Abstract
Studies have investigated the effects of androgen deprivation therapy (ADT) use on the incidence and clinical outcomes of coronavirus disease 2019 (COVID-19); however, the results have been inconsistent. We searched the PubMed, Medline, Cochrane, Scopus, and Web of Science databases from inception to March 2022; 13 studies covering 84 003 prostate cancer (PCa) patients with or without ADT met the eligibility criteria and were included in the meta-analysis. We calculated the pooled risk ratios (RRs) with 95% confidence intervals (CIs) to explore the association between ADT use and the infection risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and severity of COVID-19. After synthesizing the evidence, the pooled RR in the SARS-CoV-2 positive group was equal to 1.17, and the SARS-CoV-2 positive risk in PCa patients using ADT was not significantly different from that in those not using ADT (P = 0.544). Moreover, no significant results concerning the beneficial effect of ADT on the rate of intensive care unit admission (RR = 1.04, P = 0.872) or death risk (RR = 1.23, P = 0.53) were found. However, PCa patients with a history of ADT use had a markedly higher COVID-19 hospitalization rate (RR = 1.31, P = 0.015) than those with no history of ADT use. These findings indicate that ADT use by PCa patients is associated with a high risk of hospitalization during infection with SARS-CoV-2. A large number of high quality studies are needed to confirm these results.
Keywords: androgen deprivation therapy, coronavirus disease 2019, meta-analysis, prostate cancer, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which results in damage to the pancreas, kidney, heart, brain, and especially the lungs.1 The World Health Organization (WHO) has declared that COVID-19 is a serious public health emergency of increasing international concern.2 By March 2022, SARS-CoV-2 was circulating in more than 150 countries and had affected over 464 million people, resulting in more than 5 000 000 deaths.3 Although several COVID-19 vaccines are currently available, vaccines have not increased in popularity worldwide due to substantial variation in acceptance rates among countries; additionally, some vaccines are less effective against the Delta and Omicron variants.4,5 No valid interventions have been officially identified to protect against the high morbidity and mortality rates of COVID-19 to date.6 Therefore, risk factors for SARS-CoV-2 infection and COVID-19 severity as well as the mechanism underlying disease invasion and progression have attracted extensive attention.
Acute lung injury and respiratory distress syndrome are common and serious complications in COVID-19 patients.7 These systemic effects are chiefly caused by the extensive distribution of angiotensin-converting enzyme 2 (ACE2).1 ACE2 is highly expressed in alveolar type II cells, renal tubular epithelial cells, Sertoli cells, and Leydig cells in the testis.8 SARS-CoV-2 uses ACE2 as a functional receptor to enter host cells.9 Hoffmann et al.10 reported that the entry of SARS-CoV-2 into host cells is dependent on the binding of the SARS spike (S) protein to ACE2 on cells and on SARS S protein priming by transmembrane protease serine 2 (TMPRSS2). The gene encoding TMPRSS2, an androgen-regulated protease from the family of type 2 transmembrane serine proteases,11 is characterized by several androgen receptor elements (AREs) upstream of the first intron and the transcription start site.12 The TMPRSS2 protease is principally expressed in the prostate, with relatively low expression levels in other organs, including the liver, testis, kidney, and pancreas; it is involved in multiple signaling pathways that influence the occurrence and development of tumors.13,14 In particular, TMPRSS2 plays a pivotal role in infection with influenza virus and coronavirus.12 The pathogenesis of viral spread can be summarized as a disturbance of antibody-mediated neutralization or the SARS S protein priming for cell–cell fusion/virus–cell membrane fusion via SARS S protein cleavage,12,15,16 as observed in SARS-CoV-2.17 Therefore, TMPRSS2 may be a potential target for developing new agents against COVID-19. According to in vitro evidence, inhibitors of TMPRSS2, such as camostat mesylate, are likely effective at reducing viral infections.10 Notably, the sex difference in androgen levels might be responsible for the increased susceptibility of men to COVID-19 compared with women, and androgen-driven immune modulation may be involved in SARS-CoV-2 infection. A likely mechanism is that the androgen/androgen receptor signaling pathway regulates TMPRSS2 expression and thereby mediates SARS-CoV-2 invasion.18
Moreover, the androgen-regulated protease TMPRSS2 exhibits markedly higher expression in primary and metastatic prostate tumors and plays a role in tumor differentiation, invasion, and metastasis.19,20 The growth and progression of prostate cancer (PCa), the second most common cancer in males worldwide,21 depend on androgen stimulation; inhibitors can suppress the development of PCa.22 Androgen deprivation therapy (ADT) is one of the basic methods of treating PCa. Additionally, studies have shown that androgens positively regulate the expression of TMPRSS2 not only in prostatic cells13,20 but also in lung-derived cell lines.23 Overall, exploration of methods of downregulating TMPRSS2 to target SARS-CoV-2 and thereby prevent infection and severity of COVID-19 via androgen suppression has clinical importance. Karimi et al.24 and Sari Motlagh et al.25 have performed systematic reviews and meta-analyses, but their conclusions might change substantially given the rapid publication of relevant studies. Accordingly, we have updated prior systematic reviews and meta-analyses by evaluating whether ADT for PCa exerts protective or pathogenic effects on SARS-CoV-2 infection and COVID-19 progression. We believe that this study provides an important contribution to understanding the risk and prognosis factors of COVID-19 as well as investigating potential methods for COVID-19 prevention or treatment.
MATERIALS AND METHODS
Search strategy
The protocol of our meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The corresponding checklist is shown in Supplementary Table 1. In addition, this systematic review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration ID: CRD42021260612). Further details of our meta-analysis can be found on the official website (https://www.crd.york.ac.uk/prospero). We systematically searched for publications from database inception to March 2022 in the PubMed, Medline, Cochrane, Scopus, and Web of Science databases without restrictions. The retrieval terms were as follows: “prostatic neoplasms”, “prostate neoplasms”, “cancer of prostate”, “PCa”, “coronavirus disease 2019”, “coronavirus disease-19”, “COVID-19”, “novel coronavirus 2019”, “severe acute respiratory syndrome coronavirus 2”, “SARS coronavirus 2”, “SARS-CoV-2”, “androgen deprivation therapy”, “ADT”, “endocrine therapy”, and “androgen inhibitor”. Potentially relevant records were subjected to a full-text review. Moreover, the reference lists of the selected articles were manually searched to identify other potentially relevant studies. This iterative process was continued until no additional articles could be identified.
Supplementary Table 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: Background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 1-2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS design | 2 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | 2 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 2 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 2 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 2 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 2 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 2 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 2 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 2 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 2 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 2 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | 2 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 2; Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 2-3; Table 1; Supplementary Tables 2 and 3 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 4 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | 3-4; Figures 2–5 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 3-4; Figures 2–5 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | 4; Supplementary Figure 1 (251.2KB, tif) ; Supplementary Table 4 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see item 16]) | 3-4; Figures 2–5 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 5-6 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 7 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 7 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 7 |
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PICOS: participants, interventions, comparisons, outcomes, and study
Selection criteria
The inclusion criteria were as follows: (1) population: PCa patients; (2) intervention: any type of ADT treatment; (3) comparison: no ADT treatment; (4) outcome: SARS-CoV-2 positive risk, the rates of hospitalization and intensive care unit (ICU) admission, and death risk; and (5) design: randomized controlled trials/observational studies that provided adequate data for the meta-analysis.
Data extraction
Two researchers (YBH and WLL) independently screened the abstracts and full texts of the included studies, extracted the data, and assessed the risk of bias. The data extracted from the included studies were the author, country, study design, time span, sample size, age, ADT treatment (i.e., type, frequency, duration, and dosage), duration of therapy on COVID-19, chemotherapy, castration resistance, comorbidities, reported indicators, and risk ratios (RRs). Any divergence was resolved through discussion and consensus. Further disagreement during this process was resolved by consensus through involving the third and fourth authors (MS and XCL).
Quality assessment
The most recent version of the Cochrane tool (ROBINS-I) was used to assess the risk of bias in our meta-analyses.26 The confounding bias, selection bias, intervention bias, bias due to missing data or outcome measurements, and reporting bias were all taken into account. In addition, the quality of evidence was assessed via the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.27,28 Five limitations were assessed, including the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Two researchers (YBH and WLL) individually evaluated studies based on the above domains. Then, using GRADEpro software (version 3.6; McMaster University, Hamilton, Ontario, Canada), we classified the overall quality of evidence as “very low”, “low”, “moderate”, or “high”. A higher classification corresponds to more reliable evidence.
