Abstract
There are limited treatment options for women with severely diminished ovarian reserve (DOR) who experience repeatedly failed in vitro fertilization (IVF) cycles and with persistently thin endometrial lining thickness (EMT) during frozen embryo transfer cycles. Therefore, a large majority of patients resort to using donor oocytes and gestational carriers. Data from existing animal and human studies suggest that ozone sauna therapy (OST) and pulsed electromagnetic field therapy (PEMF) are emerging as potential therapeutic adjuncts for female reproduction. This study was conducted to assess the fertility outcome of OST + PEMF in vivo in patients undergoing IVF/frozen embryo transfe and the effects of OST in vitro on human granulosa cell (GC) function. Forty-four women with DOR underwent their 1st IVF cycle (Cycle 1), and then were administered transdermal and intravaginal OST + PEMF, twice a week for 3 weeks, followed by a 2nd IVF cycle (Cycle 2) using the same protocol as in Cycle 1. GCs collected from another six women who underwent egg retrieval were equally split and cultured with OST (test) or placed in room temperature (control) outside the OST chamber in the same room. The results demonstrated that Cycles 1 and 2 had no significant difference in the number of days of stimulation, baseline hormones measured, number of oocytes retrieved or peak estradiol levels. However, the number of embryos formed after OST + PEMF in Cycle 2 was significantly higher than the Cycle 1. Furthermore, EMT measured in Cycle 2 demonstrated a significant increase compared to Cycle 1 and all patients reached a satisfactory EMT of approximately 7 mm. In vitro studies demonstrated that OST led to a 5-fold significant increase in the aromatase enzyme while a significant 50% reduction was noted in the side-chain cleavage enzyme in GCs. Both OST + PEMF are known for their vasodilatory, anti-inflammatory, and antioxidant actions, which could enhance endometrial receptivity and increase the number of formed embryos without increasing the number of oocytes retrieved, suggesting an improvement in oocyte quality. Finally, ozone can alter genes involved in steroidogenesis suggesting that it could improve ovarian function.
Keywords: diminished ovarian reserve, endometrial lining thickness, granulosa cells, in vitro fertilization, oocyte, ozone sauna therapy, pulsed electromagnetic field therapy, steroidogenesis
INTRODUCTION
The ozone gas is comprised of three atoms of oxygen in a cyclic structure and is highly water soluble.[1] It can be produced by commercial ozone generators that send a high voltage electrical gradient through a condenser containing medical grade pure oxygen,[2] and when the electrical discharge breaks oxygen into two separate molecules, these individual molecules can be later combined with another oxygen molecule to produce ozone.[3] Ozone acts via several different biological mechanisms. For instance, upon cellular exposure to ozone, a multifaceted endogenous cascade is initiated, and active biological substrates are released due to the oxidative stress induced by ozone.[4] Hydrogen peroxide can be produced by ozone reacting with cell membrane polyunsaturated fatty acids and water. The generated reactive oxygen species can in turn enhance various biological processes, such as increasing production of adenosine triphosphate and stimulating the production of enzymes that act as free radical scavengers, and cell wall protectors, such as glutathione peroxidase, superoxide dismutase, glutathione S-transferase and catalase.[1,4,5] Another observed mechanism of action for ozone is to active the immune system since administration of ozone has been shown to elevate the production of interferon, tumor necrosis factor,[6] and interleukins.[7] Finally due to its high reactivity, ozone exhibits strong antibacterial, antiviral, antifungal, and antiprotozoal activities.[1]
Ozone sauna therapy (OST) has been administered via several routes that include transdermal, intra-arterial, subcutaneous, intramuscular, vaginal, and rectal.[8] Clinically, ozone has demonstrated valuable therapeutic effects in treating several disorders that include acquired immune deficiency syndrome,[9] cancer,[10] osteoarthritis,[11] dental conditions,[12] fibromyalgia,[13] coronavirus disease 2019 (COVID-19) pneumonia,[14] diabetic foot,[15] dermatitis,[16] degenerative disorders,[17] liver diseases[18] and many others. In animal models, there are plenty of studies that demonstrated a beneficial use of OST in gynecological and fertility disorders, for example, OST was able to treat pelvic inflammatory disease-induced endometrial injury and tubal blockage in rats,[19] and also it has shown to improve fertility and reduce bacterial load in post-partum-induced clinical and subclinical endometritis in cows.[20,21]
There is compelling evidence which showed that OST protects the ovaries from injuries by diminishing ovarian ischemia and reperfusion following ovarian torsion in rat models.[22] Furthermore, beneficial effects on reproduction were also noted in vaginitis treated with ozone compared to antibiotics and ozone might lead to a lower formation of pelvic adhesions in cow and rat models.[23,24] However, studies in humans pertaining to the effect of OST on gynecological and reproductive disorders are scarce. One study conducted on tubal recanalization with catheter mediated pressure injections of ozone resulted in significantly higher postoperative tubal recanalization and pregnancy rates after 12 months in the ozone treated group compared to controls.[25] Another preliminary study carried on 12 healthy ovulating women demonstrated that intrauterine ozonated saline infusion resulted in a statistically significant increase of the columnar epithelial cell height, increase the number of endometrial blood vessels, and raised the number of stromal cells in the endometrium.[26]
