CONTEXT:
Sepsis-induced coagulopathy leading to disseminated microvascular thrombosis is associated with high mortality and has no existing therapy. Despite the high prevalence of Gram-positive bacterial sepsis, especially methicillin-resistant Staphylococcus aureus (MRSA), there is a paucity of published Gram-positive pediatric sepsis models. Large animal models replicating sepsis-induced coagulopathy are needed to test new therapeutics before human clinical trials.
HYPOTHESIS:
Our objective is to develop a pediatric sepsis-induced coagulopathy swine model that last 70 hours.
METHODS AND MODELS:
Ten 3 weeks old piglets, implanted with telemetry devices for continuous hemodynamic monitoring, were IV injected with MRSA (n = 6) (USA300, Texas Children’s Hospital 1516 strain) at 1 × 109 colony forming units/kg or saline (n = 4). Fluid resuscitation was given for heart rate greater than 50% or mean arterial blood pressure less than 30% from baseline. Acetaminophen and dextrose were provided as indicated. Point-of-care complete blood count, prothrombin time (PT), activated thromboplastin time, d-dimer, fibrinogen, and specialized coagulation assays were performed at pre- and post-injection, at 0, 24, 48, 60, and 70 hours. Piglets were euthanized and necropsies performed.
RESULTS:
Compared with the saline treated piglets (control), the septic piglets within 24 hours had significantly lower neurologic and respiratory scores. Over time, PT, d-dimer, and fibrinogen increased, while platelet counts and activities of factors V, VII, protein C, antithrombin, and a disintegrin and metalloproteinase with thrombospondin-1 motifs (13th member of the family) (ADAMTS-13) decreased significantly in septic piglets compared with control. Histopathologic examination showed minor focal organ injuries including microvascular thrombi and necrosis in the kidney and liver of septic piglets.
INTERPRETATIONS AND CONCLUSIONS:
We established a 70-hour swine model of MRSA sepsis-induced coagulopathy with signs of consumptive coagulopathy, disseminated microvascular thrombosis, and early organ injuries with histological minor focal organ injuries. This model is clinically relevant to pediatric sepsis and can be used to study dysregulated host immune response and coagulopathy to infection, identify potential early biomarkers, and to test new therapeutics.
Keywords: disseminated, intravascular coagulation, methicillin-resistant Staphylococcus aureus, organ injury, pediatrics, sepsis, swine
KEY POINTS
Questions: Numerous controlled multicenter clinical trials of therapeutic adjuvants for sepsis, based mostly on data from small animals, had failed. There is now a consensus that relevant large animal models of sepsis are needed for preclinical studies.
Findings: We established a pediatric 70-hour swine model of methicillin-resistant Staphylococcus aureus sepsis-induced coagulopathy with consumptive coagulopathy, disseminated microvascular thrombosis, and early organ injuries with focal histological organ injuries.
Meanings: This extended 70-hour model can be used to: 1) investigate mechanistic insight into sepsis-induced coagulopathy, and microvascular thrombosis causing cellular and organ injuries; 2) develop biomarkers to detect for early organ injury; and 3) test innovative therapeutic strategies.
Sepsis poses a significant healthcare burden affecting over 1.5 million people and costing over $20 billion in the United States (1). According to the 2017 U.S. Centers for Disease Control and Prevention Data & Report, about 250,000 Americans die of sepsis each year, with one in three mortality in a hospital setting due to sepsis (2). Several large studies have revealed that Staphylococcus aureus is the most common Gram-positive infecting organism in industrialized nations and that S. aureus sepsis is associated with higher mortality (3–8). Importantly, S. aureus sepsis has been associated with disseminated intravascular coagulation (DIC), thromboembolic events, and mortality (9–11).
In children, a prospective pediatric sepsis point-prevalence study reported that S. aureus is the most commonly isolated organism, and mortality due to sepsis increased with multiple organ dysfunction syndrome (MODS) (12). Furthermore, mortality was reported to increase incrementally with each additional dysfunctional organ, such that, mortality was 20% with two dysfunctional organs and more than 80% with six dysfunctional organs (12, 13). Hence, the current consensus among experts is that the future of sepsis research should be directed toward understanding and reversing sepsis-induced MODS (14, 15). Organ dysfunction is now included in the new definition of sepsis for adults—“defined as life-threatening organ dysfunction caused by a dysregulated host response to infection” (16). Syndromes of disseminated microvascular thrombosis such as DIC and thrombocytopenia-associated multiple organ failure (TAMOF) are now considered to be a result of “dysregulated host response to infection” and one of the primary pathogenic mechanisms for the development of MODS during sepsis (17–20). Persistent inflammation activates the coagulation and thrombotic pathways leading to a prothrombotic and antifibrinolytic state. The resultant disseminated fibrin deposition in small to mid-size blood vessels leads to organ ischemia and dysfunction (21). DIC can occur in 29–50% of septic patients and is a predictor of mortality (22–24). TAMOF can occur in 9–15% of pediatric severe septic patients and is associated with 40% mortality (25, 26).
Unfortunately, no therapy exists for sepsis-induced DIC nor TAMOF other than source-control, antibiotics, hemodynamic resuscitation, and supportive care. There is a consensus among experts that clinically relevant large animal models are needed in sepsis research (14, 27). This call for large animal model of sepsis prior to clinical trials is a reflection on our numerous past failures of large multicenter controlled human clinical trials of therapeutic adjuvant for sepsis, most of which have been based on small animal data (27). Large animal model of sepsis continues to be scarce and studied only for shorter duration due to resource intensive and animal welfare concerns, as evidenced by an adult methicillin-resistant S. aureus (MRSA) sepsis swine models up to 48 hours (28, 29) and a pediatric Escherichia coli sepsis model up to 24 hours (30).
