Abstract
Sepsis is a leading cause of global mortality in children, yet definitions for pediatric sepsis are outdated and lack global applicability and validity. In adults, the Sepsis-3 Definition Taskforce queried databases from high-income countries to develop and validate the criteria. The merit of this definition has been widely acknowledged; however, important considerations about less-resourced and more diverse settings pose challenges to its use globally. To improve applicability and relevance globally, the Pediatric Sepsis Definition Taskforce sought to develop a conceptual framework and rationale of the critical aspects and context-specific factors that must be considered for the optimal operationalization of future pediatric sepsis definitions. It is important to address challenges in developing a set of pediatric sepsis criteria which capture manifestations of illnesses with vastly different etiologies and underlying mechanisms. Ideal criteria need to be unambiguous, and capable of adapting to the different contexts in which children with suspected infections are present around the globe. Additionally, criteria need to facilitate early recognition and timely escalation of treatment to prevent progression and limit life-threatening organ dysfunction. To address these challenges, locally adaptable solutions are required, which permit individualized care based on available resources and the pretest probability of sepsis. This should facilitate affordable diagnostics which support risk stratification and prediction of likely treatment responses, and solutions for locally relevant outcome measures. For this purpose, global collaborative databases need to be established, using minimum variable datasets from routinely collected data. In summary, a “Think globally, act locally” approach is required.
Keywords: biology, criteria, global, pediatric, sepsis
Across the world, substantial morbidity, mortality, expense, and adverse impacts on healthcare systems are attributed to pediatric sepsis (1). Unfortunately, variability in the operationalization of pediatric sepsis definitions leads to difficulties in measuring the burden of sepsis and optimizing management and may compromise quality improvement initiatives. In many settings, “sepsis” has been used synonymously with “severe infection” resulting in substantial challenges and variability in recognition and coding for sepsis (2). Although Sepsis-3 definitions (3) were intended to unify the various definitions and terminologies, they have some limitations when applied to the global healthcare community, limitations which are even more pronounced when considering pediatric age groups. There are significant differences in the epidemiology, biology, susceptibility, and approach to sepsis management between adults and children, which are magnified between high-income country (HIC) and upper middle-income country (UMIC) settings compared with low-income country and low middle-income country (LMIC) settings, where the majority of pediatric sepsis occurs.
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, but there are no validated measures that determine when a child transitions from having an “infection” to having “sepsis” (4). The purpose of establishing criteria for pediatric sepsis is to support a structured process for the early diagnosis of sepsis, development of appropriate interventions for that diagnosis, and to facilitate pos thoc assessment of the accuracy of management decisions. It is particularly important to recognize children on a trajectory toward infection-induced organ dysfunction early, rather than when the signs are florid, especially in resource-limited settings where advanced organ support may be lacking. A recent international survey with over 2,500 respondents highlighted that the currently available diagnostic frameworks for sepsis in children fall short of the desirable requirements in terms of benchmarking, correct disease classification, clinical recognition, and outcome prognostication (5). Operationalizing criteria for pediatric sepsis may require a more context-specific approach (e.g., considering time from onset, resources available, geography, prevalent pathogens, etc.). There is therefore an urgent need for a data- and evidence-driven, approach to derive and validate criteria for sepsis in children, applicable across global settings and reflective of different clinical situations (from first contact in primary care through to ICU) (5).
Sepsis varies in its clinical manifestations over time, which necessitates different requirements for identification of sepsis across the various stages of its progression. In the early phases, clinicians may have limited information available, but require criteria to make a presumptive diagnosis that is sufficient to implement effective therapeutic approaches. At later stages, clinicians usually have access to substantive information, including results of diagnostic testing, clinical progression, and response to therapy, to allow a definitive diagnosis. This concept enables clinicians to consider parameters for early initiation of antibiotic therapy, while allowing early termination of antibiotic therapy, once additional information allows for reevaluation of the diagnosis and risk. To ensure widespread adoption and patient benefit, criteria for sepsis should correlate with clinically relevant intervention points (e.g., invasive bacterial infection = antibiotic therapy, organ dysfunction = organ support therapy), as well as the risk of mortality in each situation.
Sepsis represents a broad umbrella term covering a condition that may be caused by a wide range of pathogens (bacterial, viral, fungal, and parasitic). There are no simple and unambiguous clinical, biological, imaging, or laboratory features that uniquely identify a patient with sepsis. The lack of rapid and reliable sepsis tests hampers the ability to make a definitive diagnosis of life-threatening organ dysfunction caused by a dysregulated host response to infection (2, 3), at the time of presentation.
