Abstract
Transmasculine individuals, considering whether to undergo total hysterectomy with bilateral salpingectomy, have the option to have a concomitant oophorectomy. While studies have evaluated hormone changes following testosterone therapy initiation, most of those patients have not undergone oophorectomy. Data are currently limited to support health outcomes regarding the decision to retain or remove the ovaries. We performed a retrospective chart review of transmasculine patients maintained on high-dose testosterone therapy at a single endocrine clinic in Vancouver, British Columbia, Canada. Twelve transmasculine individuals who underwent bilateral oophorectomy and had presurgical and postsurgical serum data were included. We identified 12 transmasculine subjects as controls, who were on testosterone therapy and did not undergo oophorectomy, but additionally matched to the first group by age, testosterone dosing regimen, and body mass index. There was a statistically significant decrease in the estradiol levels of case subjects postoophorectomy, when compared to presurgical estradiol levels (P = 0.02). There was no significant difference between baseline estradiol levels between control and case subjects; however, the difference in estradiol levels at follow-up measurements was significant (P = 0.03). Total testosterone levels did not differ between control and case subjects at baseline and follow-up (both P > 0.05). Our results demonstrate that oophorectomy further attenuates estradiol levels below what is achieved by high-dose exogenous testosterone alone. Correlated clinical outcomes, such as impacts on bone health, were not available. The clinical implications of oophorectomy versus ovarian retention on endocrinological and overall health outcomes are currently limited.
Keywords: estradiol, gender-affirming surgery, hysterectomy, oophorectomy, testosterone therapy, transmasculine
INTRODUCTION
There is great variation in the social, hormonal, or surgical interventions that best suit the goals of transgender and gender-diverse individuals in their gender-affirming care.1 Transmasculine (TM) individuals, who are assigned female at birth, may choose to begin testosterone (T) hormone therapy to suppress menses, promote virilization, and help alleviate other aspects of gender incongruence.2 Gynecological gender-affirming surgery can further assist in one’s transition by removing internal reproductive organs. For individuals considering whether to undergo total hysterectomy with bilateral salpingectomy (TH/BS), there is also a decision to undergo a concomitant bilateral oophorectomy (i.e., TH/BS with oophorectomy [TH/BSO]).3,4 Currently, there are insufficient data to support health-care providers and patients in the decision-making process for ovarian retention or removal.
One important consideration in elected TH/BS with or without oophorectomy is the impact on endocrine functions as the ovaries are the main source of endogenous sex hormones in the TM population and a significant source of aromatase activity. Individuals who initiate gender-affirming T therapy experience dose-dependent changes in serum 17β-estradiol (E2) levels. Studies of TM patients with ovaries illustrate that E2 levels decrease following the initiation of T therapy, eventually reaching a steady state.5,6,7 The aromatization of T to E2 primarily occurs in the gonadal and adipose tissues, which decreases serum total T and increases serum E2 levels.7,8 However, low-dose T therapy does not inhibit the hypothalamic–pituitary–gonadal axis adequately to attenuate endogenous E2 production in individuals without gonadectomy.9,10 Neither low nor high serum E2 levels are likely indicators that individuals are able to achieve secondary amenorrhea with T therapy alone.11 As such, additional hormonal therapy for menstrual suppression or surgical intervention with TH/BSO is required for adequate management. The influence of oophorectomy itself on hormone levels has not been well investigated; studies evaluating hormone changes following T initiations have mostly focused on individuals who have not yet undergone an oophorectomy. Only one study includes a subgroup of transmasculine individuals who underwent TH/BSO before and after T initiation, with serum E2 levels reported up to 36 months after T initiation.7
One relevant clinical outcome, when TM individuals were assessed pre- and postoophorectomy, has been evaluated in the context of bone health.12,13 Lumbar spine bone mineral density (BMD) evaluated that postoophorectomy was found to decrease compared to that in preoophorectomy, despite TM individuals being maintained on high-dose T therapy. While serum E2 levels have not been directly associated with bone health or fracture outcomes in TM patients, this hormone plays an important role in bone maintenance. When conversion of serum T to E2 is impeded with the use of oral aromatase inhibitors, serum markers of bone metabolism were increased in TM individuals on T therapy.14 In cisgender men, serum E2 may play a role to protect BMD and serve as an important regulator of bone metabolism.15 In these patients, serum E2 levels lower than 37 pmol l−1 were insufficient to prevent bone resorption.15 Though the effects of E2 on BMD and bone health are difficult to independently assess from the actions of T, it is reasonable to evaluate serum hormone changes following oophorectomy as factors supporting bone health.
