Abstract
Phospholipase D (PLD) activity can be detected in response to many agonists in most cell types; however, the pathway from receptor occupation to enzyme activation remains unclear. In vitro PLD1b activity is phosphatidylinositol 4,5-bisphosphate dependent via an N-terminal PH domain and is stimulated by Rho, ARF, and PKC family proteins, combinations of which cooperatively increase this activity. Here we provide the first evidence for the in vivo regulation of PLD1b at the molecular level. Antigen stimulation of RBL-2H3 cells induces the colocalization of PLD1b with Rac1, ARF6, and PKCα at the plasma membrane in actin-rich structures, simultaneously with cooperatively increasing PLD activity. Activation is both specific and direct because dominant negative mutants of Rac1 and ARF6 inhibit stimulated PLD activity, and surface plasmon resonance reveals that the regulatory proteins bind directly and independently to PLD1b. This also indicates that PLD1b can concurrently interact with a member from each regulator family. Our results show that in contrast to PLD1b's translocation to the plasma membrane, PLD activation is phosphatidylinositol 3-kinase dependent. Therefore, because inactive, dominant negative GTPases do not activate PLD1b, we propose that activation results from phosphatidylinositol 3-kinase–dependent stimulation of Rac1, ARF6, and PKCα.
INTRODUCTION
Phosphatidic acid (PtdOH) is a lipid-signaling molecule (Hodgkin et al., 1998) implicated in the regulation of various cellular processes including the oxidative burst in neutrophils (McPhail et al., 1995), mitogenesis (Inui et al., 1994; Ghosh et al., 1996), vesicle trafficking/exocytosis (Bi et al., 1997; Chen et al., 1997; Brown et al., 1998; Siddhanta and Shields, 1998; Siddhanta et al., 2000), and actin cytoskeletal rearrangement (Ha and Exton, 1993; Cross et al., 1996; Hastie et al., 1998). The hydrolysis of phosphatidylcholine to generate PtdOH and free choline is catalyzed by PLD in response to a range of agonists in most cell types (Hodgkin et al., 1998; Exton et al., 1999). Two PLD genes have been cloned (PLD1 and PLD2), each capable of generating two splice variants (PLD1a, PLD1b, and PLD2a, PLD2b; Colley et al., 1997a, 1997b; Hammond et al., 1997). So far there is little understanding of the pathways involved in PLD activation after receptor occupation. In contrast to phospholipase C (PLC), there is no evidence for the activation of PLD through direct interaction with heterotrimeric G-protein–coupled receptors or tyrosine kinase–coupled receptors. Thus, the regulation of this phospholipase differs from PLC; indeed PLD activation is often downstream of PLC activation (Exton, 1999). In vitro studies have revealed that PLD1b activity is stimulated in the presence of any one Rho family member, any one ADP-ribosylation family (ARF) member, and protein kinase C (PKC) family members that contain a C2 domain (Hodgkin et al., 1999). Combinations of single members from each of these activator families result in a cooperative increase in PLD1 activity (Hodgkin et al., 1999). We have also recently shown that PLD1 has an essential requirement for phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) through interaction with an N-terminal PH domain (Hodgkin et al., 2000). These data suggest that in vivo PLD1b can form a multicomponent complex involving PtdIns(4,5)P2, possibly as a membrane tether, a Rho family member, an ARF family member, and a C2 domain-containing PKC family member.
Exocytosis in the rat basophilic leukemic cell line, RBL-2H3, is controlled by a signal transduction cascade after cross-linking of the high-affinity immunoglobulin E receptors (FcεRI) as a result of multivalent antigen binding (Scharenberg and Kinet, 1994) and is mediated through the activation of PI3-kinase and PLCγ1 (Barker et al., 1998; Djouder et al., 2001; Hong-Geller et al., 2001). Similarly, antigen stimulation also initiates activation of other proteins essential to exocytosis including Rho (Guillemot et al., 1997; Hong-Geller and Cerione, 2000; Djouder et al., 2001; Hong-Geller et al., 2001) and ARF family GTPases (Way et al., 2000), members of the PKC family (Apgar, 1991; Hong-Geller and Cerione, 2000), and PLD (Brown et al., 1998; Frigeri and Apgar, 1999; Field et al., 2000). We have previously shown that antigen stimulation promotes the translocation of PLD1b from the secretory granules and secretory lysosomes to the plasma membrane along with these secretory compartments (Brown et al., 1998). Because this translocation was independent of PLD activity, we concluded that PLD-dependent exocytosis was likely to proceed after PLD1b's activation at the plasma membrane (Brown et al., 1998).
Most of the data published with regard to the regulation of PLD1b at the molecular level has been produced in vitro. In the present study, using the RBL-2H3 cell line, we present the first in vivo evidence for a pathway from receptor occupation through to the direct, molecular activation of PLD1b.
MATERIALS AND METHODS
Cell Culture and Transfection
RBL-2H3 cells were cultured at 37°C in 5% CO2 in DMEM (Life Technologies) with 10% fetal calf serum. FcεRI were sensitized by the addition of 1 μg/ml antidinitrophenol (DNP) IgE (Sigma, St. Louis, MO) for 16 h. Where applicable, LY294002 (Calbiochem, La Jolla, CA) was added 10 min before stimulation. Stimulation followed the addition of 50 ng/ml DNP-HSA (Sigma) in stimulation medium (125 mM NaCl, 5 mM KCl, 1.8 mM MgCl2, 0.5 mM Na2PO4, 5 mM NaHCO3, 10 mM glucose, 0.1% BSA, and 10 mM HEPES, pH 7.2). Cells (1 × 107) were transfected by electroporation with 3 pmol of each construct (equivalent to ∼20 μg of pHA-PLD1b, a level that alone seems to have little morphological effect upon the nonstimulated cell) in a Bio-Rad Gene Pulser II (250 V, 950 μF; Richmond, CA). Experiments were performed 16 h posttransfection.
PLD Activity Assay
Cells were grown for 16 h in the presence of 5 μCi/ml [3H]palmitate (Amersham PharmaciaBiotech, Piscataway, NJ) and 1 μg/ml anti-DNP IgE. Five minutes before stimulation cells were washed and incubated in stimulation medium containing 0.3% (vol/vol) butan-1-ol. Stimulation proceeded after the addition of 50 ng/ml DNP-HSA. Lipids were extracted with chloroform-methanol and [3H]PtdBut isolated by TLC as previously described (Wakelam et al., 1995).
BIAcore Experiments
Purified proteins (glutathione S-transferase [GST], GST-PLD1b, GST-JNK2, carbonic anhydrase, and BSA) were covalently immobilized to CM5 surfaces (BIAcore Ltd., Stevenage, United Kingdom) by amine coupling using N-hydroxysuccinamide and ethyl-dimethylaminopropyl carbodiamide. Similar-sized proteins were immobilized to equal densities. After ethanolamine treatment, surfaces were equilibrated in buffer (HEPES-buffered saline) and cycled in 10 mM HCl before challenging with 100 μM BSA to assess surface integrity and nonspecific interactions. Binding events were initiated by injection of the required protein such as the small G-proteins or carbonic anhydrase in HBS flowing over the chip surface at 10 μl/min. Surfaces could be regenerated effectively with glycine, pH 2.