Dysregulation of host response includes imbalances in proinflammatory and anti-inflammatory states driven by the innate and adaptive immune systems and contributing to new or worsening organ injury and death. The adult Sepsis-3 criteria emphasize the importance of identification of organ dysfunction in patients with infection and have led to research that improves our mechanistic understanding of the pathobiology of sepsis, including infection-specific biology and responses to specific interventions (6, 7). In this context, phenotyping using biological and clinical characteristics, and specific pathophysiology, has great potential to be employed as an enrichment strategy in the evaluation of treatment responses (8).
The 2005 International Pediatric Sepsis Consensus Conference (IPSCC) (9) provided criteria for categories of increasing disease severity (infection, sepsis, severe sepsis, septic shock), and definitions of organ dysfunction in children. Since then, our understanding of organ dysfunction and the practice of pediatric critical care have changed considerably. In 2022, the Pediatric Organ Dysfunction Information Update Mandate (PODIUM) proposed 43 criteria to characterize children with single or multiple organ dysfunction, to identify phenotypes associated with poor outcomes, and to serve as entry criteria for clinical trials (10). The number of concurrent PODIUM organ dysfunction has been shown to have good-to-excellent discrimination for in-hospital mortality at two U.S. centers, and compared favorably with the IPSCC criteria (11). However, although the PODIUM criteria were designed to be feasible, minimally invasive, generalizable, and easily operationalizable (12), their application to LMIC settings could be challenging. Furthermore, these criteria have never been validated across the world and may not be applicable to children of different age groups; countries; nutritional and genetic backgrounds; and in the context of conditions rarely found in HIC settings.
More recently, a multicenter study of children with sepsis and organ dysfunction, demonstrated that mortality risk increased in children with high C-reactive protein (CRP) or ferritin alone, with greater mortality risk when CRP and ferritin were both elevated (13). Additionally, the biomarker combination stratified groups of children with differing systemic inflammation cytokine profiles. As both these markers are easily measurable and affordable, the study results open up the possibility of stratification in randomized controlled trials of novel therapies in pediatric sepsis, including in LMICs.
Ideally, future criteria for pediatric sepsis should be are as follows:
1) Sensitive enough to enable clinicians to recognize the condition early, but specific enough to not waste potentially limited resources (14), and avoid harming patients as a consequence of over- or inappropriate treatment.
2) Flexible enough to allow clinicians to consider other possibilities with the resolution/progression of signs (14).
3) Applicable globally, and adaptable locally.
4) Correlated with biologically relevant phenotypes of sepsis to ensure the correct selection of patients that will benefit from specific therapies and organ support.
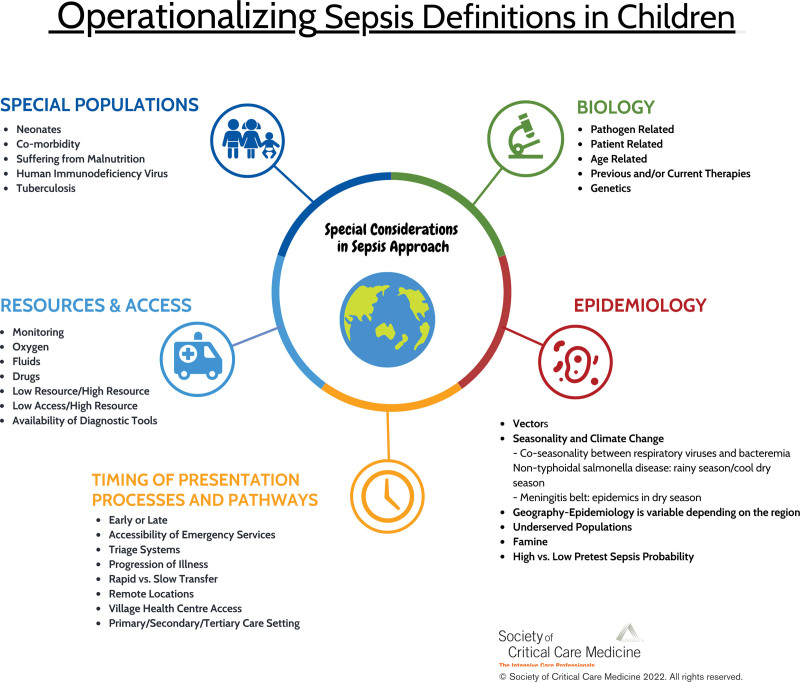
In this Special Article from the Pediatric Sepsis Definition Taskforce of the Society of Critical Care Medicine, we present a conceptual framework and a rationale of the critical aspects and context-specific factors that must be considered for the operationalization of sepsis definitions in children around the world (Fig. 1). These include biological factors, epidemiology, differences in the pathways to care, pretest probabilities of sepsis at different levels of care, and the resources that are available to provide care.