Previous studies have not specifically addressed the question whether serum E2 levels are significantly altered in TM patients postoophorectomy, when compared to their presurgical levels. Savkovic et al.16 found that 8 trans men who underwent oophorectomy had lower serum E2 levels compared to nonsurgical trans men. However, this study did not include presurgical levels for comparison. Chan et al.6 noted in their retrospective study that TM individuals that retained their ovaries had slightly elevated serum E2 levels, but they presented no quantitative data. Thus, our primary objective here is to evaluate if serum E2 and total T levels change in TM individuals after bilateral oophorectomy compared to preoophorectomy. A secondary objective of this study is to determine if there are changes in serum total T and E2 levels over time in TM individuals maintained on T therapy and who do not undergo oophorectomy. We hypothesize that serum E2 levels decrease following oophorectomy when compared to the presurgical levels, without an increase in serum total T levels if T dosing is maintained.
PATIENTS AND METHODS
A retrospective chart review was performed of all TM and gender-diverse persons (≥18 years old), who were assigned female at birth, on testosterone therapy, and were being followed at Dr. Dahl’s outpatient endocrinology clinic in Vancouver, British Columbia, Canada, between January 1, 2012, and July 31, 2020. Electronic medical records (EMR) were reviewed to identify candidates who received T therapy as part of their endocrine care. Study procedures, consent, and access to patient records were approved by the Clinical Research Ethics Board of the University of British Columbia (Vancouver, British Columbia, Canada; H20-02363).
Patients were included in the study if they (1) underwent bilateral oophorectomy after a minimum of 12 months of T therapy and (2) had serum data collected in the 12 months preceding and 24 months after the surgery. Subjects were excluded if they (1) were using an aromatase inhibitor to decrease E2 levels, (2) had ovarian surgery prior to T initiation, or (3) modified their T dosing regimen postoophorectomy. Each patient who underwent oophorectomy served as his/her own control pre- and postoophorectomy. Patients who had no laboratory measurements at baseline (n = 21), as well as those on aromatase inhibitors (n = 1), were excluded. All patients were using injectable T formulations of either testosterone cypionate or testosterone enanthate in dosages of 25 mg to 150 mg weekly, rather than a set formulation or dose for every patient. The protocol for hormone monitoring involved a requisition for blood drawn at a “midcycle” time point, which was often 4–8 days (dependent on the individual T dosing regimen) following self-injection. The serum samples were analyzed following the standard protocols of hospital and commercial laboratories using quantitative immunoassays or electrochemiluminescence. No patients included in the study had serum hormone levels that were reported below the detectable limits of the assays.17
Matched control subjects
From the pool of candidate patients, TM subjects on T therapy who did not undergo oophorectomy served as controls for E2 and T levels; they were matched by age, equivalent T dosing regimen, and calculated body mass index (BMI). These individuals were maintained on a T dosing regimen for at least 12 months, but none had an oophorectomy during the study period. Control subjects were eligible for inclusion if they had serum data collected at two time points within 24 months for a baseline and follow-up measurement.
Statistical analyses
Values were presented as mean ± standard error of the mean (s.e.m.). Individuals were used as their own controls in order to evaluate the change in serum hormone parameters over time using pairwise testing. Differences in serum hormone parameters within case or control groups were tested using the Wilcoxon matched-pairs signed rank test, when appropriate. The nonparametric multiple Mann–Whitney U test was used to test differences between the independent groups. Statistical analysis was performed with GraphPad Prism software version 9 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant.
RESULTS
There were 160 TM patients identified who were receiving exogenous T therapy. Of these patients, 45 (28.1%) had undergone bilateral oophorectomy and 115 (71.9%) did not undergo any removal of their internal reproductive organs. Only 12 patients of the 45 who underwent oophorectomy (26.7%) met all the inclusion criteria for this study (Figure 1). The most common reason for exclusion was the absence of preoophorectomy serum laboratory measurements. Twelve patients that did not undergo oophorectomy were identified as matched controls for each case subject (Table 1). In both the groups, the median weekly testosterone dose was 75 mg (Table 1).
