Abstract
This chapter discusses how the complex concept of anhedonia can be operationalized and studied in preclinical models. It provides information about the development of anhedonia in the context of early life adversity, and the power of preclinical models to tease out the diverse molecular, epigenetic and network mechanisms that are responsible for anhedonia-like behaviors.
Specifically, we first discuss the term anhedonia, reviewing the conceptual components underlying reward-related behaviors and distinguish anhedonia pertaining to deficits in motivational versus consummatory behaviors. We then describe the repertoire of experimental approaches employed to study anhedonia-like behaviors in preclinical models, and the progressive refinement over the past decade of both experimental instruments (e.g., chemogenetics, optogenetics) and conceptual constructs (salience, valence, conflict). We follow with an overview of the state of current knowledge of brain circuits, nodes and projections that execute distinct aspects of hedonic-like behaviors, as well as neurotransmitters, modulators and receptors involved in the generation of anhedonia-like behaviors. Finally, we discuss the special case of anhedonia that arises following early-life adversity as an eloquent example enabling the study of causality, mechanisms and sex dependence of anhedonia.
Together, this chapter highlights the power, potential and limitations of using pre-clinical models to advance our understanding of the origin and mechanisms of anhedonia and to discover potential targets for its prevention and mitigation.
1. The concept of anhedonia and its operationalization in preclinical models
Anhedonia denotes a transdiagnostic construct that necessitates understanding the role of the reward circuit and its altered function in the pathophysiology of mental illnesses (Bedwell et al. 2014; Lake et al. 2017). Thus, the Research Domain Criteria (RDoC) framework put forth by the National Institute of Mental Health (NIMH) in the US suggests that the neurobiological basis of anhedonia, together with empirical behavioral measures in humans and animal models are key to understanding how specific conditions including genetic make-up or early-life adversity (ELA) might lead to mental health vulnerabilities. Indeed, the common presence of anhedonia as a herald or component of many psychiatric illnesses supports the notion that the disruption of reward circuit function which characterizes anhedonia is a common mechanism for several neuropsychiatric disorders.
The concept of anhedonia and the diverse definitions of the term are well addressed in other chapters in this tome. Here we note that, in both humans and experimental models, there are multiple domains of anhedonic behaviors, and these may involve distinct neural mechanisms and processes (Der-Avakian and Markou 2012; Shankman et al. 2014; Zald and Treadway 2017). Preclinical studies have identified several behavioral components that are grouped within the concept of anhedonia (Berridge and Kringelbach 2015; Der-Avakian and Pizzagalli 2018; Levis et al. 2021). Some studies of anhedonia have focused on deficits in motivation or anticipatory reward (Sherdell et al. 2012; Bryant et al. 2017; Szczepanik et al. 2019), whereas others emphasize the importance of assessing consummatory reward (Kringelbach and Berridge 2016; Wright et al. 2020). In addition, the attenuation of reward-seeking behaviors observed in humans or preclinical models can be limited to some reinforcers but not others (e.g., social vs. food rewards), further complicating the definition of anhedonic behaviors. Yet, whereas there is a vibrant ongoing discussion of the definition and boundaries of the term anhedonia, a broad consensus is emerging regarding the brain circuitry that is involved, namely, the reward circuitry. Indeed, human and experimental animal studies conclude that distinct deficits in reward-seeking behaviors which comprise specific aspects of anhedonia all arise from selective and yet overlapping disruption of the operations of specific nodes and connections within the reward circuit.
The facts above suggest that key insights into the nature and mechanisms of anhedonia require interrogation of the reward circuits, yet the ability to do so is limited in humans. Whereas the advent of structural and functional magnetic resonance imaging has proven invaluable to visualizing the human brain and its circuits, establishing causality, teasing apart the distinct roles of genetics and environment and overcoming other uncontrolled confounders limit the capacity of human studies to fully uncover the issues revolving around anhedonia. Thus, the establishment of animal models with the goals of studying anhedonia and identifying salient projections, nodes, and circuits together with molecules and mechanisms that are disrupted, are key to elucidating the origins and trajectories of anhedonia.
1.1. Novel tools for the study of anhedonia in preclinical models
Across neuropsychiatric diagnoses, anhedonia can manifest as consummatory and/or motivational deficits (Sherdell et al. 2012; Kringelbach and Berridge 2016; Bryant et al. 2017; Szczepanik et al. 2019; Wright et al. 2020). These responses to rewarding stimuli are often relatively easy to study in rodents, yet historically, anhedonia has not been formally distinguished in rodent studies from measures of depression-like behaviors. Thus, often, repeated exposure to aversive conditions have been utilized along with reward specific tasks to model clinical depressive symptoms. Behavioral despair tests, such as the forced swim (FS) tail suspension (TS), and chronic stress (CS) and chronic mild stress (CMS) paradigms have been used to measure anhedonia and antidepressant-like behavior in rodents (Katz 1982; Willner et al. 1992; Cryan et al. 2005; Castagné et al. 2011). However, these tasks often lead to difficulties in interpretation and reproducibility and thus may not be optimal measures of the diverse facets of anhedonia. Therefore, more complex and nuanced behavioral tasks are currently used to measure aspects of anhedonia in preclinical models (Table 1), as described below.
Table 1.
