Summary
In patients with diabetic kidney disease (DKD), the estimated glomerular filtration rate (eGFR) or creatinine clearance rate (Ccr) is always used as an index of decline in renal function. However, there are few animal models of DKD that could be used to evaluate renal function based on GFR or Ccr. For this reason, it is desirable to develop animal models to assess renal function, which could also be used for the evaluation of novel therapeutic agents for DKD. Therefore, we aimed to develop such animal model of DKD by using spontaneously hypertensive rat (SHR)/NDmcr-cp (cp/cp) rats with the characteristics of obese type 2 diabetes and metabolic syndrome. As a result, we have found that unilateral nephrectomy (UNx) caused a chronic Ccr decline, development of glomerular sclerosis, tubular lesions, and tubulointerstitial fibrosis, accompanied by renal anemia. Moreover, losartan-mixed diet suppressed the Ccr decline in UNx-performed SHR/NDmcr-cp rats (UNx-SHR/cp rats), with improvement in renal anemia and histopathological changes. These results suggest that UNx-SHR/cp rats could be used as a DKD model for evaluating the efficacy of therapeutic agents based on suppression of renal function decline.
Keywords: SHR/NDmcr-cp rat, Unilateral nephrectomy, Ccr decline
Introduction
DKD is characterized by a complicated pathology that involves renal anemia, obesity, hypertension, hyperlipidemia, and hyperglycemia, accompanied by a decline in renal function. In patients with chronic kidney disease (CKD), including DKD, eGFR or Ccr, in addition to albuminuria, is always used as indices of decline in renal function to estimate the CKD risk probability based on the Kidney Disease: Improving Global Outcomes (KDIGO) classification [1]. For approximately 30 years, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) such as ramipril and losartan have been used to treat diabetic nephropathy (DN) other than hypertension [2]. However, the target patients of these agents are limited to those suffering from DN with hypertension, type 2 diabetes mellitus, and proteinuria [3,4]. In recent years, evidences are accumulating that sodium-glucose co-transporter-2 (SGLT2) inhibitors prevent disease progression in established heart failure or CKD, independent of the presence of diabetes [5], and it is likely that the basic medication used for CKD as well as DKD patients may be changed in the near future to SGLT2 inhibitors [6].
Various animal models of DKD are known, however, the complex etiological complications associated with decreased renal function in patients with DKD, develop in only a few of them [7,8]. As an example, in the widely used streptozotocin (STZ)-induced type 1 diabetes model, blood glucose levels are elevated but, unlike in humans, hypertension, albuminuria level, and the loss of renal function are often much less severe [9]. Whereas, Zucker diabetic fatty rats, Spontaneously Diabetic Torii (SDT) fatty rats, and uni-nephrectomized db/db mice, which are DKD models with such complications, developed nephropathy with histopathological changes, however, these models did not show renal function decline as indicated by Ccr or GFR during the observation period, but rather showed an increase in GFR due to glomerular hyperfiltration [10,11]. Therefore, there are currently few pharmaco-logically available animal models that exhibit renal function decline due to the complex etiopathogenic cha-racteristics of the disease that these animals suffer from.
The SHR/cp rat is an obese, type 2 diabetic model of DKD characterized by hyperglycemia, hyperlipidemia, and hypertension with the same hypertensive background as SHR, as well as a genetic mutation in the leptin receptor gene [12]. By utilizing SHR/cp rats, we attempted to establish a DKD model showing renal function decline as assessed by Ccr. To cause the decline in renal function, UNx was performed, and in addition, the efficacy of the ARB (losartan) was evaluated as a tool compound for comparison of efficacy in this model and in patients with DN.
Materials and Methods
Animals
Male SHR/cp rats and age-matched Wistar Kyoto (WKY) rats were purchased from Japan SLC (Shizuoka, Japan), and maintained in a specific pathogen-free room at a temperature of 23±3 °C and air humidity of 55±15 %, on a 12-h/12-h light/dark cycle. This animal study was conducted in accordance with the Japanese Law for the Humane Treatment and Management of Animals (Law No. 105, October 1, 1973). Prior to the initiation of the animal study, an outline of the animal study protocol had been reviewed by the Institutional Animal Care and Use Committee of the Biological/Pharmacological Research Laboratories, Central Pharmaceutical Research Institute, Japan Tobacco Inc.
Chemicals
Losartan (≥98 % purity) was purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). The losartan-mixed diet was prepared every week by mixing losartan with standard powdered chow (CRF-1 powder, Oriental Yeast Co., Ltd., Tokyo, Japan). The mixed diet containing 0.02 % losartan (approximately 10 mg/kg/day) was fed for 39 weeks from 12 to 51 weeks of age. The concentration of losartan fed at 0.02 % mixed ration was found to be sufficient to achieve the pharmacological effect indicated by the reduction in blood pressure (data not shown).
