Abstract
Aims
Hybrid atrial fibrillation (AF) ablation is a promising approach in non-paroxysmal AF. The aim of this study is to assess the long-term outcomes of hybrid ablation in a large cohort of patients after both an initial and as a redo procedure.
Methods and results
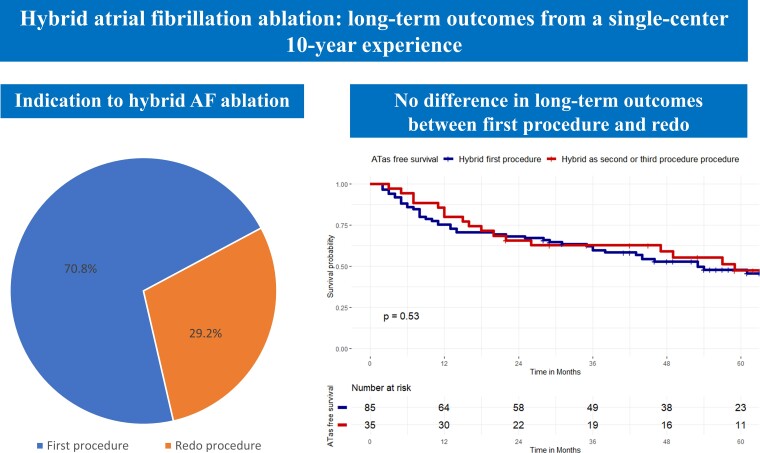
All consecutive patients undergoing hybrid AF ablation at UZ Brussel from 2010 to 2020 were retrospectively evaluated. Hybrid AF ablation was performed in a one-step procedure: (i) thoracoscopic ablation followed by (ii) endocardial mapping and eventual ablation. All patients received PVI and posterior wall isolation. Additional lesions were performed based on clinical indication and physician judgement. Primary endpoint was freedom from atrial tachyarrhythmias (ATas). A total of 120 consecutive patients were included, 85 patients (70.8%) underwent hybrid AF ablation as first procedure (non-paroxysmal AF 100%), 20 patients (16.7%) as second procedure (non-paroxysmal AF 30%), and 15 patients (12.5%) as third procedure (non-paroxysmal AF 33.3%). After a mean follow-up of 62.3 months ± 20.3, a total of 63 patients (52.5%) experienced ATas recurrence. Complications occurred in 12.5% of patients. There was no difference in ATas between patients undergoing hybrid as first vs. redo procedure (P = 0.53). Left atrial volume index and recurrence during blanking period were independent predictors of ATas recurrence.
Conclusion
In a large cohort of patients undergoing hybrid AF ablation, the survival from ATas recurrence was 47.5% at ≈5 years follow-up. There was no difference in clinical outcomes between patients undergoing hybrid AF ablation as first procedure or as a redo.
Keywords: Atrial fibrillation, Hybrid ablation, Surgical ablation, Thoracoscopic ablation, Catheter ablation
Graphical Abstract
Graphical abstract.
What’s new?
In a large cohort of patients, undergoing hybrid atrial fibrillation (AF) ablation, the long-term free survival from atrial tachyarrhythmias (ATas) was 47.5% at ≈5 years follow-up.
There was no difference in the survival free from ATas between patients undergoing hybrid AF ablation as first procedure or as a redo.
Left atrial volume index and ATas recurrence during blanking period were independent predictors of the recurrence endpoint.
Introduction
Electrical isolation of pulmonary veins (PVI) is the main invasive treatment of atrial fibrillation (AF) since Haissaguerre et al.1 demonstrated the role of atrial extrasystoles originating from the pulmonary veins (PVs). In particular, endocardial catheter ablation with circumferential PVI, by means of different sources of energy, has gained success because of its safety and efficacy.2
However, in patients with persistent and long-term persistent AF, the recurrence rate after an endocardial PVI-only procedure is higher, and no additional ablation strategy has consistently demonstrated a significant advantage in terms of clinical outcomes.3,4,5,6,7 Furthermore, another complex AF subset is represented by patients with AF recurrence and PVI confirmed at the second or third procedure.8 This is not a neglectable clinical issue, as ≈ 20% of patients are found with isolated PVs at redo.8
The suboptimal results of catheter ablation, especially in the persistent/long-standing persistent AF subgroup, have led to a quest for novel techniques. Although showing good results, surgical ablation is limited by the open chest approach.9
Hybrid AF ablation combines the advantages of both a thoracoscopic surgical ablation (direct visualization of anatomical structures to be spared and possibility to deliver epicardial lesions) and endocardial catheter ablation (possibility to confirm PVI, to check line block and, if necessary, completing additional lesions from endocardium). The results of hybrid AF ablation in clinical experience10–13 and randomized trials have been promising.14 However, long-term clinical follow-up data are scarce. Furthermore, the role of hybrid AF ablation as redo procedure is not clear.
The aim of this study is to assess the long-term outcomes of AF hybrid ablation in a large cohort of patients from a high-volume centre, over 10 years; furthermore, the role of AF hybrid ablation as a second or third procedure (re-do or re-re-do) is evaluated.
Methods
Study population
All consecutive patients diagnosed with AF who underwent a hybrid AF ablation at UZ Brussel from 2010 to 2020 were retrospectively evaluated. They were included in the current study if the following inclusion criteria were fulfilled: (i) AF diagnosis following current guidelines15 and (ii) hybrid AF ablation performed with PVI and left atrial posterior wall isolation (LAPWI).
