Abstract
Background
The COVID-19 pandemic was associated with historically low influenza circulation during the 2020–2021 season, followed by an increase in influenza circulation during the 2021–2022 US season. The 2a.2 subgroup of the influenza A(H3N2) 3C.2a1b subclade that predominated was antigenically different from the vaccine strain.
Methods
To understand the effectiveness of the 2021–2022 vaccine against hospitalized influenza illness, a multistate sentinel surveillance network enrolled adults aged ≥18 years hospitalized with acute respiratory illness and tested for influenza by a molecular assay. Using the test-negative design, vaccine effectiveness (VE) was measured by comparing the odds of current-season influenza vaccination in influenza-positive case-patients and influenza-negative, SARS-CoV-2–negative controls, adjusting for confounders. A separate analysis was performed to illustrate bias introduced by including SARS-CoV-2–positive controls.
Results
A total of 2334 patients, including 295 influenza cases (47% vaccinated), 1175 influenza- and SARS-CoV-2–negative controls (53% vaccinated), and 864 influenza-negative and SARS-CoV-2–positive controls (49% vaccinated), were analyzed. Influenza VE was 26% (95% CI: −14% to 52%) among adults aged 18–64 years, −3% (−54% to 31%) among adults aged ≥65 years, and 50% (15–71%) among adults aged 18–64 years without immunocompromising conditions. Estimated VE decreased with inclusion of SARS-CoV-2–positive controls.
Conclusions
During a season where influenza A(H3N2) was antigenically different from the vaccine virus, vaccination was associated with a reduced risk of influenza hospitalization in younger immunocompetent adults. However, vaccination did not provide protection in adults ≥65 years of age. Improvements in vaccines, antivirals, and prevention strategies are warranted.
Keywords: influenza, vaccine effectiveness, antigenic drift, SARS-CoV-2
During the 2021–2022 US influenza season, circulating A(H3N2) viruses were antigenically different than the vaccine. Vaccine effectiveness against hospitalized illness was 26% (95% CI: −14–52%) for adults 18–64-years and −3% (95% CI: −54–31) for adults ≥65-years.
The coronavirus disease 2019 (COVID-19) pandemic resulted in dramatic declines in global influenza virus circulation. The 2019–2020 US influenza season was characterized by early predominance of influenza B/Victoria lineage viruses from a newly emerged V1A.3 subclade [1], followed by an antigenically drifted A(H1N1)pdm09 virus [2]. During the early COVID-19 pandemic, the adoption of nonpharmaceutical interventions intended to reduce the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was associated with historically low influenza circulation, which continued through the 2020–2021 influenza season [3].
Some increase in circulation of influenza was observed during the 2021–2022 US influenza season, although circulation remained low compared with pre-pandemic years [4]. Most viruses belonged to the 2a.2 subgroup of the influenza A(H3N2) 3C.2a1b subclade [5]. This subgroup of A(H3N2) viruses is genetically similar to, but antigenically distinct from, the Northern Hemisphere 2021–2022 vaccine strain, which contains a 2a.1-like A(H3N2) component [6]. Influenza A(H3N2) viruses have typically been associated with reduced vaccine effectiveness (VE) due to antigenic mismatch, egg-adaptive mutations in the vaccine component, and age cohort effects in which older persons may have less protection against A(H3N2) viruses due to early exposures to non–A(H3N2) influenza viruses [7, 8].
Interim estimates of 2021–2022 influenza VE in the US Influenza Vaccine Effectiveness Network found low VE against medically attended influenza illness in outpatient settings due to these emerging H3N2 viruses [9]. However, subsequent evaluations have shown that, during periods of increased SARS-CoV-2 circulation, VE studies that enroll test-negative controls with acute respiratory illness (ARI) due to SARS-CoV-2 infection are likely to underestimate VE because use of these SARS-CoV-2–positive patients as controls may introduce bias due to the correlated likelihood of receiving influenza and SARS-CoV-2 vaccination [10, 11].
The Influenza and Other Viruses in the Acutely Ill (IVY) Network is a multistate network of hospitals that enrolls adults hospitalized with ARI to evaluate the effectiveness of influenza and COVID-19 vaccines. The objectives of this analysis were to evaluate the effectiveness of the 2021–2022 influenza vaccine against hospitalized influenza illness with the use of SARS-CoV-2–negative controls and to explore potential bias in VE associated with the use of SARS-CoV-2–positive controls.