Confocal Immunofluorescence Microscopy
Transfected cells grown on poly-l-lysine–coated glass coverslips were fixed for 7 min with ice-cold 4% paraformaldehyde and permeabilized using 0.5% CHAPS (Sigma) for 2 min. After blocking for 1 h with 20% heat-inactivated goat serum in PBS the hemaglutinin (HA) tag was detected with clone 12CA5 antibody (Roche, Indianapolis, IN), endogenous Rac1 was detected using clone 23A8 antibody (Upstate Biotechnology, Lake Placid, NY), endogenous PKCα was detected using clone 3 antibody (BD Transduction Laboratories, Lexington, KY), and ARF6 was detected using a specific antibody supplied by Julie Donaldson (NIH, Bethesda, MD), each for 1 h. Subsequently, subclass specific Texas Red– (Southern Biotechnology Associates, Birmingham, AL) or TRITC (Sigma)-conjugated secondary antibodies were added for 1 h to fluorescently label primary antibody-bound proteins. All antibodies were diluted in 0.2% saponin/20% heat-inactivated goat serum. Coverslips were mounted onto slides in Prolong (Molecular Probes, Eugene, OR). Images in Figures 1, 2, and 5 are confocal sections acquired using a Nikon TE 300 microscope/PCM2000 system (Garden City, NY) and the accompanying EZ2000 software. Images in Figure 3 were acquired using a Zeiss Axioskop 50 microscope (Thornwood, NY) and a Hamamatsu Orca camera (Bridgewater, NJ). These were subsequently deconvolved to confocal images using Openlab 2.2 software (Improvision, Coventry, United Kingdom). Z-series were obtained using a step size of 0.4 μm.
Figure 1.
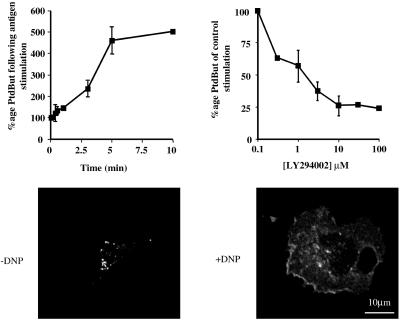
Antigen stimulation of RBL-2H3 cells induces a PI3-kinase–dependent increase in PLD activity. PLD activity was determined in the absence (A) and presence (B) of increasing concentrations of LY294002. [3H]palmitate–labeled RBL-2H3 cells were preincubated for 16 h with 1 μg/ml anti-DNP IgE and for 5 min with 0.3% butan-1-ol before receptor cross-linking by the addition of 50 ng/ml DNP-HSA. In A, the generation of [3H]PtdBut is expressed as a percentage of that at zero time. In B, LY294002 was added 10 min before stimulation, cells were stimulated for 5 min, and the generation of [3H]PtdBut is expressed as a percentage of the total in the absence of LY294002. Error bars indicate SD. (C) RBL-2H3 cells were transfected with HA-tagged PLD1b. Images were captured before stimulation (−DNP) and after stimulation for 5 min (+DNP). Similar results were obtained in at least three other experiments.
Figure 2.
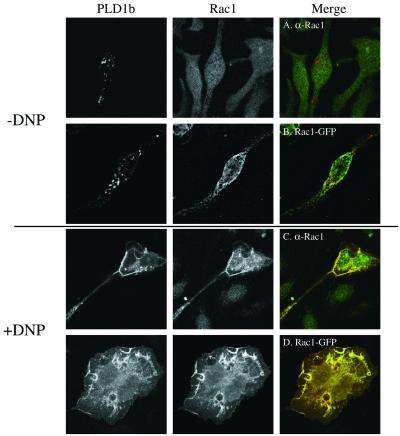
Antigen-stimulated colocalization of PLD1b with Rac1, ARF6, and PKCα. The FcεRIs of RBL-2H3 cells were sensitized by the addition of 1 μg/ml anti-DNP IgE for 16 h. Confocal images were obtained from cells before receptor cross-linking with DNP (−DNP; A, B, E, G, H, K, M, and N) or after addition of 50 ng/ml DNP-HSA for up to 8 min (+DNP; C, D, F, I, J, L, O, and P). Transfected PLD1b is indicated by the red staining. Transfected or endogenous, antibody-detected regulators of PLD1b are indicated by the green staining. Colocalization of PLD1b and its regulator is indicated by yellow staining. The first part of this figure shows the Rac1 experiments and is used as an example of the staining for individual proteins. In the rest of this figure only the merged images are shown. Identical results were obtained with PLD1b HA- or GFP-tagged and Rac1- and ARF6 both tagged with either HA- or GFP (our unpublished results). Similar results were obtained in at least three experiments.
Figure 5.
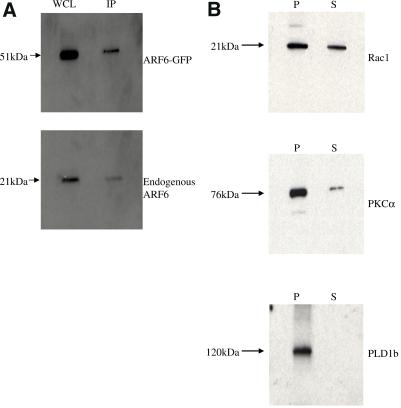
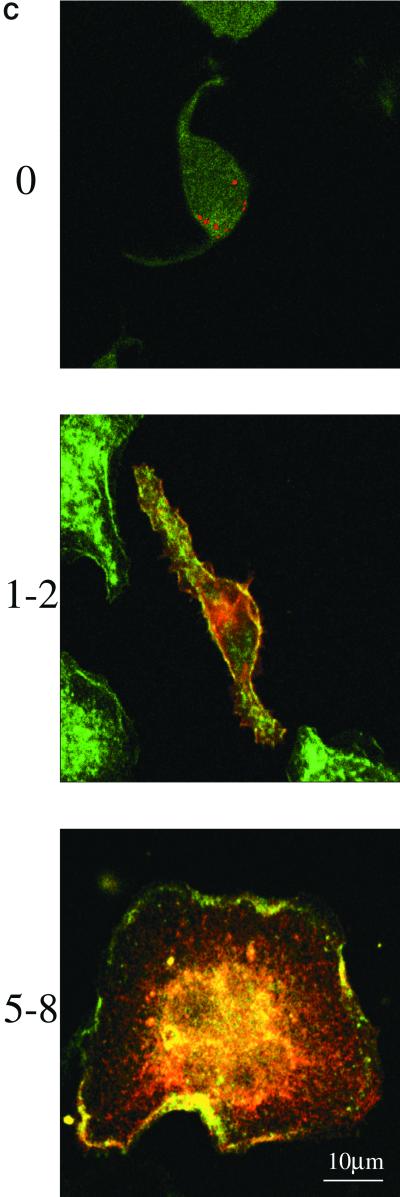
The majority of PLD1b is Triton insoluble. (A) HA-tagged PLD1b was immunoprecipitated from antigen-stimulated RBL-2H3 cells cotransfected with HA-tagged PLD1b and GFP-tagged ARF6. After electrophoresis the gel was probed with an anti-ARF6 antibody. WCL, whole cell lysate; IP, immunoprecipitated sample (The IP lane is equivalent to ∼9 times more loading than the WCL lane). (B) RBL-2H3 cells were cotransfected with HA-tagged PLD1b and GFP-tagged versions of both Rac1 and PKCα. After separation on a 4–20% gradient gel, Rac1 was detected with an anti-Rac1 antibody (Upstate Biotechnology) PKCα with an anti-PKCα antibody (Affinity BioReagents, Golden, CO) and PLD1b with the anti-HA antibody. P, Pellet/Triton-insoluble fraction; S, Supernatant/Triton-soluble pellet (The P lane is equivalent to ∼9 times more loading than the S lane). Only the bands corresponding to endogenous proteins are shown in the cases of Rac1 and PKCα (overexpressed proteins showed a similar pattern). (C) RBL-2H3 cells were transfected with HA-tagged PLD1b as indicated by the red staining. F-actin was detected with FITC-conjugated phalloidin as indicated by the green staining. Yellow staining indicates regions of colocalization between PLD1b and F-actin. Numbering down the left-hand side indicates time after addition of 50 ng/ml DNP-HSA. Similar results were obtained in at least three experiments.