Exclusion criteria were the following: presence of intracavitary thrombus, decompensated heart failure, presence of severe coronary artery disease, and moderate or severe valvular disease.16
The study cohort was divided in three groups as follows: (i) patients with hybrid AF ablation as first procedure; (ii) patients with hybrid AF ablation as second procedure; and (iii) patients with hybrid AF ablation as third procedure.
The following data were collected: (i) clinical history including: demographic, race/ethnicity and biometric data; (ii) clinical cardiovascular data: renal function, cardiovascular risk factors, CHA2DS2-VASc score, echocardiographic data; and (iii) antiarrhythmic drugs (AADs).
The study complied with the Declaration of Helsinki as revised in 2013; the ethic committee approved the study. All patients signed an informed consent that had been approved by our institutional review board.
Hybrid atrial fibrillation ablation procedure
Hybrid AF ablation procedure has been previously described in detail by our group.10
A transthoracic echocardiogram (TTE) was performed within 1 week prior to the procedure to assess the left ventricular ejection fraction (LVEF) and to rule out any significant structural and/or valvular disease. On the day of the procedure, transoesophageal echocardiography (TOE) was performed to rule out intracardiac thrombus. Pulmonary function testing was also performed as pre-procedure routine.
Oral anticoagulation therapy (OAC) with vitamin K antagonists was interrupted 2 days prior the procedure and replaced by low-molecular-weight heparin (LMWH) therapy, based on INR values.
For patients on direct anticoagulants (DOAC), the management was as follows: (i) the last dose of rivaroxaban or edoxaban was given in the morning 1 day prior to the procedure and (ii) the last dose of dabigatran or apixaban was given in the evening 1 day prior.
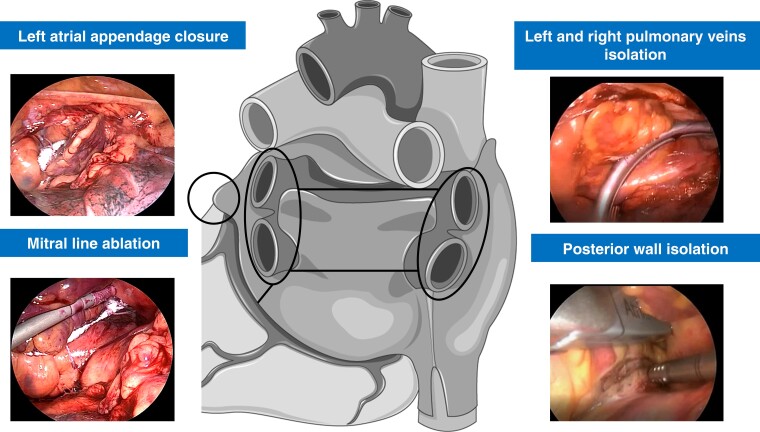
The hybrid AF ablation was performed in two times during a one-step procedure: (i) thoracoscopic ablation followed by (ii) endocardial mapping and eventual ablation. The procedure was performed in the electrophysiology laboratory as previously described.10 General anaesthesia was administered with a double-lumen endobronchial tube for selective lung ventilation. In all patients, bilateral thoracoscopic access with 5 mm ports was used. If hybrid AF ablation was performed as first (index) procedure, antral isolation of both pairs of PVs was performed with four to six applications using a bipolar radiofrequency (RF) clamp (Atricure, Inc., West Chester, OH, USA). After clamping, PVI was assessed by epicardial pacing through a quadripolar catheter (exit block). For redo procedures, PVI was assessed with epicardial pacing (exit block), and re-PVI was performed if necessary. LAPWI was performed epicardially in all patients with two lines: (i) a roof line (connecting both superior PVs) and (ii) inferior line (connecting both inferior PVs). A bipolar RF pen or a linear pen device (Isolator Pen and Coolrail, Atricure Inc.) was used for LAPWI. Furthermore, if deemed indicated by surgeon, two additional ablation lines were added epicardially: one encircling the superior vena cava (SVC) using the bipolar RF clamp and the other connecting cava veins using the pen or the RF clamp (bicaval line). A posterior mitral line, connecting mitral annulus to left inferior PV, could be performed epicardially, if deemed indicated. Left atrial appendage (LAA) clipping was performed in 91 patients (75.8%) with the AtriClip device (AtriCure Inc.) (Figure 1).
Figure 1.
Epicardial thoracoscopic stage in hybrid atrial fibrillation ablation. Left and right pulmonary veins isolation and posterior wall isolation were performed in all patients. Left atrial appendage closure and posterior mitral line epicardial ablation were performed if clinically indicated.
Percutaneous endocardial mapping and ablation was performed via femoral venous approach. A decapolar catheter (Biosense Webster, Inc., Diamond Bar, CA, USA or EnSite EP System, Abbott, St. Paul, MN) was placed in the coronary sinus under fluoroscopy, and a single or double transseptal puncture was performed with a long sheath (SL0, St Jude Medical) and BRK needle under TOE and fluoroscopy guidance. Right after the first transseptal puncture, full heparinization (1000 U heparin per 10 kg body weight) was given with targeted activated clotting time of 300 s. Electroanatomic map of the left atrium (LA) was performed with (CARTO system; Biosense Webster, Inc.) using the circular mapping catheter (Lasso, Biosense Webster, Inc.) and the open-irrigated 3.5-mm tip RF ablation catheter (NaviStar ThermoCool; Biosense Webster, Inc.) until 2015. From 2015 to 2020, a high density LA map was obtained with Pentaray mapping catheter (CARTO system; Biosense Webster, Inc.) or with Advisor HD Grid Mapping Catheter, Sensor Enabled (Abbott, St. Paul, MN). PVI and LAPWI were assessed by mapping (atrial bipolar voltage) and pacing manoeuvres (entrance and exit block). Re-PVI and/or re LAPWI were performed with 3.5-mm tip RF ablation catheter (NaviStar ThermoCool; Biosense Webster, Inc.), until 2015 and with ThermoCool SmartTouch (Biosense Webster, Inc.) from 2016 to 2020 or with TactiCath Contact Force Ablation Catheter, Sensor Enabled (Abbott, St. Paul, MN) from 2019 to 2020. Additional lesions were performed endocardially based on clinical indication and physician judgement, including the following: (i) Cavotricuspid isthmus (CTI); (ii) anterior mitral line connecting mitral annulus to right superior PV; (iii) posterior mitral line, connecting mitral annulus to left inferior PV; and (iv) left and right continuous fractionated atrial electrogram (CFAE) mapping and ablation. In patients who did not convert to sinus rhythm during the ablation, electrical cardioversion was performed. Bidirectional block was confirmed for all ablation lines with pacing manoeuvres.