METHODS
Participants and Sites
IVY is a surveillance network of 21 hospitals in 18 states that estimates VE against influenza and COVID-19 [12]. IVY sites enrolled hospitalized adults aged 18 years and older who met a prespecified ARI definition of having 1 or more of the following: fever, cough, shortness of breath, use of respiratory support for the acute illness, or new pulmonary findings on chest imaging consistent with pneumonia. Sites with 5 or more enrolled, laboratory-confirmed influenza cases during the 2021–2022 influenza season surveillance period contributed to this analysis and surveillance personnel at all sites were trained and followed a common surveillance protocol. From daily reviews of hospital admissions logs or electronic medical records, patients with ARI who received clinical testing for SARS-CoV-2 and/or influenza using a molecular or antigen assay were enrolled. Research upper respiratory specimens were also collected and shipped to Vanderbilt University Medical Center (Nashville, TN) for central reverse transcription–polymerase chain reaction (RT-PCR) testing for influenza and SARS-CoV-2 and respiratory syncytial virus (RSV). We included patients who received clinical testing within 10 days of illness onset and were admitted to the hospital within 14 days of illness onset. Enrollment began 31 January 2022, when RT-PCR testing was expanded in the surveillance protocol from exclusively SARS-CoV-2 to include influenza.
Case-patients were those with ARI who tested positive for influenza by molecular or antigen assay through clinical or molecular assay through research central laboratory testing. Control-patients had ARI and tested negative for influenza on all tests performed. Sites attempt to enroll all case-patients and target an enrollment ratio of test-positive cases (influenza, SARS-CoV-2, and/or RSV) to test-negative controls of 1:1, with randomly selected control-patients enrolled within 2 weeks of case-patients. Other than enrollment date and site, cases and controls were not matched based on individual characteristics.
At enrollment, information on patient demographics, clinical characteristics including symptoms and date of illness onset, and receipt of current season influenza vaccination was collected through patient or proxy interviews and in-depth reviews of medical records. Current-season influenza vaccination status was determined by electronic medical record and local Immunization Information System (IIS) searches performed around the time of patient enrollment or by plausible self-report that included the date and location of vaccine receipt. A patient was considered vaccinated if they received influenza vaccination on or after 1 August 2021, with a date of administration 14 days or more before illness onset. A patient was considered unvaccinated if they received no influenza vaccine doses between 1 August 2021 and the date of illness onset.
We excluded patients who (1) withdrew from the program; (2) did not receive influenza testing; (3) had an illness-onset date after admission (to exclude possible nosocomial cases) or more than 14 days before admission date; (4) received influenza testing before illness-onset date or more than 10 days after onset date for the index illness; (5) had influenza and SARS-CoV-2 coinfection; (6) were enrolled at a site that enrolled 5 or fewer patients with influenza during the surveillance period; (7) were enrolled as controls with testing before the first influenza case or after the last influenza case at a site; (8) had incomplete influenza vaccination status documentation; (9) received an influenza vaccine 0–13 days after illness onset; (10) had a reported history of influenza vaccine receipt by self-report only (ie, without verification through source documentation) and without an exact or approximate date and/or known location of vaccination; or (11) were missing covariate data on age, sex, or race/ethnicity used in VE models.
Laboratory Analysis
Standardized protocols were used for centralized pathogen RT-PCR testing at Vanderbilt University Medical Center and influenza virus sequencing at the University of Michigan (Supplementary Appendix B).
Statistical Analysis
We described demographic and clinical characteristics of influenza case-patients and test-negative controls, as well as vaccinated and unvaccinated patients, using counts and percentages or medians and interquartile ranges. Case versus control and vaccinated versus unvaccinated groups were compared using the Pearson's chi-square test for categorical variables or Wilcoxon rank-sum testing for continuous variables.
Logistic regression models were constructed to examine the association between influenza vaccination (primary exposure) and case status (outcome). Vaccine effectiveness was estimated by comparing the odds of vaccination in cases versus controls adjusting for prespecified potential confounders, calculated as VE = (1− adjusted odds ratio) × 100%. Potential confounders included age group (18–49, 50–64, ≥ 65 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic of any race, other or unknown), admitting hospital, and calendar time of admission (monthly intervals). In the primary VE model, we excluded SARS-CoV-2–positive controls due to the association between influenza and COVID-19 vaccine receipt [11]. To examine the influence of including SARS-CoV-2–positve controls, we also generated secondary VE estimates including both SARS-CoV-2–positive and SARS-CoV-2–negative controls. The primary analysis included patients who had vaccination status available by source documentation or self-report. In a sensitivity analysis, the population was restricted to patients with both source documentation and self-reported information for influenza vaccination. Furthermore, models were stratified by age group (18–64 and ≥65 years), presence of immunocompromising conditions, and time since vaccination. For time since vaccination, separate models were constructed comparing unvaccinated patients with patients vaccinated at 14–150 days before illness onset and unvaccinated patients with patients vaccinated more than 150 days before illness onset, with models adjusted for calendar month and other covariates in the primary VE model. Those without immunocompromising conditions were further stratified by age group. VE models stratified by age groups were adjusted for continuous age in years.