Therefore, the aim of our current study was to develop a hemodynamically and clinically relevant conscious pediatric swine model with human MRSA sepsis-induced coagulopathy, disseminated microvascular thrombosis, and organ injuries that can last for 70 hours.
MATERIALS AND METHODS
Anesthesia and Surgery for Piglets
Five male and five female domestic piglets (Sus domesticus) (4 wk old, 8 kg body weight [BW], approximately early toddler age equivalent [1–3 yr old] in human [31]) were obtained from a local commercial swine farm in Texas and acclimated for 7 days to the experimental facility. All animal procedures were reviewed and approved by the Baylor College of Medicine Animal Care and Use Committee (study number: AN-6148 Arginine Metabolism in Sepsis; approved May 1, 2014, to August 24, 2022). All piglets, controls (n = 4) and septic (n = 6), underwent identical surgical procedures, blood draws, and interventions (when needed), irrespective of the treatment. Four days prior to MRSA/saline inoculation, all piglets underwent general isoflurane anesthesia to have a telemetry device implanted in the left femoral artery, a central venous line cannulated in the left external jugular vein, and an arterial catheter cannulated in the left carotid artery (catheter inner diameter = 1 mm, and outer diameter = 1.7 mm). Anesthesia for piglets was induced and maintained with isoflurane (5% and 1–2%, respectively) via face mask. Buprenorphine sustained release (SR) was given subcutaneously to manage postoperative pain. Vital signs were monitored, including respiration rate, heart rate, oxygen saturation, and rectal temperature throughout the surgical procedure with pulse oximeter and thermometer. After the surgery, the piglets were monitored with telemetry device for the next 4 days as they recovered to achieve baseline temperature, heart rate, and blood pressure (BP). After randomization, six piglets were inoculated IV with MRSA, while four piglets acted as healthy controls (IV saline).
Inoculation of MRSA/Saline
Each piglet was kept in its own cage, awake, and free access to food in the animal facility. To minimize pain, discomfort and distress prior to MRSA or saline inoculation, all piglets were subcutaneously provided with buprenorphine SR (0.12–0.24 mg/kg). For these studies, we used a MRSA strain (USA300-HOU-MR-TCH 1516) that was isolated from a critically ill septic child admitted at Texas Children’s Hospital in Houston (32). This strain was chosen because its genomes were fully sequenced and highly representative of isolates causing invasive community acquired S. aureus infection in Houston. This strain contributed to an increased severity in invasive cases including sepsis-induced MODS, osteomyelitis often associated with a contiguous venous thrombosis, pyomyositis, and complicated pneumonia (33–35). The piglets were inoculated with this MRSA strain intravenously via the external jugular central venous catheter over 5 minutes with a dose of 1 × 109 colony forming units (CFU)/kg of BW at a concentration of 1 × 109 CFU/mL. Control piglets were administered normal saline (1 mL/kg BW). In establishing our model, the piglets did not develop disseminated microvascular thrombosis at 48 or 60 hours after MRSA inoculation at the dose of 1 × 108–9 CFU/kg BW (unpublished data), as reported by Soerensen et al (28). Instead, the piglets progressed into significant coagulopathy with DIC, disseminated microvascular thrombosis, and early histological organ injuries at 70 hours with a dose of 1 × 109 CFU/kg BW.
Telemetry Device
A surgically implantable PhysioTel Digital blood pressure and biopotential telemetry device (Data Sciences international, New Brighton, MN) was used in all the piglets to monitor real time hemodynamics.
Neurologic and Respiratory Scores
We examined the piglets and recorded the neurologic and respiratory scores before MRSA/saline inoculation and every 6 hours after MRSA/saline inoculation. For this, we modified a published swine neurologic examination score (36) by adding a respiratory examination score (Supplementary Table 1, http://links.lww.com/CCX/B192).
Fluids Resuscitation for Septic Shock and Other Clinical Managements
Piglets were resuscitated with normal saline bolus (10 mL/kg) when heart rate was greater than 50% or mean arterial BP was less than 30% from established individual baseline values. IV acetaminophen (15 mg/kg) was given as needed every 6 hours if the piglets’ temperature was greater than 41°C for more than 1 hour. Maintenance IV fluid composed of 5% dextrose in normal saline was given when the piglets were unable to eat independently. Blood sugar (Nova StatStrip, Waltham, MA) and arterial blood gas (ABG) (VetScan i-STAT1, San Diego, CA) were measured with scheduled blood draws at 0, 24, 48, 60, and 70 hours or when dictated by clinical signs and symptoms.
Coagulation Assays and Complete Blood Count
We used a commercially available point-of-care clinical coagulation analyzer system (Sysmex CA-600; Siemens Healthcare Diagnostics, Muenchen, Germany) along with Siemens kits to measure human plasma samples for prothrombin time (PT), activated thromboplastin time (aPTT), fibrinogen, d-dimer, and activities of factor V, factor VII, antithrombin, and protein C. Activities of Factors V, VII, antithrombin, and protein C were set to 100% in the pre-MRSA inoculated piglets, and the values for control and post-MRSA piglets were normalized to pre-MRSA values, respectively. Complete blood count including platelet count, WBC count, and hemoglobin was measured using point-of-care scil Vet ABC Hematology Analyzer (SCIL Animal Care Company, Gurnee, IL) with pig-specific smart card. A disintegrin and metalloproteinase with thrombospondin-1 motifs (13th member of the family) (ADAMTS-13) activity was assessed using Technozym ADAMTS-13 activity kit (DiaPharma, West Chester Township, OH).