After the procedure, all patients underwent to continuous telemetric monitoring until discharge from the hospital. Before discharge, TTE is performed in all patients in order to exclude post-procedural pericardial effusion. LMWH was started the same evening following the ablation, and on the third postoperative day, OAC was reinitiated. Patients were restarted with previous AADs within one week after ablation. Oral anticoagulation and AADs were continued for at least 3 months. AADs were maintained or stopped after 3 months, based on clinical judgement and following current guidelines.17 Indication to hybrid AF ablation was analysed. Hybrid AF ablation was considered as an alternative to endocardial ablation based on the expected risk and benefit of the procedure and patient preference after careful discussion following current guidelines.18
Follow-up
After discharge, patients were scheduled for follow-up visits with baseline electrocardiogram (ECG) and 24 h Holter recordings at 3, 6, 12 months, and every 6 months after 1 year. Furthermore, a 7-day ECG Holter monitoring was recorded at 3, 6, and 12 months for the first year and then every 6 months. Primary endpoint was recurrence of any atrial tachyarrhythmias (ATas) defined as episodes >30 s after a 90-day post-ablation blanking period BP off AADs. Recurrence was assessed with standard ECG or 24 h ECG Holter monitoring or with implantable loop recorders or implanted devices interrogation if applicable. Moreover, a 24 h Holter monitoring was performed if any symptom following ablation was deemed as prompting further clinical investigation. Complications were adjudicated and analysed following current AF guidelines.15
Statistical analysis
The analysis was performed using R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
All variables were tested for normality with Shapiro–Wilk test. Normally distributed variables were described as mean ± standard deviation and the groups were compared through ANOVA, paired or unpaired t-test as appropriate, while the non-normally distributed variables were described as median (interquartile range) and compared by Mann–Whitney test or Wilcoxon signed-rank test as appropriate. The categorical variables were described as frequencies (percentages) and compared by χ2 test or Fisher’s exact test as appropriate.
Kaplan–Meier’s curves were drawn to describe the patients’ freedom from ATas during the follow-up period, and Log-Rank test or Pairwise Log-Rank test was used.
Cox’s proportional hazard model was performed to identify risk factors for ATas. The covariates entered in the univariate and multivariate Cox model were chosen according to their clinical significance. Variables with P < 0.10 were then entered in the multivariate model and selected with a backward stepwise approach.
Survival analysis was performed with survival19 and survminer20 packages on R software.
A P-value of less than 0.05 was considered statistically significant.
Results
Study population characteristics
A total of 120 consecutive patients were included in the study, 85 patients (70.8%) underwent hybrid AF ablation as first (index) procedure, 20 patients (16.7%) as second procedure (first redo), and 15 patients (12.5%) as third procedure (second redo).
All 85 patients (100%) undergoing hybrid AF ablation as first procedure had non-paroxysmal AF, 17 patients (20%) with persistent AF, and 68 patients (80%) with long-standing persistent AF. Paroxysmal AF was more frequent in patients with hybrid AF ablation as second or third procedure compared with patients with an index hybrid AF ablation [14 patients (70%) vs. 10 patients (66.7%) vs. 0 patients (0%) respectively, P < 0.001].
The three cohorts had no significant differences for cardiovascular risk factors, except for diabetes. CHA2DS2-VASc score was 2.2 ± 5.8 and left atrial volume index (LAVI) was 42.4 mL/m2 ± 18.3.
Complete patient characteristics are summarized in Table 1. Indication to hybrid AF ablation has been summarized in Table 2.
Table 1.
