Changes in GI symptoms over time were illustrated with Sankey diagrams (http://www.sankeymatic.com/). Participants involved in this analysis are illustrated using a Venn diagram (https://www.meta-chart.com/). Differences between metformin formulation groups were assessed by one-way analysis of variance and chi-squared tests. A post-hoc analysis to explore the relationship between GI symptoms and metformin adherence (or HbA1c) was conducted using chi-squared tests. Statistical analyses were performed with STATA, v16 (College Station, Texas) and P-values <0.05 were considered statistically significant.
RESULTS
Demographic and participant characteristics in the total group and by metformin formulation at their most recent visit during the study period are shown in Table 1. Patients were predominantly female (64%), had a diagnosis of T2DM (77%), and were of African American descent (64%). In the overall sample, 88% were obese (BMI≥95th percentile), 9% were overweight (BMI 85–94.9th percentile), and 3% were normal weight (BMI <85th percentile). Compared to youth with T2DM, youth with pre-diabetes were younger (17 (15–18) vs. 15 (13–17) years, had lower Hemoglobin a1cs (7 (6–10.3) vs. 5.8 (5.6–6.1)%), and were taking lower doses of metformin (1500 (1000–2000) vs 1000 (750–1500) mg). Of the 488 eligible patients, 202 (41.4%) were on metformin SR, 103 (21.1%) on metformin XR and 183 (37.5%) were not on metformin therapy (Table 1). All patients on metformin were taking tablets, except 3 patients who were prescribed Riomet® SR (liquid metformin); these 3 individuals are included in the SR group. The majority of patients taking metformin SR were prescribed a total daily dose of 2000mg (50%) or 1000mg (31%). Most patients prescribed metformin XR were taking ≥1500mg/day (59.4%). Documented patterns of reported adherence were not different between those taking metformin SR and XR – 53.2% vs 55.3% missed only 0–1 doses per week, respectively (Table 1). Youth who were not on metformin were younger, had lower BMI, lower HbA1c and were more likely to have a diagnosis of prediabetes (all P<0.04, Table 1). Otherwise there were no differences in demographic or clinical characteristics among the 3 groups.
Table 1:
Demographic and participant characteristics of youth at their most recent clinic visit in a multidisciplinary diabetes clinic (2016–2019)
| All Patients (n=488) | Standard Release Metformin (n=202) | Extended Release Metformin (n=103) | No Metformin (n=183) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 16.5 (14.3–18.1) | 16.8 (14.8–18.5) | 16.8 (14.8–18.0) | 15.9 (13.5–17.9) | 0.008 |
| Race | |||||
| African American | 314 (64.3) | 131 (64.9) | 65 (63.1) | 118 (64.5) | |
| White | 35 (7.2) | 15 (7.4) | 6 (5.8) | 14 (7.7) | 0.993 |
| Asian | 12 (2.5) | 5 (2.5) | 3 (2.9) | 4 (2.2) | |
| Other/unknown | 127 (26.0) | 51 (25.3) | 29 (28.2) | 47 (25.7) | |
| Hispanic/latino ethnicity | 100 (20.5) | 36 (17.8) | 26 (25.2) | 38 (20.8) | 0.314 |
| Female sex | 309 (63.5) | 127 (62.9) | 67 (65.1) | 115 (63.2) | 0.919 |
| BMI (kg/m2) | 35.5 (30.9–41.1) | 35.6 (31.7–42.3) | 36.6 (32.3–41.4) | 34.5 (29.5–39.7) | 0.036 |
| Blood pressure (mmHg) | |||||
| Systolic | 126 (118–134) | 126 (118–133) | 126 (120–133) | 125 (117–135) | 0.909 |
| Diastolic | 75 (68–82) | 76 (69–82) | 76 (67–83) | 74 (67–82) | 0.586 |
