Summary
Mobile wireless communication technologies have now become an everyday part of our lives, 24 hours a day, 7 days a week. Monitoring the autonomous system under exposition to electromagnetic fields may play an important role in broading of our still limited knowledge on their effect on human body. Thus, we studied the interaction of the high frequency electromagnetic field (HF EMF) with living body and its effect on the autonomic control of heart rate using Heart Rate Variability (HRV) linear and nonlinear analyses in healthy volunteers. A group of young healthy probands (n=30, age mean: 24.2 ± 3.5 years) without any symptoms of disease was exposed to EMF with f=2400 MHz (Wi-Fi), and f=2600 MHz (4G) for 5 minutes applied on the chest area. The short-term heart rate variability (HRV) metrics were used as an indicator of complex cardiac autonomic control. The evaluated HRV parameters: RR interval (ms), high frequency spectral power (HF-HRV in [ln(ms2)]) as an index of cardiovagal control, and a symbolic dynamic index of 0V %, indicating cardiac sympathetic activity. The cardiac-linked parasympathetic index HF-HRV was significantly reduced (p =0.036) and sympathetically mediated HRV index 0V % was significantly higher (p=0.002) during EMF exposure at 2400 MHz (Wi-Fi), compared to simulated 4G frequency 2600 MHz. No significant differences were found in the RR intervals. Our results revealed a shift in cardiac autonomic regulation towards sympathetic overactivity and parasympathetic underactivity indexed by HRV parameters during EMF exposure in young healthy persons. It seems that HF EMF exposure results in abnormal complex cardiac autonomic regulatory integrity which may be associated with higher risk of later cardiovascular complications already in healthy probands.
Keywords: Heart rate variability, Electromagnetic field, Exposure Wi-Fi, 4G network
Introduction
Electromagnetic smog is an everyday part of the environment around us and its effect on the human body has not yet been sufficiently explained. Since 1990, the world has seen a rapid increase of wireless communication technology working on wide range of different frequency bands (GSM, UMTS, Wi-Fi, etc.). Current research indicates the possible influence of HF EMF on human body, in particular in autonomic nervous system (ANS) as regulatory “orchestra” modulating body functions including cardiovascular system which is extremely sensitive to autonomic inputs. Generally, mobile phones and all routinely used electrical wireless devices can represent a potential source of electromagnetic interference [1–3]. Long-term studies referred that the high-frequency interfaces in working or living environments can cause subjective symptoms of headaches, lack of concentration, excessive irritability, forgetfulness, slowing of reflexes, inflammation of the eyes, tearing, whistling in the ears, etc. [1, 4–5]. These and other objective health disorders (e.g., syndrom of electromagnetic hypersensitivity) may result from body exposure to high- and low-frequency EMFs [1].
The short-term heart rate variability (HRV), i.e., oscillations of heart rate around its mean value, in humans is predominantly mediated by parasympathetic regulation. From the time series analysis tools, linear methods are traditionally used, especially the spectral power in the high frequency band (HF-HRV) reflecting the cardiovagal modulation [6–10, 46–48]. In this context, symbolic dynamics - as nonlinear HRV analysis - is considered as a promising tool to study cardiac sympathetic regulation. Visnovcova et al. firstly described that 0V % is sufficiently sensitive to detect cardiac-linked sympathetic excitation in response to cognitive stressors in healthy subjects concluding that 0V % can be considered as cardiac sympathetic index [35]. From clinical aspect, this assumption was confirmed in mental disorders [11,12,13]. Notably, reduced HRV is associated with higher risk of cardiovascular complications, as it was documented in several studies [14].
At present, there is a limited number of studies examining the effect of electromagnetic radiation on heart rate variability in healthy persons. Misek et al. [15] found that short-term intermittent radiofrequency (HF EMF) radiation targeting the head of healthy volunteers affected ANS leading to a significant increase in HRV indicators such as HF band spectral power and rMSSD (root mean square of successive differences between normal heartbeats), and a decrease in heart rate. The obtained data indicate that exposure of head to HF EMF under given conditions increases parasympathetic nerve activity. Other results reported the stimulation of sympathetic nerve activity [3] or even no effect on ANS [16]. Thus it seems that findings and conclusions on this issue are rather contradictory, and need next research. The discrepancies may result from different measurement protocols, e.g., head or chest exposure, intensity of exposure, total exposure time or measurement of intervals on ECG periods [15,16,17]. Thus, we aimed to study the interaction of HF EMF with living body under chest exposure (simulated signal with frequency 2400 MHz for Wi-Fi and 2600 MHz for 4G) using HRV short-term linear and nonlinear measures in young persons.
