Abstract
Occludin is an integral membrane protein that is tyrosine phosphorylated when localized at tight junctions. When Ca2+ was depleted from the culture medium, occludin tyrosine phosphorylation was diminished from Madin-Darby canine kidney epithelial cells in 2 min. This dephosphorylation was correlated with a significant reduction in transepithelial electrical resistance (TER), indicating a global loss of the tight junction barrier function. Reconstitution of Ca2+ resulted in a robust tyrosine rephosphorylation of occludin that was temporally associated with an increase in TER. Moreover, we demonstrate in this study that occludin was colocalized with the nonreceptor tyrosine kinase c-Yes at cell junction areas and formed an immunoprecipitable complex with c-Yes in vivo. This complex dissociated when the cells were incubated in medium without Ca2+ or treated with a c-Yes inhibitor, CGP77675. In the presence of CGP77675 after Ca2+ repletion, occludin tyrosine phosphorylation was completely abolished and both tight junction formation and the increase of the TER were inhibited. Our study thus provides strong evidence that occludin tyrosine phosphorylation is tightly linked to tight junction formation in epithelial cells, and that the nonreceptor tyrosine kinase c-Yes is involved in the regulation of this process.
INTRODUCTION
A number of tight junction integral membrane proteins and tight junction-associated proteins have been identified in the last decade: occludin, the claudin family, junctional adhesion molecule, zonula occludens (ZO)-1, ZO-2, ZO-3, cingulin, symplekin, and AF6 (reviewed in Stevenson and Keon, 1998; Goodenough, 1999; Tsukita et al., 1999). Among these, occludin is an integral membrane protein localized within the freeze fracture fibrils that has been shown to be required for normal tight junction physiology (Furuse et al., 1993; Fujimoto, 1995; Balda et al., 1996; McCarthy et al., 1996; Chen et al., 1997; Wong and Gumbiner, 1997). Occludin has a predicted tetraspanning membrane topology with two extracellular loops and three cytoplasmic domains (Furuse et al., 1993; Ando-Akatsuka et al., 1996). It has been shown that the extracellular loops are important for occludin localization in culture (Wong and Gumbiner, 1997; Lacaz-Vieira et al., 1999; Medina et al., 2000). The C terminus of occludin directly interacts with ZO-1 in vitro and is required for tight junction function (Furuse et al., 1994; Balda et al., 1996; Chen et al., 1997; Matter and Balda, 1998; Mitic et al., 1999).
Occludin migrates as a tight cluster of multiple bands on SDS gels resulting from multiple phosphorylation of serine and threonine residues (Sakakibara et al., 1997; Wong, 1997; Cordenonsi et al., 1999; Farshori and Kachar, 1999). It has been suggested that the phosphorylation of occludin is important for tight junction assembly because highly phosphorylated occludin is selectively concentrated at tight junctions in a detergent-insoluble form (Sakakibara et al., 1997). However, in epithelia of Xenopus embryos, occludin dephosphorylation was correlated with the de novo assembly of tight junctions (Cordenonsi et al., 1999), suggesting that occludin phosphorylation may play different roles in different biological systems, or that phosphorylation of specific residues has different functional consequences.
Studies of tyrosine phosphorylation have revealed contradictory data on tight junction physiology. Inhibition of phosphatase activity has been reported to result in decreases in transepithelial electrical resistance (TER) and increases in paracellular permeability (Staddon et al., 1995; Collares-Buzato et al., 1998; Atkinson and Rao, 2001). However, other studies have shown that tyrosine phosphorylation can be positively temporally correlated with tight junction assembly and function (Kurihara et al., 1995; Tsukamoto and Nigam, 1999). Tsukamoto and Nigam (1999) provided the first evidence that occludin was tyrosine phosphorylated and that tyrosine kinase activity is necessary for tight junction reassembly during ATP repletion.
The signaling pathways and kinases involved in occludin phosphorylation and tight junction function remain unclear. Overexpression of protein kinase C (PKC)-α (Mullin et al., 1998) or phorbol ester activation of PKC (Clarke et al., 2000) leads to the dephosphorylation of occludin and increased tight junction permeability in LLC-PK1 renal epithelial cells. It has been shown that epidermal growth factor induces tyrosine phosphorylation and reorganization of ZO-1 in A431 human epidermal carcinoma cells (Van Itallie et al., 1995). Small GTPases, such as Rho and Rac, are also involved in regulating tight junction structure and function (Nusrat et al., 1995; Gopalakrishnan et al., 1998; Jou et al., 1998). Other signaling molecules reported to influence the function of the tight junction include G proteins, phospholipase C, Raf-1, calmodulin, and glucocorticoids (Balda et al., 1991; Singer et al., 1994; Denker et al., 1996; Saha et al., 1998; Li and Mrsny, 2000).
Our previous work demonstrated that in ras-transformed Madin-Darby canine kidney (MDCK) cells tight junction structure is absent, and occludin, claudin-1, and ZO-1 are present only in the cytoplasm (Chen et al., 2000). Under these conditions, occludin is not tyrosine phosphorylated. When Ras signaling is attenuated by the inhibition of the mitogen-activated protein kinase pathway, tight junctions are restored. Occludin, claudin-1, and ZO-1 are concomitantly recruited to the cell-cell contact areas and occludin becomes tyrosine phosphorylated. In the present study, we have used normal MDCK II cells to investigate whether occludin tyrosine phosphorylation is required for tight junction formation in epithelial cells. Using a calcium-switch model, we found that occludin tyrosine phosphorylation and dephosphorylation were temporally highly correlated with tight junction assembly and disassembly. Furthermore, we demonstrate that occludin was colocalized and formed an immunoprecipitable complex with the nonreceptor tyrosine kinase c-Yes in vivo. Treatment of MDCK cells with a c-Yes inhibitor, CGP77675, disrupted the occludin/c-Yes complex and completely abolished the tyrosine phosphorylation of occludin. Our study provides strong evidence that occludin tyrosine phosphorylation is required for tight junction formation in epithelial cells, and that the nonreceptor tyrosine kinase c-Yes is involved in the regulation of tight junction formation and function.