Finally, we examined in-hospital outcomes for influenza case-patients, including intensive care unit (ICU) admission, receipt of respiratory support including low-flow supplemental oxygen, high-flow oxygen, noninvasive ventilation, or invasive mechanical ventilation (IMV), and in-hospital death in vaccinated and unvaccinated patients. In-hospital outcomes were censored at 28 days from the date of admission if the patient was still hospitalized. Analyses were conducted using Stata version 16 (StataCorp, College Station, TX) [13]. This analysis was determined to be a public health surveillance activity by the Centers for Disease Control and Prevention (CDC) and each participating site and conducted in a manner consistent with applicable federal law and CDC policy.
RESULTS
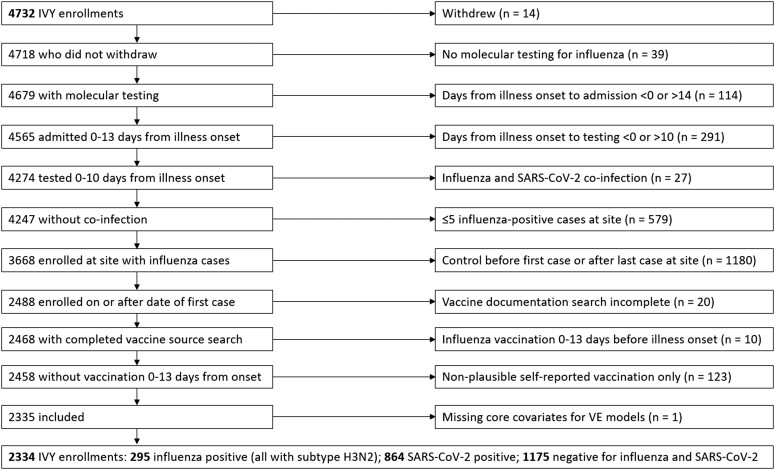
Overall, 4732 patients were enrolled during the surveillance period between 31 January 2022 and 15 June 2022 (Figure 1). Of these, 2398 (51%) were excluded from the analysis, with the most common reasons being enrolled as a control before the first or after the last influenza case at the site (n = 1180), 5 or fewer cases of influenza observed at the site (n = 579), or not receiving testing within the 10 days following illness onset (n = 291) (Figure 1). A total of 2334 patients were included in the analysis from 16 hospitals in 14 states (295 influenza cases, 1175 influenza- and SARS-CoV-2–negative controls, and 864 influenza-negative and SARS-CoV-2–positive controls for the secondary bias exploration analysis).
Figure 1.
Exclusion flowchart. Abbreviations: IVY, Influenza and Other Viruses in the Acutely Ill; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness.
For the primary analysis excluding SARS-CoV-2–positive patients (Table 1), the median age was 64 years, 756 (51%) patients were female, 829 (56%) non-Hispanic White, 303 (21%) non-Hispanic Black, 243 (17%) Hispanic of any race, and 1376 (94%) had 1 or more chronic underlying condition including 337 (23%) with an immunocompromising condition; 813 (55%) had 1 or more hospitalization in the prior year. Among influenza case-patients enrolled during the surveillance period, all cases (202) with subtype information were A(H3N2) viruses; 129 influenza A viruses were processed for sequencing, 114 (88%) of which yielded hemagglutinin sequences of sufficient quality for clade identification. All of these were A(H3N2) viruses within the 2a.2 subgroup of A(H3N2) 3C.2a1b viruses. The median number of influenza case-patients contributed by site in the analysis was 13.5 (interquartile range: 9.5–24.5).
Table 1.
