Abstract
Pre-exposure prophylaxis (PrEP) reduces human immunodeficiency virus (HIV) transmission through sexual contact by at least 90% when taken as prescribed. This retrospective cohort study evaluated differences in adherence to PrEP medication and monitoring between the physician- and nurse practitioner (NP)-led in-person setting and the pharmacist-led telehealth setting among patients followed by the infectious diseases clinic at the VA Eastern Colorado Health Care System from July 2012 to February 2021. The primary outcomes were PrEP tablets filled per person-year, serum creatinine (SCr) tests per person-year, and HIV screens per person-year. Secondary outcomes included sexually transmitted infection (STI) screens per person-year and patients lost to follow-up.
149 patients were included in the study, with 167 person-years in the in-person cohort and 153 person-years in the telehealth cohort. Adherence to PrEP medications and monitoring was similar between in-person and telehealth clinics. PrEP tablets filled per person-year was 324 in the in-person cohort and 321 in the telehealth cohort (RR = 0.99; 95% CI, 0.98-1.00). SCr screens per person-year was 3.51 in the in-person cohort and 3.37 in the telehealth cohort (RR = 0.96; 95% CI, 0.85–1.07). HIV screens per person-year was 3.55 in the in-person cohort and 3.38 in the telehealth cohort (RR = 0.95; 95% CI, 0.85–1.07). There were no new HIV infections. Additionally, patients were less likely to be lost to follow-up when followed via telehealth (11.9% vs. 30.0%), Χ2 (1, N = 149) = 6.85, p = 0.009. These findings indicate that pharmacist-driven delivery of PrEP via telehealth can be used to increase access to PrEP without sacrificing quality of care.
Keywords: Pre-exposure prophylaxis (PrEP), HIV, Adherence, Medication possession ratio, Telehealth, Truvada, Descovy
Background
Pharmacotherapy for pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) first entered the health care market in the United States on July 16, 2012 when the US Food and Drug Administration (FDA) approved emtricitabine/tenofovir disoproxil fumarate (FTC/TDF), a once-daily oral tablet that can prevent HIV infection in at-risk individuals [1]. Additionally, FTC/tenofovir alafenamide (TAF) was FDA-approved for HIV PrEP in 2019, making it the second preventative agent available for patients at risk for HIV [2]. Oral PrEP with either FTC/TDF or FTC/TAF is greater than 90% effective for preventing HIV transmission via sex when taken daily [3–7]. Since its introduction, PrEP utilization has expanded globally, with over 600,000 people across 76 countries receiving PrEP at least once in 2019—a 70% increase from 2018 [8].
Despite continual growth in access, barriers to PrEP access still exist, resulting in disparities and inequities [9–11]. These barriers include, but are not limited to, individual awareness of PrEP, geographical distance to care, and cost [12]. Providers have also cited insufficient time and lack of familiarity with guidelines as barriers to prescribing PrEP [13]. Each of these barriers may independently limit initiation of, adherence to, and efficacy of PrEP. Throughout the past decade, advocates have worked to address these barriers through education of the public and providers, lobbying for insurance coverage of PrEP and developing new models for delivery of care. In recent years, many innovative clinic models have been developed to increase access to PrEP. Most of these clinics use telehealth technologies—including synchronous or asynchronous provider-patient interactions via video, telephone, or messaging systems to overcome geographic barriers to care [12]. The National HIV/AIDS Strategy for the United States for 2022–2025 includes a goal to increase the diversity of the workforce of providers who deliver HIV prevention, testing, and supporting services [14]. As the most accessible health care professionals, pharmacists are uniquely positioned to increase access to PrEP [15–17].
According to CDC recommendations for prescribing of PrEP, patients should receive quarterly HIV screening, in addition to other routine monitoring at various frequencies (Table 1) [18, 19]. Patients prescribed PrEP through telehealth largely report that quarterly lab visits are not bothersome, [20] indicating that telehealth is a reasonable vehicle for prescription of PrEP. However, little-to-no data exists evaluating the comparative outcomes associated with prescription of PrEP between telehealth and traditional in-person appointments.
Table 1.
