Abstract
Aims
Although myocardial scar assessment using late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) imaging is frequently indicated for patients with implantable cardioverter defibrillators (ICDs), metal artefact can degrade image quality. With the new wideband technique designed to mitigate device related artefact, CMR is increasingly used in this population. However, the common clinical indications for CMR referral and impact on clinical decision-making and prognosis are not well defined. Our study was designed to address these knowledge gaps.
Methods and results
One hundred seventy-nine consecutive patients with an ICD (age 59 ± 13 years, 75% male) underwent CMR using cine and wideband pulse sequences for LGE imaging. Electronic medical records were reviewed to determine the reason for CMR referral, whether there was a change in clinical decision-making, and occurrence of major adverse cardiac events (MACEs). Referral indication was the most common evaluation of ventricular tachycardia (VT) substrate (n = 114, 64%), followed by cardiomyopathy (n = 53, 30%). Overall, CMR resulted in a new or changed diagnosis in 64 (36%) patients and impacted clinical management in 51 (28%). The effect on management change was highest in patients presenting with VT. A total of 77 patients (43%) experienced MACE during the follow-up period (median 1.7 years), including 65 in patients with evidence of LGE. Kaplan–Meier analysis showed that ICD patients with LGE had worse outcomes than those without LGE (P = 0.006).
Conclusion
The clinical yield from LGE CMR is high and provides management changing and meaningful prognostic information in a significant proportion of patients with ICDs.
Keywords: cardiac magnetic resonance, late gadolinium enhancement, implantable cardioverter defibrillators
Graphical Abstract
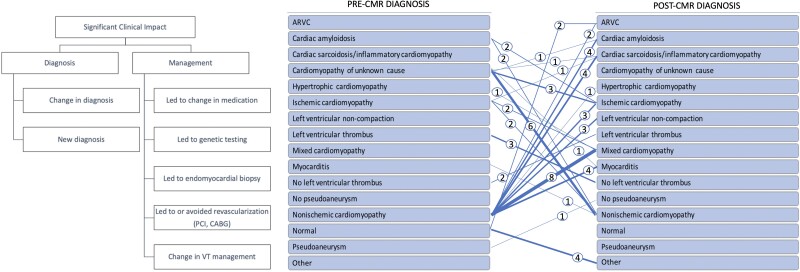
Graphical Abstract.
Left: Definition of significant clinical impact. Right: Change in diagnosis after CMR study.
See the editorial comment for this article ‘Wideband cardiac magnetic resonance for myocardial tissue characterization in patients with implantable cardioverter defibrillators (ICDs): comment on Patel et al.'s Impact of wideband cardiac magnetic resonance on diagnosis, decision-making, and outcomes in patients with ICD’, by B.L. Gerber, https://doi.org/10.1093/ehjci/jeac230.
Introduction
Cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) imaging is frequently employed in the assessment of patients with known or suspected cardiac disease. Well recognized as the reference standard for assessing cardiac function, CMR also has the ability to characterize myocardial tissue properties and thus provide insight in the determination of cardiomyopathy aetiologies and aid in the assessment of arrhythmogenic substrate burden by LGE.
Although the clinical utility of CMR has been extensively studied, it has only recently been shown that CMR can be safely performed in most patients with implantable cardioverter defibrillators (ICDs), including legacy devices that have not been formally designated for conditional use in the magnetic resonance environment,1–3 even in the presence of abandoned or retained leads.4 Moreover, newer pulse sequences, ensuring the ability to acquire high-quality LGE images free of significant ICD-associated imaging artefacts, are now validated across a spectrum of devices and scanner types.5,6 To date, only a handful of studies have reported upon the clinical impact of CMR imaging in patients with ICDs.7–9 Thus, the clinical and prognostic utility of CMR in this important cohort of patients has yet to be established. Elucidation of the clinical value of a CMR exam in these patients is relevant, given that an estimated 50% to 75% of patients with an ICD will have a potential indication for at least one CMR examination during their lifetime.10,11
Often, the aetiology of a cardiomyopathy or sudden cardiac death is not fully defined prior to ICD implantation. In this setting, it is unknown how often CMR imaging may change the clinical diagnosis or management. Furthermore, since patients with ICD can develop ventricular tachycardia, there may be a need to determine the location and burden of myocardial scar to guide ablation approach for ventricular tachycardia (VT).12 CMR additionally plays a role in predicting the post-ablation long-term VT-free survival.13 Recent studies showed that the use of wideband LGE significantly improves image quality, compared to conventional LGE imaging, and can accurately localize myocardial scar,5,14 but artefact reduction by the use of wideband LGE is not uniform across the different common types of ICD.15
Accordingly, our current study aimed to determine in patients with an ICD: (i) the current referral patterns of CMR, (ii) how often CMR results alter the clinical diagnosis, (iii) whether CMR impacts clinical decision-making, and (iv) whether CMR findings remain prognostically significant even in the presence of advanced heart disease already treated with an ICD.
Methods
Data and materials used in this study will not be made publicly available.