Characteristics of Patients Vaccinated and Unvaccinated With Influenza Vaccines: IVY Network
| Overall | Influenza Vaccinateda |
Influenza Unvaccinated |
P b | |
|---|---|---|---|---|
| No. (%) | 1470 (100) | 761 (52) | 709 (48) | |
| Census region | ||||
| Northeast | 439 (30) | 212 (28) | 227 (32) | <.001 |
| South | 420 (29) | 199 (26) | 221 (31) | |
| Midwest | 322 (22) | 199 (26) | 123 (17) | |
| West | 289 (20) | 151 (20) | 138 (19) | |
| Baseline characteristics | ||||
| Demographics and behavioral risk factors | ||||
| ȃAge, median (IQR), years | 64 (53–74) | 67 (58–77) | 61 (47–70) | <.001 |
| ȃAge group | ||||
| ȃ18–49 years | 311 (21) | 115 (15) | 196 (28) | <.001 |
| ȃ50–64 years | 452 (31) | 213 (28) | 239 (34) | |
| ȃ65+ years | 707 (48) | 433 (57) | 274 (39) | |
| ȃFemale | 756 (51) | 418 (55) | 338 (48) | .005 |
| Race/ethnicity | ||||
| ȃWhite, non-Hispanic | 829 (56) | 491 (65) | 338 (48) | <.001 |
| ȃBlack, non-Hispanic | 303 (21) | 149 (20) | 154 (22) | |
| ȃHispanic, any race | 243 (17) | 78 (10) | 165 (23) | |
| ȃOther race, non-Hispanic | 69 (5) | 34 (4) | 35 (5) | |
| ȃUnknown | 26 (2) | 9 (1) | 17 (2) | |
| ȃInsured | 1422 (97) | 755 (99) | 667 (94) | <.001 |
| ȃCurrent tobacco smoking | 261/1331 (20) | 97/697 (14) | 164/634 (26) | <.001 |
| Health status indicatorsc | ||||
| ȃ≥1 Underlying chronic medical condition | 1376 (94) | 731 (96) | 645 (91) | <.001 |
| ȃChronic respiratory condition | 615 (42) | 360 (47) | 255 (36) | <.001 |
| ȃChronic immunocompromising conditiond | 337 (23) | 207 (27) | 130 (18) | <.001 |
| ȃ≥1 Hospitalization in prior year | 813/1469 (55) | 467/760 (61) | 346/709 (49) | <.001 |
| Admission characteristics | ||||
| Days from illness onset to testing, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (0–4) | .025 |
| Days from illness onset to hospitalization, median (IQR) | 2 (0–3) | 2 (0–4) | 1 (0–3) | .016 |
Data are presented as n (%) unless otherwise indicated. Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; IVY, Influenza and Other Viruses in the Acutely Ill.
Vaccination defined as documented and/or plausible self-report of influenza vaccine received 1 August 2021 or later.
P value for test of difference across case and control groups based on chi-square statistic for categorical variables and Wilcoxon rank-sum test for continuous variables.
Health indicators obtained through chart review and included chronic cardiovascular, neurological, pulmonary, gastrointestinal, endocrine, renal, hematologic, autoimmune, or immunocompromising conditions.
Immunocompromising conditions include any of the following: active solid-organ cancer, active hematologic cancer, solid-organ transplant, bone marrow/stem cell transplant, HIV infection, congenital immunodeficiency syndrome, use of an immunosuppressive medication within the past 30 days, splenectomy, graft-versus-host disease (currently or in the past), or any other condition that causes moderate or severe immunosuppression.
Current-season influenza vaccination was received by 139 of 295 (47%) influenza case-patients, 622 of 1175 (53%) influenza- and SARS-CoV-2–negative controls, and 424 of 864 (49%) influenza-negative but SARS-CoV-2–positive controls. Restricting patients to influenza cases and primary controls (both influenza- and SARS-CoV-2–negative) (Table 2), the median time from influenza vaccination to illness onset among those who received vaccination was 166 days (interquartile range: 129–200 days). Cases and controls were similar with respect to most baseline characteristics but differed in race and ethnicity distribution (P = .003) and had slightly longer delays from illness onset to hospital admission and influenza testing (P < .05 for both). Among cases and controls combined, those who received influenza vaccination were older (median age: 67 vs 61 years; P < .001), more likely to be non-Hispanic White (65% vs 48%; P < .001 for race/ethnicity distribution), more likely to have 1 or more underlying medical condition (96% vs 91%; P < .001) including immunocompromising conditions (27% vs 18%; P < .001), and more likely to have 1 or more hospitalization in the prior year (61% vs 49%; P < .001) (Table 1).
Table 2.