Swine Cytokine and Cystatin C Assays
Commercially available swine cytokine immunoassays (EPX090-60829-901; Invitrogen, ThermoFisher Scientific, Waltham, MA) and Cystatin C (LS-F36706; LSBio, LifeSpan BioSciences, Seattle, WA) were used to measure plasma interleukin-6 (IL-6), interleukin-1β (IL-1β), and cystatin C concentrations.
Euthanasia
An early euthanasia criterion was established to manage a balance between animal welfare issues and the scientific goals of the study. Pigs were euthanized immediately if they became moribund (lying laterally recumbent and unresponsive to stimuli), showed severe acute respiratory distress, or suffered from inability to tolerate appropriate intake due to abdominal distention, emesis, or decreased consciousness or physical injury. No early euthanasia was necessary in the present study, and pigs were euthanized according to the scientific protocol (70-hr post-inoculation).
Necropsies and Histology
Immediately after euthanasia, kidney and liver tissues were harvested for analysis. Tissues were fixed in either 10% or 20% neutral buffered formalin (VWR, Radnor, PA) prior to processing and paraffin embedding. Paraffin blocks were sectioned into 5 μm and stained with hematoxylin and eosin (H&E) (Richard-Allan Scientific, Kalamazoo, MI) to evaluate microthrombi, microhemorrhages, and cellular/organ injuries. A staff pathologist and a research scientist who were blinded to the treatments reviewed independently the tissue slides.
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Assay
Commercially available terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-based assay of Trevigen Apoptotic Cell System 2 terminal deoxynucleotidyl transferase-diaminobenzidine In Situ Apoptosis Detection (4810-30-K; R&D Systems, Minneapolis, MN) was used to detect apoptosis. Before processing to the labeling reaction, deparaffinization and rehydration of fixed pig kidney and liver sections were performed with the standard protocol. TUNEL assay were done by following the manufacturer’s instruction. TUNEL-positive nuclei images were recorded using a brightfield microscope equipped with digital camera (Olympus 60X).
Statistics
Prism by GraphPad Software and Sigma Plot by Systat Software (La Jolla, CA) were used to perform statistical analyses including two-factor repeated measures analysis of variance (2F-RM-ANOVA) and t test. Values shown are means ± se. We considered p value of less than 0.05 significant.
RESULTS
All ten piglets (six septic and four control) survived through the 70 hours of the study. All septic piglets developed fever (41°C) and tachycardia by 12 hours after the bacterial insult. At 24 hours, a drip of 5% dextrose in normal saline solution was started at a rate of 4 mL/kg per hour in septic piglets because they were prostrated and unable to eat. Feed was removed from the control piglets, which also received 5% dextrose in normal saline solution at 4 mg/kg/hr. Of the six septic piglets, the condition of three piglets (50%) necessitated the use of one normal saline fluid bolus (10 mL/kg) each at 40, 60, and 64 hours timepoints for persistent heart rate greater than 50% from baseline, and one dose of IV acetaminophen (15 mg/kg) each at 29, 36, and 60 hours timepoints for sustained body temperature greater than 41°C. However, none of the piglets developed the predetermined hypotension defined as less than 30% of mean arterial BP from established individual baseline values. Septic piglets had clinically significant tachycardia over the 70 hours study period compared with control piglets (Supplementary Fig. 1A, http://links.lww.com/CCX/B192). Whereas, the mean arterial BP was not clinically different over time between the septic and control piglets (Supplementary Fig. 1B, http://links.lww.com/CCX/B192). At 36 hours, all septic piglets received one dose of 50% dextrose bolus (0.5 g/kg) for blood sugar level less than 40 mg/dL and the 5% dextrose in normal saline solution infusion rate was increased to 8 mL/kg/hr.