Pulsed electromagnetic field (PEMF) therapy apparatus induces a pulsed magnetic field when a current passes through a wire coil.[27] This method was approved by the U.S. Food and Drug Administration for clinical applications and has been largely used to successfully treat different orthopedic disorders.[28] PEMF has shown to cause strong anti-inflammatory effects by leading to movement of ions across the cell membrane and through restoration of cell membrane calcium ATPase activity.[29] A study related to female reproductive hormones in women with polycystic ovary syndrome has shown that PEMF can significantly improve the abnormal serum levels of luteinizing hormone and follicular stimulating hormone (FSH) and significantly lowered the serum C-reactive protein levels compared to the control group.[30] An animal study conducted on Japanese quail breeder hatching eggs demonstrated significantly reduced embryonic mortality and significantly high total and fertile hatchability performance in the PEMF treated group compared to the controls.[31] A case series showed that 2 out of 3 infertile patients with extremely thin endometrial lining and multiple failed in vitro fertilization (IVF) cycles, were able to get pregnant after IVF following the treatment with ozone and PEMF (by the use of the hyperthermic ozone & carbonic acid transdermal therapy (HOCATT) machine) that significantly improved the endometrial lining thickness (EMT).[32]
Women with severely diminished ovarian reserve (DOR) with repeatedly failed IVF cycles and women with persistently thin EMT during frozen embryo transfer (FET) cycles have limited treatment options with a large majority of patients resorting to using donor oocytes and surrogacy. Given the existing data pertaining to the potential advantageous effect of OST and PEMF on the female reproductive system, we hypothesized that OST and PEMF in combination using the HOCATT machine improves overall reproductive outcome in infertile women with severe DOR undergoing IVF. In order to test our hypothesis, we exposed a selected group of infertile women undergoing IVF to OST (transdermal and intravaginal) and PEMF, then we assessed their ovarian response to controlled ovarian hyperstimulation including the number of oocytes collected, number of embryos formed and EMT in the IVF cycle before and the IVF cycle immediately after OST and PEMF. Additionally, we treated primary human GCs from women who underwent IVF with OST in order to assess any changes in vital genes involved in steroidogenesis and folliculogenesis.
SUBJECTS AND METHODS
Subjects
Fifty women (aged 39.7 ± 1.1 years) undergoing infertility treatment at SUNY Downstate Health Sciences University Affiliated Private Fertility Center were recruited. Infertility was defined as inability to conceive with unprotected intercourse after 1 year for women aged < 35 years, and after 6 months for women aged > 35 years.[33] Women with any medical condition that interferes with fertility treatment were excluded from the study. Inclusion criteria included women with DOR as defined by serum anti-Mullerian hormone < 1 ng/mL, serum FSH > 10 mIU/mL, and antral follicle count < 10 measured by transvaginal ultrasound.
Forty-four of them underwent two IVF cycles: the first before using the HOCATT (Cycle 1), followed by 3 weeks of HOCATT use, followed by a second IVF cycle (Cycle 2). The informed consent was obtained from all patients and the study was approved by New England Institutional Review Board (NEIRB; IRB# 120180241) on November 30, 2018.
OST and PEMF using the HOCATT machine
Between Cycle 1 and Cycle 2, all patients underwent 30-minute session of HOCATT machine (HOCATT, Weatherford, TX, USA) twice weekly for a total of 3 weeks. The patients were required to complete a medical history questionnaire to confirm that there were no contraindications for using the HOCATT machine. Contraindications included pregnancy, allergies, fever, uncontrolled diabetes, hyper- or hypotension, cardiac diseases, renal diseases, malignancy, mental problems, history of ozone allergy, blood coagulation or bleeding disorders, stroke, hypo- or hyperthyroidism, glucose-6-phosphate dehydrogenase deficiency, acute alcohol intoxication, dehydration, vitiligo, epilepsy or seizures, uncontrolled dyslipidemia, chronic obstructive pulmonary disease, and cutaneous porphyria. For each session, the participant sat naked in the machine and was required to insert herself a silicone vaginal catheter around 1–2 inches internally to deliver intravaginal ozone. The participant's head remained outside of the machine to avoid any breathing of ozone.
The machine delivers ozone transdermally (ozone 1 setting at 500 mL/min and vaginally (ozone 2 setting at 200 mL/min). The machine was set to infuse CO2 to the chamber at 5 L/min at the beginning, which combined with steam to produce carbonic acid (CA). The temperature of the machine was set at 37°C and carbon infra-ray pads coupled with the steam was used to raise the patient's body temperature. The participants were instructed to hydrate well before the session as high temperature inside the machine can induce dehydration. During the first 3–8 minutes of the session, the patient was exposed to CA in the chamber. After the CA cycle completed, the ozone cycle was activated. At the end of the session, all ozone produced by the machine and oxygen used were extracted to the destructor and were destroyed. The CO2 and ozone concentrations delivered to the patient were determined on her tolerability and then generally increased with each session. CO2 started at 3 minutes, with 1-minute increments at each subsequent session maximum up to 8 minutes. Ozone session 1 started at 50% and generally increased up to 80% with 10% increment at each subsequent session until session 6.