Influences of unilateral nephrectomy on renal function and related parameters in SHR/cp rats and effect of losartan on the model
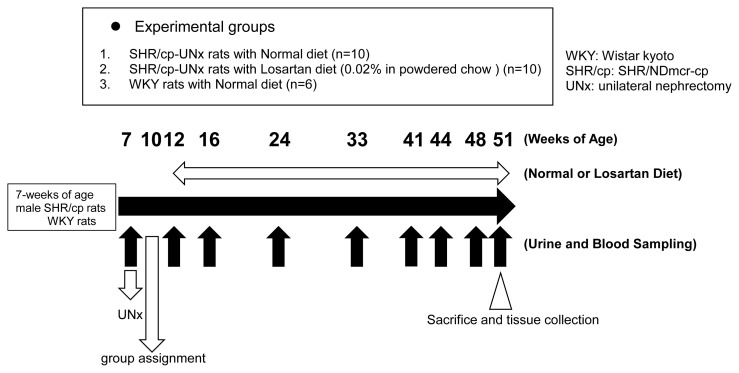
The study design is shown in Figure 1. UNx of left kidney for SHR/cp rats (UNx-SHR/cp rats) at 7 weeks of age (twice as many rats used in the study) was performed as previously described [7]. Three weeks later, half of the rats with 24-hour urine protein (UP) excretion closest to the mean of all rats on UNx were selected. Then these rats were allocated to two groups with 10 rats in each group so as to balance the means of the UP. The six WKY rats serving as a normal group were not underwent nephrectomy. Two more weeks later, starting at 12 weeks of age, these rats were fed a standard powder chow or mixed diet containing losartan for 39 weeks. The groups were: 1) UNx-SHR/cp rats fed a normal diet; 2) UNx-SHR/cp rats fed the 0.02 % losartan-mixed diet; 3) non-nephrectomized WKY rats fed a normal diet. The reason for selecting WKY rats as control animals was to understand how Ccr changed in normal WKY rats and to compare it with UNx-SHR/cp rats to determine its specificity as a DKD model.
Fig. 1.
Experimental design.
During the experimental period, body weight was measured sequentially, and blood and urine samples were collected from the tail vein and using metabolic cages, respectively. As a parameter of renal function, Ccr was measured at 16, 24, 33, 41, 44, 48, and 51 weeks of age after starting at 12 weeks of age. Ccr was calculated based on plasma and urine creatinine levels using the formula: Ccr (ml/min/100 g body weight) = urine creatinine (mg/dl) × 24-hour urine volume (ml)/1440 (min) × 1/serum creatinine (mg/dl) × 1/body weight (g) × 100. After the last sampling at 51 weeks of age, the rats were euthanized and their right kidneys were excised and processed for histological evaluation. Blood glucose, triglyceride (TG), total cholesterol (TC), plasma creatinine (pCre), blood urea nitrogen (BUN), and urine creatinine levels were measured using an automatic biochemical analyzer (Model 7180, Hitachi High-Tech Corporation, Tokyo, Japan). UP levels were measured using a commercially available kit (Tonein-TPII; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). The erythrocyte-related parameters (hemoglobin concentration [Hb], red blood cell [RBC] count, hematocrit [Hct]) were measured using a hematology analyzer (ADVIA® 120, Siemens Healthcare Diagnostics Ltd., Tokyo, Japan).
For histological analysis, the 10 % neutral formalin-fixed right kidneys were sectioned and stained with hematoxylin and eosin (HE), periodic acid-Schiff (PAS), and Sirius red. PAS staining was used to evaluate the degree of glomerular alteration; HE staining and PAS staining were used to evaluate the degree of tubulointerstitial alteration, and Sirius red staining was used to evaluate the degree of tubulointerstitial fibrosis. The histological evaluation was assessed in high-power fields by the following 7 parameters defined in the preliminary examination: increased mesangial matrix, glomerular crescent formation/adhesion, glomerular sclerosis/atrophy, tubular hyaline casts, tubular dilatation, regeneration/degeneration of tubular epithelium, and tubulointerstitial fibrosis. In the evaluation of increased mesangial matrix with observed glomerular hypertrophy and mesangial hyperplasia (the population of PAS-positive material in the glomerular and mesangial regions), 25 glomeruli were randomly selected from the kidney in each animal, scored on a 5-point scale ranging from 0 to 4 (Score 0: normal; Score 1: minimal, solitary [very small] lesion; Score 2: mild, focal [small] lesion [<25 %]; Score 3: moderate, sporadic lesion [<50 %]; and Score 4: marked, diffuse lesion [≥50 %]) based on the severity and distribution of the changes.
In the evaluation of glomerular crescent formation/adhesion, glomerular sclerosis/atrophy, tubular hyaline casts, tubular dilatation, regeneration/degeneration of tubular epithelium, and tubulointerstitial fibrosis, observation of the whole kidney was assessed to score the severity of each histological change on a 5-point scale ranging from 0 to 4 as mentioned above and the mean of these was used for each animal.
Statistical analysis
Data are expressed as the mean and standard deviation (S.D.) of the indicated numbers of animals or samples (Fig. 1). All statistical analyses were performed using StatLight 2000 (Yukms Co., Ltd, Kanagawa, Japan). In a two-group comparison, the statistical significance was assessed using the Student’s t-test (for homoscedastic data) or Aspin-Welch’s t-test (for heteroscedastic data) after homoscedasticity analysis by an F-test. For histological scores, the statistical significance was assessed by Wilcoxon rank sum test for two-group comparison. For survival rate analysis, the statistical significance was assessed using the Log-rank test. All statistical analyses were two-sided, and statistically significant level was set at p < 0.05.
