In Brief
Researchers performed an extensive literature review to investigate findings regarding the relationship between progesterone, estrogen, and androgen receptors and patient and meningioma characteristics, given evidence of the sensitivity of meningiomas to gonadal steroid hormones. Hormone receptor status was found to have strong associations with patient age and sex and tumor WHO grade, histology, and anatomical location. The findings from this comprehensive study may increase understanding of receptor heterogeneity and lay the groundwork for revisiting and improving patient stratification for meningioma treatment based on proper targeting of hormonal therapy.
Keywords: meningioma, hormone receptors, meta-analysis, estrogen, androgen, progesterone, systematic review, oncology, tumor
ABBREVIATIONS : Agg-g = aggregated data group, AR = androgen receptor, ER = estrogen receptor, HR = hormone receptor, IHC = immunohistochemistry, IPD-g = individual participant data group, LB = ligand-binding, PR = progesterone receptor
Abstract
OBJECTIVE
The relationship between patient and meningioma characteristics and hormone receptors (HRs) of progesterone, estrogen, and androgen remains poorly defined despite literature suggesting that meningiomas are sensitive to gonadal steroid hormones. Therefore, the authors sought to collect and compare data on this topic by performing a systematic review and meta-analysis of reported studies of HR status in meningiomas.
METHODS
A MEDLINE PubMed literature review conducted for articles published between January 1, 1951, and December 31, 2020, resulted in 634 unduplicated articles concerning meningiomas and HRs. There were 114 articles that met the criteria of detailed detection protocols for progesterone receptor (PR), estrogen receptor (ER), and/or androgen receptor (AR) using immunohistochemistry (IHC) or ligand-binding (LB) assays and simultaneous reporting of HR status with at least one variable among age, sex, histology, location, grade, or recurrence. Between-study heterogeneity and risk of bias were evaluated using graphical and statistical methods. The authors performed a multilevel meta-analysis using random-effects modeling on aggregated data (n = 4447) and individual participant data (n = 1363) with subgroup results summarized as pooled effects. A mixed-effects meta-regression using individual participant data was performed to analyze independently associated variables.
RESULTS
The 114 selected articles included data for 5810 patients with 6092 tumors analyzed to determine the expression of three HRs in human meningiomas: PRs, ARs, and ERs. The proportions of HR+ meningiomas were estimated to be 0.76 (95% CI 0.72–0.80) for PR+ and 0.50 (95% CI 0.33–0.66) for AR+ meningiomas. ER+ meningioma detection varied depending on the measurement method used and was 0.06 (95% CI 0.03–0.10) with IHC and 0.11 (95% CI 0.06–0.20) with LB assays. There were associations between age and PR and ER expression that varied between male and female patients. PR+ and AR+ were more common in female patients (OR 1.84, 95% CI 1.47–2.29 for PR and OR 4.16, 95% CI 1.62–10.68 for AR). Additionally, PR+ meningiomas were enriched in skull base locations (OR 1.89, 95% CI 1.03–3.48) and meningothelial histology (OR 1.86, 95% CI 1.23–2.81). A meta-regression showed that PR+ was independently associated with age (OR 1.11 95% CI 1.09–1.13; p < 0.0001) and WHO grade I tumors (OR 8.09, 95% CI 3.55–18.44; p < 0.0001). ER+ was negatively associated with meningothelial histology (OR 0.94, 95% CI 0.86–0.98; p = 0.044) and positively associated with convexity location (OR 1.12, 95% CI 1.05–1.18; p = 0.0003).
CONCLUSIONS
The association between HRs and meningioma features has been investigated but unexplained for decades. In this study the authors demonstrated that HR status has a strong association with known meningioma features, including WHO grade, age, female sex, histology, and anatomical location. Identifying these independent associations allows for a better understanding of meningioma heterogeneity and provides a foundation for revisiting targeted hormonal therapy in meningioma on the basis of proper patient stratification according to HR status.
Deciphering the contribution of gonadal steroid hormones to meningioma growth and development has been of interest to clinicians since Dr. Harvey Cushing noted the predominance of these tumors among female patients in 1938.1 Numerous studies have demonstrated a higher incidence of meningiomas in female patients, particularly patients with a history of uterine fibroids, breast cancer, or endometriosis; those with an elevated BMI; and transfeminine individuals undergoing hormonal gender affirmation therapy.2–10 Despite these findings, clinical trials of the selective estrogen receptor (ER) modulator tamoxifen and of the progesterone receptor (PR) antagonist mifepristone for treatment of meningioma have failed to show consistent benefit.11,12
While it is generally accepted that PRs are expressed in most meningiomas, the expression and importance of ERs and androgen receptors (ARs) remain inconclusive, and associations between PRs and features such as tumor grade and patient sex have remained controversial.13–15 Recently, it has been reported that meningiomas in patients taking cyproterone acetate, a synthetic form of progesterone that acts as a PR agonist and AR antagonist, are associated with certain anatomical locations and genetic mutations,16,17 features in meningiomas that have been shown to be clinically important.18,19 Hormone receptors (HRs) have a complex feedback network, which can lead to opposing regulation of biological targets based on receptor profiles, making it crucial to identify the presence of specific receptors before treatment.20 To address the limitations of previous studies, we conducted what is to our knowledge the largest study to date synthesizing current data regarding PR, ER, and AR status in meningiomas.21 In this study, we used both individual participant data and aggregated data collected from 114 publications reporting 6092 tumors in 5810 patients. Our findings show that HR status varies in accordance with well-established patient and tumor characteristics and provide insight into previously failed clinical trials, informing future patient stratification for treatment.
