Abstract
Background
Higher doses of rifampicin may improve treatment outcomes and reduce the duration of tuberculosis (TB) therapy. However, drug–drug interactions with antiretroviral therapy (ART) and safety in people with human immunodeficiency virus (HIV) have not been evaluated.
Methods
This was a randomized, open-label trial where newly diagnosed TB patients were randomized to higher (35 mg/kg) or standard (10 mg/kg) daily-dose rifampicin. ART treatment–naive patients were randomized to dolutegravir- or efavirenz-based ART. At week 6, trough dolutegravir or mid-dose efavirenz plasma concentrations were assayed. HIV viral load was measured at week 24.
Results
Among 128 patients randomized, the median CD4 count was 191 cells/mm3. The geometric mean ratio (GMR) for trough dolutegravir concentrations on higher- vs standard-dose rifampicin was 0.57 (95% confidence interval [CI], .34–.97; P = .039) and the GMR for mid-dose efavirenz was 0.63 (95% CI, .38–1.07; P = .083). There was no significant difference in attainment of targets for dolutegravir trough or efavirenz mid-dose concentrations between rifampicin doses. The incidence of HIV treatment failure at week 24 was similar between rifampicin doses (14.9% vs 14.0%, P = .901), as was the incidence of drug-related grade 3–4 adverse events (9.8% vs 6%). At week 8, fewer patients remained sputum culture positive on higher-dose rifampicin (18.6% vs 37.0%, P = .063).
Conclusions
Compared with standard-dose rifampicin, high-dose rifampicin reduced dolutegravir and efavirenz exposures, but HIV suppression was similar across treatment arms. Higher-dose rifampicin was well tolerated among people with HIV and associated with a trend toward faster sputum culture conversion.
Clinical Trials Registration
Keywords: TB-HIV, high-dose rifampicin, antiretroviral therapy, dolutegravir, efavirenz
We demonstrated that high-dose rifampicin (35 mg/kg) reduced dolutegravir and efavirenz exposures without compromising virological suppression in patients with human immunodeficiency virus (HIV). High-dose rifampicin was well tolerated among people with HIV and associated with a trend toward faster sputum culture conversion.
Of approximately 9.9 million people who fell ill with active tuberculosis (TB) in 2020, 8% with human immunodeficiency virus (HIV) [1]. Current international guidance recommends early initiation and coadministration of antiretroviral therapy (ART) alongside TB treatment; therefore, advances in anti-TB chemotherapy must be compatible with ART [2].
Shortening TB treatment from the existing duration of 6 months could substantially improve patient outcomes [3]. Rifamycins are pivotal to successful therapy because of their sterilizing effect and their ability to provide durable cure [4]. Rifampicin is the most widely used rifamycin worldwide, at a currently recommended dose of 10 mg/kg daily. Accumulative data show that higher doses are safely tolerated, provide greater bactericidal activity, and may facilitate shorter TB treatment [5–7]. A 4-month regimen that incorporates optimized dosing of rifapentine has been reported as noninferior to standard 6-month therapy [8] and granted provisional support from the World Health Organization (WHO) [9].
Most clinical rifamycin dose-escalation studies have been conducted among patients not with HIV or patients with HIV without severe immunosuppression. This is partly because drug–drug interactions and adverse drug reactions are more common in advanced HIV disease [10]. Rifamycins, including rifampicin, induce activity of cytochrome P450 (CYP) enzymes, which alter the hepatic metabolism of other medicines [11]. With standard-dose rifampicin, induction of CYP2B6 accelerates clearance of efavirenz, but a 600-mg daily dose preserves plasma exposure and HIV viral suppression [12, 13]. Induction of UDP glucuronosyltransferase (UGT) 1A1 and CYP3A4 increases clearance of dolutegravir [14], but this can be overcome by doubling the daily dose from 50 mg once to 50 mg twice daily [15]. The effect of higher doses of rifampicin on first-line ART is unknown.
We conducted a study, the SAfety and EFficacy in high-dose RIFampicin (SAEFRIF trial), an open-label, randomized clinical trial among patients with drug-sensitive pulmonary TB to explore the impact of higher-dose rifampicin (35 mg/kg) vs a standard dose (10 mg/kg) on the pharmacokinetics of efavirenz and dolutegravir. The safety and microbiological efficacy of differing rifampicin doses were also described, and HIV virologic suppression was evaluated.
METHODS
Study Design and Site
This 4-arm, open-label, phase 2b clinical trial was conducted at the integrated TB-HIV outpatient clinic of the Infectious Diseases Institute, Makerere University, Uganda. The full study design has been published [16]. The Joint Clinical Research Centre Ethics Committee and the Uganda National Council for Science and Technology approved the protocol. All participants provided informed consent, and the trial was conducted in accordance with the principles of the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice guidelines.
Participants
Participants were aged ≥18 years, with HIV, and scheduled to initiate therapy for newly diagnosed TB. TB diagnosis was confirmed using sputum Xpert Mycobacterium tuberculosis/rifampin (MTB/RIF; Cepheid), urinary lipoarabinomannan detection (Alere), and sputum culture. All participants were receiving, or planning to commence, efavirenz- or dolutegravir-based ART. Exclusion criteria included rifampicin resistance, pregnancy, aminotransferases >5 times the upper limit of normal (ULN), and glomerular filtration rate (eGFR) <50 mL/min. Patients were withdrawn in the event of treatment toxicity or pregnancy.
Randomization and Treatment
Eligible participants were randomly assigned in a 1:1 ratio to higher- (35 mg/kg) or standard-dose (10 mg/kg) daily rifampicin, alongside standard doses of isoniazid, pyrazinamide, and ethambutol for 8 weeks, followed by standard doses of rifampicin and isoniazid for 16 weeks. Anti-TB treatment was dosed according to weight bands using fixed-dose combinations. Those on higher-dose rifampicin received additional 300 mg rifampicin capsules (the dosing table is included in the Supplementary Materials). Adherence was monitored using patient self-reporting and pill counts.