Summary of CDC-recommended monitoring in patients taking oral PrEP
| Baseline | Every 3 months | Every 6 months | Every 12 months | |
|---|---|---|---|---|
| 2017 CDC Guidelines |
• HIV • SCr • Bacterial STI* • HBV serology • HCV serology∫ • Pregnancy‡ |
• HIV • Bacterial STI† • Pregnancy‡ |
• SCr • Bacterial STI† |
|
| 2021 CDC Guidelines |
• HIV • SCr • Bacterial STI • HBV serology • HCV serology∫ • Pregnancy‡ • Lipid panel¶ |
• HIV • Bacterial STI† • Pregnancy‡ |
• SCr◊ • Bacterial STI† |
• SCr◊ • HCV serology∫ • Lipid panel¶ |
HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; MSM = men who have sex with men; PWID = people who inject drugs; SCr = serum creatinine; STI = sexually transmitted infection; TGW = transgender women
*Gonorrhea, chlamydia, syphilis; testing for gonorrhea and chlamydia in MSM should be performed using samples from the urine, pharynx, and rectum (“three-site testing”)
∫In MSM, TGW, and PWID
†2017 and 2021 CDC Guidelines recommend quarterly (every three months) STI screening for sexually active persons with signs or symptoms of infection and screening for asymptomatic MSM at high risk for recurrent bacterial STI (e.g., those with history of bacterial STI or multiple sex partners) and semiannual (every six months) STI screening for all other sexually active patients
‡In people who may become pregnant
¶In patients taking F/TAF only
◊2021 CDC Guidelines recommend semiannual (every six months) renal function testing in patients age ≥ 50 years or estimated creatinine clearance (eCrCl) < 90 mL/min at PrEP initiation, and annual (every 12 months) renal function testing in all other patients
Within the Veterans Health Administration (VHA), telehealth technologies have been used to provide a variety of clinical services to patients in a way that is both cost-effective and well-received [21]. At the same time as utilization of telehealth has been increasing, providers within the VHA have worked to increase patient access to PrEP through provider education, social media outreach and awareness campaigns, and clinical support tools to identify patients who would be appropriate for PrEP [22].
Providers at the VA Eastern Colorado Heath Care System (ECHCS) utilize telehealth for prescribing PrEP to veterans in Colorado who are at-risk of contracting HIV. Over recent years, PrEP management has transitioned from exclusively in-person (where PrEP is provided by physicians and nurse practitioners) to majority telehealth (where PrEP follow-up is provided by clinical pharmacist practitioners via telephone) (Fig. 1). Additionally, the Annie App for Veterans—a VA service that sends automated text messages to veterans—is often utilized in the VA ECHCS to increase adherence to medication, laboratory monitoring, and appointments [23]. This study aimed to evaluate differences in outcomes between patients who received PrEP from an infectious diseases physician or NP via in-person encounters and those who received PrEP from an infectious diseases clinical pharmacist via telehealth.
Fig. 1.
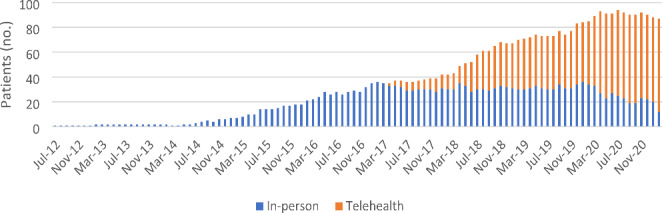
VA ECHCS PrEP clinic enrollment by month. Patients who were not included in the study are not represented in this figure
Methods
Study Design
The study was a retrospective cohort study of patients initiated on PrEP on or after July 16, 2012 through February 28, 2021. The study was approved by the institutional review boards at the primary and affiliate sites.
Study Setting
The telePrEP clinic is run by two clinical pharmacists through the infectious diseases clinic at VA ECHCS in Aurora, Colorado. The pharmacists have 30 years’ experience between them, as well as certifications within pharmacotherapy specialties and infectious diseases. Both have scopes of practice approved by the infectious diseases service line, which includes prescribing of PrEP medications, ordering of associated labs, as well as STI screening and treatment. Scopes of practice are maintained through regular professional practice competency and clinical peer review. The pharmacists have access to 8.5 full-time employee infectious diseases physicians and 2 full-time employee infectious diseases nurse practitioners.
When a patient engages with the PrEP clinic, the initial encounter is in-person with a physician or NP, at which the patient decides whether to continue with in-person care with the physician or NP, or to engage with a clinical pharmacist via the telehealth clinic.
During each telehealth encounter, a 90-days’ supply of PrEP is prescribed with no refills and labs are ordered for the following appointment. Patients are encouraged to get labs checked when they have 30 days’ worth of medication left. When labs result, the ordering provider is notified via the electronic health record, and the patient is contacted by the pharmacist via telephone for a follow-up visit. An appointment is not required. If the patient cannot be reached, they remain on the pharmacist panel for further outreach attempts. Lab due dates are also tracked so that the pharmacist can intervene if labs are not collected at the appropriate time.
Patients
Patients at the VA ECHCS were included if they were at least 18 years old and had at least two 90-day prescription fills for FTC/TDF or FTC/TAF within a continuous six-month time frame during the study period. Patients were excluded if PrEP was prescribed outside the VA ECHCS infectious diseases clinic or if FTC/TDF or FTC/TAF were being used for an indication other than PrEP.