Methods
Search Strategy and Study Selection
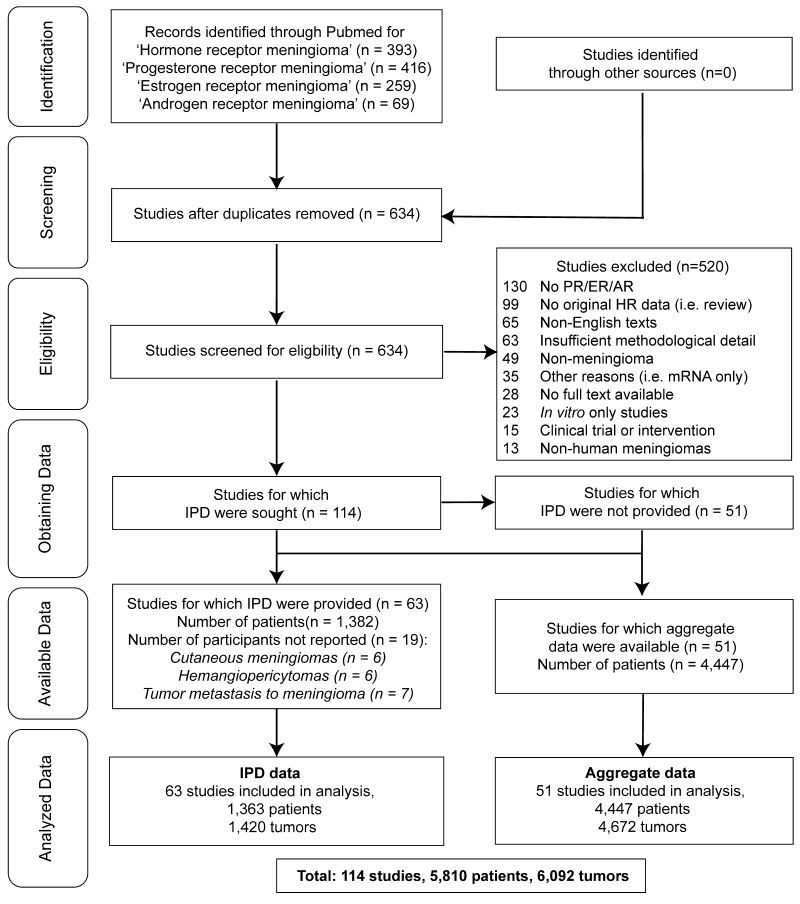
We conducted a systematic review and meta-analysis according to PRISMA-IPD 2015 guidelines using publications indexed in MEDLINE PubMed with the specific terms "hormone receptor meningioma," "estrogen receptor meningioma," "progesterone receptor meningioma," and "androgen receptor meningioma" (Fig. 1).22 The search was conducted from January 1, 1951, to December 31, 2020, and resulted in a total of 634 unduplicated articles screened. To be included, publications had to be written in the English language; assess the presence of HRs in human meningiomas using either ligand-binding (LB) or protein immunohistochemistry (IHC) assays for PR, ER, and/or AR; and report HR expression with at least one variable among age, sex, anatomical location, grade, recurrence, or histology. The IHC threshold for HR+ samples was defined as "any staining." If a study with individual participant data included quantified LB per tumor, only tumors with LB ≥ 10 fmol/mg were considered HR+ as that was the criterion set by most authors and was the most common threshold for breast cancer studies.21,23 Thirteen tumors with individual participant data and 5 studies with aggregated data were excluded because the meningiomas were in locations outside the central nervous system or there was tumor metastasis to meningiomas. Fifty-four meningiomas reported between 1992 and 2001 were removed from histological analyses because the notations were incompatible with WHO 2016 criteria (e.g., grade II meningothelial meningioma), and 6 tumors were omitted due to being classified as "hemangiopericytic." To prevent duplicate patients, we compared all publications by the same first and last authors and only included the most recent article in cases for which there was suspected overlap in patients and receptors tested. After inclusion and exclusion criteria were applied, 114 articles were included and divided into two groups: the individual participant data group (IPD-g) and the aggregated data group (Agg-g). The IPD-g consisted of 63 articles reporting 1363 total patients, and the Agg-g consisted of 51 articles reporting 4447 total patients (Supplemental Table 1, Supplemental Data).
FIG. 1.
PRISMA-IPD flowchart. Reproduced with permission of the PRISMA-IPD Group, which encourages sharing and reuse.
Defining Variables
There were 46 studies that included location variables of meningiomas with receptor status. The locations were simplified and grouped into four categories: convexity, skull base, ventricular, and spinal. Meningiomas overlying a cerebral lobe or corpus callosum, falx, parafalcine, or parasagittal location were categorized as "convexity." Tumors located in the cavernous sinus region, cerebellopontine angle, clivus, dorsum sellae, foramen magnum, infratentorial region, olfactory groove, petrous, planum sphenoidale, posterior fossa, spheno-orbital region, sphenoid, tuberculum sellae, or parasellar region, and those described as penetrating orbital structures were considered part of the "skull base." There were 76 articles that included tumor histological data. To update outdated terminologies, terms such as "syncytial" and "meningotheliomatous" were converted to "meningothelial," "fibroblastic" was converted to "fibrous," and "angioblastic" was converted to "angiomatous." Only the predominant subtype was recorded if multiple histologies were reported. Sixty-eight articles included information on WHO grade. Meningiomas were recorded as WHO grade I if they were explicitly reported by the authors as such or if the study was published after 2007 and included histology known to be synonymous with grade I (e.g., "meningothelial," "transitional," "fibrous," etc.). Cases reporting "atypical," "clear cell," or "chordoid" meningiomas were designated as WHO grade II, and those reported as "anaplastic," "malignant," "papillary," or "rhabdoid" were designated as WHO grade III.24 There were 199 female patients with reported data about menopausal status or other details such as hysterectomies or pregnancy during presentation or onset. The average age of menopause worldwide is 51.4 years, with 88% of women achieving menopause by this age, while the entire gaussian distribution of perimenopause occurs between 40 and 58 years.25,29 As such, female patients without explicit menopausal data were categorized as premenopausal (aged 18–40 years), perimenopausal (aged 40–60 years), or postmenopausal (aged > 60 years).
Quality Assessment
We conducted a risk of bias assessment with customized screening criteria tailored to the method of receptor identification, receptor quantification, group bias, and missingness of variables assessed. The assessment used ordinal classifiers (low, medium, high, and critical) to qualitatively rate the level of risk of bias in each category. We converted the ordinal classifiers into numeric scores to determine an overall measurement of the risk of bias. The scores for each of the four categories (receptor identification, receptor quantification, cohort bias, and missingness of variables assessed) were summed and averaged to generate an overall score. An overall score ≥ 3 was classified as critical (n = 6), an overall score ≥ 2.5–2.9 was classified as high (n = 24), an overall score ≥ 2–2.4 was classified as medium (n = 45), and an overall score ≥ 1–1.9 was classified as low (n = 39).