Figure 1.
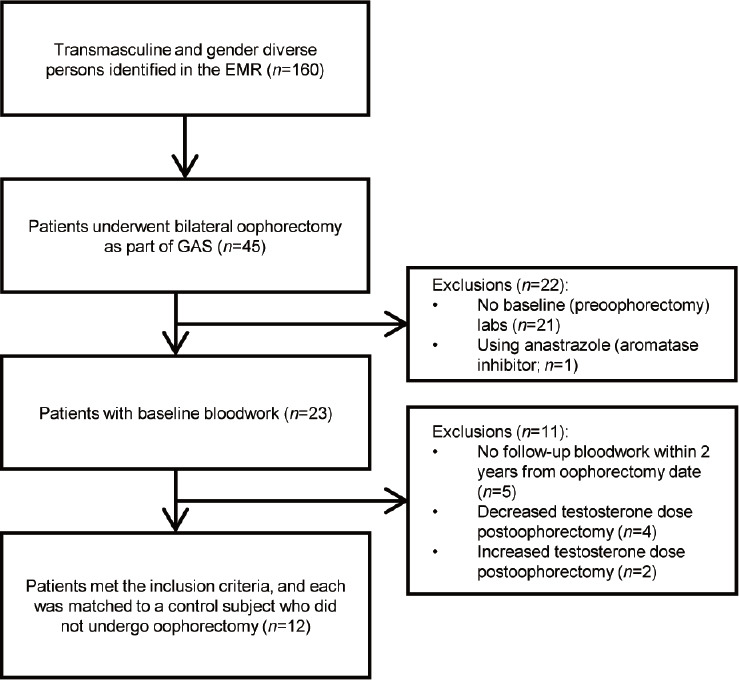
Flow diagram of study overview with inclusion and exclusion criteria. Twelve patients met the inclusion criteria and were matched to transmasculine and gender-diverse control subjects (assigned female at birth) who remained on testosterone therapy but did not undergo oophorectomy during the study period. The most common reason for exclusion included no preoophorectomy serum laboratory measurements available in the patient’s chart. EMR: electronic medical record; GAS: gender-affirming surgery.
Table 1.
Comparative characteristics of study case and control subjects
| Characteristic | Oophorectomy group (cases) | Nonoophorectomy group (controls) |
|---|---|---|
| Patient (n) | 12 | 12 |
| Age (year), mean±s.e.m. (range) | 32±3 (20–51) | 32±3 (21–51) |
| Time to follow-up (day), mean±s.e.m. | 316±55 | 302±27 |
| BMI (kg m−2), mean±s.e.m. | 27.2±6.0 | 27.2±7.8 |
| Weekly testosterone dose (mg), median (IQR) | 75 (50–75) | 75 (50–81.25) |
All P values were nonsignificant and >0.4 (oophorectomy group vs nonoophorectomy group). BMI: body mass index; IQR: interquartile range; s.e.m.: standard error of the mean
For individuals who underwent oophorectomy, the 12 subjects aged (mean ± s.e.m.) 32 ± 3 years at the time of surgery. There was a statistically significant decrease in the serum E2 levels within 2 years postoophorectomy when compared to presurgical serum E2 levels (Figure 2). The serum E2 level (mean ± s.e.m.) at baseline (preoophorectomy) was 239.3 ± 49.0 pmol l−1, whereas the serum E2 level (mean ± s.e.m.) measured at a follow-up (postoophorectomy) was 133.6 ± 17.5 pmol l−1 (P = 0.02; Figure 2a). Serum total T levels were not significantly different postoophorectomy (P = 0.47; Figure 2b).
Figure 2.