Study protocol
We identified from our CMR registry 179 patients with ICDs who underwent clinically indicated CMR examinations including LGE imaging with the wideband technique between February 2016 and July 2020. The study was approved by the Institutional Review Board, and each patient signed an informed consent to be included in the registry. In patients with repeat/follow-up examinations, only the initially performed CMR dataset was used for analysis. Inclusion criteria were as follows: (i) age >18 years, (ii) glomerular filtration rate >30 mL/min/1.73 m2, and (iii) ability to tolerate CMR protocol. In accordance with the institutional policy during the above time period, patients with the following conditions were excluded from undergoing clinical CMR examination: (i) ongoing unstable ventricular arrhythmia, (ii) ICD implantation within 30 days, or (iii) abandoned and/or retained leads or suspected lead malfunction and/or fracture. The approach to our screening process and safety algorithm (before, during, and after CMR) followed Heart Rhythm Society recommendations. Specifically, a chest X-ray was performed prior to the CMR examination to assess the location and number of leads, and a pre- and post-CMR device interrogation was performed to ensure stability of ICD parameters and inhibit inappropriate tachyarrhythmia therapies during imaging. Patients were continuously monitored by an advanced cardiac life support certified advanced nurse practitioner or physician, with continuous heart rhythm monitoring using the CMR vector-gating system in conjunction with pulse oximetry and blood pressure assessments every 5 min. No issues with ICD parameters were identified using post-study interrogation.
Clinical data including demographics, medical history, indication for CMR referral, whether there was a change in clinical diagnosis/decision-making, and outcomes were obtained from the patient and from the electronic medical records. Diagnostic utility and impact on clinical management were assessed by consensus of experts (detailed below).
CMR imaging protocol and analysis
All CMR studies were performed using a 1.5 T scanner (Achieva, Philips) with a five-channel surface coil using a standardized protocol, inclusive of cine and LGE imaging using the wideband technique as previously described.5 When possible, the protocol involved raising the arm above the head on the ipsilateral side of the ICD to increase the distance between the pulse generator and the heart in order to minimize imaging artefacts. The wideband LGE protocol modified the standard inversion radiofrequency (RF) pulse bandwidth from the vendor’s default of 1.8 kHz setting to a fixed 3.8 kHz, based on previously published works.6,15 This modified inversion RF was used in three CMR pulse sequences: first, in a series of pre-contrast wideband inversion RF scans to determine the optimal wideband RF centre frequency shift for minimizing the extent of exhibited device artefacts; second, in a wideband LGE Look-Locker T1 scout to determine the optimal inversion time for myocardial nulling, and lastly for wideband LGE imaging of the suspected myocardial scar. The pre-contrast images were acquired in the four- and two-chamber views at three frequency shifts: −1500, 0, and +1500 Hz. The frequency shift with minimal ICD-related artefact was visually identified and selected as optimal frequency shift. Next, a gadolinium-based contrast agent was administered (0.1– 0.2 mmol/kg, depending upon renal function), followed by short-axis cine images spanning the left ventricle (LV) from base to apex (temporal resolution ∼40 ms) using either a segmented steady-state free precession or gradient recalled echo technique. Ten minutes after contrast administration, wideband LGE images with a phase sensitive reconstruction were acquired in the two-, three-, and four-chamber views, as well as in the short-axis stack, using the selected optimal frequency shift and inversion time. Images were acquired using the following parameters: acquisition matrix: 192 × 192; voxel size: 2 × 2 × 10 mm; TR/TE (repetition time and echo time) = 4.1–4.5/2.0–2.2 ms; default bandwidth per pixel = 479 Hz/pixel; flip angle: 30°; and using gradient echo pulse sequence with parallel imaging (SENSE) acceleration factor of R = 2.
All CMR studies were analysed off-line using a workstation with a dedicated software (Medis Suite, Leiden, Netherlands). LV and right ventricular (RV) volume, mass, and ejection fraction (EF) were measured by use of the method of disks applied to the short axis stack of cine images. LV endocardial and epicardial borders on cine images were manually traced to define the myocardium, taking care to exclude papillary muscles and the intertrabecular blood pool. Volumes and mass were normalized to body surface area.
LGE analysis
The presence and/or absence of LGE was visually determined on post-contrast images by a level 3 trained CMR expert. LGE was defined as having a signal intensity greater than 5 SD of the normal remote myocardium and confirmed on either two contiguous slices or in two distinct imaging planes. When possible, oblique images were used to confirm that an area of suspected LGE was not due to partial volume effect from the LV outflow tract or other structure. The predominant patterns of LGE (used for categorizing diagnosis) were recorded as follows: (i) subendocardial (n = 68), (ii) mid-wall (n = 30), (iii) subepicardial (n = 15), (iv) combined (ischaemic and nonischaemic pattern, n = 6), (v) diffuse (n = 11), (vi) patchy (n = 12), and (vii) RV insertion points (n = 9), including patients with more than one of these patterns. In addition, LGE was quantified per slice of myocardium by manually tracing around its visually apparent perimeter, with the total LGE area of all slices summed over the entire LV myocardium to calculate total LGE burden.
Artefacts were assessed in each short-axis slice for wideband LGE images, and each slice was interrogated for the presence of device-related artefacts (off-resonance, signal void, or susceptibility). Studies without device-related artefact affecting the myocardium were categorized as ‘no artefact’.
