Abstract
A patient with B-cell acute lymphoblastic leukemia (ALL) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had persistent, progressive pneumonia with viremia after 5 months of infection despite monoclonal antibodies, intravenous (IV) remdesivir and prolonged oral steroids. Twenty days of nirmatrelvir/ritonavir and 10 days of IV remdesivir led to full recovery.
Keywords: SARS-CoV-2, nirmatrelvir/ritonavir, severe immunocompromise, persistent viral infection, emergency single use investigational new drug
Immunocompromised individuals, especially those with hematological malignancies, have an elevated risk of prolonged infection, clinical failure of available therapeutics, progressive lower respiratory tract infection (LRTI) and mortality far in excess of age-matched immune competent persons [1, 2]. Prolonged infection is associated with within-host viral evolution and represents a source of global variants [3–5].
Current standard practice of coronavirus disease 2019 (COVID-19) treatment in immunocompromised individuals is to use either a monoclonal antibody or a direct-acting antiviral approved under emergency use authorization (EUA) within 1 week from symptom onset. Remdesivir is currently the only antiviral to achieve Food and Drug Administration (FDA) approval to treat hospitalized patients with COVID-19 and hypoxemia [6], when administered early it is highly efficacious [7]. Despite this approach, the emergence of Omicron variant has been associated with increased acquisition and prolonged viral illness in immunocompromised patients. When progression to LRTI occurs after the first week of symptoms patients are no longer eligible for EUA-available therapeutics.
Nirmatrelvir/ritonavir (Paxlovid) is a combination antiviral with nirmatrelvir, a direct-acting 3CL protease inhibitor, and ritonavir, a strong CYP3A4 inhibitor without direct antiviral activity. Outpatient clinical trials have shown high efficacy when used early in infection [8]. Studies in patients hospitalized with severe infection or patients with prolonged infection have not been performed.
CASE REPORT
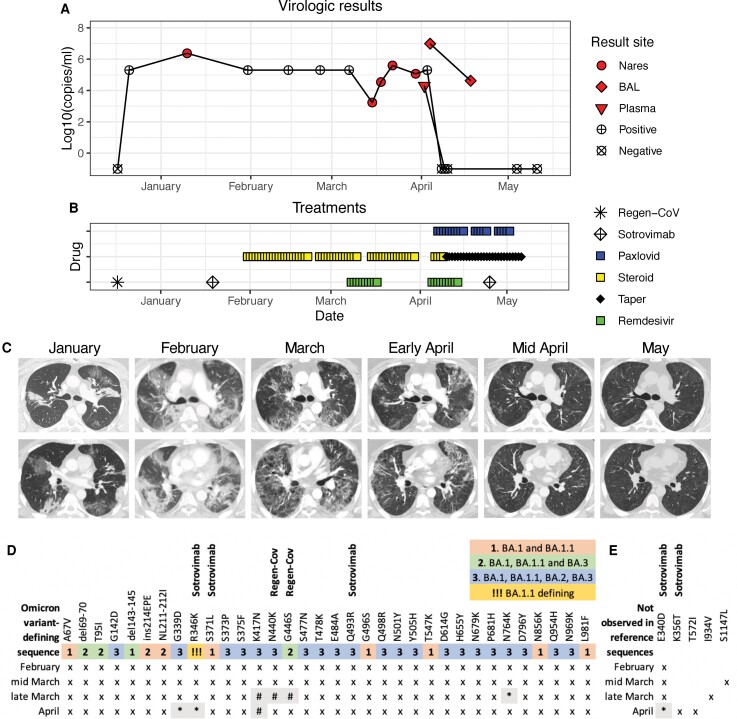
A 40-year-old man with B-cell ALL received rituximab and hyper-CVAD in 2021 with complete remission and no evidence of minimal residual disease after 6 months and 8 cycles of treatment including 8 infusions of rituximab, 375 mg/m2 in 2021. He had transitioned to maintenance chemotherapy in December 2021 when multiple family members developed polymerase chain reaction (PCR)-proven COVID-19. He received prophylactic casivirimab and imdevimab (Regen-Cov). He experienced rhinorrhea and did not present for care, but at 20 days after exposure a nares swab was positive for SARS-CoV-2 at 106 copies/mL [9] and chemotherapy was held (Figure 1A). A week later he presented to his local hospital with fevers and cough and received sotrovimab (Figure 1B). Over the next 6 weeks he was admitted 3 times to his local hospital with fever and hypoxemia. With each hospitalization he received steroids to treat worsening pulmonary infiltrates, which were initially multifocal and dense but evolved into diffuse, ground glass opacities (GGO) and interstitial disease; bronchoalveolar lavage (BAL) was not performed but nares swabs were positive (Figure 1C). In mid-March he was admitted to our hospital with oxygen saturations <90% and nares swab again positive for SARS-CoV-2. Chest computed tomography (CT) showed diffuse GGO, consistent with viral LRTI. Sequencing revealed variant of concern (VOC) BA.1.1, consistent with a nares sample from February (Figure 1D). He received 10 days of intravenous (IV) remdesivir and a dexamethasone taper (Figure 1B). He remained on 2–4 L of supplemental oxygen by nasal cannula (NC) but was afebrile and discharged home.