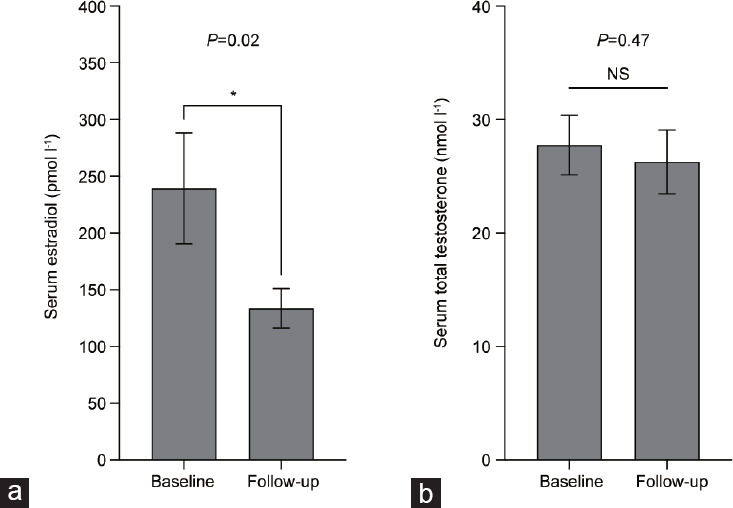
(a) Serum estradiol and (b) serum total testosterone levels of subjects who underwent oophorectomy. Investigations were performed at baseline and at a postoophorectomy follow-up visit within the first 2 years (n = 12). Serum estradiol level was significantly decreased at the follow-up period postoophorectomy. Subjects were maintained on the same injectable testosterone dose pre- and postoophorectomy. *P < 0.05. Data are presented as mean ± standard error of the mean. NS: not significant.
The age (mean ± s.e.m.) of the 12 control subjects was also 32 ± 3 years. The time on T therapy (mean ± s.e.m.) for control subjects was 707 ± 17 days. The time (mean ± s.e.m.) between the baseline and follow-up for case and control subjects was similar (316 ± 55 days vs 302 ± 27 days, P = 0.86; Table 1).
While the baseline serum E2 levels (mean ± s.e.m.) between cases and controls did not differ (239.3 ± 49.0 pmol l−1 vs 234.9 ± 53.3 pmol l−1, P = 0.54), the difference in serum E2 level (mean ± s.e.m.) between the case and control subjects at follow-up was significant (133.6 ± 17.5 pmol l−1 vs 290.1 ± 68.4 pmol l−1, P = 0.03; Figure 3a). There was no significant difference between baseline and follow-up serum E2 levels for control subjects (P = 0.34; Figure 3a). Serum total T levels were similar at baseline and follow-up measurements between case and control subjects (P = 0.50 at baseline, and P = 0.89 at follow-up; Figure 3b).
Figure 3.
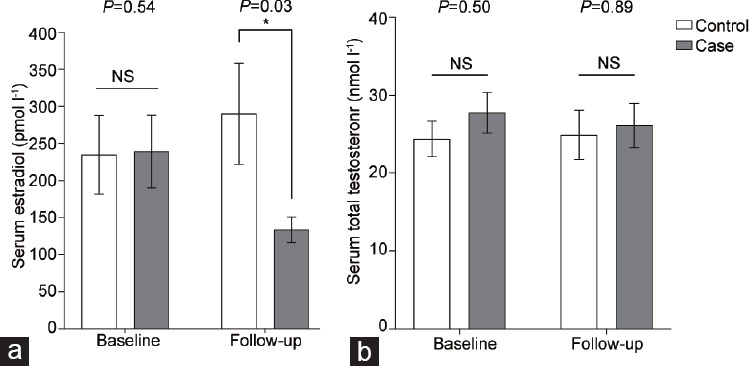
(a) Serum estradiol levels and (b) serum total testosterone levels investigated at baseline and follow-up visit for control (white bars) and case (gray bars) subjects (n=12 for each group). Control subjects did not undergo oophorectomy and were maintained on the same testosterone dose. Serum estradiol level was significantly decreased at the follow-up period postoophorectomy compared to transmasculine individuals who did not undergo oophorectomy (*P = 0.03). No significant difference was found between testosterone levels at baseline or follow-up. *P < 0.05. Data are presented as mean ± standard error of the mean. NS: not significant.
DISCUSSION
Our study suggests that serum E2 levels in TM patients decrease postoophorectomy, when compared to levels of this hormone preoophorectomy. No significant change in E2 was seen in the matched controls who did not undergo oophorectomy. Our results demonstrate that oophorectomy can attenuate serum E2 levels below what is achieved by high-dose exogenous T alone. However, our analysis revealed that oophorectomy was not associated with changes in serum total T levels, when exogenous T dosage was kept constant.