Assessment of CMR impact on clinical diagnosis, management, and outcomes
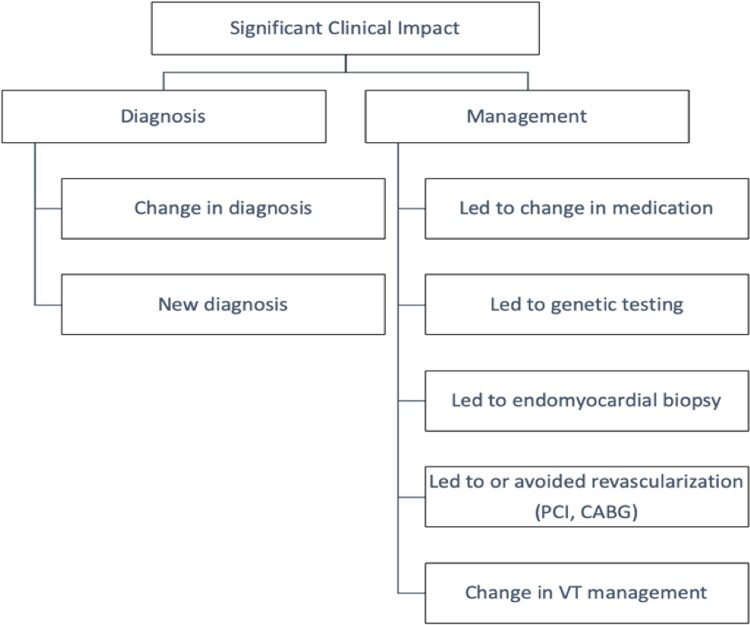
Designations of ‘significant clinical impact’ of CMR were defined prior to data collection and evaluated by direct review of electronic medical records (Figure 1). Significant clinical impact criteria were met in the presence of a ‘changed or new diagnosis’, or if a ‘change in management’ occurred. Two physicians blinded to ascertainment of ‘significant clinical impact’ independently interpreted a random sample of the patients. A third physician adjudicated any discrepancy between the interpreters.
Figure 1.
Definition of significant clinical impact.
Changed or new diagnosis/change in management
A ‘new diagnosis’ was defined as occurring only if it was previously unknown by the referring healthcare team. For example, a patient referred for CMR assessment of non-ischaemic cardiomyopathy found instead to have evidence of myocardial infarction would be defined as having had a change in diagnosis. Another example for a new diagnosis would be detection of a previously unknown LV thrombus. For CMR-based imaging, CAD was considered present if sub-endocardial based LGE was identified in a typical coronary artery distribution.
‘Change in Management’ was defined as occurring when one of the following criteria were met: CMR results led directly to either the performance or avoidance of an invasive procedure [such as endomyocardial biopsy, angiography, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or ablation]; CMR findings led to a change in ablation approach; CMR findings led to changes in medication; or CMR findings led to genetic testing of the potential proband or cascade family screening (Figure 1).
A patient with both ‘changed or new diagnosis’ and ‘change in management’ (e.g. when CMR findings led to diagnosis of intracardiac thrombus with subsequent initiation of anticoagulation) was only counted once towards ‘significant clinical impact’.
Clinical outcomes
Clinical outcomes were defined as a composite endpoint of all-cause death and major adverse cardiovascular events (MACEs): cardiovascular death, recurrent ventricular arrhythmia, heart transplantation, CABG, and LV assist device implantation. When more than 1 event occurred in a patient, the first event was used.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation if normally distributed or as median and interquartile range if not normally distributed. Categorical variables were expressed as frequency and percentages. Intergroup comparisons were performed using Student t-tests or Mann–Whitney U tests for numeric variables and χ2 tests of association or Fisher exact tests for categorical variables, as appropriate. The area under the curve in the receiver operating characteristic (ROC) analysis was estimated to determine the optimal cut-off point for LGE area to predict clinical outcome. Kaplan–Meier analysis plotted MACE survival curves according to the presence of LGE, burden of LGE, and LVEF, and compared using the log-rank test. All P-values were two-sided, and a P-value ≤0.05 was considered statistically significant. All statistical analyses were performed using STATA version 16 (Stata Corporation, College Station, TX, USA).
Results
Baseline clinical characteristics
Baseline characteristics are summarized in Table 1. Seventy-seven (43%) patients had ICD implantation for secondary prevention. Of the 179 study patients, 119 had pacemaker ICD, 47 cardiac resynchronization therapy ICD, and 13 subcutaneous devices. Overall, image quality was good. One hundred thirty studies had no significant device-related artefact (73%); 40 had device-related artefact but remained diagnostic (22%); only 9 were non-diagnostic (5%) and were excluded from LGE analysis, which consisted of the remaining 170 studies. Biventricular dysfunction was common in this cohort, with average LVEF and RVEF values of 35% and 42%, respectively. LGE was present in 134 of 170 (78.8%) studies.
Table 1.