Preclinical models of anhedonia
| Task | Behavioral component |
Important circuits/substrates |
Reference |
|---|---|---|---|
| Forced swim, tail suspension, Chronic stress, Chronic mild stress | behavioral despair; depression-like behavior; antidepressant actions | prefrontal cortex, extended amygdala, hippocampus, nucleus accumbens, serotonin/norepinephrine systems | (Katz 1982; Willner et al. 1992; Cryan et al. 2002; Castagné et al. 2011) |
| Taste reactivity | motivational valence (appetitive/aversive); incentive "liking"; core hedonic process | nucleus accumbens, ventral pallidum, amygdala, endogenous opioid, endocannabinoid systems | (Smith et al. 2010) |
| Social interaction, Urine sniff test | social, sexual motivation; motivational anhedonia | nucleus accumbens, hypothalamus, amygdala, prefrontal cortex, olfactory circuits, oxytocin, dopamine, endogenous opioid, endocannabinoid systems | (Malkesman et al. 2010; Roberts et al. 2010; Trezza et al. 2010) |
| Sucrose preference | hedonic capacity; incentive "liking"; consummatory anhedonia | nucleus accumbens, amygdala, nucleus of the solitary tract, prefrontal cortex, paraventricular nucleus of the thalamus, endocannabinoid systems, CRH | (Mahler et al. 2007; Bolton et al. 2018a; Tan et al. 2020) |
| Conditioned place preference | incentive motivation / "wanting"; motivational learning; motivational anhedonia | amygdala, striatum, hippocampus, mesolimbic dopamine | (Everitt et al. 1991) |
| Intracranial self-stimulation | incentive motivation / "wanting"; motivational anhedonia | striatum, medial forebrain bundle, basal forebrain, mesolimbic dopamine | (Olds and Fobes 1981) |
| Economic demand | discriminate hedonic set point (low-cost consumption) from essential value / motivation (high-effort consumption) | striatum, pallidum, extended amygdala more critical for regulating hedonic set point; mesolimbic dopamine more critical for high-effort consumption | (Koob 1999; Bentzley et al. 2013, 2014; Salamone et al. 2016) |
| Reinforcement learning | associate outcome with previous experience/choice | ventral tegmental area, amygdala, ventral striatum, hippocampus, prefrontal cortex, dopamine | (Der-Avakian et al. 2013; Huys et al. 2013; Costa et al. 2016; Kangas et al. 2021) |
Reward circuit dysfunction, which is thought to underlie aspects of anhedonia, is often studied using tasks involving motivated behavior, such as drug seeking, food seeking and the seeking of sex-reward cues. Notably, reward consumption at low effort can be distinguished from highly motivated, effortful reward seeking. These two types of tasks have dissociable underlying neural processes (Berridge and Robinson 2003; Vanderschuren et al. 2005; Baldo and Kelley 2007; Bentzley et al. 2013; Berridge and Kringelbach 2015; Salamone et al. 2016; Volkow et al. 2017) that may therefore be differentially susceptible to disruption and the production of anhedonia. These distinct types of reward seeking behaviors can be individually measured using tasks such as taste reactivity assays (Smith et al. 2010), intracranial self-stimulation (Olds and Milner 1954; Carlezon and Chartoff 2007), drug or food self-administration and relapse (Berridge and Aldridge 2009), reinforcement learning (Der-Avakian et al. 2013; Kangas et al. 2021), and others.
In both humans and rodents, an eloquent behavioral tool for simultaneously studying both consummatory and motivational aspects of reward in the setting of anhedonia capitalizes on behavioral economic theory, which stipulates that consumption of any commodity is sensitive to increasing price. Relative sensitivity to increasing price is referred to as “demand elasticity” (Hursh 1980). Inelastic demand, or relative insensitivity to price, is a feature of the excessive reward seeking associated with substance use disorders (Bickel et al. 2014), whereas relatively high sensitivity to increasing price, or a lack of motivation to obtain a reward at high cost, may be a feature of anhedonia. This behavior is distinct from reward intake when required effort is very low. Specifically, while consumption that persists at high cost is more reliant on motivational processes, drug consumption when cost is low corresponds to hedonic value, or “liking” of the drug, governed by a so-called “hedonic setpoint” (Hursh and Silberberg 2008; Bickel et al. 2014; Strickland et al. 2019). Anhedonia may therefore manifest as reduced “liking”, or decreased hedonic setpoint for a given reinforcer, independent of changes to demand elasticity. Thus, behavioral economic tasks allow for the dissociation of anhedonic behaviors resulting from motivational deficits from those resulting from deficits in the hedonic aspects of reward consumption. Indeed, the neural substrates of demand elasticity and hedonic setpoint for drug rewards appear to be distinct (Bentzley and Aston-Jones 2015; Bolton et al. 2018b; Salamone et al. 2018; Levis et al. 2019).
In rodents, demand elasticity and hedonic setpoint for rewards such as palatable food or abused drugs can be modeled by examining intake at different “prices,” operationalized as the amount of effort required to receive one unit of the reward (e.g. a single drug infusion or a single food pellet) (Hursh and Silberberg 2008; Oleson and Roberts 2008, 2009; Bentzley et al. 2013, 2014; Newman and Ferrario 2020). For example, using this method, early-life adversity (ELA) leads to reduced hedonic setpoint for cocaine in male rats (Bolton et al. 2018b), suggestive of anhedonia. Strikingly, in ELA-reared female rodents there is a distinct lack of anhedonia. Rather, females have enhanced motivation (reduced demand elasticity) to obtain both opioid drugs and palatable food rewards, and no alteration in hedonic setpoint (Levis et al. 2019). Interestingly, in humans, there are no compelling data identifying sex differences in the prevalence and pathophysiology of anhedonia, although sex-specific effects have not been extensively probed. Yet, these disparate findings in preclinical models demonstrate the power of sophisticated tests to tease out distinct, sex-specific disruptions of reward circuits by ELA or other insults that are associated with vulnerability to anhedonia in humans.
1.2. The reward circuit and its study in experimental models
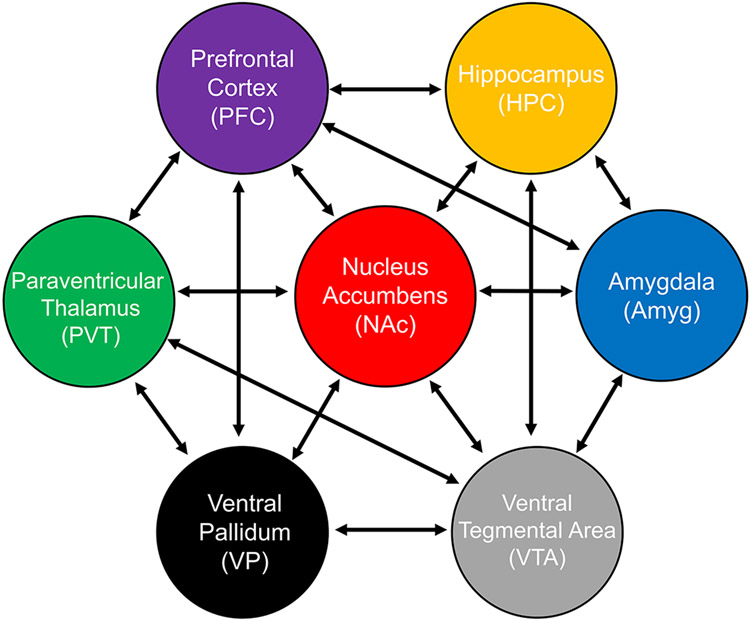
The reward circuitry is complex, encompassing nodes including the nucleus accumbens (NAc), prefrontal cortex (PFC), ventral tegmental area (VTA), ventral pallidum (VP), amygdala (Amyg), hippocampus (HPC) and paraventricular nucleus of the thalamus (PVT) (Fig. 1). These are engaged during reward processing and related choices. The NAc modulates the response to reward-related cues, as well as the value of expected versus actual reward outcomes. Studies to date have largely focused on glutamatergic and dopaminergic input pathways to the NAc and have demonstrated its role in integrating excitatory and inhibitory input to signal the salience of rewarding stimuli. These stimuli, in turn are encoded via projections to and from limbic structures (Figure 1) (Ballard et al. 2011; Tritsch et al. 2012; Britt et al. 2012; Bagot et al. 2015; Ferenczi et al. 2016; Robbins 2016).