Methods
Subjects
A total of 30 healthy persons (15 women) aged 20–30 years (age mean: 24.2 ± 3.5 years, body mass index (BMI): 23.3± 3.5 kg/m2) were examined. The inclusion criteria were following: no respiratory, neurological, cardiovascular or other diseases potentially influencing activity of the ANS, for their selection included good health (no subjective complaints or symptoms of immune, cardiac or neurological disorders or diseases), neither drugs nor abuse that would affect HRV. Volunteers were medical students informed in detail about the course of the investigation. All of them agreed with the study and endorsed the informed consent, as well as the consent to the processing of his/her personal data. Each subject obtained a personal number due to personal data protection. The study was performed with the consent of the Ethics Committee of JFM CU in Martin. Basic characteristics of the studied group are summarized in the Table 1.
Table 1.
Basic physiological data of probands (Mean x ± SD)
| Parameter | Total number of probands | ||
|---|---|---|---|
| Male (n=15)* | Female (n=15)* | ||
| Age (years) | 23.14 ± 5.76 | 24.26 ± 3.3 | |
| Weight (kg) | 79.28 ± 12.14 | 61.34 ± 9.53 | |
| Height (cm) | 180.84 ± 7.81 | 164.69 ± 5.48 | |
| BMI (kg/m 2 ) | 24.4 ± 3.99 | 22.11 ± 3.02 | |
| Blood pressure (mmHg) | Systolic | 121.25 ± 6.45 | 113.38 ± 15.02 |
| Diastolic | 68.75 ± 10.14 | 66.25 ± 11.18 | |
χ̄ ± SD=Mean x ± SD
Protocol
Each person was tested only once due to the longer time required for one measurement. Prior to each examination, the basic physiological parameters as height, weight, systolic/diastolic blood pressures were taken with a digital OMRON M300 sphygmomanometer (Omron, Kyoto, Japan). The anthropometric characteristics were evaluated by body composition analyser (InBody 120, Biospace Co., Ltd. South Korea). The formula BMI is: mass/height2 [kg/m2] [2].
Measurement procedure
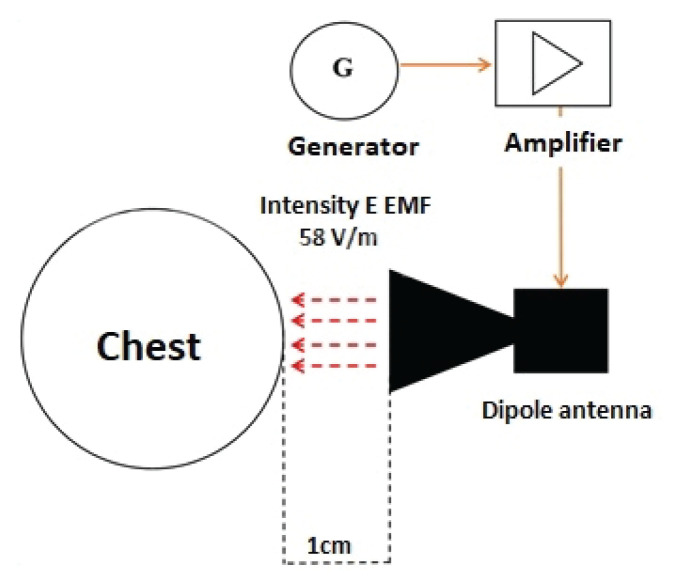
The aim of the measurement was to determine the changes in HRVafter exposure of the selected part of human body (chest) to EMF with accepted limit value of E=58V/m and to comment on the activation (inhibition) of the sympathetic resp. parasympathetic parts of the ANS after exposure. Also, our interest was to determine the differences in HRV during EMF exposure at the frequencies 2400 MHz (frequency of Wi-Fi signal) and 2600 MHz (frequency of 4G network).
All measurements were performed in uniform mode according to the particular procedures valid for exposures with f=2400 MHz and f=2600 MHz, given to chest of the probands. During the measurement, we tried to eliminate an optic perception of persons, because it can significantly affect HRV. For this reason, we used a mask to cover the eyes. The temperature in the laboratory was kept at 20 ± 1 °C and the relative humidity within the range of 50–60 %. The study took place in a normal (stable temperature and humidity, minimization of disturbing sound, visual and smell stimuli) biophysical laboratory room that is not electromagnetically shielded in any way. The trials were carried out in the morning in total time range from 8.00 a.m. to 03.00 p.m.
Prior to each examination, the basic physiological parameters height, weight, blood pressure were taken with a digital OMRON M300 sphygmomanometer. The Body Mass Index (BMI Tab.1) of probands was also measured with the IN BODY device (InBody J10, Biospace, Korea) in the physiological laboratory of JLF UK in Martin.