Figure 3.
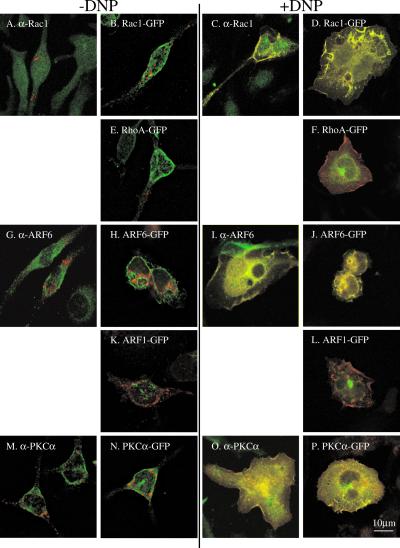
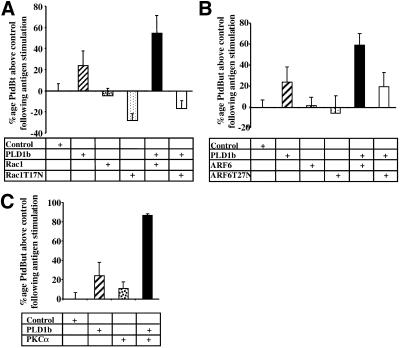
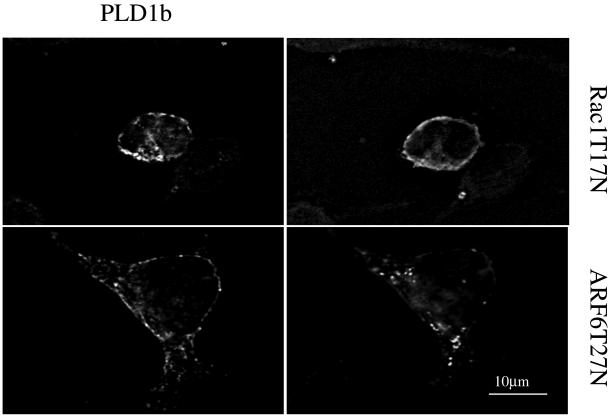
Cooperative activation of PLD1b in RBL-2H3 cells. [3H]palmitate–labeled RBL-2H3 cells, transfected with HA-tagged PLD1b, GFP-tagged Rac1, ARF6, PKCα, Rac1T17N, or ARF6T27N as stated (A–C), were preincubated for 16 h with 1 μg/ml anti-DNP IgE and for 5 min with 0.3% butan-1-ol before receptor cross-linking by the addition of 50 ng/ml DNP-HSA for 5 min. PLD activity is expressed as a percentage of the increase or decrease of generated [3H]PtdBut over vector alone transfected cells (Control). Stimulation of control cells generally resulted in a three- to fourfold increase in PLD activity. Transfections had little effect on nonstimulated activity. All transfections consisted of 3 pmol of pHA constructs and 3 pmol of pEGFP constructs. Therefore, transfections with PLD1b alone or regulators alone were performed in the presence of the opposite empty vector. Expression levels were similar for PLD1b and regulators and also between experiments (checked by Western blotting). Error bars indicate SD. (D) RBL-2H3 cells, cotransfected with HA-tagged PLD1b and GFP-tagged Rac1T17N or ARF6T27N were stimulated for 5 min. Images in the left-hand column show PLD1b, and those in the right-hand column show the indicated regulator. Comparative images of PLD1b and the indicated regulator from the same cell are adjacent in the same row. Similar results were obtained in at least three experiments.
Immunoprecipitation and Western Blotting
Cells (1 × 107) were lysed for 10 min in ice-cold 50 mM HEPES, pH 7.4, 0.5% Triton X-100, 2 mM sodium orthovanadate, 50 mM NaF, PMSF, and protease inhibitor tablets (Roche). The Triton-insoluble fraction was removed by centrifugation at 16,000 × g for 5 min. Antibody, 1 μg, was added to the lysates, incubated with mixing at 4°C for 2 h, and captured with 25 μl bed-volume of Protein G Sepharose (Amersham PharmaciaBiotech) for 2 h. The Protein G Sepharose was washed five times in lysis buffer, and bound proteins were eluted into 25 μl of 2× Lamelli buffer. Proteins were separated on 4–20% gradient gels (Novex, Encinitas, CA) and transferred to PVDF and blocked in 5% Milk (Marvel, Richmond, IN; in PBS and 0.1% Tween 20). Proteins were blotted and detected as described previously (Hodgkin et al., 1999). Antibodies used were the same as used for immunofluorescence.
RESULTS
PLD Activity Is PI3-kinase Dependent
In the presence of a short-chain primary alcohol PLD preferentially catalyzes the production of a stable phosphatidylalcohol rather than rapidly metabolized PtdOH. Therefore, we measured PLD activity in the presence of butan-1-ol by quantifying the production of phosphatidylbutan-1-ol (PtdBut). After antigen stimulation of RBL-2H3 cells, PLD activity increased rapidly up to 5 min after which point it began to level off (Figure 1A). We have previously shown that this activity is essential for exocytosis (Brown et al., 1998), a process that it also known to be PI3-kinase dependent in RBL-2H3 cells (Barker et al., 1998). Stimulation of the RBL-2H3 cells in the presence of the PI3-kinase inhibitor, LY294002, reduced PLD activity, indicating that its regulation is also PI3-kinase dependent (Figure 1B). Coincident with PLD-dependent exocytosis from the RBL-2H3 cells, PLD1b translocates to the plasma membrane, indicating that PLD1b activation at the plasma membrane may control exocytosis (Brown et al., 1998). An inactive mutant of PLD1b still translocates to the plasma membrane, suggesting that PLD activity is not necessary for its translocation (Brown et al., 1998). Similarly, here we find that in the presence of LY294002, upon stimulation (+DNP), a HA-tagged version of PLD1b still translocates to the plasma membrane (Figure 1C). Because in vitro studies indicate that PLD1b alone has little basal activity (Hodgkin et al., 1999), it is therefore likely that it becomes activated upon its arrival at the plasma membrane and that these subsequent steps are PI3-kinase dependent.