Figure 1.
Cross-species nodes and connectivity of the reward circuit.
Several neurotransmitters and neuromodulators convey information from and to the NAc (Figure 1). Specifically, glutamatergic projections from cortical, thalamic, hippocampal and amygdalar regions terminate in the NAc and mediate behavioral effects via AMPA, NMDA and metabotropic glutamate (mGluR) receptors (Krystal et al. 2003; Cozzoli et al. 2012; Wang et al. 2012). For instance, reward seeking can be restricted via glutamatergic VP neurons increasing the activity of GABA VTA neurons (Tooley et al. 2018). Dopamine plays a role in motivation and reward and has been extensively studied. Dopamine influences incentive salience and instrumental behaviors in cue reward tasks (Peciña et al. 2003), and blocking dopamine receptors in the NAc reduces the effort expended to obtain a reward (Aberman et al. 1998). Serotonin also plays a role: dorsal raphe serotonin transporter (SERT) terminals, which synapse onto VTA dopaminergic neurons, increase rewarding behaviors (Wang et al. 2019).
Beyond the classically known neurotransmitters, several peptides and neuromodulators are expressed in reward circuit nodes to influence behaviors. Opioids and endocannabinoids are well-established major neurochemical mediators of reward responsiveness (Pecina and Berridge 2005; Mahler et al. 2007). Importantly, focusing on consummatory anhedonia, several peptides are expressed and function within the reward circuit. These include orexin and neuropeptide Y which modulate food intake (Van Den Heuvel et al. 2015; Castro et al. 2016), and corticotropin-releasing hormone (CRH), a stress responsive peptide and its receptors. (Peciña et al. 2006; Lemos et al. 2012; Walsh et al. 2014; Peng et al. 2017; Bolton et al. 2018a).
Whereas the complexity of the neuroanatomical and molecular interactions described above is daunting, animal models allow the use of novel and evolving instruments and techniques to address these intricacies. Specifically, they enable both mapping and cell-type-specific and projection-specific manipulation of select components of the reward circuit. Anterograde and retrograde tracers have been extensively used to visualize the connectivity between circuit nodes (Nassi et al. 2015; Tervo et al. 2016; Itoga et al. 2019; Engelke et al. 2021). These connections can be interrogated further, with the use of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) (Mahler et al. 2014, 2019) and channelrhodopsins (Miyazaki et al. 2014; Cole et al. 2018) to dissect brain region and cell type specific functional control of behavior. For instance, in rats and mice, anterograde and retrograde tracing has identified novel CRH expressing projection sources to the NAc from regions integrating reward and stress circuits (Birnie et al. 2020a), and optogenetic activation of a CRH+ PVT-NAc projection reduced food seeking behavior (Engelke et al. 2021), identifying a novel role for a stress-regulated peptide that influences reward behaviors.
2. Anhedonia and early-life adversity (ELA)
2.1. Why study ELA and anhedonia? The human landscape
In humans, ELA is linked to several mental health disorders that indicate dysfunction of the operation of the reward circuit. Functional magnetic resonance imaging (fMRI) studies have probed the functional activation of components of the reward circuitry in individuals who have experienced early life adversity and identified several deficits (Boecker et al. 2014; Corral-Frías et al. 2015). ELA and subsequent emotional problems differ in men and women, with women with a history of early-life trauma being more likely to crave comfort food and opioids (Mason et al. 2013). These differences between the sexes are a result of both intrinsic differences in the operation of the reward circuit, as well as sex dimorphism in the response to ELA and to stress in general (Davis and Pfaff 2014; Walker et al. 2017).
2.2. What is ELA and how do we model it?
Early life adversity is a heterogenous concept. In humans, it typically denotes low socioeconomic level, poverty, parental depression, neglect or abuse and a violent, chaotic environment (Short and Baram 2019; Luby et al. 2020). These complex contexts generate and convey numerous types of signals to the developing infant and child, including significant emotional and sometimes physical anguish. Yet, in humans and especially in experimental models, the rich array of signals from caretakers and the environment, which reach and activate selective brain regions and nodes of the stress and reward circuits are often simply summed up as ‘stress’, and considered interchangeable with activation of the neuroendocrine stress response. Instead, ELA is a multidimensional construct, and simply looking at the activation of the hypothalamic-pituitary-adrenal system may not recapitulate its numerous effects on the different functions of the brain (Molet et al. 2016a; Bolton et al., 2018a). It is also likely that different types of ELA and even the same type of ELA generated at different developmental stages might exert distinct differences to mesolimbic structures which undergo significant growth, maturation, and plasticity throughout specific sensitive periods (Tottenham 2020; Marini et al. 2020; Birnie et al. 2020b; Luby et al. 2020).