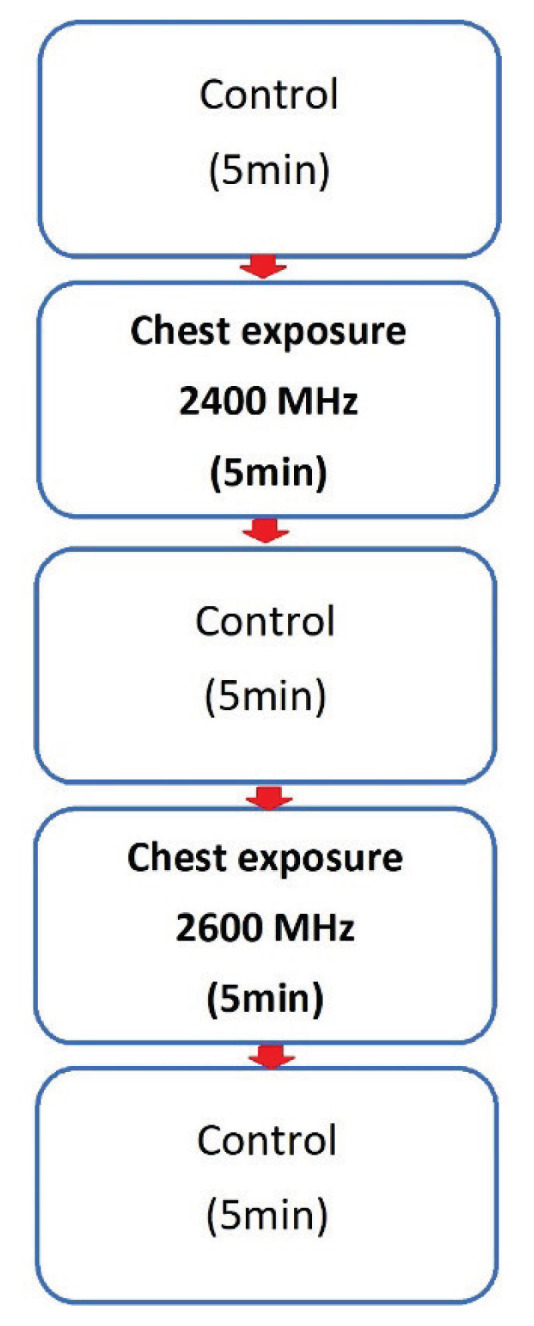
During the HRV measurement, the examined person sat on a chair with a backrest. The stool was always adjusted so that the lower limb in the examiner’s knee joint was at a 90 ° angle to the stool, feet rested on the floor. The back of the subject was leaning against the back of the chair and the back of the proband was stretched out. The examiner’s hands were placed loosely on his thighs. The proband was exposed to half a band during the measurement. Initially, a no-exposure control measurement was performed for 5 minutes. Subsequently, the irradiation dipole antenna of f=2400 MHz with simulated Wi-Fi signal, was placed at the required distance (1 cm) near the left pectoral muscle (2 cm cranially from the left nipple). The exposure time was 5 min. Exposure was followed by control measurement No.2. The control was followed by exposure with a dipole antenna with f=2600 MHz which simulated 4G (LTE) network. The measurement procedure remained the same as the exposure with the simulated Wi-Fi signal. It should be noted that, only the fundamental frequency bands where transmissions occur are harmonic and unmodulated. Fig. 1 shows a time related procedures for EMF exposure of the chest, as well as the individual controls during exposure with 2600 MHz and 2400 MHz.
Fig. 1.
Schema of chest exposure trial
Exposure system
The exposure system consisted of a frequency generator (Agilent N9310A, China) with a frequency range of 9 kHz – 3GHz, also RF signal amplifier (Amplifier Research 5S1G4, United Kingdom) with a frequency range of 800 MHz – 4.2 GHz with a power of 5 W, and directional dipole antennas with frequencies of 2600 MHz and 2400 MHz (Fig. 2).
Fig. 2.
Scheme of exposure system and affected body region
The frequency generator was set to f=2400 MHz with an amplitude of −1.4 dBm. From the output of the frequency generator, the signal was fed by the amplifier, where an intensity was set up to limit value of 58 V/m. The control level of EMF intensity was ensured by NARDA NBM-550 broadband sensor (Narda Safety Test Solutions, Germany) with an antenna with a frequency range of 100 kHz – 3 GHz. Subsequently, the signal from the output of the amplifier reached a directional dipole antenna. The directional dipole antenna was designed for EMF exposure of f=2400 MHz.
When designing the exposure antennas, we based on the technical parameters of the antennas, as well as the minimal requirements to achieve the suitable characteristics. Since we wanted to achieve a Wi-Fi signal, and simulated 4G (LTE) of current electromagnetic data transmission devices we had to established mainly carrier frequencies for these signals. It is well known that the wavelength of the EMF decreases with increasing frequency. That’s why we designed two antennas. One antenna was constructed for the carrier frequency of 2400 MHz and the other one for 2600 MHz. The antennas were made from a cylindrical aluminium tube to which an N-connector was mounted. Inside tube the advanced part was placed and through its EMF was transmitted. The wavelength of the EMF was calculated as follows:
where, v = speed of light in vacuum [m/s] and f = frequency [Hz] [21].