Results
Influences of UNx on renal function and related parameters in SHR/cp rats and the effect of losartan on the model
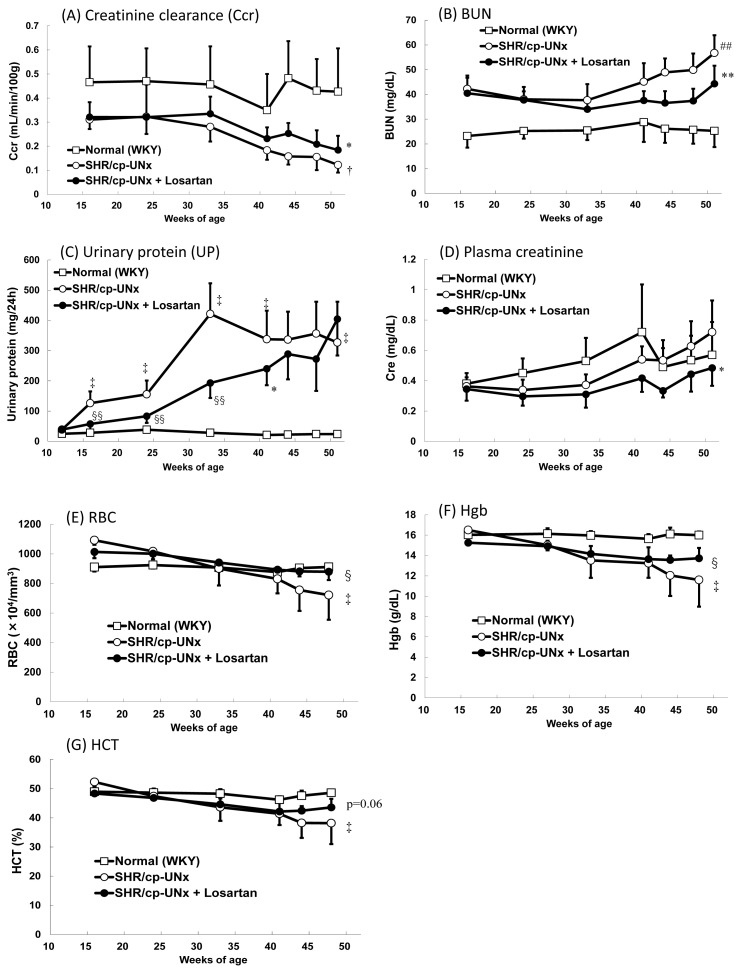
A significant decrease in Ccr was observed in UNx-SHR/cp rats compared to normal rats (WKY rats) (Fig. 2A). In addition, significant increases in BUN levels and 24-hour UP excretion were observed in UNx-SHR/cp rats during the experimental period (Fig. 2B, 2C). Furthermore, marked reductions of the erythrocyte-related parameters, including RBC count, Hb, and HCT were observed (Fig. 2E–G). On the other hand, pCre levels appeared to be similar between UNx-SHR/cp rats and WKY rats (Fig. 2D).
Fig. 2.
Influences of UNx on renal function and related parameters in SHR/cp rats and effect of losartan on the rats. Renal-related parameters (A–D) and erythrocyte-related parameters (E–G). Data points and bars represent the mean and S.D. (n=6–10). ## P<0.01 vs. Normal WKY rats group (Student’s t-test), †, ‡ P<0.05, P<0.01 vs. Normal WKY rats group (Welch’s test), *, ** P<0.05, P<0.01 vs. SHR/cp-UNx rats group (Student’s t-test), §, §§ P<0.05, P<0.01 vs. SHR/cp-UNx rats group (Welch’s test)
Investigating the effects of losartan on these renal-related parameters, the results showed that dietary administration of 0.02 % losartan significantly suppressed Ccr decline, increases of BUN, UP, and pCre, and also reduced the erythrocyte-related parameters (RBC count and Hb) in UNx-SHR/cp rats (Fig. 2).