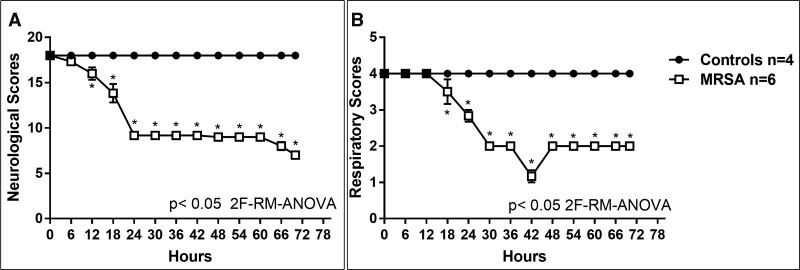
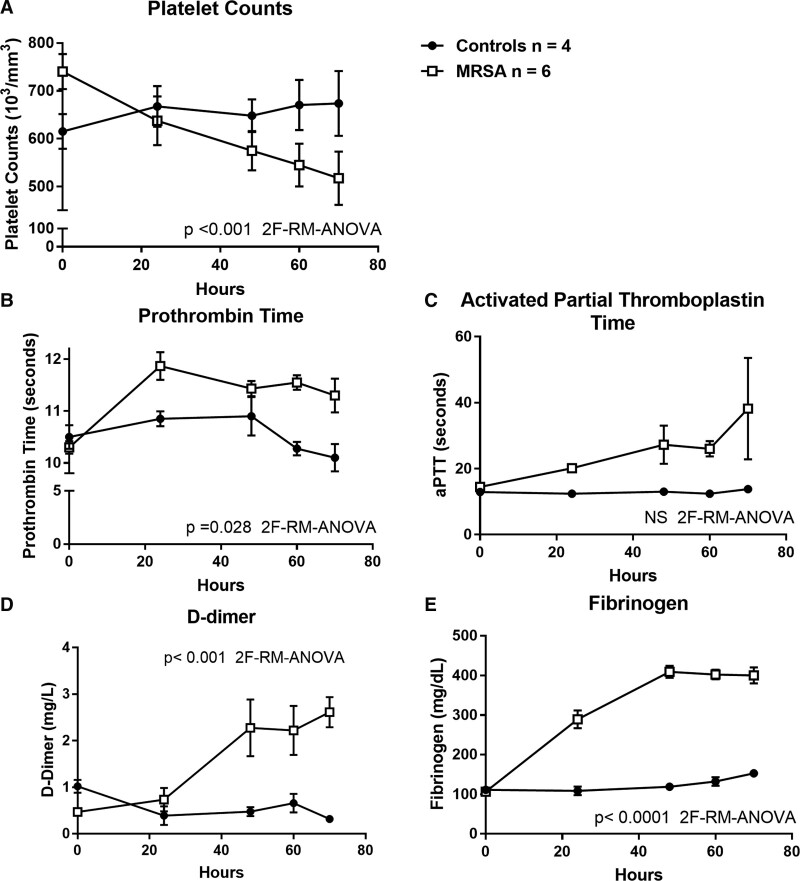
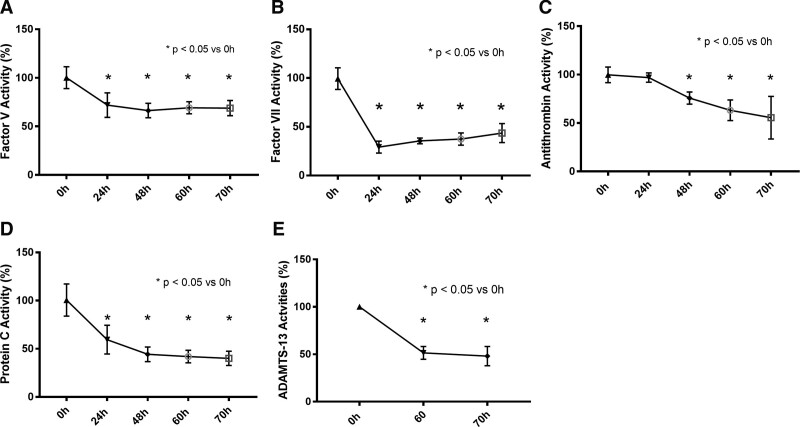
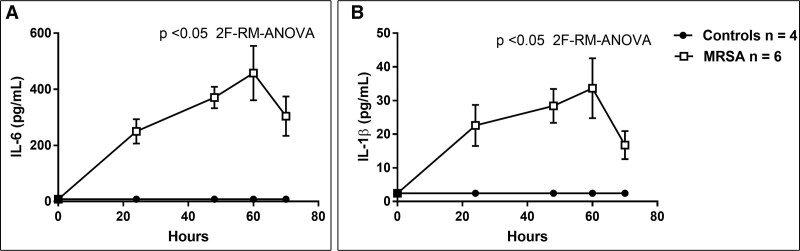
Compared with control piglets, septic piglets had significantly lower neurologic (p < 0.05; Fig. 1A) and respiratory scores (p < 0.05; Fig. 1B) by 12 and 18 hours, respectively. Platelet counts significantly decreased in the septic piglets over the 70 hours study period, compared with control (p < 0.001; Fig. 2A). Compared with the control, PT, d-dimer and fibrinogen levels were significantly increased in the septic piglets over time (p < 0.001; Fig. 2, B, D, and E). Although the septic piglets displayed a change in the aPTT compared with the control, these values did not reach statistical significance (Fig. 2C). Activities of coagulation factor V, factor VII, and protein C were significantly decreased by 24 hours post-MRSA, compared with the pre-inoculation (p < 0.05; Fig. 3, A, B, and D). Anti-coagulant activity of antithrombin was significantly decreased by 48 hours post-MRSA, compared with the pre-inoculation (p < 0.05; Fig. 3C). ADAMTS-13 activity, critical for normal von Willebrand factor/platelet hemostatic pathway, was significantly decreased by 60 hours post-MRSA, compared with pre-inoculation (p < 0.05; Fig. 3E). In contrast, inflammatory cytokines IL-6 and IL-1β levels were significantly elevated over time in MRSA septic piglets, compared with control (p < 0.05; Fig. 4, A and B).
Figure 1.
Neurologic scores (A) and respiratory scores (B) in controls (n = 4) versus methicillin-resistant Staphylococcus aureus (MRSA) septic (n = 6) piglets. X-axis = hours after MRSA inoculation; y-axis = arbitrary units; symbols are means ± se. 2F-RM-ANOVA = two-factor repeated measures analysis of variance.
Figure 2.