Clinical characteristics of hybrid atrial fibrillation ablation patients
| Hybrid first procedure (N = 85) | Hybrid second procedure (N = 20) | Hybrid third procedure (N = 15) | Total (N = 120) | P value | |
|---|---|---|---|---|---|
| Age at ablation (years) | 65.0 ± 8.5 | 62.0 ± 6.6 | 60.3 ± 7.7 | 64.0 ± 8.2 | 0.063 |
| Gender (male), n (%) | 67 (78.8) | 10 (50.0) | 11 (73.3) | 88 (73.3) | 0.042 |
| BMI (kg/m2) | 28.6 ± 4.2 | 29.9 ± 5.9 | 28.2 ± 5.0 | 28.7 ± 4.6 | 0.49 |
| Race/ethnicity, n (%) | 1.00 | ||||
| White/Caucasian, n (%) | 84 (98.8) | 20 (100.0) | 15 (100.0) | 119 (99.2) | |
| Black/African, n (%) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (0.0) | |
| Asian, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.8) | |
| Paroxysmal AF, n (%) | 0 (0.0) | 14 (70.0) | 10 (66.7) | 24 (20.0) | < 0.001 |
| Persistent AF, n (%) | 17 (20.0) | 4 (20.0) | 4 (26.7) | 25 (20.8) | 0.78 |
| Long-standing persistent AF, n (%) | 68 (80.0) | 2 (10.0) | 1 (6.7) | 71 (59.2) | < 0.001 |
| Dyslipidaemia, n (%) | 15 (17.6) | 6 (30.0) | 6 (40.0) | 27 (22.5) | 0.11 |
| Heart failure, n (%) | 11 (12.9) | 0 (0.0) | 0 (0.0) | 11 (9.2) | 0.10 |
| Hypertension, n (%) | 43 (50.6) | 9 (45.0) | 8 (53.3) | 60 (50.0) | 0.92 |
| Diabetes, n (%) | 6 (7.1) | 6 (30.0) | 1 (6.7) | 13 (10.8) | 0.021 |
| Stroke history, n (%) | 3 (3.5) | 3 (15.0) | 1 (6.7) | 7 (5.8) | 0.09 |
| Peripheral vascular disease, n (%) | 2 (2.4) | 0 (0.0) | 2 (13.3) | 4 (3.3) | 0.12 |
| CHA2DS2-VASc score | 2.5 ± 6.8 | 1.7 ± 1.4 | 1.7 ± 1.4 | 2.2 ± 5.8 | 0.81 |
| LVEF (%) | 53.7 ± 12.0 | 59.0 ± 4.5 | 60.0 ± 0.0 | 55.4 ± 10.6 | 0.025 |
| LAVI (mL/m2) | 42.8 ± 18.6 | 41.1 ± 14.7 | 42.0 ± 21.8 | 42.4 ± 18.3 | 0.94 |
| AADs, n (%) | 50 (58.8) | 13 (65.0) | 12 (80.0) | 75 (62.5) | 0.30 |
| Flecainide, n (%) | 11 (12.9) | 4 (20.0) | 5 (33.3) | 20 (16.7) | 0.12 |
| Propafenone, n (%) | 1 (1.2) | 1 (5.0) | 0 (0.0) | 2 (1.7) | 0.50 |
| Beta-blockers, n (%) | 35 (41.2) | 7 (35.0) | 3 (20.0) | 45 (37.5) | 0.30 |
| Sotalol, n (%) | 10 (11.8) | 6 (30.0) | 8 (53.3) | 24 (20.0) | < 0.001 |
| Amiodarone, n (%) | 13 (15.3) | 1 (5.0) | 0 (0.0) | 14 (11.7) | 0.23 |
| Calcium channel blockers, n (%) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 2 (1.7) | 1.00 |
| VKA, n (%) | 6 (7.1) | 0 (0.0) | 0 (0.0) | 6 (5.0) | 0.18 |
| DOAC, n (%) | 79 (92.9) | 20 (100.0) | 15 (100.0) | 114 (95.0) | 0.18 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; DOAC, direct oral anticoagulant; LAVI, left atrium volume index; LVEF, left ventricular ejection fraction; VKA, vitamin K antagonists.
Table 2.
Indication to hybrid atrial fibrillation ablation
| Hybrid first procedure (N = 85) | Hybrid second procedure (N = 20) | Hybrid third procedure (N = 15) | Total (N = 120) | |
|---|---|---|---|---|
| Persistent or long-standing persistent AF and severely dilated LA, n (%) | 79 (92.9) | 6 (30.0) | 5 (33.3) | 90 (75.0) |
| LAA closure indication, n (%) | 8 (9.4) | 2 (10.0) | 1 (6.7) | 11 (9.2) |
| Failure of previous endocardial approach, n (%) | 0 (0) | 20 (100.0) | 15 (100) | 35 (29.2) |
Each patient could have more than one indication.
AF, atrial fibrillation; LA, left atrium; LAA, left atrial appendage.
Procedural characteristics
Among 85 patients undergoing hybrid AF ablation as first procedure, all patients had PVI + LAPWI. Additional CTI was performed in 11 patients (12.9%), an anterior mitral line was performed in 11 patients (12.9%), a posterior mitral line in 8 patients (9.4%), and CFAE ablation in 22 patients (25.9%) (Table 3). A redo procedure was performed in 22 patients (25.9%): PVs were isolated in 15 patients (68.2%), and LAPWI was confirmed in 20 patients (90.9%).
Table 3.
Procedural characteristics and complications
| Hybrid first procedure (N = 85) | Hybrid second procedure (N = 20) | Hybrid third procedure (N = 15) | Total (N = 120) | P value | |
|---|---|---|---|---|---|
| Procedure time (min) | 265.7 ± 62.2 | 259.8 ± 72.7 | 258.3 ± 68.7 | 262.4 ± 65.2 | 0.87 |
| Fluoroscopy time (min) | 22.5 ± 8.1 | 23.8 ± 10.3 | 23.5 ± 10.6 | 23.4 ± 10.1 | 0.90 |
| First ablation cryoablation PVI, n (%) | 0 (0.0) | 15 (75.0) | 12 (80.0) | 27 (22.5) | NA |
| First ablation RF PVI, n (%) | 0 (0.0) | 5 (25.0) | 3 (20) | 8 (6.7) | NA |
| First ablation hybrid PVI, n (%) | 85 (100) | 0 (0.0) | 0 (0.0) | 85 (70.8) | NA |
| LAPWI (hybrid), n (%) | 85 (100) | 20 (100) | 15 (100) | 120 (100) | NA |
| CTI performed (hybrid), n (%) | 11 (12.9) | 3 (15.0) | 2 (13.3) | 16 (13.3) | 0.91 |
| Anterior mitral line performed (hybrid), n (%) | 11 (12.9) | 2 (10.0) | 3 (20.0) | 16 (13.3) | 0.90 |
| Posterior mitral line performed (hybrid), n (%) | 8 (9.4) | 3 (15.0) | 2 (13.3) | 13 (10.8) | 0.84 |
| CFAE performed (hybrid), n (%) | 22 (25.9) | 6 (30.0) | 3 (20.0) | 31 (25.8) | 0.86 |
| Complications | 11 | 2 | 2 | 15 | 0.94 |
| Left atrial perforation, n (%) | 3 | 0 | 0 | 3 | |
| Cardiac tamponade | 0 | 1 | 0 | 1 | |
| Post-operatory pericardial drainage, n (%) | 2 | 0 | 0 | 2 | |
| Pericarditis, n (%) | 3 | 1 | 1 | 5 | |
| Pleuritis, n (%) | 2 | 0 | 1 | 3 | |
| Pneumothorax requiring drainage, n (%) | 1 | 0 | 0 | 1 |
CFAE, continuous fractionated atrial electrogram; CTI, cavotricuspid isthmus; LAPWI, left atrium posterior wall isolation PVI, pulmonary vein isolation; RF, radiofrequency.