| Hemoglobin A1C (%)* | 6.4 (5.8–9.2) | 6.8 (5.9–10.3) | 6.3 (5.8–8.4) | 6.2 (5.7–7.7) | 0.011 |
| Liver Enzymes (U/L) | (n=62) | (n=31) | (n=71) | (n=164) | |
| AST | 19 (14–28) | 18 (12–25) | 19 (14–26) | 22 (16–35) | 0.178 |
| ALT | 31 (22.5–57) | 32 (25–48) | 29 (22–46) | 31 (21–63) | 0.459 |
| History of type 2 diabetes | 375 (76.8) | 181 (89.6) | 85 (82.5) | 109 (59.6) | <0.001 |
| Age of diagnosis(years) (n=349) | 14.1 (12.4–16.1) | 14.5 (12.6–16.2) | 14.0 (12.0–16.3) | 13.6 (12.0–15.2) | 0.113 |
| Insulin use | 161 (33.2) | 84 (41.6) | 28 (27.2) | 50 (27.3) | 0.004 |
| Use of other anti-hyperglycemic medications | 13 (2.7) | 2 (1.0) | 4 (3.9) | 7 (3.8) | 0.155 |
| Use of anti-hypertensive medications | 30 (6.2) | 16 (7.9) | 5 (4.9) | 9 (5.0) | 0.391 |
| Reports ≥ 1GI symptom | 70 (14.3) | 37 (18.3) | 15 (14.6) | 18 (9.8) | 0.060 |
| Reports ≥2 GI symptoms | 14 (2.9) | 7 (3.5) | 4 (3.9) | 3 (1.6) | 0.187 |
| Total Daily Dose – | N/A | <0.001 | |||
| 2000mg | 121 (24.8) | 101 (50.0) | 20 (19.4) | ||
| 1700mg | 2 (0.4) | 2 (1) | 0 | ||
| 1500mg | 55 (11.3) | 14 (6.9) | 41 (39.8) | ||
| 1000mg | 82 (16.8) | 63 (31.2) | 19 (18.5) | ||
| 850mg | 1 (0.2) | 1 (0.5) | 0 | ||
| 750mg | 17 (3.5) | 0 (0) | 17 (16.5) | ||
| 500mg | 22 (4.5) | 16 (7.9) | 6 (5.8) | ||
| Other/unknown | 3 (0.6) | 6 (1.5) | 0 (0) | ||
| Reported Missed Doses of Metformin | N/A | 0.071 | |||
| 0–1 Doses per week | 121 (39.7) | 74 (36.6)_ | 45.6) | ||
| 2–4 Doses per week | 32 (10.5) | 15 (7.4) | 17 (16.5) | ||
| ≥5 Doses per week | 71 (23.3) | 50 (24.8) | 21 (20.4) | ||
| [Not documented] | 81 (26.6) | 63 (31.2) | 18 (17.5)] |
Data are median (IQR) or n(%); Metformin groups compared with one-way analysis of variance or chi-squared tests *n=3 organic cause of GI symptom
Rates of GI Symptoms (Cross-sectional analysis)
Rates of GI symptoms by metformin formulation were assessed at patients’ last clinic visit during the study period: 14% (70/488) of youth had ≥1 GI symptom and 3% (14/488) had ≥2 GI symptoms (Table 1); these rates were similar between those with T2DM (14.4%, n=54/375) and pre-diabetes (14.2%, n=16/113). Individuals taking metformin SR had higher rates of GI symptoms compared to those not taking metformin (P=0.02) but there was no significant difference in the rate of GI symptoms in those on metformin SR vs. XR (P=0.41, Table 1). There were no differences in the rates of GI symptoms between those taking metformin XR and youth not taking any metformin (P=0.23). Only three (3) of the 488 patients had a documented organic disease at the visit encounter that could have been related to their GI symptoms, (dysmenorrhea, inflammatory bowel disease, and pancreatitis). The most common side effects documented were abdominal pain and diarrhea, while constipation was more common in patients not on metformin (Figure 2). Since constipation is not a common symptom in those taking metformin, we conducted post-hoc tests to determine if excluding constipation would change the prevalence of GI symptoms by group. In the post-hoc analysis, the prevalence of GI symptoms was: no metformin: 4% (n=8/173), SR: 17% (n=34/199), and XR: 14% (n=14/102), rates that were similar to primary analysis presented in Table 1. Patients with ≥2 GI symptoms were more likely to be on metformin (either SR or XR) (n=11/14), and diarrhea or abdominal pain was universally documented among them.
