Abstract
Background
Safe and effective respiratory syncytial virus (RSV) vaccines remain elusive. This was a phase I/II trial (NCT02927873) of ChAd155-RSV, an investigational chimpanzee adenovirus-RSV vaccine expressing 3 proteins (fusion, nucleoprotein, and M2-1), administered to 12–23-month-old RSV-seropositive children followed up for 2 years after vaccination.
Methods
Children were randomized to receive 2 doses of ChAd155-RSV or placebo (at a 1:1 ratio) (days 1 and 31). Doses escalated from 0.5 × 1010 (low dose [LD]) to 1.5 × 1010 (medium dose [MD]) to 5 × 1010 (high dose [HD]) viral particles after safety assessment. Study end points included anti–RSV-A neutralizing antibody (Nab) titers through year 1 and safety through year 2.
Results
Eighty-two participants were vaccinated, including 11, 14, and 18 in the RSV-LD, RSV-MD, and RSV-HD groups, respectively, and 39 in the placebo groups. Solicited adverse events were similar across groups, except for fever (more frequent with RSV-HD). Most fevers were mild (≤38.5°C). No vaccine-related serious adverse events or RSV-related hospitalizations were reported. There was a dose-dependent increase in RSV-A Nab titers in all groups after dose 1, without further increase after dose 2. RSV-A Nab titers remained higher than prevaccination levels at year 1.
Conclusions
Three ChAd155-RSV dosages were found to be well tolerated. A dose-dependent immune response was observed after dose 1, with no observed booster effect after dose 2. Further investigation of ChAd155-RSV in RSV-seronegative children is warranted.
Clinical Trials Registration
Keywords: immunogenicity, neutralizing antibodies, respiratory syncytial virus, safety, vaccine
Vaccination with a chimpanzee-derived replication-deficient adenovector that encodes 3 respiratory syncytial virus (RSV) proteins induced dose-dependent RSV-neutralizing antibodies that persisted over 1 year. No vaccine-related serious adverse events or RSV-related hospitalizations were reported.
Respiratory tract infections (RTIs) caused by respiratory syncytial virus (RSV) are a common cause of hospitalization among children <5 years of age. In 2019, RSV-associated infections accounted for about half (median rate, 514 per 1000) of all hospitalizations for acute lower RTIs (LRTIs) in children <5 years old across 58 countries, of whom about half were aged <1 year [1]. In 2015, 48 000–74 500 children aged <5 years died during hospitalization for RSV globally, most in developing countries [2, 3]. There is an urgent need for an effective vaccine to prevent childhood RTIs due to RSV. However, 60 years after the development of the first investigational RSV vaccine and despite numerous subsequent candidates using a range of different approaches, a safe and effective vaccine remains elusive [4].
The first investigational RSV vaccine formulation studied in pediatric field trials was a formalin-inactivated whole-virus vaccine. Seronegative vaccine recipients who later acquired natural RSV infection experienced more severe RSV disease than those who received placebo [5]. This unexpected phenomenon came to be known as vaccine-associated enhanced respiratory disease (VAERD). The underlying immunological cause of VAERD is not fully understood, but contributing factors are thought to include a magnified CD4+ T-helper (Th) 2 T-cell memory response [6, 7]. Evaluation of new candidate RSV vaccines must therefore demonstrate a balanced immune response and the absence of VAERD in seronaive populations as a critical step in development.
Since the failure of the formalin-inactivated RSV vaccine in the 1960s, development of new RSV vaccine candidates for infants has focused on alternative approaches, including the use of viral vectors [4, 8]. Viral vectors present RSV antigens to cells in a manner mimicking natural RSV infection and are therefore expected to induce robust humoral and cellular responses without risk of VAERD. Chimpanzee-derived adenoviruses (ChAd) exhibit sequence homology to human adenoviruses, are not known to cause illness, and have low levels of seroprevalence in humans [9]. The candidate vaccine studied here, ChAd155-RSV, is a chimpanzee-derived replication-deficient adenovector that encodes 3 RSV proteins: fusion (F), nucleoprotein (N) and matrix (M2-1) [10].
In a phase I study conducted in healthy adults, ChAd155-RSV was well tolerated and induced increases in RSV-A neutralizing antibody (Nab) titers, increases in RSV-F–, RSV-N–, and RSV-M2-1–specific interferon (IFN) γ–secreting T cells, and a low CD8+ IFN-γ response [11]. Based on those results, we conducted the first pediatric trial of ChAd155-RSV (NCT02927873; https://www.clinicaltrials.gov/ct2/show/NCT02927873), designed to evaluate the safety, reactogenicity, and immunogenicity of 2 doses of ChAd155-RSV (3 dose levels) administered to RSV-seropositive children aged 12–23 months. This population was selected to evaluate the tolerance to the ChAd155-RSV vaccine in a population naturally primed against RSV and, therefore, considered at low risk of VAERD, before conducting any study in seronegative infants.