Two weeks into treatment, ART-naive participants were randomly assigned in a 1:1 ratio to initiate dolutegravir or efavirenz alongside tenofovir disoproxil fumarate, zidovudine or abacavir, plus either lamivudine or emtricitabine. Participants already on ART continued their existing medications. Dolutegravir was dosed at 50 mg twice daily during TB therapy and for 2 weeks after completing TB treatment. Prior to December 2021, efavirenz was dosed at 600 mg daily; however, following a change in national guidelines, the last 6 patients enrolled received 400 mg daily. A randomization list was generated using random permuted blocks in Stata version 16.1. Treatment allocation was performed using a system programmed in Microsoft Excel.
Trial Procedures: Safety Monitoring and Microbiology
Baseline clinical and demographic characteristics were recorded. Follow-up visits were performed every 2 weeks for the first 8 weeks, then at week 24. Serum alanine transferase (ALT) and bilirubin were measured at baseline and weeks 2, 4, 6, and 8. Serum creatinine was measured at baseline and weeks 4 and 8. Anti-TB treatment was interrupted for ALT ≥3 times the ULN in the presence of hepatitis symptoms or ALT ≥5 times the ULN regardless of symptoms.
Spot sputum samples were collected at baseline and week 8. Mycobacterial cultures were conducted using standard methods for mycobacteria growth indicator tubes (Becton Dickinson) and Lowenstein–Jensen slopes.
Pharmacokinetic Sampling
Participants were requested to take their anti-TB drugs every morning after fasting overnight. Dolutegravir was taken twice daily, and efavirenz was taken nightly. Blood sampling for pharmacokinetic analysis was performed 6 weeks into TB treatment. On the pharmacokinetic visit day, participants were asked to attend without taking their morning medicines. Early morning blood draws were representative of pre-dose dolutegravir trough concentrations and mid-dose efavirenz concentrations. Quantification of drug concentrations was performed using validated high-performance liquid chromatography assays for efavirenz and mass spectrometry for dolutegravir [17–19].
Outcomes
Primary pharmacokinetic study outcomes were dolutegravir trough and efavirenz mid-dose concentrations after 6 weeks of TB treatment. Secondary pharmacokinetic outcomes were attainment of prespecified target threshold concentrations for each drug. Two target thresholds were evaluated for dolutegravir: 0.3 mg/L, which corresponds to the median trough concentrations observed with 10 mg of dolutegravir once daily that was similar to the virological response attained at week 24 in the SPRING-1 trial [14, 20], and 0.064 mg/L, which represents the in vitro protein-adjusted 90% maximal inhibitory concentration of the drug [21]. The target threshold used for efavirenz was 1 mg/L, the most commonly referenced minimum effective concentration (MEC) [22].
Other secondary outcomes were adverse events of grade 3 or higher, graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events [23]; HIV plasma viral load measurement using real-time polymerase chain reaction after 24 weeks, with virological failure defined by the WHO cutoff of >1000 copies/mL; and mycobacterial sputum culture positivity after 8 weeks.
Statistical Analyses
We calculated that 130 participants would provide 80% power to detect a 30% relative change in mean dolutegravir or efavirenz concentrations between patients on higher- vs standard-dose rifampicin with a coefficient of variation of 50%, assuming a 5% type I error and 2-sided hypothesis testing.
Participants’ characteristics, adverse events, and final TB outcomes were summarized on the intention-to-treat (ITT) population. Analysis of pharmacokinetic outcomes (dolutegravir trough and efavirenz mid-dose concentrations) were based on a pharmacokinetic per-protocol (PK-PP) population, including participants who remained in the study until blood sampling at week 6.
Concentrations below the lower limit of quantification (BLQ; <0.05 mg/L for dolutegravir and <0.2 mg/L for efavirenz) were unexpected considering the half-life of the drugs and the dosing intervals, hence, individuals with BLQ values were flagged as nonadherent and excluded from the primary analysis. However, a sensitivity analysis was performed with BLQs imputed at half their lower limits of quantification (LLOQ) cutoffs, that is, 0.025 mg/L for dolutegravir and 0.1 mg/L for efavirenz.
We compared dolutegravir trough and efavirenz mid-dose concentrations in patients on higher- (35 mg/kg) vs standard-dose (10 mg/kg) rifampicin using a geometric mean ratio (GMR) and 95% confidence intervals (CIs) generated using log-linear regression unadjusted models. For the sensitivity analysis, the Wilcoxon rank sum test was used to compare median concentrations because BLQ imputation skewed the data. In an additional sensitivity analysis, we reestimated the GMR after standardizing the concentrations by dividing concentrations with respective efavirenz dose, that is, 600 mg or 400 mg. We also used the Fisher exact test to compare proportions of patients below target thresholds for ART concentrations on different rifampicin doses.
Adverse events, HIV plasma viral loads, and mycobacterial sputum culture conversion were compared between rifampicin doses using the Pearson χ2 test. Hypotheses tests were performed at a 5% significance level.