Platelet counts (A), prothrombin time (B), activated partial thromboplastin time (aPTT) (C), d-dimer (D), and fibrinogen (E) in controls (n = 4) versus methicillin-resistant Staphylococcus aureus (MRSA) septic (n = 6) piglets. X-axis = hours after MRSA inoculation; symbols are means ± se. 2F-RM-ANOVA = two-factor repeated measures analysis of variance, NS = not significant.
Figure 3.
Activities of factors V (A), VII (B), antithrombin (C), protein C (D), and a disintegrin and metalloproteinase with thrombospondin-1 motifs (13th member of the family) (ADAMTS-13) (E) activities in methicillin-resistant Staphylococcus aureus (MRSA) septic (n = 6) piglets. X-axis = hours after MRSA inoculation; *one-way analysis of variance; symbols are means ± se.
Figure 4.
Inflammatory cytokines interleukin-6 (IL-6) (A) and interleukin-1β (IL-1β) (B) in controls (n = 4) versus methicillin-resistant Staphylococcus aureus (MRSA) septic (n = 6) piglets. X-axis = hours after MRSA inoculation; symbols are means ± se. 2F-RM-ANOVA = two-factor repeated measures analysis of variance.
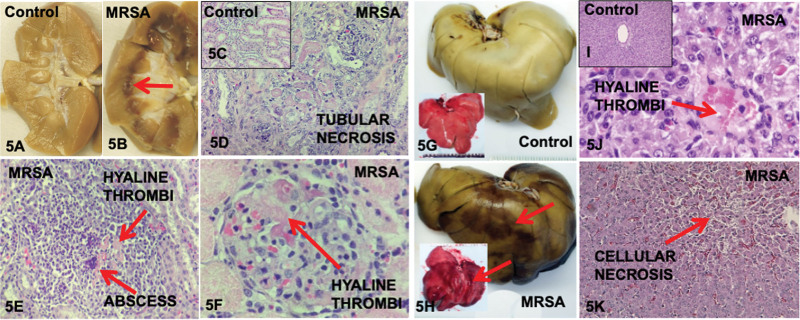
Upon gross examination of the organs during in situ necropsy of all septic piglets, we observed discreet areas of dark discoloration in all pre-formalin and post-formalin fixation of the kidneys (100%) especially in the medulla (Fig. 5B) and also in the livers (100%) (Fig. 5H), compared with none seen in control (Fig. 5, A and G). Histology analysis of the these darkened discolored areas with H&E staining revealed focal areas of tubular necrosis in kidneys (Fig. 5D) and focal areas of cellular necrosis in the liver (Fig. 5K) of all six septic piglets (100%) compared with none observed in control (Fig. 5, C and I). These (H&E) areas of necrosis occur in a minor proportion of the tissues inspected. We did not find cellular injuries in the nondiscolored areas of the tissues. In two septic and two control piglets, TUNEL staining was performed which sparingly showed apoptosis in the discolored areas of in the kidney and liver of the septic piglets compared none seen in the control (Supplementary Fig. 2, http://links.lww.com/CCX/B192). Microabscesses were observed sparingly in the kidneys and livers of all septic piglets (Fig. 5E). Microvascular thrombosis as evidenced by hyaline thrombi was observed in five kidneys (83%) (Fig. 5, E and F) and six livers (100%) (Fig. 5J). Microhemorrhages were seen in three kidneys (50%) from septic piglets. Serum levels of liver enzymes (alanine transaminase [AST], aspartate aminotransferase [ALT]), serum creatinine, and cystatin C were not significantly elevated over time in this 70 hours MRSA sepsis model (Supplementary Tables 2 and 3, http://links.lww.com/CCX/B192). In an earlier set of MRSA dose and duration titration experiments, serum Cr, AST, and ALT levels were not elevated at earlier timepoints (24, 36, 48, 60, and 70 hr post-inoculation) in three septic piglets with 1 × 109 CFU of MRSA/kg BW inoculation (Supplementary Table 4, http://links.lww.com/CCX/B192). Throughout the 70 hours sepsis experiments, piglets progressed to moderate respiratory distress, but the measured ABGs did not indicate significant hypoxic nor hypercarbic condition (Fig. 1; and Supplementary Table 5, http://links.lww.com/CCX/B192).
Figure 5.
Kidneys and livers of control and methicillin-resistant Staphylococcus aureus (MRSA) septic piglets shown pre-/post-formalin fixation and under microscopy. Post-formalin fixation kidneys revealed discoloration especially in the medulla of MRSA septic piglets (B) compared with control (A). Hematoxylin & eosin (H&E) staining of control showed normal renal tubules (C) compared with tubular necrosis (D), hyaline thrombi and abscess (E), and glomerular hyaline thrombi (F) in MRSA septic piglets. Pre-/post-formalin fixation livers showed discoloration in MRSA septic piglets (H) compared with control (G). H&E staining showed normal liver structure in control (I) compared hyaline thrombi (J) and cellular necrosis (K) in MRSA septic piglets.
DISCUSSION
Over the past decades, numerous multicenter clinical trials of therapeutic adjuvants for sepsis, based mostly on data from small animals had failed (27). There is now a consensus that relevant large animal models of sepsis are needed for preclinical studies (14, 37). Here, we have developed a 70 hours pediatrics swine model using human MRSA, which caused sepsis-induced coagulopathy and showed signs of disseminated microvascular thrombosis and early organ injuries with histological evidence of focal areas of organ injuries. This extended 70 hours model has the potential to be used in future studies to: 1) investigate mechanistic insight into sepsis-induced coagulopathy, and microvascular thrombosis causing cellular and organ injuries; 2) develop biomarkers to detect for early organ injury; and 3) test innovative therapeutic strategies.