Statistical analyses
In this study, PCa cases were classified as those with or without ADT. Forest plots were created to illustrate the incidence and severity of COVID-19 in ADT and non-ADT groups, as described by RRs and 95% confidence intervals (CIs). Heterogeneity was assessed by the I² statistic and categorized as low, medium, or high; the lower limits of I² in each category were 25%, 50%, and 75%, respectively. We applied a random-effects model if there was significant heterogeneity; otherwise, we used a fixed-effects model. The heterogeneity in each study was evaluated using the leave-one-out sensitivity analysis method. Potential publication bias was evaluated by Begg’s and Egger’s tests. All analyses were conducted using Stata 13.0 statistical software (Stata Corporation, College Station, TX, USA). P < 0.05 was considered statistically significant.
RESULTS
Description of study selection and characteristics
In total, 714 potentially relevant studies were identified using the search terms described above. After excluding duplicated studies, 168 publications remained. Subsequently, we excluded irrelevant studies by screening titles and abstracts (n = 96); afterward, 72 studies remained and their full texts were reviewed. Then, 59 publications were excluded for the following reasons: (1) review articles or comments without data (n = 20); (2) animal or molecular biology studies (n = 4); (3) insufficient data reported to calculate RR and 95% Cl (n = 21); and (4) no relevant outcomes (n = 14). Finally, 13 articles met our eligibility criteria.11,29,30,31,32,33,34,35,36,37,38,39,40 The characteristics of these studies are described in detail in Table 1, and a flowchart of study selection following the PRISMA guidelines is provided in Figure 1.
Table 1.
Main characteristics of eligible studies included in the meta-analysis concerning impact of androgen deprivation therapy on risk of incidence and severity of COVID-19 in PCa patients
| Study | Country | Study type | Recruitment period | Total cases (n) | Median or range of age (year) | Chemotherapy cases (n) | Castration resistance cases (n) | Reported indicators | Adjusted RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Kwon et al.29 2021 | USA | Cohort study; retrospective | February 2020–December 2020 | 5211 | NR | NR | NR | SARS-CoV-2 positive | 1.18 (0.70–1.99) |
| Death | 0.56 (0.07–4.88) | ||||||||
| Duarte et al.30 2021 | Brazil | Cohort study; retrospective | January 2020–April 2021 | 199 | 75 | NR | NR | Death | 0.63 (0.29–1.37) |
| Gedeborg et al.31 2022 | Sweden | Case-control study; retrospective | December 2019–December 2020 | 24 174 | 51–97 | NR | NR | Death | 5.05 (4.18–6.10) |
| Welén et al.32 2022 | Sweden | Cohort study; retrospective | July 2020–November 2020 | 5824 | 50–81 | NR | NR | Hospitalization | 1.24 (0.97–1.59) |
| ICU admission | 0.87 (0.51–1.48) | ||||||||
| Death | 0.89 (0.54–1.46) | ||||||||
| Kazan et al.33 2021 | Turkey | Cohort study; retrospective | August 2020–June 2021 | 365 | NR | NR | NR | SARS-CoV-2 positive | 0.68 (0.34–1.36) |
| Hospitalization | 3.36 (0.61–18.58) | ||||||||
| Jiménez-Alcaide et al.34 2021 | Spain | Cohort study; retrospective | March 2020–May 2020 | 1349 | 64.2 | 1 | 1 | Death | 0.67 (0.26–1.74) |
| Patel et al.352021 | USA | Cohort study; retrospective | March 2020–May 2020 | 465 | 61–81 | NR | NR | Hospitalization | 1.07 (0.61–1.87) |
| Death | 1.28 (0.79–2.08) | ||||||||
| Schmidt et al.362021 | USA | Cohort study; retrospective | May 2020–February 2021 | 1228 | 73 | 266 | NR | Death | 0.77 (0.42–1.42) |
| Tucker et al.37 2021 | USA | Cohort study; retrospective | December 2019–July 2020 | 589 | NR | NR | NR | Death | 1.41 (0.99–2.01) |
| Koskinen et al.38 2020 | Finland | Cohort study; retrospective | March 2020–May 2020 | 352 | 51–96 | NR | NR | SARS-CoV-2 positive | 0.88 (0.32–2.44) |
| Klein et al.39 2021 | USA | Cohort study; prospective | March 2020–June 2020 | 1779 | 38–101 | NR | NR | SARS-CoV-2 positive | 0.90 (0.54–1.61) |
| Hospitalization | 2.00 (0.57–7.32) | ||||||||
| ICU admission | 1.30 (0.38–4.09) | ||||||||
| Death | 2.90 (0.86–9.81) | ||||||||
| Montopoli et al.11 2020 | Italy | Cohort study; retrospective | December 2019–April 2020 | 42 434 | NR | NR | NR | SARS-CoV-2 positive | 4.05 (1.55–10.59) |
| Hospitalization | 3.93 (1.31–11.77) | ||||||||
| ICU admission | 4.40 (0.76–25.50) | ||||||||
| Caffo et al.40 2020 | Italy | Cohort study; retrospective | February 2020–June 2020 | 34 | 75 | 9 | 34 | Death | 1.17 (0.17–8.09) |
No studies reported the duration of therapy on COVID-19. COVID-19: coronavirus disease 2019; RR: risk ratio; NR: not reported; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit; CI: confidence interval
Figure 1.
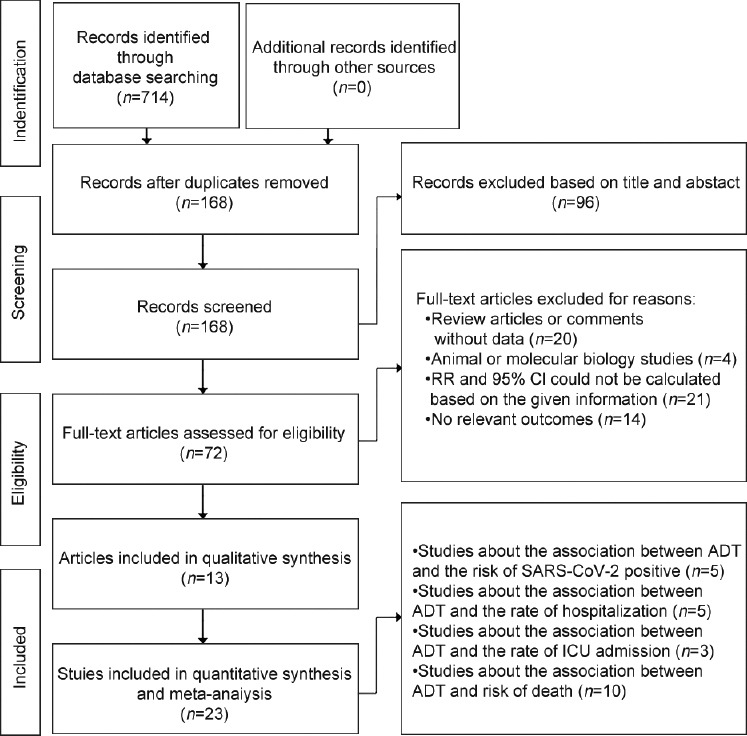
The PRISMA flowchart of the systematic review and meta-analysis. RR: risk ratio; CI: confidence interval; ADT: androgen deprivation therapy; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The studies included in our meta-analysis encompassed a total of 84 003 PCa patients. Regarding region, 5 studies were from the USA29,35–37,39 (including 9272 PCa patients), 2 studies were from Sweden31,32 (29 998 PCa patients), 2 studies were from Italy11,40 (42 468 PCa patients), 1 study was from Brazil30 (199 PCa patients), 1 study was from Turkey33 (365 PCa patients), 1 study was from Spain34 (1349 PCa patients), and 1 study was from Finland38 (352 PCa patients). All the studies were retrospective cohort studies, except one prospective cohort study conducted by Klein et al.39 and one case–control study conducted by Gedeborg et al.31 In total, 276 PCa patients underwent chemotherapy, and 35 PCa patients progressed to castration resistance. Additionally, 7 studies reported ADT administration details (Supplementary Table 2).29,30,31,32,35,36,38 Of these, most (n = 4) reported that ADT involved a gonadotrophic-releasing hormone (GnRH) analog/antagonist, surgical castration, and anti-androgen/anti-androgen receptor drugs.31,32,35,38
Supplementary Table 2.