RESULTS
Study Population
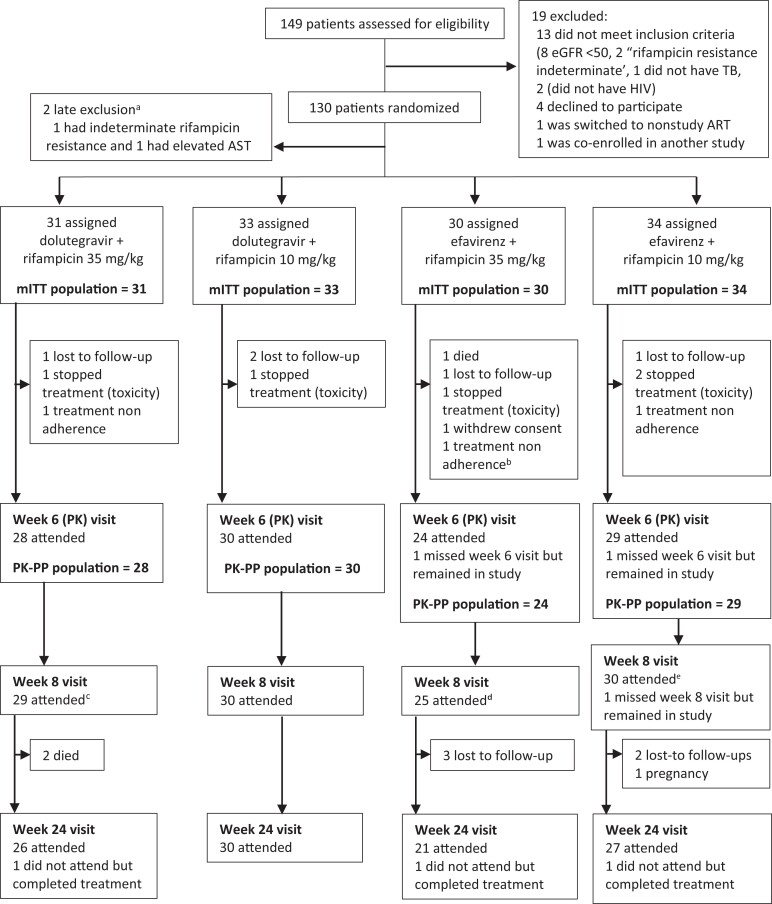
From April 2019 to February 2021, 149 participants were screened, and 130 were enrolled (Figure 1). Nineteen (12.8%) were excluded; 8 had eGFR <50 mL/min, 1 was deemed by the study team to not have TB, 2 did not have HIV, 2 had indeterminate rifampicin-resistance results on Xpert MTB/RIF, 4 declined to participate, 1 was being switched to study-ineligible second-line ART, and 1 was enrolled in another interventional study. Two enrolled participants did not fit the inclusion criteria and were withdrawn before initiating treatment. The ITT population included 128 patients, 81 (63.3%) were male with a median age of 36 years (interquartile range [IQR], 30–43) and a median body mass index of 19.3 kg/m2 (IQR, 17.7–21.7). Fifty-nine (46.1%) were on ART at baseline. The median CD4 cell count was 191 cells/µL (IQR, 80–418). Eight (22.2%) of the 36 patients who had been on ART for >3 months at enrollment had baseline viral loads >1000 copies/mL, 7 (87.5%) of those were on efavirenz (Table 1). Apart from 12 (9.4%) patients who missed ≥2 ART doses prior to the week 6 visit, all participants reported no missed ART doses.
Figure 1.
Eligibility assessment, randomization, treatment, and follow-up. aThese patients were excluded from the study before any study medication was provided. bThis patient subsequently died before week 8. cIncludes 1 patient not included in the PK-PP population because was nonadherent. d Includes 1 patient who had missed week 6 visit but came back at week 8. Abbreviations: ART, antiretroviral therapy; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; mITT, modified intention to treat population after 2 late exclusions; PK-PP, pharmacokinetic per-protocol; TB, tuberculosis.
Table 1.
Baseline Characteristics of Patients in the Intention-to-Treat Population
| Characteristic | Treatment Arm | Overall N = 128 |
|||
|---|---|---|---|---|---|
| DTG | EFV | ||||
| Arm 1A DTG + RIF35 n = 31 |
Arm 1B DTG+ RIF10 n = 33 |
Arm 2A EFV + RIF35 n = 30 |
Arm 2B EFV + RIF10 n = 34 |
||
| Demographics | |||||
| ȃMale sex, n (%) | 22 (71.0) | 22 (66.7) | 17 (56.7) | 20 (58.8) | 81 (63.3) |
| ȃMedian age (IQR), years | 37 (33–45) | 36 (29–43) | 31 (28–38) | 38 (34–43) | 36 (30–43) |
| ȃ Median BMI (IQR), kg/m2 | 18.5 (17.5–21.7) | 19.3 (17.7–21.7) | 19.7 (18.9–22.1) | 19.2 (16.8–20.9) | 19.3 (17.7–21.7) |
| ȃ BMI <18.5 kg/m2, n (%) | 15 (48.4) | 11 (33.3) | 7 (23.3) | 15 (44.1) | 48 (37.5) |
| Human immunodeficiency virus parameters | |||||
| ȃMedian CD4 count (IQR),acells/µL | 288 (154–423) | 176 (39–475) | 206 (104–496) | 149 (67–340) | 191 (80–418) |
| ȃCD4 count <200 cells/µL, n (%)a | 13 (44.8) | 18 (58.1) | 14 (46.7) | 19 (55.9) | 64 (51.6) |
| ȃOn ART at baseline, n (%) | 18 (58.1) | 20 (60.6) | 8 (26.7) | 13 (38.2) | 59 (46.1) |
| ȃOn ART for ≥3 months, n (%)b | 12 (66.7) | 11 (55.0) | 5 (62.5) | 10 (76.9) | 38 (64.4) |
| ȃViral load ≥1000 copies/mL, n (%)c | 1 (8.3) | 0 | 2 (50.0) | 5 (55.6) | 8 (22.2) |
RIF35 denotes high-dose RIF, 35 mg/kg. RIF10 denotes standard-dose RIF, 10 mg/kg.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; DTG, dolutegravir; EFV, efavirenz; IQR, interquartile range; RIF, rifampicin.
Four patients had CD4 count tests missed in error (2 in each of the DTG arms).
Denominator is number on ART at baseline in each treatment arm.
Baseline viral load measurements done for patients who had been on ART for ≥3 months. Baseline viral load not done for 2 patients (1 in each of the EFV arms) because they were off ART prior to enrollment.
Effect of High-Dose Rifampicin on Dolutegravir Trough Concentrations
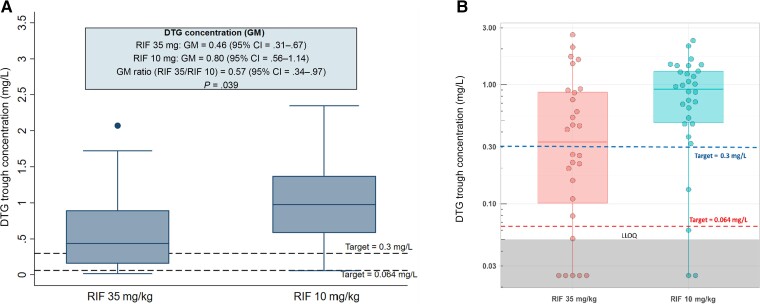
In the PK-PP population, 7 participants on dolutegravir with BLQ trough concentrations were excluded from the primary analysis (5 on higher-dose and 2 on standard-dose rifampicin). It was suspected that self-administration of their prior dose, which was not observed, had been forgotten or mistimed. The GMR (95% CI) of dolutegravir trough concentrations for patients on higher-dose rifampicin was lower than for those receiving standard-dose rifampicin: 0.46 (95% CI, .31–.67) mg/L vs 0.80 (95% CI, .56–1.14) mg/L, with a GMR of 0.57 (95% CI, .34–.97; P = .039; Table 2, Figure 2A). Using a threshold of 0.3 mg/L, a higher proportion of participants on higher-dose rifampicin failed to attain the target dolutegravir trough concentration compared with those on standard-dose (9, 39.1% vs 2, 7.1%; P = .014). Using a lower target threshold of .064 mg/L, the difference in target attainment between higher- and standard-dose rifampicin was not statistically significant (1, 4.4% vs 1, 3.6%; P = .999). No patient with dolutegravir concentrations below either target thresholds had a detectable HIV viral load at week 24.