Figure 1.
Operationalizing sepsis definitions in children.
BIOLOGICAL FACTORS IMPACTING SEPSIS
Pathogen-Related Biology
The pathogens responsible for severe infections and illnesses in children are often different from those in adults. Even responses to the same viral infections may be different, as highlighted by the manifestations of severe acute respiratory syndrome coronavirus 2 infections in adults and children (15). The pathogens responsible for severe pediatric infections in LMIC settings, where most deaths from infection occur, are different than those in HIC settings.
The Fluid Expansion As Supportive Therapy (FEAST) trial included many patients with malaria and anemia and no access to ICU (16) and demonstrated that fluid boluses significantly increased 48-hour mortality in critically ill children. Skepticism by many clinicians in HIC settings to findings of the FEAST trial may have been based on their perception that the spectrum of disease differed from what they considered to be “sepsis,” specifically, bacterial sepsis. This conclusion has major implications for future criteria of pediatric sepsis, as a large proportion of the world’s children are in countries where infections like malaria and dengue are common. In children, there are many conditions caused by nonbacterial pathogens such as viruses (e.g., enterovirus shock, dengue hemorrhagic fever/dengue shock syndrome, severe bronchiolitis, gastroenteritis with severe dehydration), and parasites (e.g., severe malarial anemia), which result in organ dysfunction causing severe morbidity and mortality, yet clinicians may not consider these to be “sepsis.” Host response profiles and endotypes may be influenced by co-infection with organisms such as HIV, malaria, and helminths, severe acute malnutrition, and immunization against vaccine-preventable invasive bacterial infections (17, 18). As per the pediatric Surviving Sepsis Campaign, patients with shock caused by bacterial sepsis require broad-spectrum antibiotics within an hour (4). Children with nonbacterial sepsis may require specific antimicrobials/immunosuppressives or only supportive care. Difficulties in differentiation, especially during the early stages, often lead to the prescription of broad-spectrum antimicrobials to all patients with a presumed infection and shock/organ dysfunction and could have far-reaching consequences in terms of antimicrobial resistance (19). For example, based on endemicity (e.g., monsoon season) and the typical pattern of illness, clinicians in certain geographic regions may correctly suspect dengue in patients with shock following defervescence, hemoconcentration, and thrombocytopenia, as opposed to malaria in patients with fever with mental status changes, pallor, and metabolic acidosis.
Patient-Related Biology
Primary or secondary immunodeficiencies, and genetic conditions such as sickle cell disease, influence predisposition to infection and host responses to infection and disease severity (20–22) and are fundamentally different in children. Malnutrition, an invasive bacterial disease, and HIV co-infection are risk factors for death among children with moderate-to-severe diarrhea. The presence of malarial anemia and/or malnutrition may profoundly affect the response to therapy such as bolus fluid administration. Finally, there is growing awareness of the significance of specific genetic common (polymorphisms) and rare variations in the manifestations and treatment of infection-associated critical illness (23, 24).
Age-Related Biology
The term “pediatric” encompasses a range of age groups, from neonates to young adults, which are characterized by sometimes profound variations in physiology, immune maturation, and specific responses to infection. The fine balance between proinflammation and anti-inflammation and the interconnectedness between inflammation and other host responses (neuroendocrine, metabolic, coagulation, endothelial, and immunosuppression) have been shown to vary substantially across age groups. Prematurity, low birth weight, and younger age remain major risk factors for sepsis and poor outcome (25).
Because of their ability to initially maintain blood pressure in septic shock, overall, infants and young children have lesser reserves to compensate for serious illness compared with adults; thus shortening the window of opportunity for clinicians to recognize signs, respond quickly, and avoid the progression of organ deterioration. However, in the early phases of infection, children who progress to life-threatening illness may be virtually indistinguishable clinically from those who will not progress (26).
Previous and Current Therapies
Vaccine-preventable infections have substantially decreased in HIC and UMIC settings but may still be present in some LMIC settings. Morbidity and mortality from childhood pneumonia have decreased in LMIC settings because of the more widespread availability of conjugate vaccines, but a considerable burden remains from viruses, Staphylococcus aureus, Escherichia coli, and other bacteria for which vaccines are not available (27). Antimicrobial-resistant infections remain highly prevalent in many LMIC settings, due to underlying complex and multifactorial problems. In the Burden of Antibiotic Resistance in Neonates from Developing Societies neonatal sepsis study, only a third of Gram-negative isolates were susceptible to the World Health Organization recommended first-line regimen (28). Poor sanitation and hygiene, malnutrition, and overcrowding also contribute to the increased transmission of multiresistant pathogens (29).