Figure 1.
Virologic test results (A), treatments (B), chest imaging (C), and SARS-CoV-2 spike sequencing (D, E) from exposure to COVID-19 through symptom resolution. A, Filled symbols indicate quantitative results of SARS-CoV-2 PCRs from nasal or nasopharyngeal swabs (circle), bronchoscopy (BAL, diamond) or plasma (triangle). Positive (+) or negative (x) marked symbols indicate qualitative results. Nasal swab was first negative 2 days after starting Paxlovid, and plasma was negative the next day. B, Treatments included three monoclonal infusions (Regen-Cov and Sotrovimab), 2 courses of remdesivir and 20 days of Paxlovid. Steroid doses below 0.3 mg/kg prednisone equivalents are indicated with taper. C, Serial chest imaging by computed tomography (CT). D, Results of SARS-CoV-2 spike sequencing from 3 nares swabs (Feb–March) and BAL (April). Numbers and shading indicate the mutations where each Omicron variant (BA.1-BA.3) differs from the reference sequence NC_045512.2 (Wuhan-Hu-1). Symbols indicate sequencing results at each locus: (x) mutation shown describes the consensus sequence (>50% allelic frequency), (*) mutation describes the consensus sequence in low coverage area (<50 reads), (#) region without sufficient sequencing coverage. Mutations associated with resistance to sotrovimab (E340D, K356T, S371F) and mutations previously seen at high frequency in sotrovimab-treated patients (R346K, Q493R) were observed at multiple timepoints. Mutations associated with escape from REGN10987 (Regen-Cov, N440K, G446S) are present in Omicron variants. E, Non-Omicron-identified consensus mutations. Both E340D and K356T are associated with resistance to sotrovimab. No BA.2-defining sequence changes were observed. Abbreviations: BAL, bronchoalveolar lavage; COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
At completion of the dexamethasone taper in early April he again developed cough, fevers, and dyspnea and had an oxygen saturation of 71% on 3 L supplemental oxygen with temperature >39°C. On admission, nares swab was still positive for SARS-CoV-2 (Figure 1A) but chest imaging by CT was slightly improved (Figure 1C). Off steroids, he required 8–10 L by NC of supplemental oxygen at rest to 10–15 L by face mask with exertion or sleep on hospital day (HD) 3–5. Fluid from BAL on HD3 was positive for SARS-CoV-2 at 107 copies/mL. BAL fluid galactomannan was 1.1 (confirmation 0.8), with no mold detected by PCR or in culture. Pneumocystis stain by direct fluorescent antibody was negative. SARS-CoV-2 was detected by PCR in serum. He was diagnosed with persistent SARS-CoV-2 pneumonia with viremia. He was started on IV remdesivir with prednisone at 0.5 mg/kg and ambisome at 5 mg/kg on HD4 due to potential contributions of organizing pneumonia and invasive pulmonary aspergillosis (IPA). He continued to require supplemental oxygen of 4–8 L by NC. Sequencing from the nares and BAL revealed the same BA.1.1 variant (Figure 1D). A single patient emergency investigational new drug (eIND) authorization was obtained for 10 days of treatment with nirmatrelvir/ritonavir.