Among 20 patients undergoing hybrid AF ablation as second procedure, 15 patients (75.0%) had a previous PVI with cryoablation and 5 patients (25.0%) had a previous PVI with RF. PVs were isolated in 14 patients (70.0%). Hybrid re-PVI (if reconnected PVs) + LAPWI was performed in all patients. Additional CTI was performed in three patients (15.0%), an anterior mitral line was performed in two patients (10.0%), a posterior mitral line in three patients (15.0%), and CFAE ablation in six patients (30.0%) (Table 3).
Among 15 patients undergoing hybrid AF ablation as third procedure, 12 patients (80.0%) had a previous PVI with cryoablation and 3 patients (20.0%) had a previous PVI with RF. PVs were isolated in 15 patients (100%). Hybrid LAPWI was performed in all patients. Additional CTI was performed in two patients (13.3%), an anterior mitral line was performed in three patients (20.0%), a posterior mitral line in two patients (13.3%), and CFAE ablation in three patients (20.0%) (Table 3).
Complications occurred in 15 patients (12.5%). There were no procedure-related deaths. Three patients experienced left atrial perforation during the thoracoscopic stage. Two patients had a vein lesion (right inferior and left superior, respectively) requiring conversion to sternotomy. One patient had an intraprocedural perforation of the left atrial appendage which was treated by clipping. One patient experienced a late tamponade (7 days after the procedure) treated with pericardial drainage and ECMO support.
Complications are summarized in Table 3.
Follow-up and predictors of the primary endpoint
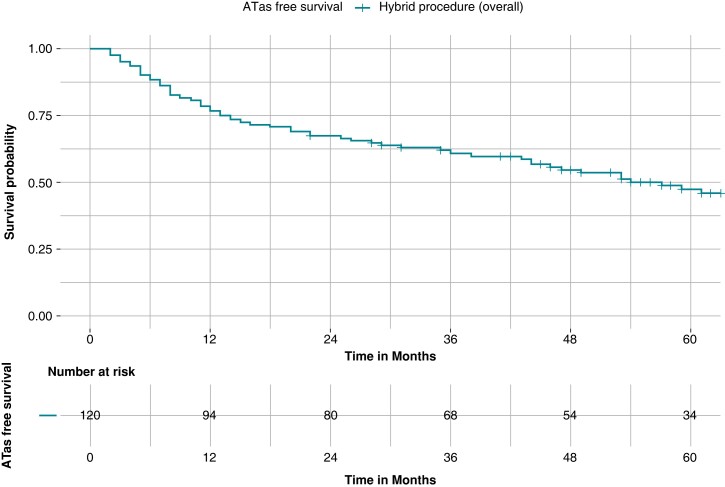
After a mean follow-up of 62.3 months ± 20.3 a total of 63 patients (52.5%) experienced an ATas recurrence. A first ATas recurrence occurred at a mean follow-up of 23.5 months ± 20.5. ATas were adjudicated as AF in 35 patients (55.5%) and atrial tachycardia in 28 patients (44.4%). ATas free survival without AADs was as follows: 76.7% at 12 months, 67.5% at 24 months, 60.5% at 36 months, 53.6% at 48 months, and 46.1% at 60 months (Figure 2). During follow-up, no patients experienced a stroke.
Figure 2.
Kaplan–Meier curves of survival free from any atrial tachyarrhythmias recurrence in the whole cohort. Kaplan–Meier curve of survival free from any atrial tachyarrhythmias (ATas) occurrence in the whole (120-patient) cohort. After a mean follow-up of 62.3 months ± 20.3, freedom from ATas recurrence was obtained in 47.5% of patients.
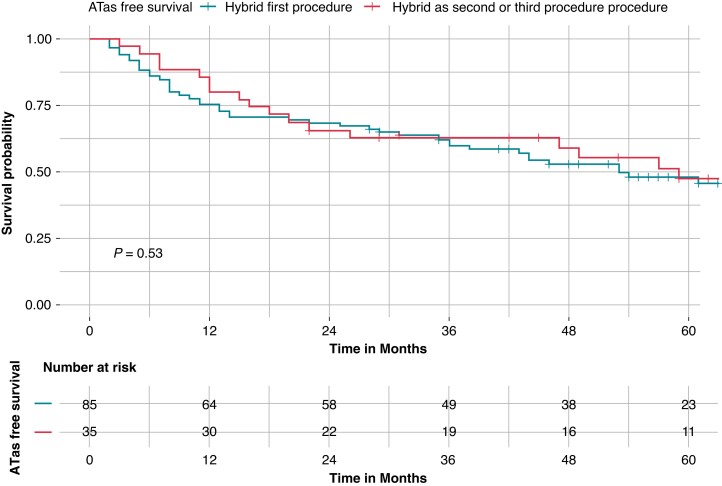
At survival analysis, there was no difference in ATas-free survival between patients with hybrid as first procedure and patients with hybrid as second or third procedure (45.9% vs. 51.4%, Log-Rank P = 0.53) (Figure 3). There was no difference in ATas free survival between patients with paroxysmal AF and patients with persistent or long-standing persistent AF (54.2% vs. 45.8%, Log-Rank P = 0.33).