Figure 2:
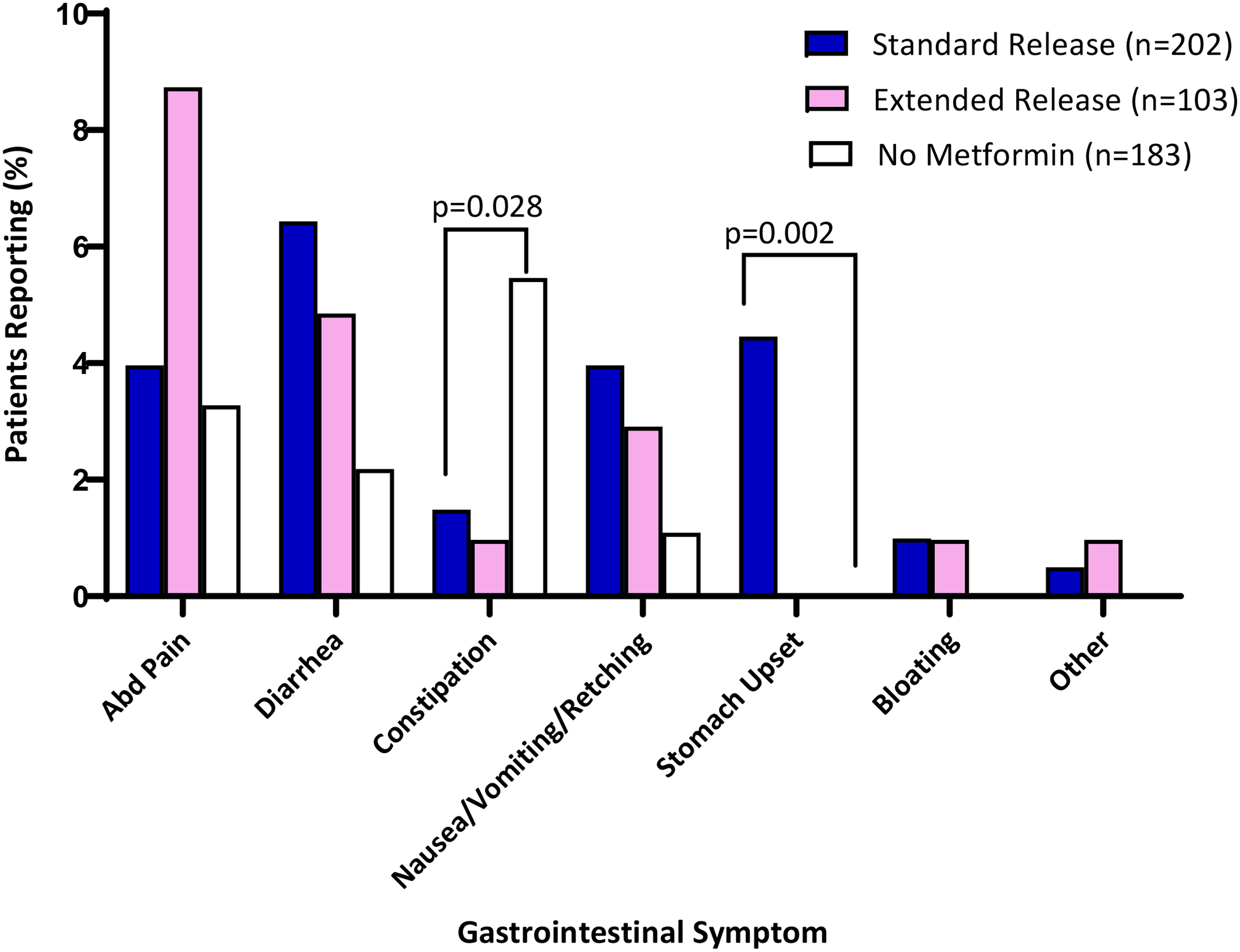
Specific gastrointestinal symptoms reported by patients at their most recent clinic visit in a multidisciplinary diabetes clinic (2016–2019). Bar graphs represent percent of patients reporting symptoms who were taking standard release (blue, n=202), extended release (pink, n=103) or no metformin (white, n=183). The category “Other” encompasses the following patient-reported complaints: “stomach upset”, “GI upset”, “GI symptoms - does not tolerate well”, “abdominal discomfort”, and “decreased appetite”. One-way analysis of variance with Bonferroni corrections were used to compare the three groups.
Longitudinal analysis
Among the 488 patients, 30% of patients (147/488) had a GI symptom documented during at least 1 visit during the 3-year study period. Metformin therapy was initiated in 99 patients (72 started taking metformin SR; 27 started taking metformin XR) during the study period (Figure 3A); a higher proportion of patients reported symptoms after starting metformin compared to baseline (33% vs 13%, P=0.001). In the 50 patients who discontinued metformin during the study period (Figure 3B), there was a trend to symptom resolution (28% while taking metformin vs 12% once discontinued, n=50, P=0.076). Additionally, 60 patients switched from SR to XR, but there was no significant change in the prevalence of symptoms (17% on SR vs 14% on XR, n= 58, P=0.6). Among those that initiated metformin (Figure 3A), 9 individuals reported constipation before metformin. After metformin, all but one patient had resolution of constipation and 4 reported other GI symptoms (e.g. diarrhea). Constipation was not reported in youth before or after discontinuing metformin and was reported in 1 patient after switching from SR to XR metformin.