Clinical data collection
IVF Cycle 1 and IVF Cycle 2 performed in the same participants used similar ovarian stimulation protocols. In brief in each cycle, after oral contraceptive pill pre-treatment for approximately 2–3 weeks and adequate suppression, minimal/mild ovarian stimulation was started with an extended regimen (from cycle day 3 until the day before triggering) of clomiphene citrate (50 mg/day orally) in conjunction with letrozole (2.5 mg/day orally) with low dose of gonadotropin (75 IU daily) injections (Follistim, Merck, White House Station, NJ, USA; or Gonal-F, EMD Serono, Rockland, MA, USA) starting on cycle days 4–7. Hypothalamic-pituitary suppression using gonadotropin releasing hormone antagonist was used to prevent ovulation. The final maturation of oocytes was induced by a gonadotropin releasing hormone agonist or by human chorionic gonadotropin trigger when the lead follicle was > 18 mm. Retrieved oocytes were fertilized by intra-cytoplasmic sperm injection as clinically indicated. All embryos were cultured until the cleavage stage and then vitrified to be transferred in subsequent FET cycle.
Granulosa cell culture
Granulosa cells (GCs) of other six participants who underwent IVF were collected after identification of the cumulus–oocyte complex in the follicular aspirate. The GCs were split equally into two groups: one group of GCs was placed in the HOCATT machine (test cells with OST only) while the other group of cells were placed on room temperature (control cells). Ozone was infused to the chamber at 500 mL/min. Cumulus GCs were mechanically collected by cutting the cumulus layer from each oocyte. Cumulus GCs were aspirated from the mineral oil media and transferred to labeled eppendorf tubes and centrifuged at 5000 × g for 3 minutes. After centrifugation, the supernatant was removed carefully without disturbing the cell pellets. The cells were treated with 300 μL of 0.8 U/mL hyaluronidase, flushed up and down for 30 seconds until the cell clumps dissolved, the cell-hyaluronidase mix was then immediately diluted in Dulbecco's modified Eagle medium and centrifuged for 3 minutes at 5000 × g. Supernatant fluid was aspirated without disturbing cells and 600 μL of Dulbecco's modified Eagle medium was added to eppendorf tubes and pipetted up and down to evenly distribute the cells. A 300 μL of cell suspension was then added to each well respectively into two 96 well plates, which were pretreated with poly-L-lysine for 5 minutes and then washed with phosphate buffered saline. Plates were incubated overnight under a humidified atmosphere of 95% air and 5% CO2 at 37°C. On day 2 (after 24 hours of incubation), cells in both groups were checked under the microscope for viability and the media was changed and the test cells plate was kept in the HOCATT machine for 30 minutes (without PEMF) with the plate lid open in order to expose the cells to ozone gas while the control cells were set aside in the same room with the plate lid open for 30 minutes. Plates were incubated overnight under a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Real time-polymerase chain reaction
On day 3 of cell culture, RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and chloroform extraction according to the manufacturer's instructions. The cells were incubated with Trizol for 15 minutes at room temperature and then mixed with chloroform, followed by centrifugation at 4°C and 12,000 × g for 15 minutes. The aqueous phase containing RNA was separated. Total RNA was precipitated with propranolol by centrifugation at 4°C and 12,000 × g for 10 minutes, washed with 75% ethanol followed by centrifugation at 4°C and 7500 × g for 5 minutes, air-dried and reconstituted in diethylpyrocarbonate-treated water. The RNA was stored at –20°C until further analysis. RNA quality was assessed by a Nanodrop Spectrophotometer (Thermo Fisher Scientific, CA, USA) and Agilent Bioanalyzer (Santa Clara, CA, USA). Only samples with a minimum concentration of 10 ng/μL and with an optical density 260 nm/280 nm ratio of 1.8–2.0 was used for evaluation of CYP19A1 (aromatase), side-chain cleavage enzyme (SCC, also called CYP11A1), steroidogenic acute regulatory protein, 3 beta-hydroxysteroid dehydrogenase mRNA expression levels. Real time-polymerase chain reaction (RT-PCR) was achieved by using the SYBR Green I chemistry. The primers utilized in the study are listed in Table 1 and was synthesized by Fisher (Pittsburg, PA, USA). The PCR protocol used in this study is described somewhere else.[34] The mRNA expression levels for each steroidogenesis and folliculogenesis gene was calculated using ΔΔCT cycle threshold method relative to glyceraldehyde-3-phosphate which was used as the loading control.