Antigen–stimulated Colocalization of PLD1b with Rac1, ARF6, and PKCα
It has been shown in vitro that PLD1b is cooperatively activated by a Rho family member, an ARF family member and PKCα or δ (Hodgkin et al., 1999). Considerable evidence points to the activation of Rho, ARF, and PKC family proteins through PI3-kinase (Apgar, 1991; Guillemot et al., 1997; Stam et al., 1997; Hong-Geller and Cerione, 2000; Venkateswarlu and Cullen, 2000; Way et al., 2000; Djouder et al., 2001; Hong-Geller et al., 2001). In antigen-stimulated RBL-2H3 cells, it is therefore possible that PI3-kinase–dependent PLD activity (Figure 1) results from PLD1b's association with PI3-kinase–dependent regulators.
In nonstimulated cells (−DNP), confocal sections revealed that PLD1b primarily localized to vesicular compartments, which we have previously identified as secretory granules and secretory lysosomes (Brown et al., 1998; Figure 2, A, B, E, G, H, K, M, and N). At this time, both endogenous (Figure 2A) and transfected (Figure 2B) Rac1 were predominantly detected at a diffuse intracellular localization, distinct from these secretory compartments. After antigen stimulation (+DNP), however, PLD1b colocalized with both endogenous (Figure 2C) and transfected (Figure 2D) Rac1 at the plasma membrane within structures resembling lamellipodia and membrane ruffles, as the cells began to flatten and spread out, and also within intracellular vesicle-like structures. In contrast little colocalization was observed with another Rho family member, RhoA, before (Figure 2E) or after antigen stimulation (Figure 2F).
Previous studies have highlighted a close functional relationship between Rac1 and ARF6 in the control of actin-rich structures at the plasma membrane (D'Souza-Schorey et al., 1997; Franco et al., 1999; Honda et al., 1999; Radhakrishna et al., 1999; Tolias et al., 2000; Santy and Casanova, 2001). In the present study, confocal sections revealed that the distribution of ARF6 in nonstimulated cells (−DNP) was distinct from that of PLD1b, being detectable at both the plasma membrane and an intracellular compartment (Figure 2, G and H). However, after antigen stimulation (+DNP) as these cells began to flatten and spread out, both endogenous (Figure 2I) and transfected (Figure 2J) ARF6 colocalized with PLD1b at the plasma membrane, within lamellipodia and membrane ruffles and also intracellularly. The colocalization of PLD1b with ARF6 is not unique to this cell line because we have observed similar results, for example, in HT1080 fibrosarcoma cells (our unpublished results). Equivalent experiments performed with ARF1 highlighted little colocalization with PLD1b before (Figure 2K) or after antigen stimulation (Figure 2L) either within the cell or at the plasma membrane.
As with Rac1 and ARF6, the PKC family of PLD1b regulators have also been shown to regulate actin cytoskeletal reorganizations (Apgar, 1991; Frank et al., 1998; Job and Lagnado, 1998). In correlation with these observations, the PKC family activating compound, phorbol 12-myristate 13-acetate, initiated translocation of PLD1b to the plasma membrane and subsequent exocytosis by the RBL-2H3 cells (Brown et al., 1998; Hong-Geller and Cerione, 2000; Hong-Geller et al., 2001). Similarly to both Rac1 and ARF6, in confocal sections little colocalization was observed between PLD1b and endogenous (Figure 2M) or transfected (Figure 2N) PKCα in nonstimulated cells (−DNP). However, after antigen stimulation (+DNP) as the cells began to flatten and spread out, both enzymes colocalized at the plasma membrane, within structures resembling lamellipodia and membrane ruffles and also intracellularly (Figure 2, O and P).
Control experiments performed in the absence of transfected PLD1b showed that Rac1, ARF6, and PKCα still translocated to lamellipodia and membrane ruffles at the plasma membrane, indicating that this phenomenon was not induced by overexpression of PLD1b (our unpublished results). In contrast, transfection of PLD1b and either empty vector did not result in phospholipase and tag colocalization at the plasma membrane, indicating that the colocalization of PLD1b with Rac1, ARF6, and PKCα was a specific event (our unpublished results).
Cotransfection of PLD1b and either Rac1, ARF6, or PKCα Results in Cooperative Increases in Activity
The results in Figure 2 show that after antigen stimulation of RBL-2H3 cells PLD1b colocalizes with its activators at the plasma membrane. Colocalization is predominant within actin-rich structures, such as lamellipodia and membrane ruffles, as the cell begins to flatten and spread out. Because each of PLD1b and these activators have been shown to control actin cytoskeletal reorganizations, it appears that colocalization may be functionally significant to the flattening and spreading of the cell. Accordingly, increased PLD activity is coincident with this colocalization, suggesting that it may result from the direct, molecular stimulation of PLD1b by these activators (Figure 1A). Because PLD1b colocalized with transfected as well as endogenous regulators, we therefore hypothesized that transfection of PLD1b and/or its regulators would result in increased, stimulatable PLD activity. Figure 3 shows that in comparison to control cells, PLD1b transfected cells exhibited increased activity, whereas cells transfected with either Rac1, ARF6, or PKCα alone exhibited no such increase. In contrast, antigen stimulation of cells cotransfected with PLD1b and either Rac1, ARF6, or PKCα resulted in a further, cooperative increase in PLD activity, above that seen for cells transfected with PLD1b alone.
The specificity of this activation was confirmed using dominant negative mutants of Rac1 and ARF6. Members of the Rho and ARF GTP binding proteins are predicted to be active in the GTP-bound state and inactive in the GDP-bound state. Transfection of PLD1b with either Rac1 or ARF6 dominant negative mutants trapped in the inactive, GDP-bound conformation (Rac1T17N and ARF6T27N) did not result in the same cooperative increases in PLD activity as seen with their wild-type counterparts; indeed, an inhibition was observed with Rac1T17N (Figure 3A). Analysis of these cells using confocal microscopy revealed that after antigen stimulation, PLD1b translocated to the plasma membrane where it partially colocalized with Rac1T17N (Figure 3D), in keeping with the apparent dominant negative effects of Rac1T17N (Figure 3A) but to a lesser extent with ARF6T27N (Figure 3D). Although ARF6T27N-transfected cells exhibited pseudopodia-like extensions, cells transfected with either dominant negative mutant lacked lamellipodia or membrane ruffles and did not flatten and spread to the same extent as wild-type cells after antigen stimulation (Figure 3D). Transfections in the presence of PLD1b and constitutively active mutants of Rac1 and ARF6 (Rac1Q61L and ARF6Q67L) resulted in a higher degree of cell death than other transfections such that it was impossible to accurately assess their effects.
