Abstract
Migraine is thought to involve sensitization of the trigeminal nociceptive system. In preclinical pain models, activation of MNK-eIF4E signalling contributes to nociceptor sensitization and the development of persistent pain. Despite these observations, the role of MNK signalling in migraine remains unclear. Here, we investigate whether activation of MNK contributes to hypersensitivity in two rodent models of migraine. Female and male wild-type (WT) and MNK1 knock-out mice were subjected to repeated restraint stress or a dural injection of interleukin-6 (IL-6) and tested for periorbital hypersensitivity and grimacing. Upon returning to baseline thresholds, stressed mice were administered a low dose of the nitric oxide donor sodium nitroprusside and mice previously injected with IL-6 were given a second dural injection of pH 7.0 to test for hyperalgesic priming.
MNK1 knock-out mice were significantly less hypersensitive than the WT following dural IL-6 and did not prime to pH 7.0 or sodium nitroprusside. Furthermore, treatment with the selective MNK inhibitor, eFT508, in WT mice prevented hypersensitivity caused by dural IL-6 or pH 7.0. Together, these results implicate MNK-eIF4E signalling in the development of pain originating from the dura and strongly suggest that targeting MNK inhibition may have significant therapeutic potential as a treatment for migraine.
Keywords: migraine, MNK, eIF4E, trigeminal, headache
Using preclinical models, Lackovic et al. demonstrate a role for MNK-eIF4E signalling in migraine-related mechanisms and show efficacy of eFT508, an MNK inhibitor currently in Phase III trials for cancer. These findings provide a rationale for targeting MNK inhibition as a novel therapeutic intervention for this disorder.
Introduction
Sensitization of the trigeminal sensory system has been strongly implicated in the pathophysiology of migraine, a disorder in which patients suffer from a variety of symptoms including intense headaches, and where attacks are often triggered by stimuli which are not noxious to healthy individuals.1 In preclinical headache models, repeated or intense activation of intracranial meningeal afferents results in the release of a wide-range of pro-inflammatory mediators and excitatory neurotransmitters from trigeminal ganglia (TG) sensory neurons, degranulated mast cells, and dural fibroblasts, which can directly contribute to the sensitization of primary sensory neurons and their central projections in the trigeminal nucleus caudalis (TNC).1,2 In humans, sensitization of neurons in the TNC and thalamus is thought to at least partially underlie the development of wide-spread allodynia experienced by many migraine patients.3,4 Based on these observations, it is likely that mechanisms underlying peripheral and central sensitization are key to the development and persistence of migraine pathophysiology.
The translational control of nascent protein synthesis is a key mechanism mediating neuronal plasticity and provides an efficient way for neurons to quickly respond and adapt to harmful stimuli.5 In dorsal root ganglia (DRG) and TG sensory neurons, endogenous ligands act via their receptors to stimulate mRNA translation via mitogen-activated protein kinases (MAPK), a family of intracellular signalling molecules that are known to promote nociceptor hyperexcitability and persistent pain.6 MAPKs act via MAPK interacting kinases (MNKs) 1/2 to phosphorylate eukaryotic initiation factor (eIF) 4E at serine 209 and promote translation through assembly of the eIF4F complex, a tri-subunit complex which is critical for recruitment of the pre-initiation complex to the 5′ cap of mRNAs.7 Importantly, this pathway promotes the translation of a specific subset of eIF4E-dependent mRNAs, some of which code proteins that are involved in pain processing.8
In preclinical pain models, activation of MNK-eIF4E is critical to the development of mechanical and thermal hypersensitivity and persists long after the initial injury has resolved, suggesting that dysregulation of this pathway contributes to nociceptor sensitization and, ultimately, the development of chronic pain.8 In preclinical models of migraine, phosphorylation of eIF4E is increased in TG sensory neurons following repeated exposure to stress, a translationally relevant observation given that stress is the number one reported trigger of migraine in humans.9 Additionally, rodent studies across numerous pain models have demonstrated that disrupting MNK-eIF4E signalling attenuates nociceptor excitability, hypersensitivity, and injury-induced cognitive impairments.7,10,11 Consistent with this, we have recently demonstrated that periorbital hypersensitivity caused by repeated stress or dural application of the pro-inflammatory cytokine interleukin-6 (IL-6) is reduced in eIF4ES209A mice, thus, strongly implicating eIF4E-mediated translation in the development of headache.9
Despite these observations, it is currently unknown to what extent MNK-eIF4E signalling or translation dysregulation in general contributes to pain hypersensitivity in migraine. Thus, the goal of this study was to test the hypothesis that MNK activation contributes to the development of periorbital hypersensitivity in rodent models of migraine headache.