Table 1:
Primers used for real time-polymerase chain reaction in the study
| Genes in GCs important in steroidogenesis and folliculogenesis | Sequence primers (5'–3') | Product length (bp) |
|---|---|---|
| CYP19A1 (aromatase) | Forward: GAC GGA AGG TCC TGT GCT C | 164 |
| Reverse: GGG GGC AAT TTA GAG TCC ACA | ||
| Side-chain cleavage enzyme | Forward: TGG GTC GCC TAT CAC CAG TA | 173 |
| Reverse: TGC AGG ACA CTG ACG AAG TC | ||
| Steroidogenic acute regulatory protein | Forward: AGG ACG AAG AAC CAC CCT TG | 180 |
| Reverse: CAT CAC AGC CTG TTG CCT CA | ||
| 3 Beta-hydroxysteroid dehydrogenase | Forward: GCT TCT GGG TCA GAG GAT CG | 184 |
| Reverse: CTG GCA GGC TCT TTT CAG GA | ||
| Glyceraldehyde-3-phosphate (housekeeping gene) | Forward: ACC CAC TCC TCC ACC TTT GA | 100 |
| Reverse: TGT TGC TGT AGC CAA ATT CGT T |
Statistical analysis
Sample size calculation was performed to detect a 50% difference in the number of embryos with 80% power and two-tailed alpha error of 0.05.
The data are presented as mean ± standard error from mean (SEM). The data of Cycle 1 and Cycle 2 use were analyzed and compared. The parameters collected include serum FSH, luteinizing hormone, estradiol, and progesterone levels, antral follicle count by ultrasound, the dose of fertility medications used for ovarian stimulation, the number of days of stimulation, the number of follicles in the ovaries observed by transvaginal ultrasound, EMT, number of oocytes collected as well as the number of embryos formed. The Kolmogorov-Smirnov test was used methods to test the normality of the data and the Levene's test was used to assess the homogeneity of variance.
A paired t-test was used to compare both clinical and RT-PCR results if the data were normally distributed. The number of follicles observed by the transvaginal ultrasound was the main outcome of the study, which denoted the efficacy of HOCATT use on folliculogenesis. The statistical analysis was conducted using GraphPad Prism statistical software (version 9.2.0, GraphPad Software, CA, USA, www.graphpad.com) and P value of < 0.05 was considered statistically significant.
RESULTS
Clinical data
There were no significant differences between Cycle 1 and Cycle 2 in the number of days of stimulation or in the dose of medications (Gonal-F or Follistim, clomiphene citrate and letrozole) used per cycle during the ovarian stimulation cycles (P > 0.05 for all). Additionally, there were no significant differences in baseline FSH, luteinizing hormone, progesterone, and estradiol serum levels as well as the antral follicle count between Cycle 1 and Cycle 2 (P > 0.05 for all). However, even though the number of oocytes collected and peak estradiol levels were similar, the number of embryos formed in Cycle 2 was significantly higher than the number of embryos formed in Cycle 1 (P = 0.02; Figure 1). Additionally, the EMT in Cycle 2 was significantly higher than the EMT in Cycle 1 in all participants (P = 0.03; Figure 1).
Figure 1:
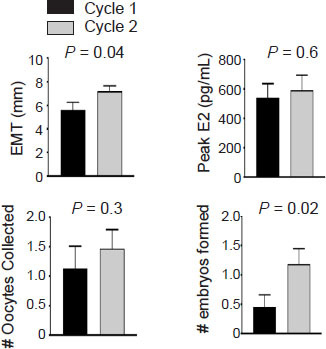
Ozone therapy and pulsed electromagnetic field therapy improve the female reproductive system.
Note: Women (n = 44) with severe diminished ovarian reserve underwent 1st in vitro fertilization cycle (Cycle 1), followed by hyperthermic ozone & carbonic acid transdermal therapy machine use for 3 weeks followed by a 2nd in vitro fertilization cycle (Cycle 2) using the same protocol as Cycle 1. There was no significant difference in the number of oocytes collected or peak serum estradiol (E2) levels. However, the endometrial lining thickness (EMT) and number of embryos formed were significantly higher in Cycle 2 compared to Cycle 1. Data are presented as mean ± SEM and were analyzed by paired t-test. The detailed statistical analysis is shown in Additional Table 1.
In vitro GCs results
Six patients who underwent oocyte retrieval had their GCs split equally and cultured, then placed in the HOCATT machine or in room temperature (control) in the same room as the HOCATT machine (OST without PEMF). RT-PCR was performed for genes involved in steroidogenesis: CYP19A1, SCC, steroidogenic acute regulatory protein, and 3 beta-hydroxysteroid dehydrogenase in order to quantify their mRNA expression levels. The results showed that OST exposure caused a significant 5 times increase in CYP19A1 and 50% significant decrease in SCC (Figure 2). There were no statistically significant differences in steroidogenic acute regulatory protein or 3 beta-hydroxysteroid dehydrogenase mRNA expression levels (P > 0.05).