Characteristics of Influenza-Positive Patients (Cases) and Influenza-Negative Patients (Controls): IVY Network
| Overall | Influenza-Positive | Influenza-Negative | P a | |
|---|---|---|---|---|
| No. (%) | 1470 (100) | 295 | 1175 | |
| Census region | ||||
| ȃNortheast | 439 (30) | 81 (27) | 358 (30) | .29 |
| ȃSouth | 420 (29) | 79 (27) | 341 (29) | |
| ȃMidwest | 322 (22) | 66 (22) | 256 (22) | |
| ȃWest | 289 (20) | 69 (23) | 220 (19) | |
| Baseline characteristics | ||||
| Demographics and behavioral risk factors | ||||
| Age, median (IQR), years | 64 (53–74) | 66 (51–76) | 64 (53–73) | .28 |
| Age group | ||||
| ȃ18–49 years | 311 (21) | 70 (24) | 241 (21) | .009 |
| ȃ50–64 years | 452 (31) | 69 (23) | 383 (33) | |
| ȃ65+ years | 707 (48) | 156 (53) | 551 (47) | |
| ȃFemale | 756 (51) | 157 (53) | 599 (51) | .49 |
| Race/ethnicity | ||||
| ȃWhite, non-Hispanic | 829 (56) | 142 (48) | 687 (58) | .003 |
| ȃBlack, non-Hispanic | 303 (21) | 63 (21) | 240 (20) | |
| ȃHispanic, any race | 243 (17) | 63 (21) | 180 (15) | |
| ȃOther race, non-Hispanic | 69 (5) | 22 (7) | 47 (4) | |
| ȃUnknown | 26 (2) | 5 (2) | 21 (2) | |
| ȃInsured | 1422 (97) | 282 (96) | 1140 (97) | .22 |
| ȃCurrent tobacco smoking | 261/1331 (20) | 54/269 (20) | 207/1062 (19) | .83 |
| Health status indicatorsb | ||||
| ≥1 Underlying chronic medical condition | 1376 (94) | 275 (93) | 1101 (94) | .76 |
| Chronic respiratory condition | 615 (42) | 124 (42) | 491 (42) | .94 |
| Chronic immunocompromising conditionc | 337 (23) | 62 (21) | 275 (23) | .38 |
| ≥1 Hospitalization in prior year | 813/1469 (55) | 149/295 (51) | 664/1174 (57) | .062 |
| Admission characteristics | ||||
| Days from illness onset to testing, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (0–4) | .023 |
| Days from illness onset to hospitalization, median (IQR) | 2 (0–3) | 2 (1–4) | 1 (0–3) | <.001 |
Data are presented as n (%) unless otherwise indicated. Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; IVY, Influenza and Other Viruses in the Acutely Ill.
P value for test of difference across case and control groups based on chi-square statistic for categorical variables and non-parametric Wilcoxon rank-sum test for continuous variables.
Health indicators obtained through chart review and included chronic cardiovascular, neurological, pulmonary, gastrointestinal, endocrine, renal, hematologic, autoimmune, or immunocompromising conditions.
Immunocompromising conditions include any of the following: active solid-organ cancer, active hematologic cancer, solid-organ transplant, bone marrow/stem cell transplant, HIV infection, congenital immunodeficiency syndrome, use of an immunosuppressive medication within the past 30 days, splenectomy, graft-versus-host disease (currently or in the past), or any other condition that causes moderate or severe immunosuppression.
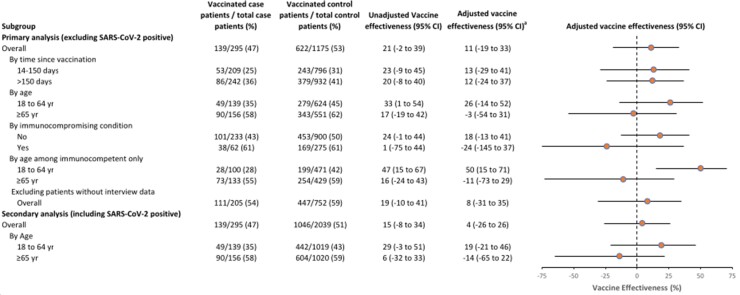
Overall VE against influenza-associated hospitalizations in the primary analysis (ie, excluding SARS-CoV-2–positive patients) was 11% (95% confidence interval [CI]: −19% to 33%). In stratified analyses, VE was 26% (95% CI: −14% to 52%) among all adults aged 18–64 years and −3% (95% CI: −54% to 31%) among adults 65 years of age and older. Vaccine effectiveness was 18% (95% CI: −13% to 41%) among all patients without immunocompromising conditions and −24% (95% CI: −145% to 37%) among patients with immunocompromising conditions (Figure 2). In contrast, VE was 50% (95% CI: 15% to 71%) among younger adults (18–64 years) without immunocompromising conditions. No evidence of waning vaccine protection was observed during the season, with VE similar for those vaccinated 14–150 days and more than 150 days prior to illness onset. Post hoc analyses found similar findings restricted to patients tested for influenza within 5 days of illness onset (overall VE: 10%; 95% CI: −23% to 34%) and adjusting for calendar week rather than calendar month (overall VE: 10%; 95% CI: −20% to 32%).