Effect on metabolic parameters
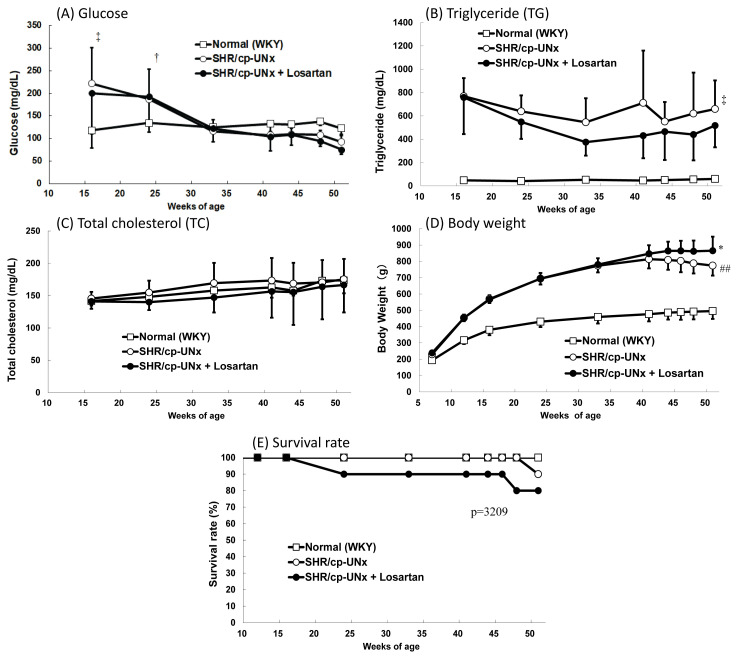
Higher blood glucose levels were observed in the UNx-SHR/cp rats until 24 weeks of age (Fig. 3A), thereafter the levels were comparable to those of WKY rats. Plasma TG levels in the UNx-SHR/cp rats were elevated compared to WKY rats, whereas TC levels were similar during the experimental period (Fig. 3B, 3C). Body weight gains of UNx-SHR/cp rats were significant compared to those of WKY rats up to 40 weeks of age, thereafter no further gains were observed (Fig. 3D).
Fig. 3.
Effect on metabolic parameters. A) Blood glucose. B) Triglyceride (TG). C) Total cholesterol (TC). D) Body weight. E) Survival rate. Data points and bars represent the mean and S.D. (n = 6). ## P < 0.01 vs. Normal WKY rats group (Student’s t-test), †, ‡ P<0.05, P<0.01 vs. Normal WKY rats group (Welch’s test), * P<0.05 vs. SHR/cp-UNx rats group (Student’s t-test)
Administration of losartan had no effect on these parameters, except that significantly higher body weight gains were observed at 51 weeks of age (Fig. 3A–D). In the survival rate, there were no significant differences between the UNx-SHR/cp rats group and the UNx-SHR/cp with losartan rats group (Fig. 3E).
Effect on renal histopathology
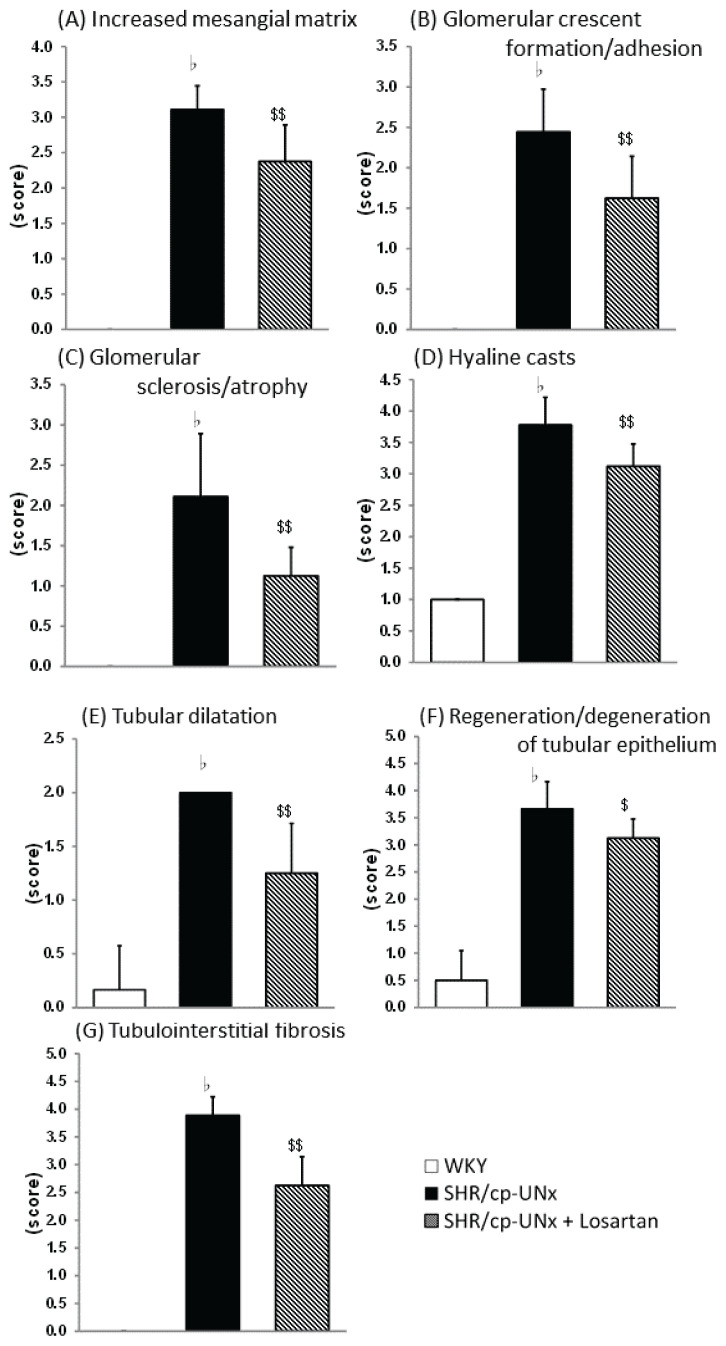
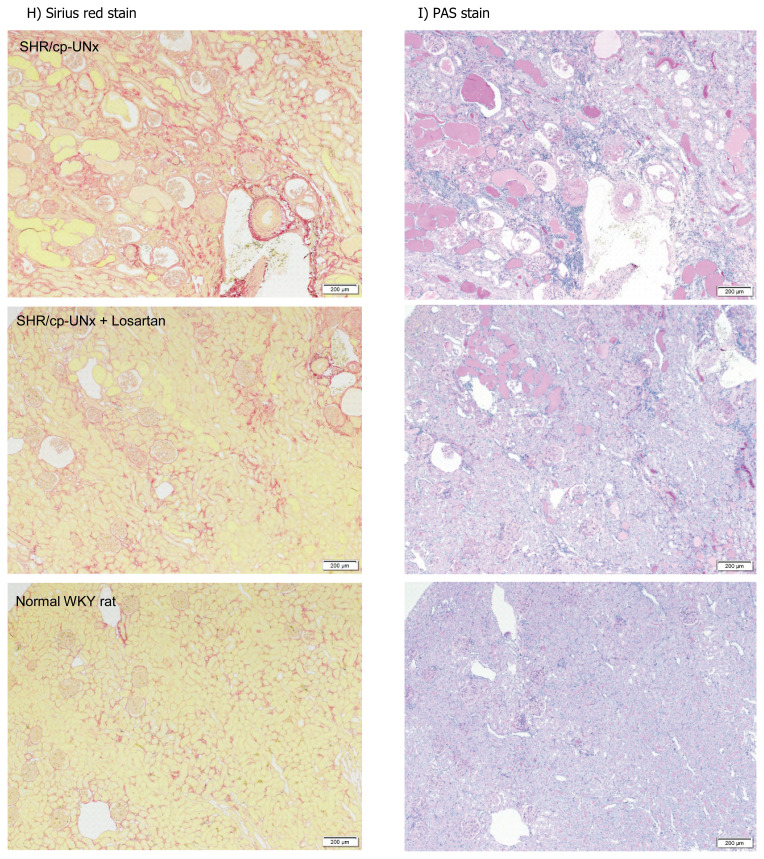
Histopathologically, some glomerular, tubular, and tubulointerstitial lesions were prominently observed in UNx-SHR/cp rats at 51 weeks of age. Administration of losartan reduced glomerular lesions (mesangial matrix increase, adhesion/crescent, sclerosis/atrophy), tubular lesions (hyaline casts, dilatation, regeneration/degeneration of epithelium), and tubulointerstitial lesions (fibrosis) (Fig. 4).