Figure 2:
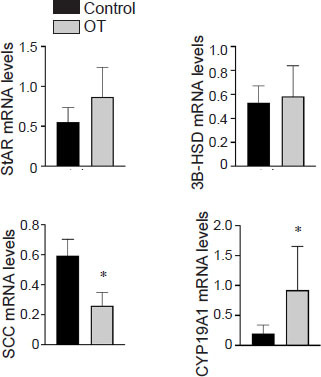
Ozone therapy and pulsed electromagnetic field therapy improve the genes involved in steroidogenesis of primary human granulosa cells.
Note: Six participants underwent oocyte retrieval had their granulosa cells split equally and cultured, then half of the cells placed in the hyperthermic ozone & carbonic acid transdermal therapy (HOCATT) machine (ozone therapy for 30 minutes, without pulsed electromagnetic field therapy) versus half of the cells places on room air temperature (control) in the same room where the HOCATT machine was located. Real time-polymerase chain reaction was performed on both groups of cells and the results showed that cells placed in the HOCATT showed a significant 5 times increase in CYP19A1 mRNA levels and 50% significant decrease in SCC mRNA levels (*P < 0.05 for both). Data are presented as mean ± SEM, and were analyzed by paired t-test. The detailed statistical analyses are shown in Additional Table 1.3B-HSD: 3 beta-hydroxysteroid dehydrogenase; SCC: side-chain cleavage enzyme, also called CYP11A1; StAR: steroidogenic acute regulatory protein.
DISCUSSION
In this study, we assessed the clinical outcome in women with severe DOR undergoing IVF before and after a total of six sessions of HOCATT use (OST + PEMF) over a 3-week period. The data showed improvement in the number of embryos formed even though the number of oocytes collected was similar, thus indicating improvement in oocyte quality. Additionally, there was improvement in EMT which is a significant predictor of pregnancy success in IVF/FET cycles. Finally, GCs treatment with OST in vitro showed upregulation of CYP19A1, which is the aromatase enzyme responsible for estradiol production, and showed downregulation in SCC, which is an enzyme that catalyzes the conversion of cholesterol to pregnenolone: the first reaction in the process of steroidogenesis for the production of various steroid hormones.
The EMT is a significant predictor of pregnancy outcome in IVF/FET cycles thus the pregnancy rate is usually significantly higher in women with EMT of more than 7 mm.[35] A study carried out to evaluate the effects of ozonated saline on endometrial irrigation by histological and ultra-sonographic parameters on healthy ovulating women demonstrated a favorable effect towards successful implantation.[26] In accordance with our findings, a meta-analysis carried out by Momeni et al.[36] on 14 studies including 2204 pregnant and 2718 nonpregnant women suggested that there is a significant difference in the mean EMT between pregnant and nonpregnant groups (P < 0.001).
As follicles grow and develop each month, they produce estradiol. Animal studies conducted with aromatase inhibitors suggest that estrogens are important for oocyte function.[37] Estrogen also has been shown to induce the transcription factor, early growth response 1, to positively regulate the actions in uterine epithelial cells that are responsible for improving endometrial receptivity for embryo implantation.[38] He et al.[39] suggested that oil sands process water may decrease the estrogen production, and ozonation reduced the naphthenic acids concentrations, the primary toxic compound in oil sands process water without impacting and altering the steroidogenesis pathway and aromatase mRNA expression in cell culture studies. In our study, our results showed that OST altered GC gene expression by showing a five-fold increase in the mRNA expression levels of CYP19A1 gene which is a critical enzyme in estrogen production. Our results also showed a significant 50% reduction in the SCC gene which produces the enzyme responsible for the rate limiting step in steroidogenesis. There are studies demonstrating that overexpression can lead to reduced fertility ability while complete loss of the SCC gene was associated with neonatal deaths.[40,41] Women with polycystic ovarian syndrome, when compared to age-matched controls, demonstrated significantly higher SCC mRNA and protein levels in their ovarian tissue.[42] CYP11A1 (SCC) transgenic female mice exhibited reduced pregnancy rates, impaired implantation and placentation and reduced litter size in the uterus.[43] The authors thus inferred that the overexpression of the SCC gene leads to progesterone insufficiency in the early pregnancy.[43] On the other hand, mice with SCC knockout gene produced no steroids and presented with severe adrenal defects or resulted in neonatal deaths.[44] According to the findings above, we hypothesized that the downregulation of SCC gene in GCs following OST exposure may be related to a better fertility outcome.
There are several limitations to our study. Even though we have found significant improvement in the number of embryos formed, we did not assess the pregnancy rates in our participants as most of them are still banking more embryos due to the severe nature of their low ovarian reserve. Additionally, even though we have found significant increase in EMT, we did not assess the implantation rate for similar reasons. Another limitation is that most of our patients decided to freeze their embryos on the cleavage stage rather than blastocyst stage due to fear of embryo arrest. Additionally, we only assessed 4 genes in GCs while there are many other genes involved in steroidogenesis that need to be assessed in future studies. Finally, we evaluated mRNA levels which do not always translate into protein expression. Thus ideally, the quantification of steroids (estradiol and progesterone) in the GC culture media would have added a large value to our findings.