Figure 2.
Unadjusted and adjusted influenza vaccine effectiveness, IVY Network. aAdjusted for calendar time (in monthly intervals), enrollment site, age, female sex, and race and ethnicity. Abbreviations: CI, confidence interval; IVY, Influenza and Other Viruses in the Acutely Ill; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; yr, years.
In a secondary analysis evaluating the magnitude of potential bias associated with including SARS-CoV-2–positive patients as controls (representing 864/2039 [42%] of potential influenza-negative controls), we observed a downward bias in estimated overall VE compared with the primary analysis (4% vs 11%) among patients aged 18–64 years (19% vs 26%) and among patients aged 65 years and older (−14% vs −3%).
Among 291 of 295 (99%) influenza case-patients with available in-hospital outcomes, 38 (13%) were admitted to the ICU, 176 (60%) received any supplemental oxygen support including 12 (4%) receiving IMV, and 6 (2%) died in-hospital within 28 days of admission (Table 3). A lower proportion of vaccinated compared with unvaccinated case-patients required ICU admission (9% vs 17%; P = .045).
Table 3.
In-Hospital Outcomes of Patients Hospitalized With Influenza Infection (Cases) Who Were Vaccinated and Unvaccinated
| Characteristic | Overall (N = 291) | Vaccinated (n = 136) | Unvaccinated (n = 155) | P |
|---|---|---|---|---|
| Intensive care unit admission | 38 (13) | 12 (9) | 26 (17) | .045 |
| Low-flow supplemental oxygen | 176 (60) | 79 (58) | 97 (63) | .43 |
| High-flow nasal cannula | 19 (7) | 7 (5) | 12 (8) | .37 |
| Noninvasive positive-pressure ventilation | 28 (10) | 14 (10) | 14 (9) | .72 |
| Invasive mechanical ventilation | 12 (4) | 4 (3) | 8 (5) | .34 |
| Vasopressor receipt | 15 (5) | 5 (4) | 10 (6) | .29 |
| In-hospital death within 28 days | 6 (2) | 2 (1) | 4 (3) | .51 |
Data are presented as n (%).
DISCUSSION
During the 2021–2022 season when a subclade of A(H3N2) viruses predominated that were antigenically distinct from the vaccine virus, we found no significant effectiveness of seasonal influenza vaccine against influenza hospitalization among older adults and adults with immunocompromising conditions. However, modest VE was observed among younger (18–64 years), non-immunocompromised adults, even in the setting of drifted A(H3N2) viruses. These findings highlight heterogeneity across adult populations and limits to the generalizability of pooled VE estimates to the broader population. They also suggest a general need for improved influenza vaccines and therapeutics as well as a potential role for nonpharmaceutical prevention strategies during periods of high influenza circulation.
Our findings align with repeated observations of relatively low VE against influenza A(H3N2) virus infection [14, 15] and hospitalization [16] during recent influenza seasons. Low overall VE against A(H3N2) may be due to the vaccine strain being antigenically distinct from the clade of the subsequently circulating virus [17], as well as possibly ongoing egg adaptation mutations in A(H3N2) during vaccine manufacturing, such that the vaccine strain may elicit antibodies to sites that are unavailable in circulating viruses [17, 18]. In addition, we found moderate VE in younger adults without immunocompromising conditions but null VE for persons aged 65 years and older that cannot be explained by differences in virus subtype since nearly all infections were related to the same 2a.2 subclade. This finding could be related to factors that affect A(H3N2)-specific immune response to vaccination among younger versus older adults. The concept of original antigenic sin suggests that the human body will condition, or “imprint,” antibody response to the first influenza virus infection that one experiences in life [19]. Older adults were imprinted with A(H1N1) since it was the only subtype circulating in humans from 1918–1957; consequently, their immune response may be biased toward A(H1N1) and away from A(H3N2) viruses, which emerged in 1968 [8]. In contrast, younger adults were more likely to have been imprinted by A(H3N2) viruses. Other factors for the lower VE in older adults might be related to immune senescence [20], “inflamm-aging” [21] (ie, a chronic low-level state of inflammation from multiple underlying conditions and frailty suspected to impair immune responses), and diminished effects of repeated vaccination that might occur among older adults [22]. Differences in vaccine type are unlikely to explain lower VE in older US adults who typically receive high-dose inactivated influenza vaccine as compared with younger US adults who largely receive the standard-dose vaccine [23].