Baseline patient clinical characteristics
| Baseline characteristics | All subjects, n = 179 |
|---|---|
| Age (years ± SD) | 59 (± 13) |
| Sex | |
| ȃMale | 135 (75.4%) |
| ȃFemale | 44 (24.6%) |
| Race | |
| ȃWhite | 115 (64.3%) |
| ȃBlack | 55 (30.7%) |
| ȃHispanic | 4 (2.2%) |
| ȃAsian | 4 (2.2%) |
| ȃOther | 1 (0.6%) |
| BMI (kg/m2) | 29 ± 6 |
| ICD indication | |
| ȃPrimary prevention | 101 (56.7%) |
| ȃSecondary prevention | 77 (43.3%) |
| CMR study indication | |
| ȃVentricular arrhythmia | 114 (63.7%) |
| ȃCardiomyopathy evaluation | 53 (29.6%) |
| ȃViability | 10 (5.6%) |
| ȃOther | 2 (1.1%) |
| Referral setting | |
| ȃInpatient | 62 (34.8%) |
| ȃOutpatient | 116 (64.8%) |
| Comorbidities | |
| ȃHypertension | 122 (68.2%) |
| ȃDyslipidaemia | 112 (62.6%) |
| ȃDiabetes mellitus | 56 (31.3%) |
| ȃSmoking | |
| ȃȃNever | 114 (64.0%) |
| ȃȃCurrent | 60 (33.7%) |
| ȃȃFormer | 4 (2.3%) |
| ȃChronic kidney disease | 54 (30.3%) |
| ȃPeripheral arterial disease | 16 (9.0%) |
| ȃStroke | 19 (10.7%) |
| ȃCoronary artery disease | 87 (48.9%) |
| ȃHeart failure | 151 (84.8%) |
| ȃRheumatoid arthritis | 3 (1.7%) |
| ȃAmyloidosis | 7 (4.0%) |
| ȃSarcoidosis | 8 (4.5%) |
| ȃArrhythmogenic right ventricular cardiomyopathy | 7 (3.9%) |
| ȃFamily history of atherosclerotic cardiovascular disease | 65 (36.5%) |
| ȃPrior ventricular ablation | 36 (20.9%) |
The major CMR findings are summarized in Table 2. Overall, the most common referral indication was for evaluation of ventricular arrhythmia substrate (n = 114, 63.7%), followed by assessment or follow-up of cardiomyopathy (n = 53, 29.6%). All patients had a prior echocardiogram within the last 12 months. Sixty-two (34.8%) were inpatient referrals (Table 1).
Table 2.
CMR characteristics
| CMR characteristics | All patients |
|---|---|
| LVEF (%) | 35 (25–46) |
| LVEDV index (mL/m2) | 113 (92–153) |
| LVEDV (mL) | 237 (190–308) |
| LVESV index (mL/m2) | 78 (50–107) |
| LVESV (mL) | 153 (100–235) |
| LV mass index (g/m2) | 71 (60–90) |
| LV mass (g) | 154 (114–192) |
| RVEF (%) | 43 (33–51) |
| RVEDV index (mL/m2) | 90 (71–112) |
| RVEDV (mL) | 188 (146–233) |
| RVESV index (mL/m2) | 50.5 (37–67) |
| RVESV (mL) | 99.5 (77–140) |
| Artefact | |
| ȃȃNo artefact, n (%) | 140 (75.3) |
| ȃȃArtefact + diagnostic, n (%) | 36 (19.4) |
| ȃȃArtefact + nondiagnostic, n (%) | 10 (5.4) |
| LGE present, n (%) | 134 (78.7) |
| LGE area, cm2 (136) | 13.9 (0.56–44) |
Values are median (25–75th percentile).
LVEDV, LVESV - left ventricular end-diastolic/end-systolic volumes; RVEDV, RVESV - right ventricular end-diastolic/end-systolic volumes; LVEF, RVEF - left/right ventricular ejection fractions.
Impact of CMR on diagnosis
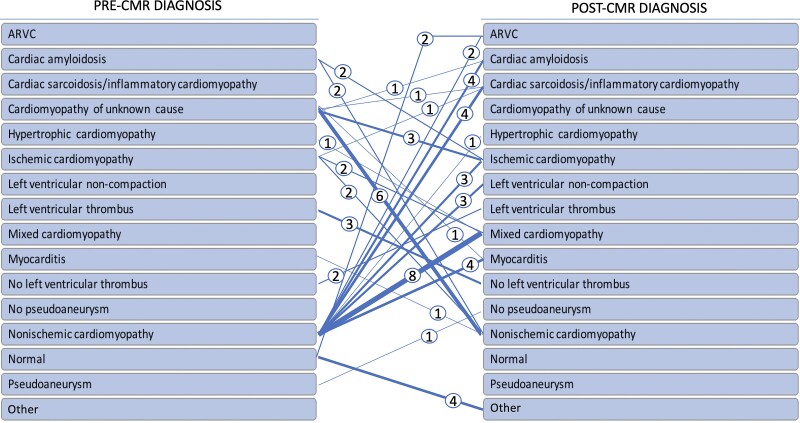
CMR resulted in a new or changed diagnosis in 64 (36%) studies (Figures 2 and 3). A changed diagnosis based on CMR findings occurred in 33% (38/114) patients referred for ventricular arrhythmias and in 36% (19/53) referred for cardiomyopathy evaluation. Impact of CMR on diagnosis was also seen in all (2/2) patients referred for thrombus/pseudoaneurysm evaluation and in 30% (3/10) of patients referred for viability assessment. In addition, this impact was seen in 31% of patients with primary prevention ICD, vs. 34% with secondary ICD. The most frequent new diagnoses were related to determination of cardiomyopathy aetiology, specifically in recognition of mixed aetiology (n = 10) and non-ischaemic aetiology (n = 11), followed by a diagnosis of unrecognized or silent myocardial infarction (n = 8) based on typical LGE patterns which had previously gone undetected by coronary angiography or echocardiography. LV thrombus was found in two patients not seen on echocardiography and excluded in three patients with inconclusive echocardiography findings. CMR led to a new diagnosis of cardiac sarcoidosis in six patients, all of whom had been referred for CMR prior to VT evaluation, with the diagnosis confirmed by subsequent positron emission tomography-computed tomography (PET-CT) or biopsy. Cardiac amyloidosis was detected in an additional five patients, who were all referred for cardiomyopathy evaluation. CMR findings diagnosed hypertrophic cardiomyopathy in three patients (2 referred for cardiomyopathy and 1 for VT evaluation) based on the characteristic pattern of hypertrophy and scar—one in a patient with a prior myocardial infarction and presumed ischaemic cardiomyopathy and two with a history of nonischaemic cardiomyopathy. Acute myocarditis was diagnosed in five patients (4 referred for cardiomyopathy and 1 referred for VT assessment) based on mid-myocardial or sub-epicardial LGE patterns in combination with the clinical context. Three patients were strongly suspected of LV non-compaction as an aetiology for their cardiomyopathy after characteristic findings were noted on CMR (not previously seen on echocardiography). CMR findings led to a new diagnosis of arrhythmogenic RV cardiomyopathy (ARVC) in four patients who were all referred for VT evaluation (2 previously normal and 2 with nonischaemic cardiomyopathy). CMR findings of fibrosis in four patients modified the diagnosis from normal heart to other conditions that not clearly fit into any one of the above categories. Pseudoaneurysm was excluded in one patient with suggestion of this diagnosis on prior echocardiography. Overall, the discovery of a new diagnosis led to a change in patient management in 37% of cases as detailed in the following section.