Materials and methods
Animals
Female and male MNK1 KO mice were a gift from the Sonenberg Laboratory at McGill University and bred at the University of Texas at Dallas to generate experimental animals.12 Experimental C57BL6/J wild-type (WT) animals were obtained from an internally maintained C57BL/6 J colony at the University of Texas at Dallas. For pharmacological studies, female and male ICR (CD-1) mice aged 6–8 weeks (∼25–30 g) were outbred and purchased from Envigo. All experimenters were blinded during testing and data analysis. See Supplementary material for detailed information. All procedures were conducted with prior approval of the Institutional Animal Care and Use Committee at the University of Texas at Dallas.
Drugs and compounds
Human recombinant interleukin-6 (IL-6) protein was prepared as previously described.9 To test for the presence of hyperalgesic priming, sodium nitroprusside (SNP) or a synthetic interstitial fluid (SIF) solution that was adjusted to pH 7.0 was prepared and administered as previously described.9 eFT508 was provided by eFFECTOR under a Material Transfer Agreement with The University of Texas at Dallas and was dissolved in 10% 1-methyl-2-pyrrolidinone, anhydrous (NMP; Sigma Cat. No. 328634), 90% propylene glycol (PG; Fisher Cat. No. P355–1) with ∼1 eq. of HCl. eFT508 was given orally to WT mice at 10 mg/kg approximately 1 h prior to dural IL-6 or dural pH 7.0. See Supplementary material for detailed information.
Mouse dural injections
Mouse dural injections were performed as previously described.13,14 See Supplementary material for detailed information.
Repeated restraint stress
Mice were stressed between the hours of 10:00 AM to 12.00 PM for 2 h per day for three consecutive days as previously described.15 See Supplementary material for detailed information.
Measuring mechanical hypersensitivity and grimace
Mice were tested for mechanical hypersensitivity and spontaneous pain using von Frey and grimace measures, respectively.13 See Supplementary material for detailed information.
Statistical analysis
All behavioural data were analysed for multiple comparisons at each time point via two-way ANOVA followed by Bonferroni post hoc analysis. F-values for these analyses are presented in Supplementary Table 1. All data are represented in the results and figures as mean ± SEM. All analyses were performed using Prism version 9.2 for Mac OS X.
Data availability
All information necessary to evaluate the findings of the paper are included in the paper. Additional data can be provided by the authors upon request.