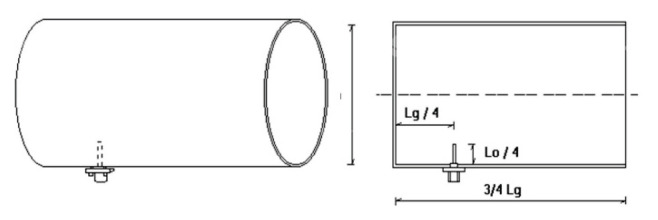
The wavelength in this case is given in units of (m−1). By recalculation we got λ=11.54 cm for f=2600 MHz and λ=12.5 cm for f=2400 MHz. When designing the antennas, an impact was devoted to inner surface of the antenna, which had to correspond to a smooth surface in order to eliminate reflections inside them. Fig. 3 shows the dimensions of a directional dipole antenna for its proper function.
Fig. 3.
Scheme of exposure antenna. Lg/4 – distance from the antenna tip to the rear wall of the antenna, L0/4 – antenna tip height, 3/4 Lg – antenna length, D – antenna diameter.
Antenna dimensions:
• for Wi-Fi signal antenna (2400 MHz) – D=85 mm, L0=125 mm, Lg=247 mm, L0/4=31 mm, Lg/4=62 mm
• for LTE (4G) signal antenna (2600 MHz) – D=85 mm, L0=115 mm, Lg=191 mm, L0/4=29 mm, Lg/4=48 mm.
The dimensions of the antennas must be strictly adhered to, as on their inner sides (due to reflections), so-called standing waves can appear which may change the signal intensity. The distance between the transmitting antenna and the irradiated segment was 1 cm. The limit value for intensity E of EMF at 2600 MHz frequency was 58 V/m [21–23].
Heart rate variability analysis
We used an automated DiANS PF8 HRV system (Dimea Group, Ltd., Czech Republic) to detect QRS complex and RR intervals, the same as Misek et al. used [15]. According to the recommendations for HRV, the artifact-free, 5 min of RR-intervals were analysed by software package HRVAS [12, 18]. Continuous recording of RR intervals for conventional spectral analysis was performed under sampling frequency of 1 kHz using a window length of 256 samples. The HRV linear analysis allows to evaluate the faster high frequency (HF: 0.15–0.4 Hz) component reflecting respiratory sinus arrhythmia, i.e., oscillations of the heart rate during breathing mediated through parasympathetic regulation [19, 20, 45, 46]. From the non-linear HRV analysis – symbolic dynamics – 0V % index was found to be sensitive to the changes of the sympathovagal balance and could be associated with the conditions characterizing the sympathetic activation. This index may represent novel approach for non-invasive assessment of cardiac sympathetic regulation that is independent on the other effects, such as myocardial preload and afterload influencing frequently used beta-adrenergic index pre-ejection period [11, 20, 49–51]. Thus, the evaluated HRV parameters were following RR interval (ms) spectral power in high frequency (HF-HRV: 0.15 – 0.4 Hz in [ln(ms2)]) as an index of cardiovagal control, and a symbolic dynamic index 0V %, indicating cardiac sympathetic activity [35].
Statistical analysis
The measured values were processed using Jamovi 1.6 (statistical software, Australia), as well as Microsoft Office Excel 2016 (Microsoft corp., USA). The selected statistical comparisons were preceded by an informative normal distribution test (Shapiro-Wilk). Student’s paired t-test for Gaussian distribution and Wilcoxon non-parametric test were used to compare statistical significance. The confidence interval was set up at 95 % (P <0.05). The probabilities P <0.05 were considered significant. Data were expressed as mean (χ̄) ± standard deviation (SD).
Results
Statistical analysis revealed that the cardiac parasympathetic index LN HF-HRV was significantly decreased comparing to control only in response of exposure 2600 MHz (4G) (p=0.036). In contrast, sympathetically mediated HRV index 0V % was significantly increased in response of EMF exposure at 2400 MHz (Wi-Fi) compared to simulated 4G frequency 2600 MHz (p=0.002). No significant differences were found in the RR intervals. All results are summarized in the Table 2.
Table 2.
HRV parameters of probands at EMF 2400 MHz (Wi-Fi) and 2600 MHz (4G) chest exposure
| HRV parameter | f= 2400 (MHz) | p-value | HRV parameter | f= 2600 (MHz) | p-value | ||
|---|---|---|---|---|---|---|---|
| RR ms | RR ms | ||||||
| control | 955.413 ± 167.00 | control | 965.78 ± 163.83 | ||||
| exposure | 973.19 ± 189.00 | 0.389 | exposure | 960.93 ± 171.51 | 0.763 | ||
|
| |||||||
| LN HF ln(ms 2 ) | LN HF ln(ms 2 ) | ||||||
| control | 6.37 ± 0.96 | control | 6.39 ± 0.17 | ||||
| exposure | 6.20 ± 1.08 | 0.262 | exposure | 6.10 ± 0.23 | 0.036* | ||
|
| |||||||
| 0V % | 0V % | ||||||
| control | 25.62 ± 2.04 | control | 26.10 ± 11.03 | ||||
| exposure | 30.99± 2.40 | 0.002* | exposure | 27.41± 11.79 | 0.488 | ||
(Mean ± SD, n =30)
Statistical significance at p<0.05
Discussion
In our study, we studied cardiac autonomic control using HRV parameters during exposure to EMF applicated on the chest in healthy young people. The simulated harmonic and unmodulated signal meeting the minimum requirements represented the frequency band 2400 MHz for Wi-Fi data transmission and 2600 MHz for 4G (LTE) mobile networks. The intensity E of EMF reached the value 58V/m, which did not exceed the standard limiting value of 61 V/m [15,24]. This study revealed that cardiac sympathovagal balance indexed by HRV measures was shifted to the sympathetic overactivity (higher 0V %) during EMF exposure at 2400 MHz (Wi-Fi), and to cardiovagal underactivity (lower HF-HRV) in case of exposure 4G. Several mechanisms are supposed.