Fig. 4.
Effects on renal histopathology. A) Increased mesangial matrix, B) Glomerular crescent formation/adhesion, C) Glomerular sclerosis/atrophy, D) Tubular hyaline casts, E) Tubular dilatation, F) Regeneration/degeneration of tubular epithelium, G) Tubulointerstitial fibrosis. Effects on renal histopathology. H) Representative images of Sirius red staining showing tubulointerstitial fibrosis. I) Representative images of PAS staining showing glomerular sclerosis (arrow). Black scale bar: 200 μm. Data points and bars represent the mean and S.D. (n=6–10). ЬP<0.05 vs. Normal WKY rats group (Wilcoxon rank sum test), $, $$ P<0.05, P<0.01 vs. SHR/cp-UNx rats group (Wilcoxon rank sum test)
Discussion
In SHR/cp rats, UNx was able to induce a Ccr decline, accompanied by histopathological changes such as increased glomerular mesangial matrix, glomerular crescent formation/adhesion, glomerular sclerosis/atrophy, tubular hyaline casts, tubular dilatation, regeneration/degeneration of tubular epithelium, and tubulointerstitial fibrosis (Fig. 2A, Fig. 4). Furthermore, UNx performed on SHR/cp rats caused renal-related parameters such as UP and BUN to increase, in contrast to levels of these parameters in normal rats (WKY rats) (Fig. 2B–D). The hallmarks of DKD include decline in renal function as indicated by GFR or Ccr, proteinuria, and impaired renal morphology (increased glomerular basement membrane thickness, mesangial hyperplasia, interstitial fibrosis, glomerular hypertrophy, glomerulosclerosis, podocyte foot process effacement, and arterial hyalinosis) [13]. The induction of decreased renal function by UNx in SHR/cp rats was suggested to be reminiscent of the pathological condition in patients with DKD, as indicated above.