A greater understanding is needed for the risks and benefits of TM patients retaining versus removing their ovaries as part of their gender-affirming journey. Most patients on testosterone therapy at our center retain their internal reproductive organs or decide later to undergo TH/BS with or without oophorectomy as part of lower reconstructive surgeries. Their options might be limited for ovarian retention, as this reflects a dated clinical practice focused on a homogeneous surgical pathway for transgender and gender-diverse patients. Increasingly though, there has been both diversification of treatment options and a move to encourage patient engagement in decisions about their treatment. TM satisfaction with their treatment depends on the patients being effectively counseled on the hormonal outcomes they may experience from ovarian retention versus removal.
Few publications have evaluated serum E2 and serum total T levels in TM individuals who have undergone TH/BSO.6,7,18 Only one previous study evaluated serum E2 in TM individuals with or without TH/BSO, who had initiated T therapy.7 The authors reported that there was an observed difference in serum E2 levels during the 1st year of T therapy, but this normalized to the levels of those without TH/BSO within 18 months. T dosing, however, may have been adjusted over this time period making it difficult to compare those results to ours. Furthermore, our study included individuals who were maintained on T for a longer duration before TH/BSO and were followed up for 2 years postoophorectomy at the same T dosage. Our results did not demonstrate a normalizing trend in E2 after TH/BSO.
The optimal range for E2 in TM individuals is unknown. Currently, the Endocrine Society treatment guidelines do not include recommendations for serum E2 concentration in TM individuals.1 The serum E2 level of cisgender females after surgical menopause can fall as low as 36.7 pmol l−1 (10 pg ml−1) and are similar to the levels observed in individuals after normal menopause.19 Target serum E2 levels for cisgender females on oral estradiol replacement therapy have been reported in the literature to range from 110 pmol l−1 to 404 pmol l−1 (30 pg ml−1 to 110 pg ml−1) on a standard daily dose.20 Moreover, the typical laboratory reference range for serum E2 in cisgender eugonadal males is 36.7–147 pmol l−1 (10–40 pg ml−1).21 In our study, the mean serum E2 level postoophorectomy was 133.6 pmol l−1 (range: 40–231 pmol l−1). The clinical implication of the reduction in serum E2 postoophorectomy warrants further research. It is interesting to note, however, that our patients’ postoophorectomy serum E2 level was substantially higher than what has been reported for cisgender females with surgical or natural menopause and consistent with targets for cisgender females on exogenous E2 supplements.
BMD is supported by the action of sex hormones, either directly by T or by E2 derived from T via aromatization.1,14 There is conflicting evidence published over the last few decades, though, regarding BMD after TH/BSO in the TM population, where density has been reported to either decrease, increase, or remain the same.12,13,22,23 Regrettably, our patients did not undergo BMD scans pre- or postoophorectomy. Such data would have supported a clinical outcome for bone health and its correlation to sex hormone levels in TM individuals undergoing TH/BSO versus ovarian retention.
There is a common belief that oophorectomy may allow for decreased exogenous testosterone dosing in TM individuals wishing to establish T levels within the cisgender male reference range from 8.4 nmol l−1 to 28.8 nmol l−1. In our cohort, 17 out of 23 individuals who underwent oophorectomy maintained their presurgical T dosage (Figure 1). Only 6 patients changed their T dosing regimens: 4 decreased and 2 increased it. Similar to our result, Rachlin et al.24 found that 44% (50 out of 114) of respondents in their survey maintained their presurgery T dose postoophorectomy, and 23% increased their dosage. Individuals in that study who decreased their dose (25%) cited concerns about increased scalp hair loss, unstable mood, significantly altered lipid profile, and possible medication-induced liver damage.24 Others reported just following a health-care provider’s recommendation. Our results did not support a consistent decrease in testosterone dosing postoophorectomy and there is little evidence that most individuals make such a change postsurgically.