Table 2.
Dolutegravir Trough Concentrations in Patients on Higher- vs Standard-Dose Rifampicin at Week 6
| Variable | Arm 1A DTG + RIF35 |
Arm 1B DTG+ RIF10 |
P Value |
|---|---|---|---|
| Randomized (intention-to-treat population), number | 31 | 33 | ... |
| Number in PK-PP population | 28 | 30 | ... |
| ȃ DTG Ctrough BLQ, n | 5 | 2 | ... |
| Primary PK analysis: PK-PP population, patients with BLQ DTG trough concentrations excluded | |||
| ȃNumber analyzed | 23 | 28 | |
| Primary PK outcome: DTG trough concentrations | |||
| Geometric mean (95% CI) | 0.46 (.31–.67) | 0.80 (.56–1.14) | ... |
| Geometric mean ratio (95% CI) | 0.57 (.34–.97) | .039 | |
| Secondary PK outcome: attainment of target threshold DTG trough concentration of 0.3 mg/L | |||
| <0.3 mg/L, n (%) | 9 (39.1) | 2 (7.1) | .014a |
| ≥0.3 mg/L, n (%) | 14 (60.9) | 26 (92.9) | |
| Secondary PK outcome: attainment of target threshold DTG trough concentration of 0.064 mg/L | |||
| <0.064 mg/L, n (%) | 1 (4.4) | 1 (3.6) | .999a |
| ≥0.064 mg/L, n (%) | 22 (95.6) | 27 (96.4) | |
| Sensitivity PK analysis: PK-PP population, patients with BLQ DTG trough concentration imputed to 0.025 mg/Lb | |||
| ȃNumber analyzed | 28 | 30 | ... |
| Primary PK outcome: DTG trough concentrations | |||
| Median (interquartile range) | 0.34 (0.09–0.88) | 0.92 (0.47–1.30) | .020c |
| Secondary PK outcome: attainment of target threshold DTG trough concentration of 0.3 mg/L | |||
| <0.3 mg/L, n (%) | 14 (50.0) | 4 (13.3) | .004a |
| ≥0.3 mg/L, n (%) | 14 (50.0) | 26 (86.7) | |
| Secondary PK outcome: attainment of target threshold DTG trough concentration of 0.064 mg/L | |||
| <0.064 mg/L, n (%) | 6 (21.4) | 3 (10.0) | .290a |
| ≥0.064 mg/L, n (%) | 22 (78.6) | 27 (90.0) | |
Geometric mean ratio estimate is a ratio of the geometric mean in the higher-dose arm (RIF, 35 mg/kg) compared with the standard-dose arm (RIF, 10 mg/kg). Geometric means and their 95% CIs and P values obtained using log-linear regression model.
Abbreviations: BLQ, below limit of quantification; CI, confidence interval; DTG, dolutegravir; PK, pharmacokinetics; PP, per protocol; RIF, rifampicin.
Fisher exact P value.
Single value imputation by assigning BLQs to 0.5 × BLQ cutoff (0.5 × 0.05 = 0.025).
Wilcoxon rank sum test P value.
Figure 2.
DTG trough/minimum concentrations in participants on high-dose vs standard-dose RIF. A, Primary analysis that excludes values below the limit of quantification. B, Sensitivity analysis with values below the level of quantification included. The upper and lower limit of the boxes are the 25th and 75th percentiles. Abbreviations: CI, confidence interval; DTG, dolutegravir; GM, geometric mean; LLOQ, lower limits of quantification; RIF, rifampicin.
In the sensitivity analysis (Figure 2B) with imputation to 0.025 mg/L (LLOQ/2) for BLQ dolutegravir results, the median dolutegravir concentrations were significantly lower in the higher-dose compared with the standard-dose arm (median [IQR]: 0.34 [0.09–0.88] mg/L vs 0.92 [0.47–1.30] mg/L; P = .019), and a significant proportion of patients on higher-dose rifampicin failed to attain the target trough concentration of 0.3 mg/L compared with those on standard-dose rifampicin (median [IQR]: 0.34 [0.09–0.88] mg/L vs 0.92 [0.47–1.30] mg/L; P = .020). However, similar to the primary analysis, using the lower 0.064-mg/L threshold, the difference in target attainment between higher- and standard-dose rifampicin was not significant (6, 21.4% vs 3, 10%; P = .290). No patients with dolutegravir concentrations below either target had a detectable HIV viral load at week 24.
Effect of High-Dose Rifampicin on Efavirenz Concentrations
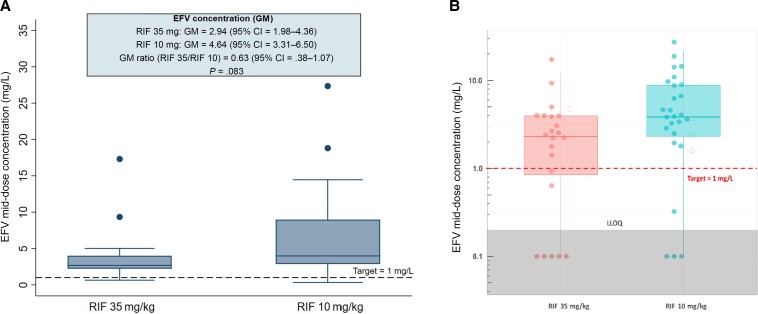
In the PK-PP population, 8 participants on efavirenz (5 on higher-dose and 3 on standard-dose rifampicin) were excluded from the primary analysis due BLQ mid-dose concentrations. As with doltegravir, dose omission or mistiming was suspected; Figure 3B shows that they were outliers. The geometric mean for efavirenz mid-dose concentrations for patients on higher-dose rifampicin was 37.0% lower than those receiving the standard dose: 2.94 (95% CI, 1.98–4.36) mg/L vs 4.64 (95% CI, 3.31–6.50) mg/L, with a GMR of 0.63 (95% CI, .38–1.07; P = .083; Table 3, Figure 3A). Five patients received efavirenz 400 mg (2 in the higher-dose and 3 in the standard-dose arm), and the GMR remained the same: 0.63 (95% CI, .38–1.05; P = .078).