Pigs and humans share a greater similarity in genetics, anatomy, immunology, and physiology, when compared with mice and humans (38–40). Pig models, in particular, have been suggested for preclinical studies in sepsis research (27). Accordingly, investigators have reported adult MRSA sepsis swine models up to 48 hours (28, 29), juvenile MRSA swine model up to 30 hours (41), and pediatric E. coli sepsis model up to 24 hours (30). However, these models were studied for a shorter period (24–48 hr), in part, due the substantial resources needed to carrying out a large animal model, and due to animal welfare issues (41).
Our swine model exhibited several differences compared with these published large animal models. First, we titrated the dose of MRSA (unpublished) to achieve significant coagulopathy and disseminated intravascular thrombosis which resulted in our 70 hours model. This extended model allows time for immune dysregulation to drive the development of coagulopathy compared with shorter models which depends on hemodynamic compromised with resultant ischemia to drive the development of coagulopathy. Our approach incorporated individualized care, similar to the one used for critically ill patients in a hospital setting. Specifically, the use of telemetry devices provided vital signs of each piglet continuously in real time. This allowed us to intervene as needed with fluid resuscitation for predefined shock, and IV acetaminophen for persistent fever. The piglets were continuously monitored with dedicated staff outside the room monitoring vital signs, conducting neurologic/respiratory scores every 6 hours, performing point-of care assays, and placing calls for backup help, if needed. Second, unlike previous pig models which used swine MRSA strain, we used human pathologic MRSA strain (32–35). Third, piglets were conscious throughout the 70 hours and did not have significant hemodynamic instability. As a result, our model was not lethal as observed in other endotoxemia or Gram-negative bacterial sepsis models with significant hemodynamic instability and deaths (42, 43).
Systemic inflammatory response syndrome started approximately 12-hour post-MRSA inoculation with fever, tachycardia, and decreased neurologic scores. Only three septic piglets met our criteria for fluid resuscitation (total of 10 mL/kg each due to tachycardia > 50% from baseline) starting at 40 hours. By 24 hours post-MRSA, DIC panels demonstrated that all septic piglets were progressing toward consumptive coagulopathy as suggested by international definition of DIC (44). Over time, platelet counts decreased and PT/aPTT was prolonged. The rising d-dimer level supported the notion of ongoing fibrin mesh formation that was likely fibrinolysed by endogenous plasmin. Despite the high d-dimer, fibrinogen levels did not decrease in septic piglets at 70 hours. We speculated that for fibrinogen levels to decrease, a septic model beyond 70 hours would be needed. Of note, Wada et al (45) reported that 47% of 560 patients diagnosed with DIC had elevated fibrinogen level. Furthermore, in patients with DIC due to sepsis, the frequency of organ failure increased concomitantly with increasing fibrinogen level suggesting that fibrinogen level may not be a sensitive marker for DIC. Our septic piglets progressed to consumptive coagulopathy with significant decrease in factors V, VII, antithrombin, protein C, and ADAMTS13 activities (21, 46). Indeed, acquired ADAMTS-13 deficiency is well described in sepsis-induced TAMOF phenotype (19, 25). Lastly, inflammatory cytokines IL-6 and IL-1β were significantly elevated in septic piglets. Thus, our model had three key elements that supported the pathogenesis of DIC: 1) systemic inflammation; 2) impaired anticoagulant pathway; and 3) impaired fibrinolytic pathway (21).
The consumptive coagulopathy and histological evidence of focal organ injuries observed in this model were not due to hypotension because the continuous mean arterial BP measurements were never below 30% from baseline. We speculated that the observed coagulopathy and histological organ injuries seen in this model were likely due to the dysregulated host immune response to MRSA infection. This was an early organ injuries model as it importantly revealed that current bedside laboratory evidence of organ dysfunction such as elevated AST, ALT, and creatinine levels was a late sign of cellular and organ injuries. This lengthened model mimicked the real world setting as patients often did not seek medical attention until they became much sicker when the pathology of organ injuries had already initiated. This perhaps reflected the observation that even when clinicians successfully resuscitated septic shock by abiding to international consensus guidelines (47, 48), a subset of patients continued to progress into DIC and MODS.
Necropsies demonstrated heterogeneous dark color discoloration on gross examination in the liver and in the kidney medulla of septic piglets. These discolored organs displayed heterogeneous focal areas of abnormalities including tubular necrosis, microthrombi, microhemorrhages, infarct, congestion, and lymphocytic infiltration in septic piglets. The significance of discoloration in kidney medulla is unclear, but is consistent with a previous report of decreased medullary oxygenation and perfusion compared with preserved cortical oxygenation and perfusion in a 48 hours sheep model of E. coli sepsis-induced acute kidney injury (49). However, the sheep model did not observe abnormal histological changes in the kidney medulla (50). It remains unclear whether the regional histological injuries with concomitant discoloration seen in the kidney medulla of our model is due to the use of 4-week-old piglets with MRSA instead of adult sheep’s with E. coli.