Subgroups of patients included those followed during the period of April 2017 (i.e., the launch of the telehealth clinic) through the end of the study period, rural veterans (as identified by the RUCA2.0 code, which assigns a rurality code based on the veteran’s zip code) and veterans enrolled in the Annie App for Veterans. While any provider can enroll a veteran in the Annie App, it was only utilized in the telehealth clinic during the study period.
Procedures
Patients were identified using prescription data for FTC/TDF and FTC/TAF. Inclusion in the study was confirmed using information from the Computerized Patient Record System (CPRS), the electronic medical record system used at the VA. Data (e.g., demographic, appointment, prescription, and laboratory) were collected from CPRS and organized using Microsoft Excel. To account for the large proportion of patients who transitioned from in-person to telehealth clinic, incidence densities were utilized to estimate adherence to medication and monitoring (Eq. 1). Time spent in both in-person and telehealth clinics (i.e., the treatment period) was determined by CPRS progress note titles and authors—which differ between clinics—and was recorded in months. Number of PrEP tablets filled and number of screens for SCr, HIV, and STI were recorded and separated into those ordered while being followed by in-person PrEP providers and those ordered while being followed by telehealth PrEP providers. Non-routine (e.g., duplicate, repeat, hospital) labs were not included. Patients were determined to be lost to follow up if there was a period greater than 6 months since the last PrEP-related provider visit or PrEP fill date without documentation that the patient would be continuing PrEP (whichever occurred later). For patients who transferred care outside of the infectious diseases clinic, study enrollment ended at the time transfer of care was documented in the health record, or 3 months after the last PrEP fill (whichever occurred later).
Equation 1:
Incidence density equation used to estimate adherence to medication and monitoring
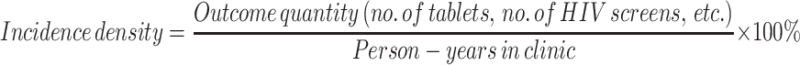 |
Outcomes
The primary outcomes were PrEP tablets filled per person-year, HIV screens per person-year, and SCr tests per person-year. Secondary outcomes included STI screens per person-year and patients lost to follow-up. Patients who transitioned from FTC/TDF to FTC/TAF were evaluated as an exploratory outcome.
Statistical Analysis
Primary outcomes were reported as incidence rates and compared as rate ratios with 95% confidence intervals, employing exact Poisson method. Loss to follow-up was assessed using chi-square analysis. P-values of less than 0.05 were considered to indicate statistical significance in all analyses.
Results
Patient Characteristics
Between July 16, 2012 and February 28, 2021, 488 patients were prescribed FTC/TDF or FTC/TAF. Of the 488 patients identified using prescription data, 176 were on FTC/TDF or FTC/TAF as part of an HIV treatment regimen. 147 patients had fewer than two 90-day prescription fills within a 6-month time frame during the study period. Nine patients were prescribed PrEP outside the VA ECHCS infectious diseases clinic. Seven patients were prescribed FTC/TDF or FTC/TAF for either post-exposure prophylaxis (PEP) or hepatitis B treatment. In total, 149 patients were included in the study. Demographics and clinical characteristics of the patients are summarized in Table 2.
Table 2.
Demographic and clinical characteristics of study patients
| Characteristic | Overall (N = 149) |
|---|---|
| Treatment setting - no. (%) | |
| All in-person Multiple in-person, then telehealth Single in-person, then telehealth All telehealth |
40 (26.8) 48 (32.9) 49 (32.2) 12 (8.1) |
| PrEP Indication - no. (%) | |
| Men who have sex with men (MSM) High-risk heterosexual activity |
141 (94.6) 8 (5.4) |
| Serodiscordant Relationship - no. (%) | 25 (16.8) |
| Age - no. (%) | |
|
18–29 yr 30–39 yr 40–49 yr 50–65 yr > 65 yr |
7 (4.7) 57 (38.3) 38 (25.5) 38 25.5) 9 (6.0) |
| Gender - no. (%) | |
|
Cisgender man Transgender man Cisgender woman Transgender woman |
145 (97.3) 2 (1.3) 1 (0.7) 1 (0.7) |
| Race - no. (%) | |
|
White Black or African American Asian American Indian or Alaska Native Native Hawaiian or Other Pacific Islander Unknown or Declined to Answer |
107 (71.8) 23 (15.4) 2 (1.3) 2 (1.3) 2 (1.3) 13 (8.7) |
| PrEP History - no. (%) | |
|
New start Continuation from outside clinic |
103 (69.1) 46 (31.5) |
| Annie App Enrollment - no. (%) | 39 (26.2) |
Clinic Treatment Periods
149 unique patients spent 167.4 person-years in the in-person PrEP clinic and 153.1 person-years in the telehealth PrEP clinic. The median time followed in clinic was 0.83 years for in-person and 1.08 years for telehealth. 55 patients transitioned from in-person to telehealth during the study period, excluding patients who were only seen in-person for the first encounter. No patients transitioned from telehealth to in-person during the study period.