Over the past few decades, various animal models of DKD have been developed. However, complications due to complex etiologies associated with renal dysfunction in DKD patients appear in only a few of them. As an example, in the widely used STZ-induced type 1 diabetes model, blood glucose levels are elevated, however unlike in humans, hypertension, level of albuminuria, and the loss of renal function are often much less severe[9]. The Dahl salt-sensitive hypertensive rat is a model of hypertension, however it is not a model for studying the risk factors typical of DKD, such as diabetes, obesity, and abnormal lipid metabolism. SDT fatty rats and db/db mice, which are known diabetic models, show signs of DKD, such as abnormal lipid metabolism and obesity, however, during the lifetime of the animals, renal function is not influenced or in a hyperfiltration state, and infrequently declines as measured by Ccr [14–16]. To promote renal dysfunction by reducing the number of nephrons, 5/6 nephrectomy and UNx in mice and rats are widely used to generate experimental models of CKD [17,18]. Therefore, to accelerate the decline of renal function, we performed UNx in SHR/cp rats, and this model exhibited a Ccr decline and might mimic the pathogenesis of DKD in patients.
In the present study, we performed UNx in the rats, which allowed us to establish a model that would lead to steady Ccr decline, hypertension and worsened renal function parameters, accompanied by renal histopathological changes. It was considered that the decreased number of nephrons due to nephrectomy causes an increased single nephron GFR, and the persistent hypertension due to hyperfiltration leads to glomerulosclerosis and enhances medullary hypoxia, resulting in further reduction in the number of nephrons and hypoxia due to interstitial fibrosis [19]. Furthermore in our model, it was possible to observe renal anemia (Fig. 2E–G), which is associated with a decline in renal function. Renal anemia could reflect impaired erythropoietin (Epo) production by Epo-producing cells in the renal cortex and outer medulla region, and it could be correlated with declining renal function [20]. In addition, plasma TG levels were elevated in UNx-SHR/cp rats in this study (Fig. 3B). Plasma TGs could accumulate as lipid droplets and become toxic within cells such as renal tubular epithelial cells [21,22]. It has also been reported that TGs and free fatty acids (FFAs) bound to albumin accumulated in the proximal tubules could cause damage and induce inflammation [21]. Furthermore, our model is characterized by hypertension. In the preliminary study, systolic blood pressure levels (mmHg) of WKY rats, UNx-treated SHR/cp rats and losartan-treated rats were 113±7, 167±29 and 116±8 after 9 weeks of treatment, and 110±14, 166±30 and 111±16 after 26 weeks of treatment, respectively, measured by the indirect tail cuff method [17].
In this study, decreases in blood glucose levels were observed after 33 weeks of age. (Fig. 3A). The reason for the decrease might be related to the upward trend in the plasma insulin concentration of UNx-SHR/cp rats, as observed in a preliminary study. The preliminary data were as follows; blood insulin levels (ng/mL) at 9, 17, 29, and 41 weeks of age, respectively, were 50±10, 135±72, 234±150, and 25±19 for UNx-SHR/cp rats and 2.7±0.6, 3.0±0.8, 2.6±0.8, and 2.1±0.5 for WKY rats.
The SHR/cp rat is genetically mutated in the leptin receptor gene and is known as spontaneous model for type 2 diabetes mellitus. Thus, the rat exhibits a wide range of metabolic abnormalities (obesity, hyperglycemia, hyperlipidemia, and hyperinsulinemia) similar to those associated with overeating and human type 2 diabetes, leading to nephropathy. These metabolic abnormalities, together with increased blood pressure, result in a heterogeneous condition called metabolic syndrome [14].
In our model, hyperglycemia should be a prerequisite factor for nephropathy. Initial duration of high glucose exposure has been suggested to cause long-term negative effects on the kidney, whereas its physiological mechanisms are not yet well defined [23–25]. Possibilities include epigenetic programming, remodeling, and persistent post-translational modifications such as advanced glycation end-products [25]. Furthermore, other factors of metabolic abnormalities might be involved in the impaired renal function. Moreover, the data indicated that insulin resistance was a feature of this model, since we observed obvious hyperinsulinemia from the beginning of the disease.
The hyperinsulinemia was also reported to be involved in the pathogenesis of DN [26]. Therefore, our model was considered to be a type 2 diabetes mellitus model that is complicated by multiple pathological conditions, similar to those in humans. Further understanding of the molecular basis would certainly provide new targets for interventions to reduce the symptom burden of DKD patients, and our model might be helpful in identifying them.
In addition, proteinuria, which increased in the earlier phase of the study period, was observed as a decreasing trend in the latter phase. We speculate that the decrease in proteinuria in the later phase of the model might be due to the disruption of glomerular hyperfiltration. In this model, after undergoing UNx, the remaining one kidney was responsible for the glomerular filtration function, and although the apparent Ccr was the same as that of normal animals, the kidney may have remained a state of glomerular hyperfiltration until the renal function declined.
This study confirmed the effect of losartan, which is widely used for the treatment of DN [27]. The results showed that dietary administration of losartan attenuated the Ccr decline and attenuated renal anemia induced by UNx in SHR/cp rats (Fig. 2). Even the results of histopathological evaluation were substantially improved by losartan. These results suggest that this model might mimic the pre-phase of the later stages of DKD, when ACE inhibitors and ARBs are effective in clinical treatment. The study was conducted for a long term, and therefore some animals died (Fig. 3E). There were no significant differences between the groups in survival rates during the experimental period.