Results
MNK1 KO mice exhibit reduced periorbital hypersensitivity and do not prime following dural IL-6 or repeated stress
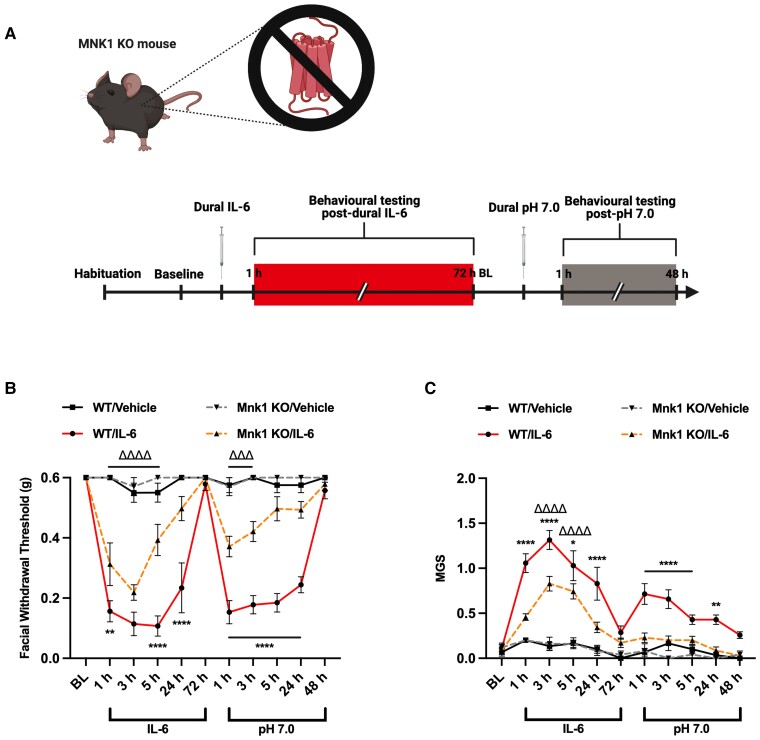
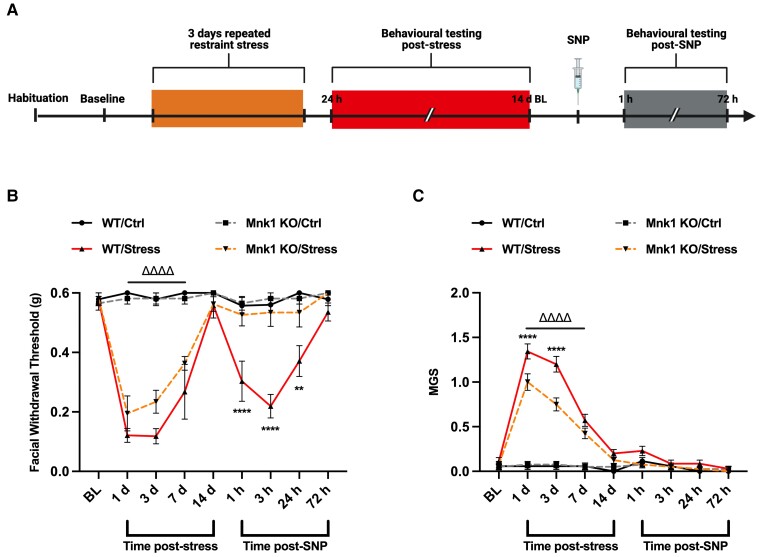
We have previously validated and published two novel mouse models of migraine headache, in which stimulation of the dura mater with pronociceptive agents or exposure to 2 days (2 h per day) of restraint stress causes robust periorbital hypersensitivity and induces a primed state in which the mice are sensitized to non-noxious stimuli.13,15 In order to assess whether MNK signalling through eIF4E is essential to the development of periorbital hypersensitivity in these models, we utilized a full body knockout (KO) mouse strain for MNK1, which is believed to be the key isoform underlying pain development. In WT and MNK1 KO mice, we administered 5 µl of IL-6 (0.1 ng) or vehicle onto the dura mater and measured periorbital von Frey thresholds and grimace scores to assess for periorbital hypersensitivity and grimacing (Fig. 1). Following resolution of acute hypersensitivity, we delivered a second dural injection of SIF solution at pH 7.0, which is not noxious in naïve mice, to test for the presence of hyperalgesic priming. Interestingly, MNK1 KO mice were significantly less hypersensitive than WT mice following dural IL-6. Likewise, contrary to WTs, MNK1 KO mice that received IL-6 did not prime to dural pH 7.0, demonstrating a key role for MNK signalling in the development of acute periorbital hypersensitivity and priming. In a separate experiment, WT and MNK1 KO mice were subjected to the repeated stress paradigm, upon which both strains developed significant periorbital hypersensitivity compared to controls (Fig. 2). Upon returning to baseline withdrawal thresholds, mice were administered a non-noxious dose of the NO donor, SNP [0.1 mg/kg, intraperitoneally (IP)], to test for priming. While WT mice became sensitized to SNP, MNK1 KO mice did not transition to a primed state, further suggesting a role for MNK1 activation in the development of priming.