Primary Outcomes
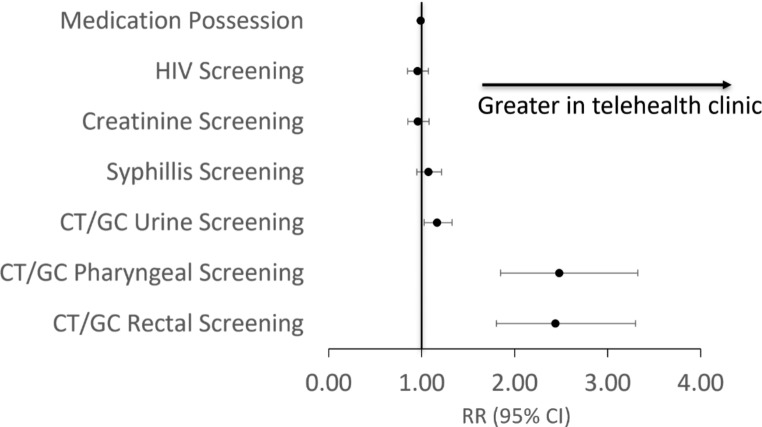
Primary and secondary outcomes are summarized in Table 3; Fig. 2. PrEP tablets filled per person-year was 324.4 in the in-person clinic, compared to 320.6 in the telehealth clinic (RR = 0.99; 95% CI, 0.98-1.00). These incidence rates correspond with estimated medication possession rates of 89% and 88%, respectively. HIV screens per person-year was 3.6 in the in-person clinic, compared to 3.4 in the telehealth clinic (RR = 0.95; 95% CI, 0.85–1.07). Similarly, SCr tests per person-year was 3.5 in the in-person clinic, compared to 3.4 in the telehealth clinic (RR = 0.96; 95% CI, 0.85–1.08). There were no HIV infections across either clinic.
Table 3.
Primary and secondary outcomes
| In-person | Telehealth | Rate ratio (95% CI) | |
|---|---|---|---|
| Time in clinic (PY): | 167.4 | 153.08 | |
| Primary Outcomes | |||
|
Tablets prescribed per PY (no.) Estimated medication possession (%) HIV screens per PY (no.) Creatinine tests per PY (no.) |
324.4 89 3.55 3.51 |
320.6 88 3.38 3.37 |
0.99(0.98-1.00) 0.95 (0.85–1.07) 0.96 (0.85–1.08) |
| Secondary Outcomes | |||
|
Syphilis screens per PY (no.) CT/GC (urine) screens per PY (no.) CT/GC (pharyngeal) screens per PY (no.) CT/GC (rectal) screens per PY (no.) |
3.11 2.68 0.38 0.36 |
3.33 3.13 0.94 0.88 |
1.07 (0.95–1.21) 1.17 (1.03–1.33) 2.46 (1.83–3.30) 2.42 (1.79–3.27) |
| Loss to Follow-up | |||
|
Clinic of most recent PrEP appointment (no.) Lost to follow up (no. [%]) |
40 12 (30) |
109 13 (11.9) |
Χ [2] (1, N = 149) = 6.85 P = 0.009 |
PY = Person-Year
Fig. 2.
Comparison of medication possession and monitoring between in-person and telehealth PrEP clinics
Secondary and Exploratory Outcomes
There were 3.11 syphilis screens per person-year in the in-person clinic, compared to 3.33 in the telehealth clinic (RR = 1.07; 95% CI, 0.95–1.21). Urine screens for chlamydia and gonorrhea occurred at a slightly lower rate in the in-person clinic, compared to telehealth, with 2.68 and 3.33 screens per person-year, respectively (RR = 1.17; 95% CI, 1.03–1.33). Pharyngeal and rectal screens for chlamydia and gonorrhea occurred at a significantly lower rate in the in-person clinic, compared to telehealth (RR = 2.46; 95% CI, 1.83–3.30 [pharyngeal]; RR = 2.42; 95% CI, 1.79–3.27 [rectal]), but occurred less frequently in either clinic compared to other STI screens, at 0.38 pharyngeal and 0.36 rectal screens per person-year in the in-person clinic, vs. 0.94 pharyngeal and 0.88 rectal screens per person-year in the telehealth clinic.