PLD1b Binds Directly and Independently to Rho, ARF, and PKC Family Proteins
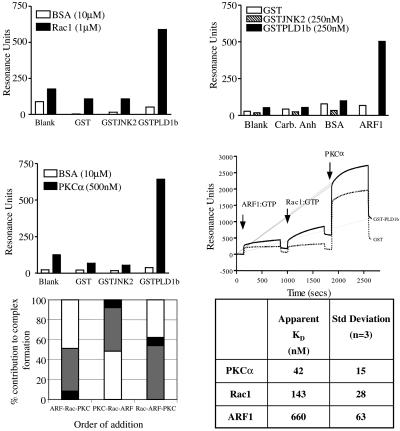
Colocalization of PLD1b with each of Rac1, ARF6, and PKCα was coincident with cooperatively increasing PLD activity (Figures 2 and 3). Because in vitro studies using purified recombinant proteins have also shown activation of PLD1b by single Rho and ARF family members and PKCα or δ (Hodgkin et al., 1999), we hypothesized that this activation is mediated through physical interaction between PLD1b and each regulator. Therefore, the interactions of purified, recombinantly expressed proteins were assessed by surface plasmon resonance (SPR). Figure 4A shows that recombinant Rac1 (1 μM) bound specifically to immobilized PLD1b in the presence of GTPγS (30 μM). Rac1 did not interact with recombinant GST or GST-JNK2, a GST-fusion protein of a size similar to PLD1b. The binding of Rac1 to PLD1b was observed at flow rates of between 5 and 20 μl/min and varying concentrations of Rac1. BSA (10 μM) did not interact with any of the immobilized protein surfaces. Analysis of the kinetics of the interaction between Rac1 and PLD1b using Biaevaluation 3.0 software (Bioevaluation 3, Stevenage, U.K.) gave an apparent KD of ∼0.15 μM (Figure 4F). Both Cdc42 and RhoA showed very similar specificity for PLD1b compared with control proteins and similar kinetic parameters (our unpublished results). Figure 4B shows that the binding of PLD1b to control surfaces and ARF1 in the presence of GTPγS was specific. Measurement affinity of the interaction between Arf1 and PLD1 over a range of concentrations gave an apparent KD of ∼0.7 μM (Figure 4F). PLD1b did not interact with immobilized carbonic anhydrase (a protein of similar size to ARF1) or with BSA. Neither GST nor GST-JNK2 interacted with the immobilized ARF1 or the control proteins. The binding of PKCα to PLD1b was observed in the absence of exogenous phorbol ester, ATP, or GTPγS and was independent of changes in flow rate. Figure 4C illustrates that recombinant PKCα (500 nM) bound specifically to the PLD1b surface but did not interact with GST or GST-JNK2 surfaces. Kinetic analysis of the binding of PKCα to PLD1b over a range of concentrations gave an apparent KD of ∼50 nM. Both specificity data and kinetic data were confirmed at a range of flow rates.
Figure 4.
The formation of a multicomponent complex consisting of PLD1b, ARF1, Rac1, and PKCα. The interactions between Rac1 (A), ARF1 (B), and PKCα (C) in the presence of GTPγS and PLD1b were quantified using SPR. In A and C PLD1b was immobilized on one channel of a CM5 chip surface: GST-JNK2 and BSA were similarly immobilized, and one surface was treated with the chemistry alone. The binding of 1 μM Rac1 or 500 nM PKCα to all the surfaces was quantified. In B, ARF1 was immobilized to a single channel of a CM5 chip: carbonic anhydrase and BSA were similarly immobilized, and one surface was treated with the chemistry alone. The binding of 250 nM PLD1b in the presence of GTPγS to all surfaces was quantified. (D) Purified recombinant ARF1, Rac1 (both 1 μM in the presence of excess GTPγS), or PKCα (100 nM) were injected onto surfaces of covalently immobilized GST (dotted line) or GST-PLD1b (line), and the interaction was quantified by SPR. The arrows indicate when the regulator proteins were injected. The initial vertical increase in resonance is caused by differences in refractive index between the buffer composition in the regulator preparations and the flow buffer. This is particularly evident for the PKCα injection because of glycerol in the PKCα storage buffer. Association is evident by the upward curve in resonance after injection. Injections were stopped after 600 s (indicated by a vertical drop in resonance), and dissociation was allowed to proceed for a short time before the next injection was performed. The specific binding of each of the regulator proteins to PLD1b was between three and four times the amount of binding to GST alone. The formation of a complex between PLD1b and ARF1 is indicated by the increased resonance from the point of injection to the end of the injection of ARF1 as dissociation begins and are similar for Rac1 and PKCα. (E) The order of addition of the regulatory proteins did not affect the formation of a complex between PLD1b and its regulators. (F) The affinities of Rac1, ARF1, and PKCα for PLD1 were determined by fitting experimental data to expected data using Biaevaluation 3.0 analysis software. The expected data assumed a simple 1:1 binding model consistent with independent experimental evidence. For each regulator, three sets of experimental data at different regulator concentrations were analyzed and averaged.
PLD1b is cooperatively activated by single members from each of the Rho, ARF, and PKC families. In subsequent SPR experiments, purified, recombinant GTPases (1 μM) and purified, recombinant PKCα (100 nM) were sequentially injected across both PLD1b and GST surfaces and interactions monitored by SPR. Under these conditions, the activating regulatory proteins bound directly and independently to PLD1b and a multicomponent complex was generated (Figure 4D). Figure 4E illustrates that the order of addition of each activating protein does not affect their binding to PLD1b during the formation of this complex, and Figure 4F indicates the apparent affinities of the interactions between each regulator and PLD1b.
The Majority of PLD1b Is Triton Insoluble
Because PLD1b can directly and independently interact with members from each of its regulator families (Figure 4), the antigen-stimulated colocalization of PLD1b with each of Rac1, ARF6, and PKCα (Figure 2) coupled to the coincident, cooperative increase in PLD activity (Figures 1A and 3) indicates a direct interaction between PLD1b and its regulators in RBL-2H3 cells. To further examine this, RBL-2H3 cells were cotransfected with HA-tagged PLD1b and either of the GFP-tagged versions of Rac1, ARF6, or PKCα. After 5 min of antigen stimulation, the cells were lysed in Triton X-100–containing buffer, and PLD1b was immunoprecipitated from the Triton-soluble fraction using the anti-HA antibody, 12CA5. Western blotting of these samples using the respective Rac1-, ARF6-, or PKCα-specific antibodies revealed that only ARF6 coimmunoprecipitated with PLD1b in both its GFP-tagged and endogenous forms, indicating the possibility of a direct interaction in RBL-2H3 cells (Figure 5A).