METHODS
Study Design and Population
This phase I/II randomized, controlled, dose escalation study was conducted across 29 sites located in Canada, Italy, Mexico, Panama, Spain, Taiwan, and the United States. Three formulations of vaccine were tested using a dose escalation design, allowing sequential advancement to the next dose following data reviews by both an internal safety review committee and an independent data monitoring committee. The study was observer blind; the investigational vaccine was administered by unblinded team members who did not participate in any of the study evaluation procedures.
The primary study objective was to evaluate the safety and reactogenicity of the 3 increasing dose levels of ChAd155-RSV up to 30 days after dose 2 (day 61). Secondary objectives included evaluation of safety, occurrence of RSV RTIs throughout the entire study period (through day 731), and vaccine-associated humoral and cell-mediated immune (CMI) responses through day 366.
Study participants were children aged 12–23 months who were seropositive for RSV as determined by enzyme-linked immunosorbent assay (IBL International; see Supplement). Written informed consent was obtained from parents or legal representatives before enrollment. Inclusion and exclusion criteria are provided in the Supplement.
Vaccines were randomized (1:1) in blocks using MATerial Excellence (MATEX), a program developed for use in Statistical Analysis System (SAS®) (Cary, NC, USA) by GSK. Participant allocation to each treatment group was performed at the investigator site using an online randomization system with a minimization procedure accounting for the study center. The study was approved by local independent ethics committees (see Supplement).
Vaccines
Three dose levels of ChAd155-RSV were evaluated: low dose (LD; 0.5 × 1010 viral particles), medium dose (MD; 1.5 × 1010), and high dose (HD; 5 × 1010), suspended in formulation buffer S9b (containing disodium hydrogen phosphate, monopotassium phosphate, sodium chloride, potassium chloride, and magnesium chloride). Placebo consisted of formulation buffer S9b alone. The study vaccines, in volumes of 0.5 mL for the LD and HD groups and 0.15 mL for the MD group, were administered as 2 doses of placebo or 2 doses of active vaccine given intramuscularly 30 days apart.
Safety Monitoring
Local and general symptoms were solicited using diary cards for 7 days after each dose. All other (unsolicited) adverse events (AEs) were collected for 30 days after each dose, and serious AEs (SAEs) were captured until the study conclusion. An RSV LRTI occurring at any time during the study was an AE of special interest (AESI). Blood samples were collected for biochemical testing at baseline and on days 31 and 61 and for hematological testing at baseline and on days 2, 31, 32, and 61 and on days 8 and 38 in children with a grade ≥1 abnormality detected at day 2 and/or day 32. As transient non–clinically significant decreases in platelet counts (maximal decrease observed 24 hours after vaccination) were noted in preclinical studies, hematological and clinical monitoring was conducted at each visit through day 61, with special attention given to the detection of potential spontaneous bleeding, easy bruising, and/or petechiae induced by the tourniquet. Parents were instructed to report any symptoms of spontaneous bleeding, easy bruising, or rash that occurred within 30 days after each vaccination.
Monitoring for RSV Disease
Active surveillance for RSV RTIs began immediately after dose 1 and included weekly parental contact during RSV season and monthly contact outside the RSV season. Passive surveillance throughout the study duration consisted of parents notifying study staff as soon as new RTI symptoms occurred. Monthly nasal swab samples were collected during RSV season. Parents were given RTI episode cards on which to record the occurrence and duration of cough, runny nose, blocked nose, difficulty breathing, or wheezing.
Episodes of RSV RTI were classified by the investigator as RTI, LRTI, severe LRTI, or very severe LRTI (Supplementary Table 1), based on World Health Organization case definitions [12]. RSV infection was confirmed based on nasal swab samples positive for RSV-A or RSV-B with quantitative reverse transcription polymerase chain reaction.
Immunogenicity
Nab responses against RSV-A and total (prefusion and postfusion) anti–RSV-F antibody concentrations were measured in serum samples collected from all participants at baseline and on days 31, 61, and 366. CMI responses were measured on days 8, 31, 38, 61, and 366 in participants at sites with a suitable laboratory in proximity. Detailed descriptions of each of the assays and response definitions are provided in the Supplement.
Statistical Analysis
The study analysis was descriptive. The safety analysis was conducted using data collected from all vaccinated participants. The immunogenicity analyses were performed on the per-protocol cohort at defined time points and included all vaccinated participants who complied with protocol-defined procedures and had immunogenicity data available.
The frequency of CD3+/CD4+ and CD3+/CD8+ T cells expressing ≥2 markers among CD40 ligand (CD40L), interleukin 2, tumor necrosis factor α, and IFN-γ on stimulation with F, N, and M2-1 peptide pools was calculated. CMI responses after exposure to RSV-F, RSV-N, and RSV-M2-1 peptide pools were grouped to indicate Th1 (CD4+ T cells expressing at least IFN-γ but not interleukin 13), Th2 (CD4+ T cells expressing at least interleukin 13 but not IFN-γ), or Th17 (CD4+ T cells expressing at least interleukin 17) profiles. For humoral immune responses, geometric mean concentrations or geometric mean titers (GMTs) were calculated with 95% confidence intervals (CIs).