Twelve of 40 (30%) patients most recently seen in the in-person clinic were lost to follow-up, compared to 13 of 109 (11.9%) patients most recently seen in the telehealth clinic, Χ [2] (1, N = 149) = 6.85, p = 0.009. Eleven (7.4%) patients changed therapy from FTC/TDF to FTC/TAF during the study period. Nine (6.0%) patients changed due to renal dysfunction and two (1.3%) changed due to decreased bone mineral density.
April 2017 to February 2021 Subgroup
In patients followed for any duration during the period of April 2017 to February 2021, the average number of PrEP tablets filled per person-year was 311.3 in the in-person clinic, compared to 321.3 in the telehealth clinic (RR = 1.03; 95% CI, 1.02–1.05). Screens for HIV and SCr were similar between clinics (RR = 0.98; 0.85–1.11 and RR = 0.99; CI 0.87–1.14, respectively).
Annie App Subgroup
Of 109 patients followed—at any point—via telehealth, 39 were enrolled in the Annie App for Veterans. PrEP tablets filled per person-year was 332.7 in the Annie subgroup, compared to 304.7 in the non-Annie subgroup (RR = 1.09; 95% CI, 1.07–1.11). HIV screens per person-year was 3.61 in the Annie subgroup, compared to 3.04 in the non-Annie subgroup (RR = 1.19; 95% CI, 1.0-1.42). Serum creatinine tests per person-year was 3.57 in the Annie subgroup, compared to 3.11 in the non-Annie subgroup (RR = 1.15; 95% CI, 0.96–1.37). These results are summarized in Table 4.
Table 4.
Outcomes for Annie App for Veterans subgroup
| Non-Annie | Annie | Rate ratio (95% CI) | |
|---|---|---|---|
| Time in clinic (PY): | 66.2 | 86.9 | |
| Primary Outcomes: | |||
|
Tablets prescribed per PY (no.) Estimated medication possession (%) HIV screens per PY (no.) Creatinine tests per PY (no.) |
305 83 3.04 3.11 |
332.7 91 3.61 3.56 |
1.09 (1.07–1.11) 1.19 (1.00-1.42) 1.15 (0.96–1.37) |
PY = Person-Year
Rural Veterans
This subgroup analysis was not performed due to an inadequate number of rural veterans enrolled in the study. 139 (93.3%) veterans included in the study live in a metropolitan area.
Discussion
This study was developed to identify whether PrEP could be prescribed by clinical pharmacists via telehealth without compromising quality of care when compared to in-person prescribing by physicians or NP, thus reducing geographic barriers to PrEP and increasing prescribing capacity. Using markers of adherence to medication and CDC-recommended monitoring of HIV status and renal function, we identified that there is no difference in engagement in care between the two healthcare modalities. Medication adherence, as measured by prescription fill rates, was high in both clinics—near 90%. A pharmacokinetics study of men who have sex with men (MSM) taking FTC/TDF for PrEP found that HIV risk was reduced by 99% for individuals who took an average seven doses per week, 96% for four doses per week, and 76% for two doses per week [24]. Data from clinical trials support this finding, indicating that an average of four or more doses per week is sufficient to prevent HIV infection [25].
Additionally, we found that patients are less likely to be lost to follow-up when receiving care via telehealth. This finding is consistent with other studies that evaluated patient retention in non-PrEP telehealth clinics [26, 27]. Reasons for improved patient retention in our telehealth clinic is likely multifactorial. First, telehealth minimizes the geographic barrier to care, with patients only having to present for labs, rather than returning for a scheduled appointment. Second, our pharmacist-driven outreach model and frequently monitored patient panel allows for active intervention to maintain patient engagement. Third, the Annie App services utilized by a subset of patients in the telehealth clinic provided active reminders of upcoming labs and recommended follow-up. The latter may be a confounding factor to consider when interpreting the results of this study, as the Annie App was only offered to patients in the telehealth clinic.
STI screening was more variable between clinics, likely due to changes in standard practices over time. Prior to 2017, PrEP was prescribed entirely via in-person care when STI screening was recommended every six months. As PrEP care has evolved to become majority telehealth in our facility, recommendations for frequency of bacterial STI screening have also evolved and now, it is standard practice in our facility to screen all patients prescribed PrEP for bacterial STI every three months. Additionally, self-collection of samples for extragenital CT/GC testing became standard at our facility in December 2019 following a targeted quality improvement project. Since, we have seen pharyngeal and rectal CT/GC testing increase dramatically and in an exploratory analysis of our data after December 2019, we saw similar rates of STI screening between clinic settings. The increase in standard frequency of bacterial STI screening over time and availability of self-collection kits for extragenital testing likely skewed the results of this study in favor of the telehealth clinic.