EPIDEMIOLOGY
Pneumonia, malaria, HIV, tuberculosis, dengue, meningitis, and neonatal infection represent a large proportion of emergency presentations in LMIC settings, whereas self-limiting (mainly viral) infections are common presentations in pediatric emergency departments (EDs) in HIC/UMIC settings. In South-East Asia, common causes of childhood sepsis include dengue, rickettsia, typhoid, nontyphoidal Salmonella, Streptococcus suis, and Burkholderia pseudomallei, all of which are rarely seen in North America and Europe, and most of which do not respond to standard antimicrobial interventions. Mixed infections are also common in LMICs and may be difficult to diagnose because of rudimentary laboratory support.
The highest burden of bacterial meningitis occurs in the “Meningitis belt” in Africa, with increased incidence and epidemics in the dry season (from October to March) (30). Colder weather also promotes higher transmission because individuals spend more time indoors, or in close proximity outdoors, with exposure to smoke from wood fires (31). The co-occurrence of viral respiratory infections further facilitates invasive disease. Climate change has a significant influence on the incidence and severity of infections through environmental effects of floods, heat, drought, and freshwater decline (32). For example, the incidence of dengue, a mosquito-borne viral disease that may present as septic shock, has increased due to global warming, and increasing wetland areas facilitating transmission (Fig. 1).
PROCESSES AND PATHWAYS INFLUENCING TIMING OF PRESENTATION
In HIC settings, many presentations to primary and emergency care settings are for those who are “worried well” (requiring reassurance), or early presentations who may require a period of observation. In LMIC settings, health facilities may be in remote locations with inaccessible emergency and specialized critical care transport services, and consequently are often faced with mostly late presentations. In early presentations, symptoms and signs may relate to the direct effects of the pathogen, but in late presentations, they may be related to organ dysfunction as consequences and the progression of infection and the host response.
The experience and specialization of healthcare professionals assessing children with suspected infection influence how sepsis criteria are operationalized. In primary care, there are fewer specialist assessments, fewer investigations, and potentially more antibiotics prescribed (33). Some EDs have co-located primary care physicians, which allows for sicker children to be referred to more specialized ED physicians onsite. In an LMIC setting, children might first present to a village health center before referral to a district/tertiary hospital, and there may be delays in securing IV access and administering antibiotics, or IV fluids and vasoactive-inotropic drugs if available at all. In the latter setting, florid multiple organ dysfunction may be established by the time the child receives advanced care.
RESOURCES AND ACCESS
Classification of countries or regions into high-, middle-, or low-income settings has significant limitations: the resources available to a particular child who presents to a healthcare facility with suspected infection may vary significantly. In certain HIC/UMIC settings, some disadvantaged populations may show features otherwise seen in LMIC, and yet some LMIC settings have highly resourced tertiary ICU facilities depending on the location. Differential resources and access thus relate to: a) within institution differences (ED, ward, PICU), b) within country differences (urban, rural, academic, general/district), and c) within high-, middle-, or low-income country differences (34). Literature on the epidemiology of presentations to the ED in LMIC is scant. Many PICUs in LMICs do not have specialized transport teams, so many children will die of sepsis outside PICUs. Under-reporting of sepsis incidence and limited research funding for sepsis in LMIC countries further limits the impact of any quality improvement initiatives (35). In many LMIC settings, facilities are challenged by patient load and inadequate human resources, as well as limited availability of oxygen saturation and blood pressure monitoring, oxygen, balanced IV fluids, vasoactive-inotropic drugs, and staff with expertise in skilled IV/arterial access and intubation/ventilation. Conversely, in many HIC/UMIC settings, a variety of fluid types, antibiotics, vasoactive-inotropic drugs, renal replacement therapy, electronic data capture, and even sepsis algorithms embedded into the electronic patient record, are routinely available.
New Considerations
Although concepts such as sepsis and pediatric acute respiratory distress syndrome have enabled the development of management strategies, standardized research cohorts, and research protocols, our understanding of sepsis is evolving thanks to more detailed phenotyping, providing opportunities for the enrichment of cohorts toward precision medicine (8). As we focus on criteria for sepsis across the world, we need to develop global databases which are granular enough in content to examine the details of specific communities, and broad enough to contain information on multiple additional factors. Databases ideally will capture the full “narrative” of illness (not just presentation), and incorporate concepts of probability rather than definitive “certainty of diagnosis.” Although the Pediatric Sepsis Definition Taskforce has included primary care and emergency practitioners in the “definition” process, during the implementation of new criteria it will be necessary to reach out to other practitioners such as those who provide long-term care, laboratory teams, and those with expertise in implementation science. Social determinants of health (SDOH) in pediatric sepsis studies are not commonly reported, with marked variability in categorizations and definitions of SDOH variables. Standardized reporting of SDOH in pediatric sepsis studies is needed to understand the role these factors play in the development, progression, and recovery of sepsis, to implement policies aimed at addressing socioeconomic conditions related to sepsis (36).