Figure 3:
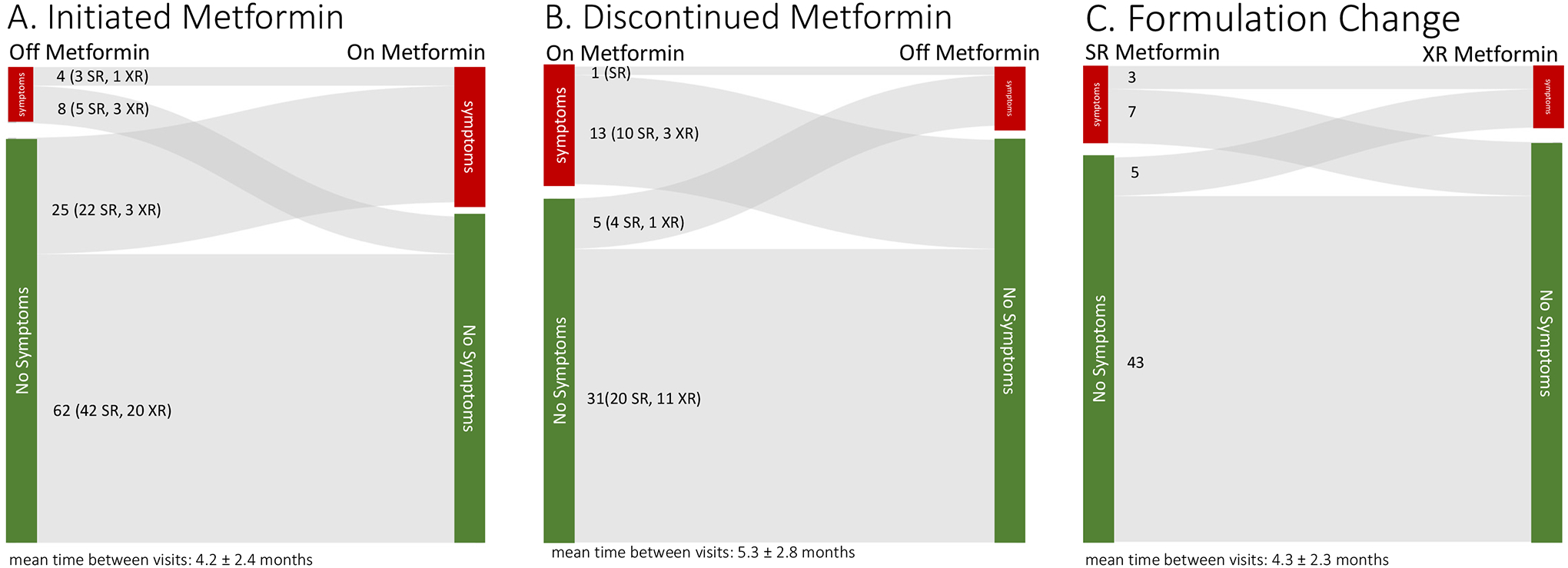
Sankey diagrams displaying changes in reporting of GI symptoms for patients who (A) initiated metformin, (B)discontinued metformin, and (C) changed from standard release (SR) to extended release (XR) metformin. Total n=170 in the analysis, though some experienced more than one of these changes during the study period (supplemental figure 1).
Patient reported adherence was also compared between the groups of those who had changes in their metformin prescriptions. After metformin initiation, GI symptoms were associated with number of missed doses per week (P=0.038, Figure 4A). However, there was no association between GI symptoms and adherence in those who discontinued metformin (Figure 4B) or youth who switched from SR to XR (Figure 4C). Hemoglobin A1c was not different between those with symptoms and those without (P≥0.5, data not shown).
Figure 4:
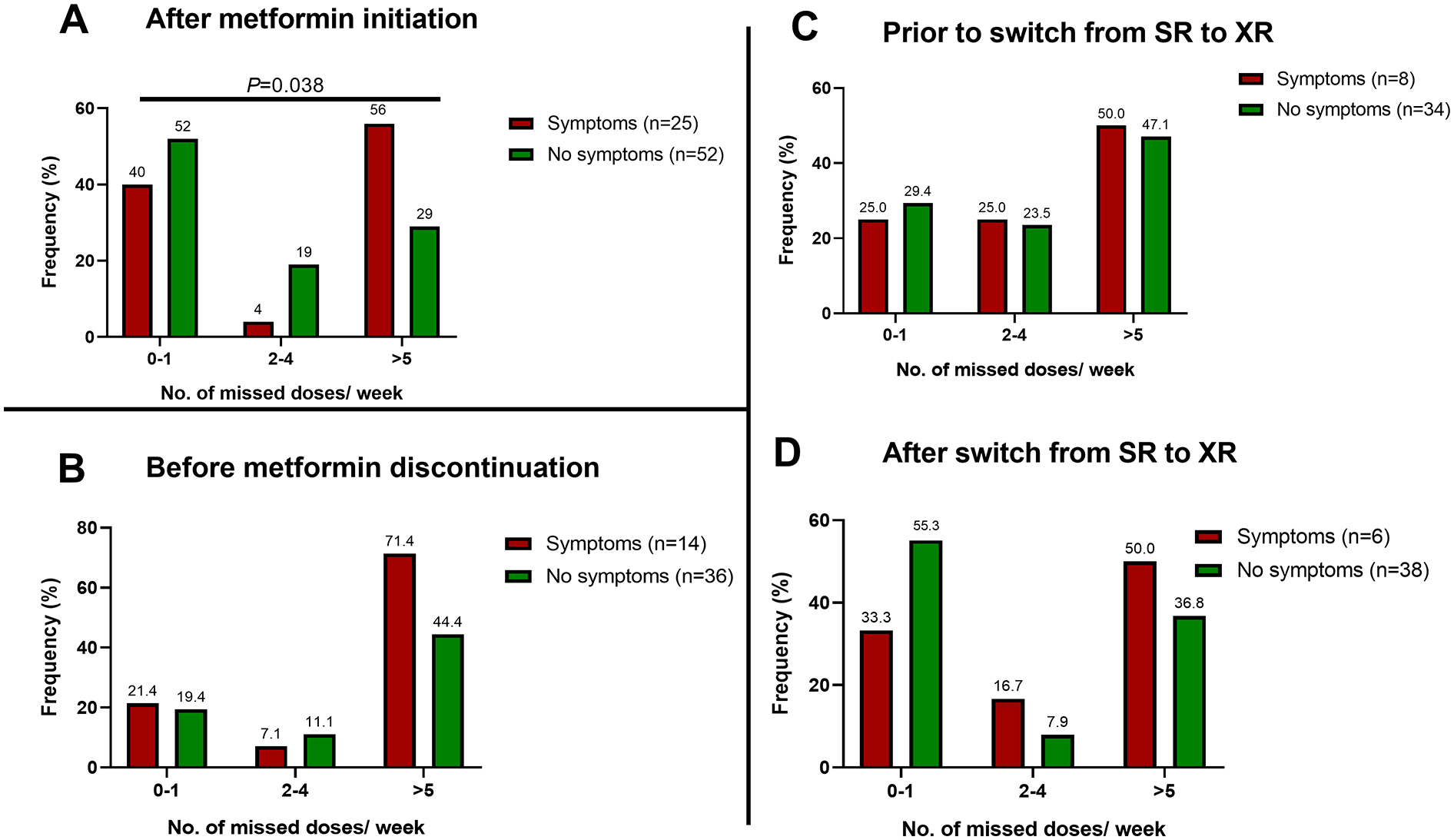
Metformin adherence at the visit immediately following (A) metformin initiation, (B) at the visit where metformin was discontinued, and (C) at the visits before and after a switch from standard release (SR) to extended release (XR). Bar graphs represent the percent of patients with symptoms (red) and without symptoms (green) who reported missing doses per week. Chi-squared analysis was used to compare the groups. No: number
DISCUSSION
Prevalence of GI symptoms in youth taking metformin
This study evaluated the prevalence and progression of GI symptoms in youth treated in a multi-disciplinary diabetes clinic to provide new insight into metformin tolerability in the clinical setting. Our contemporary findings confirm that metformin use for the management of prediabetes and diabetes in youth was associated with a high prevalence of GI symptoms, affecting almost a third of patients at some point during the study period. The longitudinal analysis demonstrated that initiating