Figure 3.
Kaplan–Meier curves of survival free from any atrial tachyarrhythmias recurrence stratified by hybrid ablation timing: hybrid as first procedure vs. hybrid as second or third procedure. Kaplan–Meier curve of survival free from any atrial tachyarrhythmias (ATas) occurrence. There was no difference in ATas free survival between patients with hybrid as first procedure (blue curve) and patients with hybrid as second or third procedure (red curve), (45.9% vs. 51.4%, Log-Rank P = 0.53).
At survival analysis stratified for hybrid timing, there was no difference in ATas-free survival between patients with hybrid as first procedure and patients with hybrid as second procedure (45.9% vs. 55.0%, pairwise Log-Rank P = 0.60) or patients with hybrid as third procedure (45.9% vs. 46.7%, Pairwise Log-Rank P = 0.81).
At Cox multivariate analysis, independent predictors of ATas recurrence were the following: LAVI (HR = 1.02, 95% CI 1.01–1.03, P = 0.004) and recurrence during blanking period (HR = 5.1, 95% CI 2.12–10.23, P = 0.001) (Table 4).
Table 4.
Cox regression analysis
| Cox univariate analysis HR (95% CI), P value |
Cox multivariate analysis HR (95% CI), P value |
|
|---|---|---|
| Age at ablation | 1.01 (0.98–1.04), P = 0.55 | — |
| BMI | 0.98 (0.93–1.04), P = 0.66 | — |
| Gender (male) | 0.96 (0.55–1.67), P = 0.87 | — |
| Duration of symptoms | 1.02 (1.01–1.04), P = 0.02 | — |
| Hypertension | 1.21 (0.74–1.99), P = 0.44 | — |
| LAVI | 1.03 (1.01–1.04), P = 0.002 | 1.02 (1.01–1.03), P = 0.004 |
| Coronary artery disease | 1.24 (0.49–3.1), P = 0.64 | — |
| Blanking period recurrence | 5.2 (2.31–10.52), P < 0.001 | 5.1 (2.12–10.23), P = 0.001 |
| Persistent or long standing persistent AF | 1.39 (0.72–2.66), P = 0.32 | — |
AF, atrial fibrillation; BMI, body mass index; LAVI, left atrium volume index.
Discussion
The main findings of this study can be summarized as follows: (i) In a large cohort of patients undergoing hybrid AF ablation, the long-term free survival from ATas was 47.5%; (ii) There was no difference in the survival free from ATas between patients undergoing hybrid AF ablation as first procedure or as a redo; and (iii) LAVI and ATas recurrence during blanking period were independent predictors of the primary endpoint.
Long-term outcomes of hybrid atrial fibrillation ablation
Wolf et al.21 reported the first patients treated with PVI by means of a minimally invasive approach to. They described the feasibility of video-assisted thoracoscopic PVI with a new promising minimally invasive technique. Since the first report, minimally invasive surgery experience has increased and hybrid procedure for AF ablation has been developed. Mahapatra et al.22 described their experience with a staged hybrid AF ablation on 15 patients with non-paroxysmal AF and previous failed ablation. A sequential epicardial surgical ablation, through bilateral thoracoscopic approach, followed by endocardial catheter-based ablation, was used. Freedom from ATas in the hybrid group vs. a matched catheter ablation group at 20 months of follow-up was 87% vs. 53%, respectively.
The current study is the first evaluating the long-term outcomes after hybrid one-step AF ablation. One-stage hybrid AF ablation has the advantage to reduce hospitalizations while potentially enhancing clinical outcomes.23,24 Indeed, in a pre-clinical model from our group, simultaneous epi–endo ablation produced broader and deeper lesions.25 Two-stages hybrid AF ablation might be associated with shorter procedural time and the possibility to assess lesions after oedema resolution.