MATERIALS AND METHODS
Antibodies and Reagents
The rabbit polyclonal anti-occludin, anti-claudin-1, and anti-ZO-1 antibodies were purchased from Zymed Laboratories (South San Francisco, CA). The mouse anti-phosphotyrosine and mouse anti-c-Yes monoclonal antibodies (mAbs) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Transduction Laboratories (Lexington, KY), respectively. Genistein, a broad-range tyrosine kinase inhibitor that inhibits epidermal growth factor receptor tyrosine kinase and p60v-src kinase, was obtained from Calbiochem (San Diego, CA). The c-Yes inhibitor CGP77675 was kindly provided by Dr. Mira Susa (Novartis Pharma AG, Basel, Switzerland). Fluorescein isothiocyanate-conjugated goat anti-rabbit IgG was purchased from Roche Applied Sciences (Indianapolis, IN). Rhodamine-conjugated goat anti-mouse IgG was obtained from Cappel (Malvern, PA).
Cell Culture and Calcium-Switch Assay
MDCK II cells were grown in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified air, 5% CO2 atmosphere at 37°C. During the calcium-switch experiments, the monolayers were washed three times with Ca2+/Mg2+-free phosphate-buffered saline (PBS) and incubated in Ca2+-free DMEM, 10% dialyzed fetal bovine serum for 1 h or overnight depending on the experiments, and then switched to normal culture medium. At the time of switching back to normal medium the cells were either not treated (control), or treated with 50 μM genistein, or with 200 nM CGP77675 for various lengths of time before fixing for immunohistochemistry or lysed for immunoprecipitation.
Immunofluorescence and Confocal Microscopy
Cells grown on glass coverslips were fixed with 100% methanol at −20°C for 5 min. Cells were blocked with 2% bovine serum albumin (BSA) for 30 min at room temperature and incubated with primary antibodies against occludin (1:300 dilution), claudin-1 (1:100), ZO-1 (1:500), and c-Yes (1:500) for 1 h. All antibodies were diluted in 2% BSA in PBS. After washing, cells were incubated with secondary antibody for 50 min at room temperature. The secondary antibody used for occludin, claudin-1, and ZO-1 was fluorescein isothiocyanate-conjugated anti-rabbit IgG (1:400). The rhodamine- (for double labeling) conjugated anti-mouse IgG was used for c-Yes (1:400). Coverslips were mounted with ProLong antifade kit (Molecular Probes, Eugene, OR). Samples were analyzed and photographed using either a Zeiss Axiophot or Zeiss Axiovert S100 (Carl Zeiss, Thornwood, NY). The z-axial images were collected using a Zeiss LSM 510 laser confocal scanning microscopy (Carl Zeiss).
Immunoprecipitation and Western Blot Analysis
MDCK II cells without treatment, with 50 μM genistein, or with 200 nM CGP77675 were washed three times in PBS, and then lysed in radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100; 0.5% sodium deoxycholate; 0.2% SDS; 150 mM NaCl; 10 mM HEPES, pH 7.3; 2 mM EDTA; 10 μg/ml each of chymostatin, leupeptin, and pepstatin A; 20 μM phenylmethylsulfonyl fluoride; 2 mM sodium orthovanadate; 10 mM sodium pyrophosphate; and 20 mM sodium fluoride). After 20-min incubation at 4°C, the lysates were homogenized on ice by passing 20 times through a 22-gauge needle, and centrifuged at 15,000 × g for 30 min at 4°C. The total protein concentration of each sample was measured by bicinchoninic acid protein assay kit (Pierce Chemical, Rockford, IL) and adjusted to equal concentration (1 mg/ml). After preclearing with protein A- or protein G-Sepharose beads the supernatants were incubated with polyclonal anti-occludin or anti-ZO-1 or monoclonal anti-c-Yes antibody at 4°C overnight. The supernatants were incubated with either protein A-Sepharose (for occludin and ZO-1) or protein G (for c-Yes) for additional 2 h. The beads were washed three times with RIPA buffer, one time with high salt (0.5 M NaCl), and one time with Tris buffer (10 mM Tris, pH 7.4). Bound protein was eluted from the beads in SDS sample buffer and boiled for 5 min.
Cell lysates or immunoprecipitates in SDS sample buffer were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membrane was then blocked in 5% nonfat dried milk or 5% BSA (for anti-phosphotyrosine detection) in Tris-buffered saline plus 0.1% Tween 20, and incubated with primary antibodies for 1.5 h followed by incubation with appropriate secondary antibodies for 1 h. The dilutions for the primary antibodies were as follows: anti-occludin, 1:1000; anti-claudin-1, 1:500; anti-ZO-1, 1:2000; and anti-phosphotyrosine, 1:300. After blotting, the signals were detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) on X-OMAT film. When necessary, blots were stripped (100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 7.6) for 30 min at 55°C. The blots were then washed in Tris-buffered saline containing 0.1% Tween 20 before reprobing with specific antibodies.
Measurement of TER
For TER measurements, cells were plated on Transwell filters with a pore size of 0.4 μm (Corning Glassworks, Cambridge, MA). A Millicell-ERS volt-ohm meter (Millipore, Bedford, MA) was used to determine the TER value (McCarthy et al., 1996). A pair of Ag/AgCl electrodes was placed into the transwell (one electrode was inserted into up-chamber and the other electrode was inserted into low-chamber) and an AC square wave current from the volt-ohm meter was passing across the cell monolayer to obtain the TER values. All the TER values were determined after the background subtraction (contributed by filter and bath solution) and multiplied by the surface area of the filter.