Figure 1.
Genetic inhibition of MNK1 partially attenuates facial hypersensitivity and hyperalgesic priming caused by dural IL-6. (A) Female and male WT or MNK1 KO mice were administered 5 µl of the proinflammatory cytokine, IL-6 (0.1 ng), or vehicle onto their dura mater and tested for acute facial hypersensitivity (B) and grimace measures (C). Following resolution of acute allodynia, all mice were tested for hyperalgesic priming by administering 5 µl of SIF (pH = 7.0) and tested again. WT mice were observed to have significantly reduced withdrawal thresholds and increased grimacing compared to MNK1 KO mice, in which these effects were partially attenuated. Likewise, contrary to WT mice, MNK1 KO mice did not prime to dural pH 7.0. No sex differences were observed. Comparisons were made via two-way ANOVA followed by Bonferroni post hoc analysis. Significance between WT/IL-6 and MNK1 KO/IL-6 groups (denoted by asterisk) and between MNK1 KO/Veh and MNK1KO/IL-6 groups (denoted by delta symbol) is shown. n ≥ 6 for all groups; *P ≤ 0.05; **,ΔΔP ≤ 0.01; ΔΔΔP ≤ 0.001; ****,ΔΔΔΔP ≤ 0.0001.
Figure 2.
MNK1 KO mice do not prime to low-dose NO donor following repeated stress. (A) Following three consecutive days of restraint stress, female and male WT and MNK1 KO mice were tested for acute facial hypersensitivity and grimace measures. Upon resolution of hypersensitivity, mice were administered a low dose of the NO donor, SNP (0.1 mg/kg, IP), to test for the presence of priming. Following stress, WT and MNK1 KO mice showed similar levels of facial hypersensitivity (B) lasting up to 14 days, with MNK1 KO mice exhibiting significantly lower grimace scores (C) in the early time points measured. Interestingly, despite becoming acutely hypersensitive, MNK1 KO mice do not prime to low-dose SNP compared to WTs, suggesting a role for MNK1 activation in the development of stress-induced hyperalgesic priming. No sex differences were observed. Comparisons were made via two-way ANOVA followed by Bonferroni post hoc analysis. Significance between WT/Stress and MNK1 KO/Stress groups (denoted by asterisk) and MNK1KO/Ctrl and MNK1KO/Stress groups (denoted by delta symbol) is shown. n ≥ 7 for all groups; **P ≤ 0.01; ****,ΔΔΔΔP ≤ 0.0001.
