Abstract
Sleep has attracted extensive attention due to its significance in health. However, its association with erectile dysfunction (ED) is insufficiently investigated. To investigate the potential causal links between sleep traits (insomnia, sleep duration, and chronotype) and ED, this study was performed. The single-nucleotide polymorphisms (SNPs) associated with insomnia, sleep duration, and chronotype were retrieved from previous genome-wide association studies (GWAS). A conventional two-sample Mendelian randomization (MR) was used to estimate the causal links between sleep traits and ED. The summary statistics of ED were from individuals of European ancestry (6175 cases vs 217 630 controls). As shown by the random effect inverse-variance-weighting (IVW) estimator, genetically predicted insomnia was causally associated with a 1.15-fold risk of ED (95% confidence interval: 1.07–1.23, P < 0.001). Sleep duration and morningness were not causally associated with ED, as indicated by the IVW (all P > 0.05). These findings were consistent with the results of sensitivity analyses. Based on genetic data, this study provides causal evidence that genetically predicted insomnia increases the risk of ED, whereas sleep duration and chronotype do not.
Keywords: causal association, chronotype, erectile dysfunction, insomnia, Mendelian randomization, sleep duration
INTRODUCTION
Erectile dysfunction (ED) is defined as the inability to attain or maintain a penile erection sufficient for successful vaginal intercourse.1 According to the investigation by Nguyen et al.,2 the prevalence of ED in the young is up to 30%. This figure is higher in the aged and patients with diabetes and end-stage renal disease.3,4,5 ED exerts substantial adverse effects on patients, leading to poor mental state and quality of life. It was observed by Liu et al.6 that ED patients had a 2.92-fold risk of depression than their non-ED counterparts. Measures should be taken to suppress the high incidence of ED.
Previous studies have reported several risk factors of ED, including depression, anxiety, and chronic kidney disease.7,8 The connection between sleep and ED has been gradually noted with the deepening understanding of the role of sleep in heath.9 Cross-sectional surveys have reported a positive association between ED and obstructive sleep apnea (OSA), shift work, and insomnia.10,11 Xiong et al.10 disclosed that OSA can causally increase the risk of ED. Further randomized control trial showed that the adherent continuous positive airway pressure (CPAP) therapy could improve the sleep-related erectile function in OSA patients, possibly via attenuating OSA-induced hypoxia.12,13 Another cross-sectional study enrolling 754 males reported that male night shift workers had lower International Index of Erectile Function (IIEF) scores than male non-night shift workers.11 Additionally, according to Seehuus and Pigeon,14 insomnia severity was significantly associated with sexual dysfunction and sleep disorders may function in the occurrence of ED. However, Seftel et al.15 reported that there was no correlation between insomnia and ED. The inconsistency may be attributed to the confounding derived from the cross-sectional or retrospective design and limited sample size, which impede the yielding of unbiased causal estimates. In addition, it has been observed that insomnia and OSA often co-exist. The effect of insomnia alone is hard to be assessed with the observational design.16 The association between sleep disorder and ED remains unclear and requires further study.
As of now, few studies have comprehensively examined the causal association between ED and sleep traits, including insomnia, sleep duration, and chronotype. Due to the defects of observational design, Mendelian randomization (MR) is selected to overcome the endogeneity. MR is a method using single nucleotide polymorphisms (SNPs) as genetic instruments to examine the causal effects of exposures (i.e., insomnia, sleep duration, and chronotype) on the outcomes (i.e., ED).17 SNPs are assorted randomly during gestation, which helps to avoid reversed causality and confounding.17 Therefore, a natural randomized controlled trial (RCT) is mimicked using MR. In this study, we used the data from the Integrative Epidemiology Unit (IEU) Open genome-wide association studies (GWAS) database (https://gwas.mrcieu.ac.uk/; last accessed on March 18, 2022) to evaluate the causal association between sleep traits (insomnia, sleep duration, and chronotype) and ED. The results may facilitate further clinical intervention.
MATERIALS AND METHODS
Data sources and study samples
Four summary-level datasets regarding insomnia, sleep duration, chronotype, and ED were extracted from four cohorts.18,19,20,21 Detailed data sources and relevant information are displayed in Supplementary Table 1.