Coimmunoprecipitation of PLD1b with Rac1 or PKCα may have been impossible because Western blotting revealed that the majority of PLD1b was present in the nonimmunoprecipitatable, Triton-insoluble fraction of the RBL-2H3 cells. In contrast, Rac1 and PKCα were found within both the Triton-soluble and -insoluble fractions (Figure 5B). In many hematopoetic cell lines, including RBL-2H3s, the actin cytoskeleton is a major constituent of the Triton-insoluble fraction (Frigeri and Apgar, 1999; Hodgkin et al., 1999; Iyer and Kusner, 1999). Previously it has been shown that reorganizations of the actin cytoskeletal can be controlled by PLD (Ha and Exton, 1993; Cross et al., 1996; Hastie et al., 1998) as well as each of Rac1, ARF6, and PKC (Apgar, 1991; D'Souza-Schorey et al., 1997; Frank et al., 1998; Hall, 1998; Job and Lagnado, 1998; Franco et al., 1999; Radhakrishna et al., 1999; Tolias et al., 2000). Furthermore, Figure 2 shows that as these cells begin to flatten and spread out, PLD1b colocalizes with Rac1, ARF6, and PKCα in structures resembling lamellipodia and membrane ruffles. These structures are rich in filamentous actin (F-actin), and consistent with these data, after antigen stimulation we found that in confocal sections PLD1b colocalized with F-actin at the cell cortex and also on the dorsal surface of the RBL-2H3 cells (Figure 5C, 1–2 and 5–8 min).
DISCUSSION
In this report we provide the first in vivo evidence for a pathway from receptor occupation at the cell surface to the direct, molecular activation of PLD1b. Antigen stimulation of RBL-2H3 cells promotes the selective colocalization of PLD1b with Rac1, ARF6, and PKCα in actin-rich structures at the plasma membrane as PLD activity increases. When PLD1b is cotransfected with each of these regulators, colocalization is coincident with a further specific, cooperative increase in PLD activity. Consequently, the demonstration here that PLD1b binds directly and independently to its regulators indicates that this activity increase results from PLD1b's physical interaction with and subsequent activation by Rac1, ARF6, and/or PKCα.
The results in Figure 4 illustrate that the interactions between PLD1b and its activators are not only specific and direct (Figure 4, A–C) but also independent of each other (Figure 4, D and E). These data highlight that conserved regions from each family interact with distinct but as yet incompletely defined domains of PLD1b; it has been suggested that Rho proteins interact between residues K946 and K962 and at I870 (Du et al., 2000; Cai and Exton, 2001), PKC proteins interact around residue E87 (Zhang et al., 1999), whereas the location of the ARF binding site is unclear, but they do not interact within the N-terminal or the loop region (Sung et al., 1999). Here we show that the propensity for such interactions to occur in vivo is determined by spatially directed and temporally coordinated targeting (Figure 2). The specificity profile of interaction is consistent with previous experiments illustrating that PLD1 is stimulated by Rho, ARF, and PKC proteins (Hodgkin et al., 1999; for review see Exton, 1999). Between 1 and 5 μM of any Rho family member is required to maximally stimulate PLD1 activity in vitro, and our data regarding the affinity of the interactions between PLD1b and Rho family members are consistent with this (Hodgkin et al., 1999; Figure 4). The binding of Rho and ARF proteins to PLD1b, detected by SPR, was surprisingly not dependent on their GTP loading (our unpublished results); however, the in vitro activation of PLD1b requires GTP (Hodgkin et al., 1999). It is possible that the GTPases bind GTP when associated with PLD1b and this induces activation, mediated through a conformational change. The concept that the binding of a GTPase to its effector increases its GTP binding has been previously demonstrated for ARF proteins both in vivo and in vitro (Zhu et al., 2000). The results in Figure 3 support this possibility because GDP-ligated Rac1 and ARF6 mutants inhibited PLD activation, presumably by binding to the phospholipase.
Although our data show PLD1b's initial regulation after FcεRI-ligation of RBL-2H3 cells, it is also possible that after activation of different signaling pathways in response to diverse stimuli, more prolonged stimuli or in other cell types, PLD1b is regulated by different regulators than those identified here. The activation of PLD1b by different regulatory proteins may help explain the many cellular functions attributed to this enzyme/activity. Consistent with this, in COS-7 cells PLD1b directly interacts with a constitutively active mutant of RhoA (Cai and Exton, 2001).
In stimulated cells, transfected PLD1b colocalizes with endogenous Rac1, ARF6, and PKCα (Figure 2, C, I, and O, respectively). Coincidently PLD activity increases to a level above that seen for control cells (Figure 3). In contrast, the PLD activity of Rac1-, ARF6-, or PKCα-transfected cells was similar to that of control cells (Figure 3). Because PLD1b has little basal activity, these results indicate that transfected PLD1b was accessible to endogenous activators, which must therefore be in excess of endogenous, available PLD1b. Accordingly, cotransfection of PLD1b with either Rac1, ARF6, or PKCα resulted in a further cooperative increase in PLD activity, above that seen for cells transfected with PLD1b alone (Figure 3) and coincidently with their colocalization (Figure 2D, 2J and 2P). Thus the increase in PC hydrolysis results from specific activation of PLD1b by these transfected regulators as dominant negative versions of Rac1 and ARF6 (Rac1T17N and ARF6T27N) did not result in a similar increase (Figure 3, A and B); indeed, Rac1T17N mediated a decrease in endogenous PLD activity (Figure 3A). From these findings we consider that PLD1b levels in the cell are a limiting factor controlling the magnitude of PLD activation, whereas the endogenous levels of the regulatory proteins could support further PLD activation. It is only when PLD1b is overexpressed and its levels are in excess of endogenous, available Rac1, ARF6, and PKCα that overexpression of these regulators can mediate a further increase in PLD activity. Therefore the excess level of endogenous regulators in RBL-2H3s perhaps suggests that under the appropriate conditions, they may be able to stimulate larger PLD responses after an increase in transcription/translation of PLD1b.
Although our experiments do not allow us to define the precise makeup of PLD1b/regulator complexes at a given time or location, SPR indicates that PLD1b can simultaneously bind a member from all three regulators (Figure 3, D and E). In the presence of all three regulators in vitro, activation is 40-fold higher than in the presence of individual regulators (Hodgkin et al., 1999). If the in vivo activation of PLD1b results in similar activity increases, the formation of different PLD1b/regulator complexes would provide a highly amplifiable mechanism for regulating the PLD1b activity necessary for different functions and may be one reason why cells normally only require such relatively low levels of PLD1b.
Previously we found that a catalytically inactive mutant of PLD1b was still able to translocate to the plasma membrane (Brown et al., 1998). Similarly, although we find that PLD activity is diminished in the presence of the PI3-kinase inhibitor LY294002, PLD1b was still able to translocate to the plasma membrane (Figure 1C). Therefore, because activity is not required for translocation, PLD1b must be activated when at the plasma membrane. In RBL-2H3 cells, stimulation promotes the activation of Rho, ARF, and PKC family proteins (Apgar, 1991; Guillemot et al., 1997; Way et al., 2000; Hong-Geller et al., 2001). Here, subsequent to stimulation, PLD1b colocalizes with Rac1, ARF6, and PKCα as its activity increases (Figure 2). In the case of Rac1 and ARF6, activation results from the exchange of GDP for GTP (Djouder et al., 2001). Our data showing that PLD activity is not stimulated in the presence of GDP-ligated, mutants of Rac1 and ARF6 (Figure 3, A and B) indicate that these GTPases must be able to become active by cycling through the GTP-bound conformation in order to activate PLD1b. Because the exchange of GDP for GTP on these GTPases has been shown to be PI3-kinase dependent, their stimulation presumably accounts for the PI3-kinase dependence of PLD1b activation.