Figure 3.
Box plot showing EFV mid-dose concentrations in patients on higher-dose vs standard-dose RIF. A, Primary analysis that excludes values below the limit of quantification. B, Sensitivity analysis with values below the level of quantification included; the filled circles denote patients who received EFV 600 mg and the empty circles denote 400 mg. The upper and lower limit of the boxes are the 25th and 75th percentiles. Abbreviations: CI, confidence interval; EFV, efavirenz; GM, geometric mean; LLOQ, lower limits of quantification; RIF, rifampicin.
Table 3.
Efavirenz Mid-Dose Concentrations in Patients on Higher- vs Standard-Dose Rifampicin at Week 6
| Variable | Arm 2A EFV + RIF35 |
Arm 2B EFV + RIF10 |
P Value |
|---|---|---|---|
| Randomized (intention-to-treat population), number | 30 | 34 | ... |
| Number in PK-PP population | 24 | 29 | ... |
| EFV Cmid−dose BLQ, n | 5 | 3 | ... |
| Primary PK analysis: PK-PP population, patients with BLQ EFV concentrations excluded | |||
| Number analyzed | 19 | 26 | ... |
| Primary PK outcome: EFV mid-dose concentration | |||
| Geometric mean (95% CI) | 2.94 (1.98–4.36) | 4.64 (3.31–6.50) | ... |
| Geometric mean ratio (95% CI) | 0.63 (.38–1.07) | .083 | |
| Secondary PK outcome: attainment of target threshold EFV trough concentration of 1 mg/L | |||
| <1 mg/L, n (%) | 2 (10.5) | 1 (3.9) | .565a |
| ≥1 mg/L, n (%) | 17 (89.5) | 25 (96.1) | |
| Sensitivity PK analysis: PK-PP population, patients with BLQ EFV mid-dose concentrations imputed to 0.1 mg/Lb | |||
| Number analyzed (PP population) | 24 | 29 | ... |
| Primary PK outcome: EFV mid-dose concentration | |||
| Median (interquartile range) | 2.32 (0.78–3.97) | 3.84 (2.3–8.77) | .039c |
| Secondary PK outcome: attainment of target threshold EFV trough concentration of 1 mg/L | |||
| <1 mg/L, n (%) | 7 (29.2) | 4 (13.8) | .194a |
| ≥1 mg/L, n (%) | 17 (70.8) | 25 (86.2) | |
Geometric mean ratio estimate is a ratio of the geometric mean in the higher-dose arm (RIF, 35 mg/kg) compared with the standard-dose arm (RIF, 10 mg/kg). Geometric means and their 95% CIs and P values obtained using log-linear regression model.
Abbreviations: BLQ, below limit of quantification; CI, confidence interval; EFV, efavirenz; PK, pharmacokinetic; PP, per protocol; RIF, rifampicin.
Fisher exact P value.
Single value imputation by assigning BLQs to 0.5 × BLQ cutoff value (=0.5 × 0.2 = 0.1).
Wilcoxon rank sum test P value.
However, the proportion of patients who failed to attain a target mid-dose efavirenz concentration of 1 mg/L was not significantly different between arms: 2 (10.5%) on higher-dose compared with 1 (3.9%) on standard-dose rifampicin (P = .565). No patients with efavirenz concentrations below the target had a detectable HIV viral load at week 24.
In the sensitivity analysis, the median mid-dose concentrations on higher-dose rifampicin were significantly lower than on standard-dose rifampicin: 2.32 (IQR, 0.78–3.97) mg/L vs 3.84 (IQR, 2.33–8.77) mg/L (P = .039; Figure 3B). The difference in target attainment was not significantly different between rifampicin doses (7, 29.2% vs 4, 13.8%; P = .194), and no patients with efavirenz concentrations below the target had a detectable HIV viral load at week 24.
Safety
Thirty-six (59%) participants on higher-dose rifampicin (19 on dolutegravir and 17 on efavirenz) experienced adverse events, similar to 39 (58.2%) on the standard dose (23 on dolutegravir and 16 on efavirenz; Table 4). Six (9.8%) participants on higher-dose rifampicin (4 on dolutegravir and 2 on efavirenz) and 4 (6.0%) on the standard dose (2 on dolutegravir and 2 on efavirenz) developed grade 3–4 drug-related adverse events that included elevated total bilirubin in 5 patients on higher-dose rifampicin and grade 3–4 elevation of ALT in 3 patients on standard-dose rifampicin. Six of 8 participants with drug-related liver injury had a CD4 cell count <200 cells/µL. Five patients discontinued study medications due to drug-liver injury (2 in the efavirenz + standard-dose rifampicin arms and 1 in each of the other arms).
Table 4.