Actionable solutions to the considerations above are described in Table 1 and include individualized approaches dependent on setting, affordable diagnostics for accurate risk stratification, optimization of data capture from routinely collected datasets to support collaborative registries, and development of locally relevant outcome measures.
TABLE 1.
Actionable Solutions to Support Operationalization of Pediatric Sepsis Criteria
| Approach | Solution |
|---|---|
| Tailored approaches for different settings | Consider pretest probability of sepsis, available resources, and prevalence of other diseases with “sepsis-like” presentations |
| Sepsis bundle components individualized for specific settings | Impact of individual components on improving outcomes should be evaluated |
| Develop minimum variable datasets using routinely collected data | Minimum variable datasets and a common dictionary for data labeling can allow the merging of data for collaborative development and assessment of data-driven criteria and patient-relevant outcomes |
| Develop datasets from multiple settings around the world | The datasets should include information on patients through the continuum of care (primary care, emergency department, ward, and PICU). Ideally, the datasets should originate from diverse settings (geographical, sociodemographic, resource availability, etc.) |
| Determine locally relevant time zero | Depending on setting, the first presentations of sepsis may be to local health center, primary or secondary care |
| Determine locally relevant clinical decision support systems | Clinical decision support systems can be incorporated into electronic patient records in the high-income country and upper middle-income country settings, and mobile phone apps in low middle-income countries |
| Address antimicrobial stewardship | Structured clinical assessments and investigations can help determine treatment urgency of antimicrobials, to balance patient safety and antimicrobial stewardship |
| Develop locally relevant outcome measures | Outcome measures may include mortality, disability, critical care admission, or organ dysfunction depending on setting |
| Develop affordable and accurate diagnostics | Affordable host response and/or pathogen diagnostics can support risk stratification and stratification of likely response to novel therapies |
CONCLUSIONS
Subsequent to the wide uptake of adult data-driven Sepsis-3 criteria, it is important to address the practical challenges in developing pediatric sepsis criteria which capture manifestations of illness in a range of settings and with vastly different etiologies and underlying mechanisms. The criteria will need to be unambiguous yet flexible, capable of adapting to the widely different contexts in which children with suspected infections are present around the globe. These criteria should facilitate early recognition of patients at risk, improved diagnosis, and risk stratification to enable timely escalation of treatment to prevent progression. At the same time, we must endeavor to avoid the problems associated with overdiagnosis and treatment.
In summary, there are challenges and opportunities in operationalizing sepsis in the global and diverse settings in which children present, but we present potential actionable solutions that can be applied locally to identify, manage and study children with sepsis.
ACKNOWLEDGMENTS
We thank the World Federation of Pediatric Intensive and Critical Care Societies, the Society of Critical Care Medicine (SCCM), and the European Society of Pediatric and Neonatal Intensive Care for their support in facilitating this project. We thank Lori Harmon and Lynn Retford from the SCCM for their support with SCCM’s Pediatric Sepsis Definitions Task Force.