Characteristics of androgen deprivation therapy usage in eligible studies
| First author (year) | ADT types | ADT selection | Duration of ADT use | Assessed variables | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ① | ② | ③ | ④ | ⑤ | ||||
| BAT | GnRH analogue | GnRH antagonist | Surgical castration | Anti-androgen/anti-AR | ||||
| Kwon et al.29 2021 | NR | √ | √ | NR | NR | ②+③ | ≤6 months | SARS-CoV-2 positive Death |
| Duarte et al.30 2021 | NR | √ | NR | √ | NR | ②+④ | NR | Death |
| Gedeborg et al.31 2022 | NR | √ | √ | √ | √ | ②+③+④+⑤ | NR | Death |
| Welén et al.32 2022 | NR | √ | √ | √ | √ | ②+③+④+⑤ | NR | Hospitalization ICU admission Death |
| Kazan et al.33 2021 | NR | NR | NR | NR | NR | NR | NR | SARS-CoV-2 positive Hospitalization |
| Jiménez-Alcaide et al.34 2021 | NR | NR | NR | NR | NR | NR | NR | Death |
| Patel et al.35 2021 | NR | √ | √ | √ | √ | ②+③+④+⑤ | ≤6 months | Hospitalization Death |
| Schmidt et al.36 2021 | NR | √ | √ | √ | NR | ②+③+④ | ≤3 months | Death |
| Tucker et al.37 2021 | NR | NR | NR | NR | NR | NR | NR | Death |
| Koskinen et al.38 2020 | NR | √ | √ | √ | √ | ②+③+④+⑤ | NR | SARS-CoV-2 positive |
| Klein et al.39 2021 | NR | NR | NR | NR | NR | NR | NR | SARS-CoV-2 positive Hospitalization ICU admission Death |
| Montopoli et al.11 2020 | NR | NR | NR | NR | NR | NR | NR | SARS-CoV-2 positive Hospitalization ICU admission |
| Caffo et al.40 2020 | NR | NR | NR | NR | NR | NR | 50 months | Death |
ADT: androgen deprivation therapy; BAT: bipolar androgen therapy; GnRH: gonadotropin-releasing hormone; AR: androgen receptor; NR: not reported; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit
Ten studies reported comorbidities (Supplementary Table 3),29,30,31,32,33,34,36,38,39,40 and six of these enrolled ADT and non-ADT populations with various comorbidities.30,33,34,36,38,39 For example, in the study of Duarte et al.,30 patients receiving ADT were more likely to have kidney disease (RR = 0.37, 95% CI: 0.17–0.81; P = 0.010), and in the study of Klein et al.,39 patients receiving ADT were more likely to have a respiratory disease (RR = 0.65, 95% CI: 0.44–0.94; P = 0.020), a history of smoking (RR = 1.15, 95% CI: 1.05–1.25; P = 0.002), or immunosuppressive diseases (RR = 1.25, 95% CI: 1.04–1.49; P = 0.010).
Supplementary Table 3.
Stratified analysis between androgen deprivation therapy and comorbidities in eligible studies
| First author (year) | Comorbidities | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Diabetes mellitus | Kidney disease | Respiratory disease | Obesity | Smoking | Immune suppressive disease | Hypertension | Cardiovascular disease | Neurological disease | |
| Kwon et al.29 2021 | 763 | 658 | 321 | 340 | NR | NR | NR | 577 | NR |
| Duarte et al.30 2021 | ADT (+): 37 ADT (−): 12 RR: 0.85 95% CI: 0.49–1.48 P=0.570 | ADT (+): 12 ADT (−): 9 RR: 0.37 95% CI: 0.17–0.81 P=0.010 | ADT (+): 17 ADT (−): 6 RR: 0.78 95% CI: 0.33–1.86 P=0.580 | NR | NR | NR | NR | ADT (+): 60 ADT (−): 21 RR: 0.79 95% CI: 0.55–1.13 P=0.200 | ADT (+): 8 ADT (−): 3 RR: 0.74 95% CI: 0.20–2.65 P=0.640 |
| Gedeborg et al.31 2022 | 2458 | NR | 1320 | NR | NR | NR | NR | 2184 | NR |
| Welén et al.32 2022 | 8 | NR | 3 | NR | NR | NR | 13 | 6 | NR |
| Kazan et al.33 2021 | ADT (+): 38 ADT (−): 60 RR: 1.04 95% CI: 0.74–1.47 P=0.820 | NR | ADT (+): 16 ADT (−): 13 RR: 2.02 95% CI: 1.00–4.08 P=0.050 | NR | ADT (+): 90 ADT (−): 145 RR: 1.02 95% CI: 0.87–1.19 P=0.790 | NR | ADT (+): 70 ADT (−): 107 RR: 1.08 95% CI: 0.87–1.33 P=0.500 | ADT (+): 31 ADT (−): 39 RR: 1.31 95% CI: 0.86–1.99 P=0.210 | NR |
| Jiménez-Alcaide et al.34 2021 | NR | NR | ADT (+): 0 ADT (−): 13 RR: 0.16 95% CI: 0.01–2.47 P=0.19 | ADT (+): 1 ADT (−): 9 RR: 0.51 95% CI: 0.07–3.59 P=0.49 | ADT (+): 3 ADT (−): 11 RR: 1.24 95% CI: 0.41–3.71 P=0.70 | NR | ADT (+): 6 ADT (−): 41 RR: 0.55 95% CI: 0.29–1.07 P=0.08 | ADT (+): 0 ADT (−): 9 RR: 0.22 95% CI: 0.01–3.58 P=0.29 | NR |
| Patel et al.35 2021 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Schmidt et al.36 2021 | ADT (+): 40 ADT (−): 80 RR: 0.91 95% CI: 0.66–1.27 P=0.580 | ADT (+): 24 ADT (−): 54 RR: 0.81 95% CI: 0.52–1.26 P=0.350 | ADT (+): 21 ADT (−): 46 RR: 0.83 95% CI: 0.51–1.35 P=0.450 | NR | ADT (+): 86 ADT (−): 151 RR: 1.04 95% CI: 0.86–1.25 P=0.700 | NR | NR | ADT (+): 70 ADT (−): 114 RR: 1.12 95% CI: 0.89–1.41 P=0.340 | NR |
| Tucker et al.37 2021 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Koskinen et al.38 2020 | ADT (+): 16 ADT (−): 17 RR: 1.53 95% CI: 0.80–2.93 P=0.200 | NR | ADT (+): 8 ADT (−): 12 RR: 1.08 95% CI: 0.46–2.58 P=0.850 | NR | ADT (+): 18 ADT (−): 17 RR: 1.72 95% CI: 0.92–3.22 P=0.090 | NR | ADT (+): 30 ADT (−): 47 RR: 1.04 95% CI: 0.69–1.56 P=0.850 | ADT (+): 51 ADT (−): 71 RR: 1.17 95% CI: 0.88–1.56 P=0.290 | NR |
| Klein et al.39 2021 | ADT (+): 107 ADT (−): 466 RR: 1.11 95% CI: 0.94–1.32 P=0.210 | NR | ADT (+): 28 ADT (−): 210 RR: 0.65 95% CI: 0.44–0.94 P=0.020 | NR | ADT (+): 207 ADT (−): 875 RR: 1.15 95% CI: 1.05–1.25 P=0.002 | ADT (+): 104 ADT (−): 405 RR: 1.25 95% CI: 1.04–1.49 P=0.010 | ADT (+): 252 ADT (−): 1194 RR: 1.02 95% CI: 0.97–1.08 P=0.410 | ADT (+): 186 ADT (−): 925 RR: 0.98 95% CI: 0.88–1.08 P=0.620 | NR |
| Montopoli et al.11 2020 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Caffo et al.40 2020 | 4 | 2 | 3 | NR | NR | NR | 20 | 12 | NR |
ADT: androgen deprivation therapy; +: use; −: no use; RR: risk ratio; CI: confidence interval; NR: not reported
Association between ADT use and the risk of SARS-CoV-2 positive in PCa patients
To evaluate the impact of ADT use to treat PCa on the risk of SARS-CoV-2 positive, we included five studies11,29,33,38,39 on 50 141 PCa patients. The results of the heterogeneity test showed moderate heterogeneity (I2 = 60.1%; P = 0.040); thus, a random-effects model was applied. The individual heterogeneity of each study is shown in Figure 2a. There was no significant difference in SARS-CoV-2 positive between the ADT and non-ADT groups (P = 0.544), indicating that ADT use did not protect the patients against infection with SARS-CoV-2 (RR = 1.17, 95% CI: 0.71–1.92; Figure 2b).
Figure 2.