metformin was related to increased reporting of GI symptoms, and appeared to resolve on discontinuing metformin therapy. Metformin SR was the most commonly prescribed formulation with only 20% of youth taking Metformin XR. The prevalence of GI symptoms was similar in youth treated with metformin SR and XR - a finding that, to our knowledge, has not been previously reported. Notably, GI symptoms were observed in 1 in 10 youth who were not on metformin treatment--though the most prevalent symptom in this group was constipation, a common complaint in youth with obesity. Constipation was infrequent among those taking metformin. This study extends the findings of controlled metformin treatment trials [7, 16, 17] by documenting the large GI symptom burden in youth observed at varying stages of treatment with both standard and extended release formulations in youth with prediabetes and diabetes. Further, both the cross-sectional and longitudinal analyses suggest that switching to XR formulation may not completely mitigate the symptom burden. Due to the retrospective study design, prescribing practices for prediabetes and metformin use may have varied by provider. Nevertheless, these data suggest that GI symptoms complicating metformin use are experienced across the spectrum of glucose intolerance, even in youth on a low doses. These observations are clinically important as practitioners weigh the risk benefit ratio of metformin’s GI side effects with its glucose-lowering properties. Documenting the magnitude of the GI symptom burden describes a potentially modifiable barrier to maximal dose escalation and treatment optimization, as a high prevalence of GI symptoms is linked to poor quality of life metrics [17, 18] and medication non-adherence [17, 19]. Larger studies in youth, stratified by diagnosis and metformin dose, would be needed to assess the risk: benefit ratio of metformin treatment in youth with prediabetes.