Coimmunoprecipitation experiments in the RBL-2H3 cells revealed PLD1b in complex with ARF6 (Figure 5A). The proportion of total ARF6 immunoprecipitated with PLD1b was low, probably because the majority of PLD1b was present in the Triton-insoluble fraction of the RBL-2H3 cells such that it cannot be immunoprecipitated (Figure 5B). This may also be the reason that PLD1b did not coimmunoprecipitate with Rac1 and PKCα. As with PLD1b in RBL-2H3 cells, a Rho-, ARF-, and PKC-regulated PLD has been identified in the Triton-insoluble fraction of HL60 and U937 cells (Hodgkin et al., 1999; Iyer and Kusner, 1999). Rac1, ARF6, PKC, and PLD all have the ability to mediate actin reorganization, and therefore it is consistent for the majority of the F-actin to also be located within the Triton-insoluble fraction of these three cell lines (Frigeri and Apgar, 1999; Hodgkin et al., 1999; Iyer and Kusner, 1999).
In RBL-2H3 cells, actin polymerization proceeds with kinetics similar to those of PLD1b activation (Frigeri and Apgar, 1999; Figures 1A and 3). Coincidently, as the cells flatten and spread out, PLD1b colocalizes with F-actin at the plasma membrane within actin-rich lamellipodia and membrane ruffles (Figures 2 and 5C). These structures are synonymous with spreading and migration (Hall, 1998), and here it was observable that PLD1b-transfected cells spread out more rapidly than vector alone or nontransfected cells. Consistent with this, cotransfection of PLD1b with dominant negative Rac1 or ARF6 caused a reduction in both PLD activity and the ability of these cells to flatten and spread out after stimulation (Figure 3). Fittingly therefore, inhibition of PLD after the addition of butan-1-ol or the PI3-kinase inhibitor, LY294002, is also known to negatively regulate the spreading and migration processes (Powner and Wakelam, unpublished observations; Santy and Casanova, 2001).
Previous studies have indicated that Rac1 and ARF6 control actin cytoskeletal rearrangements via distinct pathways (D'Souza-Schorey et al., 1997; Boshans et al., 2000; Santy and Casanova, 2001). Because Rac1, ARF6, and PKCα can all independently activate PLD1b (Hodgkin et al., 1999), this phospholipase may be a point of convergence for each of its regulators' respective abilities to control these rearrangements. Indeed, in the absence of a stimulus, the addition of PtdOH alone mediates actin reorganizations (Cross et al., 1996). PtdIns(4,5)P2 is known to be a major factor governing actin cytoskeletal rearrangements and each of Rac1-, ARF6-, and PLD-produced PtdOH have been implicated in production of this lipid through the activation of type I α phosphatidylinositol 4-phosphate 5-kinase (Jenkins et al., 1994; Divecha et al., 2000; Tolias et al., 2000). Hence, we hypothesize that the localized production of PtdIns(4,5)P2 may be responsible for at least some of Rac1, ARF6, PKCα, and PLD1b's effects on the actin cytoskeleton.
In conclusion, we formulate the first in vivo model for PLD1b activation. In RBL-2H3 cells, receptor cross-linking initiates a tyrosine kinase cascade, through Lyn and Syk leading to activation of class Ia PI3-kinase (Scharenberg and Kinet, 1994; Barker et al., 1998). The subsequent production of PtdIns(3,4,5)P3 contributes to the stimulation of PLCγ1 through its PH domains (Barker et al., 1998), while also activating PH-domain–containing GEFs for both Rac1 and ARF6. The generation of diacylglycerol and release of calcium as a consequence of PLCγ1 stimulation leads to PKCα activation, whereas GEF stimulation catalyzes the GTP-loading and therefore activation of Rac1 and ARF6. PLD1b translocates to the plasma membrane and becomes associated with PtdIns(4,5)P2 via its PH domain. Spatially directed and temporally coordinated targeting of the activated regulators to the plasma membrane results in their binding to and activation of PLD1b.
ACKNOWLEDGMENTS
We thank M. Aggarwal for help with early experiments. ARF cDNAs were a gift from N. Thompson (GlaxoWellcome). PKCα cDNA was a gift from Peter Parker (ICRF). Rho cDNAs were a gift from N. Akhtar (Biosciences, Birmingham University). The ARF6 antibody was a gift from J.G. Donaldson (NIH). This study was supported by a grant from the Wellcome Trust. M.N.H. is a Beit Memorial Research Fellow.
Abbreviations used:
- ARF
ADP-ribosylation factor
- DNP
dinitrophenol
- FcεRI
high-affinity immunoglobulin E receptor
- F-actin
filamentous actin
- HA
hemaglutinin
- PtdOH
phosphatidic acid
- PtdBut
phosphatidylbutan-1-ol
- PKC
protein kinase C
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- PLC
phospholipase C
- PLD
phospholipase D
- RBL
rat basophilic leukemia
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–05–0235. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–05–0235.