We evaluated the categories based on the following considerations. 1) Receptor identification was an identification method based on the likelihood of detecting all valuable HR genes (i.e., ESR1 [ERα], ESR2 [ERβ], and GPER1). We also considered the compartment studied (nucleus or cytosol), with less risk of bias given to studies that assessed both compartments. Studies that assessed ER with only one method for one gene were given a high score, while studies that assessed both nuclear and cytoplasmic compartments were given a low score. 2) Receptor quantification included ratings of low for quantitative studies, medium for semiquantitative studies, and high for qualitative studies. Seventeen studies were given a high rating for using a cutoff that was arbitrarily more stringent than those used in other studies (> 2% of nuclei). Twelve studies that lacked specific details of their method to determine HR+ tumors were designated as critical. 3) Cohort bias, for which a low rating was given to studies that stated that their cohorts were selected consecutively/sequentially, prospectively, for case-control studies, or randomly from all cases. A high rating was given to case reports or narrowly selected patients (i.e., only angiomatous or skull base), and a medium rating to all other cases. 4) Missingness among variables was evaluated based on the number of variables reported for a possible 6 characteristics: age, sex, location, histology, recurrence, and grade. A critical rating was given when 0 or 1 variable was reported, a high rating for 1 or 2 variables, a medium rating for 3 or 4 variables, and a low rating for 5 or 6 variables reported. Study heterogeneity was evaluated using graphical methods of analysis, such as a Baujat plot, funnel plot asymmetry (Peter’s linear regression test), and a chi-square test of heterogeneity between subgroups. Sensitivity was measured using an I2-ranked leave-one-out cross-validation.
Statistical Analysis
We employed a two-step approach to data evaluation. The first step involved the exploratory analysis of IPD-g, and the second step involved a pooled estimate and meta-analysis with Agg-g and IPD-g. All analyses were done using R version 4.0.3 (The R Foundation for Statistical Computing) and the packages metafor (version 3.9-21), meta (version 6.1-0), stats (version 3.6.2), epitools (version 0.09), and forestploter (version 1.0.0).26,27 Cases with missing variables were omitted from individual analyses. A multilevel meta-analysis was performed with a Hartung-Knapp adjustment for random effects and heterogeneity estimates as I2 to synthesize data between IPD-g and Agg-g and separately between the detection methods LB and IHC for all studies. HR proportions and meta-regression were calculated using a logit-transformed, random intercept regression model with a maximum-likelihood estimator for τ2 and Clopper-Pearson confidence intervals for individual studies. For comparisons of HR status between sexes and WHO grade (low vs high), we used an inverse variance method with the Paule-Mandel estimator for τ2 with a Q-profile for confidence intervals,28 for which the prediction interval was reported as an estimate of the between-study variance, which was calculated using the metafor package.28 Effect measures were computed as odds ratios and reported with 95% confidence intervals. For categorical variables, exploratory univariate analysis using the chi-square test without correction was conducted except in cases where n ≤ 5 and the Fisher exact test was used. For the univariate analysis of covariates, one-way ANOVA or two-tailed t-tests were used when appropriate. Similarly, Spearman correlation coefficients were reported when appropriate. For regressions, we calculated the variance inflation factor to exclude covariates > 5.
Results
We conducted a systematic review and multilevel meta-analysis with pooled effects for 6092 tumors from 5810 patients. There were 4672 tumors in 4447 patients from 51 articles (Agg-g) and individual participant data from 1420 tumors in 1363 patients in 63 articles (IPD-g) identified from a total of 634 unduplicated, full-text articles screened (Fig. 1).
Study Characteristics
Patients in the IPD-g had a mean age of 52.42 years (SD 17.86 years; Table 1). Expectedly, meningiomas were reported in a higher proportion of females (65%) than males. Race or ethnicity was only reported in 28 cases and was thus excluded from our analyses. Most cases were first-time diagnoses (78%) and WHO grade I (67%). The predominant histology was meningothelial (36%), followed by transitional (29%) and fibrous (14%). Anatomically, tumors were located at the convexity (50%), skull base (35%), spinal (13%), and intraventricular (3%). The Agg-g had a mean age of 53.76 years (SD 6.41 years; Table 1) and also showed female predominance (70%). Most of the tumors were primary diagnoses (91%) and WHO grade I (76%). The most common histology was meningothelial (35%), followed by transitional (22%) and fibrous (15%). Tumor locations included convexity (50%), skull base (38%), spinal (11%), and intraventricular (1%).
TABLE 1.
Cohort characteristics
| IPD-g (n = 63) | Agg-g (n = 51) | |
|---|---|---|
| Pt characteristics |
|
|
| No. of pts |
1363 |
4447 |
| No. of tumors |
1420 |
4672 |
| Age at op, yrs |
52.42 ± 17.86 |
53.76 ± 6.41 |
| Sex |
|
|
| Female |
849 (65) |
2706 (70) |
| Male |
451 (35) |
1168 (30) |
| WHO grade |
|
|
| I |
403 (67) |
2652 (76) |
| II |
126 (21) |
559 (16) |
| III |
70 (12) |
269 (8) |
| Diagnosis |
|
|
| Primary |
354 (78) |
1881 (91) |
| Recurrent |
102 (22) |
186 (9) |
| Tumor characteristics |
|
|
| Histology |
|
|
| Anaplastic |
31 (3) |
63 (2) |
| Angiomatous |
23 (2) |
165 (6) |
| Atypical |
54 (5) |
367 (13) |
| Fibrous |
142 (14) |
418 (15) |
| Meningothelial |
371 (36) |
995 (35) |
| Psammomatous |
37 (4) |
126 (4) |
| Transitional |
294 (29) |
615 (22) |
| Secretory |
62 (6) |
28 (1) |
| Clear cell |
16 (2) |
26 (1) |
| Anatomical location |
|
|
| Convexity |
328 (50) |
790 (50) |
| Skull base |
230 (35) |
607 (38) |
| Spinal |
83 (13) |
167 (11) |
| Intraventricular | 20 (3) | 21 (1) |
Pt = patient.
Values are presented as number (%) or mean ± SD.