The GWAS summary dataset for insomnia was extracted from the UK Biobank (UKB) version 2 (n = 386 533) and 23andMe project (n = 944 477).18 By combining the two cohorts, 1 331 010 participants were enrolled for further analyses (397 454 cases and 933 556 controls). The diagnosis of insomnia was based on questionnaires evaluating sleep status. In the UKB cohort, insomnia cases were participants with a sleep disorder making it difficult to fall asleep at night and get back to sleep after waking up at midnight. In the 23andMe cohort, participants who admitted any phenotypic concepts regarding inferior sleep status were considered insomnia cases. Specific information regarding the phenotype and quality control process can be accessed in the previous study.18
The genetic association estimates for sleep duration in participants of European ancestry were retrieved from the UKB (n = 446 118).19 Sleep duration was recorded according to the self-report of the respondents and was then categorized into three groups (short sleep duration: ≤6 h; normal sleep duration: 7–8 h; and long sleep duration: ≥9 h). Respondents with extremely short or long sleep duration (<3 h and >18 h) and who had any sleep medication were excluded. In this GWAS, 78 loci associated with self-reported habitual sleep duration were identified.19
Another GWAS combined the participants from the UKB (n = 449 734) and the 23andMe cohort (n = 248 098) to investigate the genetic loci associated with being a morning person.20 In the UKB cohort, participants were divided into six groups: definitely morningness, more morningness-oriented, do not know, more eveningness-oriented, definitely eveningness, and all, according to their self-reports. As for the 23andMe cohort, subjects were asked, “Are you naturally a night person or a morning person?” and then they were labeled according to their answers. Participants giving a discordant or neutral answer were excluded as described by Jones et al.20
Summary-level statistics of ED in participants of European ancestry (6175 cases vs 217 630 controls) were derived from three cohorts: UKB, the Estonian Genome Center of the University of Tartu (EGCUT) cohort, and hospital-recruited Partners HealthCare Biobank (PHB) cohort.21 The diagnosis of ED was based on the codes of International Classification of Diseases version 10 (N48.4 and F52.2), oral medication history (e.g., sildenafil), surgical intervention, or self-report of the respondents. The prevalence of ED was 25.4%, 7.0%, and 1.5% in the PHB, EGCUT, and UKB, respectively.
Ethical review and approval were waived for this study, all the data from MR are publicly accessible (https://gwas.mrcieu.ac.uk/; last accessed on March 18, 2022). Informed consent was obtained from all subjects in the original genome-wide association studies.
Genetic instrument selection
All the genetic instruments associated with insomnia, sleep duration, long sleep duration (≥9 h), short sleep duration (≤6 h), and morningness reached a significance level at a genome-wide statistical threshold of P < 5 × 10−8. Further, the linkage disequilibrium (LD) of selected SNPs was calculated to identify independent SNPs (LD r2 < 0.01 at a window size of 10 000 Kb) and exclude dependent SNPs using the PLINK clumping approach. Additionally, Steiger-MR was employed to affirm that the SNPs explained significantly more variance in exposures than in outcome (the opposite may indicate reverse causation).22 The insignificant SNPs were removed to avoid the violation of MR assumptions. Further, radial-MR and MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) approaches were adopted to explore the outlier SNPs accounting for the possible pleiotropy.23 If the outlier SNPs were identified, they were excluded to reduce the heterogeneity and horizontal pleiotropy. Finally, a total of 196, 68, 5, 22, and 293 qualified SNPs of insomnia, sleep duration, long sleep duration, short sleep duration, and morningness, respectively, were used as genetic instruments, as shown in Supplementary Table 2–6.