The development of preclinical models for ELA provides basic and translational scientists with the opportunity to understand complex neural mechanisms using techniques and approaches that are not possible in humans (Baram et al. 2012). Indeed, several approaches have been used to generate adversity early in life. Maternal separation has been used extensively to study the effects of such adversity/stress, and several variants exist including daily short (3-4 hour) separation or a single prolonged deprivation. These models have generally yielded deficits in cognitive abilities as well as anxiety-like and depression-like and addiction-like behaviors, yet the development of anhedonia has not been consistent (Matthews et al. 1996; Leventopoulos et al. 2009; Nelson et al. 2009; Andersen 2019). More recently, with the aim of generating a naturalistic, highly reproducible model for early life adversity, a paradigm of simulated poverty, using cages with limited bedding and nesting material (LBN) in rodents, has been devised and used extensively around the world. This environment stresses the dams and leads to disruption of maternal caring behaviors (Ivy et al. 2008; Molet et al. 2016a). This translates into unpredictable and fragmented sensory signals received by the developing pups. Whereas the overall duration and quality of maternal care remain unaltered, the pattern of their signals to pups is aberrant. Sensory signals from the environment are required for normal maturation of brain circuits including visual and auditory systems (Espinosa and Stryker 2012; Short and Baram 2019). It is believed that aberrant patterns of tactile signals from the dam may interfere with the maturation of reward-and stress-related circuits in the pups (Short and Baram 2019; Birnie et al. 2020b; Luby et al. 2020). Indeed, aberrant brain circuit maturation is generated in the pups, evidenced with MRI (Molet et al. 2016b; Bolton et al. 2018a) and manifesting as impaired memory as well as deficits in emotional-like and reward-executed behaviors, measured by food consumption, social play, and hedonic setpoint for cocaine. A third model of ELA involves cross-fostering, aiming to model early postnatal instability in humans. Starting during the first two days of birth, pups are cross-fostered with a lactating dam. This intervention, allows for distinguishing between relatedness and environment, thus allowing researchers to recognize how genes and environment interact to influence developmental processes and later adult behaviors (Hager et al. 2009; Ventura et al. 2013; McCarty 2017). Comprehensive reviews of these preclinical models have been published (Molet et al., 2014; Walker et al., 2017).
2.3. Anhedonia following ELA involves a developing reward circuit
In the context of the mechanisms by which ELA generates anhedonic behaviors, it is useful to consider that the reward circuity overlaps with nodes of the stress circuit, and that both are in a state of maturation and heightened plasticity during the developmental epochs comprising ELA (Avishai-Eliner et al. 2002; Birnie et al. 2020b).
Robust evidence exists for profound, likely permanent changes in brain reward and stress systems of individuals experiencing ELA, including mesolimbic and extended amygdala circuits, and neurotransmitter/neuromodulator systems including dopamine, endogenous opioids, and CRH. These enduring changes may promote vulnerability to anhedonia. At the circuit level, amygdala-PFC structural connectivity was augmented in adult male rats that had experienced ELA relative to controls (Bolton et al. 2018a). Pre-weaning LBN males, but not females, have reduced BLA-PFC, and altered PFC-striatum resting state functional connectivity (Guadagno et al. 2018b, a), a finding that persists into adulthood and is accompanied by reduced sucrose preference and social interaction (Yan et al. 2017). Disruption of the early maturation of BLA-PFC connections has been reported after maternal separation as well (Brenhouse et al. 2013; Honeycutt et al. 2020; Nieves et al. 2020), further implicating this circuit in the effects of ELA. Notably, human studies suggest that ELA’s impact on amygdala development is critical for the resulting depression and anxiety (Callaghan and Tottenham 2016; Fareri and Tottenham 2016), and potentially for anhedonia as well.
Changes in neurotransmitter and neuromodulators in both reward and stress circuitries result from ELA. For example, endogenous opioid systems are enduringly altered by ELA, a fact that may impact pleasure or other reward-relevant processes (Peciña et al. 2019). Maternal separation persistently alters endogenous opioid peptides, as well as opioid and dopamine receptor expression in reward and stress related areas, including striatum, midbrain, hippocampus, and hypothalamus in both a sex- and ELA timing-dependent manner (Ploj et al. 1999, 2001, 2003; Gustafsson et al. 2008; Chang et al. 2019). The development of the mesolimbic dopamine system is also strongly impacted by ELA (Rodrigues et al. 2011; Ventura et al. 2013; Peña et al. 2014; Bonapersona et al. 2018), thereby potentially disrupting dopamine-dependent incentive motivation and learning.
ELA leads to enduring changes in the expression levels of the stress-sensitive neuropeptide CRH in the amygdala central nucleus (CeA) (Dubé et al. 2015) and hippocampus (Ivy et al. 2010), and is associated with major changes in circuit functions (Brunson et al. 2005; Ivy et al. 2010). Such changes in circuit function are apparent from both neuronal activation and from performance in tasks probing the reward circuits and specifically anhedonia. For example, palatable food, social play, and acute cocaine reward induce a stronger Fos response in CeA of ELA males than of control males, an effect accompanied by anhedonia-like behavioral responses to those same rewards (Bolton et al. 2018a, b). These findings suggest that ELA promotes aberrant activation of stress-circuit nodes during tasks that typically engage the reward circuit exclusively.
2.4. Anhedonia after ELA: manifestations and sex specificity
The expression of anhedonia following ELA in preclinical models appears to involve interactions among the timing and nature of the ELA paradigm, biological sex, and the nature of the behavioral tests (Matthews and Robbins 2003; Rüedi-Bettschen et al. 2005; Der-Avakian and Markou 2010; Leussis et al. 2012; Molet et al. 2016a; Lukkes et al. 2017; Di Segni et al. 2019; Luby et al. 2020). For example, in male rodents, ELA imposed via rearing for one week in cages with limited bedding and nesting materials leads to enduring anhedonia for both natural and drug rewards. This includes blunted sucrose and palatable food preference, and reduced interest in social play (Molet et al. 2016a; Rincón-Cortés and Sullivan 2016; Yan et al. 2017; Bolton et al. 2018a, b). Using a behavioral economic assay of cocaine seeking, ELA does not change motivation to obtain drug at high effort (demand elasticity), but leads to reduced hedonic setpoint for cocaine in male rats when the drug is “free” (Bolton et al. 2018b). This suggests that, in addition to anhedonia for natural rewards (Bolton et al. 2018a), these animals are anhedonic for drug rewards. Such anhedonia is not observed in female rats after LBN (Levis et al. 2019), however others have identified an age-dependent reduction of sucrose preference and depressive-like behaviors in female mice following LBN (Goodwill et al. 2019). Using a maternal separation model of ELA, both male and female rats have reduced sucrose preference later in life (Matthews et al. 1996; Leventopoulos et al. 2009; Coccurello et al. 2014). Anhedonia has also been reported in nonhuman primates exposed to maternal deprivation and maltreatment (Rosenblum and Paully 1987; Paul et al. 2000; Pryce et al. 2004; Kaufman et al. 2007; Glynn and Baram 2019), including reduced sucrose preference (Paul et al. 2000) and lack of interest in social interaction (Coplan et al. 1996). However, others have found increased sucrose drinking in juvenile males following ELA (Nelson et al. 2009).