Investigating Heterogeneity
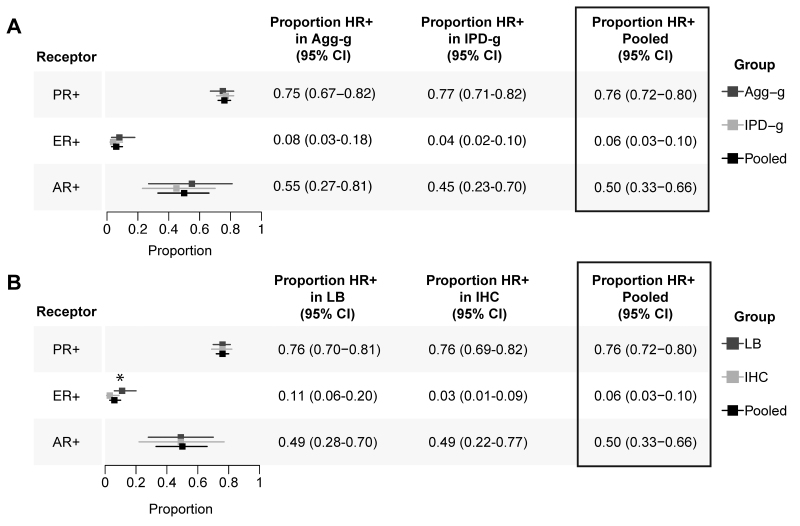
To assess the impact of heterogeneity between the studies, we evaluated the pooled effects between the IPD-g and Agg-g data sets and found that, for all HRs, the heterogeneity was reduced within IPD-g compared with Agg-g (I2 for IPD-g vs Agg-g: PR 49% vs 92%, ER 36% vs 91%, and AR 38% vs 93%; Supplemental Figs. 1–3). We created risk of bias criteria and conducted a graphical and statistical evaluation for bias (Supplemental Figs. 4–7). To examine the impact of the methodology used for HR detection, we pooled the effects based on IHC versus LB (Supplemental Figs. 8–10). Our results showed that the method used significantly influenced ER detection, with LB having higher rates of detection and lower heterogeneity (81% for IHC and 71% for LB, between-subgroup difference χ2 = 4.31, p = 0.03; Supplemental Fig. 8). There were no significant differences in detection of PR and AR when comparing IHC and LB methods (Supplemental Figs. 9 and 10). There was a large amount of heterogeneity across all studies with no single study deemed an outlier in all evaluations; therefore, no studies were excluded for risk of bias.
Exploratory Analysis Using Individual Participant Data
To determine which variables to include in the meta-analysis and meta-regression, we used the IPD-g to analyze co-occurring features in an exploratory univariate analysis of sex, grade, recurrence, histology, location, and age for each receptor (Supplemental Table 2). We found significant associations between PR+ and older age (p = 0.022), female sex (p < 0.0001), tumor grade (p < 0.0001), and primary meningiomas (p = 0.014). Relative to convexity, according to individual participant data analyses, skull base (p = 0.0011) and spinal tumors (p = 0.032) were enriched for PR+, as were meningothelial meningiomas compared with anaplastic (p = 0.00039), atypical (p = 0.0079), fibrous (p = 0.00065), and transitional (p = 0.0043) histologies. For ER+ meningiomas, we found associations between meningothelial and psammomatous (p = 0.033) and secretory (p = 0.046) meningiomas, as well as between convexity and skull base (p = 0.017) and spinal (p = 0.010) locations. We found associations between AR+ meningiomas and female sex (p = 0.002) and fibrous histology (p = 0.02).
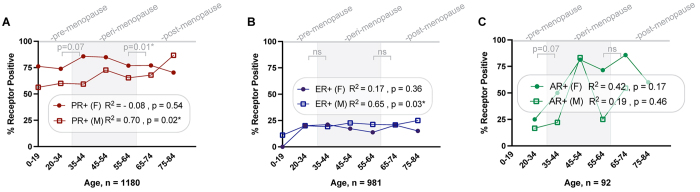
To investigate the relationship between HR status, age, and patient hormonal states, we conducted several analyses. We first divided the patient data into two groups based on sex and found that in male patients there was a positive linear association between increasing age and the proportion of meningiomas that were PR+ (R2 = 0.70, p = 0.02; Fig. 2A). We divided the female patient data into groups based on menopausal status and found that postmenopausal women were less likely to have a PR+ tumor than perimenopausal women (p = 0.013; Supplemental Table 2). A positive association was seen between male sex, increasing age, and ER+ status (R2 = 0.65, p = 0.03; Fig. 2B), with no difference in ER expression in females, regardless of their menopausal status (Supplemental Table 2). No statistically significant linear relationship was seen between AR+ meningiomas and age in either sex or between menopausal states (Fig. 2C, Supplemental Table 2).
FIG. 2.
Associations between age and receptor status across the lifespan of males and females. Data for individual patients were divided into seven groups according to age, and the proportion of patients with PR+ (A), ER+ (B), and AR+ (C) meningiomas were calculated for each bin. These data were then tested using a simple linear regression with R2. Additionally, an assessment based on categorical evaluation of hormonal status in women was performed for each receptor. ns = not significant. *p < 0.05.
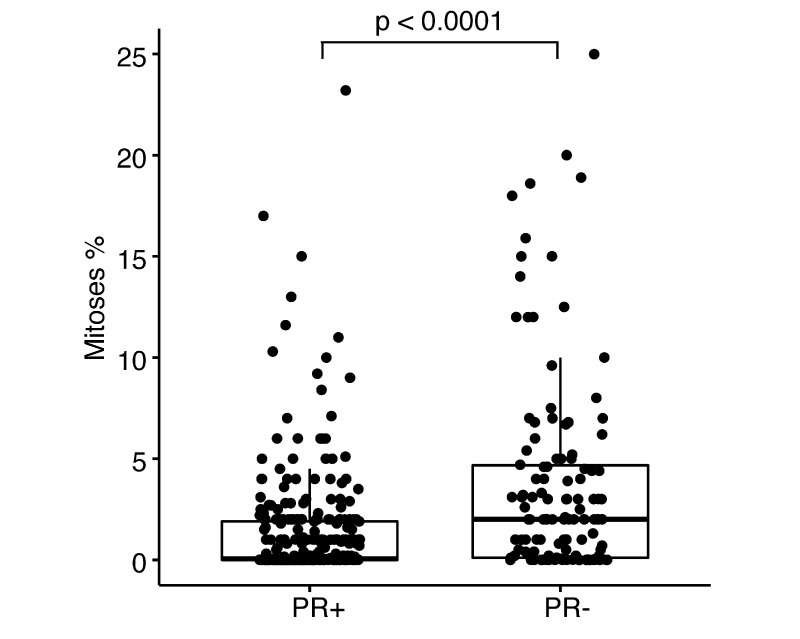
We then analyzed the quantitative measurements of receptor LB to assess its relationship with age. Our findings show a weak correlation between the cytoplasmic LB of ER+ meningiomas and increasing age (n = 340; Spearman’s r = 0.12, p = 0.029). However, no such relationship was found between nuclear ER and age, and neither were nuclear or cytoplasmic associations seen for PR+ meningiomas and age. To further explore the relationship between receptor and grade, we evaluated the relationship between LB and mean mitoses, as measured by authors using Ki-67/MIB-1 markers. The results showed that PR+ samples had lower mean mitoses per tumor than PR− samples (1.37% ± 2.77% vs 3.71% ± 5.01%; two-sided t-test, p < 0.0001; Fig. 3). No significant difference in mitoses was observed between ER status. Because of the small number of AR+ meningiomas with quantitative LB (n = 8), we did not perform a complete analysis for these receptors.