Figure 2.
Change in diagnosis after CMR study. Weighted lines represent number of patients (also numerically represented within the circle).
Figure 3.
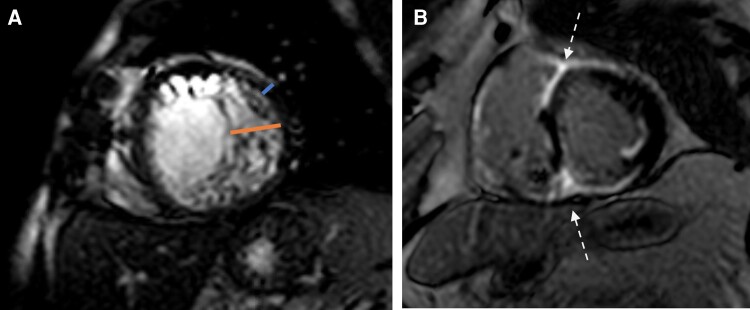
Examples of a change in or new diagnosis. (A) A 58-year-old male with heart failure with reduced ejection of unclear aetiology was referred for cardiomyopathy evaluation, with findings suggestive of left ventricular non-compaction cardiomyopathy. (B) A 67-year-old male with non-ischaemic cardiomyopathy and recurrent VT referred for scar evaluation prior to ablation. LGE in the anteroseptal and inferoseptal segments with extension to the RV (‘hook sign’ in anteroseptum and ‘triangle sign’ in inferoseptum) was seen. Findings were highly probable for cardiac sarcoidosis, which was confirmed on biopsy.
Impact of CMR on management
CMR findings impacted clinical management in 51 (28%) studies (Table 3). Six patients underwent revascularization with PCI or CABG after LGE imaging demonstrated myocardial viability. Conversely, coronary revascularization was deferred in four patients due to the absence of viability on CMR. In nine patients with suspected or known cardiomyopathy, findings suggested hypertrophic cardiomyopathy, cardiac amyloidosis, or ARVC, prompting genetic testing. Four patients underwent PET-CT and myocardial biopsy after LGE imaging demonstrated abnormalities suggesting sarcoidosis.
Table 3.
Impact of CMR on patient management
| Impact of CMR on patient management | n |
|---|---|
| Impact on revascularization | 10 |
| ȃLed to PCI/CABG | 6 |
| ȃAvoided PCI/CABG | 4 |
| Impact on medications | 8 |
| ȃStarted anticoagulation | 1 |
| ȃStopped anticoagulation | 2 |
| ȃStarted immunosuppressive therapy | 4 |
| ȃStarted ASA/statin | 1 |
| Impact on diagnostics | 13 |
| ȃLed to genetic testing | 9 |
| ȃLed to endomyocardial biopsy | 4 |
| Impact on electrophysiology procedures | 20 |
| ȃAvoided ablation | 3 |
| ȃChange in ablation approach | 17 |
| TOTAL | 51 |
Medication management was also influenced in a number of patients. Antiplatelet and statin therapy was commenced in one patient after diagnosis of unsuspected myocardial infarction; dual antiplatelet therapy was modified with systemic anticoagulation after confirming LV thrombus in another patient with known CAD. Systemic anticoagulation was stopped in two patients due to absence of LV thrombus on CMR, despite being suspected by recent echocardiography. LGE findings of inflammatory cardiomyopathy or cardiac amyloidosis led to initiation of immunosuppressive therapy or tafamidis in four patients.
CMR imaging depicted LGE in 85 of the 114 patients referred for ventricular arrhythmia assessment (75%). Seventy-six (67% of VT referral) patients underwent VT ablation following CMR. Of these, LGE imaging identified a structural substrate in 58 patients (76%). As it is our institutional practice pattern to simultaneously perform epicardial and endocardial mapping in most patients during VT ablation, CMR results did not impact whether an epicardial approach was used, with the exception of those with isolated scarring in the septum. In this scenario, an epicardial approach was not performed (17/58, 29%) thus impacting ablation strategy. In addition, LGE findings led to a deferral of ablation in 2 of 58 (3%) patients, who were instead referred for coronary angiogram and genetic testing, respectively.