Similar to Veternik et al. during monitoring, we focused on the basic parameters for each frequency in the time and frequency domain, mainly the average duration of RR intervals and spectral power [25]. By using the linear (spectral) analysis of HRV we evaluated the faster oscillations in the HF band (LN HF-HRV), which is primarily influenced by vagal activity [18,26,33].
The HRV short-term spectral analysis represents a promising tool to evaluate predominantly cardiac vagal control. More specifically, the HF EMF (0.15–0.40 Hz) is mainly affected by fluctuations in cardiac vagal activity. The HF band reflects more the modulation of parasympathetic entry than its tone, due to immediate changes in acetylcholine concentration. The HF component is highlighted under physiological circumstances during parasympathetic activation manoeuvres, together with its connection to the central autonomous network [19]. Thus, the HF-HRV is considered as an index of cardiovagal control, especially in psychophysiological research [14,46]. Further, HRV signal is generally considered as a chaotic non-harmonic component, so the method of nonlinear analysis is also used to analyse the complexity of the cardiovascular system. Symbolic dynamics is a suitable method for the quantification of cardiac time series complexity independent of its magnitude [27,28,49]. When the complexity (qualitative indicator) of the heart rate variability series is reduced, the ability of the regulatory subsystems to interact is reduced. This in turn leads to the inability of the physiological system to adapt [29–30] Notably, several studies have referred those symbolic dynamics analysis is superior to conventional spectral indices due to its sensitivity to sympathetically mediated heart rate fluctuations [31]. More specifically, the symbolic dynamics index 0V % was assessed due to the fact that several studies revealed 0V % sufficient sensitivity to detect sympathetically mediated changes [31,32,35,46,52].
Our study found that the 0V % index increased during Wi-Fi exposure (2400 MHz), without significant changes in cardiac vagal control indicating impaired complex sympathetically mediated cardiac control. Although ANS activity appears to be affected by EMF, it shows a different pattern of change depending on some parameters (e.g., signal type, time, modulation, incidence area, etc). The sympathetic nervous system thus plays a key role in the stress reaction from the external environment (EMF exposure) and its increased activity results in an increase in heart rate and suppression of the parasympathetic effect [19–20]. Therefore, we assume that the high-frequency electromagnetic field given to the chest of volunteers can act as a form of stress for the human body, affecting the electrical activity of the heart, resulting in a shift in autonomous dynamic heart rate regulation towards sympathetic hyperactivity. The same changes in the results were also observed by Misek et al. in their study where they declared that changes in HRV associated with stress are represented by a decrease in parasympathetic nerve activity, increase in HR, and a higher level of sympathovagal balance [15]. Moreover, the same effect on ANS was documented after exposure of head to HF EMF in rabbits [41]. Then we propose that the place of exposure to EMF (chest or head) can be of importance for particular type of response of ANS.
Increasing evidence suggests that EMF such as Wi-Fi, 4G have many bioeffects that could affect cardiovascular system and induce oxidative stress [34,36,37] In general, an EMF field source located near the chest can easily interact with biological tissues. The higher the frequency of the signal, the shorter EM waves penetrate the body. EM waves can thus interfere and modulate the cardiac pacemaker – sinoatrial node causing the beat-to-beat short-term dysregulation of the cardiac chronotropic control. Moreover, we can suggest discrete abnormalities of brain areas involved in central autonomic network (i.e., insular cortex, amygdala, hypothalamus, periaqueductal gray matter, parabrachial complex, nucleus of the tractus solitarius, and ventrolateral medulla) that may subsequently alter cardiovascular control towards sympathetic overactivity [39,42]. Otherwise, reduced prefrontal activity may lead to compensatory increased activity of subcortical areas resulting in persistent sympathetic area activation. The faultless functioning of inhibitory-excitatory regulatory mechanisms is crucial for the physiological adaptability and flexibility of the organism. Disruption of the proper functioning of regulatory influences prefrontal-subcortical areas may be associated with psychopathology with manifestations internalized and externalized mental disorders [40,43]. We therefore assume a disruption of the neurocardiac integrity towards sympathicotonia during close chest exposure at 2400 MHz simulated Wi-Fi signal.