In conclusion, this study is the first to assess the effect of OST and PEMF in infertile women with severe DOR undergoing IVF. The preliminary findings showed promising improvement in ovarian (both in vivo and in vitro) and uterine environment. These results could be helpful for women with severe DOR who had repeated failed IVF cycles due to poor oocyte quality and for women who have persistently thin EMT who cannot use donor oocytes or a gestational carrier due to personal, ethical, religious or financial reasons. There is a definite need for well-designed large cohort studies and even randomized trials in order to better assess the efficacy of OST and PEMF in female reproduction.
Author contributions
Conceptualization and supervision: ZM; investigation and original draft: ZM, ARD; methodology and manuscript review & editing: ZM, ARD, CB, AHS, DE, LB. All authors approved the final manuscript before submission for publication.
Conflicts of interest
ARD and CB have nothing to disclose. AHS is the founder and Co-CEO of HOCATT USA. LB and DE are affiliated with HOCATT, SA. ZM acts as a paid consultant for HOCATT, USA, but he was not paid to write this manuscript.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file
Additional Table 1: Statistics for Figures 1 and 2.
Additional Table 1.
| Item | Data |
|---|---|
| Figure 1 | |
| Number of oocytes collected | P=0.4168 |
| t=0.8367, df=14 | |
| 95% confidence interval -0.5212 to 1.188 | |
| R2 (partial eta squared)= 0.04762 | |
| Correlation coefficient (r)=0.3567 | |
| Number of embryos | P=0.0236 |
| t=2.667, df=10 | |
| 95% confidence interval 0.1196 to 1.335 | |
| R2 (partial eta squared)= 0.4156 | |
| Correlation coefficient (r)=0.3480 | |
| EMT | P=0.0341 |
| t=2.367, df=13 | |
| 95% confidence interval 0.1447 to 3.165 | |
| R2 (partial eta squared)= 0.3012 | |
| Correlation coefficient (r)=0.2858 | |
| Peak E2 | P=0.6756 |
| t=0.4310, df=10 | |
| 95% confidence interval -216.5 to 320.4 | |
| R2 (partial eta squared)= 0.01824 | |
| Correlation coefficient (r)=0.2658 | |
| Figure 2 | |
| Steroidogenic acute regulatory protein | P=0.4617 |
| t=0.7655, df=10 | |
| Mean cells treated with OT 0.5525 | |
| Mean control cells 0.8684 | |
| 95% confidence interval -0.6035 to 1.235 | |
| R2 (eta squared)= 0.05535 | |
| F=4.044, DFn=5, Dfd=5 | |
| 3 Beta-hydroxysteroid dehydrogenase | P=0.8551 |
| t=0.1874, df=10 | |
| Mean cells treated with OT 0.5301 | |
| Mean control cells 0.5848 | |
| 95% confidence interval -0.5956 to 0.7050 | |
| R2 (eta squared)= 0.003498 | |
| F=3.247, DFn=5, Dfd=5 | |
| Side-chain cleavage enzyme | P=0.0474 |
| t=2.341, df=8 | |
| Mean cells treated with OT 0.5926 | |
| Mean control cells 0.2598 | |
| 95% confidence interval -0.6608 to -0.004936 | |
| R2 (eta squared)= 0.4065 | |
| F=1.537, DFn=4, Dfd=4 | |
| CYP19A1 | P=0.0242 |
| t=2.9752, df=4 | |
| Mean cells treated with OT 0.1961 | |
| Mean control cells 0.9218 | |
| 95% confidence interval 1.340 to 2.792 | |
| R2 (eta squared)= 0.1921 | |
| F=26.11, DFn=2, Dfd=2 |
REFERENCES
- 1.Elvis AM, Ekta JS. Ozone therapy: a clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta G, Mansi B. Ozone therapy in periodontics. J Med Life. 2012;5:59–67. [PMC free article] [PubMed] [Google Scholar]
- 3.Nogales CG, Ferrari PH, Kantorovich EO, Lage-Marques JL. Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 2008;9:75–84. [PubMed] [Google Scholar]
- 4.Smith NL, Wilson AL, Gandhi J, Vatsia S, Khan SA. Ozone therapy: an overview of pharmacodynamics, current research, and clinical utility. Med Gas Res. 2017;7:212–219. doi: 10.4103/2045-9912.215752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocci V, Zanardi I, Travagli V. Oxygen/ozone as a medical gas mixture. A critical evaluation of the various methods clarifies positive and negative aspects. Med Gas Res. 2011;1:6. doi: 10.1186/2045-9912-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larini A, Bocci V. Effects of ozone on isolated peripheral blood mononuclear cells. Toxicol In Vitro. 2005;19:55–61. doi: 10.1016/j.tiv.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Bocci V, Valacchi G, Corradeschi F, Fanetti G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediators Inflamm. 1998;7:313–317. doi: 10.1080/09629359890820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madej P, Antoszewski Z, Madej JA. Ozonotherapy. Mater Med Pol. 1995;27:53–56. [PubMed] [Google Scholar]