Adverse Events That Occurred Between Baseline and Week 24
| Characteristic | Treatment Arm | Higher-Dose RIF (35 mg/kg)a | Standard-Dose RIF (10 mg/kg)b | P Valuec | |||
|---|---|---|---|---|---|---|---|
| DTG | EFV | ||||||
| Arm 1A DTG + RIF35 n = 31 |
Arm 1B DTG+ RIF10 n = 33 |
Arm 2A EFV + RIF35 n = 30 |
Arm 2B EFV + RIF10 n = 34 |
n = 61 | n = 67 | ||
| Patients with any AE, n (%) | 19 (61.3) | 23 (69.7) | 17 (56.7) | 16 (47.1) | 36 (59.0) | 39 (58.2) | .926 |
| Patients with grade 3–4 AE, n (%) | 5 (16.1) | 2 (6.1) | 5 (16.7) | 4 (11.8) | 10 (16.4) | 6 (9.0) | .204 |
| Patients with drug-related grade 3–4 AE, n (%) | 3 (9.7) | 2 (6.1) | 2 (6.7) | 2 (5.9) | 5 (8.1) | 4 (6.0) | ... |
| Patients discontinued due to toxicities, n (%) | 1 (3.2) | 1 (3.0) | 1 (3.3) | 2 (5.9) | 2 (3.3) | 3 (4.5) | ... |
| Total AEs | 50 | 58 | 37 | 40 | 87 | 98 | ... |
| Total number of grade 3–4 AEs | 7 | 2 | 5 | 4 | 12 | 6 | ... |
| Total number of drug-related grade 3–4 AEs | 4 | 2 | 2 | 2 | 6 | 4 | ... |
| Grade 3–4 events listing, number | … | … | … | … | … | … | ... |
| ȃElevated total bilirubin | 3 | 0 | 2 | 0 | 5 | 0 | ... |
| ȃElevated alanine transferase | 0 | 1 | 0 | 2 | 0 | 3 | ... |
| ȃOtherd | 4 | 1 | 3 | 2 | 7 | 3 | ... |
| Patients with at least 1 SAE, n (%) | 4 (12.9) | 2 (6.1) | 3 (10.0) | 1 (2.9) | 7 (11.5) | 3 (4.5%) | .140 |
| Total SAEs, number | 5 | 2 | 3 | 1 | 8 | 3 | ... |
| ȃFatal | 2 | 0 | 2 | 0 | 4 | 0 | ... |
| ȃLife-threatening | 0 | 1 | 0 | 0 | 1 | 0 | ... |
| ȃResulted in prolonged hospitalization | 2 | 1 | 1 | 1 | 3 | 2 | ... |
| ȃAssociation with persistent major disability or incapacity | 1 | 0 | 0 | 0 | 1 | 0 | ... |
| Number of drug-related SAEs | 2 | 2 | 0 | 0 | 2 | 2 | ... |
RIF35 denotes high-dose RIF, 35 mg/kg. RIF10 denotes standard-dose RIF, 10 mg/kg.
Abbreviations: AE, adverse event; DTG, dolutegravir; EFV, efavirenz; RIF, rifampicin; SAE, serious adverse event.
Higher-dose RIF obtained by combining the 2 treatment arms: DTG + RIF35 and EFV + RIF35.
Standard-dose RIF obtained by combining the 2 treatment arms: DTG + RIF10 and EFV + RIF10.
P values based on the Pearson χ2 test comparing higher-dose RIF vs standard-dose RIF combined treatment groups.
Other grade 3–4 AEs included worsening anemia, gastroenteritis, hearing loss, and bronchopneumonia in 1A; worsening anemia in 1B; 2 cases of worsening anemia and gastroenteritis in 2A; and genital ulcers and transient ischemic attack in 2B.
Ten participants (7.8%) experienced severe adverse events; 7 (22.9%) on higher-dose rifampicin and 3 (9%) on the standard dose. However, only 2 on higher-dose rifampicin + dolutegravir and 2 on standard-dose rifampicin + dolutegravir were drug related. Four deaths occurred on higher-dose rifampicin, but none were attributed to study medicines: 1 had disseminated Kaposi’s sarcoma, 1 died after developing sudden difficulty breathing 5 months into TB treatment, 1 had severe sepsis, and 1 had severe anemia since baseline.
Virological Outcomes
At week 24, 15 of 104 patients with viral load measured had virological failure (12 on efavirenz and 3 on dolutegravir; Table 5). Six of the 8 patients who had virological failure at baseline still had virological failure at week 24 despite adherence counseling (5 on efavirenz and 1 on dolutegravir). There was no difference in the proportion of patients with virological failure at week 24 between those on higher- and standard-dose rifampicin (7 of 47, 14.9% vs 8 of 57, 14.0%; P = .901). As noted, no patients with trough dolutegravir or mid-dose efavirenz concentrations below any of the target thresholds experienced virological failure.
Table 5.
Week 8 Sputum Conversion, Human Immunodeficiency Virus Type 1 Viral Load at Week 24, and Tuberculosis Treatment Outcomes
| Outcome | Treatment Arm | Higher-Dose RIF (35 mg/kg) | Standard-Dose RIF (10 mg/kg) | P Valuea | |||
|---|---|---|---|---|---|---|---|
| DTG | EFV | ||||||
| Arm 1A DTG + RIF35 n = 31 |
Arm 1B DTG+ RIF10 n = 33 |
Arm 2A EFV + RIF35 n = 30 |
Arm 2B EFV + RIF10 n = 34 |
n = 61 | n = 67 | ||
| Human immunodeficiency virus type 1 RNA level at week 24,b n (%) | |||||||
| <1000 copies/mL | 24 (92.3) | 29 (96.7) | 16 (76.2) | 20 (74.1) | 40 (85.1) | 49 (86.0) | .901 |
| ≥1000 copies/mL | 2 (7.7) | 1 (3.3) | 5 (23.8) | 7 (25.9) | 7 (14.9) | 8 (14.0) | |
| Week 8 sputum conversion,c n (%) | |||||||
| Negative | 17 (80.9) | 16 (61.5) | 18 (81.8) | 13 (65.0) | 35 (81.4) | 29 (63.0) | .063 |
| Positive | 4 (19.1) | 10 (38.5) | 4 (18.2) | 7 (35.0) | 8 (18.6) | 17 (37.0) | |
| Unable to produce sputum at week 8 | 7 | 4 | 3 | 10 | 10 | 14 | .562 |
| Tuberculosis treatment outcomes at week 24,d,e n (%) | |||||||
| Cured | 17 (54.8) | 23 (69.7) | 15 (50.0) | 20 (58.8) | 32 (52.2) | 43 (64.2) | ... |
| Completed | 9 (29.0) | 6 (18.2) | 5 (16.7) | 6 (17.7) | 14 (23.0) | 12 (17.9) | ... |
| Treatment failure | 0 | 1 (3.0) | 1 (3.3) | 1 (2.9) | 1 (1.6) | 2 (3.0) | ... |
| Died | 2 (6.5) | 0 | 2 (6.7) | 0 | 4 (6.6) | 0 | ... |
| Lost to follow-up | 2 (6.5) | 2 (6.1) | 4 (13.3) | 4 (11.8) | 6 (9.8) | 6 (9.0) | ... |
| Not assessed | 1 (3.2) | 1 (3.0) | 3 (10.0) | 3 (8.8) | 4 (6.6) | 4 (6.0) | ... |
RIF35 denotes high-dose RIF, 35 mg/kg. RIF10 denotes standard-dose RIF, 10 mg/kg.