In preclinical research, the lack of animal models that mimic the pathophysiological characteristics of patients with DKD has been a hurdle to precisely addressing clinical needs [28]. Animal models might offer new insights into the development and progression of nephropathy in patients with DKD, and help us better understand the etiology of the disease. In addition, animal models could be used to explain how novel therapies might function, identify alternative pathways other than current therapies, and even help validate potentially adverse side effects.
In patients with DKD, risk factors such as dyslipidemia, hyperuricemia, and hypertension, in addition to diabetes mellitus, are considered to be complex pathophysiological determinants of the condition. Therefore, we considered it noteworthy that our model is also characterized by these risk factors in addition to diabetes. In contrast, it is unclear which of these risk factors, including the diabetes mellitus, contribute more specifically to the pathogenesis of DKD.
Previously, the ACE inhibitors and ARBs, two antihypertensive drugs that slow the progression of DN, have been extensively studied in numerous experimental DN models. However, not all of the typical features of DN are exhibited in many of these models. As an example, the mouse model has several limitations. Indeed, the classical DN model exhibits only early-stage DKD features such as moderate albuminuria, glomerular hypertrophy, and slight expansion of the mesangial matrix [29]. Glomerulosclerosis, tubular atrophy, or tubulointerstitial fibrosis is rarely observed in these animals. The present model of DKD developed by uni-nephrectomizing SHR/cp rats is a novel animal model that exhibits many of the same features observed in patients with DKD and therefore might be helpful in better defining the mechanisms involved in this disease.
Our model is a diabetic model complicated by various pathological conditions. We consider that the factors involved in renal decline are complex. In order to establish a model that promotes a decline in Ccr, at first, a unilateral nephrectomy is performed, leading to a state of glomerular hyperfiltration possibly until the middle of the experimental period, followed by a slow decline in renal function. By intervening in this model with agents or other treatments at various experimental time points, in addition to obtaining a better understanding of the agents’ properties, information about the characteristics of this model can be acquired. Accumulation of such knowledge will lead to more accurate use of the model and pharmaceutical intervention methods. Based on the results of the evaluation of a tool compound (losartan), we have determined that the model is feasible to represent CKD stage G3a or milder stages in the patient. This is because losartan is indicated for the treatment of DN with elevated serum creatinine and proteinuria (urinary ACR [UACR] > 300 mg/g) in patients with type 2 diabetes and a history of hypertension [3,4].
In recent years, preclinical and clinical studies have accumulated evidence for the efficacy of non-steroidal mineralocorticoid receptor antagonists (MRAs) and SGLT2 inhibitors in treating CKD and DN [5,30–32].
In the future, we expect to use our model to evaluate the effects of MRA or SGLT2 inhibitor alone or in combination and to understand the characteristics and limitations of the model by comparing the pathology of the DKD in our animal model with that of DKD in patients, which will allow us to further characterize pathogenic factors other than the current ones used as therapeutic targets in our model. Ultimately, we would like to establish our model as useful in evaluating the effects of many other agents with various mechanisms of action.
Conclusions
We found that UNx in SHR/cp rats could induce a decline of glomerular filtration, and that UNx-SHR/cp rats might be a potentially useful model corresponding to early stages of DKD in patients, by showing that treatment of these rats with ARB improved the histopathology of the kidney and prevented renal function decline.
Acknowledgements
We would like to thank ASCA Corporation (https://www.asca-co.com/) for editorial assistance. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.KDIGO Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. ACE inhibitors or ARBs for diabetic nephropathy: the unrelenting debate. Diabetes Metab Syndr. 2012;6:215–217. doi: 10.1016/j.dsx.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R, Investigators RS. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–1507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P Irbesartan in Patients with Type D, Microalbuminuria Study G. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjostrom CD, Toto RD, Langkilde AM, Wheeler DC Committees D-CT Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle KR, Brosius FC, 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, Perreault L, Rosas SE, Rossing P, Sola L, Vallon V, Wanner C, Perkovic V. SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021;77:94–109. doi: 10.1053/j.ajkd.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Shinozaki Y, Katayama Y, Yamaguchi F, Suzuki T, Watanabe K, Uno K, Tsutsui T, Sugimoto M, Shinohara M, Miyajima K, Ohta T. Salt loading with unilateral nephrectomy accelerates decline in glomerular filtration rate in the hypertensive, obese, type 2 diabetic SDT fatty rat model of diabetic kidney disease. Clinical and experimental pharmacology & physiology. 2022;49:492–500. doi: 10.1111/1440-1681.13621. [DOI] [PubMed] [Google Scholar]