No hypothesis-driven sample size calculation was conducted owing to the descriptive nature of the study objectives. A sample size of 32 participants per dose cohort randomized to vaccine or placebo was planned based on conventional study designs for phase I/II trials. If no AE was observed among the 16 ChAd155-RSV recipients included in each cohort, the exact 2-sided 95% CI rules out an AE rate of ≥20%.
Supplementary Material
Contributor Information
Javier Díez-Domingo, Vaccine Research Department, FISABIO Public Health, Valencia, Spain.
Xavier Sáez-Llorens, Department of Infectious Diseases, Hospital del Niño Dr José Renán Esquivel and Cevaxin Clinical Research Center, Panama City, Panama; National Investigation System, Senacyt, Panama City, Panama.
Miguel A Rodriguez-Weber, Department of Neonatology, Instituto Nacional de Pediatría, Mexico City, Mexico.
Cristina Epalza, Pediatric Infectious Diseases Unit, Department of Pediatrics, Hospital Universitario 12 de Octubre, Madrid, Spain; Research and Clinical Trials Unit, Madrid, Spain; Pediatric Research and Clinical Trials Unit (UPIC), Instituto de Investigación Sanitaria Hospital 12 de Octubre, Madrid, Spain; RITIP (Traslational Research Network in Pediatric Infectious Diseases), Fundación para la Investigación Biomédica del Hospital 12 de Octubre, Madrid, Spain.
Archana Chatterjee, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, USA.
Cheng-Hsun Chiu, Department of Pediatrics, Chang Gung Memorial Hospital, Chang Gung University Taoyuan, Taoyuan, Taiwan.
Chien-Yu Lin, Department of Pediatrics, Hsinchu Mackay Memorial Hospital, Hsinchu City, Taiwan.
Andrea A Berry, Department of Pediatrics and Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Federico Martinón-Torres, Translational Pediatrics and Infectious Diseases, Pediatrics Department, Hospital Clínico Universitario de Santiago, Santiago de Compostela, Spain; Genetics, Vaccines, Infectious Diseases and Pediatrics Research Group, Spain, Instituto de Investigación Sanitaria de Santiago, Universidad de Santiago de Compostela, Galicia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Spain.
Fernando Baquero-Artigao, Hospital Universitario Infantil La Paz, Department of Infectious Diseases and Tropical Pediatrics, Spain; CIBERINFEC, Instituto de Salud Carlos III, Madrid, Spain; CIBERINFEC, Instituto de Salud Carlos III, Madrid, Spain.
Joanne M Langley, Canadian Center for Vaccinology, Iwk Health Centre and Nova Scotia Health Authority, Dalhousie University, Halifax, Canada.
José T Ramos Amador, Departamento De Salud Pública Y Materno-infantil, Hospital Universitario Clínico San Carlos, Madrid, Spain.
Joseph B Domachowske, Department of Pediatrics, SUNY Upstate Medical University, Syracuse, New York, USA.
Li-Min Huang, Department of Pediatrics, National Taiwan University Hospital, Taipei City, Taiwan.
Nan-Chang Chiu, Department of Pediatrics, Mackay Memorial Hospital, Taipei City, Taiwan.
Susanna Esposito, Pietro Barilla Children's Hospital, University of Parma, Pediatric Clinic, Parma, Italy.
Philippe Moris, GSK, Rixensart, Belgium.
Thi Lien-Anh Nguyen, GSK, Wavre, Belgium.
Vanja Nikic, GSK, Rockville, Maryland, USA.
Wayne Woo, GSK, Rockville, Maryland, USA.
Yingjun Zhou, GSK, Rockville, Maryland, USA.
Ilse Dieussaert, GSK, Rockville, Maryland, USA.
Amanda Leach, GSK, Rockville, Maryland, USA.
Antonio Gonzalez Lopez, GSK, Rockville, Maryland, USA.
Nicolas Vanhoutte, GSK, Rixensart, Belgium.
Data Availability
The study report, including the protocol, is available on the GSK Clinical Study Register (https://www.gsk-studyregister.com/). Anonymized individual participant data and study documents for further research can be requested from www.clinicalstudydatarequest.com (study 204838).
RESULTS
Study Participant Demographics
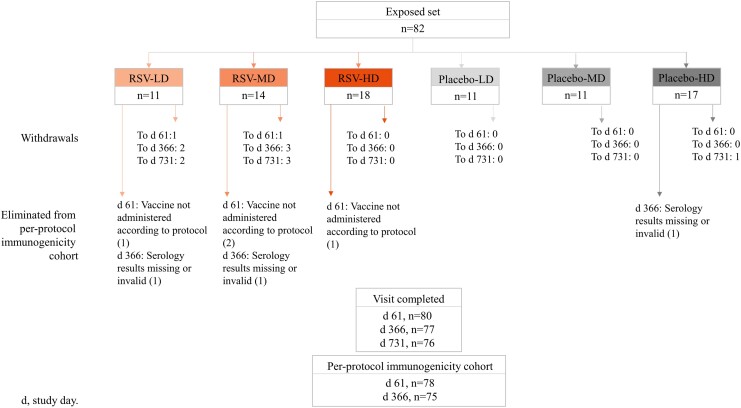
The study was conducted from 11 January 2017 until 26 November 2020. Of the 82 participants who were enrolled and vaccinated (Figure 1), 78 (95.1%) received both doses. There were no study withdrawals due to AEs. The mean (standard deviation) age of participants at the time of dose 1 was 16.1 (3.2) months, and 50% of participants were female.