This study examined VE both with and without the inclusion of patients with SARS-CoV-2 as controls. A recent simulation study has shown that the inclusion of SARS-CoV-2–positive controls can bias influenza VE estimates downwards due to correlation between COVID-19 and influenza vaccination coverage [11]. In test-negative VE studies, including SARS-CoV-2–positive patients among controls for influenza cases may introduce confounding bias when COVID-19 and influenza vaccine receipt are strongly correlated [10]. Because COVID-19 vaccines are protective, controls with COVID-19 are less likely to be vaccinated against COVID-19 compared with the source population. If COVID-19 and influenza vaccination rates are strongly correlated, including patients with COVID-19 as controls in influenza VE studies will enrich the control population with participants who are unvaccinated against influenza compared with the source population from which the cases were generated, and thus bias VE down. Doll et al [10] found that, while this bias was low when patients with COVID-19 represented 10% or less of controls, higher proportions of controls who were SARS-CoV-2–positive likely increased the magnitude of the bias. This analysis provides a practical illustration of this problem, showing a nearly 10% difference in point estimates for VE when SARS-CoV-2–positive patients were included as controls, indicating that investigators should consider excluding SARS-CoV-2–positive controls from future VE analyses.
This analysis was subject to several limitations. First, enrollment did not start until several months into the 2021–2022 influenza season (influenza activity was observed in the United States by October 2021 [24], although activity decreased in December 2021 with the emergence of the SARS-CoV-2 Omicron variant before increasing again in February 2022), the median time from influenza vaccination to illness onset was more than 5 months, and the influenza season was long, with ongoing circulation at many sites through June 2022. This may have led to lower VE in the setting of waning vaccine protection [25], although evidence of waning was not observed. Second, vaccination coverage among hospitalized adults was lower than typically observed in prior influenza seasons [26]. Under-capture of influenza vaccination could lead to VE estimates biased toward the null. However, the COVID-19 pandemic as well as lower influenza activity in the United States since 2020 may have influenced vaccination coverage [11]. Further, among patients with patient or proxy interview data, influenza vaccination coverage was generally similar to that in the full cohort. A sensitivity analysis including only patients with interview data yielded similar VE estimates, lessening concerns about the primary results being heavily biased by underreporting of vaccination coverage. Third, low levels of influenza activity limited statistical power. Fourth, although the analysis controlled for several relevant potential confounders such as calendar time and geographic location, residual confounding is possible. Patients hospitalized with ARI are predominantly older adults and have multiple underlying medical conditions. Therefore, findings may not be generalizable to the entire US population. Finally, influenza clinical testing practice may have varied across participating sites, resulting in a varied number of influenza-positive cases. However, selection bias is not substantial because all patients met ARI criteria and testing is unlikely to be influenced by vaccination status [27]. In addition, all upper respiratory specimens were tested centrally for influenza by RT-PCR.
CONCLUSIONS
During a season where circulating influenza A(H3N2) was antigenically different from the vaccine virus, we did not observe significant overall VE against hospitalized influenza, but some protection was seen among younger, non-immunocompromised adults. Vaccine effectiveness was lowest in older adults in whom severe influenza burden is substantially higher, warranting strategies such as improvements in vaccines, antivirals, and other prevention strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (grant number 75D30121F00002). The REDCap data tool was supported by a Clinical and Translational Science Award (UL1 TR002243) from the National Center for Advancing Translational Sciences.
Supplementary Material
Contributor Information
Mark W Tenforde, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Manish M Patel, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Nathaniel M Lewis, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine Adams, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Manjusha Gaglani, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
Jay S Steingrub, Department of Medicine, Baystate Medical Center, Springfield, Massachusetts, USA.
Nathan I Shapiro, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Abhijit Duggal, Department of Medicine, Cleveland Clinic, Cleveland, Ohio, USA.
Matthew E Prekker, Departments of Emergency Medicine and Medicine, Hennepin County Medical Center, Minneapolis, Minnesota, USA.
Ithan D Peltan, Department of Medicine, Intermountain Medical Center, Murray, Utah and University of Utah, Salt Lake City, Utah, USA.
David N Hager, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michelle N Gong, Department of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, USA.
Matthew C Exline, Department of Medicine, The Ohio State University, Columbus, Ohio, USA.
Adit A Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Nicholas M Mohr, Department of Emergency Medicine, University of Iowa, Iowa City, Iowa, USA.
Christopher Mallow, Department of Medicine, University of Miami, Miami, Florida, USA.
Emily T Martin, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
H Keipp Talbot, Departments of Medicine and Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kevin W Gibbs, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
Jennie H Kwon, Department of Medicine, Washington University, St Louis, Missouri, USA.
James D Chappell, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Natasha Halasa, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Adam S Lauring, Departments of Internal Medicine and Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan, USA.