- 9.Cespedes-Suarez J, Martin-Serrano Y, Carballosa-Peña MR, Dager-Carballosa DR. The immune response behavior in HIV-AIDS patients treated with ozone therapy for two years. J Ozone Ther. 2019:2. [Google Scholar]
- 10.Rodionova OG, Gusareva MA, Sheiko EA, et al. Combination ozone therapy as an effective method of radiomodification in chemoradiation treatment of patients with cervical cancer. J Clin Oncol. 2021;39:e17515. [Google Scholar]
- 11.Raeissadat SA, Rayegani SM, Forogh B, Hassan Abadi P, Moridnia M, Rahimi Dehgolan S. Intra-articular ozone or hyaluronic acid injection: Which one is superior in patients with knee osteoarthritis? A 6-month randomized clinical trial. J Pain Res. 2018;11:111–117. doi: 10.2147/JPR.S142755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh Y, Patel S, Kaitlyn R, et al. Clinical utility of ozone therapy in dental and oral medicine. Med Gas Res. 2019;9:163–167. doi: 10.4103/2045-9912.266997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirelli U, Cirrito C, Pavanello M, Piasentin C, Lleshi A, Taibi R. Ozone therapy in 65 patients with fibromyalgia: an effective therapy. Eur Rev Med Pharmacol Sci. 2019;23:1786–1788. doi: 10.26355/eurrev_201902_17141. [DOI] [PubMed] [Google Scholar]
- 14.Hernández A, Viñals M, Isidoro T, Vilás F. Potential role of oxygen-ozone therapy in treatment of COVID-19 pneumonia. Am J Case Rep. 2020;21:e925849. doi: 10.12659/AJCR.925849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao L, Li T, Wang S, Wang J. Comprehensive treatment of diabetic hallux gangrene with lower extremity vascular disease: A case report. J Int Med Res. 2019;47:6374–6384. doi: 10.1177/0300060519886993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J, Dou J, Gao L, et al. Topical ozone therapy restores microbiome diversity in atopic dermatitis. Int Immunopharmacol. 2020;80:106191. doi: 10.1016/j.intimp.2020.106191. [DOI] [PubMed] [Google Scholar]
- 17.Braidy N, Izadi M, Sureda A, et al. Therapeutic relevance of ozone therapy in degenerative diseases: Focus on diabetes and spinal pain. J Cell Physiol. 2018;233:2705–2714. doi: 10.1002/jcp.26044. [DOI] [PubMed] [Google Scholar]
- 18.Zaky S, Kamel SE, Hassan MS, et al. Preliminary results of ozone therapy as a possible treatment for patients with chronic hepatitis C. J Altern Complement Med. 2011;17:259–263. doi: 10.1089/acm.2010.0016. [DOI] [PubMed] [Google Scholar]
- 19.Wei A, Feng H, Jia XM, Tang H, Liao YY, Li BR. Ozone therapy ameliorates inflammation and endometrial injury in rats with pelvic inflammatory disease. Biomed Pharmacother. 2018;107:1418–1425. doi: 10.1016/j.biopha.2018.07.137. [DOI] [PubMed] [Google Scholar]
- 20.ZOBEL R. Endometritis in Simmental cows: incidence, causes, and therapy options. Turk J Vet Anim Sci. 2013;37:134–140. [Google Scholar]
- 21.Mali SS, Rangnekar MN, Amle MB, Khillare KP, Mali AB, Mhase PP. Efficacy of intrauterine ozone therapy in repeat breeder cows with subclinical uterine infection. Haryana Vet. 2020;59:S83–86. [Google Scholar]
- 22.Sayar I, Bicer S, Gursul C, Gürbüzel M, Peker K, Işik A. Protective effects of ellagic acid and ozone on rat ovaries with an ischemia/reperfusion injury. J Obstet Gynaecol Res. 2016;42:52–58. doi: 10.1111/jog.12858. [DOI] [PubMed] [Google Scholar]
- 23.Uysal B, Demirbag S, Poyrazoglu Y, et al. Medical ozone therapy decreases postoperative uterine adhesion formation in rats. Arch Gynecol Obstet. 2012;286:1201–1207. doi: 10.1007/s00404-012-2435-y. [DOI] [PubMed] [Google Scholar]
- 24.Zobel R, Tkalčić S, Stoković I, Pipal I, Buić V. Efficacy of ozone as a novel treatment option for urovagina in dairy cows. Reprod Domest Anim. 2012;47:293–298. doi: 10.1111/j.1439-0531.2011.01857.x. [DOI] [PubMed] [Google Scholar]
- 25.He C, Ma X. Distal fallopian tube recanalization using ozone treatment: a clinical study in two hundred tubal obstruction Chinese patients. Int J Clin Exp Med. 2015;8:2958–2961. [PMC free article] [PubMed] [Google Scholar]
- 26.Calderon I, Cohen M, Sagi-Dain L, Artzi O, Bejar J, Sagi S. The effect of ozonated sterile saline irrigation on the endometrium - A preliminary study. J Obstet Gynaecol. 2016;36:635–640. doi: 10.3109/01443615.2015.1133579. [DOI] [PubMed] [Google Scholar]