The patient's hypoxemia resolved quickly after initiation of nirmatrelvir/ritonavir on HD6. Nares swab SARS-CoV-2 PCR was negative on HD7 and serum PCR on HD8. By HD14 he was weaned off supplemental oxygen and chest CT showed persistent but improved areas of GGO (Figure 1C). To further investigate whether GGO persistence was related to COVID or organizing pneumonia, repeat bronchoscopy was performed on HD15. SARS-CoV-2 was again detected in BAL, though at much lower concentration (104 copies/ml). BAL fluid galactomannan decreased to 0.5. Serum nucleocapsid antibodies were undetectable.
In the first week after hospital discharge his oxygen requirement recurred and he was treated with nirmatrelvir/ritonavir for an additional 10 days via eIND extension and a second dose of sotrovimab, also under eIND, due to persistent BA.1.1 virus. By day 20 of nirmatrelvir/ritonavir he was off oxygen. He tolerated twenty days of nirmatrelvir/ritonavir well, only reporting abdominal bloating. No laboratory abnormalities arose during treatment and the transaminase elevation, thrombocytopenia and lymphopenia observed during his viral illness resolved. Chest CT one week after completion of nirmatrelvir/ritonavir revealed substantial improvement with no suggestion of IPA (Figure 1C). Prednisone taper was completed 10 days after discontinuation of nirmatrelvir/ritonavir. Isavuconazole was used in place of ambisome after cessation of ritonavir to prevent IPA and was stopped after discontinuation of prednisone. At 4 months after discontinuation of nirmatrelvir/ritonavir he is clinically stable without evidence of recrudescent infections.
Whole SARS-CoV-2 genome sequencing of nares and BAL fluid samples revealed a consistent BA.1.1 sequence without evidence of novel SARS-CoV-2 exposures (Figure 1D) [10]. The first nasopharyngeal sample sequenced from 2 months after virus acquisition revealed changes associated with resistance or escape to sotrovimab (E340D, S371F) and mutations previously seen at high frequency in sotrovimab-treated patients (R346K, Q493R) that were consistently detected in subsequent samples [11–14]. K356T, also associated with reduced susceptibility to sotrovimab, was observed in the BAL sample [13]. These likely contributed to the ineffective clinical response associated with its administration. The sequence from BAL after 10 days of nirmatrelvir/ritonavir was not of sufficient quality to detect mutations that might be associated with nirmatrelvir/ritonavir resistance.
DISCUSSION
We present a case of prolonged, persistent, and severe lower respiratory tract COVID-19 in a profoundly immunocompromised person who was cured with 20 days of nirmatrelvir/ritonavir. While multiple therapies were tried, including monoclonal antibodies and IV remdesivir, the clinical and virological improvement we observed was most closely associated with nirmatrelvir/ritonavir. Virus remained detectable by BAL and oxygen requirement returned after 10 days of nirmatrelvir/ritonavir in combination with remdesivir but a full twenty days of nirmatrelvir/ritonavir led to sustained improvement and clinical cure.
Our case illustrates the importance of the abrogation of viral replication for effective treatment of persistent LRTI in the profoundly immunocompromised patient. Our patient had severe clinical progression with evidence of profound respiratory insufficiency and disseminated SARS-CoV-2, approaching hypoxemic respiratory failure despite sotrovimab and IV remdesivir therapy. The SARS COV2 variant BA.1.1 was sequenced from a nares sample from February and persisted with minimal changes throughout his course. Although many sequence changes are shared across Omicron variants BA.1 – BA.3, no BA.2-defining mutations were observed at any time point [10]. Nirmatrelvir/ritonavir treatment was associated with rapid clearance of SARS-CoV-2 from nares and serum, which became negative by days 2 and 3 respectively of nirmatrelvir/ritonavir therapy (days 5, 6 of his second 10-day course of IV remdesivir). A high viral load in the lower respiratory tract required a longer duration of therapy: SARS-CoV-2 was still detected in the BAL at 104 copies/ml after 10 days of nirmatrelvir/ritonavir, indicating that only partial virologic control had been achieved. Although we did not repeat BAL after 20 days to confirm lower airway clearance, his resumption of daily activities off supplemental oxygen and marked resolution of infiltrates by chest CT provide ample evidence of the resolution of his illness after prolonged antivirals.