- 8.Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, Crabtree M, Gross SS, Goligorsky MS. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15:2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- 9.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology. 2007;12:261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa M, Maekawa T, Sasase T, Takagi K, Takeuchi S, Kitamoto M, Nakagawa T, Toyoda K, Konishi N, Ohta T, Yamada T. Pathophysiological analysis of uninephrectomized db/db mice as a model of severe diabetic kidney disease. Physiological research. 2022;71:209–217. doi: 10.33549/physiolres.934784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano R, Ishii Y, Yamanaka M, Yasui Y, Kemmochi Y, Kuroki F, Sugimoto M, Fukuda S, Sasase T, Miyajima K, Nakae D, Ohta T. Glomerular hyperfiltration with hyperglycemia in the spontaneously diabetic Torii (SDT) fatty rat, an obese type 2 diabetic model. Physiological research. 2021;70:45–54. doi: 10.33549/physiolres.934533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohtomo S, Izuhara Y, Nangaku M, Dan T, Ito S, van Ypersele de Strihou C, Miyata T. Body weight control by a high-carbohydrate/low-fat diet slows the progression of diabetic kidney damage in an obese, hypertensive, type 2 diabetic rat model. Journal of obesity. 2010:2010. doi: 10.1155/2010/136502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nangaku M, Izuhara Y, Usuda N, Inagi R, Shibata T, Sugiyama S, Kurokawa K, van Ypersele de Strihou C, Miyata T. In a type 2 diabetic nephropathy rat model, the improvement of obesity by a low calorie diet reduces oxidative/carbonyl stress and prevents diabetic nephropathy. Nephrol Dial Transplant. 2005;20:2661–2669. doi: 10.1093/ndt/gfi096. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MP, Lautenslager GT, Shearman CW. Increased urinary type IV collagen marks the development of glomerular pathology in diabetic d/db mice. Metabolism. 2001;50:1435–1440. doi: 10.1053/meta.2001.28074. [DOI] [PubMed] [Google Scholar]
- 16.Gartner K. Glomerular hyperfiltration during the onset of diabetes mellitus in two strains of diabetic mice (c57bl/6j db/db and c57bl/ksj db/db) Diabetologia. 1978;15:59–63. doi: 10.1007/BF01219330. [DOI] [PubMed] [Google Scholar]
- 17.Katsuda Y, Kemmochi Y, Maki M, Sano R, Toriniwa Y, Ishii Y, Miyajima K, Kakimoto K, Ohta T. Effects of unilateral nephrectomy on renal function in male Spontaneously Diabetic Torii fatty rats: a novel obese type 2 diabetic model. J Diabetes Res. 2014;2014:363126. doi: 10.1155/2014/363126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Sullivan J, Finnie SL, Teenan O, Cairns C, Boyd A, Bailey MA, Thomson A, Hughes J, Benezech C, Conway BR, Denby L. Refining the Mouse Subtotal Nephrectomy in Male 129S2/SV Mice for Consistent Modeling of Progressive Kidney Disease With Renal Inflammation and Cardiac Dysfunction. Front Physiol. 2019;10:1365. doi: 10.3389/fphys.2019.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyman SN, Khamaisi M, Zorbavel D, Rosen S, Abassi Z. Role of Hypoxia in Renal Failure Caused by Nephrotoxins and Hypertonic Solutions. Seminars in nephrology. 2019;39:530–542. doi: 10.1016/j.semnephrol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N. Erythropoietin gene expression: developmental-stage specificity, cell-type specificity, and hypoxia inducibility. The Tohoku journal of experimental medicine. 2015;235:233–240. doi: 10.1620/tjem.235.233. [DOI] [PubMed] [Google Scholar]
- 21.Bobulescu IA. Renal lipid metabolism and lipotoxicity. Current opinion in nephrology and hypertension. 2010;19:393–402. doi: 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afshinnia F, Nair V, Lin J, Rajendiran TM, Soni T, Byun J, Sharma K, Fort PE, Gardner TW, Looker HC, Nelson RG, Brosius FC, Feldman EL, Michailidis G, Kretzler M, Pennathur S. Increased lipogenesis and impaired beta-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI insight. 2019:4. doi: 10.1172/jci.insight.130317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas MC. Glycemic exposure, glycemic control, and metabolic karma in diabetic complications. Advances in chronic kidney disease. 2014;21:311–317. doi: 10.1053/j.ackd.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990;87:404–408. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper ME, El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circulation research. 2010;107:1403–1413. doi: 10.1161/CIRCRESAHA.110.223552. [DOI] [PubMed] [Google Scholar]
- 26.Michel O, Heudes D, Lamarre I, Masurier C, Lavau M, Bariety J, Chevalier J. Reduction of insulin and triglycerides delays glomerulosclerosis in obese Zucker rats. Kidney Int. 1997;52:1532–1542. doi: 10.1038/ki.1997.483. [DOI] [PubMed] [Google Scholar]
- 27.Tuttle KR, Cherney DZ Diabetic kidney disease task force of the American Society of N. sodium glucose cotransporter 2 inhibition heralds a call-to-action for diabetic kidney disease. Clin J Am Soc Nephrol. 2020;15:285–288. doi: 10.2215/CJN.07730719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noshahr ZS, Salmani H, Khajavi Rad A, Sahebkar A. Animal models of diabetes-associated renal injury. J Diabetes Res. 2020;2020:9416419. doi: 10.1155/2020/9416419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soler MJ, Riera M, Batlle D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy. Exp Diabetes Res. 2012;2012:616313. doi: 10.1155/2012/616313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel V, Joharapurkar A, Jain M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug development research. 2021;82:341–363. doi: 10.1002/ddr.21760. [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE Investigators E-RO. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 32.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]