Treatment with eFT508, attenuates periorbital hypersensitivity and hyperalgesic priming caused by dural IL-6
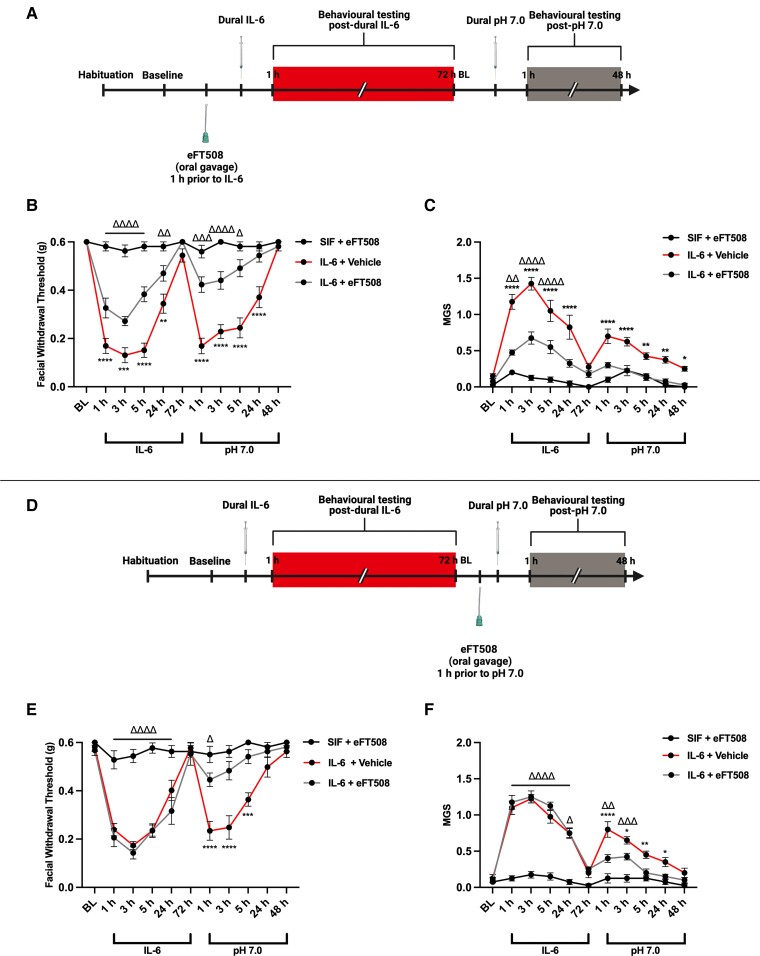
Because the prior studies used a global MNK KO strain to show a role for MNK signalling in these models, we examined whether pharmacological inhibition of MNK had the same effect. Female and male ICR mice were administered 100 µl of the MNK inhibitor, eFT508 (10 mg/kg), or vehicle via oral gavage approximately 1 h prior to dural IL-6 and tested for periorbital hypersensitivity and grimacing (Fig. 3A). Mice that received eFT508 prior to IL-6 were significantly less hypersensitive and had reduced grimace measures in both the acute and priming phases compared to those that received vehicle (Fig. 3B–C). Additionally, mice which were administered eFT508 1 h prior to dural pH 7.0 did not respond to this stimulus, despite being acutely hypersensitive following dural IL-6 (Fig. 3D–F). Together, these results show that pharmacological inhibition of MNK causes similar effects to global MNK KO mice and demonstrate the therapeutic potential of targeting MNK for the treatment of migraine headache.
Figure 3.
The MNK inhibitor, eFT508, reduces dural IL-6 -induced facial hypersensitivity and prevents priming to pH 7.0. Female and male WT mice were administered 5 µl of dural IL-6 (0.1 ng) or vehicle and tested for acute facial hypersensitivity and grimacing. Upon returning to baseline thresholds, mice were tested for priming with a 5 µl dural injection of SIF (pH = 7.0). Prior to IL-6 (A) or pH 7.0 (D), mice received 100 µl of the MNK inhibitor, eFT508 (10 mg/kg), or vehicle via oral gavage. In mice that received eFT508, dural IL-6 induced facial hypersensitivity (B) and grimace measures (C) were significantly attenuated compared to the vehicle group and these mice did not prime to dural pH 7.0. Similarly, mice that received eFT508 prior to dural pH 7.0 did not prime to pH 7.0 compared to the vehicle group (E–F), suggesting that activation of MNK is critical to transition to a primed state. No sex differences were observed. Comparisons were made via two-way ANOVA followed by Bonferroni post hoc analysis. Significance between IL-6/Vehicle and IL-6/eFT508 groups (denoted by asterisk) and SIF/eFT508 and IL-6/eFT508 groups (denoted by delta symbol) is shown. n = 7 for all groups; *,ΔP ≤ 0.05; **,ΔΔP ≤ 0.01; ***,ΔΔΔP ≤ 0.001; ****,ΔΔΔΔP ≤ 0.0001.