Clinical outcomes
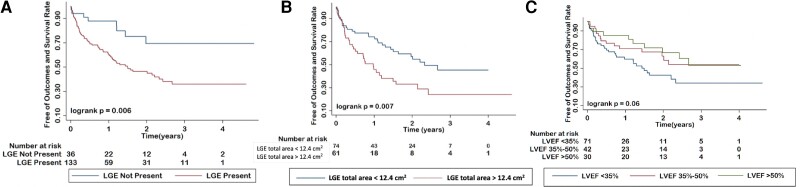
A total of 77 patients (43%) experienced MACE during the follow up period (median, 1.7 years), including: all-cause death (n = 30), LV assist device implantation (n = 8), heart transplantation (n = 7), recurrent ventricular arrhythmias (n = 31), and CABG (n = 1). Of these outcomes, 65 of 77 (84%) occurred in patients with LGE and 12 of 77 (16%) occurred in patients without LGE. Sixty-six percent (51/77) occurred in patients referred for VT evaluation, 25% (19/77) for CM, 6.5% (5/77) for viability, and 2.6% (2/77) for other reasons (Table 4). Kaplan–Meier analysis revealed that ICD patients with LGE had worse outcomes than those patients with no LGE (Figure 4A; P = 0.006). The median total area of LGE extent in LGE positive patients was 13.9 cm2. ROC curve was created to determine the best discriminator value of LGE extent for predicting event free survival. From this, a value of LGE area of 12.4 cm2 was obtained as the best cut-off value for predicting MACE in this cohort. LGE positive patients were then subdivided into LGE area < and ≥ 12.4 cm2 for Kaplan-Meier analysis. The burden of LGE also demonstrated prognostic association with MACE (Figure 4B). Lastly, LVEF as measured using CMR also demonstrated prognostic association with MACE (Figure 4C).
Table 4.
Clinical outcome according to CMR study referral indication
| All patients | CMR indication | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VT (n = 114) | CM (n = 53) | Viability (n = 9) | Other (n = 2) | |||||||
| LGE (+) | LGE (−) | LGE (+) | LGE (−) | LGE (+) | LGE (−) | LGE (+) | LGE (−) | |||
| (n = 44) | (n = 7) | (n = 19) | (n = 0) | (n = 5) | (n = 0) | (n = 1) | (n = 1) | |||
| Outcome | Death | 30 | 13 | 2 | 10 | 0 | 4 | 0 | 0 | 1 |
| OHT | 7 | 3 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | |
| LVAD | 8 | 3 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | |
| Recurrent VT | 31 | 25 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | |
| CABG | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
CM, cardiomyopathy; LVAD, left ventricular assist device; OHT, orthotopic heart transplantations.
Figure 4.
Kaplan–Meier survival curves by presence of LGE (A), by total LGE area (B), and by LVEF (C).
Discussion
Due to a growing understanding that CMR can be safely performed in most patients with an ICD while maintaining diagnostic quality images,5,16 CMR is increasingly utilized in this patient population. However, patients it is unknown how frequently the use of CMR in patients with ICDs alters the clinical diagnosis and/or the treatment plan, nor is it known whether CMR provides prognostic value in these patients. The primary findings of our study are as follows: (i) the most common indications for CMR referral at our centre were for evaluation of ventricular arrhythmia substrate (64%) or for assessment or follow-up of cardiomyopathy (30%), (ii) CMR testing resulted in a change in diagnosis in 36% of the overall population, (iii) CMR testing resulted in a change in clinical decision-making in 28% of patients, and (iv) the presence and extent of LGE was associated with worse outcomes. These data indicate that CMR is valuable as a diagnostic and prognostic imaging tool in appropriately selected patients with ICDs.
CMR has the distinctive ability for tissue characterization, with particular emphasis on its ability to detect myocardial injury patterns. CMR detection of myocardial injury in both ischaemic and non-ischaemic cardiomyopathies provide valuable prognostic information, and can help guide appropriate patient selection for device therapies and pre-procedural planning for VT ablation. While the number of patients with ICDs is steadily rising, CMR based LGE assessment in this population remains limited due to residual apprehensions about safety and ICD-induced artefacts that can render LGE assessment non-diagnostic. Despite these concerns, a growing body of literature suggests that it is safe to perform CMR in the presence of cardiac devices by taking several precautions during patient selection and image acquisition. By adhering to a safety algorithm to ensure appropriate patient selection and monitoring, as well as pre- and post-CMR interrogation of the device to maintain the specific absorption rate below 2 W/kg, we obtained diagnostic quality LGE imaging in patients with an ICD without any adverse events. We also used a modified sequence with a widened RF bandwidth inversion pulse, which reduced the hyperintensity artefact and resulted in diagnostic-quality LGE images in 95% of subjects at our institution. Efforts are currently underway to develop additional CMR techniques using wideband pulse sequences, including T1- and T2-mapping, as well as stress perfusion imaging,17,18 but the principal value of CMR in patients with ICDs remains in LGE assessment. Moreover, 3D LGE might be useful for guiding ablation procedures.