Corticosteroids treat the inflammatory manifestations of COVID-19 including organizing pneumonia, which presents as ground glass opacities and may be indistinguishable from viral pneumonia. This patient may not have survived 4 months of COVID-19 LRTI without the use of steroids; during his last hospitalization and prior to initiation of nirmatrelvir/ritonavir, steroids were held and he progressed to requiring 100% oxygen by non-rebreathing face mask over a period of 4–5 days. However, we suspect that corticosteroids facilitated his persistent viral infection and contributed to his risk of invasive fungal infection.
This case demonstrates the need to evaluate therapies including prolonged, potent antiviral therapy and combination therapies in immunocompromised patients with persistent COVID-19. In this patient, a 20-day course of nirmatrelvir/ritonavir led to clinical cure. Use of high dose corticosteroids likely compromises the potency of antiviral therapy, but how to manage corticosteroids and other immunomodulatory agents in profoundly immunocompromised patients is not yet clear. The authors deeply appreciate the eIND process and the capacity to provide cure for this patient, but the need for expanded availability of nirmatrelvir/ritonavir and clinical trials for its use in profoundly immunocompromised patients, including in combination with other antivirals and immunomodulatory agents, is emergent.
Notes
Financial support. This work was supported by NIAID/NIH K08 AI148588 to E. S. F.
Contributor Information
Emily S Ford, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
William Simmons, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Ellora N Karmarkar, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Leah H Yoke, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Ayodale B Braimah, Division of General Internal Medicine, Department of Medicine, University of Washington, Seattle, Washington, USA.
Johnnie J Orozco, Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Medical Oncology, Department of Medicine, University of Washington, Seattle, Washington, USA.
Cristina M Ghiuzeli, Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Hematology, Department of Medicine, University of Washington, Seattle, Washington, USA.
Serena Barnhill, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington, USA.
Coralynn L Sack, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington, USA.
Joshua O Benditt, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington, USA.
Pavitra Roychoudhury, Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Laboratory Medicine, University of Washington, Seattle, Washington, USA.
Alexander L Greninger, Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Laboratory Medicine, University of Washington, Seattle, Washington, USA.
Adrienne E Shapiro, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
Jennifer L Hammond, Pfizer, New York, NY, USA.
James M Rusnak, Pfizer, New York, NY, USA.
Mikael Dolsten, Pfizer, New York, NY, USA.
Michael Boeckh, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Catherine Liu, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Guang-Shing Cheng, Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington, USA.
Lawrence Corey, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Laboratory Medicine, University of Washington, Seattle, Washington, USA.
References
- 1. Zhang Y, Luo W, Li Q, et al. . Risk factors for death among the first 80,543 coronavirus disease 2019 (COVID-19) cases in China: relationships between age, underlying disease, case severity, and region. Clin Infect Dis 2022; 74:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun J, Zheng Q, Madhira V, et al. . Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022; 182:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021; 385:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi S, Klein J, Robertson AJ, et al. . De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat Commun 2022; 13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nussenblatt V, Roder AE, Das S, et al. . Yearlong COVID-19 infection reveals within-host evolution of SARS-CoV-2 in a patient with B-cell depletion. J Infect Dis 2022; 225:1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottlieb RL, Vaca CE, Paredes R, et al. . Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond J, Leister-Tebbe H, Gardner A, et al. . Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perchetti GA, Huang ML, Mills MG, Jerome KR, Greninger AL. Analytical sensitivity of the abbott BinaxNOW COVID-19 ag card. J Clin Microbiol 2021; 59:e02880–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar S, Karuppanan K, Subramaniam G. Omicron (BA.1) and sub-variants (BA.1.1, BA.2, and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. J Med Virol 2022; 94:4780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Destras G, Bal A, Simon B, Lina B, Josset L. Sotrovimab drives SARS-CoV-2 omicron variant evolution in immunocompromised patients. Lancet 2022; 3:e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacco MD, Hu Y, Gongora MV, et al. . The P132H mutation in the main protease of omicron SARS-CoV-2 decreases thermal stability without compromising catalysis or small-molecule drug inhibition. Cell Res 2022; 32:498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. UK Health Security Agency. SARS-CoV-2 therapeutics technical briefing 3: Genomic surveillance 10-May -2022. 2022. Available at: https://www.gov.uk/government/publications/covid-19-therapeutic-agents-technical-briefings.
- 14. Vellas C, Trémeaux P, Del Bello A, et al. . Resistance mutations in SARS-CoV-2 omicron variant in patients treated with sotrovimab. Clin Microbiol Infect 2022; 28:1297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]