- 27.Yuan J, Xin F, Jiang W. Underlying signaling pathways and therapeutic applications of pulsed electromagnetic fields in bone repair. Cell Physiol Biochem. 2018;46:1581–1594. doi: 10.1159/000489206. [DOI] [PubMed] [Google Scholar]
- 28.Waldorff EI, Zhang N, Ryaby JT. Pulsed electromagnetic field applications: a corporate perspective. J Orthop Translat. 2017;9:60–68. doi: 10.1016/j.jot.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvam R, Ganesan K, Narayana Raju KV, Gangadharan AC, Manohar BM, Puvanakrishnan R. Low frequency and low intensity pulsed electromagnetic field exerts its antiinflammatory effect through restoration of plasma membrane calcium ATPase activity. Life Sci. 2007;80:2403–2410. doi: 10.1016/j.lfs.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Abd Al Samea GA, Mahmoud NF, Hamada HA, Gabr AA. Influence of pulsed electromagnetic field on dermatological symptoms of hyperandrogen in obese women with polycystic ovarian syndrome. J Clin Anal Med. 2018;9:493–497. [Google Scholar]
- 31.Pandian C, Omprakash AV, Selvan ST, Sundaresan A. Effect of pulsed electro magnetic field (PEMF) exposure on hatchability performance of japanese quail hatching eggs. Indian J Vet Sci Biotechnol. 2015;11:42–44. [Google Scholar]
- 32.Merhi Z, Moseley-LaRue R, Moseley AR, Smith AH, Zhang J. Ozone and pulsed electro-magnetic field therapies improve endometrial lining thickness in frozen embryo transfer cycles: Three case reports. Medicine (Baltimore) 2019;98:e16865. doi: 10.1097/MD.0000000000016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Merhi Z, Buyuk E, Berger DS, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28:1661–1669. doi: 10.1093/humrep/det072. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Gao X, Lu X, et al. Endometrial thickness affects the outcome of in vitro fertilization and embryo transfer in normal responders after GnRH antagonist administration. Reprod Biol Endocrinol. 2014;12:96. doi: 10.1186/1477-7827-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momeni M, Rahbar MH, Kovanci E. A meta-analysis of the relationship between endometrial thickness and outcome of in vitro fertilization cycles. J Hum Reprod Sci. 2011;4:130–137. doi: 10.4103/0974-1208.92287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afonso LO, Iwama GK, Smith J, Donaldson EM. Effects of the aromatase inhibitor Fadrozole on plasma sex steroid secretion and ovulation rate in female coho salmon, Oncorhynchus kisutch, close to final maturation. Gen Comp Endocrinol. 1999;113:221–229. doi: 10.1006/gcen.1998.7198. [DOI] [PubMed] [Google Scholar]
- 38.Kim HR, Kim YS, Yoon JA, et al. Estrogen induces EGR1 to finetune its actions on uterine epithelium by controlling PR signaling for successful embryo implantation. FASEB J. 2018;32:1184–1195. doi: 10.1096/fj.201700854RR. [DOI] [PubMed] [Google Scholar]
- 39.He Y, Wiseman SB, Zhang X, et al. Ozonation attenuates the steroidogenic disruptive effects of sediment free oil sands process water in the H295R cell line. Chemosphere. 2010;80:578–584. doi: 10.1016/j.chemosphere.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Strauss JF, Barbieri RL. Philadelphia: W.B. Saunders; 2014. Yen & Jaffe 's reproductive endocrinology: physiology, pathophysiology, and clinical management. [Google Scholar]
- 41.Andersen CY, Ezcurra D. Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod Biol Endocrinol. 2014;12:128. doi: 10.1186/1477-7827-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Jiang H, He LY, Huang WJ, He XY, Xing FQ. Abnormal expression of uncoupling protein-2 correlates with CYP11A1 expression in polycystic ovary syndrome. Reprod Fertil Dev. 2011;23:520–526. doi: 10.1071/RD10266. [DOI] [PubMed] [Google Scholar]
- 43.Chien Y, Cheng WC, Wu MR, Jiang ST, Shen CK, Chung BC. Misregulated progesterone secretion and impaired pregnancy in Cyp11a1 transgenic mice. Biol Reprod. 2013;89:91. doi: 10.1095/biolreprod.113.110833. [DOI] [PubMed] [Google Scholar]
- 44.Hsu HJ, Hsu NC, Hu MC, Chung BC. Steroidogenesis in zebrafish and mouse models. Mol Cell Endocrinol. 2006;248:160–163. doi: 10.1016/j.mce.2005.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