Our study included a cohort of 120 patients, of whom the majority of first-do hybrid procedures were performed for long-standing persistent AF (80%). The long-term free survival from ATas was 47.5% at ≈5 years follow-up. At 2 years follow-up freedom from ATas was 67.5%. This is consistent with previous literature from our group10 and other groups.26 Pison et al. reported 87% of ATas free survival at 2 years follow-up.13 However, 37.2% of patients had paroxysmal AF compared with 20.0% in the current study. The hereby reported ATas freedom at 3 years was 60.5%. The only study reporting 3 years follow-up after hybrid AF ablation found ≈80% ATas freedom in a cohort with 47% of paroxysmal AF patients.11
In the current study, freedom from ATas in patients with persistent or long-standing persistent AF undergoing hybrid AF ablation after a single procedure was 45.8%. This is higher than some studies on conventional transcatheter AF ablation. Wynn et al.27 at a mean follow-up of 46 months reported a 25% survival free from ATas after AF ablation in patients with persistent AF. At 5 years follow-up, arrhythmia-free survival rates were 29% from a large single-centre cohort of 100 patients and patients with long-standing persistent AF experienced a higher recurrence rate than those with paroxysmal or persistent AF.28 In a meta-analysis by Ganesan et al., for patients with non-paroxysmal AF, single-procedure success was 50.8% at 1 year and 41.6% at 3 years.29 More recently, Kawaji et al.30 reported a 5-year freedom from ATas of 42.5% in persistent AF after first procedure and 70.8% after last procedure, with 14% of patients on AADs. The use of contact-force catheters was associated with improved outcomes and a 5-years freedom from AF recurrence after first procedure of 52.0% and 37.0% in patients with persistent and long-standing persistent AF, respectively.31
Redo hybrid ablation and safety outcomes
Hybrid ablation was performed as a redo procedure in 35 patients, of whom 29 patients (82.8%) with isolated PVs. All paroxysmal AF patients were in the redo group. ATas free survival for redo procedures was 55.0% at ≈5 years follow-up. Recurrence of AF after first PVI especially with isolated PVs represents a challenging scenario and no ablation strategy has consistently demonstrated to improve clinical outcomes.32 In a recent randomized trial, the addition of LAPWI to re-PVI at first AF redo ablation did not improve clinical outcomes7; furthermore ATas free survival in this population remained poor with 30.7% of survival free from ATas at 17 months follow-up (both with or without LAPWI). The addition of SVC isolation at redo AF ablation has also not consistently shown a net clinical benefit. In a study on 276 patients (31.5% paroxysmal AF), after 12 months, there was no significant difference in ATas free survival between SVC isolation (73%) vs. No-SVC isolation (74%, P = 0.85).33
A complication occurred in 15 patients (12.5%) in the current cohort. This is lower compared to our historical cohort10 (20.3%) and might be explained by both the learning curve and an optimized medical therapy workflow. In particular, the reduction in complication rate was driven by a reduction in rate of pericarditis and pleuritis after the introduction of a standard anti-inflammatory drugs regimen post hybrid ablation in our centre. Our results are consistent with other previous studies reporting a complication rate of 8–24% after hybrid AF ablation.13,34–36
Strenghts and limitations
Limitations include referral bias due to the inclusion of patients from a tertiary centre specialized in hybrid ablation. One single experienced surgeon (M.L.M.) performed all the cases (thoracoscopic step). The current study is a retrospective single-centre study. There is no comparison between single-step and staged hybrid AF ablation as a single-step procedure is the standard in our centre. The three groups of patients analysed are heterogenous, and this might hamper sub-group analysis. However, the paper is intended as a real-world analysis of hybrid AF ablation outcomes in a wide patient population cohort.
Conclusions
In a large cohort of patients undergoing hybrid AF ablation, the long-term free survival from ATas recurrence was 47.5%. Complications occurred in 12.5% of patients. There was no difference in clinical outcomes between patients undergoing hybrid AF ablation as first procedure or as a redo, thus prompting further clinical evaluation for hybrid ablation in this complex setting.
Contributor Information
Luigi Pannone, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Sahar Mouram, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Domenico Giovanni Della Rocca, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Antonio Sorgente, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Cinzia Monaco, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Alvise Del Monte, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Anaïs Gauthey, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Antonio Bisignani, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium; Arrhythmology Unit, Ospedale Fatebenefratelli Isola Tiberina-Gemelli Isola, Rome, Italy.
Rani Kronenberger, Cardiac Surgery Department, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, Brussels, Belgium.
Gaetano Paparella, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Robbert Ramak, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Ingrid Overeinder, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Gezim Bala, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Alexandre Almorad, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Erwin Ströker, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Juan Sieira, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Pedro Brugada, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Gian Battista Chierchia, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Mark La Meir, Cardiac Surgery Department, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, Brussels, Belgium.
Carlo de Asmundis, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou Get al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339(10):659-666. [DOI] [PubMed] [Google Scholar]
- 2. Marini M, Pannone L, Della Rocca DG, Branzoli S, Bisignani A, Mouram Set al. Hybrid ablation of atrial fibrillation: a contemporary overview. J Cardiovasc Dev Dis 2022;9(9):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verma A, Jiang C, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372(19):1812-1822. [DOI] [PubMed] [Google Scholar]
- 4. Bisignani A, Pannone L, Bala G, Kazawa S, Calburean P, Overeinder Iet al. Repeat procedures for recurrent persistent atrial fibrillation: a propensity-matched score comparison between left atrial linear ablation with radiofrequency and posterior wall isolation with the cryoballoon. J Arrhythmia 2021;37(5):1287-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bisignani A, Pannone L, Miraglia V, Sieira J, Iacopino S, Bala Get al. Feasibility and safety of left atrial posterior wall isolation with a new cryoballoon technology in patients with persistent atrial fibrillation. Pacing Clin Electrophysiol 2022;45(5):605-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kistler PM, Chieng D, Sugumar H, Ling L, Segan L, Azzopardi Set al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation the CAPLA randomized clinical trial. JAMA. 2023;329(2):127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim D, Yu HT, Kim TH, Uhm JS, Joung B, Lee MHet al. Electrical posterior box isolation in repeat ablation for atrial fibrillation: a prospective randomized clinical study. JACC Clin Electrophysiol 2022;8:582–92. [DOI] [PubMed] [Google Scholar]
- 8. Kuck KH, Albenque JP, Chun KRJ, Fürnkranz A, Busch M, Elvan Aet al. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE and ICE trial. Circ Arrhythmia Electrophysiol 2019;12:1–9. [DOI] [PubMed] [Google Scholar]
- 9. Genev IK, Tompkins LA, Khare MM, Farokhi F. Comparison of the efficacy and complication rates of the hybrid maze, complete cox-maze and catheter ablation in the treatment of atrial fibrillation. J Atr Fibrillation 2017;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Asmundis C, Chierchia GB, Mugnai G, van Loo I, Nijs J, Czapla Jet al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: a single-centre experience. Europace 2017;19:58–65. [DOI] [PubMed] [Google Scholar]
- 11. Maesen B, Pison L, Vroomen M, Luermans JG, Vernooy K, Maessen JGet al. Three-year follow-up of hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg 2018;53(suppl_1):i26-i32. [DOI] [PubMed] [Google Scholar]
- 12. Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60(1):54-61. [DOI] [PubMed] [Google Scholar]
- 13. Pison L, Gelsomino S, Lucà F, Parise O, Maessen JG, Crijns HJGMet al. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg 2014;3(1):38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delurgio DB, Crossen KJ, Gill J, Blauth C, Oza SR, Magnano ARet al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythmia Electrophysiol 2020;13(12):e009288. [DOI] [PubMed] [Google Scholar]
- 15. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021:42(5):373-498. [DOI] [PubMed] [Google Scholar]
- 16. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs Jet al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022:43(7):561-632. [DOI] [PubMed] [Google Scholar]
- 17. Dan GA, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita Fet al. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) working group on cardiovascular pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace 2018;20(5):731-732an. [DOI] [PubMed] [Google Scholar]
- 18. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018:20(1):157-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Therneau TM. survival: A Package for Survival Analysis in R. R Packag version 238 2021.