Among patients followed via telehealth, estimated adherence to PrEP was statistically significantly higher in patients enrolled in the Annie App for Veterans, compared to those not enrolled with the app. Annie sends automated text messages to veterans that can include health-related notifications, reminders, or motivational messages. The app has been utilized by VA ECHCS PrEP providers to remind veterans of due laboratory tests. While there was no significantly significant difference in adherence to CDC-recommended monitoring, a higher proportion of medication possession during the study period may indicate that automated reminders can improve patient care. Limited evidence suggests that text messaging services and mobile applications are low burden tools that can improve adherence to PrEP [28, 29]. Patients are generally willing to utilize these applications to streamline their care [30]. As previously mentioned, the fact that only veterans in the telehealth clinic were enrolled in the Annie App may confound the results of this study.
To our knowledge, this is the first study to compare in-person and telehealth delivery of PrEP, as well as physician/NP-prescribed and pharmacist-prescribed PrEP within the same health care system. VA ECHCS is consistently in the top ten providers of PrEP within the VHA and has been utilizing telehealth for PrEP delivery since 2017, making this facility an ideal place to perform this study. Our findings support the United States National HIV/AIDS Strategy for 2022–2025 of increasing the diversity of the workforce of providers who deliver HIV prevention, testing, and supportive services.
This study has several limitations, namely its small size (n = 176) despite a 9-year study period. Other limitations of this study stem largely from the evolution of PrEP prescribing from majority in-person to majority telehealth in recent years. Changes in CDC PrEP prescribing recommendations and recent standardization of self-collection of samples for extragenital STI screens likely skewed results in favor of telehealth clinic. Several observational studies describe decreased PrEP utilization during the COVID-19 pandemic due to limited access to PrEP-related healthcare services and reduced number and frequency of sexual encounters [31–34]. In our clinic, to limit COVID-19 exposure, we reduced the frequency of laboratory monitoring to every 6 months in most patients, as opposed to quarterly. Decreased utilization and reduced frequency of monitoring during the COVID-19 pandemic would likely skew our results in favor of the in-person clinic since most patients were followed via telehealth during the pandemic.
Additionally, utilizing prescription fill data as a marker of adherence–and therefore treatment efficacy—presents a margin for error since it was not confirmed that patients took each dose as prescribed. However, it is commonplace to measure medication adherence using prescription fill rates (medication possession), as direct observation of medication administration is not pragmatic in most scenarios. Furthermore, medication adherence is assessed during each PrEP-related patient encounter.
Generalizability of these results may be limited for several reasons. First, high medication adherence in both PrEP clinics is likely aided by a lower cost barrier to PrEP within the VHA. Veterans pay $0-$11 per month for medications and since the VHA is the payer for medications, lack of insurance is not an issue for veterans enrolled in our PrEP clinics. Second, the patients included in this study were majority white MSM, which may limit the generalizability of these results to other genders or those with other indications for PrEP. Lastly, nondaily (also called event-driven, intermittent, or “on-demand”) PrEP, which has demonstrated efficacy for preventing HIV infection, [35–38] is not commonly prescribed at our facility. The prescribing patterns for nondaily PrEP caused these patients to be excluded from this study. Thus, the results of this study may not be generalizable to patients prescribed nondaily PrEP.
As PrEP care continues to change, continued research and evaluation of delivery models will be needed. Other areas for study include evaluating PrEP delivery between infectious diseases and primary care clinics, outcomes between oral PrEP (i.e., FTC/TDF and FTC/TAF) and injectable PrEP (cabotegravir), which received FDA approval in 2021 [39, 40].
In conclusion, adherence to PrEP medications and routine monitoring was similar between in-person prescribing by physicians/NP and telehealth prescribing by clinical pharmacists. We also found that patients were less likely to be lost to follow-up when followed via telehealth. These findings indicate that pharmacist-driven delivery of PrEP via telehealth can be used to increase prescribing capacity and eliminate geographic barriers to PrEP without sacrificing the quality of care.
Authors’ Contribution
Kellen Greenwell: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing (original draft). Randolph Fugit: conceptualization, methodology, resources, supervision, validation, writing (reviewing and editing). Lindsay Nicholson: methodology, resources, validation, writing (reviewing and editing). Jason Wright: conceptualization, investigation, methodology, resources, supervision, validation, writing (reviewing and editing).