RESULTS
Tyrosine Phosphorylation of Occludin Was Diminished in Ca2+-free Medium
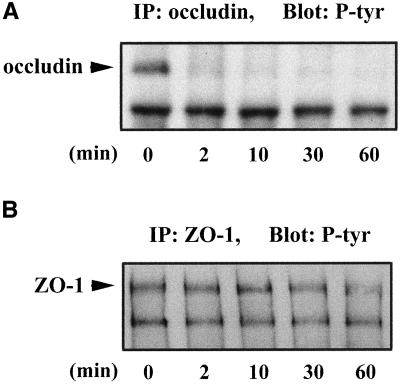
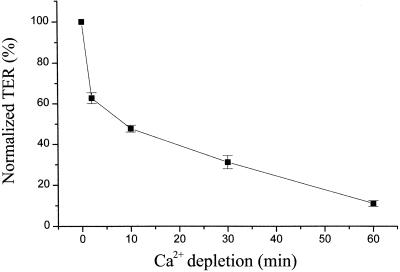
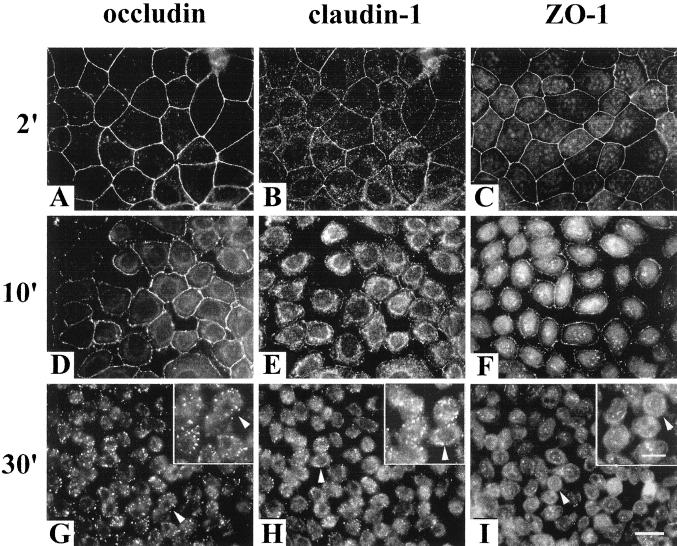
Occludin was tyrosine phosphorylated in confluent MDCK II cells when cultured in Ca2+-containing medium as shown in Figure 1A, lane 0. However, after switching to the medium without Ca2+, occludin tyrosine phosphorylation was diminished within 2 min (Figure 1A, lane 2). In contrast, ZO-1 tyrosine phosphorylation was unchanged until 60 min after Ca2+ depletion (Figure 1B). Occludin remained tyrosine dephosphorylated for as long as the cells remained in the Ca2+-free medium (Figure 1A, lanes 10, 30, and 60). An initial sharp drop in TER was closely correlated with the time of occludin tyrosine dephosphorylation (Figure 2). Each TER measurement was normalized to the initial value (the TER before switching to the Ca2+-free medium, indicated as 100% in Figure 2). TER continuously decreased over the next hour until reaching its minimum. Immunohistochemistry showed that the tight junction proteins occludin, claudin-1, and ZO-1 were all still localized at the cell-cell contact area after 2 min of Ca2+ depletion, although the TER had dropped ∼40% at this time point (Figure 3, A–C). After 10 min of Ca2+ depletion, cells detached from each other and the cell junctions were disrupted (Figure 3, D–F). The fluorescence intensities of occludin, claudin-1, and ZO-1 on the cell membrane were reduced and the staining pattern became discontinuous. On the other hand, the cytoplasmic staining of occludin, claudin-1, and ZO-1 increased. By 30 min of Ca2+ depletion, cells were all dissociated from each other, and the fluorescence staining patterns associated with each of the tight junction proteins appeared punctate (Figure 3, G and H), or dispersed in the cytoplasm (Figure 3I). The inserts provided in Figure 3, G–I, are enlarged images from the same field.
Figure 1.
Changes in tyrosine phosphorylation of occludin and ZO-1 during Ca2+ depletion. Cells were grown in Ca2+-containing medium until they were confluent (0 min). Then cells were switched to the medium without Ca2+ for 2, 10, 30, and 60 min. After Ca2+ depletion at desired time points, cells were lysed in RIPA buffer, immunoprecipitated with anti-occludin (A) or anti-ZO-1 (B), and immunoblotted with anti-phosphotyrosine antibody.
Figure 2.
TER measurements during Ca2+ depletion. TER was measured at 0, 2, 10, 30, and 60 min after cells were switched from Ca2+-containing medium to the medium without Ca2+. Each TER measurement was normalized to the TER value before switching to the Ca2+-free medium and was obtained after background subtraction (contributed by the filter and bath solution). The data represent the average ± SE from five experiments.
Figure 3.
Immunolocalization of occludin, claudin-1, and ZO-1 after Ca2+ was removed from the medium. Cells were grown in normal culture medium until they were confluent, and then the cells were switched to the medium without Ca2+ for 2, 10, and 30 min. The results from immunofluorescence staining showed that 2 min after Ca2+ was depleted from the medium, cell-cell contacts were still intact and occludin, claudin-1, and ZO-1 were all localized at cell-cell contact area (A–C). However, after 10 min of Ca2+ depletion, the cell junctions were disrupted and the immunostaining of the tight junction proteins became discontinued (D–F). By 30 min of Ca2+ depletion, cells were detached from each other and the fluorescence staining patterns appeared either punctate (G and H) or dispersed in the cytoplasm (I). Bar, 20 μm. The inserts in G–I are enlarged images selected in the same fields (arrowheads indicate the same cells) to show the localization of the signals. Bar, 10 μm.
Tyrosine Phosphorylation of Occludin Was Associated with Tight Junction Formation during Ca2+ Repletion
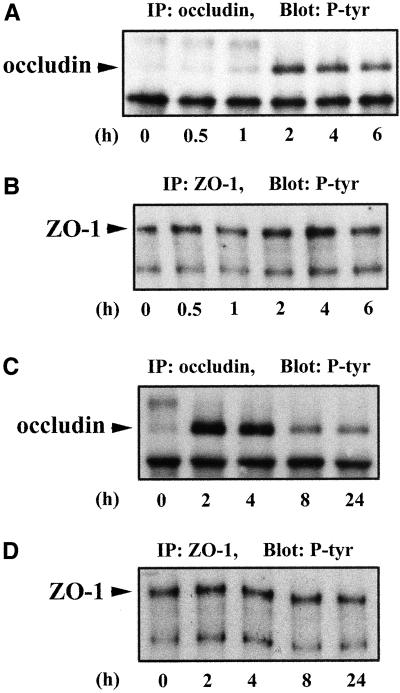
To correlate occludin tyrosine rephosphorylation with tight junction assembly, cells were switched back to Ca2+-containing culture medium after 1 h of Ca2+ depletion. Tyrosine phosphorylation of occludin appeared clearly at the 1-h time point after Ca2+ repletion and reached its maximum at 2 and 4 h (Figure 4A). The more slowly migrating band seen at the 0-, 0.5-, and 1-h time points was not occludin related because it could not be stained when this membrane was stripped and reprobed with anti-occludin antibody (our unpublished data). The total amount of occludin at each time point in Figure 4A was similar as determined by densitometry of anti-occludin immunoblots (our unpublished data). The changes in ZO-1 tyrosine phosphorylation during Ca2+ repletion were much less significant and not correlated with the tight junction assembly (Figure 4B). The band below ZO-1 was most likely ZO-2 because these molecules have been shown to coimmunoprecipitate with ZO-1 under similar experimental conditions (Gumbiner et al., 1991; Jesaitis and Goodenough, 1994). Figure 4C documents that occludin tyrosine phosphorylation reached a maximum at 2 and 4 h and then declined to a steady-state level at 8 h. The level of ZO-1 tyrosine phosphorylation did not show a detectable change over the time course of these experiments (Figure 4D).