Figure 1.
Study participant disposition. Numbers represent numbers of participants. Abbreviations: HD, LD, and MD, high, low, and medium dosage of the respiratory syncytial virus (RSV) investigational vaccine or placebo, respectively.
Safety and Reactogenicity of ChAd155-RSV Through Day 61
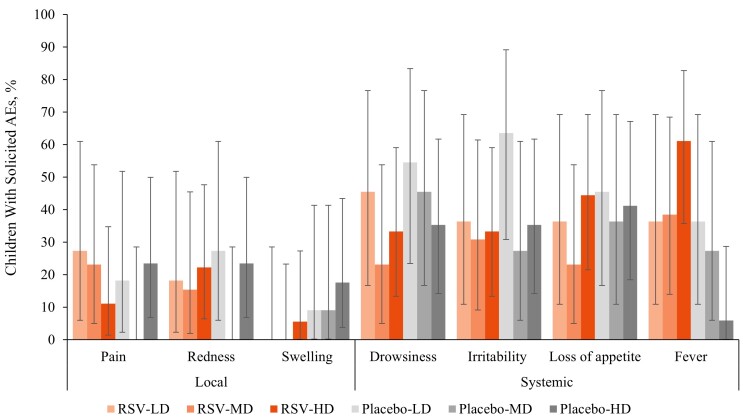
ChAd155-RSV was generally well tolerated. No increases in the incidence or intensity of symptoms were observed after dose 2 or with dose escalation. Pain and redness were the most commonly reported local symptoms (Figure 2). There were no cases of grade 3 pain or redness in any of the study groups. Systemic symptoms were reported with similar frequency in the study groups, except for fever (any grade), which was reported more frequently in the RSV-HD group overall (61.1% for the RSV-HD vs 38.5% and 36.4% for the RSV-MD and RSV-LD groups, respectively), and after each dose (Supplementary Figure 1). Fever >39.5°C (axillary) was uncommon, reported for 1 RSV-LD and 1 RSV-HD recipient, and 2 placebo recipients after any dose (Supplementary Figure 1).
Figure 2.
Percentage of children with solicited local and systemic adverse events (AEs; any severity) for 7 days after any vaccine. Abbreviations: HD, LD, and MD, high, low, and medium dosage of the respiratory syncytial virus (RSV) investigational vaccine or placebo; error bars represent 95% confidence intervals.
Unsolicited AEs until 30 days after each vaccine dose were reported for 81.8% of participants in the RSV-LD, 64.3% in the RSV-MD, and 72.2% in the RSV-HD group versus 63.6%, 90.9%, and 76.5% in the respective placebo groups. Unsolicited AEs reported by ≥2 participants were nasopharyngitis (RSV-LD, 2 cases; RSV-MD, 6; RSV-HD, 7; placebo-MD, 4; and placebo-HD, 5), cough (RSV-HD, 2; placebo-MD, 2; placebo-HD, 1), upper RTI (RSV-LD, 1; RSV-MD, 1; placebo-MD, 1), gastroenteritis (RSV-HD, 4; placebo-MD, 3; RSV-MD, 1; RSV-HD, 1), conjunctivitis (RSV-LD, 3), pyrexia (RSV-LD, 2; placebo-MD, 2; placebo-LD, 1; placebo-HD, 1), and diarrhea (RSV-LD, 2; RSV-MD, 2; placebo-LD, 2; placebo-MD, 2; placebo-HD, 3). The single grade 3 unsolicited AE was gastroenteritis in the placebo-MD group. AEs considered by the blinded investigators to be causally related to vaccination include 1 case of diarrhea and 1 case of rash, both in placebo groups.
One RSV LRTI (AESI) was reported in the RSV-MD group 6 months after dose 2. The event did not require hospitalization, nor did it meet the World Health Organization definition of RSV LRTI [12]. Three other potential cases of RSV LRTI reported as AESIs occurred in participants who received placebo. None required hospitalization.
There were no reports of spontaneous bleeding, easy bruising, petechiae, thrombocytopenia, or lymphopenia. One RSV-LD recipient experienced mild neutropenia, reported as an AE corresponding to a grade 3 laboratory abnormality (neutrophil count 688 U/L) 31 days after dose 2, which resolved within 5 weeks and was not considered causally related by the blinded investigator. Two other participants had grade ≥3 abnormally decreased neutrophil counts that were not reported as AEs (RSV-MD, 500 U/L 1 day after dose 2; RSV-HD, 450 U/L 32 days after dose 2). Both counts were back above 750 U/L (grade ≤2) within 4–6 weeks at the last follow-up visit. One RSV-HD recipient was reported with AEs for moderate elevations in aspartate aminotransferase and alanine aminotransferase levels, corresponding to grade 2 laboratory abnormalities (70 and 119 U/L, respectively), 31 days after dose 2; these AEs were considered by the blinded investigator as possibly related to vaccination.