Funding
No funding was received for conducting study.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Declarations
Conflicts of interest/Competing interests
The authors have no financial or proprietary interests in any material discussed in this article.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted with the IRBs of VA Eastern Colorado Health Care System and University of Colorado who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the IRBs of VA Eastern Colorado Health Care System and University of Colorado.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Food and Drug Administration Approves Gilead’s Truvada® for Reducing the Risk of Acquiring HIV. (2012). Gilead Sciences, Inc. Accessed 1 Sep 2021. https://www.gilead.com/news-and-press/press-room/press-releases/2012/7/us-food-and-drug-administration-approves-gileads-truvada-for-reducing-the-risk-of-acquiring-hiv.
- 2.U.S. Food and Drug Administration Approves Descovy® for HIV Pre-Exposure Prophylaxis (PrEP). (2019). Gilead Sciences, Inc. Accessed 1 Sep 2021 https://www.gilead.com/news-and-press/press-room/press-releases/2019/10/us-food-and-drug-administration-approves-descovy-for-hiv-preexposure-prophylaxis-prep.
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer KH, Molina J-M, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomized, double-blind, multicentre, active-controlled, phase 3, noninferiority trial. The Lancet. 2020;396(10246):239–54. doi: 10.1016/S0140-6736(20)31065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomized, double blind, placebo-controlled phase 3 trial. Lancet Jun. 2013;15(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. (2021, March 16). Global data shows increasing PrEP use and widespread adoption of WHO PrEP recommendations. Accessed 15 Sep 2021. https://www.who.int/news-room/feature-stories/detail/global-data-shows-increasing-prep-use-and-widespread-adoption-of-who-prep-recommendations.
- 9.Lelutiu-Weinberger C, Golub S. Enhancing PrEP Access for black and latino men who have sex with men. J Acquir Immune Defic Syndr. 2016;73(5):547–55. doi: 10.1097/QAI.0000000000001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page K, Martinez O, Nieves-Lugo K, et al. Promoting Pre-Exposure Prophylaxis to prevent HIV infections among sexual and gender minority Hispanics/Latinxs. AIDS Educ Prev. 2017;29(5):389–400. doi: 10.1521/aeap.2017.29.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanny D, Jeffries W, Chapin-Bardales J, et al. Racial/Ethnic disparities in HIV Preexposure Prophylaxis among Men who have sex with men — 23 Urban Areas, 2017. MMWR Morb Mortal Wkly Rep 2019. 2019;68:801–6. doi: 10.15585/mmwr.mm6837a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touger R, Wood BR. A review of telehealth innovations for HIV pre-exposure prophylaxis (PrEP) Curr HIV/AIDS Rep. 2019;16(1):113–9. doi: 10.1007/s11904-019-00430-z. [DOI] [PubMed] [Google Scholar]
- 13.Babiarz J, Nix CD, Bowden S, Roberts R. (2023). Insufficient PrEParation: an assessment of primary care prescribing habits and use of pre-exposure prophylaxis in patients at risk of HIV acquisition at a single medical centre. Sexually transmitted infections, sextrans-2022-055551. Advance online publication. 10.1136/sextrans-2022-055551. [DOI] [PubMed]
- 14.Office of National AIDS Policy (ONAP). Date last updated: December 14 2021. National HIV/AIDS Strategy (2022–2025). Accessed 22 Jul 2022. https://www.hiv.gov/federal-response/national-hiv-aids-strategy/national-hiv-aids-strategy-2022-2025.
- 15.Farmer EK, Koren DE, Cha A, Grossman K, Cates DW. The pharmacist’s expanding role in HIV pre-exposure prophylaxis. AIDS patient care and STDs. 2019 May 1;33(5):207–13. doi:10.1089/apc.2018.0294. [DOI] [PubMed]
- 16.Zhao A, Dangerfield DT, Nunn A, Patel R, Farley JE, Ugoji CC, Dean LT. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS and Behavior 2021 Oct 20:1–6. doi:10.1007/s10461-021-03494-4. [DOI] [PMC free article] [PubMed]
- 17.Crawford ND, Myers S, Young H, Klepser D, Tung E. The role of pharmacies in the HIV prevention and care continuums: a systematic review. AIDS and Behavior 2021 Jun;25(6):1819–28. doi:10.1007/s10461-020-03111-w. [DOI] [PMC free article] [PubMed]
- 18.Preexposure prophylaxis for the prevention of HIV infection in the United States. – 2017 update. U.S. Public Health Service, Centers for Disease Control and Prevention. Accessed 15 Sep 2021. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf.
- 19.Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update. U.S. Public Health Service, Centers for Disease Control and Prevention. Accessed 1. Sep 2021. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf.