Statistical analyses
The random effect and fixed effect inverse-variance-weighting (IVW) methods were used to test the causal links between genetically predicted sleep traits and ED. The IVW method assumes that all the SNPs in the MR analysis are valid and then combine the Wald ratio of each SNP into an overall weighted effect.24 Results obtained from IVW method were deemed as the main findings.25
Further, we used four other approaches to perform sensitivity analyses, including MR robust adjusted profile score (MR.RAPS), MR-PRESSO, weighted median, and MR-Egger. MR.RAPS can yield robust causal estimates by modeling and then assuming the normal distribution of pleiotropic effects.26 The results produced by MR.RAPS remain robust when weak genetic instruments and systematic and idiosyncratic pleiotropy exist. MR-PRESSO was applied in this study to detect the outlier SNPs, which were then excluded for horizontal pleiotropy.23 Therefore, a more robust estimate was produced by the MR-PRESSO estimator. By combining data on multiple instruments into one single causal estimate, the weighted median estimator can produce consistent results even when 50% of instruments are invalid.27 The MR-Egger approach is adapted from Egger regression by introducing an intercept term into the regression model.28 It can be used to detect directional pleiotropy and yield valid causal effect estimates even when all the instruments are invalid.28
The intercept of MR-Egger regression and MR-PRESSO approaches were used to confirm the absence of pleiotropy. The Cochran’s Q test was used to evaluate the heterogeneity between the genetic instruments. The leave-one-out analysis was employed to verify that no influential SNPs existed in the sleep traits-ED causal links, indicating the robustness of the casual estimation. The strength of genetic instruments was represented by F-statistics calculated using the following formula: F-statistics = (Beta/Se)2. Here, Beta represents the correlation coefficient between SNPs and traits (i.e., insomnia and ED), and Se represents the standard error. The mean of F-statistics was deemed as the overall statistics. F-statistics >10 was an indicator of statistical robustness.29
After Bonferroni correction (0.05/5), the threshold of statistical significance was set as P < 0.01 (two-sided). MR analyses were performed using the R software version 3.6.5 (http://www.R-project.org; The R Foundation, Vienna, Austria), with the “TwoSampleMR”, “RadialMR”, and “mr.raps” packages.
RESULTS
Causal effects of genetically predicted insomnia on ED
The results obtained from the random effect IVW method are shown in Figure 1a. It can be found that genetically predicted insomnia leads to a 1.15-fold risk of ED (95% confident interval [CI] = 1.07–1.23, P < 0.001). The scatter plot in Figure 1b discloses that with the increase of the SNP effect on insomnia, the SNP effect on ED is intensified. The causal association between insomnia and ED still exist (fixed effect IVW: odds ratio [OR] = 1.15, P < 0.001; MR.RAPS: OR = 1.12, P < 0.001; and MR-PRESSO: OR = 1.12, P < 0.001; Figure 2).
Figure 1.
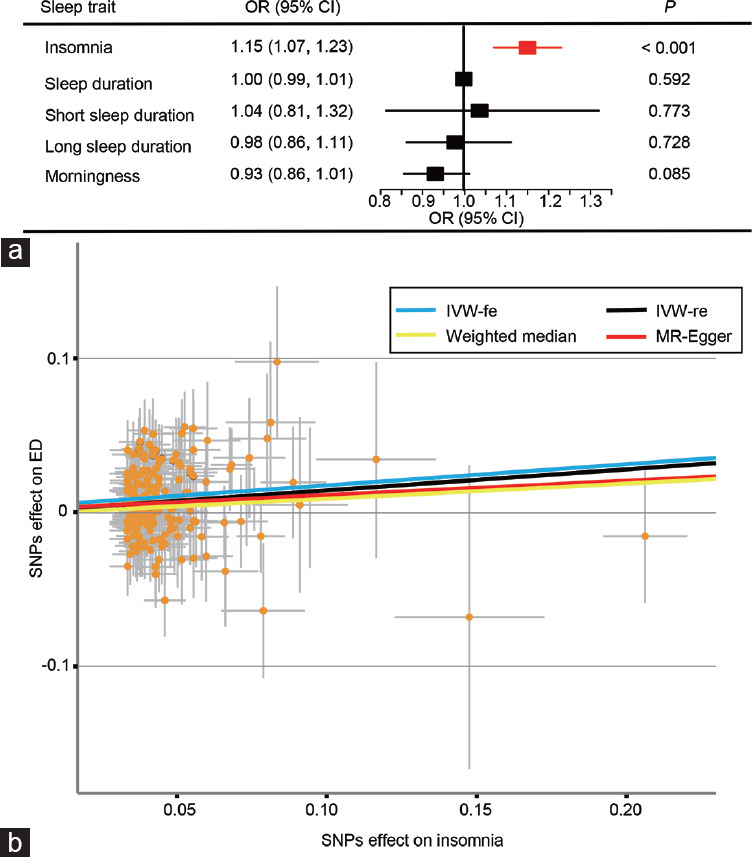
The causal effects of sleep traits on ED. (a) The causal links between sleep traits and ED results obtained by the random effect IVW. (b) The scatter plot of the effect size for each SNP on insomnia and ED. ED: erectile dysfunction; IVW: inverse-variance-weighting; IVW-fe: fixed effect inverse-variance-weighting; IVW-re: random effect inverse-variance-weighting; MR: Mendelian randomization; SNP: single nucleotide polymorphisms; OR: odds ratio; CI: confidence interval.