Safety Until Day 731
By day 731, 6 participants reported 12 SAEs: 1 in the RSV-LD group, 2 in the RSV-HD group, and 3 in placebo groups. Three SAEs were reported before day 61; none was considered by the blinded investigator to be related to vaccination. One participant reported 2 SAEs of urinary tract infection; 1 reported concurrent SAEs of acute gastroenteritis and febrile convulsion; 1 reported concurrent SAEs of coronavirus infection, enterovirus infection, pyrexia, rhinovirus infection, and unresponsiveness to stimuli; and 3 reported 1 SAE each, including gastroenteritis, pneumonia, and herpangina. AESIs reported through day 731 were RSV bronchitis (1 case), RSV infection (2 cases). and concurrent bronchitis, tonsillitis with coronavirus, and RSV infection (1 case). One case of RSV infection met the definition of RSV LRTI. These cases are also included in the section below.
RSV Surveillance
From day 1 until day 731, 17 ChAd155-RSV recipients (39.5%) experienced 22 episodes of RSV infection (13 symptomatic and 9 asymptomatic) and 15 placebo recipients (38.5%) experienced 20 episodes (15 symptomatic and 5 asymptomatic) (Table 1). Among ChAd155-RSV recipients, 8 cases (in 7 participants) occurred in the RSV-LD group, 11 (in 7 participants) in the RSV-MD group, and 3 (in 3 participants) in the RSV-HD group. There were no cases of RSV LRTI (any, severe, or very severe) in ChAd155-RSV recipients and 1 case of RSV severe LRTI in a placebo recipient. There were no RSV-related hospitalizations through day 731.
Table 1.
Participants With Respiratory Syncytial Virus Infection, by World Health Organization Severity Level, From Vaccination Dose 1 Through Day 731a
| Infection Category | Participants in Pooled RSV Vaccine Groups (n = 43) | Participants in Pooled Placebo Groups (n = 39) | ||
|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | |
| No infection | 26 | 60.5 (44.4–75.0) | 24 | 61.5 (44.6–76.6) |
| Any RSV infection | 17 | 39.5 (25.0–55.6) | 15 | 38.5 (23.4–55.4) |
| ȃAsymptomatic | 7 | 16.3 (6.8–30.7) | 2 | 5.1 (.6–17.3) |
| ȃRTI | 10 | 23.3 (11.8–38.6) | 12 | 30.8 (17.0–47.6) |
| ȃAny LRTI | 0 | 0.0 (.0–8.2) | 1 | 2.6 (.1–13.5) |
| ȃSevere LRTI | 0 | 0.0 (.0–8.2) | 1 | 2.6 (.1–13.5) |
| ȃVery severe LRTI | 0 | 0.0 (.0–8.2) | 0 | 0.0 (.0–9.0) |
| ȃHospitalization | 0 | 0.0 (.0–8.2) | 0 | 0.0 (.0–9.0) |
| All-cause LRTIb | 8 | 18.6 (8.4–33.4) | 10 | 25.6 (13.0–42.1) |
Abbreviations: CI, confidence interval; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus; RTI, respiratory tract infection.
World Health Organization categories as outlined in reference [12].
Meeting the case definition for LRTI (Supplementary Table 5).
Immunogenicity
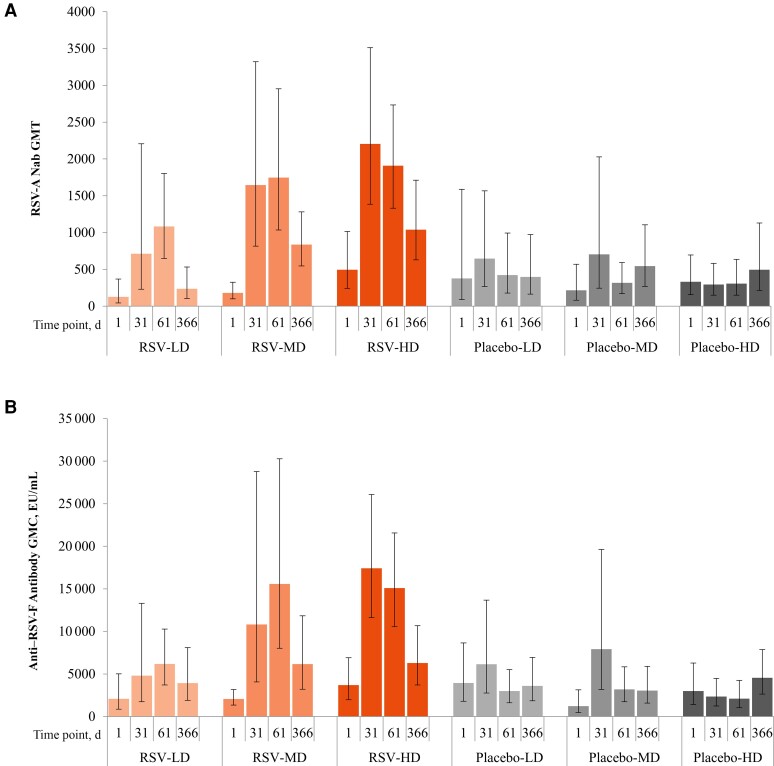
After dose 1, RSV-A Nab GMTs increased across the ChAd155-RSV treatment groups but not in placebo recipients, with the highest titers observed in the RSV-MD and RSV-HD groups (Figure 3A). At baseline, the RSV-A Nab GMT ranged from 32 to 9778 across participants. At day 31, RSV-A Nab GMTs were 711.7 (95% CI, 229.7–2204.9) in the RSV-LD, 1646 (816–3321) in the RSV-MD, and 2204 (1384–3510) in the RSV-HD group, with 5.6-, 8.1-, and 4.2-fold increases, respectively, compared with prevaccination GMTs. No further increases in GMTs were observed in any group after dose 2 (at day 61). At day 366, RSV-A Nab GMTs remained higher than baseline in all ChAd155-RSV treatment groups. Day 366 RSV-A Nab GMTs were 1.5–2-fold higher in the RSV-MD and RSV-HD groups than in the placebo groups.