Together, the findings in rodents and non-human primates suggest the manifestations of reward circuit disruption by ELA may vary by the timing, duration, and nature of the ELA, and may be further modulated by sex. Whereas deficits in reward seeking behaviors are observed in males, such deficits are not as commonly found in females. Rather, in females, the prevailing phenotype includes the enhanced consumption palatable food and drugs of abuse. Furthermore, the variable consequences of ELA on distinct assays of reward-seeking behaviors in animal models (e.g., reduced “hedonic setpoint” but unchanged “demand elasticity”) demonstrate that reward processing is not a singular entity; rather, individuals may express different and dissociable anhedonia phenotypes that suggest potentially discrete mechanisms of reward circuit disruption. Thus, further investigation into how ELA alters specific aspects of reward processing, and the underlying neural substrates will be critical for understanding the biological processes that contribute to the risk anhedonia, a key harbinger and component of several psychiatric disorders.
3. General conclusions
Anhedonia is a transdiagnostic construct which is both a herald and a core component of several mental illnesses, yet the identification and characterization of the neural mechanisms that contribute to its emergence remain challenging. Strides have been made in understanding of the origins of anhedonia though both human studies and the use of preclinical animal models. In rodents, researchers can induce anhedonia-like behaviors, enabling the identification and characterization of individual reward-circuit nodes and projections that influence these behaviors, and further characterize them at the cellular and molecular level. The study of the emergence of anhedonia-like behaviors following experimental ELA is an exciting avenue for two reasons. First, it recapitulates the human association, allowing delineation of trajectories and life-long progression. Second, it enables the use of powerful modern technologies including chemo- and optogenetics for the interrogation and functional identification of neural projections and molecules that may mediate anhedonia. Critically, with the use of animal models and asking the right questions, clinical queries can be translated to lab-based mechanistic studies, which in turn can lead to novel discoveries for the prevention of anhedonia or its mitigation.
Funding and Disclosures:
Authors’ work supported by National Institute of Health Grant Nos. P50 MH096889, MH73136, F30 DA051137, T32 GM008620; and the Hewitt Foundation for Biomedical Research.
The authors thank Dr. Annabel Short for excellent discussions.
Footnotes
MTB, SCL, SVM and TZB report no biomedical financial interests or conflicts of interest.
References:
- Aberman JE, Ward SJ, Salamone JD (1998) Effects of Dopamine Antagonists and Accumbens Dopamine Depletions on Time-Constrained Progressive-Ratio Performance. Pharmacol Biochem Behav 61:341–348. 10.1016/S0091-3057(98)00112-9 [DOI] [PubMed] [Google Scholar]
- Andersen SL (2019) Stress, sensitive periods, and substance abuse. Neurobiol Stress 10:. 10.1016/J.YNSTR.2018.100140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ (2002) Stressed-out, or in (utero)? Trends Neurosci 25:518–524. 10.1016/S0166-2236(02)02241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Peña CJ, et al. (2015) Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun 6:7062. 10.1038/ncomms8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE (2007) Discrete neurochemical coding of distinguishable motivational processes: Insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 191:439–459. 10.1007/S00213-007-0741-Z [DOI] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, et al. (2011) Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci 31:10340–6. 10.1523/JNEUROSCI.0895-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, et al. (2012) Fragmentation and Unpredictability of Early-Life Experience in Mental Disorders. Am J Psychiatry 169:907–915. 10.1176/appi.ajp.2012.11091347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell J, Gooding D, Chan C, Trachik B (2014) Anhedonia in the age of RDoC. Schizophr Res 160:226–227. 10.1016/J.SCHRES.2014.10.028 [DOI] [PubMed] [Google Scholar]
- Bentzley B, Aston-Jones G (2015) Ore7xin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41:1149–1156. 10.1111/EJN.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley B, Fender K, Aston-Jones G (2013) The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226:113–125. 10.1007/S00213-012-2899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley B, Jhou T, Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111:11822–11827. 10.1073/PNAS.1406324111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW (2009) Decision Utility, Incentive Salience, and Cue-Triggered “Wanting.” Oxford Ser Soc Cogn Soc Neurosci 2009:509. [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2015) Pleasure Systems in the Brain. Neuron 86:646–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513. 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, et al. (2014) The Behavioral Economics of Substance Use Disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol 10:641. 10.1146/ANNUREV-CLINPSY-032813-153724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie M, Short A, Itoga C, et al. (2020a) A novel CRH-specific projection from basolateral amygdala to nucleus accumbens depresses reward-seeking behaviors. Neuropsychopharmacology 45:29231597159 [Google Scholar]
- Birnie MT, Kooiker CL, Short AK, et al. (2020b) Plasticity of the Reward Circuitry After Early-Life Adversity: Mechanisms and Significance. Biol. Psychiatry 87:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker R, Holz NE, Buchmann AF, et al. (2014) Impact of Early Life Adversity on Reward Processing in Young Adults: EEG-fMRI Results from a Prospective Study over 25 Years. PLoS One 9:e104185. 10.1371/journal.pone.0104185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Regev L, et al. (2018a) Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biol Psychiatry 83:137–147. 10.1016/j.biopsych.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Ruiz CM, Rismanchi N, et al. (2018b) Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress 8:57–67. 10.1016/J.YNSTR.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapersona V, Joëls M, Sarabdjitsingh R (2018) Effects of early life stress on biochemical indicators of the dopaminergic system: A 3 level meta-analysis of rodent studies. Neurosci Biobehav Rev 95:1–16. 10.1016/j.neubiorev.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Lukkes JL, Andersen SL (2013) Early Life Adversity Alters the Developmental Profiles of Addiction-Related Prefrontal Cortex Circuitry. Brain Sci 3:143. 10.3390/BRAINSCI3010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, et al. (2012) Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 76:790–803. 10.1016/J.NEURON.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, et al. (2005) Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 25:9328–9338. 10.1523/JNEUROSCI.2281-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J, Winer ES, Salem T, Nadorff MR (2017) Struggling toward reward: Recent experience of anhedonia interacts with motivation to predict reward pursuit in the face of a stressful manipulation. PLoS One 12:. 10.1371/JOURNAL.PONE.0173439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N (2016) The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology 41:163–176. 10.1038/npp.2015.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2007 211 2:2987–2995. 10.1038/nprot.2007.441 [DOI] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD (2011) Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr Protoc Neurosci 55:. 10.1002/0471142301.ns0810as55 [DOI] [PubMed] [Google Scholar]