FIG. 3.
Association between PR status and mitotic index. A two-tailed t-test was used to evaluate the differences in mitotic index percentages (y-axis) and PR status (x-axis). Each dot represents 1 tumor. The bar represents the 95% confidence interval, with the mean represented by the horizontal black bar in the middle of each bar plot.
To measure the association between variables, we used the chi-square test to evaluate sex, grade, recurrence, histology, and location and a two-tailed t-test to assess the relationship between age and these variables (Supplemental Table 3). The analysis confirmed associations between sex and grade (p < 0.0001) and sex and recurrence (p = 0.0007), as well as sex and age (p < 0.0001). Meningothelial histology was associated with grade (p < 0.0001), skull base location (p = 0.0006), and age (p = 0.0026). Grade was associated with skull base location (p = 0.0014) and recurrence (p = 0.0063). To evaluate the features independently associated with female sex, we performed a logistic regression that included the significant variables on univariate analysis: PR status, age, year of publication, detection method, and recurrence (Supplemental Table 4), which determined that age was independently associated with female sex (p < 0.02). Next, we summarized the co-expression breakdown of tumors simultaneously assessed for PR and ER (n = 991), which revealed a variety of receptor combinations: PR+/ER− was the largest percentage of cases (64%), followed by PR−/ER− (22%), PR+/ER+ (11%), and PR−/ER+ (3%; Supplemental Fig. 11D). There were 63 tumors that were assessed for PRs, ERs, and ARs simultaneously. The tumors had many receptor combinations, including "triple negative" (13%) and "triple positive" (6%), and equal percentages of meningiomas with PR+/ER−/AR+ and PR+/ER−/AR− (35%). There were 6% PR−/ER−/AR+, 3% PR+/ER+/AR−, and 2% PR−/ER+/AR– meningiomas (Supplemental Fig. 11E).
Synthesized Analysis of HR Associations With Patient and Tumor Features
We conducted a meta-analysis by synthesizing individual participant data (IPD-g) and aggregated data (Agg-g) with a pooled effect estimate by group for the proportion of HR+ tumors (Fig. 4), HR status between males and females, and the relationship between HR status and grade. The proportion of receptors was estimated to be 0.76 for PR+ (95% CI 0.72–0.80; n = 5391; Supplemental Fig. 1), 0.06 for ER+ (95% CI 0.03–0.10; n = 3249; Supplemental Fig. 2), and 0.50 for AR+ (95% CI 0.33–0.66; n = 761; Supplemental Fig. 3). We found that relative to male patients, female patients were more likely to have PR+ (OR 1.84, 95% CI 1.47–2.29, prediction interval 95% CI 1.47–2.29; n = 2429; Supplemental Fig. 12) and AR+ (OR 4.16, 95% CI 1.62–10.68, prediction interval 95% CI 1.57–11.03; n = 195; Supplemental Fig. 13) meningiomas, but these results were not seen for ER+ meningiomas (Supplemental Fig. 14). We then analyzed the relationship between PR+ status and meningiomas graded as WHO grade I (low grade) or WHO grade II or III (high grade) and found that PR+ status was associated with low-grade tumors (n = 2893; OR 4.13, 95% CI 2.47–6.90; Supplemental Fig. 15). There was no difference associated with WHO grade seen for ER (Supplemental Fig. 16) or AR (Supplemental Fig. 17) status.
FIG. 4.
Summary of HR expression meta-analysis. Simplified forest plots representing the proportion of each receptor analysis by aggregated subgroup. Results by study can be found in the Supplemental Figures as indicated in the text. The pooled estimate of each subgroup is represented by the square and 95% confidence intervals by the horizontal line for each square. A: Subgroup comparison summaries of Agg-g (dark gray) and IPD-g (light gray) with pooled effects indicated with black. B: Subgroup comparison summaries by detection method: LB (dark gray) and IHC (light gray). *p = 0.03 for difference between detection methods.
For a more in-depth examination of histological and location features identified in the individual participant data univariate exploratory analysis, we chose to conduct a meta-analysis with only individual participant data studies (n = 63 articles, n = 1420 tumors) due to missing data and excess heterogeneity seen in Agg-g. We found that PR+ status was associated with meningothelial histology (n = 866; OR 1.86, 95% CI 1.23–2.81, prediction interval 95% CI 1.01–3.40; Supplemental Fig. 18) and skull base location (n = 585; OR 1.89, 95% CI 1.03–3.48, prediction interval 95% CI 0.49–7.30; Supplemental Fig. 19). While spinal location was enriched on univariate analysis, no difference was shown in the meta-analysis (Supplemental Fig. 20). In the analysis of ER meningiomas, we found an association with meningothelial histology (n = 669; OR 1.84, 95% CI 1.18–2.86; Supplemental Fig. 21) and convexity location (n = 472; OR 1.84, 95% CI 0.99–3.42; Supplemental Fig. 22). The analysis of AR status revealed a wide confidence interval for fibrous histology, likely due to an insufficient number of tumors (n = 17; OR 7.12, 95% CI 0.32–160.16; Supplemental Fig. 23).