Figure 4.
Tyrosine phosphorylation of occludin and ZO-1 during tight junction assembly after Ca2+ repletion. Cells were incubated in Ca2+-free medium for 1 h (designated as 0 h) and then switched to the Ca2+-containing medium for 0.5, 1, 2, 4, and 6 h in A and B, or for 2, 4, 8, and 24 h in C and D. After Ca2+ repletion at designated time points, cells were lysed in RIPA buffer, immunoprecipitated with anti-occludin (A and C) or anti-ZO-1 (B and D), and immunoblotted with anti-phosphotyrosine antibody. Tyrosine phosphorylation of occludin appeared clearly at 1-h time point after Ca2+ repletion (A) and reached maximum at 2- and 4-h time points (A and C). Tyrosine phosphorylation of ZO-1 did not change significantly over the time course of these experiments (B and D).
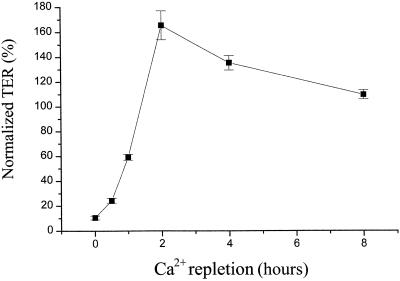
TER measurements revealed a close temporal correlation between occludin tyrosine phosphorylation and tight junction formation during Ca2+ repletion (Figure 5). Cells were incubated in the Ca2+-free medium for 1 h and then switched to Ca2+-containing culture medium. As presented previously (Figure 2), each TER measurement was normalized to the initial TER value (as 100%) before Ca2+ depletion. As shown in Figure 5, TER overshot its initial value at 2 and 4 h, at which time occludin tyrosine phosphorylation was also at a maximum (Figure 4, A and C). TER then declined to its initial value by 8 h (Figure 5) at which time the occludin tyrosine phosphorylation had also returned to its steady-state level (Figure 4, A and C).
Figure 5.
Time course of TER recovery during Ca2+ repletion. Cells were incubated in Ca2+-free medium for 1 h and then switched to the Ca2+-containing medium for 0, 0.5, 1, 2, 4, and 8 h at which time points the TER were measured. Each TER measurement was normalized to the initial TER value (before switching to the Ca2+-free medium) and was obtained after background subtraction (contributed by filter and bath solution). The data represent the average ± SE from five experiments.
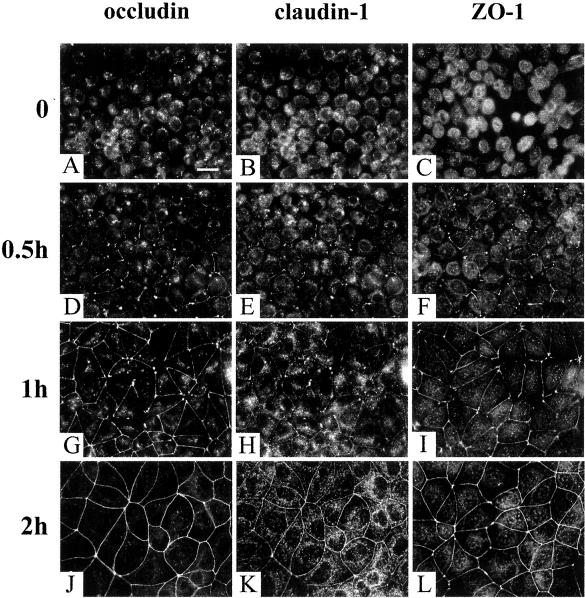
Immunohistochemistry was consistent with the TER measurements. There was no cell membrane staining with the three tight junction markers after 1 h of Ca2+ depletion when the cells were rounded up (Figure 6, A–C). After 0.5 h of Ca2+ repletion, occludin, claudin-1, and ZO-1 started to appear at some of the cell-cell contact areas (Figure 6, D–F). The junctional staining of occludin, claudin-1, and ZO-1 was more obvious at the 1-h time point as shown in Figure 6, G–I. After the cells were switched back to Ca2+-containing culture medium for 2 h, the tight junction was fully assembled as indicated both by immunofluorescent staining (Figure 6, J–L) and TER measurement (Figure 5).
Figure 6.
Recruitments of occludin, claudin-1, and ZO-1 to the cell junction during Ca2+ repletion. After incubated in Ca2+-free medium for 1 h, cells were dissociated from each other, and occludin, claudin-1, and ZO-1 were largely present in the cytoplasm (A–C). After 0.5 h of Ca2+ repletion, occludin, claudin-1, and ZO-1 started to be recruited to some of the cell-cell contact area (D–F). At 1-h time point, tight junction proteins were well accumulated at cell junctions (G–I). The tight junction staining of occludin, claudin-1, and ZO-1 completely returned to normal by 2 h of Ca2+ repletion (J–L). Bar, 20 μm.