- Castro DC, Terry RA, Berridge KC (2016) Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose “Liking” and Intake but Scopolamine in Caudal Shell Shifts “Liking” Toward “Disgust” and “Fear.” Neuropsychopharmacology 41:2101–2111. 10.1038/npp.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kigar S, Ho J, et al. (2019) Early life stress alters opioid receptor mRNA levels within the nucleus accumbens in a sex-dependent manner. Brain Res 1710:102–108. 10.1016/J.BRAINRES.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R, Bielawski A, Zelek-Molik A, et al. (2014) Brief maternal separation affects brain α1-adrenoceptors and apoptotic signaling in adult mice. Prog Neuro-Psychopharmacology Biol Psychiatry 48:161–169. 10.1016/j.pnpbp.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Cole SL, Robinson MJF, Berridge KC (2018) Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLoS One 13:e0207694. 10.1371/journal.pone.0207694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, et al. (1996) Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci 93:1619–1623. 10.1073/pnas.93.4.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, et al. (2015) Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med 45:2605–17. 10.1017/S0033291715000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Dal Monte O, Lucas DR, et al. (2016) Amygdala and Ventral Striatum Make Distinct Contributions to Reinforcement Learning. Neuron 92:505–517. 10.1016/j.neuron.2016.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, et al. (2012) Nucleus Accumbens mGluR5-Associated Signaling Regulates Binge Alcohol Drinking Under Drinking-in-the-Dark Procedures. Alcohol Clin Exp Res 36:1623–1633. 10.1111/j.1530-0277.2012.01776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245. 10.1016/S0165-6147(02)02017-5 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625. 10.1016/j.neubiorev.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Davis EP, Pfaff D (2014) Sexually dimorphic responses to early adversity: Implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology 49:11–25. 10.1016/j.psyneuen.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A (2013) Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry 3:e297–e297. 10.1038/tp.2013.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A (2010) Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience 170:1189–1198. 10.1016/j.neuroscience.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A (2012) The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35:68–77. 10.1016/J.TINS.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Pizzagalli DA (2018) Translational Assessments of Reward and Anhedonia: A Tribute to Athina Markou. Biol Psychiatry 83:932–939. 10.1016/J.BIOPSYCH.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Segni M, Andolina D, D’Addario SL, et al. (2019) Sex-dependent effects of early unstable post-natal environment on response to positive and negative stimuli in adult mice. Neuroscience 413:1–10. 10.1016/j.neuroscience.2019.06.016 [DOI] [PubMed] [Google Scholar]
- Dubé CM, Molet J, Singh-Taylor A, et al. (2015) Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiol Stress 2:10–19. 10.1016/j.ynstr.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke DS, Zhang XO, O’Malley JJ, et al. (2021) A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats. Nat Commun 12:2517. 10.1038/s41467-021-22730-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP (2012) Development and Plasticity of the Primary Visual Cortex. Neuron 75:230. 10.1016/J.NEURON.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW (1991) The basolateral amygdala-ventral striatal system and conditioned place preference: Further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42:1–18. 10.1016/0306-4522(91)90145-E [DOI] [PubMed] [Google Scholar]
- Fareri D, Tottenham N (2016) Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci 19:233–247. 10.1016/J.DCN.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, et al. (2016) Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science (80- ) 351:. 10.1126/science.aac9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Baram TZ (2019) The influence of unpredictable, fragmented parental signals on the developing brain. Front. Neuroendocrinol 53:100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, Gallo M, et al. (2019) Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 44:711–720. 10.1038/s41386-018-0195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A, Kang MS, Devenyi GA, et al. (2018a) Reduced resting-state functional connectivity of the basolateral amygdala to the medial prefrontal cortex in preweaning rats exposed to chronic early-life stress. Brain Struct Funct. 10.1007/s00429-018-1720-3 [DOI] [PubMed] [Google Scholar]
- Guadagno A, Wong T, Walker C (2018b) Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Prog Neuropsychopharmacol Biol Psychiatry 81:25–37. 10.1016/J.PNPBP.2017.09.025 [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Oreland S, Hoffmann P, Nylander I (2008) The impact of postnatal environment on opioid peptides in young and adult male Wistar rats. Neuropeptides 42:177–191. 10.1016/J.NPEP.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Hager R, Cheverud JM, Wolf JB (2009) Change in maternal environment induced by cross-fostering alters genetic and epigenetic effects on complex traits in mice. Proc R Soc B Biol Sci 276:2949–2954. 10.1098/RSPB.2009.0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JA, Demaestri C, Peterzell S, et al. (2020) Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. Elife 9:. 10.7554/ELIFE.52651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115:186–198. 10.1037/0033-295X.115.1.186 [DOI] [PubMed] [Google Scholar]
- Hursh SR (1980) Economic concepts for the analysis of behavior. J Exp Anal Behav 34:219. 10.1901/JEAB.1980.34-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P (2013) Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord 3:12. 10.1186/2045-5380-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoga CA, Chen Y, Fateri C, et al. (2019) New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J Comp Neurol 527:2474–2487. 10.1002/cne.24676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ (2008) Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience 154:1132–1142. 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, et al. (2010) Hippocampal Dysfunction and Cognitive Impairments Provoked by Chronic Early-Life Stress Involve Excessive Activation of CRH Receptors. J Neurosci 30:13005–13015. 10.1523/JNEUROSCI.1784-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Short AK, Luc OT, et al. (2021) A Cross-species Assay Demonstrates that Reward Responsiveness is Enduringly Impacted by Adverse, Unpredictable Early-Life Experiences. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ (1982) Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav 16:965–968. 10.1016/0091-3057(82)90053-3 [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, et al. (2007) Early-Life Stress and the Development of Obesity and Insulin Resistance in Juvenile Bonnet Macaques. Diabetes 56:1382–1386. 10.2337/db06-1409 [DOI] [PubMed] [Google Scholar]