Paradoxically, 4G signal caused us a shift in the cardiac parasympathetic component - a decrease in cardiovagal regulation indexed by LN HF-HRV. It suggests that cardiac vagal regulation is more sensitive to 4G compared to Wi-Fi signal. We consider that a directional dipole antenna placed at a very close distance from the chest represents a type of stressor affecting neurocardiac integrity resulting in altered parasympathetic control. The short-term action of the HF EMF at 2600 MHz causes changes in the cells of the neurocardiac transmission system. Thus, cardiovagal dysregulation can be caused directly by the influence of regulatory medullary centers (nc. ambiguous) leading to altered sinoatrial node activity. Moreover, several psychophysiological theories, such as polyvagal theory [44] or neurovisceral integration model [38] suggest that vagus nerve is associated with emotional, social and cognitive abilities. Thus, in addition to impaired prefrontal-subcortical inhibitory functioning, we can also suggest potential effect of psychological features associated with 4G exposure such as anticipatory anxiety on cardiovagal regulation. More specifically, neural networks implicated in autonomic, emotional, and cognitive self-regulation are also involved in the control of cardiac autonomic activity. Behavioural and neuroimaging studies have identified several pathways by which cardiac vagal tone is linked to neural networks implicated in emotional and cognitive self-regulation. According to this neurovisceral integration model, the reduced cardiovagal modulation can lead to decreased ability of the organism to track the rapid changes in environmental demands and to organize appropriate response [38]. Thus, we can assume impact of both – neurobiological and psychological processes leading to impaired complex cardiac autonomic control evoked by EMF at young healthy probands. The question is also time - will sympathetic/parasympathetic regulation change over longer time?
Taken together, this study for the first time revealed a distinct shift in heart rate autonomic regulation towards complex sympathetic overactivity (Wi-Fi) and cardiovagal underactivity (4G) in young healthy subjects after exposure to EMF on the chest of persons. It seems that EMF exposure results in abnormal complex cardiac autonomic regulatory integrity which may be associated with higher risk of later cardiovascular complications already in healthy probands.
Study Limitations
The limitation of the study is relatively small population sample of healthy volunteers (n=30). We suggest to validate the study on a larger number of probands with respect to gender. Moreover, further research with continual recording of other parameters evaluating autonomic nervous system (e.g., blood pressure variability) is necessary to obtain more knowledge about other autonomic effectors’ responses.
Conclusions
Our study revealed discrete abnormalities of complex cardiac autonomic regulation indexed by HRV measures dependent on the effects of 4G and Wi-Fi networks. It seems that direct exposure to high radiofrequency EMF may be associated with a higher risk of cardiovascular complications already in healthy youth. However, the results need to be validated in a future study on a larger number of young probands under the same conditions.
Acknowledgements
This study was supported by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sports of the Slovak Republic and the Slovak Academy of Sciences within the project VEGA - 1/0190/20 (Prof. Tonhajzerova) and the Slovak Agency for Research and Development under the project APVV 19-0214 (Prof. Jakus).
Funding Statement
This study was supported by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sports of the Slovak Republic and the Slovak Academy of Sciences within the project VEGA - 1/0190/20 (Prof. Tonhajzerova) and the Slovak Agency for Research and Development under the project APVV 19-0214 (Prof. Jakus).
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Jakušová V. Ultraviolet radiation and mobile communication: physical properties, biological effects and health protection. (In Slovak) Bratislava: Samosato; 2009. p. 97 s. [Google Scholar]
- 2.Ekici B, Tanindi A, Ekici G, Diker E. The effects of the duration of mobile phone use on heart rate variability parameters in healthy subjects. Anatol J Cardiol. 2016:833–838. doi: 10.14744/AnatolJCardiol.2016.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barutcu I, Esen AM, Kaya D, Turkmen M, Karakaya O, Saglam M, Melek M, Çelik A, Kilit C, Onrat E, Kirma C. Do mobile phones pose a potential risk to autonomic modulation of the heart? Pacing Clin Electrophysiol. 2011;34:1511–1514. doi: 10.1111/j.1540-8159.2011.03162.x. [DOI] [PubMed] [Google Scholar]