Metformin XR vs. SR to mitigate GI symptoms
Data describing methods to reduce symptomatology in youth chronically treated with metformin are scarce. Current treatment paradigms recommend initiating metformin therapy at a low dose (typically 500mg daily) and gradually increasing to minimize side effects. Though this incremental dose escalation was implemented in our clinic, this strategy was insufficient to completely alleviate the side effect burden. Our observation that 1 in 6 youth who were on chronic SR or XR therapy continued to have side effects fills a gap in the youth literature and posits that the side effect burden in youth is comparable to the 10–30% of adults who continue to have GI symptoms on long term therapy [19].
To reduce the metformin GI symptom burden, it is first important to understand the factors that contribute to the metformin side effect profile. Emerging data suggests that one of the main mechanistic targets of metformin is the gut itself, rather than the liver as previously believed [20, 21]. While metformin increases short chain fatty acids and incretins, thereby improving glycemia, other changes to the intestinal milieu -- including changes to bile acids, lactic acid, and gas producing species of bacteria -- may contribute to the adverse GI side effects commonly seen with metformin use by causing temporary mucosal injury [22–24]. Immediate release (over 1–2 hours) provides high peak levels, so formulations with slower release should lower the metformin concentration in the GI tract at a single point in time, but provide the same drug delivery over a 24 hour period [25]. The reasons for the theoretical improved symptomatology with XR formulations have not been definitively proven but may be related to this delayed absorption and lower peak exposure of the drug in gut [26].
Prescribing metformin XR (at drug initiation or after SR has been tried) is a commonly recommended strategy to reduce GI symptoms, but there are no rigorous trials in youth [14, 27]. Based on adult data, metformin XR is assumed to improve compliance and tolerability in youth and is often prescribed off-label [28]. Some, but not all, studies in adults show a clear difference in GI side effects between metformin SR and XR [9]. We now provide novel data in youth demonstrating no overall difference in GI symptom rates between XR and SR, findings substantiated by data from controlled trials in adults [8, 10, 11, 13]. Yet GI symptomatology reporting is complex. An adult study has documented an improvement in diarrhea for those taking XR metformin compared to those taking SR [8]. Another adult study found a reduction in overall GI symptoms for those that switched from SR to XR metformin, indicating that metformin XR formulations may reduce GI symptoms in those already experiencing side effects from SR formulations [9]. Of note, we found that stomach upset was frequently reported in those taking metformin SR but was not observed in those on XR. However, there were no significant differences in the rates of diarrhea and abdominal pain between the 2 groups. Although limited by small sample size, data from our longitudinal analysis suggest that those individuals who have symptoms with metformin SR might benefit from switching to XR formulation −7/10 individuals on SR had resolution of their symptoms when switched to ER. Interestingly, a small number (n=5) youth who did not have symptoms on SR developed symptoms on XR indicating that other factors may play a role. These data support clinical practice recommendations that switching from one formulation to another is a reasonable strategy to reduce side effect burden and improve adherence.
A rigorous randomized controlled trial comparing XR vs. SR formulations is needed to assess quality of life metrics and the cost-benefit ratio in youth, since a major obstacle and deterrent to prescribing metformin XR is medication cost and insurance coverage. Multiple XR formulations are available and cost varies widely; some generic XR formulations are orders of magnitude more expensive than others (Table 2). All XR formulations employ variations of slow-release technology that theoretically improve tolerability. Among the XR formulations, the side effect profile is comparable, though no studies have been undertaken in youth. For example, Glucophage® XR (500 and 750mg tablets) are the least expensive and are slowly released in a pH dependent manner by diffusion through a 2 layer gel matrix that is broken up by peristalsis [29]. However, maximal dosing with Glucophage ® XR requires four of the 500mg tablets. By contrast, Fortamet and Glutmetza (1000mg tablets) are more convenient for maximizing dosage but are significantly more expensive. Fortamet® uses a patented “single-composition osmotic technology” with an outer membrane that is permeable only to water and two laser drilled exit holes that allow for a consistent slow delivery of medication using an osmotic gradient such that the membrane remains intact through GI transit [30]. Glumetza® utilizes a “gastric-retentive” tablet that is designed to deliver slowly released (over 8–9 hours) metformin to the upper GI tract, and provides lower peak exposure of the intestinal mucosa metformin, thereby theoretically reducing discomfort [25, 31]. Lastly, Riomet®, a liquid version of standard release metformin, has long been an alternative to swallowing multiple pills that are often large. Riomet® ER, recently approved by the FDA, contains a delivery system whereby active drug can be slowly released from a 200–300μm pellet vehicle to allow once daily maximal dosing in a solution form [32]. Additionally, the flavoring available for Riomet® ER was found to be more palatable than either the standard release oral solution or crushed SR tabs of metformin [32].