We performed a mixed-effects multiple meta-regression to identify the covariates that were independently associated with receptor status (Table 2), followed by a multimodal inference analysis to quantify the relative importance of each variable in the models (Supplemental Fig. 24). The meta-regression was carried out separately for each receptor (PR, ER, and AR). To avoid multicollinearity, we calculated the variance inflation factor for each variable used in each model (Supplemental Table 5). For PR expression, the model included the covariates age, sex, meningioma grade, meningothelial histology, location at the skull base, year of article publication, and detection method. The model included 293 tumors and had a residual heterogeneity I2 of 79.62%. The results indicated that increased age (OR 1.11, 95% CI 1.09–1.13; p < 0.00001), WHO grade I (OR 8.09, 95% CI 3.55–18.44; p < 0.00001), and year published (OR 1.21, 95% CI 1.16–1.27; p < 0.00001) were independently associated with PR+ expression. For ER expression, the model included 427 tumors, and the residual heterogeneity I2 was 0.00%. The covariates included age, sex, meningothelial histology, convexity location, receptor detection method, and year published. The results indicated that the detection method used had an independent association with ER expression, with a decrease in detection when evaluating IHC compared with LB analyses (OR 0.47, 95% CI 0.42–0.52; p < 0.00001). Other covariates that were independently associated with ER expression were convexity location (OR 1.12, 95% CI 1.05–1.18; p = 0.0003), meningothelial histology (OR 0.94, 95% CI 0.86–0.98; p = 0.044), and year published (OR 1.01, 95% CI 1.01–1.02; p < 0.00001). The model for AR included only 68 tumors and the covariates sex, age, fibrous histology, receptor detection method, and year published. The model resulted in 0.00% residual heterogeneity, but only year published (OR 1.24, 95% CI 1.19–1.29; p < 0.00001) and receptor detection method (OR 4.63, 95% CI 3.06–7.00; p < 0.00001) were associated with AR expression.
TABLE 2.
Summary and synthesis of results based on HR status and meningioma characteristics
| 1-Way ANOVA |
Subgroup Meta-Analysis |
IPD-g–Only Meta-Analysis |
Mixed-Effects Meta-Regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | OR | 95% CI | OR | 95% CI | p Value | |
| PR+ |
|
|
|
|
|
|
|
|
|
|
| Pt age |
1.00 |
1.00–1.00 |
0.02
|
— |
— |
— |
— |
1.11 |
1.09–1.13 |
<0.0001
|
| Female sex |
1.15 |
1.09–1.211 |
<0.0001
|
1.84 |
1.47–2.29 |
— |
— |
0.80 |
0.41–1.54 |
0.50 |
| Low grade |
1.28 |
1.19–1.39 |
<0.0001
|
3.64 |
2.22–5.96 |
— |
— |
8.09 |
3.55–18.44 |
<0.0001
|
| Meningothelial |
1.12 |
1.06–1.19 |
<0.0001
|
— |
— |
1.86 |
1.23–2.81 |
1.28 |
0.63–2.59 |
0.49 |
| Skull base |
1.12 |
1.04–1.20 |
0.0028
|
— |
— |
1.89 |
1.03–3.48 |
1.08 |
0.54–2.16 |
0.83 |
| Spinal |
1.09 |
0.98–1.20 |
0.122 |
— |
— |
1.23 |
0.21–7.40 |
0.68 |
0.31–1.46 |
0.32 |
| Detection method (IHC) |
0.98 |
0.94–1.03 |
0.54 |
*
|
*
|
— |
— |
— |
— |
— |
| Year published |
0.99 |
1.00–1.00 |
0.44 |
— |
— |
— |
— |
1.21 |
1.16–1.27 |
<0.0001
|
| ER+ |
|
|
|
|
|
|
|
|
|
|
| Pt age |
1.00 |
1.00–1.00 |
0.44 |
— |
— |
— |
— |
0.99 |
0.98–1.00 |
0.48 |
| Female sex |
1.04 |
0.86–1.05 |
0.84 |
1.20 |
0.70–2.06 |
— |
— |
1.05 |
0.92–1.12 |
0.09 |
| Meningothelial |
1.04 |
0.99–1.10 |
0.11 |
— |
— |
1.84 |
1.18–2.86 |
0.94 |
0.86–0.98 |
0.044
|
| Convexity |
1.06 |
1.03–1.10 |
0.0008
|
— |
— |
1.84 |
0.99–3.42 |
1.12 |
1.05–1.18 |
0.0003
|
| Detection method (IHC) |
0.88 |
0.85–0.92 |
<0.0001
|
*
|
*
|
— |
— |
0.47 |
0.42–0.52 |
<0.0001
|
| Year published |
0.99 |
0.98–1.00 |
0.08 |
— |
— |
— |
— |
1.01 |
1.01–1.02 |
<0.0001
|
| AR+ |
|
|
|
|
|
|
|
|
|
|
| Pt age |
1.01 |
1.00–1.01 |
0.071 |
— |
— |
— |
— |
1.00 |
0.99–1.01 |
0.71 |
| Female sex |
3.53 |
1.61–7.74 |
0.002
|
4.16 |
1.62–10.69 |
— |
— |
1.12 |
0.90–1.41 |
0.32 |
| Fibrous |
8.57 |
1.80–40.80 |
0.002
|
— |
— |
7.12 |
0.32–160.16 |
1.05 |
0.80–1.38 |
0.72 |
| Detection method (IHC) |
1.13 |
0.95–1.35 |
0.18 |
*
|
*
|
— |
— |
4.63 |
3.06–7.00 |
<0.0001
|
| Year published | 0.99 | 0.98–1.00 | 0.08 | — | — | — | — | 1.24 | 1.19–1.29 | <0.0001 |
Boldface type indicates statistical significance.
Test for subgroup differences when assessing PR+ proportion of IHC compared to LB (χ2 = 0.01, p = 0.91), ER+ (χ2 = 4.87, p = 0.03), or AR+ (χ2 = 0, p = 0.99).
Discussion
In this meta-analysis and systematic review of previously published data from studies on the relationship between patient and meningioma characteristics and progesterone, estrogen, and androgen HRs, the significant differences in receptor expression identified between male and female patients over the lifespan may be related to hormonal changes that occur in the hypothalamic-pituitary-gonadal axis during aging. Specifically, for PR expression, the highest levels were observed in perimenopausal female patients, while in male patients there was a positive linear association between PR and ER expression with increasing age. These findings are particularly notable because the entire gaussian distribution of menopause in women worldwide occurs between the ages of 40 and 58 years, and this perimenopausal period is known to result in significant metabolic changes in the female brain driven by hormonal fluctuations.29 Similarly, men have a gradual decrease in testosterone with age, resulting in metabolic changes.30 Notably, this effect of age also corresponds to the highest female-to-male ratio in meningiomas, which may be explained by the sum of the combination of differences observed in receptor expression with age and sex.10 Moreover, arachnoid granulations, derived from the proposed cells of origin for meningiomas, increase in number along the sagittal sinus and peak around age 40 years, regardless of sex,31 and this same age range is when diagnostic imaging reveals the highest number of incidental meningiomas, which are estimated to have prevalence rates as high as 1%–3% of the population on autopsy.32,33 This information suggests that hormonal changes during aging may provide an opportunity for meningioma development; however, further research is needed to better understand the relationship between hormones, meninges, and aging. Importantly, very little is known about the role of hormones in normal meningeal physiology or the expression of PRs, ERs, and ARs in the meninges.