- 20.Koester KA, Hughes SD, Grant RM. A good habit”: telehealth PrEP users find benefit in quarterly monitoring requirements. J Int Association Providers AIDS Care (JIAPAC) 2020;19:2325958220919269. doi: 10.1177/2325958220919269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perdew C, Erickson K, Litke J. Innovative models for providing clinical pharmacy services to remote locations using clinical video telehealth. Am J Health-System Pharm. 2017;74(14):1093–8. doi: 10.2146/ajhp160625. [DOI] [PubMed] [Google Scholar]
- 22.Chartier M, Gylys-Cowell I, Van Epps P, et al. Accessibility and uptake of pre-exposure prophylaxis for HIV prevention in the Veterans Health Administration. Fed Practitioner. 2018;35(Suppl 2):42. [PMC free article] [PubMed] [Google Scholar]
- 23.Annie app for Veterans. VA Mobile. Accessed 15 Sep 2021. https://mobile.va.gov/app/annie-app-veterans.
- 24.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson PL, Glidden DV, Liu A et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012 Sep 12;4(151):151ra125. doi:10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed]
- 26.Saifu HN, Asch SM, Goetz MB, et al. Evaluation of human immunodeficiency virus and hepatitis C telemedicine clinics. Am J Manag Care. 2012;18(4):207–12. [PubMed] [Google Scholar]
- 27.Boshara AI, Patton ME, Hunt BR, Glick N, Johnson AK. Supporting Retention in HIV Care: comparing In-Person and telehealth visits in a Chicago-Based infectious Disease Clinic. AIDS Behav. 2022;1–7. 10.1007/s10461-022-03604-w. [DOI] [PMC free article] [PubMed]
- 28.Siegler AJ, Steehler K, Sales JM, Krakower DS. A review of HIV pre-exposure prophylaxis streamlining strategies. Curr HIV/AIDS Rep. 2020 Dec;17(6):643–53. 10.1007/s11904-020-00528-9. [DOI] [PMC free article] [PubMed]
- 29.Moore DJ, Jain S, Dubé MP et al. Randomized controlled trial of daily text messages to support adherence to preexposure prophylaxis in individuals at risk for human immunodeficiency virus: the TAPIR study. Clinical Infectious Diseases. 2018 May 2;66(10):1566-72. doi:10.1093/cid/cix1055. [DOI] [PMC free article] [PubMed]
- 30.Bundy C, Hall X, Foran CD, Jozsa JE, Newcomb K, Mustanski B. Reassessing the importance of PrEP use given reduced sex during the COVID-19 pandemic: perspectives from a sample of young sexual minority men. AIDS Educ prevention: official publication Int Soc AIDS Educ. 2022;34(6):441–52. doi: 10.1521/aeap.2022.34.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pampati S, Emrick K, Siegler AJ, Jones J. Changes in sexual behavior, PrEP adherence, and Access to sexual Health Services because of the COVID-19 pandemic among a cohort of PrEP-Using MSM in the South. J Acquir Immune Defic Syndr. 2021;87(1):639–43. doi: 10.1097/QAI.0000000000002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong C, Horvath KJ, Stephenson R, Nelson KM, Petroll AE, Walsh JL, John SA. PrEP use and persistence among young sexual minority men 17–24 Years Old during the COVID-19 pandemic. AIDS Behav. 2022;26(3):631–8. doi: 10.1007/s10461-021-03423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goedel WC, Rogers BG, Li Y, Nunn AS, Patel RR, Marshall BDL, Mena LA, Ward LM, Brock JB, Napoleon S, Zanowick-Marr A, Curoe K, Underwood A, Johnson CJ, Lockwood KR, Chan PA. Pre-exposure Prophylaxis Discontinuation during the COVID-19 Pandemic among Men who have sex with men in a Multisite Clinical Cohort in the United States. J Acquir Immune Defic Syndr. 2022;91(2):151–6. doi: 10.1097/QAI.0000000000003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan PS, Driggers R, Stekler JD et al. Usability and acceptability of a mobile comprehensive HIV prevention app for men who have sex with men: a pilot study. JMIR mHealth and uHealth. 2017 Mar 9;5(3):e7199. doi:10.2196/mhealth.7199. [DOI] [PMC free article] [PubMed]
- 35.Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–46. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 36.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9):e402–10. doi: 10.1016/S2352-3018(17)30089-9. [DOI] [PubMed] [Google Scholar]
- 37.Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. The Lancet HIV. 2020;7(2):e113. doi: 10.1016/S2352-3018(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 38.Dimitrov D, Moore JR, Wood D, et al. Predicted effectiveness of daily and non-daily PrEP for MSM based on sex and pill-taking patterns from HPTN 067/ADAPT. Clin Infect Dis. 2019;71(2):249–55. doi: 10.1093/cid/ciz799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration. (2021). FDA approves first injectable treatment for HIV pre-exposure prevention. FDA. gov. Updated December, 20. Accessed 1 May 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.