Molecular Interaction of Occludin with Nonreceptor Tyrosine Kinase c-Yes
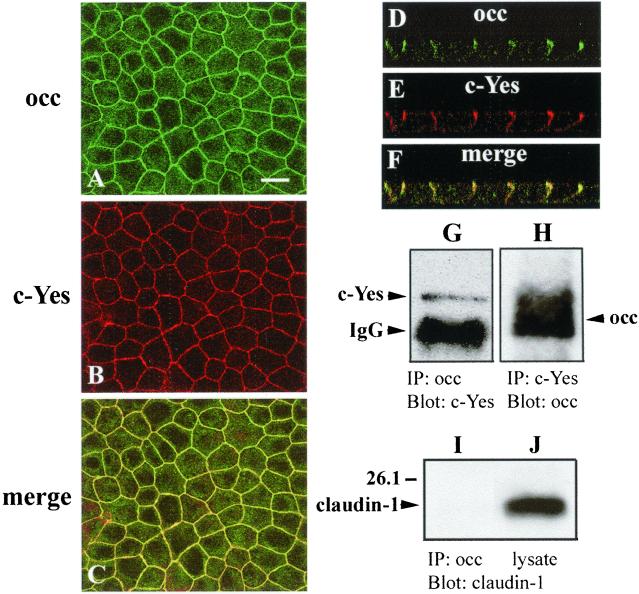
Immunohistochemistry and immunoprecipitation were used to identify an upstream tyrosine kinase of occludin. Nusrat et al. (2000) demonstrated previously that a 27 amino-acid peptide of the human occludin sequence interacts in vitro with occludin itself, ZO-1, PKC-ζ, c-Yes, the regulatory subunit of phosphatidylinositol 3-kinase, and the gap junction component connexin26. Pursuing these findings, we found that nonreceptor tyrosine kinase c-Yes was colocalized with occludin at tight junctions and along basolateral membranes in MDCK cells as demonstrated in Figure 7, A–C. Confocal microscopy confirmed z-axis colocalization of occludin and c-Yes to within confidence levels of the light microscope (Figure 7, D–F). To determine whether occludin and c-Yes form a stable complex in vivo, coimmunoprecipitation experiments were performed. Figure 7G shows that when MDCK II cell lysates were immunoprecipitated with anti-occludin antibody, c-Yes was present in the complex as revealed by immunoblot with an anti-c-Yes antibody. Reciprocal immunoprecipitation with anti-c-Yes antibody followed by immunoblot with anti-occludin confirmed the interaction of these two proteins in vivo (Figure 7H). This complex was disrupted in Ca2+-free medium (see below). We were unable to detect claudin-1 in this complex as indicated in Figure 7I, although claudin-1 was robustly present in the cell lysates (Figure 7J). Both Src and phosphatidylinositol 3-kinases were also undetectable in this complex (our unpublished data).
Figure 7.
Colocalization and coimmunoprecipitation of occludin with nonreceptor tyrosine kinase c-Yes in canine kidney epithelial cells. Confluent cells were fixed in methanol and processed for double immunofluorescence by using the rabbit anti-occludin (A) polyclonal antibody and mouse anti-c-Yes (B) mAb antibody. Images of A and B were superimposed as C. Confocal fluorescence microscopy showed the z-axis colocalization of occludin (D) and c-Yes (E) and their superimposed image (F). Occludin and c-Yes formed a complex in vivo. Cells were lysed with RIPA buffer and immunoprecipitated with either anti-occludin and blotted with anti-c-Yes (G), or immunoprecipitated with anti-c-Yes and blotted with anti-occludin (H). As a negative control, cell lysates were also either immunoprecipitated with anti-occludin and blotted with anti-claudin-1 (I) or directly probed with anti-claudin-1 antibody (J). Bar, 20 μm.
Inhibition of c-Yes Results in Dissociation of Occludin/c-Yes Complex and Disruption of Tight Junction Formation
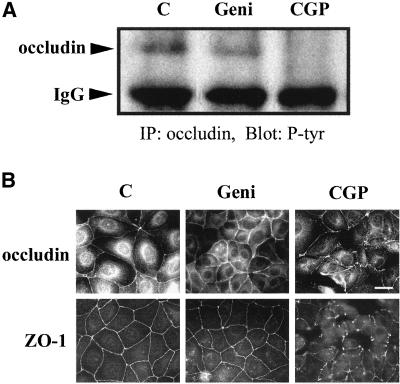
MDCK II cells were recovered from low Ca2+ condition in the presence of the tyrosine kinase inhibitors genistein and CGP77675 to demonstrate a temporal correlation between the presence of an occludin/c-Yes complex and tight junction reassembly. To completely dissociate cells from each other and reduce overall background the overnight Ca2+ depletion condition was used in tyrosine kinase inhibitor experiments. Cells were grown overnight in Ca2+-free medium, and then changed to Ca2+-containing medium without (Figure 8A, lane C), or with the addition of 50 μM genistein (Figure 8A, lane Geni), or 200 nM CGP77675 (Figure 8A, lane CGP). After 2.5 h, cells were lysed and immunoprecipitated with anti-occludin antibody. All the immunoprecipitates were then probed with anti-phosphotyrosine antibody. Figure 8A shows that genistein treatment only slightly reduced the amount of occludin that was tyrosine phosphorylated after repletion of Ca2+. In contrast, CGP77675 treatment completely abolished the tyrosine phosphorylation of occludin under the identical conditions. Indirect immunofluorescence light microscopy data revealed that CGP77675 treatment interfered with tight junction assembly and denied monolayer formation after switching to Ca2+-containing medium for 2.5 h (Figure 8B, CGP). In contrast, the control cells or the cells treated with genistein formed confluent monolayers with the characteristic honeycomb-like staining of occludin and ZO-1 (Figure 8B, C and Geni).
Figure 8.
Effects of CGP77675, a c-Yes inhibitor, on tyrosine phosphorylation of occludin and tight junction formation. (A) Treatment of MDCK II cells with CGP77675 led to occludin tyrosine dephosphorylation. Confluent cells were cultured in Ca2+-free medium overnight and then switched to Ca2+-containing medium without (C) or with the addition of 50 μM genistein (Geni) or 200 nM CGP77675 (CGP). After 2.5 h of treatment, cells were lysed in RIPA buffer, immunoprecipitated with rabbit anti-occludin polyclonal antibody, and blotted with anti-phosphotyrosine antibody. (B) CGP77675 treatment disrupted tight junction formation. Same as in A, cells were incubated in Ca2+-free medium overnight and switched to Ca2+-containing medium for 2.5 h without (C), or with the addition of 50 μM genistein (Geni) or 200 nM CGP77675 (CGP). Cells were fixed in methanol at −20°C and detected by indirect immunofluorescence staining of anti-occludin antibody or anti-ZO-1 antibody. Bar, 20 μm.