- 4.Balikci K, Cem Ozcan I, Turgut-Balik D, Balik HH. A survey study on some neurological symptoms and sensations experienced by long term users of mobile phones. Pathol Biol (Paris) 2005;53:30–34. doi: 10.1016/j.patbio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Taheri M, Mortazavi SMJ, Moradi M, Mansouri S, Hatam GR, Nouri F. Evaluation of the effect of radiofrequency radiation emitted from wi-fi router and mobile phone simulator on the antibacterial susceptibility of pathogenic Bacteria Listeria monocytogenes and Escherichia coli. Dose-Response: An Int J. 2017;15:1–8. doi: 10.1177/1559325816688527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 7.Acharya RU, Lim C, Joseph P. Heart rate variability analysis using correlation dimension and detrended fluctuation analysis. ITBM-RBM. 2002;23:333–339. doi: 10.1016/S1297-9562(02)90002-1. [DOI] [Google Scholar]
- 8.Joseph P, Acharya UR, Poo Ch, Chee J, Lim Ch, Iyengar S, Wei H. Effect of reflexological stimulation on heart rate variability. ITBM-RBM. 2004;25:40–45. doi: 10.1016/j.rbmret.2004.02.002. [DOI] [Google Scholar]
- 9.Ahamed TVI, Karthick NG, Joseph PK. Effect of mobile phone radiation on heart rate variability. Computers in Biology and Medicine. 2008;38:709–712. doi: 10.1016/j.compbiomed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Levy MN, Schwartz PJ. Vagal control of the heart: experimental basis and clinical implications. Future. Armonk. 1994 [Google Scholar]
- 11.Tonhajzerova I, Ondrejka I, Javorka K, Turianikova Z, Farsky I, Javorka M. Cardiac autonomic regulation is impaired in girls with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:613–8. doi: 10.1016/j.pnpbp.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Visnovcova Z, Mestanik M, Gala M, Mestanikova A, Tonhajzerova I. The complexity of electrodermal activity is altered in mental cognitive stressors. Comput Biol Med. 2016;79:123–129. doi: 10.1016/j.compbiomed.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Thorat KD, Shelke V. Effects of mobile phone radiation on heart rate variation in healthy volunteers. Res J Pharmaceut. Biol Chem Sci. 2013;4:840–845. [Google Scholar]
- 14.Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research - recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;8:213. doi: 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misek J, Belyaev I, Jakusova V, Tonhajzerova I, Barabas J, Jakus J. Heart rate variability affected by radiofrequency electromagnetic field in adolescent students. Bioelectromagnetics. 2018;39:277–288. doi: 10.1002/bem.22115. [DOI] [PubMed] [Google Scholar]
- 16.Wallace J, Andrianome S, Ghosn R, Blanchard ES, Telliez F, Selmaoui B. Heart rate variability in healthy young adults exposed to global system for mobile communication (GSM) 900-MHz radiofrequency signal from mobile phones. Environ Res. 2020;191:110097. doi: 10.1016/j.envres.2020.110097. [DOI] [PubMed] [Google Scholar]
- 17.Ramshur JT. Design, evaluation, and application of heart rate variability analysis software (HRVAS) The University of Memphis; 2010. [DOI] [Google Scholar]
- 18.Camm A. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Tasks force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996. Circulation. 93:1043–1065. [PubMed] [Google Scholar]
- 19.Javorka K, Čalkovská A, Danko J, Dókuš K, Funiak S, Gwozdziewicz M, et al. Heart rate variability (in Slovak) Martin: Osveta; 2008. p. 204p. [Google Scholar]
- 20.Bujnakova I, Ondrejka I, Mestanik M, Visnovcova Z, Mestanikova A, Hrtanek I, Fleskova D, Calkovska A, Tonhajzerova I. Autism spectrum disorder is associated with autonomic underarousal. Physiol Res. 2016;65(Suppl 5):S673–S682. doi: 10.33549/physiolres.933528. [DOI] [PubMed] [Google Scholar]
- 21.Navrátil L, Rosina J. Medicínská biofyzika. (in Czech) Praha: Grada; 2005. p. 524s. [Google Scholar]
- 22.Hampton RCh. The fundamentals of Wi-Fi Antennas. Technical Article. 2015 [Google Scholar]
- 23.Yang Ming, Sun Yufa, Li Fan. A Compact Wideband Printed Antenna for 4G/5G/WLAN Wireless Applications. Int J Antennas Propagation. 2019;2019:3209840. doi: 10.1155/2019/3209840. [DOI] [Google Scholar]
- 24.Dasdag S, Akdag MZ. The link between radiofrequencies emitted from wireless technologies and oxidative stress. J Chem Neuroanatomy. 2016;75:85–93. doi: 10.1016/j.jchemneu.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Veternik M, Tonhajzerova I, Misek J, Jakusova V, Hudeckova H, Jakus J. The impact of sound exposure on heart rate variability in adolescent students. Physiol Res. 2018;67:695–702. doi: 10.33549/physiolres.933882. [DOI] [PubMed] [Google Scholar]
- 26.Oliver F, Acharya UR, Krishnan SM, Min LC. Analysis of cardiovascular signals using spatial filling index and time-frequency domain. Biomed Online J USA. 2004;3:30. doi: 10.1186/1475-925X-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzzetti S, Borroni E, Garbelli PE, Ceriani E, Della Bella P, Montano N, Cogliati C, Somers VK, Malliani A, Porta A. Symbolic dynamics of heart rate variability. A probe to investigate cardiac autonomic modulation Circulation. 2005;112:465–470. doi: 10.1161/CIRCULATIONAHA.104.518449. [DOI] [PubMed] [Google Scholar]