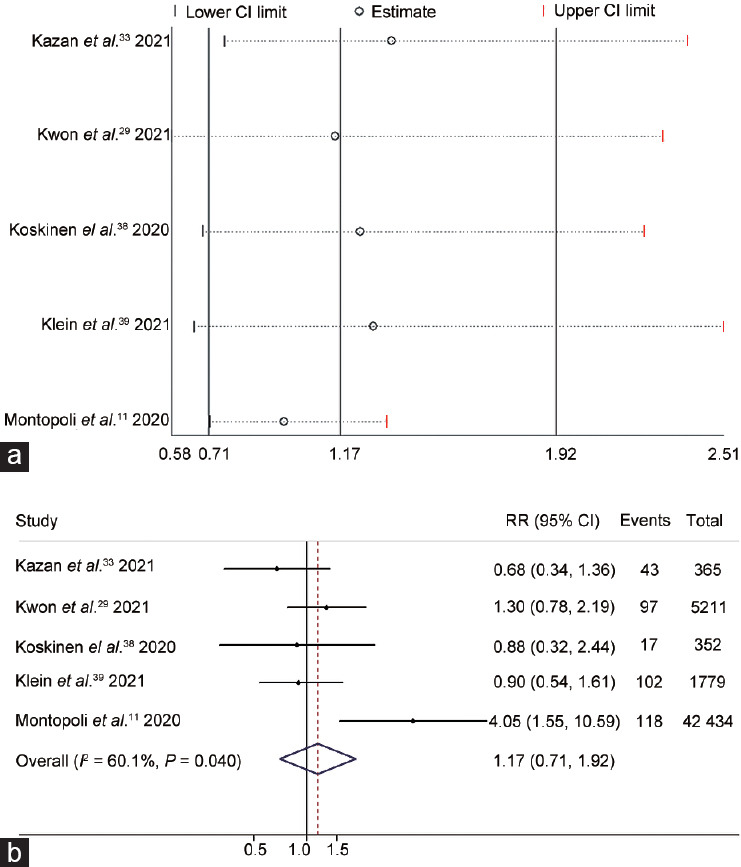
The correlation between androgen deprivation therapy and SARS-CoV-2 positive risk was illustrated by (a) leave-one-out sensitivity analysis and (b) a forest plot. The best models were applied to estimate each meta-analysis. For the forest plot, the line perpendicular to the X-axis indicates an RR equal to 1 (baseline). The RR values and 95% CIs of individual studies are represented by each enclosed circle and horizontal line parallel to the X-axis, respectively. Overlap between the baseline and a diamond indicates no statistical significance. CI: confidence interval; RR: risk ratio; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Association between ADT use and the rate of COVID-19 hospitalization in PCa patients
A total of 50 867 PCa patients from 5 studies11,32,33,35,39 concerning the relationship between ADT and the rate of COVID-19 hospitalization were included in the meta-analysis. As shown in Figure 3a, we detected no significant individual heterogeneity. The overall heterogeneity of these studies (I2 = 34.7%; P = 0.190) suggested that a fixed-effects model could be used for analyses. According to the combined outcomes, there was a significant association between ADT use and a high rate of COVID-19 hospitalization in PCa patients (RR = 1.31, 95% CI: 1.05–1.62; P = 0.015; Figure 3b).
Figure 3.

The correlation between androgen deprivation therapy and the rate of COVID-19 hospitalization was analyzed by (a) leave-one-out sensitivity analysis and (b) forest plot. CI: confidence interval; RR: risk ratio; COVID-19: coronavirus disease 2019.
Association between ADT use and the rate of ICU admission for COVID-19 in PCa patients
Additionally, we collected data from 50 037 patients from three eligible studies11,32,39 and conducted a meta-analysis to explore whether the use of ADT correlated with the rate of emergency medical care necessitated for COVID-19. The heterogeneity test and leave-one-out sensitivity analysis did not reveal significant overall or individual heterogeneity (I2 = 36.6%; P = 0.206; Figure 4a). Our meta-analysis applied a fixed-effects model, revealing that ADT use had no significant effect on ICU admission resulting from COVID-19 (95% CI: 0.65–1.66; P = 0.872), with the pooled RR of 1.04 (Figure 4b).
Figure 4.
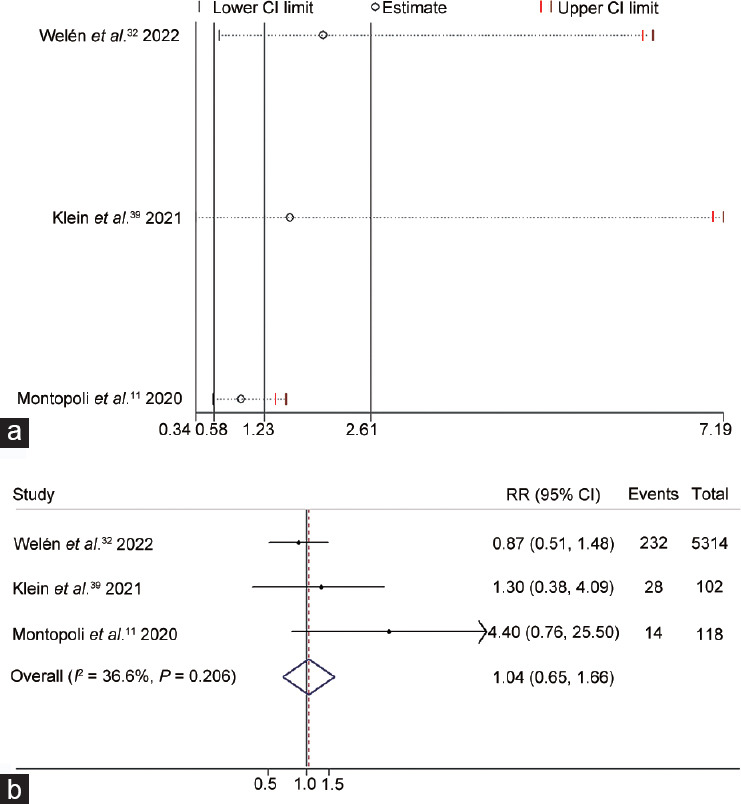
The correlation between androgen deprivation therapy and the rate of COVID-19 ICU admission was shown in (a) leave-one-out sensitivity analysis and (b) forest plot. CI: confidence interval; RR: risk ratio; COVID-19: coronavirus disease 2019; ICU: intensive care unit.
Association between ADT use and the death risk of COVID-19 in PCa patients
Death is the most unfavorable outcome when assessing the severity of COVID-19; the death risk due to COVID-19 was explored in 10 studies29,30,31,32,34,35,36,37,39,40 including 40 852 PCa patients. Among these studies, we found high heterogeneity (I2 = 92.7%; P < 0.001); therefore, we utilized a random-effects model. The outcomes of the leave-one-out sensitivity analysis are shown in Figure 5a. No significant difference (RR = 1.23, 95% CI: 0.64–2.38; P = 0.53) in the death risk was observed between the ADT and non-ADT groups (Figure 5b).
Figure 5.
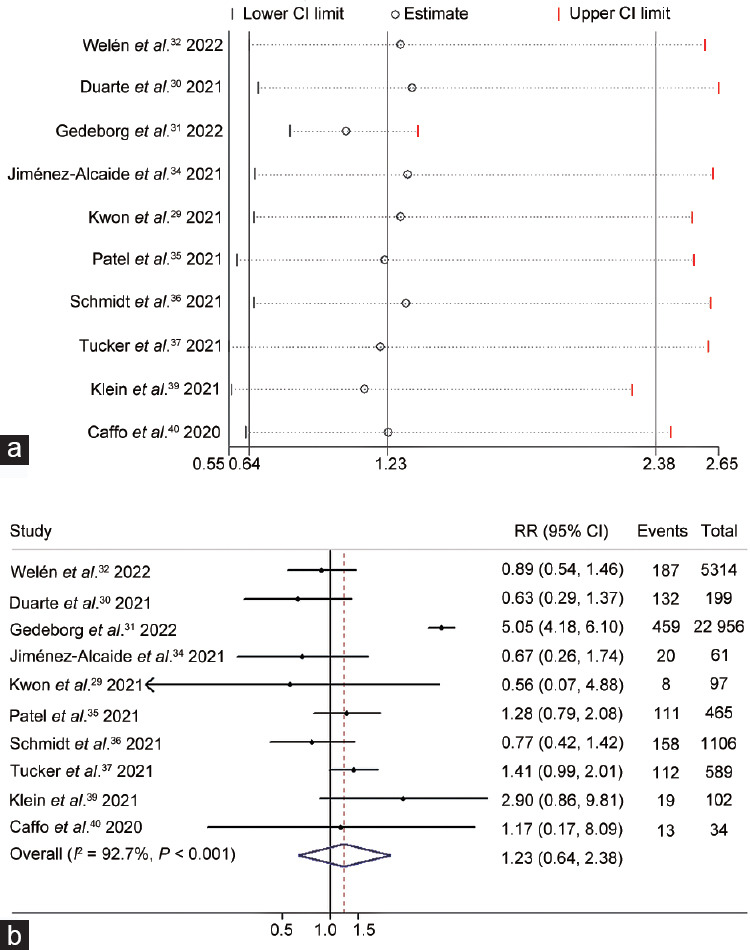
The correlation between androgen deprivation therapy and the death risk of COVID-19 was illustrated by (a) leave-one-out sensitivity analysis and (b) forest plot. CI: confidence interval; RR: risk ratio; COVID-19: coronavirus disease 2019.