Inadequate HR detection has implications beyond the observational associations identified in this study and specifically establishes a need for a critical reevaluation of previous clinical trials related to steroid hormones. We previously reported that more than 50% of patients included in these trials did not have positive HR expression before being treated with hormone therapies.34 The lack of receptor detection was detrimental to these trials as HRs have varying biological effects, thought to be a consequence of the different recruitment of coregulatory and epigenetic elements.20 Inadequate detection of receptors, along with their ligand-specific activity, calls into question the utility of treatment with hormonal therapies without characterized receptor expression. The clinical relevance of these considerations is particularly evident with selective receptor modulators, the most well studied of which is tamoxifen, which can induce cell death in breast cancer while promoting cell growth in the endometrium.20,35 The importance of proper characterization of receptor activity in meningiomas is further exemplified by the finding that PR+ expression is independently associated with WHO grade I and enriched in meningiomas with a relatively low proliferation index. The only phase III clinical trial of a gonadal steroid hormone–related therapy used mifepristone, a PR and glucocorticoid receptor antagonist and weak androgen agonist. The results of this study call into question whether PR as a therapeutic target needs to be suppressed, or whether doing so may lead to worse long-term outcomes by disinhibition of proliferation. On the other hand, the observation that a history of treatment with the progestin and androgen antagonist cyproterone acetate may be associated with meningiomas of certain genetic backgrounds should be investigated further.17
Inadequate receptor detection may also explain the differences between the LB and IHC detection of ER in meningiomas that we identified in our investigation of reported data. Specifically, there were no included studies that sufficiently differentiated between the protein expression of receptors encoded from the distinct ER genes ESR1 (ERα), ESR2 (ERβ), and GPER1. Further investigation of this finding is important, as each of these receptors has unique LB affinities and non–cross-reactivity with antibody detection and may form receptor complexes resulting in different pathway activation when expressed simultaneously, leading to distinct cellular responses to therapy.36–38 To gain a complete understanding of the impact that combinatorial receptor expression has in these tissues, parallel assessments of AR, PR, ERα, ERβ, and GPER1 should be prioritized, especially considering their complex network of feedback regulation. Further research comparing HRs present in meningiomas and meninges over the lifespan, as well as the effects of hormonal agents on both tissues, must be conducted before further clinical implications can be considered or clinical trials are reattempted.
Strengths and Limitations
This study has several strengths, including its well-defined inclusion criteria and large number of patients in the reviewed articles, many of which included individual participant data. These strengths are significant, particularly given the long-standing debate on the topic. However, the study is not without limitations. The large heterogeneity between studies, missing variables, and nonrandom selection of patients by authors pose major limitations, as does the impact of IHC analysis on the detection of the receptors. The limited number of studies that included AR hindered the analysis of several variables. Despite efforts at standardization, the WHO grades and histological classifications are based on notation by different pathologists over many decades. These limitations should be taken into account when designing future investigations of HRs in meningioma.
Conclusions
This meta-analysis and systematic review is to our knowledge the largest and most comprehensive study undertaken about gonadal steroid HR expression in human meningiomas and included 6092 tumors from 5810 patients across 114 published investigations. We found that PR expression is enriched in WHO grade I tumors and is associated with age in a sex-specific manner. Significant differences in tumor location and histology between PR+ and ER+ meningiomas were also found. By synthesizing data from decades of investigation, this study provides groundwork for reevaluating hormonal therapy as a precision medicine approach to treatment for patients stratified by receptor status.
Acknowledgments
This study was supported by the Gregory M. Kiez and Mehmet Kutman Foundation, Connecticut Brain Tumor Alliance, and Yale School of Medicine funds; NIH/NCI (no. F31CA254426); and NIH–Medical Scientist Training Program (no. T32GM007205).
Disclosures
Dr. Moliterno reported being a consultant for BK Medical outside the submitted work. Dr. Günel reported personal fees from AI Therapeutics, Hyperfine, and 4C Companies outside the submitted work.
Author Contributions
Conception and design: Günel, Claus, Miyagishima. Acquisition of data: Günel, Miyagishima, Gutierrez, Barak. Analysis and interpretation of data: Günel, Miyagishima, Sundaresan, Yeung, Claus. Drafting the article: Günel, Miyagishima, Sundaresan, Gutierrez, Claus. Critically revising the article: all authors. Reviewed submitted version of manuscript: Günel, Miyagishima, Sundaresan, Gutierrez, Barak, Yeung, McGuone, Claus. Approved the final version of the manuscript on behalf of all authors: Günel. Statistical analysis: Miyagishima, Sundaresan. Administrative/technical/material support: Günel.
Supplemental Information
- Supplemental Data. https://thejns.org/doi/suppl/10.3171/2023.3.JNS221838.
Previous Presentations
Portions of this article were presented virtually as "A Systematic Review and Meta-analysis of Sex-Hormone Receptors in Meningioma" at the 2021 Brain Tumor Epidemiology Consortium Conference, held on June 23, 2021.