Figure 3.
Respiratory syncytial virus (RSV) type A (RSV-A) neutralizing antibody (Nab) geometric mean titers (GMTs) (A) and anti–RSV fusion protein (RSV-F) antibody geometric mean concentrations (GMCs) (B) through day 366 (per-protocol set) after baseline (day 1). Error bars represent 95% confidence intervals. Abbreviations: EU, enzyme-linked immunosorbent assay units; HD, LD, and MD, high, low, and medium dosage of the RSV investigational vaccine or placebo.
Similar trends were noted for anti–RSV-F binding antibody concentrations (Figure 3B). Owing to challenges in obtaining blood samples in infants, very few samples were available for CMI assessment from the MD (0–3 RSV-MD participants at each time point and 0–2 placebo-MD recipients at each time point) or HD (0–1 RSV-HD recipient and 0–1 placebo-HD recipients at each time point) groups.
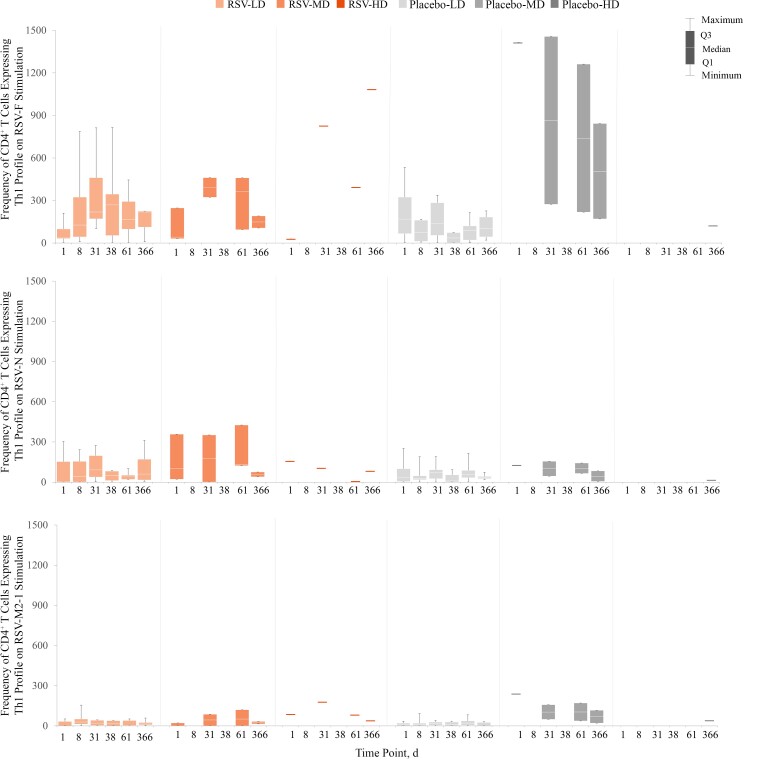
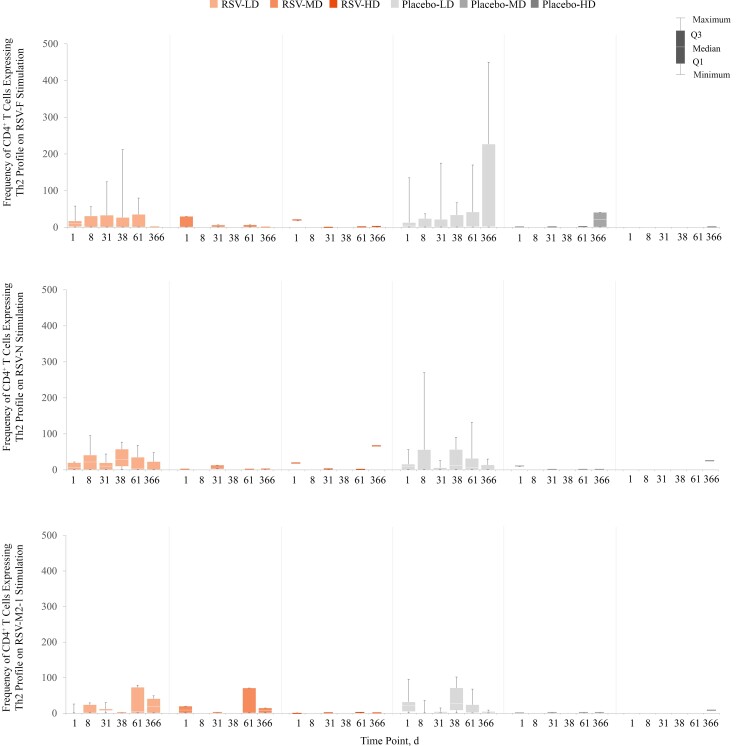
Compared with prevaccination levels, there was some evidence of a CD4+ Th1 response at day 31 in the unique RSV-HD recipient who provided whole blood for CMI assessment. This was observed after stimulation with the RSV-F peptide pool in recipients of ChAd155-RSV but not after stimulation with the RSV-N or RSV-M2-1 peptide pools (Figure 4). No RSV infection was detected during the study for this child. The single individual in the placebo-MD group who provided a sample for CMI testing had a high frequency of CD4+ Th1 cells (1415 per million CD4+ T cells at the prevaccination time point), likely owing to a recent natural RSV exposure before enrollment. No RSV infection was detected during the study for this individual. No CD4+ Th2 or Th17 responses were observed (Figure 5 and Supplementary Figure 2). The frequency of CD8+ T cells expressing ≥2 markers (including ≥1 cytokine) increased on stimulation with the RSV-N peptide pool and to a lesser degree with the RSV-M2-1 peptide pool (Supplementary Figure 3).
Figure 4.
Box plots of individual data for CD4+ T cells (per million CD4+ T cells) expressing T-helper (Th) 1 profile on stimulation with respiratory syncytial virus (RSV) peptide pools (fusion [F], nucleoprotein [N], and matrix [M2-1]), through day 366 after baseline (day 1; per-protocol set). Th1 cells are CD4+ T cells expressing at least interferon γ but not interleukin 13. Abbreviations: HD, LD, and MD, high, low, and medium dosage of the RSV investigational vaccine or placebo; Q1, quartile; Q3, quartile 3. Graph shows data at each time point for the following numbers of participants: RSV-LD, 3–9; RSV-MD, 0–3; RSV-HD, 0–1; placebo-LD, 4–10; placebo-MD, 0–2; placebo-HD, 0–1.
Figure 5.