Publication bias and quality assessment
Begg’s and Egger’s tests were performed in the four groups of studies, and none exhibited significant publication bias (all P > 0.05 for the SARS-CoV-2 positive, COVID-19 hospitalization, ICU admission, and death groups). The ROBINS-I checklists of individual studies included in these four meta-analyses (the above groups) are shown in Supplementary Figure 1 (251.2KB, tif) . We determined that our meta-analyses had a low-to-moderate risk of bias. Moreover, details on the quality of evidence for each meta-analysis according to the GRADE approach are summarized in Supplementary Table 4. In the meta-analysis of the risk of SARS-CoV-2 positive, a low quality of evidence was found due to high overall heterogeneity. In the meta-analysis of the risk of COVID-19 hospitalization, the range of values was excessively large; therefore, the evidence of outcomes related to the rate of hospitalization was determined to be of low quality. For the meta-analysis of ICU admissions due to COVID-19, the range of true values in each pooled study was excessively large and variable, which severely impacted the GRADE score and resulted in a very low quality of evidence. Similarly, in the meta-analysis exploring the relationship between ADT use and the death risk, imprecision and inconsistency resulted in very low quality of evidence.
Supplementary Table 4.
The overall quality of evidence in pooled findings from eligible studiesa
| Outcomes | Number of studies | Design | Certain quality assessment | Summary of findings | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative effect, RR (95% CI) | Quality | |||
| Risk of SARS-CoV-2 positive | 5 | Observational studies | Not serious | Seriousb | Not serious | Not serious | None | 1.17 (0.71–1.92) | Low |
| Rate of hospitalization | 5 | Observational studies | Not serious | Not serious | Not serious | Seriousc | None | 1.31 (1.05–1.62) | Low |
| Rate of ICU admission | 3 | Observational studies | Not serious | Not serious | Not serious | Very seriousc | None | 1.04 (0.65–1.66) | Very low |
| Death risk | 10 | Observational studies | Not serious | Seriousb | Not serious | Seriousc | None | 1.23 (0.64–2.38) | Very low |
aThe quality assessment was based on GRADE approach; bHigh levels of heterogeneity in pooled findings; cThe range of true values in each pooled studies was too large in varying degrees. GRADE: Grading of Recommendations assessment, Development and Evaluation, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit; CI: confidence interval; RR: risk ratio
DISCUSSION
The sheer magnitude of the COVID-19 outbreak and pandemic has triggered an unprecedented global health emergency. The potential role of testosterone and other androgens in COVID-19 occurrence and development has been highly controversial. Worse clinical outcomes and higher mortality of COVID-19 have been reported among the male population in many countries,41 and this discrepancy may be largely due to hormonal differences. The expression of TMPRSS2 is mediated by testosterone, and TMPRSS2 plays a vital role in proteolysis of the SARS S protein and binding of SARS-CoV-2 to ACE2, possibly explaining the increased severity of COVID-19 in males.42 Additionally, Kissick et al.43 indicated that high levels of circulating testosterone had immunosuppressive effects, as it inhibited B-cell growth and the differentiation of CD4+ T cells, thereby impairing the immune response and reducing the production of immunoglobulin. The effects could increase the probability of SARS-CoV-2 invasion.
However, the level of circulating preoperative testosterone may also predict biochemical recurrence (BCR) in patients who received radical prostatectomy (RP);44 thus, beneficial effects of testosterone on COVID-19 have been reported by an increasing number of studies. High testosterone levels are associated with a shorter duration of SARS-CoV-2 viral positive, while low testosterone levels can lead to poor prognosis, worse clinical COVID-19 phenotype, and higher mortality rate in SARS-CoV-2 positive male patients.45,46,47 Rastrelli et al.48 indicated that lower baseline levels of testosterone predicted higher likelihood of poor prognosis and mortality rate by analyzing male patients with COVID-19 pneumonia. Montaño et al.49 found that sufficient levels of testosterone exerted a protective effect on the respiratory system, improving pulmonary ventilation and respiratory muscle contraction; in contrast, low levels of testosterone during hospitalization may be a risk factor for clinical disease severity and inflammation.50,51,52 These findings can be explained by the inhibitory effect of testosterone on proinflammatory cytokine production. In acute multiorgan damage induced by COVID-19, crucial proinflammatory cytokines, including interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α), rapidly lead to a severe inflammatory cytokine storm.53 Testosterone exerts a protective effect by inhibiting these cytokines.54 Papadopoulos et al.55 and Sun et al.56 indicated that a high level of IL-6 could lead to increased COVID-19 severity but testosterone inhibited IL-6 synthesis and released and downregulated IL-6 receptor expression. In addition, testosterone inhibits the nuclear factor kappa-B (NF-κB) inflammatory signaling pathway, thus reducing lung inflammation and fibrosis.57 In sum, the possible effects of testosterone on COVID-19 are very complicated and span a wide spectrum.
The relationship between androgen levels and PCa has been fully elucidated.58 ADT, which reduces androgen levels, is the mainstay of therapy in advanced or metastatic PCa patients.59 However, routine ADT therapies, such as GnRH analogs, GnRH antagonists, and surgical castration, cannot permanently prevent the progression of castration-resistant PCa. Therefore, androgen receptor signaling blocking agents, such as enzalutamide and abiraterone, have been developed and applied in the treatment of metastatic castration-resistant PCa.60 Enzalutamide has antagonism against the overexpression of androgen receptor, and abiraterone downregulates the expression of cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1), which is an androgen synthesis enzyme.61,62 Both of these agents inhibit androgen receptor signaling, interfering with the malignant biological behavior of metastatic castration-resistant PCa. Inspired by the findings above, we investigated whether ADT can regulate the androgen receptor pathway, mediate TMPRSS2 transcription, and impede SARS-CoV-2 from infecting host cells. Montopoli et al.11 found that PCa patients who received ADT had a lower risk of SARS-CoV-2 positive and ICU admission than patients who did not receive ADT, indicating that ADT may protect against SARS-CoV-2 infection in PCa patients. However, a subsequent study conducted by Kwon et al.,29 including 5211 PCa patients with COVID-19, observed the opposite pattern, with COVID-19 more prevalent in PCa patients who received ADT. In addition, several studies did not find that ADT was associated with reductions in the mortality rates of PCa patients who were SARS-CoV-2 positive.39,40 Given these contradictory results, a systematic review is needed to verify the findings and update the prior hypothesis. We established inclusion and exclusion criteria and conducted a comprehensive literature search, extracting data from 13 eligible studies to conduct four meta-analyses. Significant differences in the hospitalization rates (RR = 1.31, P = 0.015) were identified between PCa patients who received ADT and those who did not. Our findings indicated that ADT use by PCa patients was associated with a high risk of COVID-19-related hospitalization.