APPENDIX. Society of Critical Care Medicine’s Pediatric Sepsis Definition Taskforce
SCCM’s Pediatric Sepsis Definition Taskforce: Luregn J. Schlapbach (Co-Chair), Department of Intensive Care and Neonatology, and Children’s Research Center, University Children’s Hospital Zurich, Zurich, Switzerland, and Child Health Research Centre, The University of Queensland, Brisbane, QLD, Australia; R. Scott Watson (Co-Chair), Center for Child Health, Behavior and Development, Seattle Children’s Research Institute, Seattle Children’s Hospital, Seattle, WA; Andrew Argent (Vice-Chair), Department of Pediatrics and Adolescent Health, Red Cross War Memorial Children’s Hospital and University of Cape Town, Cape Town, South Africa; Lauren R. Sorce (Vice-Chair), Ann & Robert H. Lurie Children’s Hospital of Chicago and Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL; Samuel Akech, KEMRI Wellcome Trust Research Programme, Nairobi, Kenya; Elizabeth R. Alpern, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Fran Balamuth, Children’s Hospital of Philadelphia, Philadelphia, PA; Tellen D. Bennett, University of Colorado and Children’s Hospital of Colorado, Aurora, CO; Paolo Biban, Verona University Hospital, Verona, Italy; Joe Carcillo, Department of Critical Care Medicine, UPMC Children’s Hospital, Pittsburg, PA; Enitan D Carrol, University of Liverpool, Liverpool, United Kingdom; Kathleen Chiotos, Children’s Hospital of Philadelphia, Philadelphia, PA; Mohammod Jobayer Chisti, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh; Idris Evans, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA; Lu Guoping, Children’s Hospital of Fudan University, Shanghai, China; Mark W. Hall, Nationwide Children’s Hospital, Columbus, OH; David Inwald, Addenbrooke’s Hospital, Cambridge University Hospital NHS Trust, Cambridge, United Kingdom; Paul Ishimine, University of California, San Diego School of Medicine, La Jolla, CA; Michael Levin, Imperial College London, London, United Kingdom; Niranjan Tex Kissoon, British Columbia Women and Children’s Hospital and the University of British Columbia, Vancouver, BC, Canada; Rakesh Lodha, All India Institute of Medical Sciences, New Delhi, India; Kathryn Maitland, Imperial College, London, United Kingdom; Simon Nadel, St. Mary’s Hospital, London, United Kingdom; Satoshi Nakagawa, National Center for Child Health & Development, Tokyo, Japan; Claudio Flauzino Oliveira, Associação de Medicina Intensiva Brasileira, São Paulo, Brazil; Mark Peters, University College London Great Ormond Street Institute of Child Health, London, United Kingdom; Adrienne G. Randolph, Boston Children’s Hospital, Boston, MA; Suchitra Ranjit, Apollo Children’s Hospital, Chennai, India; L. Nelson Sanchez-Pinto, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Halden F. Scott, Children’s Hospital of Colorado, Denver, CO; Daniela C. Souza, University Hospital of The University of São Paulo, Sao Paulo, Brazil; Pierre Tissieres, Hospital de Bicetre, Paris, France; Juliane Bubeck Wardenburg, Department of Pediatrics Washington School of Medicine St. Louis, MO; Scott L. Weiss, Children’s Hospital of Philadelphia, Philadelphia, PA; Wilson Milton Were, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, Geneva, Switzerland; Matthew O. Wiens, University of British Columbia, Vancouver, BC, Canada; James L. Wynn, University of Florida, Gainesville, FL; and Jerry J. Zimmerman, Seattle Children’s Hospital, Seattle, WA.
Footnotes
Drs. Kissoon and Argent contributed equally.
This project was sponsored by the Society of Critical Care Medicine.
Dr. Carrol received support for article research from the U.K. National Institute for Health and Care Research. Dr. Bennett’s institution received funding from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Drs. Bennett and Zimmerman’s institutions received funding from the National Institute of Child Health and Human Development. Drs. Bennett and Randolph received support for article research from the National Institutes of Health. Dr. Zimmerman’s institution received funding from Immunexpress; he received funding from Elsevier Publishing. Dr. Sorce disclosed she is a Society of Critical Care Medicine (SCCM) Executive Board Member. Dr. Randolph’s institution received funding from the U.S. Centers for Disease Control and Prevention and the National Institute of Allergy and Infectious Diseases; she received funding from UpToDate; she disclosed she is Chair of the International Sepsis Forum. Ms. Harmon disclosed she is an employee of SCCM. Dr. Argent received travel, accommodation, and registration fees to attend a number of conferences as an invited guest speaker and received fees for provision of expert opinion in a number of medicolegal cases (from several legal firms in South Africa). The remaining authors have disclosed that they do not have any potential conflicts of interest..
The Society of Critical Care Medicine’s Pediatric Sepsis Definition Taskforce is listed in the Appendix.
Contributor Information
Luregn J. Schlapbach, Department of Intensive Care and Neonatology, and Children’s Research Center, University Children’s Hospital Zurich.
R. Scott Watson, Center for Child Health, Behavior and Development, Seattle Children’s Research Institute, Seattle Children’s Hospital.
Andrew Argent, Department of Pediatrics and Adolescent Health, Red Cross War Memorial Children’s Hospital and University of Cape Town.
Lauren R. Sorce, Ann & Robert H. Lurie Children’s Hospital of Chicago and Department of Pediatrics, Northwestern University Feinberg School of Medicine.
Samuel Akech, KEMRI Wellcome Trust Research Programme.
Elizabeth R. Alpern, Ann & Robert H. Lurie Children’s Hospital of Chicago
Fran Balamuth, Children’s Hospital of Philadelphia.
Tellen D. Bennett, University of Colorado and Children’s Hospital of Colorado
Paolo Biban, Verona University Hospital.
Joe Carcillo, Department of Critical Care Medicine, UPMC Children’s Hospital.