- 28.Brosschot JF, Verkuil B, Thayer JF. Generalized unsafety theory of stress: unsafe environments and conditions, and the default stress response. Int J Environ Res Public Health. 2018;15:464. doi: 10.3390/ijerph15030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porta A, Guzzeti S, Furlan R, Gnecchi-Ruscone T, Montano N, Malliani A. Complexity and nonlinearity in short-term heart rate variability: comparison of methods based on local nonlinear prediction. Trans Biomed Eng. 2007;54:94–106. doi: 10.1109/TBME.2006.883789. [DOI] [PubMed] [Google Scholar]
- 30.Slavikova M, Sekaninova N, Bona O, Visnovcova Z, Tonhajzerova I. Biofeedback - A promising non-pharmacological tool of stress - related disorders. Acta Medica Martiniana. 2020;20:1–8. doi: 10.2478/acm-2020-0001. [DOI] [Google Scholar]
- 31.Voss A, Schulz S, Schroeder R, Baumert M, Caminal P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos Trans A Math Phys Eng Sci. 2009;367:277–96. doi: 10.1098/rsta.2008.0232. [DOI] [PubMed] [Google Scholar]
- 32.Porta A, Gnecchi-Ruscone T, Tobaldini E, Guzzetti S, Furlan R, Malliani A, Montano N. Symbolic analysis of short-term heart period variability during graded head-up tilt. Computers in Cardiology. 2006;33:109–112. [Google Scholar]
- 33.Mann K, Röschke J, Connemann B, Beta H. No effects of pulsed high-frequency electromagnetic fields on heart rate variability during human sleep. Neuropsychobiology. 1998;38:251–256. doi: 10.1159/000026549. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed FA, Ahmed AA, El-Kafoury BMA, Lasheen NN. Study of the cardiovascular effects of exposure to electromagnetic field. Life Sci J. 2011;8:260–275. [Google Scholar]
- 35.Visnovcova Z, Mestanik M, Javorka M, Mokra D, Gala M, Jurko A, Calkovska A, Tonhajzerova I. Complexity and time asymmetry of heart rate variability are altered in acute mental stress. Physiol Meas. 2014;35:1319–1334. doi: 10.1088/0967-3334/35/7/1319. [DOI] [PubMed] [Google Scholar]
- 36.Gmitrov J. Static magnetic field effect on the arterial baroreflex-mediated control of microcirculation: implications for cardiovascular effects due to environmental magnetic fields. Radiat Environ Biophys. 2007;46:281–290. doi: 10.1007/s00411-007-0115-2. [DOI] [PubMed] [Google Scholar]
- 37.Salah MB, Abdelmelek H, Abderraba M. Effects of olive leave extract on metabolic disorders and oxidative stress induced by 2.45 GHz WIFI signals. Environ Toxicol Pharmacol. 2013;36:826–834. doi: 10.1016/j.etap.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 39.Wehrwein EA, Orer HS, Barman SM. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol. 2016;6:1239–1278. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 40.Tonhajzerová I. Psychofyziológia: Od stresovej odpovede po Biofeedback. Martin. 2016. et al; p. 164s. [Google Scholar]
- 41.Misek J, Veterník M, Tonhajzerova I, Jakusova V, Janousek L, Jakus J. Radiofrequency electromagnetic field affects heart rate variability in rabbits. Physiol Res. 2020;69:633–643. doi: 10.33549/physiolres.934425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/S0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 43.Tonhajzerová I. Psychophysiology: Respiratory sinus arrhythmia in the context of the polyvagal theory. (in Slovak) Martin. 79s. 2015. [Google Scholar]
- 44.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonhajzerova I, Ondrejka I, Ferencova N, Bujnakova I, Grendar M, Olexova LB, Hrtanek I, Visnovcova Z. Alternations in the Cardiovascular Autonomic Regulation and Growth Factors in Autism. Physiol Res. 2021;70:551–561. doi: 10.33549/physiolres.934662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mestanik M, Mestanikova A, Langer P, Grendar M, Jurko A, Sekaninova N, Visnovcova N, Tonhajzerova I. Respiratory sinus arrhythmia - testing the method of choice for evaluation of cardiovagal regulation. Respir Physiol Neurobiol. 2019;259:86–92. doi: 10.1016/j.resp.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Young Benton. Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health? Behav Pharmacol. 2018;29(Spec issue 2–3):140–151. doi: 10.1097/FBP.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porta A, Tobaldini E, Guzzetti S, Furlan R, Montano N. Gnecchi-Ruscone TAssessment of cardiac autonomic modulation during graded head-up tilt by symbolic analysis of heart rate variability. Am J Physiol Heart Circ Physiol. 2007;293:H702–H708. doi: 10.1152/ajpheart.00006.2007. [DOI] [PubMed] [Google Scholar]
- 50.Silva L, Geraldini VR, De Olivera BP, Silva CAA, Porta A, Fazan R. Comparison between spectral analysis and symbolic dynamics for heart rate variability analysis in the rat. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-08888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther. 2020;24:91–102. doi: 10.1016/j.bjpt.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moura-Tonello SCG, Carvalho VO, Godoy MF, Porta A, Leal ÂMO, Bocchi EA, Catai AM. Evaluation of cardiac autonomic modulation using symbolic dynamics after cardiac transplantation. Braz J Cardiovasc Surg. 2019;34:572–580. doi: 10.21470/1678-9741-2019-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]