REFERENCES
- Apgar J. Regulation of the antigen-induced F-actin response in rat basophilic leukemia cells by protein kinase C. J Cell Biol. 1991;112:1157. doi: 10.1083/jcb.112.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Pfeiffer JR, Wilson BS. Wortmannin-sensitive phosphorylation, translocation and activation of PLCγ1, but not PLCγ2, in antigen-stimulated RBL-2H3 mast cells. Mol Biol Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi K, Roth MG, Ktistakis NT. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- Boshans RL, Szanto S, van Aelst L, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol Biol Cell. 2000;20:3685–3694. doi: 10.1128/mcb.20.10.3685-3694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Thompson N, Saqib KM, Clark JM, Powner DJ, Thompson NT, Solari R, Wakelam MJO. Phospholipase D1 localizes to secretory granules and lysosomes and is plasma membrane translocated on cellular stimulation. Curr Biol. 1998;8:835–838. doi: 10.1016/s0960-9822(98)70326-4. [DOI] [PubMed] [Google Scholar]
- Cai S, Exton JH. Determination of interaction sites of PLD1 for RhoA. Biochem J. 2001;355:779–785. doi: 10.1042/bj3550779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley WC, Altshuller YM, Sue-Ling CK, Copeland NG, Gilbert DJ, Jenkins NA, Branch KD, Tsirka SE, Bollag RJ, Bollag WB, Frohman MA. Cloning and expression analysis of murine phospholipase D1. Biochem J. 1997a;326:745–753. doi: 10.1042/bj3260745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley WC, Sung T-C, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganisation. Curr Biol. 1997b;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung T-C, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:459–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MJ, Roberts S, Ridley AJ, Hodgkin MN, Stewart A, Claesson-Welsh L, Wakelam MJO. Stimulation of actin stress fiber formation mediated by activation of phospholipase D. Curr Biol. 1996;6:588–597. doi: 10.1016/s0960-9822(02)00545-6. [DOI] [PubMed] [Google Scholar]
- Divecha N, Roefs M, Halstead JR, D'Andrea S, Fernandez-Borga M, Oomen L, Saqib KM, Wakelam MJO, D'Santos C. Interaction of the Type I α PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4,5-bisphosphate in the regulation of PLD2 activity. EMBO J. 2000;19:5440–5449. doi: 10.1093/emboj/19.20.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouder N, Schmidt G, Frings M, Cavalie A, Thelen M, Aktories K. Rac, and phosphotidylinositol 3-kinase regulate the protein kinase B in FcεRI signaling in RBL-2H3 mast cells. J Immunol. 2001;166:1627–1634. doi: 10.4049/jimmunol.166.3.1627. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Klausner RD. ARF: a key regulatory switch in membrane traffic and organelle structure. Curr Opin Cell Biol. 1994;6:527–532. doi: 10.1016/0955-0674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Altshuller YM, Kim Y, Han JM, Ryu SH, Morris AJ, Frohman MA. Dual requirement for Rho and protein kinase C in direct activation of PLD1 through G protein-coupled receptor signaling. Mol Biol Cell. 2000;11:4359–4368. doi: 10.1091/mbc.11.12.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- Field K, Apgar JR, Hong-Geller E, Siraganian RP, Baird B, Holowka DH. Mol. Biol Cell. 2000;11:3661–3673. doi: 10.1091/mbc.11.10.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Peters PJ, Boretto J, van Donselaar E, Neri A, D'Souza-Schorey C, Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SR, Hatfield JC, Casanova JE. Remodeling of the actin cytoskeleton is coordinately regulated by protein kinase C and the ADP-ribosylation factor nucleotide exchange factor ARNO. Mol Biol Cell. 1998;9:3133–3146. doi: 10.1091/mbc.9.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri L, Apgar JR. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. J Immunol. 1999;162:2243–2250. [PubMed] [Google Scholar]
- Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. J Biol Chem. 1996;271:8472–8480. doi: 10.1074/jbc.271.14.8472. [DOI] [PubMed] [Google Scholar]
- Guillemot J-C, Montcourrier P, Vivier E, Davoust J, Chavrier P. Selective control of membrane ruffling and actin plaque assembly by the Rho GTPases Rac1 and Cdc42 in FcεR.I-activated rat basophilic leukemic (RBL-2H3) cells. J Cell Sci. 1997;110:2215–2225. doi: 10.1242/jcs.110.18.2215. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Ha KS, Exton JH. Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J Cell Biol. 1993;123:1789–1796. doi: 10.1083/jcb.123.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganisation. J Biol Chem. 1997;271:3860–3868. [Google Scholar]
- Hastie LE, Patton WF, Hechtman HB, Sherpo D. Metabolites of the phospholipse D pathway regulate H2O2-induced filamin redistribution in endothelial cells. J Cell Biochem. 1998;68:511–524. [PubMed] [Google Scholar]
- Hodgkin MN, Clark JM, Rose S, Saqib KM, Wakelam MJO. Characterization of protein kinase C and small G-protein regulated phospholipase D activity in the detergent-insoluble fraction of HL60 cells. Biochem J. 1999;339:87–93. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin MN, Masson MR, Powner DJ, Saqib KM, Ponting CP, Wakelam MJO. Phospholipase D regulation and localization is dependent upon a phosphatidylinositol 4,5-bisphosphate-specific PH domain. Curr Biol. 2000;10:43–46. doi: 10.1016/s0960-9822(99)00264-x. [DOI] [PubMed] [Google Scholar]
- Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJO. Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem Sci. 1998;23:200–204. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaha Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Cerione RA. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP3/calcium pathway in RBL-2H3 mast cells. J Cell Biol. 2000;148:481–493. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Geller E, Holowka D, Siraganian RP, Baird B, Cerione RA. Activated Cdc42/Rac reconstitutes FcεRI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proc Natl Acad Sci USA. 2001;98:1154–1159. doi: 10.1073/pnas.98.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui H, Kitami Y, Tani M, Kondo T, Inagami T. Differences in signal transduction between platelet-derived growth factor (PDGF) alpha and beta receptors in vascular smooth muscle cells. PDGF-BB is a potent mitogen, but PDGF-AA promotes only protein synthesis without activation of DNA. synthesis. J Biol Chem. 1994;269:30546–30552. [PubMed] [Google Scholar]
- Iyer SS, Kusner DJ. Association of phospholipase D activity with the detergent-insoluble cytoskeleton of U937 promonocytic leukocytes. J Biol Chem. 1999;274:2350–2359. doi: 10.1074/jbc.274.4.2350. [DOI] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and PKC regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail LC, Qualliotine-Mann D, Waite KA. Cell-free activation of neutrophil NADPH oxidase by a phosphatidic acid-regulated protein kinase. Proc Natl Acad Sci USA. 1995;92:7931–7935. doi: 10.1073/pnas.92.17.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg AM, Kinet JP. Early events in mast cell signal transduction. Chem Immunol. 1994;61:72–76. [PubMed] [Google Scholar]
- Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- Siddhanta A, Backer JM, Shields D. Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J Biol Chem. 2000;275:12023–12031. doi: 10.1074/jbc.275.16.12023. [DOI] [PubMed] [Google Scholar]
- Stam JC, Sander EE, Michiels F, van Leeuwen FN, Kain HET, van der Kammen RA, Collard JG. Targetting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal Pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272:28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- Sung TC, Zhang Y, Morris AJ, Frohman MA. Structured analysis of Human Phospholipase D1. J Biol Chem. 1999;274:3659–3666. doi: 10.1074/jbc.274.6.3659. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type Iα phosphatidylinositol 4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu K, Cullen PJ. Signaling via ADP-ribosylation factor 6 lies downstream of phosphatidylinositide 3-kinase. Biochem J. 2000;345:719–724. [PMC free article] [PubMed] [Google Scholar]
- Wakelam MJO, Hodgkin MN, Martin A. The measurement of phospholipase D-linked signaling in cells. In: Kendall DA, Hill SJ, editors. Signal Transduction Protocols. Totowa, NJ: Humana Press; 1995. pp. 271–278. [DOI] [PubMed] [Google Scholar]
- Way G, O'Luanaigh N, Cockcroft S. Activation of exocytosis by cross-linking of the IgE receptor is dependent on ADP-ribosylation factor 1-regulated phospholipase D in RBL-2H3 mast cells: evidence that the mechanism of activation is via regulation of phosphatidylinositol 4,5-bisphosphate synthesis. Biochem J. 2000;346:63–70. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Altshuller YM, Hammond SM, Morris AJ, Frohman MA. Loss of receptor regulation by a PLD1 mutant unresponsive to protein kinase C. EMBO J. 1999;18:6339–6348. doi: 10.1093/emboj/18.22.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Boman AL, Kuai J, Cieplak W, Kahn RA. Effectors increase the affinity of ADP-ribosylation factor for GTP to increase binding. J Biol Chem. 2000;275:13465–13475. doi: 10.1074/jbc.275.18.13465. [DOI] [PubMed] [Google Scholar]