Figure 2.
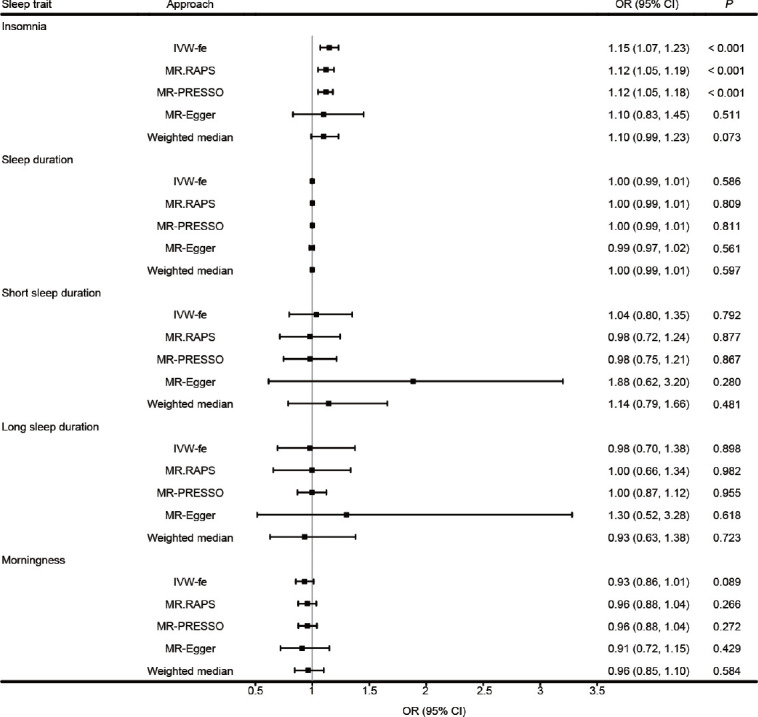
Sensitivity analysis for the causality of sleep traits with the risk of ED. ED: erectile dysfunction; IVW-fe: fixed effect inverse-variance-weighting; MR: Mendelian randomization; OR: odds ratio; CI: confidence interval; MR.RAPS: MR robust adjusted profile score; MR-PRESSO: MR Pleiotropy RESidual Sum and Outlier.
There are no signs of heterogeneity according to the results of Cochran’s Q tests shown in Table 1 (P > 0.05) and the funnel plot in Supplementary Figure 1. Additionally, as revealed in Table 1, the MR-Egger regression and MR-PRESSO global test indicate no pleiotropy (P > 0.05). Outliers are not found, either, verifying the absence of unknown pleiotropic effects of the genetic instruments.
Table 1.
Results of heterogeneity and pleiotropy tests
| Sleep trait | P value of MR-PRESSO | MR-Egger intercept | Q-df value by IVW | Q-df value by MR-Egger |
|---|---|---|---|---|
| Insomnia | 0.714 | 0.00198* | 195* | 194* |
| Sleep duration | 0.451 | 0.00475* | 67* | 66* |
| Short sleep duration | 0.979 | −0.02125* | 4* | 3* |
| Long sleep duration | 0.655 | −0.02150* | 21* | 20* |
| Morningness | 0.382 | 0.00070* | 292* | 291* |
*P>0.05. IVW: inverse-variance-weighting; MR: Mendelian randomization; MR-PRESSO: MR Pleiotropy RESidual Sum and Outlier; df: degree of freedom
The leave-one-out analysis in Supplementary Figure 2 shows no influential SNP in the insomnia-ED causal association. The results are still robust when excluding any one of the SNPs. The forest plot visualizing the estimates of the effect of each SNP on ED is displayed in Supplementary Figure 3.
Causal effect estimates of genetically predicted sleep duration and morningness on ED
As shown in Figure 1a, sleep duration, long sleep duration, short sleep duration, and morningness are not causally associated with ED (all P > 0.05). Figure 2 shows that, similar to random effect IVW, sensitivity analyses do not support the causal effect of sleep duration and morningness either (P > 0.05 in the fixed effect IVW, MR.RAPS, MR-PRESSO, MR-Egger, and the weighted median estimators). There are no signs of heterogeneity according to the results of Cochran’s Q tests shown in Table 1 (P > 0.05). The MR-Egger intercept and MR-PRESSO global test reveal neither pleiotropy (P > 0.05) nor outliers.