Table 2:
Generic Metformin formulations in the US and their approximate costs [36]
| Release | Dosages | Brand equivalent | Retail Unit Price |
|---|---|---|---|
| Standard Release | Glucophage® | ||
| 1000 | $0.06–0.07 | ||
| Extended Release | Glucophage® XR | ||
| 750 | $0.06–0.95 | ||
| Extended Release | Fortamet® | ||
| 1000 | $5–18 | ||
| Extended Release | Glumetza® | ||
| 1000 | $21–102 | ||
| Solution/SR | 500mg/5mL | Riomet® | $1.22 (per mL) |
| Solution/XR | 500mg/5mL | Riomet® ER | $1.25 (per mL) |
Despite mixed data on improvement in GI side effects, these XR formulations of metformin are still a valuable tool for our patients as they allow once daily administration, with greater chances of maximizing adherence and total tolerated dose. In adults, switching to a once daily metformin XR formulation improved adherence and was also associated with a reduction in HbA1c [12]; also the ability to have fewer pills to take has been shown to improve adherence [33, 34]. An improvement in adherence after switching from SR to XR was not seen in our study; however, this exploratory analysis was limited by missing data and based on documentation of patient reports. Additionally, since some youth will not tolerate either formulation, alternative medications or adjuncts to improve GI symptomatology in those taking metformin are urgently needed for the treatment of type 2 diabetes. Dietary prebiotic fiber supplements, that modulate the gut bacteria, were effective in small studies in adults with type 2 diabetes for improving metformin tolerability and glycemia [35]. While this may be a promising adjunct to metformin therapy, the mechanisms by which prebiotics influence metformin-induced GI side effects in adults and youth are unknown.
Limitations
Study limitations include the retrospective single-center study design in predominantly African American youth with type 2 diabetes. Although the retrospective nature of data collection is subject to recall bias and underreporting of GI symptoms, we expect that this would equally affect patients taking both formulations of metformin. Self-reported adherence reporting is a suboptimal instrument but, in this study, it represented a consistent form of documentation in a majority of charts; 73% of youth on metformin had documented adherence. Among those who had documented adherence, up to ½ of individuals reported missing >1 dose/ week. Since self-reporting is subject to recall bias, we hypothesize that metformin adherence may have been even lower. Therefore, in this analysis we cannot determine an association between metformin adherence and GI symptoms. However, we posit that the rates of GI symptoms in this analysis reflect real-world reporting in a tertiary care clinic but were likely an underestimate of true rates if metformin adherence was rigorously assessed. This type of study design may be of benefit as the participants’ responses were not biased by knowing that their data on GI symptoms was of research interest. Other limitations include the lack of reliable documentation of clinical factors that may contribute to the prescribing practices in our clinic, lack of information on number of tablets dispensed, and GI symptoms were broadly defined. For example, constipation is not likely to be causally linked to metformin treatment and the highest rates of constipation were observed in those not on metformin therapy. Nevertheless, this systematic analysis of GI symptoms fills an important gap in the literature especially since metformin efficacy is lowest in African American youth and identifying reasons for reduced metformin responsiveness, including medication factors such as metformin tolerability, is critical.
CONCLUSION
GI symptoms are common in youth with type 2 diabetes especially among those who take metformin therapy. This study demonstrated that the burden of GI symptoms is not eliminated by gradual dose escalation of metformin XR and SR in a real world setting. Rigorous controlled studies would be needed to determine whether metformin XR may improve overall GI tolerability and prevent treatment discontinuation in youth. Adjuncts to mitigate GI symptoms in youth on metformin therapy are needed to improve quality of life and medication adherence.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to thank our patients and their families for allowing us to participate in their clinical care.
FUNDING:
The Division of Intramural Research supports AGM (National Institute of Child Health and Development), STC, CKC, STM, AVP (National Institute of Diabetes & Digestive & Kidney Diseases). The Office of Dietary Supplements also supports AGM.