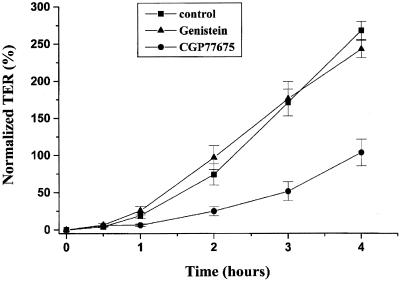
TER measurements also demonstrated the impairment of tight junction formation after the cells were treated with c-Yes inhibitor compared with the control cells and genistein-treated cells (Figure 9). After the cells were switched to Ca2+-containing medium for 3 h, the TER measured from the control cells and the genistein-treated cells reached ∼175% of the initial TER value, whereas the TER from CGP77675-treated cells had only 50% of the initial TER value. The effect of CGP77675 was time limited because TER values from all three conditions were similar by 8 h (our unpublished data).
Figure 9.
Recovery of TER after Ca2+ repletion was inhibited by CGP77675 treatment. Cells were incubated in Ca2+-free medium overnight and then switched to the Ca2+-containing medium for 0, 0.5, 1, 2, 3, and 4 h. At these time points TER measurements were taken. Each TER measurement was normalized to the initial TER value (before incubated in the Ca2+-free medium) and was obtained after background subtraction (contributed by filter and bath solution). The data represent the average ± SE from five experiments.
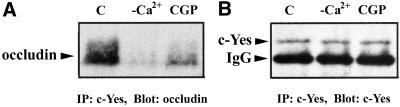
We examined the interaction of occludin with c-Yes tyrosine kinase in Ca2+-free medium and CGP77675-treated conditions. Figure 10A shows that c-Yes and occludin were not associated with each other in Ca2+-free medium. In addition, when cells were treated with CGP77675 for 2.5 h after switching to Ca2+-containing medium, the interaction of occludin and c-Yes was greatly reduced compared with the control cells. Figure 10B demonstrates that equivalent amounts of c-Yes were immunoprecipitated under all three conditions. These data were consistent with those shown in Figures 8A and 9 in which occludin tyrosine phosphorylation was undetectable after CGP77675 treatment (Figure 8A) and the TER value in the presence of CGP77675 was much lower compared with the control and genistein-treated conditions (Figure 9).
Figure 10.
Occludin/c-Yes complex was absent in minus calcium condition and was disrupted by the CGP77675 treatment. (A) Cells were incubated in Ca2+-free medium for overnight and then switched to the Ca2+-containing medium for 2.5 h without (C), or with the addition of 200 nM CGP77675 (CGP). Lane -Ca2+ indicated the cells without switching back to Ca2+-containing medium. Cells were lysed in RIPA buffer, immunoprecipitated by mouse anti-c-Yes mAb, and blotted with rabbit anti-occludin antibody. (B) Same blot as in A, but was stripped by 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl pH 7.6 for 30 min at 55°C, and then reprobed with anti-c-Yes antibody to show the equal amount of c-Yes immunoprecipitated in all three conditions.
DISCUSSION
In the present study we found that occludin tyrosine phosphorylation tightly correlated with the tight junction formation in a kidney epithelial cell line. During Ca2+ depletion, the initial quick drop of TER was accompanied by a parallel tyrosine dephosphorylation of occludin. The occurrence of occludin tyrosine rephosphorylation after Ca2+ repletion was closely associated with an increase in TER. These changes in tyrosine phosphorylation of occludin are significantly more rapid than occludin turnover (Chen et al., 2000), indicating recycling of the occludin molecules at the tight junction rather than degradation followed by de novo synthesis. The intensity of occludin tyrosine phosphorylation correlated with the TER value, because both showed a parallel overshoot 2–4 h after Ca2+ repletion. In addition, we demonstrated for the first time that the nonreceptor tyrosine kinase c-Yes was colocalized with occludin at the tight junction and along basolateral membranes and that occludin and c-Yes formed a complex in vivo by reciprocal coimmunoprecipitation assay. These data do not reveal, however, that occludin is the direct substrate for c-Yes tyrosine kinase activity or that the tyrosine phosphorylation of occludin is a necessary or sufficient condition for tight junction assembly. Because tight junctions are known to form in mice depleted of occludin (Saitou et al., 2000), more specific assays of occludin function in the tight junction are required to dissect the functional meaning of occludin tyrosine phosphorylation.
When the cells were treated with the c-Yes inhibitor CGP77675 after Ca2+ repletion, tight junction formation and the establishment of the TER were inhibited or significantly delayed. CGP77675 also disrupted the occludin/c-Yes complex, and completely abolished the tyrosine rephosphorylation of occludin. Because CGP77675 inhibits both c-Src and c-Yes (Missbach et al., 1999; Susa et al., 2000), we performed our CGP77675 inhibition assays in parallel with genistein, an inhibitor of both the epidermal growth factor receptor kinase and p60v-src kinase (Akiyama et al., 1987; Nakanishi et al., 1993; Laniyonu et al., 1995) and herbimycin A, a c-Src tyrosine kinase inhibitor (Yoneda et al., 1993; Kuo et al., 1997). We found that CGP77675, but not genistein or herbimycin A (our unpublished data), was able to inhibit both occludin tyrosine phosphorylation and tight junction reassembly, indicating that only the inhibition of c-Yes by CGP77675 was relevant. Moreover, we were unable to immunoprecipitate c-Src kinase with occludin antibodies (our unpublished data).