Discussion
Here we demonstrate that genetic or pharmacological inhibition of MNK(1/2), the essential kinase responsible for eIF4E phosphorylation, results in the attenuation of periorbital hypersensitivity and prevents hyperalgesic priming in two preclinical migraine models. This expands on our previous work implicating eIF4E-dependent nascent protein synthesis in migraine pathophysiology, pointing to a clear therapeutic intervention point for migraine: inhibition of MNK signalling. As stated earlier, we previously demonstrated that eIF4ES209A mice do not prime to a low-dose NO donor following repeated restraint stress. In the present study, we found that these effects are recapitulated in MNK1 KO mice, thereby implicating the MNK-eIF4E signalling pathway in the development of stress-induced priming to NO donors.9 This observation is translationally relevant, as stress is the number one reported trigger of migraine in humans and NO donors are one of the most consistent pharmacological triggers in both patients and rodent models.16
It is not clear how stress engages the MAPK-MNK-eIF4E pathway. In the context of migraine, acute or chronic stress enhances the release of proinflammatory mediators, increases NO synthase expression, alters immune cell properties in the dura, decreases the threshold for cortical spreading depression, and regulates hypothalamic modulation of the TNC.17,18 NO is capable of phosphorylating eIF4E via activation of the mechanistic target of rapamycin and MAPK pathways and has been shown to stimulate expression of hypoxia-inducible factor-1 (HIF-1ɑ), a transcription factor that is expressed in DRG sensory neurons and plays a critical role in regulating hypersensitivity following sciatic nerve injury.19,20 Because a low dose of SNP was used in this study, it may be true that shutting down NO-induced eIF4E phosphorylation via MNK inhibition is robust enough to attenuate any changes in nociceptor excitability that may be caused by activation of this pathway. Because our previous and current findings strongly underscore a key role for eIF4E activation in the development of hypersensitivity, future studies focused on investigating how these pathways are regulated, or regulate each other, following stress could provide valuable insight into understanding the role of these mechanisms in the context of pain. Regardless, while it is not yet clear how MNK-eIF4E signalling is impacted following stress or NO donor administration, the current observations provide valuable insight into the contributions of this pathway to stress-induced hypersensitivity.
In the dural stimulation model, MNK1 KO mice had significantly reduced periorbital hypersensitivity and spontaneous pain following IL-6 and did not prime to pH 7.0, suggesting that MNK activation is critical to the establishment of hyperalgesic priming caused by IL-6. IL-6 is an key cytokine released by cells following injury or inflammation and has been highly implicated in migraine, with patients exhibiting significantly elevated levels of the cytokine in their serum compared to healthy controls.21 We have shown recently that IL-6 promotes neuronal hyperexcitability in DRG sensory neurons via activation of the MNK-eIF4E pathway, an effect that is attenuated in eIF4ES209A and MNK1 KO mice as well as in mice treated with eFT508.7,10 Similarly, treatment with eFT508 one hour prior to dural IL-6 or pH 7.0 in WT mice resulted in attenuation of acute periorbital hypersensitivity and blocked the development of hyperalgesic priming, providing the first evidence in support for the use of eFT508 as a therapeutic intervention for migraine. eFT508 is a highly potent and selective MNK inhibitor which was developed by eFFECTOR therapeutics for the treatment of cancer. eFT508 has excellent oral bioavailability and has been demonstrated to significantly reduce eIF4E phosphorylation in the DRG and sciatic nerve, as well as in the CNS.22 Likewise, our data indicate that eIF4E phosphorylation is reduced in the dura of MNK1 KO mice and in both the TG and dura of mice treated with eFT508 (Supplementary Fig. 1). Currently, eFT508 is in phase III clinical trials for the treatment of cancer, adding to the clinical potential of MNK inhibitors as migraine therapeutics.