Footnotes
CONFLICT OF INTEREST DISCLOSURE: I AGM certify that neither I nor my co-authors have any conflicts of interest
REFERENCES
- 1.Sharma M, Nazareth I, and Petersen I, Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open, 2016. 6(1): p. e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescription data source: Medical Expenditure Panel Survey (MEPS), A.f.H.R.a.Q. (AHRQ), Editor. 2005–2015: Rockville, MD. [Google Scholar]
- 3.Nambam B, et al. , A cross-sectional view of the current state of treatment of youth with type 2 diabetes in the USA: enrollment data from the Pediatric Diabetes Consortium Type 2 Diabetes Registry. Pediatr Diabetes, 2017. 18(3): p. 222–229. [DOI] [PubMed] [Google Scholar]
- 4.Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and Beta-cell function in TODAY. Diabetes Care, 2013. 36(6): p. 1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE, et al. , Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med, 2006. 355(23): p. 2427–43. [DOI] [PubMed] [Google Scholar]
- 6.Candler TP, et al. , Treatment adherence and BMI reduction are key predictors of HbA1c one year after diagnosis of childhood Type 2 Diabetes in UK. Pediatr Diabetes, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care, 2013. 36(6): p. 1765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blonde L, et al. , Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin, 2004. 20(4): p. 565–72. [DOI] [PubMed] [Google Scholar]
- 9.Derosa G, et al. , Effects of metformin extended release compared to immediate release formula on glycemic control and glycemic variability in patients with type 2 diabetes. Drug Des Devel Ther, 2017. 11: p. 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal N, et al. , Metformin extended-release versus immediate-release: An international, randomized, double-blind, head-to-head trial in pharmacotherapy-naive patients with type 2 diabetes. Diabetes Obes Metab, 2018. 20(2): p. 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz S, et al. , Efficacy, tolerability, and safety of a novel once-daily extended-release metformin in patients with type 2 diabetes. Diabetes Care, 2006. 29(4): p. 759–64. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly LA, Morris AD, and Pearson ER, Adherence in patients transferred from immediate release metformin to a sustained release formulation: a population-based study. Diabetes Obes Metab, 2009. 11(4): p. 338–42. [DOI] [PubMed] [Google Scholar]
- 13.Ji L, et al. , Comparative effectiveness of metformin monotherapy in extended release and immediate release formulations for the treatment of type 2 diabetes in treatment-naive Chinese patients: Analysis of results from the CONSENT trial. Diabetes Obes Metab, 2018. 20(4): p. 1006–1013. [DOI] [PubMed] [Google Scholar]
- 14.13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care, 2020. 43(Suppl 1): p. S163–s182. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, et al. , Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 2009. 42(2): p. 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanovski JA, et al. , Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes, 2011. 60(2): p. 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florez H, et al. , Impact of metformin-induced gastrointestinal symptoms on quality of life and adherence in patients with type 2 diabetes. Postgrad Med, 2010. 122(2): p. 112–20. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, et al. , Gastrointestinal symptoms predictors of health-related quality of life in pediatric patients with functional gastrointestinal disorders. Quality of Life Research, 2017. 26(4): p. 1015–1025. [DOI] [PubMed] [Google Scholar]
- 19.Walker EA, et al. , Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care, 2006. 29(9): p. 1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napolitano A, et al. , Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One, 2014. 9(7): p. e100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cravalho CKL, et al. , Metformin improves blood glucose by increasing incretins independent of changes in gluconeogenesis in youth with type 2 diabetes. Diabetologia, 2020. [DOI] [PubMed] [Google Scholar]
- 22.McCreight LJ, Bailey CJ, and Pearson ER, Metformin and the gastrointestinal tract. Diabetologia, 2016. 59(3): p. 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarpello JH, Hodgson E, and Howlett HC, Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet Med, 1998. 15(8): p. 651–6. [DOI] [PubMed] [Google Scholar]
- 24.Forslund K, et al. , Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature, 2015. 528(7581): p. 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gusler G, et al. , Pharmacokinetics of metformin gastric-retentive tablets in healthy volunteers. J Clin Pharmacol, 2001. 41(6): p. 655–61. [DOI] [PubMed] [Google Scholar]
- 26.Davidson JA, Scheen AJ, and Howlett HC, Tolerability profile of metformin/glibenclamide combination tablets (Glucovance): a new treatment for the management of type 2 diabetes mellitus. Drug Saf, 2004. 27(15): p. 1205–16. [DOI] [PubMed] [Google Scholar]
- 27.Zeitler P, et al. , ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes, 2018. 19 Suppl 27: p. 28–46. [DOI] [PubMed] [Google Scholar]
- 28.Arslanian S, et al. , Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care, 2018. 41(12): p. 2648–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bristol-Myers Squibb Company. Glucophage XR [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020357s031,021202s016lbl.pdf Accessed April 2020.
- 30.Actavis Laboratories FL. Fortamet [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021574s020lbl.pdf Accessed April 2020.
- 31.Schwartz SL, Wu JF, and Berner B, Metformin extended release for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother, 2006. 7(6): p. 803–9. [DOI] [PubMed] [Google Scholar]
- 32.Marshall AC, et al. , Assessment of Taste and Grittiness of Riomet((R)) ER Strawberry, Riomet((R)) ER Grape, Riomet((R)) Cherry, and Metformin Immediate-Release Tablets in Healthy Subjects. Drugs R D, 2019. 19(1): p. 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thom S, et al. , Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. Jama, 2013. 310(9): p. 918–29. [DOI] [PubMed] [Google Scholar]
- 34.Castellano JM, et al. , A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol, 2014. 64(20): p. 2071–82. [DOI] [PubMed] [Google Scholar]
- 35.Burton JH, et al. , Addition of a Gastrointestinal Microbiome Modulator to Metformin Improves Metformin Tolerance and Fasting Glucose Levels. J Diabetes Sci Technol, 2015. 9(4): p. 808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GoodRx. 05/13/2020]; Available from: https://www.goodrx.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