- Koob GF (1999) The Role of the Striatopallidal and Extended Amygdala Systems in Drug Addiction. Ann N Y Acad Sci 877:445–460. 10.1111/j.1749-6632.1999.tb09282.x [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC (2016) Neuroscience of reward, motivation, and drive. Adv Motiv Achiev 19:23–35. 10.1108/S0749-742320160000019020 [DOI] [Google Scholar]
- Krystal J, Petrakis I, Mason G, et al. (2003) N-methyl-D-aspartate glutamate receptors and alcoholism: reward, depedence, treatment, and vulnerability. Pharmacol Ther 99:79–94 [DOI] [PubMed] [Google Scholar]
- Lake JI, Yee CM, Miller GA (2017) Misunderstanding RDoC. Z Psychol 225:170. 10.1027/2151-2604/A000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, et al. (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–6. 10.1038/nature11436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, et al. (2012) Depressive-Like Behavior in Adolescents after Maternal Separation: Sex Differences, Controllability, and GABA. Dev Neurosci 34:210–217. 10.1159/000339162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, et al. (2009) Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology 56:692–701. 10.1016/j.neuropharm.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Levis SC, Baram TZ, Mahler SV. (2021) Neurodevelopmental origins of substance use disorders: Evidence from animal models of early-life adversity and addiction. Eur J Neurosci ejn.15223. 10.1111/ejn.15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis SC, Bentzley BS, Molet J, et al. (2019) On the early-life origins of vulnerability to opioid addiction. Mol Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Baram T, Rogers C, Barch D (2020) Neurodevelopmental Optimization after Early-Life Adversity: Cross-Species Studies to Elucidate Sensitive Periods and Brain Mechanisms to Inform Early Intervention. Trends Neurosci 43:744–751. 10.1016/J.TINS.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Meda S, Thompson BS, et al. (2017) Early life stress and later peer distress on depressive behavior in adolescent female rats: Effects of a novel intervention on GABA and D2 receptors. Behav Brain Res 330:37–45. 10.1016/j.bbr.2017.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, et al. (2019) Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse. J Neurosci 39:503–518. 10.1523/JNEUROSCI.0537-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC (2007) Endocannabinoid Hedonic Hotspot for Sensory Pleasure: Anandamide in Nucleus Accumbens Shell Enhances ‘Liking’ of a Sweet Reward. Neuropsychopharmacology 32:2267–2278. 10.1038/sj.npp.1301376 [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, et al. (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17:577–585. 10.1038/nn.3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, et al. (2010) The Female Urine Sniffing Test: A Novel Approach for Assessing Reward-Seeking Behavior in Rodents. Biol Psychiatry. 10.1016/j.biopsych.2009.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S, Davis KA, Soare TW, et al. (2020) Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 113:104484. 10.1016/j.psyneuen.2019.104484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SM, Flint AJ, Field AE, et al. (2013) Abuse victimization in childhood or adolescence and risk of food addiction in adult women. Obesity 21:. 10.1002/OBY.20500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW (1996) Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding,d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology (Berl) 126:75–84. 10.1007/BF02246414 [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW (2003) Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev 27:45–55. 10.1016/S0149-7634(03)00008-3 [DOI] [PubMed] [Google Scholar]
- McCarty R (2017) Cross-fostering: Elucidating the effects of gene×environment interactions on phenotypic development. Neurosci Biobehav Rev 73:219–254. 10.1016/J.NEUBIOREV.2016.12.025 [DOI] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, et al. (2014) Optogenetic Activation of Dorsal Raphe Serotonin Neurons Enhances Patience for Future Rewards. Curr Biol 24:2033–2040. 10.1016/j.cub.2014.07.041 [DOI] [PubMed] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014. Dec;56(8):1675–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, et al. (2016a) Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry 6:e702–e702. 10.1038/tp.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J, Maras PM, Kinney-Lang E, et al. (2016b) MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus 26:1618–1632. 10.1002/hipo.22661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Cepko CL, Born RT, Beier KT (2015) Neuroanatomy goes viral! Front Neuroanat 0:80. 10.3389/FNANA.2015.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Herman KN, Barrett CE, et al. (2009) Adverse Rearing Experiences Enhance Responding to Both Aversive and Rewarding Stimuli in Juvenile Rhesus Monkeys. Biol Psychiatry 66:702–704. 10.1016/j.biopsych.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M, Ferrario C (2020) An improved demand curve for analysis of food or drug consumption in behavioral experiments. Psychopharmacology (Berl) 237:943–955. 10.1007/S00213-020-05491-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves GM, Bravo M, Baskoylu S, Bath KG (2020) Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. Elife 9:1–24. 10.7554/ELIFE.55263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47:419–427. 10.1037/H0058775 [DOI] [PubMed] [Google Scholar]
- Olds M, Fobes J (1981) The central basis of motivation: intracranial self-stimulation studies. Annu Rev Psychol 32:523–574. 10.1146/ANNUREV.PS.32.020181.002515 [DOI] [PubMed] [Google Scholar]
- Oleson E, Roberts D (2008) Parsing the Addiction Phenomenon: Self-Administration Procedures Modeling Enhanced Motivation for Drug and Escalation of Drug Intake. Drug Discov Today Dis Models 5:217–226. 10.1016/J.DDMOD.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DCS (2009) Behavioral Economic Assessment of Price and Cocaine Consumption Following Self-Administration Histories which Produce Escalation of Either Final Ratios or Intake. Neuropsychopharmacology 34:796. 10.1038/NPP.2008.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul IA, English JA, Halaris A (2000) Sucrose and quinine intake by maternally-deprived and control rhesus monkeys. Behav Brain Res 112:127–134. 10.1016/S0166-4328(00)00173-X [DOI] [PubMed] [Google Scholar]
- Peciña M, Karp J, Mathew S, et al. (2019) Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry 24:576–587. 10.1038/S41380-018-0117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC (2005) Hedonic Hot Spot in Nucleus Accumbens Shell: Where Do -Opioids Cause Increased Hedonic Impact of Sweetness? J Neurosci 25:11777–11786. 10.1523/JNEUROSCI.2329-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Cagniard B, Berridge KC, et al. (2003) Hyperdopaminergic mutant mice have higher wanting but not liking for sweet rewards. J Neurosci 23:9395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Schulkin J, Berridge KC (2006) Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: Paradoxical positive incentive effects in stress? BMC Biol 4:. 10.1186/1741-7007-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña C, Neugut Y, Calarco C, Champagne F (2014) Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur J Neurosci 39:946–956. 10.1111/EJN.12479 [DOI] [PubMed] [Google Scholar]