References
- 1.Cushing H. Charles C Thomas; 1938. Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical End Result. [Google Scholar]
- 2. Bickerstaff ER, Small JM, Guest IA. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21(2):89–91. doi: 10.1136/jnnp.21.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laviv Y, Ohla V, Kasper EM. Unique features of pregnancy-related meningiomas: lessons learned from 148 reported cases and theoretical implications of a prolactin modulated pathogenesis. Neurosurg Rev. 2018;41(1):95–108. doi: 10.1007/s10143-016-0762-3. [DOI] [PubMed] [Google Scholar]
- 4. Shahin MN, Magill ST, Dalle Ore CL, et al. Fertility treatment is associated with multiple meningiomas and younger age at diagnosis. J Neurooncol. 2019;143(1):137–144. doi: 10.1007/s11060-019-03147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Claus EB, Black PM, Bondy ML, et al. Exogenous hormone use and meningioma risk: what do we tell our patients? Cancer. 2007;110(3):471–476. doi: 10.1002/cncr.22783. [DOI] [PubMed] [Google Scholar]
- 6. Lusis EA, Scheithauer BW, Yachnis AT, et al. Meningiomas in pregnancy: a clinicopathologic study of 17 cases. Neurosurgery. 2012;71(5):951–961. doi: 10.1227/NEU.0b013e31826adf65. [DOI] [PubMed] [Google Scholar]
- 7. Li Q, Coulson H, Klaassen Z, et al. Emerging association between androgen deprivation therapy and male meningioma: significant expression of luteinizing hormone-releasing hormone receptor in male meningioma. Prostate Cancer Prostatic Dis. 2013;16(4):387–390. doi: 10.1038/pcan.2013.45. [DOI] [PubMed] [Google Scholar]
- 8. Yen YS, Sun LM, Lin CL, Chang SN, Sung FC, Kao CH. Higher risk for meningioma in women with uterine myoma: a nationwide population-based retrospective cohort study. J Neurosurg. 2014;120(3):655–661. doi: 10.3171/2013.10.JNS131357. [DOI] [PubMed] [Google Scholar]
- 9. Claus EB, Calvocoressi L, Bondy ML, Wrensch M, Wiemels JL, Schildkraut JM. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg. 2013;118(3):649–656. doi: 10.3171/2012.9.JNS12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ji Y, Rankin C, Grunberg S, et al. Double-blind phase III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable meningioma: SWOG S9005. J Clin Oncol. 2015;33(34):4093–4098. doi: 10.1200/JCO.2015.61.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodwin JW, Crowley J, Eyre HJ, Stafford B, Jaeckle KA, Townsend JJ. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15(1):75–77. doi: 10.1007/BF01050266. [DOI] [PubMed] [Google Scholar]
- 13. Korhonen K, Salminen T, Raitanen J, Auvinen A, Isola J, Haapasalo H. Female predominance in meningiomas can not be explained by differences in progesterone, estrogen, or androgen receptor expression. J Neurooncol. 2006;80(1):1–7. doi: 10.1007/s11060-006-9146-9. [DOI] [PubMed] [Google Scholar]
- 14. Apra C, Roblot P, Alkhayri A, Le Guérinel C, Polivka M, Chauvet D. Female gender and exogenous progesterone exposition as risk factors for spheno-orbital meningiomas. J Neurooncol. 2020;149(1):95–101. doi: 10.1007/s11060-020-03576-8. [DOI] [PubMed] [Google Scholar]
- 15. Blankenstein MA, Blaauw G, Lamberts SW, Mulder E. Presence of progesterone receptors and absence of oestrogen receptors in human intracranial meningioma cytosols. Eur J Cancer Clin Oncol. 1983;19(3):365–370. doi: 10.1016/0277-5379(83)90134-7. [DOI] [PubMed] [Google Scholar]
- 16. Portet S, Naoufal R, Tachon G, et al. Histomolecular characterization of intracranial meningiomas developed in patients exposed to high-dose cyproterone acetate: an antiandrogen treatment. Neurooncol Adv. 2019;1(1):vdz003. doi: 10.1093/noajnl/vdz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peyre M, Gaillard S, de Marcellus C, et al. Progestin-associated shift of meningioma mutational landscape. Ann Oncol. 2018;29(3):681–686. doi: 10.1093/annonc/mdx763. [DOI] [PubMed] [Google Scholar]
- 18. Youngblood MW, Duran D, Montejo JD, et al. Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg. 2020;133(5):1345–1354. doi: 10.3171/2019.8.JNS191266. [DOI] [PubMed] [Google Scholar]
- 19. Ülgen E, Bektaşoğlu PK, Sav MA, et al. Meningiomas display a specific immunoexpression pattern in a rostrocaudal gradient: an analysis of 366 patients. World Neurosurg. 2019;123:e520–e535. doi: 10.1016/j.wneu.2018.11.201. [DOI] [PubMed] [Google Scholar]
- 20. Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437–1452. doi: 10.2147/CIA.S66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabos S, Berkel J. Meta-analysis of progestin and estrogen receptors in human meningiomas. Neuroepidemiology. 1992;11(4-6):255–260. doi: 10.1159/000110938. [DOI] [PubMed] [Google Scholar]
- 22. Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 23. Allegra JC, Lippman ME, Thompson EB, et al. Estrogen receptor status: an important variable in predicting response to endocrine therapy in metastatic breast cancer. Eur J Cancer (1965) 1980;16(3):323–331. doi: 10.1016/0014-2964(80)90348-5. [DOI] [PubMed] [Google Scholar]
- 24. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 25. Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 26.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. 1st ed. Chapman & Hall/CRC Press; 2021. Doing Meta-Analysis With R: A Hands-On Guide. [Google Scholar]
- 27. Tierney J, Stewart L, Rovers M, Clarke M, Rydzewska L. The Cochrane Individual Participant Data Meta-analysis Methods Group Cochrane. https://methods.cochrane.org/sites/methods.cochrane.org.ipdma/files/uploads/MeetTheEntities_FINAL_1.pdf Accessed March 20, 2023. [Google Scholar]
- 28.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. eds. 2022. https://training.cochrane.org/handbook/current/chapter-10 Accessed March 20, 2023. [Google Scholar]
- 29. Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22(5 suppl):110–116. [PubMed] [Google Scholar]
- 31. Radoš M, Živko M, Periša A, Orešković D, Klarica M. No arachnoid granulations—no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi: 10.3389/fnagi.2021.698865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chamoun R, Krisht KM, Couldwell WT. Incidental meningiomas. Neurosurg Focus. 2011;31(6):E19. doi: 10.3171/2011.9.FOCUS11220. [DOI] [PubMed] [Google Scholar]
- 33. Johnson MD, Abu-Farsakh S. Clinicopathologic features of incidental meningiomas: a review of the literature and the University of Rochester autopsy experience. Clin Neuropathol. 2019;38(3):118–121. doi: 10.5414/NP301160. [DOI] [PubMed] [Google Scholar]
- 34. Miyagishima DF, Moliterno J, Claus E, Günel M. Hormone therapies in meningioma—where are we? J Neurooncol. 2023;161(2):297–308. doi: 10.1007/s11060-022-04187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356(9233):881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- 36. Andersson S, Sundberg M, Pristovsek N, et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun. 2017;8(1):15840. doi: 10.1038/ncomms15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arterburn JB, Prossnitz ERG. G Protein-coupled estrogen receptor GPER: molecular pharmacology and therapeutic applications. Annu Rev Pharmacol Toxicol. 2023;63(1):295–320. doi: 10.1146/annurev-pharmtox-031122-121944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dahlman-Wright K, Cavailles V, Fuqua SA, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58(4):773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- Supplemental Data. https://thejns.org/doi/suppl/10.3171/2023.3.JNS221838.