While most of our case subjects showed a decreasing trend in their E2 levels at follow-up, three individuals had a slightly higher serum E2 levels when compared to baseline. In these three subjects, their serum total T measurements were also higher, when compared to their baseline measurements. While these individuals’ prescribed T dose did not change, there could be inherent variability in the data due to variation in patients’ self-administration of T and precisely when they had their blood drawn. Individuals who had their blood drawn either too early or past their midcycle point may have artifactually elevated or reduced total T levels, respectively.
Limitations
Limitations of this study include the retrospective study design, which restricted our ability to capture laboratory measurements for all potential candidates and to include additional investigations. Moreover, the study had a small-sized cohort as many patients did not have follow-up bloodwork available. The small sample size substantially limited the power for statistical testing. While few statistical comparisons were made, the type I error rate was not corrected, and significance was reported with α = 0.05. Future research with a larger sample size that is sufficiently powered would support the use of Bonferroni corrections for multiple statistical tests.
The study design did not attempt to capture other serum hormone markers, such as sex hormone-binding globulin (SHBG) and free T, which are not normally assessed in routine bloodwork. Data on luteinizing hormone, follicle-stimulating hormone, SHBG, and free T were not uniformly available. Such data may have helped reveal factors in the regulation of E2 and T levels in the study population.
No patients had Dual-energy X-ray absorptiometry (DEXA) scans to evaluate changes of BMD. Moreover, we did not have data for our cohort on levels of smoking and alcohol use, which may affect the metabolism of E2.25
Our data had heterogeneity due to variability in when patients had their blood drawn after self-injection, and in processing those blood samples. Preprinted requisition forms were not used in the clinic; rather, tests were manually selected or entered. Three subjects’ laboratory panel did not include serum E2, and thus no data were reported for these cases. Add-on tests were not available in certain instances. Sample collection sites included in-hospital and commercial labs, which used varying assays to determine serum hormone levels, limiting data precision.
We recognized how medical records can be incomplete or lacking with respect to endocrinological management for the trans population. More baseline and long-term endocrinological data should be collected on trans and gender-diverse individuals, so that future studies are not hampered by the lack of data, potentially relevant to the patients’ long-term health.
CONCLUSIONS
Serum E2 levels decrease postoophorectomy when compared to preoophorectomy levels for patients maintained at the same T dose. This decrease in E2 level was statistically significant in our study when compared to individuals who did not undergo oophorectomy over a similar follow-up time. Despite the decrease, serum E2 concentration remained much higher than what would be expected for a postmenopausal cisgender female and consistent with targets for serum E2 with exogenous supplementation in this population. Only a small minority of individuals were found to adjust their T dosing following oophorectomy. More research is warranted on serum E2 in the TM population to determine the implications of oophorectomy on how gender-affirming surgery may impact TM endocrinological and overall health.
AUTHOR CONTRIBUTIONS
SK carried out the methodology, investigation and visualization of data, writing, and editing. EB involved in the methodology and writing. CO, AK, and RW participated in drafting the manuscript. AGK, KG, NM, and MD helped conceptualize the project. SM and AGK supervised the project. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 2.Dahl M, Feldman JL, Goldberg JM, Jaberi A. Physical aspects of transgender endocrine therapy. Int J Transgenderism. 2006;9:111–34. [Google Scholar]
- 3.Reilly ZP, Fruhauf TF, Martin SJ. Barriers to evidence-based transgender care: knowledge gaps in gender-affirming hysterectomy and oophorectomy. Obstet Gynecol. 2019;134:714–7. doi: 10.1097/AOG.0000000000003472. [DOI] [PubMed] [Google Scholar]