DISCUSSION
In this Mendelian randomization study, it is found that insomnia exerts adverse effects on ED while other sleep traits do not. These findings provide novel evidence supporting the causal effect of insomnia on ED. The MR framework overcomes the biases from confounding in observational studies.
Sleep disorder has received plenty of attention due to its significant effects on human health.30 However, analyses of the causal association between sleep disorder and ED are lacking. There are several different forms of sleep disorders, including OSA, insomnia, shift work sleep disorder (SWSD), etc. In a single-center cross-sectional study with 129 ED patients of European ancestry, 55% of participants were reported to have OSA and they had worse IIEF-ED scores than the non-OSA ED patients.31 Similar results were also found in Israel,32 Turkey,33 and China.34 However, OSA is closely associated with recurrent apneas and hypopneas when sleeping. Hypoxia may play a more core role in linking OSA to ED than sleep disorder.35 Besides OSA, SWSD, another form of sleep disorder, is also found to be negatively correlated with erectile function. In a cross-sectional study with 754 males, Rodriguez et al.11 reported that males working night shifts had worse erectile function than males working during the day or evening. These studies disclose the potential role of OSA and SWSD in the occurrence of ED. However, to date, no prospective study has been performed, and the robustness of the conclusion has not been proved, either.
The negative association between sleep disorders and erectile function is recorded in most but not all observational studies.36 In a cross-sectional survey enrolling 734 males aged >40 years, none of the sleep indices measured by polysomnography (PSG) were correlated with ED.36 Further subgroup analysis revealed a positive association between oxygen desaturation index and ED in men aged >65 years.36 This study presented an insignificant correlation between sleep disorder and ED, which may be attributed to the confounding. Hence, we used the MR design for causal estimates to avoid confounding. Accurate causal effect estimates of sleep traits on ED will facilitate timely clinical intervention.
Insomnia is also a major form of sleep disorder.37 The relationship between insomnia and ED is inconsistent in previous literature. In a cross-sectional study enrolling 1118 males with diabetes, insomnia was found to be a risk factor for ED.38 However, given the specificity of the population, diabetes may lead to insomnia and ED concurrently, making the results less convincing. Additionally, for Americans, insomnia measured by the Insomnia Severity Index (ISI) is reported to be inversely correlated with erectile function.14 However, in a self-report survey including 285 males, no correlation is detected between insomnia and ED.15 This finding is also supported by the report from Martin et al.39 In their study with 1195 randomly selected community-dwelling men, insomnia did not increase the risk of mild ED or moderate/severe ED. Given the poor sleep status in ED patients, it is not clarified whether insomnia causes ED or reversely, which is solved in the present study.
The specific biological mechanisms linking insomnia to ED remain unknown but are possibly associated with circadian rhythm and the hypothalamic–pituitary–gonadal (HPG) axis. According to the findings of Lee et al.,40 rats suffering acute sleep deprivation (SD) had lower levels of luteinizing hormone and testosterone and a higher level of cortisol than the controls. In addition, the SD rats were found to have lower expression levels of endothelial nitric oxide synthase (NOS)/neuronal NOS mRNAs and proteins in the cavernosal tissues than the control group, indicating worse erectile function.40 HPG may function in linking insomnia to ED. The role of the circadian clock has also been noted in the occurrence of urological diseases and may also be one possible pathway.41 It was found by Flynn-Evans et al.42 that the circadian rhythm of insomnia patients was different from that of healthy controls. The disrupted circadian clock may induce hypoxia and activate HPG to impair erectile function, consequently leading to ED.43 We will perform further studies regarding the role of HPG and the circadian clock in the occurrence of ED in the future.
This study has some merits and limitations. The major strength is the MR design, which avoids residual confounding and other biases, and then yields stronger causal inferences between sleep traits and ED. Additionally, a broader range of sleep traits is included, while only one trait is explored in most previous studies. Moreover, the participants are all of the European ancestry, reducing population architecture bias. However, with the rigorous racial restriction, the generalizability of the conclusions is sacrificed. Further verification in the population of other ancestries is still required. In addition, none of the authors are involved in the creation of the original datasets analyzed in this study. Therefore, only summary statistics are used and the nonlinear association between insomnia and ED cannot be evaluated in this study, which limits the full understanding of their interaction.