Abbreviations: DTG, dolutegravir; EFV, efavirenz; RIF, rifampicin.
P values based on Pearson χ2 test comparing higher-dose rifampicin vs standard-dose rifampicin combined treatment groups.
Twenty-four participants were discontinued before viral load was measured at week 24 (5 in 1A, 3 in 1B, 9 in 2A, and 7 in 2B).
Fifteen participants discontinued before week 8, and 24 patients could not produce sputum at week 8 (7 in 1A, 4 in 1B, 3 in 2A, and 10 in 2B).
Treatment outcome could not be assessed following withdrawal from the study (5 due to toxicities, 1 withdrew consent, and 1 due to pregnancy).
Consistent with World Health Organization criteria, terms are defined as follows: cured: a pulmonary tuberculosis (TB) patient with bacteriologically confirmed TB at baseline who was sputum culture–negative at end of treatment; completed: TB patient who completed treatment with clinical recovery but no record to confirm sputum culture status at end of therapy; treatment failure: TB patient whose sputum culture was positive at end of treatment; died: death from any cause; lost to follow-up: stopped attending clinic before treatment completion; and not assessed: no outcome assigned, including patients transferred to another center for completion of therapy. Treatment success is the sum of cured and completed outcomes.
TB Treatment Response
A total of 113 patients remained in the study at week 8, but 24 could not produce a sputum sample at this visit (10 on higher- and 14 on standard-dose rifampicin). Of 89 patients who provided sputum, 8 of 43 (18.6%) remained culture positive on higher-dose rifampicin compared with 17 of 46 (37.0%) on the standard dose (P = .063; Table 5).
By the end of TB treatment, in the ITT population, treatment success had been achieved by 46 (75.4%) participants on higher- and 54 (80.6%) on standard-dose rifampicin. TB treatment failure was rare: 1 participant on higher-dose and 2 on standard-dose rifampicin (Table 5).
DISCUSSION
Higher-dose rifampicin was well tolerated when coadministered with ART but was associated with reduced dolutegravir trough concentrations. Mid-dose concentrations of efavirenz were also lower compared with standard rifampicin dosing. Higher risk of virologic failure was not observed, but patient numbers were small.
The existing literature suggests that hepatic cytochrome P450 enzyme induction by rifampicin is almost maximal at 450 mg daily [10], but our data suggest accelerated metabolism of important ART drugs at higher rifampicin doses. A clinical target value for dolutegravir trough concentrations has not been validated [14]. Using a 0.3-mg/L threshold [20, 24], nonattainment of the target in 39.1% of our higher-dose rifampicin patients appears concerning, but it is unknown how much the 0.3-mg/L exposure must be reduced in order to drive HIV treatment failure. Using a threshold of 0.064 mg/L, target nonattainment on higher-dose rifampicin was not significantly different from standard dosing. For patients on efavirenz-containing ART, we did not detect an association between rifampicin dose and attainment of the MEC of 1 mg/L, although HIV pharmacologists suggest that effective HIV suppression is maintained at even lower exposures [25, 26]. No patient with nonattainment of any dolutegravir or efavirenz threshold suffered HIV treatment failure. Overall, our data suggest that the pharmacokinetic effect of higher-dose rifampicin on dolutegravir and efavirenz exposure does not compromise HIV management. Larger studies based on HIV viral load measurements are required to confirm these findings.
In our trial, 14.4% of patients who provided a sample for analysis were failing HIV treatment at week 24, 80% of whom were on efavirenz, underlining the WHO-endorsed case for preferential first-line use of dolutegravir-based regimens [27].
Previous higher-dose rifampicin studies in Africa have mainly been conducted in patients without HIV or patients with HIV and CD4 counts >200 cells/µL [4, 5], avoiding populations at higher risk of drug-induced liver injury during TB treatment [28]. We have shown that, even in more vulnerable populations, higher-dose rifampicin does not increase the frequency of grade 3–4 drug-related adverse events. Provisional WHO endorsement [9] of 4-month anti-TB regimens that include optimized high-dose rifapentine [8] highlights the need to study higher rifamycin dosing effects in all relevant populations.
Our study was not powered to evaluate TB treatment outcomes. However, sputum culture positivity at 8 weeks was less on higher-dose rifampicin, consistent with prior studies [5–7]. It is arguable that reduced antiviral concentrations with preserved virological suppression are offset by gains in increased antimycobacterial activity and faster sputum clearance that may, in turn, reduce TB transmission.
Our study had limitations. Patients with concentrations that were BLQ were excluded from the primary pharmacokinetic analyses; missed dosing or mistiming of self-administered medications likely explains these observations. A sensitivity analyses provided results that were similar to those from the primary analyses. In addition, our sample size was too small to assess virologic efficacy and TB treatment outcomes definitively.
In conclusion, higher-dose rifampicin (35 mg/kg) reduced plasma exposure of dolutegravir and efavirenz without loss of virological control of HIV. Higher-dose rifampicin was well tolerated among patients with HIV on ART, indicating that it can be safely used in this population. Higher rates of sputum culture conversion support inclusion of people with HIV in larger efficacy trials of high-dose rifampicin.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. S. W., K. E. D., D. S., R. A., P. D., and M. L. contributed to protocol conceptualization. C. S. W. and D. S. wrote the article. C. S. W., R. N., B. O., F. A., A. B., J. N., D. O., and L. A. contributed to data collection. J. M., L. N., A. K., K. G., P. D., C. S. W., and R. N. contributed to data management and analysis. All authors read and approved the final version of the manuscript.
Acknowledgments. The authors acknowledge the contributions of the study participants, staff, and management team of the Infectious Diseases Institute.
Disclaimer. The views and opinions expressed here are the authors and do not necessarily state or reflect those of EDCTP.
Data sharing. The data that underlie the results of this study are available on request by contacting the corresponding author.