Enitan D Carrol, University of Liverpool.
Kathleen Chiotos, Children’s Hospital of Philadelphia.
Mohammod Jobayer Chisti, International Centre for Diarrhoeal Disease Research.
Idris Evans, Children’s Hospital of Pittsburgh of UPMC.
Lu Guoping, Children’s Hospital of Fudan University.
Mark W. Hall, Nationwide Children’s Hospital
David Inwald, Addenbrooke’s Hospital, Cambridge University Hospital NHS Trust.
Paul Ishimine, University of California, San Diego School of Medicine.
Michael Levin, Imperial College London.
Niranjan Tex Kissoon, British Columbia Women and Children’s Hospital and the University of British Columbia.
Rakesh Lodha, All India Institute of Medical Sciences.
Kathryn Maitland, Imperial College.
Simon Nadel, St. Mary’s Hospital.
Satoshi Nakagawa, National Center for Child Health & Development.
Claudio Flauzino Oliveira, Associação de Medicina Intensiva Brasileira.
Mark Peters, University College London Great Ormond Street Institute of Child Health.
Adrienne G. Randolph, Boston Children’s Hospital
Suchitra Ranjit, Apollo Children’s Hospital.
L. Nelson Sanchez-Pinto, Ann & Robert H. Lurie Children’s Hospital of Chicago.
Halden F. Scott, Children’s Hospital of Colorado
Daniela C. Souza, University Hospital of The University of São Paulo
Pierre Tissieres, Hospital de Bicetre.
Juliane Bubeck Wardenburg, Department of Pediatrics Washington School of Medicine.
Scott L. Weiss, Children’s Hospital of Philadelphia
Wilson Milton Were, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization.
Matthew O. Wiens, University of British Columbia
James L. Wynn, University of Florida
Jerry J. Zimmerman, Seattle Children’s Hospital, Seattle, WA
Collaborators: Luregn J. Schlapbach, R. Scott Watson, Andrew Argent, Lauren R. Sorce, Samuel Akech, Elizabeth R. Alpern, Fran Balamuth, Tellen D. Bennett, Paolo Biban, Joe Carcillo, Enitan D Carrol, Kathleen Chiotos, Mohammod Jobayer Chisti, Idris Evans, Lu Guoping, Mark W. Hall, David Inwald, Paul Ishimine, Michael Levin, Niranjan Tex Kissoon, Rakesh Lodha, Kathryn Maitland, Simon Nadel, Satoshi Nakagawa, Claudio Flauzino Oliveira, Mark Peters, Adrienne G. Randolph, Suchitra Ranjit, L. Nelson Sanchez-Pinto, Halden F. Scott, Daniela C. Souza, Pierre Tissieres, Juliane Bubeck Wardenburg, Scott L. Weiss, Wilson Milton Were, Matthew O. Wiens, James L. Wynn, and Jerry J. Zimmerman
REFERENCES
- 1.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tidswell R, Parker T, Brealey D, et al. : Sepsis—the broken code how accurately is sepsis being diagnosed? J Infect. 2020; 81:e31–e32 [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SL, Peters MJ, Alhazzani W, et al. : Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020; 21:e52–e106 [DOI] [PubMed] [Google Scholar]
- 5.Morin L, Hall M, de Souza D, et al. : The current and future state of pediatric sepsis definitions: An international survey. Pediatrics. 2022; 149:e2021052565. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Zhang G, Goyal H, et al. : Identification of subclasses of sepsis that showed different clinical outcomes and responses to amount of fluid resuscitation: A latent profile analysis. Crit Care. 2018; 22:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour CW, Kennedy JN, Wang S, et al. : Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019; 321:2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maslove D, Tang B, Shankar-Hari M, et al. : Redefining critical illness. Nat Med. 2022; 28:1141–1148 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International Pediatric Sepsis Consensus Conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 10.Schlapbach LJ, Weiss SL, Bembea MM, et al. ; Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Collaborative: Scoring systems for organ dysfunction and multiple organ dysfunction: The PODIUM consensus conference. Pediatrics. 2022; 149(Suppl_1):S23–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Pinto LN, Bembea MM, Farris RW, et al. ; Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Collaborative: Patterns of organ dysfunction in critically ill children based on PODIUM criteria. Pediatrics. 2022; 149(1 Suppl 1):S103–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bembea MM, Agus M, Akcan-Arikan A, et al. : Pediatric Organ Dysfunction Information Update Mandate (PODIUM) contemporary organ dysfunction criteria: Executive summary. Pediatrics. 2022; 149(Suppl_1):S1–S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvat CM, Fabio A, Nagin DS, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Mortality risk in pediatric sepsis based on C-reactive protein and ferritin levels. Pediatr Crit Care Med. 2022; 23:968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klompas M, Calandra T, Singer M: Antibiotics for sepsis—finding the equilibrium. JAMA. 2018; 320:1433–1434 [DOI] [PubMed] [Google Scholar]