Christopher J Lindsell, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Sydney A Swan, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kimberly W Hart, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kelsey N Womack, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Adrienne Baughman, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carlos G Grijalva, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Wesley H Self, Vanderbilt Institute for Clinical and Translational Research and Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1. Owusu D, Hand J, Tenforde MW, et al. . Early season pediatric influenza B/Victoria virus infections associated with a recently emerged virus subclade—Louisiana, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tenforde MW, Kondor RJG, Chung JR, et al. . Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clin Infect Dis 2021; 73:e4244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. . Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Fluview past weekly surveillance reports 2020–2021. Available at: https://www.cdc.gov/flu/weekly/pastreports.htm. Accessed 13 July 2022.
- 5. Delahoy MJ, Mortenson L, Bauman L, et al. . Influenza A(H3N2) outbreak on a University Campus—Michigan, October–November 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1712–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Recommendations announced for influenza vaccine composition for the 2022–2023 Northern Hemisphere influenza season. 2022. Available at: https://www.who.int/news/item/25-02-2022-recommendations-announced-for-influenza-vaccine-composition-for-the-2022-2023-northern-hemisphere-influenza-season. Accessed 18 August 2022.
- 7. Russell K, Chung JR, Monto AS, et al. . Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine 2018; 36:1272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis 2017; 215:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung JR, Kim SS, Kondor RJ, et al. . Interim estimates of 2021–22 seasonal influenza vaccine effectiveness—United States, February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doll MK, Pettigrew SM, Ma J, Verma A. Effects of confounding bias in COVID-19 and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis 2022; 75:e564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leuchter RK, Jackson NJ, Mafi JN, Sarkisian CA. Association between COVID-19 vaccination and influenza vaccination rates. N Engl J Med 2022; 386:2531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams K, Rhoads JP, Surie D, et al. . Vaccine effectiveness of primary series and booster doses against Covid-19 associated hospital admissions in the United States: living test negative design study. BMJ 2022; 379:e072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. StataCorp . Stata Statistical Software: release 17. College Station, TX: StataCorp LLC, 2021. [Google Scholar]
- 14. Rizzo C, Gesualdo F, Loconsole D, et al. . Moderate vaccine effectiveness against severe acute respiratory infection caused by A(H1N1)pdm09 influenza virus and no effectiveness against A(H3N2) influenza virus in the 2018/2019 season in Italy. Vaccines 2020; 8:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valenciano M, Kissling E, Reuss A, et al. . The European I-MOVE multicentre 2013–2014 case-control study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2). Vaccine 2015; 33:2813–22. [DOI] [PubMed] [Google Scholar]
- 16. Martin ET, Cheng C, Petrie JG, et al. . Low influenza vaccine effectiveness against A(H3N2)-associated hospitalizations in 2016–2017 and 2017–2018 of the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2021; 223:2062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flannery B, Zimmerman RK, Gubareva LV, et al. . Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis 2016; 214:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skowronski DM, Chambers C, De Serres G, et al. . Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infect Dis 2017; 215:1059–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gostic KM, Bridge R, Brady S, Viboud C, Worobey M, Lloyd-Smith JO. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog 2019; 15:e1008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McElhaney JE, Verschoor CP, Andrew MK, et al. . The immune response to influenza in older humans: beyond immune senescence. Immun Ageing 2020; 17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 2009; 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belongia EA, Skowronski DM, McLean HQ, et al. . Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:723–36. [DOI] [PubMed] [Google Scholar]
- 23. Izurieta HS, Lu M, Kelman J, et al. . Comparative effectiveness of influenza vaccines among US Medicare beneficiaries ages 65 years and older during the 2019–2020 season. Clin Infect Dis 2021; 73:e4251–9. [DOI] [PubMed] [Google Scholar]
- 24. Merced-Morales A, Daly P, Abd Elal AI, et al. . Influenza activity and composition of the 2022–23 influenza vaccine—United States, 2021–22 season. MMWR Morb Mortal Wkly Rep 2022; 71:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferdinands JM, Gaglani M, Martin ET, et al. . Waning vaccine effectiveness against influenza-associated hospitalizations among adults, 2015–2016 to 2018–2019, United States hospitalized adult influenza vaccine effectiveness network. Clin Infect Dis 2021; 73:726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tenforde MW, Chung J, Smith ER, et al. . Influenza vaccine effectiveness in inpatient and outpatient settings in the United States, 2015–2018. Clin Infect Dis 2021; 73:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldstein LR, Ferdinands JM, Self WH, et al. . Modeling the impacts of clinical influenza testing on influenza vaccine effectiveness estimates. J Infect Dis 2021; 224:2035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.