In conclusion, genetically predicted insomnia increases the risk of ED, whereas other sleep traits do not. This study demonstrates the causal link between insomnia and ED using the Mendelian randomization method and may guide clinical practice.
CONCLUSIONS
This study provides causal evidence that genetically predicted insomnia increases the risk of ED, whereas sleep duration and chronotype do not.
AUTHOR CONTRIBUTIONS
YX and JHY proposed the conception and design. YCZ, CJW, FQ, and JHY provided administrative support. YCZ, CJW, FQ, and JHY supplied the study materials. YX and FXZ collected, assessed, and analyzed the data. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (No. 81871147 and No. 82071639) and the Sichuan Science and Technology Program (No. 2022YFS0028 and No. 2022YFS0134).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Nguyen HM, Gabrielson AT, Hellstrom WJ. Erectile dysfunction in young men - a review of the prevalence and risk factors. Sex Med Rev. 2017;5:508–20. doi: 10.1016/j.sxmr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 3.McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12(Suppl 4):S6–11. doi: 10.1038/sj.ijir.3900567. [DOI] [PubMed] [Google Scholar]
- 4.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–47. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 5.Pyrgidis N, Mykoniatis I, Nigdelis MP, Kalyvianakis D, Memmos E, et al. Prevalence of erectile dysfunction in patients with end-stage renal disease:a systematic review and meta-analysis. J Sex Med. 2021;18:113–20. doi: 10.1016/j.jsxm.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Zhang Y, Wang J, Li S, Cheng Y, et al. Erectile dysfunction and depression:a systematic review and meta-analysis. italic> J Sex Med. 2018;15:1073–82. doi: 10.1016/j.jsxm.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Song Y, Lu Y, Xu Y, Liu L, et al. Associations between erectile dysfunction and psychological disorders (depression and anxiety):a cross-sectional study in a Chinese population. Andrologia. 2019;51:e13395. doi: 10.1111/and.13395. [DOI] [PubMed] [Google Scholar]
- 8.Pizzol D, Xiao T, Yang L, Demurtas J, McDermott D, et al. Prevalence of erectile dysfunction in patients with chronic kidney disease:a systematic review and meta-analysis. Int J Impot Res. 2021;33:508–15. doi: 10.1038/s41443-020-0295-8. [DOI] [PubMed] [Google Scholar]
- 9.Hale L, Troxel W, Buysse DJ. Sleep health:an opportunity for public health to address health equity. Annu Rev Public Health. 2020;41:81–99. doi: 10.1146/annurev-publhealth-040119-094412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Y, Zhong X, Zhang F, Wang W, Zhang Y, et al. Genetic evidence supporting a causal role of snoring in erectile dysfunction. Front Endocrinol. 2022;13:896369. doi: 10.3389/fendo.2022.896369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez KM, Kohn TP, Kohn JR, Sigalos JT, Kirby EW, et al. Shift work sleep disorder and night shift work significantly impair erectile function. J Sex Med. 2020;17:1687–93. doi: 10.1016/j.jsxm.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melehan KL, Hoyos CM, Hamilton GS, Wong KK, Yee BJ, et al. Randomized trial of CPAP and vardenafil on erectile and arterial function in men with obstructive sleep apnea and erectile dysfunction. J Clin Endocrinol Metab. 2018;103:1601–11. doi: 10.1210/jc.2017-02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, Shu Y, Liu X, Kong L, Li K, et al. The effects of CPAP treatment on resting-state network centrality in obstructive sleep apnea patients. Front Neurol. 2022;13:801121. doi: 10.3389/fneur.2022.801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seehuus M, Pigeon W. The sleep and sex survey:relationships between sexual function and sleep. J Psychosom Res. 2018;112:59–65. doi: 10.1016/j.jpsychores.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Seftel AD, Strohl KP, Loye TL, Bayard D, Kress J, et al. Erectile dysfunction and symptoms of sleep disorders. Sleep. 2002;25:643–7. [PubMed] [Google Scholar]
- 16.Ong JC, Crawford MR, Wallace DM. Sleep apnea and insomnia:emerging evidence for effective clinical management. Chest. 2021;159:2020–8. doi: 10.1016/j.chest.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GD, Ebrahim S. 'Mendelian randomization':can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 18.Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51:394–403. doi: 10.1038/s41588-018-0333-3. [DOI] [PubMed] [Google Scholar]