Results from the literature also deny Src kinase a role in cell junction assembly. Overexpression of v-Src in MDCK epithelial cells rapidly disrupts cell-to-cell contacts and the cells acquire a fibroblast-like morphology (Behrens et al., 1993). In Caco-2 cells, expression of v-Src induces β-catenin and p120ctn tyrosine phosphorylation, redistribution of E-cadherin to the cytosol, and disassembly of adherens junctions (Gomez et al., 1999). These studies indicate that Src kinase disrupts rather than promotes the assembly of cell-cell junctions. Finally, two groups have reported that Triton-insoluble membrane complexes isolated from MDCK cells contain only one major tyrosine-phosphorylated protein, p62c-yes (Sargiacomo et al., 1993; Arreaza et al., 1994). The closely related tyrosine kinase p60c-src is undetectable in these insoluble complexes even when this kinase is overexpressed in MDCK cells. Taken together, we believe that it is c-Yes kinase that is involved in occludin tyrosine phosphorylation and tight junction reassembly.
The effect of CGP77675 had a time window. By 8 h the cells treated with CGP77675 had a TER value similar to that of the control cells, indicating that the effect of CGP77675 was specific and not due to toxicity. The transient inhibitory activity of CGP77675 may have been due to other nonreceptor tyrosine kinases compensating for the loss of c-Yes kinase function, as has been postulated (Stein et al., 1994; Luton and Mostov, 1999).
c-Yes is a member of the Src family of tyrosine kinases and was initially identified as a homolog of v-yes, the oncogene of avian sarcoma virus Y73 (Ghysdael et al., 1981; Kitamura et al., 1982; Sukegawa et al., 1987). The c-yes gene is widely expressed in a variety of tissues, including epithelia such as kidney, liver, lung, and intestine (reviewed in Brickell, 1992; Thomas and Brugge, 1997). In the kidney, p62c-yes is found in the epithelial cells of the proximal tubules, which are engaged in transport and secretion (Sukegawa et al., 1990). c-Yes is tightly associated with the membrane and active as a nonreceptor protein tyrosine kinase (Sudol and Hanafusa, 1986; Sudol et al., 1988). Although there are studies of gene structure and expression patterns, the function of c-Yes at the cellular level has not been elucidated. Recently, it has been reported that c-Yes kinase interacts with Yes-associated protein 65 at the apical compartment of airway epithelia by association with ezrin-radixin-moesin-binding phosphoprotein 50 kDa (also called Na+/H+ exchanger regulatory factor; Mohler et al., 1999). These protein complexes may regulate apical signal transduction pathways leading to changes in ion transport, cytoskeletal organization, or gene expression in epithelial cells. Nusrat et al. (2000) demonstrated that c-Yes kinase interacts with a 27 amino-acid peptide of the human occludin sequence in vitro by using a novel bait peptide method. Although c-Src and c-Yes share high sequence homology outside of their unique domains, Summy et al. (2000) showed recently that the SH3 and SH2 domains between c-Src and c-Yes are capable of directing specificity in protein interactions. It is possible that c-Yes interacts with occludin through these specific unique sequences.
Both the occludin-deficient mice and the mice with null mutation of c-yes gene do not lead to an overt phenotype related to the tight junction formation (Luton et al., 1999; Saitou et al., 2000). It is well recognized now that the action of a protein may appear redundant in a null mutant because other related proteins compensate its function. For example, the double mutant fyn−/yes− mice develop a renal disease characterized as diffuse segmental glomerulosclerosis (Stein et al., 1994). These mice show high levels of proteinuria and hematuria. This phenotype was absent in fyn- or yes-deficient mice. Our finding that c-Yes kinase is involved in regulating tight junction formation and function by tyrosine phosphorylation of tight junction protein may contribute to the understanding of a subtype of renal pathology.
ACKNOWLEDGMENTS
We are grateful to Dr. Mira Susa for providing the CGP77675 reagent. We thank Dr. David L. Paul for advice and helpful discussion. We gratefully acknowledge support from grants F-270379 and GM-18974 to D.A.G., DK-34854 to Y.H.C. (pilot grant), and GM-37751 to D.L.P.
Footnotes
DOI: 10.1091/mbc.01–08–0423.
REFERENCES
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Furuse M, Tsukita S. Interspecies diversity of the occludin sequence - cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreaza G, Melkonian KA, LaFevre-Bernt M, Brown DA. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem. 1994;269:19123–19127. [PubMed] [Google Scholar]
- Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol. 2001;280:G1280–G1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- Balda MS, González-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M. Assembly and sealing tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell PM. The p60c-src family of protein-tyrosine kinases: structure, regulation, and function. Crit Rev Oncog. 1992;3:401–446. [PubMed] [Google Scholar]
- Chen Y-H, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in Ras-transformed Madin-Darby Canine Kidney cells. Mol Biol Cell. 2000;11:849–862. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci. 2000;113:3187–3196. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- Collares-Buzato CB, Jepson MA, Simmons NL, Hirst BH. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in M.D.C.K. epithelia. Eur J Cell Biol. 1998;76:85–92. doi: 10.1016/S0171-9335(98)80020-4. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Turco F, D'Atri F, Hammar E, Martinucci G, Meggio F, Citi S. Xenopus laevis occludin. Identification of in vitro phosphorylation sites by protein kinase CK2 and association with cingulin. Eur J Biochem. 1999;264:374–384. doi: 10.1046/j.1432-1327.1999.00616.x. [DOI] [PubMed] [Google Scholar]
- Denker BM, Saha C, Khawaja S, Nigam SK. Involvement of a heterotrimeric G protein alpha subunit in tight junction biogenesis. J Biol Chem. 1996;271:25750–25753. doi: 10.1074/jbc.271.42.25750. [DOI] [PubMed] [Google Scholar]
- Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling if intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Occludin–a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J, Neil JC, Vogt PK. A third class of avian sarcoma viruses, defined by related transformation-specific proteins of Yamaguchi 73 and Esh sarcoma viruses. Proc Natl Acad Sci USA. 1981;78:2611–2615. doi: 10.1073/pnas.78.4.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S, del Mont Llosas M, Verdu J, Roura S, Lloreta J, Fabre M, Garcia de Herreros A. Independent regulation of adherens and tight junctions by tyrosine phosphorylation in Caco-2 cells. Biochim Biophys Acta. 1999;1452:121–132. doi: 10.1016/s0167-4889(99)00124-x. [DOI] [PubMed] [Google Scholar]