There are several possible explanations for this finding. First, ADT resistance develops rapidly, either to routine therapy or enzalutamide/abiraterone, resulting in further progression of metastatic castration-resistant PCa; this could influence the rate of hospitalization of PCa patients with COVID-19. Additionally, reactivation of the androgen receptor signaling pathway is one of the main factors underlying metastatic castration-resistant PCa.63 Mediated by resistance to enzalutamide/abiraterone, overexpression and mutations of androgen receptors or high levels of androgen receptor splice variants could also cause SARS-CoV-2 invasion.64 Second, according to stratification analyses based on various comorbidities, the use of ADT was significantly associated with high incidence rates of comorbidities, such as a history of smoking and immunosuppressive diseases, in two studies.30,39 These comorbidities would also yield increased risks of hospitalization. Moreover, the patient population included in our study was mainly comprised of elderly males. A reduction in serum testosterone levels and related risky health habits or basic diseases, such as smoking and hypertension, are prevalent in populations of older male patients. These factors also cause higher rates of hospitalization for COVID-19. Third, as we discussed previously, androgen is a double-edged sword regarding the rapid progression of COVID-19. Testosterone exerts diverse roles on different components of the immune system: low levels of testosterone may have protective effects in the early stages of infection but lead to worse outcomes in advanced COVID-19 cases.46 Therefore, we hypothesized that in the early stage, COVID-19 patients exhibit a relatively weak inflammatory response. As ADT inhibits the proinflammatory effects of testosterone, it may downregulate the levels of TMPRSS2 and prevent SARS-CoV-2 from replicating in host cells. In advanced stages of COVID-19, SARS-CoV-2 has undergone massive viral replication in the host. A hyperactive inflammatory response is produced, causing a series of pneumonia-like symptoms, such as fever, coughing, sneezing, and leukocytosis. Patients are more likely to come to the hospital for treatment and hospitalization in this stage. Finally, in COVID-19 patients exhibiting acute multiorgan damage, a severe inflammatory cytokine storm is rapidly activated. At this point, control of the inflammatory response is more important than preventing viral replication.
Some studies have indicated that prostate inflammation is related to the severity of PCa. From macroscopic and clinical perspectives, inflammatory-related factors, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and eosinophil-to-lymphocyte ratio (ELR), are significantly correlated with increases in the Gleason score in PCa patients who underwent active surveillance and RP.65 Moreover, a single-center study compared histological specimens from 260 patients with or without active surveillance and prostate biopsy after RP; the outcomes revealed that systemic inflammatory markers in the prostate, consisting of the NLR, PLR, and monocyte-to-lymphocyte ratio (MLR), may be associated with a higher risk of adverse pathology.66 In addition, higher levels of testosterone predict shorter durations of SARS-CoV-2 viral positive,47 and ADT reduces testosterone secretion, possibly mitigating the damaging effects of inflammation due to COVID-19. From a microscopic, molecular biology perspective, abnormal levels of testosterone influence reactive oxygen species (ROS) production, cellular senescence, autophagy, and mitophagy, as well as vitamin D levels. Higher levels of ROS may activate nucleotide-like receptors, especially NOD-like receptor protein 3 (NLRP3), a member of the nucleotide-like receptor family, and induce pyroptosis. Steroidogenic acute regulatory protein (StAR) could act as a barrier to pyroptosis by inducing testosterone production. The crosstalk between diverse SARS-CoV-2-related immune cells in the microenvironment and inflammatory cytokines is listed in Supplementary Figure 2 (128.3KB, tif) .67,68,69,70,71,72,73 Furthermore, testosterone inhibits the hypersecretion of adipokines, including TNF-α and IL-6, in senescent adipocytes. Testosterone-related immunosenescence may alter the ratio of CD4+/CD8+ T cells; interfere with the distributions of immature T cells, memory T cells, the Th1 response (IL-2 and interferon [IFN]-γ), and the Th2 response (IL-4); and modify the production of proinflammatory cytokines, including IL-2, IL-8, IL-17, monocyte chemoattractant protein 1 (MCP1), TNF-α, and chemokine (C-C motif) ligand 3 (CCL3). Since studies have reported a significant positive association between testosterone levels and 25-hydroxyvitamin D levels, shorted as 25(OH) D levels in elderly patients with COVID-19, testosterone is also implicated in modulating 25(OH) D levels.74,75 After 25(OH) D combines with the vitamin D receptor, it can influence the autophagic activity of macrophages, increase antimicrobial products, and reduce the expression of proinflammatory cytokines, including TNF-α, IL-8, IL-13, and IFN-γ.74,75,76,77,78 Additionally, male patients with long-term low testosterone levels may have reduced muscle mass and strength, which could lead to progressively reduced lung capacity and excessive reliance on ventilators.79,80,81 This may be the primary explanation for the association between lower testosterone levels after long-term treatment with ADT and increases in the hospitalization rate of COVID-19 patients. Moreover, ADT has severe side effects, including anaphylactoid reactions, shock, diabetes, cerebral hemorrhage, and cardiac diseases, which may indirectly lead to the hospitalization of COVID-19 patients.82
We acknowledge that shortcomings and limitations in the present study may have affected our findings. First, since individuals have different rates of exposure to sources of infection during a pandemic, the actual rate of SARS-CoV-2 infection is challenging to determine. Second, although all authors participated in the systematic literature review, development of inclusion and exclusion criteria, and crosschecking of the included studies, selection bias with regard to the inclusion and exclusion of certain studies was unavoidable. Third, in terms of the inclusion criteria, studies did not control for baseline information, such as PCa stage, medical history, and the age of patients. Additionally, SARS-CoV-2 positive has a wide range of clinical manifestations, from asymptomatic cases to complicated cases. The main limitation of the statistical power of our meta-analyses was a shortage of relevant studies that reported the data of interest. Moreover, further consideration of the eligibility of studies included in our meta-analyses is needed to confirm our results. Statistical significance might be achieved with the inclusion of more studies, and longitudinal studies are needed. Additionally, as research on this topic is new and sparse, large-scale, multicenter clinical prospective and randomized controlled trial (RCT) studies have not yet to be conducted. All the included studies were retrospective and observational, directly resulting in low or very low evidence quality according to the GRADE criteria. Therefore, more cogent results might be obtained by systematic reviews following extensive study in this field and the inclusion of more high-quality studies.
In addition, we believe that unstudied confounding factors, which cannot be fully ruled out, are likely to have interfered with our findings. For example, the administration route, drug type, dosage, frequency, and treatment course of ADT were not identical among the included studies, and different effects of ADT would directly impact the progression of COVID-19. Additionally, the risk of cardiovascular adverse events varies across ADT regimens. For example, Albertsen et al.83 integrated 6 phase-III prospective randomized trials that included 2328 male patients; they found that compared with a GnRH antagonist, GnRH agonists doubled the risk of cardiovascular adverse events in men with a history of cardiovascular disease. More importantly, surgical castration significantly increases the risks of cardiovascular thrombotic events, coronary heart disease, acute myocardial infarction, and ischemic stroke compared to GnRH agonists in PCa patients.84,85 An innovative therapeutic strategy, bipolar androgen therapy (BAT), rapidly fluctuates between supraphysiological and near-castrate serum testosterone levels,86 which could restore androgen receptor sensitivity in castration-resistant PCa patients after progression on enzalutamide/abiraterone.87 Recently, Xiong et al.88 reported that BAT caused low-grade cardiovascular adverse events in castration-resistant PCa patients. Therefore, we attempted to conduct a subgroup analysis to assess the relationship between cardiovascular events and ADT regimens, as well as their potential effects on hospitalization rates for COVID-19. However, the limited number of studies with available information on the ADT type and our selection criteria restricted this subgroup analysis; therefore, large, prospective trials are needed to further confirm its value in the future. Additionally, uses of diverse supportive care interventions, such as antiviral drugs and corticosteroids, merit examination. Fatigue is one of the most common and debilitating PCa-related symptoms as well as acute COVID-19 sequelae. Fatigue and/or cognitive impairment appear in a substantial proportion of COVID-19 patients,89,90 which may lead to poor prognosis. Ferro et al.91 found that the chronic, high-dose use of corticosteroids was significantly associated with a high level of fatigue in PCa patients undergoing systemic treatment. However, complete information on the above variables could not be extracted from eligible studies. These issues will be the focus of our future study.
CONCLUSIONS
As COVID-19 continues to spread worldwide, vast swaths of populations have been infected. Therefore, strong evidence regarding the validity and safety of ADT in COVID-19 is urgently needed. According to our systematic review and updated meta-analyses, we concluded that in PCa patients, ADT use is associated with a higher rate of hospitalization for COVID-19. Our meta-analyses provide valuable contributions to the development of prevention and treatment methods for COVID-19, especially for PCa patients. Future studies with large and standardized samples, as well as those that analyze immune parameters, pathological examinations, and other variables, are needed to confirm our conclusions. Additionally, further exploration of new techniques could provide insights for repurposing and exploiting PCa and COVID-19 drugs.
AUTHOR CONTRIBUTIONS
BF and XCL conceptualized and designed this work and undertook project leadership. BF and XCL funded this work. YBH, WLL, MS, XD, YTW, LXZ, ZHX, and ZFY provided significant inputs on this manuscript. YBH, WLL, MS, and XCL collected and checked the published data; and YBH and BF performed the analysis. YBH, WLL, BF, and XCL wrote, reviewed, edited, and revised the final manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
The review authors’ judgments based on ROBINS-I checklists about risk of bias for each meta-analyses, including (a) SARS-CoV-2 positive risk, (b) rate of COVID-19 hospitalization, (c) rate of COVID-19 ICU admission, and (d) death risk of COVID-19. The different colors represent various of evaluations for each methodological quality items. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; ICU: intensive care unit.