- 19.Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10:1100. doi: 10.1038/s41467-019-08917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10:343. doi: 10.1038/s41467-018-08259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bovijn J, Jackson L, Censin J, Chen CY, Laisk T, et al. GWAS identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am J Hum Genet. 2019;104:157–63. doi: 10.1016/j.ajhg.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Fan J, Chen L, Xiong Y, Wu T, et al. Causal association of coffee consumption and total, knee, hip and self-reported osteoarthritis:a Mendelian randomization study. Front Endocrinol (Lausanne) 2021;12:768529. doi: 10.3389/fendo.2021.768529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess S, Thompson SG. Boca Raton: CRC Press; 2015. Mendelian Randomization: Methods for Using Genetic Variants in 494 Causal Estimation; p. 495. [Google Scholar]
- 26.Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat. 2018;48:1742–69. [Google Scholar]
- 27.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments:effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Yang H, Li H, He C, Yang L, et al. Insights into modifiable risk factors of cholelithiasis:a Mendelian randomization study. Hepatology. 2021;75:785–96. doi: 10.1002/hep.32183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu PY. A clinical perspective of sleep and andrological health:assessment, treatment considerations, and future research. J Clin Endocrinol Metab. 2019;104:4398–417. doi: 10.1210/jc.2019-00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalejaiye O, Raheem AA, Moubasher A, Capece M, McNeillis S, et al. Sleep disorders in patients with erectile dysfunction. BJU Int. 2017;120:855–60. doi: 10.1111/bju.13961. [DOI] [PubMed] [Google Scholar]
- 32.Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D. Association between erectile dysfunction and sleep disorders measured by self-assessment questionnaires in adult men. J Sex Med. 2005;2:543–50. doi: 10.1111/j.1743-6109.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 33.Taken K, Ekin S, Arısoy A, Günes M, Dönmez Mİ. Erectile dysfunction is a marker for obstructive sleep apnea. Aging Male. 2016;19:102–5. doi: 10.3109/13685538.2015.1131259. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XB, Lin QC, Zeng HQ, Jiang XT, Chen B, et al. Erectile dysfunction and sexual hormone levels in men with obstructive sleep apnea:efficacy of continuous positive airway pressure. Arch Sex Behav. 2016;45:235–40. doi: 10.1007/s10508-015-0593-2. [DOI] [PubMed] [Google Scholar]
- 35.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA:implications for comorbidities. Chest. 2015;147:266–74. doi: 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin SA, Appleton SL, Adams RJ, Taylor AW, Vincent A, et al. Erectile dysfunction is independently associated with apnea-hypopnea index and oxygen desaturation index in elderly, but not younger, community-dwelling men. Sleep Health. 2017;3:250–6. doi: 10.1016/j.sleh.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292–9. doi: 10.1016/j.amjmed.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki H, Yamasaki H, Ogawa K, Nanjo K, Kawamori R, et al. Prevalence and risk factors for erectile dysfunction in Japanese diabetics. Diabetes Res Clin Pract. 2005;70:81–9. doi: 10.1016/j.diabres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Martin S, Atlantis E, Wilson D, Lange K, Haren MT, et al. Clinical and biopsychosocial determinants of sexual dysfunction in middle-aged and older australian men. J Sex Med. 2012;9:2093–103. doi: 10.1111/j.1743-6109.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee DS, Choi JB, Sohn DW. Impact of sleep deprivation on the hypothalamic-pituitary-gonadal axis and erectile tissue. J Sex Med. 2019;16:5–16. doi: 10.1016/j.jsxm.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Xiong Y, Zhang F, Wu C, Zhang Y, Huang X, et al. The circadian syndrome predicts lower urinary tract symptoms suggestive of benign prostatic hyperplasia better than metabolic syndrome in aging males:a 4-year follow-up study. Front Med. 2021;8:715830. doi: 10.3389/fmed.2021.715830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flynn-Evans EE, Shekleton JA, Miller B, Epstein LJ, Kirsch D, et al. Circadian phase and phase angle disorders in primary insomnia. Sleep. 2017;40:zsx163. doi: 10.1093/sleep/zsx163. [DOI] [PubMed] [Google Scholar]
- 43.Vignozzi L, Maggi M. Circadian rhythm and erectile function:is there a penile clock? Nat Rev Urol. 2020;17:603–4. doi: 10.1038/s41585-020-00376-7. [DOI] [PubMed] [Google Scholar]