Box plots of individual data for CD4+ T cells (per million CD4+ T cells) expressing T-helper 2 (Th2) profile on stimulation with respiratory syncytial virus (RSV) peptide pools (fusion [F], nucleoprotein [N], and matrix [M2-1]), through day 366 after baseline (day 1; per-protocol set). Th2 cells are CD4+ T cells expressing at least interleukin 13 but not interferon γ. Abbreviations: HD, LD, and MD, high, low, and medium dosage of the RSV investigational vaccine or placebo; Q1, quarter 1; Q3, quarter 3. Graph shows data at each time point for the following numbers of participants: RSV-LD, 3–9; RSV-MD, 0–3; RSV-HD, 0–1; placebo-LD, 4–10; placebo-MD, 0–2; placebo-HD, 0–1.
DISCUSSION
This is the first study of a novel RSV vaccine administered to young children. All 3 ChAd155-RSV doses were well tolerated, with solicited local and systemic symptoms occurring at similar rates in placebo recipients. There was no dose-related increase in symptoms except for fever, which was more frequent among those receiving RSV-HD. However, most fevers were ≤38.5°C, and the occurrence and duration of fever did not increase after dose 2. SAEs occurred at similar rates in both ChAd155-RSV and placebo recipients, and none were considered as related to vaccination by the investigators. In the 17 children naturally infected with RSV after vaccination, there was no progression to severe disease and therefore no evidence to support a VAERD risk.
Levels of baseline RSV-A Nab varied by 306-fold among study participants, which is substantially higher than the variation observed in adults (up to 2.6-fold difference [11]), possibly owing to higher rates of recent RSV infection in the younger age group. Nevertheless, ChAd155-RSV induced specific, dose-dependent humoral responses, with the highest GMTs observed after the first dose of ChAd155-RSV in the HD group. As observed in adults given 2 doses of ChAd155-RSV (LD or HD) [11], no further increase in antibody responses was observed after administration of a second vaccine dose. The lack of a post–dose 2 response in young children suggests that a second dose may not be necessary in children who are naturally primed with RSV or that a heterologous prime-boost approach should be considered. One year after vaccination, levels of RSV-A Nabs and total antibodies to RSV-F remained higher than prevaccination levels in all RSV-MD and RSV-HD recipients. A potential limitation of the study is that we did not measure RSV-B Nab because we assumed that cross reactivity would be similar in all groups.
Interpretation of CMI data is hampered by the very low number of samples available for analysis in the RSV-MD and RSV-HD groups. Despite this limitation, the data suggest a Th1 response in ChAd155-RSV recipients, without evidence for Th2 or Th17 responses, which are thought to be deleterious [13, 14]. Strong Th1 responses accompanied by cytotoxic immunity contribute to clearance of RSV and are needed to prevent a Th2-skewed response associated with allergic desensitization and long-term development of asthma [15].