- Peng J, Long B, Yuan J, et al. (2017) A Quantitative Analysis of the Distribution of CRH Neurons in Whole Mouse Brain. Front Neuroanat 11:63. 10.3389/fnana.2017.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Pham TM, Bergström L, et al. (1999) Neonatal handling in rats induces long-term effects on dynorphin peptides. Neuropeptides 33:468–474. 10.1054/NPEP.1999.0764 [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Bergström L, Nylander I (2001) Effects of neonatal handling on nociceptin/orphanin FQ and opioid peptide levels in female rats. Pharmacol Biochem Behav 69:173–179. 10.1016/S0091-3057(01)00511-1 [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I (2003) Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides 37:149–156. 10.1016/S0143-4179(03)00043-X [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, et al. (2004) Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry 56:72–79. 10.1016/J.BIOPSYCH.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Rincón-Cortés M, Sullivan RM (2016) Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Transl Psychiatry 6:e930–e930. 10.1038/tp.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW (2016) Illuminating anhedonia. Science (80- ) 351:24–25. 10.1126/science.aad9698 [DOI] [PubMed] [Google Scholar]
- Roberts SA, Mclean L, Beynon R (2010) Darcin: A male pheromone that stimulates female memory and sexual attraction to an individual male’s odour Mecanisms of premating speciation in the house mouse View project Social suppression in female mammals and implications for captive breeding programs View project. Artic BMC Biol 8:. 10.1186/1741-7007-8-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A, Leão P, Carvalho M, et al. (2011) Potential programming of dopaminergic circuits by early life stress. Psychopharmacology (Berl) 214:107–120. 10.1007/S00213-010-2085-3 [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Paully GS (1987) Primate Models of Separation-Induced Depression. Psychiatr Clin North Am 10:437–447. 10.1016/S0193-953X(18)30553-7 [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Pedersen E-M, Feldon J, Pryce CR (2005) Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res 156:297–310. 10.1016/j.bbr.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Salamone J, Correa M, Yang J, et al. (2018) Dopamine, Effort-Based Choice, and Behavioral Economics: Basic and Translational Research. Front Behav Neurosci 12:. 10.3389/FNBEH.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Yohn SE, López-Cruz L, et al. (2016) Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology. Brain 139:1325. 10.1093/BRAIN/AWW050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Katz AC, DeLizza AA, et al. (2014) The Different Facets of Anhedonia and Their Associations with Different Psychopathologies. Anhedonia A Compr Handb Vol I Concept Issues Neurobiol Adv 3–22. 10.1007/978-94-017-8591-4_1 [DOI] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH (2012) Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol 121:51–60. 10.1037/a0024945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short A, Baram TZ (2019) Adverse early-life experiences and neurological disease: Age-old questions and novel answers. Nat Rev Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Mahler S, Pecina S, Berridge K (2010) Hedonic hotspots: Generating sensory pleasure in the brain. . Pleasures of the brain 27–49 [Google Scholar]
- Strickland J, Lile J, Stoops W (2019) Evaluating non-medical prescription opioid demand using commodity purchase tasks: test-retest reliability and incremental validity. Psychopharmacology (Berl) 236:2641–2652. 10.1007/S00213-019-05234-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanik JE, Reed JL, Nugent AC, et al. (2019) Mapping anticipatory anhedonia: an fMRI study. Brain Imaging Behav 13:1624. 10.1007/S11682-019-00084-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H-E, Sisti AC, Jin H, et al. (2020) The gut–brain axis mediates sugar preference. Nat 2020 5807804 580:511–516. 10.1038/s41586-020-2199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DGR, Hwang BY, Viswanathan S, et al. (2016) A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92:372–382. 10.1016/j.neuron.2016.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley J, Marconi L, Alipio JB, et al. (2018) Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula–Tegmental Circuitry and Constrain Reward Seeking. Biol Psychiatry 83:1012–1023. 10.1016/j.biopsych.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N (2020) Early Adversity and the Neotenous Human Brain. Biol Psychiatry 87:350–358. 10.1016/j.biopsych.2019.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ (2010) The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci 31:463–469. 10.1016/j.tips.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490:262–266. 10.1038/nature11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel JK, Furman K, Gumbs MCR, et al. (2015) Neuropeptide Y activity in the nucleus accumbens modulates feeding behavior and neuronal activity. Biol Psychiatry 77:633–641. 10.1016/j.biopsych.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Ciano P Di, Everitt BJ (2005) Involvement of the Dorsal Striatum in Cue-Controlled Cocaine Seeking. J Neurosci 25:8665–8670. 10.1523/JNEUROSCI.0925-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Coccurello R, Andolina D, et al. (2013) Postnatal aversive experience impairs sensitivity to natural rewards and increases susceptibility to negative events in adult life. Cereb Cortex 23:1606–1617. 10.1093/CERCOR/BHS145 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18:741–752. 10.1038/nrn.2017.130 [DOI] [PubMed] [Google Scholar]
- Walker CD, Bath KG, Joels M, et al. (2017) Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress 20:421–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, et al. (2014) Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 17:27–29. 10.1038/nn.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-L, Zhang S, Qi J, et al. (2019) Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep 26:1128–1142.e7. 10.1016/j.celrep.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hamida SB, Darcq E, et al. (2012) Ethanol-Mediated Facilitation of AMPA Receptor Function in the Dorsomedial Striatum: Implications for Alcohol Drinking Behavior. J Neurosci 32:15124–15132. 10.1523/JNEUROSCI.2783-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev 16:525–534. 10.1016/S0149-7634(05)80194-0 [DOI] [PubMed] [Google Scholar]
- Wright RL, Gilmour G, Dwyer DM (2020) Wistar Kyoto Rats Display Anhedonia In Consumption but Retain Some Sensitivity to the Anticipation of Palatable Solutions. Front Behav Neurosci 0:70. 10.3389/FNBEH.2020.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Rincón-Cortés M, Raineki C, et al. (2017) Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl Psychiatry 2017 71 7:e1005–e1005. 10.1038/tp.2016.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D, Treadway M (2017) Reward Processing, Neuroeconomics, and Psychopathology. Annu Rev Clin Psychol 13:471–495. 10.1146/ANNUREV-CLINPSY-032816-044957 [DOI] [PMC free article] [PubMed] [Google Scholar]