There is now widespread evidence that the translation of nascent proteins in response to stimuli occurs locally in axonal compartments rather than in the cell body.8 For example, the activation and sensitization of nociceptors at peripherally distant sites, such as in the skin, would be best explained by local translation mechanisms.23 Additionally, local translation provides a means to explain how many endogenous pain-promoting molecules induce long-lasting plasticity.8 Based on this, future work will be required to identify differences between translation in the soma versus in the axonal compartment, as insight into this will ultimately aid in drug development. Similarly, delineating the role of MNK-eIF4E signalling in the central versus peripheral nervous system will be necessary, as translation regulation mechanisms are essential to processes that depend on synaptic plasticity, such as learning and memory.24 It is also important to note that, because we did not observe a complete attenuation of hypersensitivity via targeting MNK, stimulation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway, which also leads to downstream activation of eIF4E and plays a role in pain, must also be considered to play a role in these models.25,26 Moreover, identifying the transcriptional and translational signatures of TG sensory neurons in migraine models, including which mRNAs are impacted by translation dysregulation, will also be essential to understanding the extent to which these mechanisms contribute to headache. Activation of the MNK-eIF4E pathway promotes the translation of brain-derived neurotrophic factor (BDNF), a protein that enhances plasticity in sensory neurons which has been highly implicated in migraine and other forms of pain.27,28 Additionally, eIF4E mediates the translation of several proteins involved in inflammation, including interleukin-2, tumor necrosis factor alpha, IκBɑ, and chemokine ligand 3.29 Furthermore, eIF4E may play a role in the translation of various ion channels involved in pain hypersensitivity, such as voltage-gated calcium (Cav2.2) and sodium channels (Nav1.8).30
In conclusion, these data strongly implicate MNK-eIF4E signalling in migraine-relevant mechanisms and provide a rationale for targeting MNK inhibition as a novel therapeutic intervention for this disorder. Based on the critical role that translation regulation pathways play in neuronal plasticity in the brain, we suggest the development of peripherally restricted MNK1 inhibitors in order to increase therapeutic efficacy and limit adverse effects. While this study provides a potential approach for novel therapeutics, there remain key questions that must be addressed moving forward, including defining the mRNA targets of eIF4E phosphorylation in trigeminal nociceptors.
Supplementary Material
Contributor Information
Jacob Lackovic, Department of Neuroscience, The Center for Advanced Pain Studies, The University of Texas at Dallas, Richardson, TX 75080, USA.
Theodore J Price, Department of Neuroscience, The Center for Advanced Pain Studies, The University of Texas at Dallas, Richardson, TX 75080, USA.
Gregory Dussor, Department of Neuroscience, The Center for Advanced Pain Studies, The University of Texas at Dallas, Richardson, TX 75080, USA.
Funding
National Institutes of Health Grant NS065926 to T.J.P.
Competing interests
T.J.P. is a co-founder of 4E Therapeutics. J.L., T.J.P. and G.D. have filed for patent protection based on these findings.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. [DOI] [PubMed] [Google Scholar]
- 2. Zhang XC, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. [DOI] [PubMed] [Google Scholar]
- 3. Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126:1801–1813. [DOI] [PubMed] [Google Scholar]
- 4. Burstein R, Jakubowski M, Garcia-Nicas E, et al. . Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glock C, Heumüller M, Schuman EM. mRNA transport & local translation in neurons. Current Opinion Neurobiol. 2017;45:169–177. [DOI] [PubMed] [Google Scholar]
- 6. Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. [DOI] [PubMed] [Google Scholar]
- 7. Moy JK, Khoutorsky A, Asiedu MN, et al. . The MNK-eIF4E signaling axis contributes to injury-induced nociceptive plasticity and the development of chronic pain. J Neurosci. 2017;37:7481–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khoutorsky A, Price TJ. Translational control mechanisms in persistent pain. Trends Neurosci. 2018;41:100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lackovic J, Price TJ, Dussor G. De novo protein synthesis is necessary for priming in preclinical models of migraine. Cephalalgia. 2021;41:237–246. [DOI] [PubMed] [Google Scholar]