We found a relatively high rate of diagnostic impact in patients referred for CMR for the evaluation of ventricular arrhythmia, and in particular, secondary prevention ICD patients as well as in those referred for evaluation of cardiomyopathy. There may be several reasons for this observation. Current diagnostic algorithms for patients presenting with malignant ventricular arrhythmia recommend the routine use of transthoracic echocardiography and invasive coronary angiography with selective consideration of additional imaging, such as CMR. This approach, however, cannot readily identify the entire range of myocardial tissue abnormalities that may be detectable at the time of ICD implantation. For instance, cardiac diseases mimicking LV hypertrophy may be misdiagnosed as hypertensive heart disease and remain undetected. In our cohort, cardiac sarcoidosis, ARVC, and LV non-compaction cardiomyopathy were diagnosed in patients with no prior history of these disorders. Previous data in survivors of sudden cardiac death/VT showed that the addition of CMR prior to ICD implantation yielded a high incremental diagnostic value, resulting in a new or alternate diagnosis in about 50% of patients.19 Because certain conditions, such as acute myocarditis, may be considered transient catalysts of ventricular arrhythmia, identifying them is clinically important. We also identified unsuspected myocardial infarction-LGE pattern in a group of patients presenting with ventricular arrhythmias. This finding raises important considerations, and provides a mechanistic explanation for the malignant ventricular arrhythmias experienced by these patients. The recurrence of arrhythmia in these patients with CMR evidence of myocardial infarction may present insights into the appropriate use of ICD therapy in this population.
Notably, a single baseline CMR exam may not be sufficient for identifying myocardial pathology, which may manifest over time. Given the natural evolution of myocardial diseases and variable disease stages, interval re-imaging of ICD patients could be considered if life threatening arrhythmias or worsening heart failure symptoms recur over time. Our results add to previous research showing the added value of CMR imaging on clinical decision-making in patients with CMR–conditional pacemakers20 and ICDs7 with ventricular arrhythmia.
While not directly assessed by the current study, perhaps the greatest clinical impact is related to electrophysiology procedural decision making in patients with VT. Our results support that CMR imaging has diagnostic utility in the detection of myocardial substrate in patients with malignant ventricular arrhythmia. LGE imaging identified a structural substrate in 76% of patients who underwent VT ablation following CMR. In non-ischaemic cardiomyopathy in particular, identifying a potential target for subsequent VT ablation is important as substrate-guided (‘fibrosis-guided’) ablation has become a cornerstone for catheter treatment of complex ventricular arrhythmias. LGE CMR based information can help determine the preferred route of access (i.e. endocardial or epicardial approach only or combined endo-/epicardial approach) and facilitate a more targeted ablation with knowledge about the segmental location of potential substrate beforehand.
CMR remains the gold standard for tissue characterization. In the current study, the information gained from CMR testing resulted in a change in clinical diagnosis and management in a significant proportion of ICD patients referred for cardiomyopathy assessment. Of the 53 such patients, a new or changed diagnosis was made in 36%. This increase was especially realized through more sensitive detection of ischaemic and nonischaemic forms of myocardial disease. LGE assessment additionally provided guidance in decisions regarding immunosuppression in sarcoidosis, genetic screening with suspected cardiomyopathy, and pharmacotherapy in ischaemic cardiomyopathy.
It is well known that CMR offers additional prognostic data with assessment of fibrosis and disease severity in ICD patients. Both the presence and burden of LGE was associated with worse outcomes in our study, which included patients with both primary and secondary prevention ICD.
Limitations
Our study has important limitations, the first being that our data are reflective of a single medical centre; thus, the impact of institutional referral patterns on the findings cannot be quantified. While the study cohort closely reflected the clinical spectrum typically encountered at a tertiary care centre, future work needs to consider larger study groups from multiple centres. Additionally, our CMR protocol for imaging patients with an ICD was limited to cine and wideband LGE imaging and did not include techniques such as wideband T1-mapping, T1- or T2-weighted imaging, magnetic resonance angiography, or myocardial perfusion, which are the focus of many current research endeavours. Accordingly, reclassification to non-ischaemic cardiomyopathy was based on absence of typical ischaemic LGE pattern, leaving a possibility of classification error in some patients without corroborative evaluation by other modalities.
One might question the high incidence of LGE in our study cohort, which indeed is high even for a mixed population of ischaemic and non-ischaemic cardiomyopathy. However, the high incidence of LGE is explained by the fact that this cohort is comprised of patients who have ICDs. It has been well established that patients with various non-ischaemic cardiomyopathies who have LGE are more likely to have ventricular arrhythmias21–23; thus, it is no surprise that patients with an ICD are more likely to have LGE. Additionally, the fact that a patient with an ICD was referred for CMR by itself suggests that their cardiomyopathy is not behaving as expected in some way; therefore, the higher incidence of LGE in our population is not surprising. Furthermore, two of three patients in our cohort were referred for ventricular arrhythmias (Table 1), and so these individuals would be expected to have more LGE.
Conclusion
The use of a tailored CMR protocol that includes cine and wideband LGE imaging has the potential to change the clinical diagnosis, alter treatment plan, and provide incremental prognostic value in appropriately selected patients with an ICD. Future studies in a multicentre setting are needed to fully understand the clinical impact of performing CMR imaging in this patient population.
Contributor Information
Hena N Patel, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Shuo Wang, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Swati Rao, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Amita Singh, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Luis Landeras, Department of Radiology, University of Chicago Medical Center, Chicago, IL 60637, USA.