- 20. Kassambara A, Kosinski M, Biecek P, Fabian S. Package ‘survminer’. In Drawing Survival Curves Using ‘ggplot2’.(R package version 0.3. 1.). R Packag. version 0.4.3. 2017.
- 21. Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JBet al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130(3):797-802. [DOI] [PubMed] [Google Scholar]
- 22. Mahapatra S, Lapar DJ, Kamath S, Payne J, Bilchick KC, Mangrum JMet al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91(6):1890-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pison L, Dagres N, Lewalter T, Proclemer A, Marinskis G, Blomstrm-Lundqvist C. Surgical and hybrid atrial fibrillation ablation procedures. Europace 2012;14(7):939-941. [DOI] [PubMed] [Google Scholar]
- 24. Maesen B, Luermans JGLM, Bidar E, Chaldoupi SM, Gelsomino S, Maessen JGet al. A hybrid approach to complex arrhythmias. Europace 2021;23(23 Suppl 2):ii28-ii33. [DOI] [PubMed] [Google Scholar]
- 25. Matteucci F, Maesen B, Vernooy K, De Asmundis C, Maessen JG, La Meir Met al. One-stage versus sequential hybrid radiofrequency ablation: an in vitro evaluation. Innov Technol Tech Cardiothorac Vasc Surg 2020;15(4):338-345. [DOI] [PubMed] [Google Scholar]
- 26. Magni FT, Al-Jazairi MIH, Mulder BA, Klinkenberg T, Van Gelder IC, Rienstra Met al. First-line treatment of persistent and long-standing persistent atrial fibrillation with single-stage hybrid ablation: a 2-year follow-up study. Europace 2021;23(10):1568-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wynn GJ, El-Kadri M, Haq I, Das M, Modi S, Snowdon Ret al. Long-term outcomes after ablation of persistent atrial fibrillation: an observational study over 6 years. Open Hear 2016;3(2):e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weerasooriya R, Khairy P, Litalien J, MacLe L, Hocini M, Sacher Fet al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57(2):160-166. [DOI] [PubMed] [Google Scholar]
- 29. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HSet al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J. Am. Heart Assoc 2013;2(2):e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawaji T, Shizuta S, Morimoto T, Aizawa T, Yamagami S, Yoshizawa Tet al. Very long-term clinical outcomes after radiofrequency catheter ablation for atrial fibrillation: a large single-center experience. Int J Cardiol 2017;249:204-213. [DOI] [PubMed] [Google Scholar]
- 31. Winkle RA, Mead RH, Engel G, Salcedo J, Brodt C, Barberini Pet al. Very long term outcomes of atrial fibrillation ablation. Hear Rhythm 2023;20(5):680-688. [DOI] [PubMed] [Google Scholar]
- 32. Gianni C, Anannab A, Della Rocca DG, Salwan A, MacDonald B, Mayedo AQet al. Recurrent atrial fibrillation with isolated pulmonary veins: what to do. Card Electrophysiol Clin 2020;12(2):209-217. [DOI] [PubMed] [Google Scholar]
- 33. Simu G, Deneke T, Ene E, Nentwich K, Berkovitz A, Sonne Ket al. Empirical superior vena cava isolation in patients undergoing repeat catheter ablation procedure after recurrence of atrial fibrillation. J Interv Card Electrophysiol 2022;65(2):551-558. [DOI] [PubMed] [Google Scholar]
- 34. Khoynezhad A, Ellenbogen KA, Al-Atassi T, Wang PJ, Kasirajan V, Wang Xet al. Hybrid atrial fibrillation ablation: current status and a look ahead. Circ. Arrhythmia Electrophysiol 2017;10(10):e005263. [DOI] [PubMed] [Google Scholar]
- 35. Bulava A, Mokracek A, Hanis J, Kurfirst V, Eisenberger M, Pesl L. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc 2015;4(3):e001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurfirst V, Mokráček A, Bulava A, Čanádyová J, Haniš J, Pešl L. Two-staged hybrid treatment of persistent atrial fibrillation: short-term single-centre results. Interact Cardiovasc Thorac Surg 2014;18(4):451-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.