Financial support. This publication was produced as part of the SAfety and EFficacy in high-dose RIFampicin trial, which is part of the European and Developing Countries Clinical Trials Partnership-2 (EDCTP2) Programme supported by the European Union (grant TMA2016CDF-1580). Additional support was provided by a Global Challenges Research Fund award from the Scottish Funding Council, administered via the University of St. Andrews.
Supplementary Material
Contributor Information
Christine Sekaggya-Wiltshire, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda; Department of Medicine, Mulago National Referral Hospital, Kampala, Uganda.
Ruth Nabisere, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Joseph Musaazi, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Brian Otaalo, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Florence Aber, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Lucy Alinaitwe, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Juliet Nampala, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Letisha Najjemba, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Allan Buzibye, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Denis Omali, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Kamunkhwala Gausi, Department of Medicine, Division of Clinical Pharmacology, University of Cape Town, Cape Town, South Africa.
Allan Kengo, Department of Medicine, Division of Clinical Pharmacology, University of Cape Town, Cape Town, South Africa.
Mohammed Lamorde, Infectious Diseases Institute, Makerere University College of Health Sciences, Kampala, Uganda.
Rob Aarnoutse, Department of Pharmacy and Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegene, The Netherlands.
Paolo Denti, Department of Medicine, Division of Clinical Pharmacology, University of Cape Town, Cape Town, South Africa.
Kelly E Dooley, Department of Medicine, Division of Clinical Pharmacology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Derek J Sloan, Division of Infection and Global Health, School of Medicine, University of St. Andrews, St. Andrews, United Kingdom.
References
- 1. World Health Organization . Global tuberculosis report. Geneva Switzerland: WHO, 2021. https://www.who.int/publications/i/item/9789240037021. Accessed 2 February 2022. [Google Scholar]
- 2. World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. New York: WHO, 2021. Available at: https://www.who.int/publications/i/item/9789240031593. Accessed 2 February 2022. [PubMed] [Google Scholar]
- 3. Abu-Raddad LJ, Sabatelli L, Achterberg JT, et al. . Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A 2009; 106:13980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonnett LJ, Ken-Dror G, Koh G, Davies GR. Comparing the efficacy of drug regimens for pulmonary tuberculosis: meta-analysis of endpoints in early-phase clinical trials. Clin Infect Dis 2017; 65:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boeree MJ, Diacon AH, Dawson R, et al. . A step toward an optimized rifampin dose completed. Am J Respir Crit Care Med 2015; 192:525–6. [DOI] [PubMed] [Google Scholar]
- 6. Boeree MJ, Heinrich N, Aarnoutse R, et al. . High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Svensson EM, Svensson RJ, Te Brake LHM, et al. . The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018; 67:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorman SE, Nahid P, Kurbatova EV, et al. . Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med 2021; 384:1705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . WHO treatment of drug-susceptible tuberculosis: rapid communication. New York: WHO, 2021. Available at: https://www.who.int/publications/i/item/9789240028678. Accessed 2 February 2022. [Google Scholar]
- 10. Schutz C, Ismail Z, Proxenos CJ, et al. . Burden of antituberculosis and antiretroviral drug-induced liver injury at a secondary hospital in South Africa. S Afr Med J 2012; 102:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 2003; 42:819–50. [DOI] [PubMed] [Google Scholar]
- 12. Boulle A, Van Cutsem G, Cohen K, et al. . Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA 2008; 300:530–9. [DOI] [PubMed] [Google Scholar]
- 13. Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother 2006; 58:1299–302. [DOI] [PubMed] [Google Scholar]
- 14. Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013; 52:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dooley KE, Kaplan R, Mwelase N, et al. . Dolutegravir-based antiretroviral therapy for patients coinfected with tuberculosis and human immunodeficiency virus: a multicenter, noncomparative, open-label, randomized trial. Clin Infect Dis 2020; 70:549–56. [DOI] [PubMed] [Google Scholar]
- 16. Nabisere R, Musaazi J, Denti P, et al. . Pharmacokinetics, SAfety/tolerability, and EFficacy of high-dose RIFampicin in tuberculosis-HIV co-infected patients on efavirenz- or dolutegravir-based antiretroviral therapy: study protocol for an open-label, phase II clinical trial (SAEFRIF). Trials 2020; 21:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennetto-Hood C, Tabolt G, Savina P, et al. . A sensitive HPLC-MS/MS method for the determination of dolutegravir in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 945–946:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cresswell FV, Meya DB, Kagimu E, et al. . High-dose oral and intravenous rifampicin for the treatment of tuberculous meningitis in predominantly human immunodeficiency virus (HIV)-positive Ugandan adults: a phase II open-label randomized controlled trial. Clin Infect Dis 2021; 73:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scarsi KK, Darin KM, Nakalema S, et al. . Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz-based antiretroviral therapy: a three-arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 2016; 62:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Lunzen J, Maggiolo F, Arribas JR, et al. . Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012; 12:111–8. [DOI] [PubMed] [Google Scholar]
- 21. Barcelo C, Aouri M, Courlet P, et al. . Population pharmacokinetics of dolutegravir: influence of drug-drug interactions in a real-life setting. J Antimicrob Chemother 2019; 74:2690–7. [DOI] [PubMed] [Google Scholar]
- 22. Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 2001; 15:71–5. [DOI] [PubMed] [Google Scholar]
- 23. US Department of Health and Human Services. The common terminology criteria for adverse events, version 4.03. Bethesda, MD: National Institutes of Health. 14 June 2010. Available at:https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 2 February 2022. [Google Scholar]
- 24. Stellbrink HJ, Reynes J, Lazzarin A, et al. . Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS 2013; 27:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dickinson L, Amin J, Else L, et al. . Pharmacokinetic and pharmacodynamic comparison of once-daily efavirenz (400 mg vs. 600 mg) in treatment-naive HIV-infected patients: results of the ENCORE1 study. Clin Pharmacol Ther 2015; 98:406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orrell C, Bienczak A, Cohen K, et al. . Effect of mid-dose efavirenz concentrations and CYP2B6 genotype on viral suppression in patients on first-line antiretroviral therapy. Int J Antimicrob Agents 2016; 47:466–72. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization . Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. 2018. Available at: https://apps.who.int/iris/handle/10665/273632. Accessed 2 February 2022.
- 28. Yimer G, Gry M, Amogne W, et al. . Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: a prospective four arm observational study in Ethiopian patients. PLoS One 2014; 9:e94271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.