- Goodenough DA. Plugging the leaks. Proc Natl Acad Sci USA. 1999;96:319–321. doi: 10.1073/pnas.96.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Raman N, Atkinson SJ, Marrs JA. Rho GTPase signaling regulates tight junction assembly and protects tight junctions during ATP depletion. Am J Physiol. 1998;275:C798–C809. doi: 10.1152/ajpcell.1998.275.3.C798. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N, Kitamura A, Toyoshima K, Hirayama Y, Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982;297:205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Kuo M-L, Chau Y-P, Wang J-H, Lin P-J. The role of Src kinase in the potentiation by ethanol of cytokine- and endotoxin-mediated nitric oxide synthase expression in rat hepatocytes. Mol Pharmacol. 1997;52:535–541. doi: 10.1124/mol.52.3.535. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Anderson JM, Farquhar MG. Increased tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol. 1995;37:F514–F524. doi: 10.1152/ajprenal.1995.268.3.F514. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J Membr Biol. 1999;168:289–297. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- Laniyonu A, Eto S, Wang JH, Hollenberg MD. Detection of sarcoma virus family tyrosine kinase activity in coronary arterial tissue. Can J Physiol Pharmacol. 1995;73:1552–1560. doi: 10.1139/y95-214. [DOI] [PubMed] [Google Scholar]
- Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F, Mostov KE. Transduction of basolateral-to-apical signals across epithelial cells: ligand-stimulated transcytosis of the polymeric immunoglobulin receptor requires two signals. Mol Biol Cell. 1999;10:1409–1427. doi: 10.1091/mbc.10.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F, Verges M, Vaerman JP, Sudol M, Mostov KE. The SRC family protein tyrosine kinase p62yes controls polymeric IgA transcytosis in vivo. Mol Cell. 1999;4:627–632. doi: 10.1016/s1097-2765(00)80213-0. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Biogenesis of tight junctions - the C-terminal domain of occludin mediates basolateral targeting. J Cell Sci. 1998;111:511–519. doi: 10.1242/jcs.111.4.511. [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita Sh, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Medina R, Rahner C, Mitic LL, Anderson JM, Van Itallie CM. Occludin localization at the tight junction requires the second extracellular loop. J Membr Biol. 2000;178:235–247. doi: 10.1007/s002320010031. [DOI] [PubMed] [Google Scholar]
- Missbach M, Jeschke M, Feyen J, Muller K, Glatt M, Green J, Susa M. A novel inhibitor of the tyrosine kinase Src suppresses phosphorylation of its major cellular substrates and reduces bone resorption in vitro and in rodent models in vivo. Bone. 1999;24:437–449. doi: 10.1016/s8756-3282(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Schneeberger EE, Fanning AS, Anderson JM. Connexin-occludin chimeras containing the ZO-binding domain of occludin localize at MDCK tight junctions and NRK cell contacts. J Cell Biol. 1999;146:683–693. doi: 10.1083/jcb.146.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JM, Kampherstein JA, Laughlin KV, Clarkin CEK, Miller RD, Szallasi Z, Kachar B, Soler AP, Rosson D. Overexpression of protein kinase C-delta increases tight junction permeability in LLC-PK1 epithelia. Am J Physiol. 1998;275:C544–C554. doi: 10.1152/ajpcell.1998.275.2.C544. [DOI] [PubMed] [Google Scholar]
- Nakanishi O, Shibasaki F, Hidaka M, Homma Y, Takenawa T. Phospholipase C-γ 1 associates with viral and cellular src kinases. J Biol Chem. 1993;268:10754–10759. [PubMed] [Google Scholar]
- Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight Junction. J Biol Chem. 2000;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha C, Nigam SK, Denker BM. Involvement of Gα2 in the maintenance and biogenesis of epithelial cell tight junctions. J Biol Chem. 1998;273:21629–21633. doi: 10.1074/jbc.273.34.21629. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer KL, Stevenson BR, Woo PL, Firestone GL. Relationship of serine/threonine phosphorylation/dephosphorylation signaling to glucocorticoid regulation of tight junction permeability and zo-1 distribution in nontransformed mammary epithelial cells. J Biol Chem. 1994;269:16108–16115. [PubMed] [Google Scholar]
- Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Stein PL, Vogel H, Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Sudol M, Alvarez-Buylla A, Hanafusa H. Differential developmental expression of cellular yes and cellular src proteins in cerebellum. Oncogene Res. 1988;2:345–355. [PubMed] [Google Scholar]
- Sudol M, Hanafusa H. Cellular proteins homologous to the viral yes gene product. Mol Cell Biol. 1986;6:2839–2846. doi: 10.1128/mcb.6.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa J, Akatsuka T, Sugawara I, Mori S, Yamamoto T, Toyoshima K. Monoclonal antibodies to the amino-terminal sequence of the c-yes gene product as specific probes of its expression. Oncogene. 1990;5:611–614. [PubMed] [Google Scholar]
- Sukegawa J, Semba K, Yamanashi Y, Nishizawa M, Miyajima N, Yamamoto T, Toyoshima K. Characterization of cDNA clones for the human c-yes gene. Mol Cell Biol. 1987;7:41–47. doi: 10.1128/mcb.7.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summy JM, Guappone AC, Sudol M, Flynn DC. The SH3 and SH2 domains are capable of directing specificity in protein interactions between the non-receptor tyrosine kinases cSrc and cYes. Oncogene. 2000;19:155–160. doi: 10.1038/sj.onc.1203265. [DOI] [PubMed] [Google Scholar]
- Susa M, Luong-Nguyen NH, Crespo J, Maier R, Missbach M, McMaster G. Active recombinant human tyrosine kinase c-Yes: expression in baculovirus system, purification, comparison to c-Src, and inhibition by a c-Src inhibitor. Protein Expr Purif. 2000;19:99–106. doi: 10.1006/prep.2000.1221. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Structural and signaling molecules come together at tight junctions [In Process Citation] Curr Opin Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Balda MS, Anderson JM. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J Cell Sci. 1995;108:1735–1742. doi: 10.1242/jcs.108.4.1735. [DOI] [PubMed] [Google Scholar]
- Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Lowe C, Lee CH, Gutierrez G, Niewolna M, Williams PJ, Izbicka E, Uehara Y, Mundy GR. Herbimycin A, a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J Clin Invest. 1993;91:2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]