- 4.Grimstad F, Boskey ER, Taghinia A, Ganor O. Gender-affirming surgeries in transgender and gender diverse adolescent and young adults: a pediatric and adolescent gynecology primer. J Pediatr Adolesc Gynecol. 2021;34:442–8. doi: 10.1016/j.jpag.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol. 2015;125:605–10. doi: 10.1097/AOG.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan KJ, Jolly D, Liang JJ, Weinand JD, Safer JD. Estrogen levels do not rise with testosterone treatment for transgender men. Endocr Pract. 2018;24:329–33. doi: 10.4158/EP-2017-0203. [DOI] [PubMed] [Google Scholar]
- 7.Defreyne J, Aers XP, Collet SM, Wiepjes CM, Fisher AD, et al. Lower serum estradiol levels in assigned female at birth transgender people with initiation of testosterone therapy: results from the European Network for the investigation of gender incongruence. LGBT Health. 2020;7:71–81. doi: 10.1089/lgbt.2019.0260. [DOI] [PubMed] [Google Scholar]
- 8.Cirrincione LR, Huang KJ. Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin Pharmacol Ther. 2021;110:897–908. doi: 10.1002/cpt.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub RL, Ellis SA, Neal-Perry G, Magaret AS, Prager SW, et al. The effect of testosterone on ovulatory function in transmasculine individuals. Am J Obstet Gynecol. 2020;223:229.e1–8. doi: 10.1016/j.ajog.2020.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defreyne J, Vanwonterghem Y, Collet S, Iwamoto SJ, Wiepjes CM, et al. Vaginal bleeding and spotting in transgender men after initiation of testosterone therapy:a prospective cohort study (ENIGI) Int J Transgend Health. 2020;21:163–75. doi: 10.1080/26895269.2020.1719951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimstad F, Kremen J, Shim J, Charlton BM, Boskey ER. Breakthrough bleeding in transgender and gender diverse adolescents and young adults on long-term testosterone. J Pediatr Adolesc Gynecol. 2021;34:706–16. doi: 10.1016/j.jpag.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 12.van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf) 1998;48:347–54. doi: 10.1046/j.1365-2265.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 13.Goh HH, Ratnam SS. Effects of hormone deficiency, androgen therapy and calcium supplementation on bone mineral density in female transsexuals. Maturitas. 1997;26:45–52. doi: 10.1016/s0378-5122(96)01073-0. [DOI] [PubMed] [Google Scholar]
- 14.Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, et al. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med. 2008;5:2442–53. doi: 10.1111/j.1743-6109.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126:1114–25. doi: 10.1172/JCI84137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savkovic S, Lim S, Jayadev V, Conway A, Turner L, et al. Urine and serum sex steroid profile in testosterone-treated transgender and hypogonadal and healthy control men. J Clin Endocrinol Metab. 2018;103:2277–83. doi: 10.1210/jc.2018-00054. [DOI] [PubMed] [Google Scholar]
- 17.Handelsman DJ, Ly LP. An accurate substitution method to minimize left censoring bias in serum steroid measurements. Endocrinology. 2019;160:2395–400. doi: 10.1210/en.2019-00340. [DOI] [PubMed] [Google Scholar]
- 18.Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9:2641–51. doi: 10.1111/j.1743-6109.2012.02876.x. [DOI] [PubMed] [Google Scholar]
- 19.Judd HL, Judd GE, Lucas WE, Yen SS. Endocrine function of the postmenopausal ovary:concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020–4. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, Kim SE, Lee DY, Choi D. Serum estradiol level according to dose and formulation of oral estrogens in postmenopausal women. Sci Rep. 2021;11:3585. doi: 10.1038/s41598-021-81201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadid S, Barber JR, Rohrmann S, Nelson WG, Yager JD, et al. Age-specific serum total and free estradiol concentrations in healthy men in US nationally representative samples. J Endocr Soc. 2019;3:1825–36. doi: 10.1210/js.2019-00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, et al. Body composition, bone turnover, and bone mass in trans men during testosterone treatment:1-year follow-up data from a prospective case-controlled study (ENIGI) Eur J Endocrinol. 2015;172:163–71. doi: 10.1530/EJE-14-0586. [DOI] [PubMed] [Google Scholar]
- 23.Miyajima T, Kim YT, Oda H. A study of changes in bone metabolism in cases of gender identity disorder. J Bone Miner Metab. 2012;30:468–73. doi: 10.1007/s00774-011-0342-0. [DOI] [PubMed] [Google Scholar]
- 24.Rachlin K, Hansbury G, Pardo ST. Hysterectomy and oophorectomy experiences of female-to-male transgender individuals. Int J Transgend Health. 2010;12:155–66. [Google Scholar]
- 25.Mueck AO, Seeger H. Smoking, estradiol metabolism and hormone replacement therapy. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:45–54. doi: 10.2174/1568016052773270. [DOI] [PubMed] [Google Scholar]