There are limitations in this study. This model did not meet the current widely accepted definition of organ dysfunction with elevated AST, ALT, and creatinine levels. In this awake MRSA sepsis model, the piglets progressed to early organ injuries with severe coagulopathy and did not progress to clinical MODS observed in the ICU that necessitated invasive interventions such as mechanical ventilation and continuous analgesic and sedative agents. Due to resource limitation and animal welfare concerns, we limited this awake MRSA model to 70 hours. The worsening neurologic and respiratory scores, the hematologic perturbation, the elevated cytokine levels, and the necropsy abnormalities were the only evidence of organ injuries. There were no deaths. The study used 4-week-old piglets that might respond differently than adult pigs. We used MRSA strain isolated from humans and not from pigs. We injected a bolus dose of bacteria over a short amount of time, which did not mimic real clinical infection. We did not use antibiotics, which is standard after sepsis recognition, and this may affect the course of observed coagulopathy and organ injuries. Even though we had equal males and females in our study, our study was not powered for analysis of sex differences in hemodynamics variables and biomarkers.
In summary, we have established a novel awake and hemodynamically relevant swine model of human pathogenic MRSA sepsis-induced coagulopathy with signs of DIC and early organ injuries with histological evidence of focal organ injuries. The coagulopathy and organ injuries are not due to hypotension and may be due to dysregulated host immune response to MRSA infection. This extended 70 hours model can be used in future studies to: 1) investigate mechanistic insight into sepsis-induced coagulopathy, and microvascular thrombosis causing cellular and organ injuries, 2) develop biomarkers to detect for early organ injury, and 3) test innovative therapeutic strategies.
ACKNOWLEDGMENTS
We thank Qi Da, PhD for helping with the experiments.
Supplementary Material
Footnotes
Drs. Nguyen, Vijayan, and Cruz received funding from National Institute of General Medical Sciences (NIGMS; R01GM112806). Dr. Marini received funding from NIGMS (R01GM108940). The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content of this article does not represent the views of the Department of Veterans Affairs or the U.S. Government.
Drs. Nguyen, Marini, and Cruz designed and performed the research, analyzed the data, and wrote the article. Dr. Guillory, Dr. Valladolid-Brown, Dr. Cohen, and Ms. Cirlos performed the research, analyzed the data, and wrote the article. Dr. Subramanyam, Dr. Lam, Dr. Stoll, Ms. Didelija, Ms. Vonderohe, Dr. Orellana, Dr. Saini, Dr. Pradhan, Dr. Bashir, Dr. Desai, Dr. Flores, Dr. Virk, Dr. Tcharmtchi, Dr. Navaei, Dr. Kaplan, Ms. Lamberth, Dr. Hulten, Mr. Scull, Dr. Allen, Dr. Akcan-Arikan, and Dr. Vijayan performed the research, and wrote the article and all authors checked the final version of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Hershey TB, Kahn JM: State sepsis mandates - a new era for regulation of hospital quality. N Engl J Med 2017; 376:2311–2313 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: CDC Urges Early Recognition, Prompt Treatment of Sepsis. Centers for Disease Control and Prevention 2017 Report. 2017. Available at: https://www.cdc.gov/media/releases/2017/p0831-sepsis-recognition-treatment.html Accessed May 8, 2023
- 3.Weiner LM, Webb AK, Limbago B, et al. : Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol 2016; 37:1288–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent JL, Rello J, Marshall J, et al. : International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–2329 [DOI] [PubMed] [Google Scholar]
- 5.Laupland KB: Incidence of bloodstream infection: A review of population-based studies. Clin Microbiol Infect 2013; 19:492–500 [DOI] [PubMed] [Google Scholar]
- 6.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 7.McMullan BJ, Bowen A, Blyth CC, et al. : Epidemiology and mortality of Staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatr 2016; 170:979–986 [DOI] [PubMed] [Google Scholar]
- 8.Hassoun A, Linden PK, Friedman B: Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care 2017; 21:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T, Kyle UG, Jaimon N, et al. : Coinfection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med 2012; 40:3246–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kravitz GR, Dries DJ, Peterson ML, et al. : Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis 2005; 40:941–947 [DOI] [PubMed] [Google Scholar]
- 11.Carpenter SL, Goldman J, Sherman AK, et al. : Clinical variables and Staphylococcus aureus virulence factors associated with venous thromboembolism in children. Thromb Res 2016; 138:69–73 [DOI] [PubMed] [Google Scholar]
- 12.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JC, Spinella PC, Fitzgerald JC, et al. : New or progressive multiple organ dysfunction syndrome in pediatric severe sepsis: A sepsis phenotype with higher morbidity and mortality. Pediatr Crit Care Med 2017; 18:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J, Vincent JL, Adhikari NK, et al. : Sepsis: A roadmap for future research. Lancet Infect Dis 2015; 15:581–614 [DOI] [PubMed] [Google Scholar]
- 15.Tamburro RF, Jenkins TL, Kochanek PM: Strategic planning for research in pediatric critical care. Pediatr Crit Care Med 2016; 17:e539–e542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angus DC, van der Poll T: Severe sepsis and septic shock. N Engl J Med 2013; 369:2063. [DOI] [PubMed] [Google Scholar]