- 15.Vella LA, Giles JR, Baxter AE, et al. : Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol. 2021; 6:eabf7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitland K, Kiguli S, Opoka RO, et al. ; FEAST Trial Group: Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011; 364:2483–2495 [DOI] [PubMed] [Google Scholar]
- 17.Madhi SA, Klugman KP, Kuwanda L, et al. : Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J Infect Dis. 2009; 199:1168–1176 [DOI] [PubMed] [Google Scholar]
- 18.Schachter J, Alvarinho de Oliveira D, da Silva CM, et al. : Whipworm infection promotes bacterial invasion, intestinal microbiota imbalance, and cellular immunomodulation. Infect Immun. 2020; 88:e00642-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlapbach LJ, Weiss SL, Wolf J: Reducing collateral damage from mandates for time to antibiotics in pediatric sepsis—primum non nocere. JAMA Pediatr. 2019; 173:409–410 [DOI] [PubMed] [Google Scholar]
- 20.Borghesi A, Truck J, Asgari S, et al. : Whole-exome sequencing for the identification of rare variants in primary immunodeficiency genes in children with sepsis: A prospective, population-based cohort study. Clin Infect Dis. 2020; 71:e614–e623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battersby AJ, Knox-Macaulay HH, Carrol ED: Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer. 2010; 55:401–406 [DOI] [PubMed] [Google Scholar]
- 22.Payne AB, Link-Gelles R, Azonobi I, et al. ; Active Bacterial Core Surveillance Team: Invasive pneumococcal disease among children with and without sickle cell disease in the United States, 1998 to 2009. Pediatr Infect Dis J. 2013; 32:1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumpf O, Gurtler K, Sur S, et al. : A genetic variation of lipopolysaccharide binding protein affects the inflammatory response and is associated with improved outcome during sepsis. Immunohorizons. 2021; 5:972–982 [DOI] [PubMed] [Google Scholar]
- 24.Cvijanovich NZ, Anas N, Allen GL, et al. : Glucocorticoid receptor polymorphisms and outcomes in pediatric septic shock. Pediatr Crit Care Med. 2017; 18:299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan B, Wong JJ, Sultana R, et al. : Global case-fatality rates in pediatric severe sepsis and septic shock: A systematic review and meta-analysis. JAMA Pediatr. 2019; 173:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Bruel A, Thompson M, Buntinx F, et al. : Clinicians’ gut feeling about serious infections in children: Observational study. BMJ. 2012; 345:e6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marangu D, Zar HJ: Childhood pneumonia in low- and-middle-income countries: An update. Paediatr Respir Rev. 2019; 32:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlapbach LJ, van Rossum A, Carrol ED: Antibiotics for neonatal sepsis in low-income and middle-income countries—where to go from here? Lancet Infect Dis. 2021; 21:1617–1618 [DOI] [PubMed] [Google Scholar]
- 29.Ayukekbong JA, Ntemgwa M, Atabe AN: The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob Resist Infect Control. 2017; 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agier L, Deroubaix A, Martiny N, et al. : Seasonality of meningitis in Africa and climate forcing: Aerosols stand out. J R Soc Interface. 2013; 10:20120814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper LV, Robson A, Trotter CL, et al. ; MenAfriCar Consortium: Risk factors for acquisition of meningococcal carriage in the African meningitis belt. Trop Med Int Health. 2019; 24:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirsaeidi M, Motahari H, Taghizadeh Khamesi M, et al. : Climate change and respiratory infections. Ann Am Thorac Soc. 2016; 13:1223–1230 [DOI] [PubMed] [Google Scholar]
- 33.Leigh S, Mehta B, Dummer L, et al. : Management of non-urgent paediatric emergency department attendances by GPs: A retrospective observational study. Br J Gen Pract. 2021; 71:e22–e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burstein R, Henry NJ, Collison ML, et al. : Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019; 574:353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souza DC, Jaramillo-Bustamante JC, Cespedes-Lesczinsky M, et al. : Challenges and health-care priorities for reducing the burden of paediatric sepsis in Latin America: A call to action. Lancet Child Adolesc Health. 2022; 6:129–136 [DOI] [PubMed] [Google Scholar]
- 36.Menon K, Sorce LR, Argent A, et al. ; Pediatric Sepsis Definition Taskforce: Reporting of social determinants of health in pediatric sepsis studies. Pediatr Crit Care Med. 2023; 24:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]