RSV infections rates were similar in the vaccine and placebo groups, although there were more asymptomatic infections in the vaccine group, and the only RSV LRTI (severe) during the study occurred in the placebo group. Vaccination against RSV is designed to prevent disease rather than infection. However, this study was not designed to assess vaccine efficacy, and the sample size is too small to draw conclusions.
In conclusion, ChAd155-RSV administered to young RSV-seropositive children was well tolerated and had an acceptable safety profile up to day 731. A single dose of ChAd155-RSV in the RSV-MD and RSV-HD groups induced RSV-A Nab titers, without evidence for a further increase following a second dose. Nab titers remained above baseline 1 year later. The induction of a CD4+ Th1 profile response is suggested, and no evidence for a CD4+ Th2 or Th17 response was observed. These results support further evaluation of the response to vaccination in RSV-naive infants.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the participating families and the investigators and staff at the study sites. They thank the staff at the GSK Clinical and Clinical Laboratory Science laboratories for their contribution to this study. They also thank the Modis platform for writing support, editorial assistance, and manuscript coordination, on behalf of GSK. Writing support was provided by Joanne Wolter; editorial support and publication coordination was provided by Stéphanie Deroo.
Author contributions. Study design: J. D. D., X. S. L., M. A. R. W., J. M. L., J. B. D., S. E., T. L. A. N., V. N., I. D., A. L., A. G. L., and N. V. Data acquisition: J. D. D., X. S. L., M. A. R. W., C. E., A. C., C. H. C., C. Y. L., A. A. B., F. M. T., F. B. A., J. M. L., J. T. R. A., J. B. D., L. M. H., N. C. C., S. E., P. M., T. L. A. N., V. N., W. W., I. D., A. G. L., and N. V. Data analysis: J. D. D., X. S. L., M. A. R. W., C. H. C., F. M. T., J. B. D., L. M. H., P. M., T. L. A. N., V. N., W. W., Y. Z., I. D., A. L., A. G. L., and N. V. Contribution to the conduct of the study: J. D. D., X. S. L., M. A. R. W., C. E., A. C., C. H. C., C. Y. L., A. A. B., F. M. T., F. B. A., J. M. L., J. T. R. A., J. B. D., L. M. H., N. C. C., S. E., V. N., W. W., A. G. L., and N. V. All authors reviewed and revised the manuscript and approved the final manuscript as submitted.
Disclaimer. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis and took responsibility for all costs associated with the development and the publishing of the present manuscript. Funding to pay the Open Access publication charges for this article was provided by GlaxoSmithKline SA.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA.
References
- 1. Li Y, Johnson EK, Shi T, et al. . National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9:175–85. [DOI] [PubMed] [Google Scholar]
- 2. Scheltema NM, Gentile A, Lucion F, et al. . Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health 2017; 5:e984–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, McAllister DA, O'Brien KL, et al. . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazur NI, Higgins D, Nunes MC, et al. . The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. [DOI] [PubMed] [Google Scholar]
- 5. Kim HW, Canchola JG, Brandt CD, et al. . Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. [DOI] [PubMed] [Google Scholar]
- 6. Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 2007; 39:225–39. [DOI] [PubMed] [Google Scholar]
- 7. Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol 2007; 179:5415–24. [DOI] [PubMed] [Google Scholar]
- 8. Sasso E, D'Alise AM, Zambrano N, Scarselli E, Folgori A, Nicosia A. New viral vectors for infectious diseases and cancer. Semin Immunol 2020; 50:101430. [DOI] [PubMed] [Google Scholar]
- 9. Capone S, D'Alise AM, Ammendola V, et al. . Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines 2013; 12:379–93. [DOI] [PubMed] [Google Scholar]
- 10. Boyoglu-Barnum S, Chirkova T, Anderson LJ. Biology of infection and disease pathogenesis to guide RSV vaccine development. Front Immunol 2019; 10:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cicconi P, Jones C, Sarkar E, et al. . First-in-human randomized study to assess the safety and immunogenicity of an investigational respiratory syncytial virus (RSV) vaccine based on chimpanzee-adenovirus-155 viral vector-expressing RSV fusion, nucleocapsid, and antitermination viral proteins in healthy adults. Clin Infect Dis 2020; 70:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Modjarrad K, Giersing B, Kaslow DC, et al. . WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization meeting held on 23–24 March 2015. Vaccine 2016; 34:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green CA, Scarselli E, Sande CJ, et al. . Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med 2015; 7:300ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi T, Li N, He Y, et al. . Th17/Treg cell imbalance plays an important role in respiratory syncytial virus infection compromising asthma tolerance in mice. Microb Pathog 2021; 156:104867. [DOI] [PubMed] [Google Scholar]
- 15. Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against ad vectors: humoral, cellular, and innate response, what's important? Hum Vaccin Immunother 2014; 10:2875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study report, including the protocol, is available on the GSK Clinical Study Register (https://www.gsk-studyregister.com/). Anonymized individual participant data and study documents for further research can be requested from www.clinicalstudydatarequest.com (study 204838).