- 18.Levi M, van der Poll T: Coagulation and sepsis. Thromb Res 2017; 149:38–44 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TC, Han YY, Kiss JE, et al. : Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med 2008; 36:2878–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carcillo JA, Podd B, Aneja R, et al. : Pathophysiology of pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med 2017; 18(3 Suppl 1):S32–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gando S, Levi M, Toh CH: Disseminated intravascular coagulation. Nat Rev Dis Primers 2016; 2:16037. [DOI] [PubMed] [Google Scholar]
- 22.Khemani RG, Bart RD, Alonzo TA, et al. : Disseminated intravascular coagulation score is associated with mortality for children with shock. Intensive Care Med 2009; 35:327–333 [DOI] [PubMed] [Google Scholar]
- 23.Dhainaut JF, Yan SB, Joyce DE, et al. : Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost 2004; 2:1924–1933 [DOI] [PubMed] [Google Scholar]
- 24.Zeerleder S, Hack CE, Wuillemin WA: Disseminated intravascular coagulation in sepsis. Chest 2005; 128:2864–2875 [DOI] [PubMed] [Google Scholar]
- 25.Carcillo JA, Berg RA, Wessel D, et al. : A multicenter network assessment of three inflammation phenotypes in pediatric sepsis-induced multiple organ failure. Pediatr Crit Care Med 2019; 20:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carcillo JA, Halstead ES, Hall MW, et al. : Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. Pediatr Crit Care Med 2017; 18:513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink MP, Warren HS: Strategies to improve drug development for sepsis. Nat Rev Drug Discovery 2014; 13:741–758 [DOI] [PubMed] [Google Scholar]
- 28.Soerensen KE, Olsen HG, Skovgaard K, et al. : Disseminated intravascular coagulation in a novel porcine model of severe Staphylococcus aureus sepsis fulfills human clinical criteria. J Comp Pathol 2013; 149:463–474 [DOI] [PubMed] [Google Scholar]
- 29.Leifsson PS, Iburg T, Jensen HE, et al. : Intravenous inoculation of Staphylococcus aureus in pigs induces severe sepsis as indicated by increased hypercoagulability and hepatic dysfunction. FEMS Microbiol Lett 2010; 309:208–216 [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse A, Leslie D, Bolgen D, et al. : Modified clinical monitoring assessment criteria for multi-organ failure during bacteremia and sepsis progression in a pig model. Adv Crit Care Med 2018; 1:002 [Google Scholar]
- 31.Tohyama S, Kobayashi E: Age-appropriateness of porcine models used for cell transplantation. Cell Transplant 2019; 28:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Highlander SK, Hulten KG, Qin X, et al. : Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus. BMC Microbiol 2007; 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez BE, Hulten KG, Dishop MK, et al. : Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis 2005; 41:583–590 [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. : Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 2005; 115:642–648 [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez BE, Teruya J, Mahoney DH, Jr, et al. : Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics 2006; 117:1673–1679 [DOI] [PubMed] [Google Scholar]
- 36.Rhee P, Talon E, Eifert S, et al. : Induced hypothermia during emergency department thoracotomy: An animal model. J Trauma 2000; 48:439–447; discussion 447–450 [DOI] [PubMed] [Google Scholar]
- 37.Fink MP: Animal models of sepsis. Virulence 2014; 5:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawson HD: Comparative assessment of the pig, mouse, and human genomes: A structural and functional analysis of genes involved in immunity. In: The Minipig in Biomedical Research. McAnulty PA, Dayan A, Hastings KH, et al. (Eds). Boca Raton, FL, CRC Press, 2011, pp 321–341 [Google Scholar]
- 39.Dawson HD, Smith AD, Chen C, et al. : An in-depth comparison of the porcine, murine and human inflammasomes; lessons from the porcine genome and transcriptome. Vet Microbiol 2017; 202:2–15 [DOI] [PubMed] [Google Scholar]
- 40.Meurens F, Summerfield A, Nauwynck H, et al. : The pig: A model for human infectious diseases. Trends Microbiol 2012; 20:50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen HG, Kjelgaard-Hansen M, Tveden-Nyborg P, et al. : Modelling severe Staphylococcus aureus sepsis in conscious pigs: Are implications for animal welfare justified? BMC Res Notes 2016; 9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ten Have GAM, Deutz RCI, Engelen M, et al. : Characteristics of a pseudomonas aeruginosa induced porcine sepsis model for multi-organ metabolic flux measurements. Lab Anim 2018; 52:163–175 [DOI] [PubMed] [Google Scholar]
- 43.Duburcq T, Durand A, Tournoys A, et al. : Single low dose of human recombinant Antithrombin (ATryn) has no impact on endotoxin-induced disseminated intravascular coagulation: An experimental randomized open label controlled study. Shock 2019; 52:e60–e67 [DOI] [PubMed] [Google Scholar]
- 44.Taylor FB, Jr, Toh CH, Hoots WK, et al. ; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH): Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001; 86:1327–1330 [PubMed] [Google Scholar]
- 45.Wada H, Mori Y, Okabayashi K, et al. : High plasma fibrinogen level is associated with poor clinical outcome in DIC patients. Am J Hematol 2003; 72:1–7 [DOI] [PubMed] [Google Scholar]
- 46.Levi M, Dorffler-Melly J, Reitsma P, et al. : Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein-C-deficient mice. Blood 2003; 101:4823–4827 [DOI] [PubMed] [Google Scholar]
- 47.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 48.Davis AL, Carcillo JA, Aneja RK, et al. : American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017; 45:1061–1093 [DOI] [PubMed] [Google Scholar]
- 49.Calzavacca P, Evans RG, Bailey M, et al. : Cortical and medullary tissue perfusion and oxygenation in experimental septic acute kidney injury. Crit Care Med 2015; 43:e431–e439 [DOI] [PubMed] [Google Scholar]
- 50.Langenberg C, Gobe G, Hood S, et al. : Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med 2014; 42:e58–e67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.