Summary of the connections between diverse SARS-CoV-2–related immune cells in the microenvironment and inflammatory cytokines. Authorized illustrations came from the Smart Medical Art (https://smart.servier.com). AEC: airway epithelial cell; IL: interleukin; TNF-α: tumor necrosis factor-α; TGF-β: transforming growth factor-β; IFN-γ: interferon-γ; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81972831 and 31800787), the Natural Science Foundation of Liaoning Province (502722), the “Seedling” Project of Young Scientific and Technological Talents of Liaoning Education Department (LR2019008), the United Fund of the Second Hospital of Dalian Medical University and Dalian Institute of Chemical Physics, Chinese Academy of Sciences (UF-QN-202004), the Dalian High-level Talents Innovation Support Program (2019RQ014), the Doctoral Research Startup Foundation of the Second Hospital of Dalian Medical University (DY2Y201704), and the Young Reserve Talent Project of the Second Hospital of Dalian Medical University (dy2yhbrc202010).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, et al. Is ivermectin-azithromycin combination the next step for COVID-19? Biomed Biotechnol Res J. 2020;4:101. [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pourfattah F, Wang LP, Deng W, Ma YF, Hu L, et al. Challenges in simulating and modeling the airborne virus transmission:a state-of-the-art review. Phys Fluids (1994) 2021;33:101302. doi: 10.1063/5.0061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–8. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki TK. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2022;10:e17. doi: 10.1016/S2213-2600(21)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel VG, Zhong X, Liaw B, Tremblay D, Tsao CK, et al. Does androgen deprivation therapy protect against severe complications from COVID-19. Ann Oncol. 2020;31:1419–20. doi: 10.1016/j.annonc.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lugnier C, Al-Kuraishy HM, Rousseau E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem Pharmacol. 2021;185:114431. doi: 10.1016/j.bcp.2021.114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bank S, De SK, Bankura B, Maiti S, Das M, et al. ACE/ACE2 balance might be instrumental to explain the certain comorbidities leading to severe COVID-19 cases. Biosci Rep. 2021;41:BSR20202014. doi: 10.1042/BSR20202014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2:a functional receptor for SARS coronavirus. Cell Mol Life Sci. 2004;61:2738–43. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2:a population-based study (N =4532) Ann Oncol. 2020;31:1040–5. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen LW, Mao HJ, Wu YL, Tanaka Y, Zhang W. TMPRSS2:a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin B, Ferguson C, White JT, Wang S, Vessella R, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4. [PubMed] [Google Scholar]
- 14.Bhowmick NA, Oft J, Dorff T, Pal S, Agarwal N, et al. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr Relat Cancer. 2020;27:R281–92. doi: 10.1530/ERC-20-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–34. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, et al. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93:e01815–8. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–64. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharifi N, Ryan CJ. Androgen hazards with COVID-19. Endocr Relat Cancer. 2020;27:E1–3. doi: 10.1530/ERC-20-0133. [DOI] [PubMed] [Google Scholar]
- 19.Lucas JM, True L, Hawley S, Matsumura M, Morrissey C, et al. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215:118–25. doi: 10.1002/path.2330. [DOI] [PubMed] [Google Scholar]
- 20.Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–25. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen-Nielsen M, Borre M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med. 2016;46:484–90. doi: 10.1053/j.semnuclmed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor:structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36:3–23. doi: 10.1038/aps.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer:a systematic review and meta-analysis. Urol J. 2021;18:577–84. doi: 10.22037/uj.v18i.6691. [DOI] [PubMed] [Google Scholar]
- 25.Sari Motlagh R, Abufaraj M, Karakiewicz PI, Rajwa P, Mori K, et al. Association between SARS-CoV-2 infection and disease severity among prostate cancer patients on androgen deprivation therapy:a systematic review and meta-analysis. World J Urol. 2022;40:907–14. doi: 10.1007/s00345-021-03810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, et al. ROBINS-I:a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines:a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Kwon DH, Vashisht R, Borno HT, Aggarwal RR, Small EJ, et al. Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer:findings from the University of California Health System registry. Ann Oncol. 2021;32:678–9. doi: 10.1016/j.annonc.2021.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte M, Leal F, Argenton J, Carvalheira J. Impact of androgen deprivation therapy on mortality of prostate cancer patients with COVID-19:a propensity score-based analysis. Infect Agent Cancer. 2021;16:66. doi: 10.1186/s13027-021-00406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gedeborg R, Lindhagen L, Loeb S, Styrke J, Garmo H, et al. Androgen deprivation therapy, comorbidity, cancer stage and mortality from COVID-19 in men with prostate cancer. Scand J Urol. 2022;56:104–11. doi: 10.1080/21681805.2021.2019304. [DOI] [PubMed] [Google Scholar]
- 32.Welén K, Rosendal E, Gisslén M, Lenman A, Freyhult E, et al. A phase 2 trial of the effect of antiandrogen therapy on COVID-19 outcome:no evidence of benefit, supported by epidemiology and in vitro data. Eur Urol. 2022;81:285–93. doi: 10.1016/j.eururo.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazan Ö, Çulpan M, Efiloğlu Ö, Atiş G, Yildirim A. The clinical impact of androgen deprivation therapy on SARS-CoV-2 infection rates and disease severity. Turk J Urol. 2021;47:495–500. doi: 10.5152/tud.2021.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiménez-Alcaide E, García-Fuentes C, Hernández V, De la Peña E, Pérez-Fernández E, et al. Influence of androgen deprivation therapy on the severity of COVID-19 in prostate cancer patients. Prostate. 2021;81:1349–54. doi: 10.1002/pros.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel VG, Zhong X, Shah NJ, Martina LP, Hawley J, et al. The role of androgen deprivation therapy on the clinical course of COVID-19 infection in men with prostate cancer. J Clin Oncol. 2021;39:41. [Google Scholar]
- 36.Schmidt AL, Tucker MD, Bakouny Z, Labaki C, Hsu CY, et al. Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19. JAMA Netw Open. 2021;4:e2134330. doi: 10.1001/jamanetworkopen.2021.34330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker MD, Schmidt AL, Hsu CY, Shyr Y, Armstrong AJ, et al. Severe-COVID-19 and mortality among patients (pts) with prostate cancer (PCa) receiving androgen deprivation therapy (ADT) J Clin Oncol. 2021;39:39. [Google Scholar]
- 38.Koskinen M, Carpen O, Honkanen V, Seppänen M, Miettinen PJ, et al. Androgen deprivation and SARS-CoV-2 in men with prostate cancer. Ann Oncol. 2020;31:1417–8. doi: 10.1016/j.annonc.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein EA, Li J, Milinovich A, Schold JD, Sharifi N, et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2. J Urol. 2021;205:441–3. doi: 10.1097/JU.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 40.Caffo O, Gasparro D, Di Lorenzo G, Volta AD, Guglielmini P, et al. Incidence and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with metastatic castration-resistant prostate cancer. Eur J Cancer. 2020;140:140–6. doi: 10.1016/j.ejca.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19:serendipity or opportunity for intervention. Cancer Discov. 2020;10:779–82. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A. 2014;111:9887–92. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferro M, Lucarelli G, de Cobelli O, Vartolomei MD, Damiano R, et al. Circulating preoperative testosterone level predicts unfavourable disease at radical prostatectomy in men with International Society of Urological Pathology Grade Group 1 prostate cancer diagnosed with systematic biopsies. World J Urol. 2021;39:1861–7. doi: 10.1007/s00345-020-03368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cattrini C, Bersanelli M, Latocca MM, Conte B, Vallome G, et al. Sex hormones and hormone therapy during COVID-19 pandemic:implications for patients with cancer. Cancers (Basel) 2020;12:2325. doi: 10.3390/cancers12082325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salciccia S, Del Giudice F, Eisenberg ML, Mastroianni CM, De Berardinis E, et al. Androgen-deprivation therapy and SARS-Cov-2 infection:the potential double-face role of testosterone. Ther Adv Endocrinol Metab. 2020;11:2042018820969019. doi: 10.1177/2042018820969019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salciccia S, Eisenberg ML, Maggi M, Lai S, Mastroianni CM, et al. Modeling the contribution of male testosterone levels to the duration of positive COVID testing among hospitalized male COVID-19 patients. Diagnostics (Basel) 2021;11:581. doi: 10.3390/diagnostics11040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montaño LM, Espinoza J, Flores-Soto E, Chávez J, Perusquía M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- 50.Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021;4:e2111398. doi: 10.1001/jamanetworkopen.2021.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camici M, Zuppi P, Lorenzini P, Scarnecchia L, Pinnetti C, et al. Role of testosterone in SARS-CoV-2 infection:a key pathogenic factor and a biomarker for severe pneumonia. Int J Infect Dis. 2021;108:244–51. doi: 10.1016/j.ijid.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagano MT, Peruzzu D, Busani L, Pierdominici M, Ruggieri A, et al. Predicting respiratory failure in patients infected by SARS-CoV-2 by admission sex-specific biomarkers. Biol Sex Differ. 2021;12:63. doi: 10.1186/s13293-021-00407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc. 2019;3:91–107. doi: 10.1210/js.2018-00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keilich SR, Bartley JM, Haynes L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell Immunol. 2019;345:103992. doi: 10.1016/j.cellimm.2019.103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papadopoulos V, Li L, Samplaski M. Why does COVID-19 kill more elderly men than women?Is there a role for testosterone. Andrology. 2021;9:65–72. doi: 10.1111/andr.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun X, Wang T, Cai D, Hu Z, Chen J, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Huang L, Jiang S, Cheng K, Wang D, et al. Testosterone attenuates pulmonary epithelial inflammation in male rats of COPD model through preventing NRF1-derived NF-κB signaling. J Mol Cell Biol. 2021;13:128–40. doi: 10.1093/jmcb/mjaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huggins C, Hodges CV. Studies on prostatic cancer:I. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 59.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–99. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer:a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–9. doi: 10.1016/S1470-2045(19)30688-6. [DOI] [PubMed] [Google Scholar]
- 61.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–6. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 63.Lam HM, Corey E. Supraphysiological testosterone therapy as treatment for castration-resistant prostate cancer. Front Oncol. 2018;8:167. doi: 10.3389/fonc.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferro M, Musi G, Serino A, Cozzi G, Mistretta FA, et al. Neutrophil, platelets, and eosinophil to lymphocyte ratios predict Gleason score upgrading in low-risk prostate cancer patients. Urol Int. 2019;102:43–50. doi: 10.1159/000494259. [DOI] [PubMed] [Google Scholar]
- 66.Ferro M, Musi G, Matei DV, Mistretta AF, Luzzago S, et al. Assessment of PSIM (prostatic systemic inflammatory markers) score in predicting pathologic features at robotic radical prostatectomy in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Diagnostics (Basel) 2021;11:355. doi: 10.3390/diagnostics11020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montaño LM, Sommer B, Solís-Chagoyán H, Romero-Martínez BS, Aquino-Gálvez A, et al. Could lower testosterone in older men explain higher COVID-19 morbidity and mortalities. Int J Mol Sci. 2022;23:935. doi: 10.3390/ijms23020935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matyushenko V, Isakova-Sivak I, Kudryavtsev I, Goshina A, Chistyakova A, et al. Detection of IFNγ-secreting CD4+ and CD8+ memory T cells in COVID-19 convalescents after stimulation of peripheral blood mononuclear cells with live SARS-CoV-2. Viruses. 2021;13:1490. doi: 10.3390/v13081490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adamo S, Michler J, Zurbuchen Y, Cervia C, Taeschler P, et al. Signature of long-lived memory CD8+ T cells in acute SARS-CoV-2 infection. Nature. 2022;602:148–55. doi: 10.1038/s41586-021-04280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, et al. COVID-19 and Treg/Th17 imbalance:potential relationship to pregnancy outcomes. Am J Reprod Immunol. 2020;84:e13304. doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lozano-Ojalvo D, Camara C, Lopez-Granados E, Nozal P, Del Pino-Molina L, et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell Rep. 2021;36:109570. doi: 10.1016/j.celrep.2021.109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diao B, Wang C, Tan Y, Chen X, Liu Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm:systematic review and meta-analysis. Eur J Clin Invest. 2021;51:e13429. doi: 10.1111/eci.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Infante M, Pieri M, Lupisella S, D'Amore L, Bernardini S, et al. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. Eur Rev Med Pharmacol Sci. 2021;25:5889–903. doi: 10.26355/eurrev_202110_26865. [DOI] [PubMed] [Google Scholar]
- 75.Caballero-García A, Pérez-Valdecantos D, Guallar P, Caballero-Castillo A, Roche E, et al. Effect of vitamin D supplementation on muscle status in old patients recovering from COVID-19 infection. Medicina (Kaunas) 2021;57:1079. doi: 10.3390/medicina57101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glinsky GV. Tripartite combination of candidate pandemic mitigation agents:vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020;8:129. doi: 10.3390/biomedicines8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Binder EF, Christensen JC, Stevens-Lapsley J, Bartley J, Berry SD, et al. A multi-center trial of exercise and testosterone therapy in women after hip fracture:design, methods and impact of the COVID-19 pandemic. Contemp Clin Trials. 2021;104:106356. doi: 10.1016/j.cct.2021.106356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peruzzu D, Pagano MT, Pierdominici M, Ruggieri A, Antinori A, et al. Synergy between vitamin D and sex hormones in respiratory functionality of patients affected by COVID-19. Front Pharmacol. 2021;12:683529. doi: 10.3389/fphar.2021.683529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Svartberg J, Schirmer H, Medbø A, Melbye H, Aasebø U. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromsøstudy. Eur J Epidemiol. 2007;22:107–12. doi: 10.1007/s10654-006-9095-9. [DOI] [PubMed] [Google Scholar]
- 80.Mousavi SA, Kouchari MR, Samdani-Fard SH, Gilvaee ZN, Arabi M. Relationship between serum levels of testosterone and the severity of chronic obstructive pulmonary disease. Tanaffos. 2012;11:32–5. [PMC free article] [PubMed] [Google Scholar]
- 81.Mohan SS, Knuiman MW, Divitini ML, James AL, Musk AW, et al. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin Endocrinol (Oxf) 2015;83:268–76. doi: 10.1111/cen.12738. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–36. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–73. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 84.Chen DY, See LC, Liu JR, Chuang CK, Pang ST, et al. Risk of cardiovascular ischemic events after surgical castration and gonadotropin-releasing hormone agonist therapy for prostate cancer:a nationwide cohort study. J Clin Oncol. 2017;35:3697–705. doi: 10.1200/JCO.2016.71.4204. [DOI] [PubMed] [Google Scholar]
- 85.Teoh JY, Chan SY, Chiu PK, Poon DM, Cheung HY, et al. Risk of cardiovascular thrombotic events after surgical castration versus gonadotropin-releasing hormone agonists in Chinese men with prostate cancer. Asian J Androl. 2015;17:493–6. doi: 10.4103/1008-682X.143313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denmeade SR, Isaacs JT. Bipolar androgen therapy:the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate. 2010;70:1600–7. doi: 10.1002/pros.21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide:an open-label, phase 2, multicohort study. Lancet Oncol. 2018;19:76–86. doi: 10.1016/S1470-2045(17)30906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong X, Qiu S, Yi X, Xu H, Lei H, et al. Efficacy and safety of bipolar androgen therapy in mCRPC after progression on abiraterone or enzalutamide:a systematic review. Urol Oncol. 2022;40:4.e19–28. doi: 10.1016/j.urolonc.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 89.Ceban F, Ling S, Lui L, Lee Y, Gill H, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome:a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–64. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 91.Ferro M, Di Lorenzo G, de Cobelli O, Bruzzese D, Pignataro P, et al. Incidence of fatigue and low-dose corticosteroid use in prostate cancer patients receiving systemic treatment:a meta-analysis of randomized controlled trials. World J Urol. 2019;37:1049–59. doi: 10.1007/s00345-018-2579-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The review authors’ judgments based on ROBINS-I checklists about risk of bias for each meta-analyses, including (a) SARS-CoV-2 positive risk, (b) rate of COVID-19 hospitalization, (c) rate of COVID-19 ICU admission, and (d) death risk of COVID-19. The different colors represent various of evaluations for each methodological quality items. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; ICU: intensive care unit.
Summary of the connections between diverse SARS-CoV-2–related immune cells in the microenvironment and inflammatory cytokines. Authorized illustrations came from the Smart Medical Art (https://smart.servier.com). AEC: airway epithelial cell; IL: interleukin; TNF-α: tumor necrosis factor-α; TGF-β: transforming growth factor-β; IFN-γ: interferon-γ; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.