- 10. Jeevakumar V, Al Sardar AK, Mohamed F, Smithhart CM, Price T, Dussor G. IL-6 induced upregulation of T-type Ca(2+) currents and sensitization of DRG nociceptors is attenuated by MNK inhibition. J Neurophysiol. 2020;124:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yousuf MS, Shiers SI, Sahn JJ, Price TJ. Pharmacological manipulation of translation as a therapeutic target for chronic pain. Pharmacol Rev. 2021;73:59–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burgos-Vega CC, Quigley LD, Trevisan dos Santos G, et al. . Non-invasive dural stimulation in mice: A novel preclinical model of migraine. Cephalalgia. 2019;39:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mason BN, Avona A, Lackovic J, Dussor G. Dural stimulation and periorbital von frey testing in mice as a preclinical model of headache. J Vis Exp. Published online 29 July 2021. 10.3791/62867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avona A, Mason BN, Lackovic J, et al. . Repetitive stress in mice causes migraine-like behaviors and CGRP-dependent hyperalgesic priming to a migraine trigger. Pain. 2020;161:2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olesen J, Jansen-Olesen I. Nitric oxide mechanisms in migraine. Pathol Biol (Paris). 2000; 48:648–657. [PubMed] [Google Scholar]
- 17. McIlvried LA, Borghesi LA, Gold MS. Sex-, stress-, and sympathetic post-ganglionic neuron-dependent changes in the expression of pro- and anti-inflammatory mediators in rat dural immune cells. Headache. 2015; 55:943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIlvried LA, Cruz JA, Borghesi LA, Gold MS. Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura. Cephalalgia. 2017;37:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kasuno K, Takabuchi S, Fukuda K, et al. . Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. [DOI] [PubMed] [Google Scholar]
- 20. Kanngiesser M, Mair N, Lim HY, et al. . Hypoxia-inducible factor 1 regulates heat and cold pain sensitivity and persistence. Antioxid Redox Signal. 2014;20:2555–2571. [DOI] [PubMed] [Google Scholar]
- 21. Geng C, Yang Z, Xu P, Zhang H. Aberrations in peripheral inflammatory cytokine levels in migraine: a systematic review and meta-analysis. J Clin Neurosci. 2022;98:213–218. [DOI] [PubMed] [Google Scholar]
- 22. Reich SH, Sprengeler PA, Chiang GG, et al. . Structure-based design of pyridone-aminal eFT508 targeting dysregulated translation by selective mitogen-activated protein kinase interacting kinases 1 and 2 (MNK1/2) inhibition. J Med Chem. 2018;61:3516–3540. [DOI] [PubMed] [Google Scholar]
- 23. Obreja O, Rukwied R, Nagler L, Schmidt M, Schmelz M, Namer B. Nerve growth factor locally sensitizes nociceptors in human skin. PAIN. 2018;159:416–426. [DOI] [PubMed] [Google Scholar]
- 24. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. [DOI] [PubMed] [Google Scholar]
- 25. Liang L, Tao B, Fan L, Yaster M, Zhang Y, Tao YX. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res. 2013;1513:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terenzio M, Koley S, Samra N, et al. . Locally translated mTOR controls axonal local translation in nerve injury. Science (New York, NY) 2018;359:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moy JK, Khoutorsky A, Asiedu MN, Dussor G, Price TJ. eIF4E phosphorylation influences bdnf mRNA translation in mouse dorsal root ganglion neurons. Front Cell Neurosci. 2018;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burgos-Vega CC, Quigley LD, Avona A, Price T, Dussor G. Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain. 2016;157:2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uttam S, Wong C, Price TJ, Khoutorsky A. eIF4E-Dependent translational control: A central mechanism for regulation of pain plasticity. Front Genet. 2018;9:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hudmon A, Choi JS, Tyrrell L, et al. . Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28:3190–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All information necessary to evaluate the findings of the paper are included in the paper. Additional data can be provided by the authors upon request.