Stephanie A Besser, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Spencer Carter, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Satish Mishra, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Takuro Nishimura, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Dalise Y Shatz, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Roderick Tung, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Hemal Nayak, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Keigo Kawaji, Illinois Institute of Technology, Department of Biomedical Engineering, Chicago, IL 60616, USA.
Victor Mor-Avi, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA.
Amit R Patel, Department of Medicine, University of Chicago Medical Center, Chicago, IL 60637, USA; Department of Radiology, University of Chicago Medical Center, Chicago, IL 60637, USA.
Funding
This project was indirectly supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) through Grant Number 5UL1TR002389-02 that funds the Institute for Translational Medicine (ITM). H.N.P. was funded by a T32 Cardiovascular Sciences Training Grant (2T32HL007381-41A1).
References
- 1. Nazarian S, Halperin HR. Magnetic resonance imaging and cardiac devices. N Engl J Med 2018;378:1652–3. [DOI] [PubMed] [Google Scholar]
- 2. Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RWet al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med 2017;376:755–64. [DOI] [PubMed] [Google Scholar]
- 3. Padmanabhan D, Kella DK, Deshmukh AJ, Mulpuru SK, Mehta RA, Dalzell CMet al. Safety of thoracic magnetic resonance imaging for patients with pacemakers and defibrillators. Heart Rhythm 2019;16:1645–51. [DOI] [PubMed] [Google Scholar]
- 4. Schaller RD, Brunker T, Riley MP, Marchlinski FE, Nazarian S, Litt H. Magnetic resonance imaging in patients with cardiac implantable electronic devices with abandoned leads. JAMA Cardiol 2021;6:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh A, Kawaji K, Goyal N, Nazir NT, Beaser A, O'Keefe-Baker Vet al. Feasibility of cardiac magnetic resonance wideband protocol in patients with implantable cardioverter defibrillators and its utility for defining scar. Am J Cardiol 2019;123:1329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JPet al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology 2014;270:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindemann F, Oebel S, Paetsch I, Arya A, Dagres N, Richter Set al. Clinical utility of cardiovascular magnetic resonance imaging in patients with implantable cardioverter defibrillators presenting with electrical instability or worsening heart failure symptoms. J Cardiovasc Magn Reson 2020;22:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhuva AN, Kellman P, Graham A, Ramlall M, Boubertakh R, Feuchter Pet al. Clinical impact of cardiovascular magnetic resonance with optimized myocardial scar detection in patients with cardiac implantable devices. Int J Cardiol 2019;279:72–8. [DOI] [PubMed] [Google Scholar]
- 9. Samar H, Yamrozik JA, Williams RB, Doyle M, Shah M, Bonnet CAet al. Diagnostic value of MRI in patients with implanted pacemakers and implantable cardioverter-defibrillators across a cross population: does the benefit justify the risk? A proof of concept study. JACC Clin Electrophysiol 2017;3:991–1002. [DOI] [PubMed] [Google Scholar]
- 10. Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326–8. [DOI] [PubMed] [Google Scholar]
- 11. Nazarian S, Reynolds MR, Ryan MP, Wolff SD, Mollenkopf SA, Turakhia MP. Utilization and likelihood of radiologic diagnostic imaging in patients with implantable cardiac defibrillators. J Magn Reson Imaging 2016;43:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stojanovska J, Runge M, Mahani MG, Cronin PP, Sayyouh M, Bogun Fet al. Cardiac MRI for patients with cardiac implantable electronic devices. AJR Am J Roentgenol 2020;215:374–81. [DOI] [PubMed] [Google Scholar]
- 13. Siontis KC, Kim HM, Sharaf Dabbagh G, Latchamsetty R, Stojanovska J, Jongnarangsin Ket al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm 2017;14:1487–93. [DOI] [PubMed] [Google Scholar]
- 14. Do DH, Eyvazian V, Bayoneta AJ, Hu P, Finn JP, Bradfield JSet al. Cardiac magnetic resonance imaging using wideband sequences in patients with nonconditional cardiac implanted electronic devices. Heart Rhythm 2018;15:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh A, Chen W, Patel HN, Alvi N, Kawaji K, Besser SAet al. Impact of wideband late gadolinium enhancement cardiac magnetic resonance imaging on device-related artifacts in different implantable cardioverter-defibrillator types. J Magn Reson Imaging 2021;54:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek Eet al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med 2017;377:2555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial t1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magn Reson Med 2017;77:1495–504. [DOI] [PubMed] [Google Scholar]
- 18. Hong K, Jeong EK, Wall TS, Drakos SG, Kim D. Wideband arrhythmia-insensitive-rapid (air) pulse sequence for cardiac T1 mapping without image artifacts induced by an implantable-cardioverter-defibrillator. Magn Reson Med 2015;74:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White JA, Fine NM, Gula L, Yee R, Skanes A, Klein Get al. Utility of cardiovascular magnetic resonance in identifying substrate for malignant ventricular arrhythmias. Circ Cardiovasc Imaging 2012;5:12–20. [DOI] [PubMed] [Google Scholar]
- 20. Raphael CE, Vassiliou V, Alpendurada F, Prasad SK, Pennell DJ, Mohiaddin RH. Clinical value of cardiovascular magnetic resonance in patients with mr-conditional pacemakers. Eur Heart J Cardiovasc Imaging 2016;17:1178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza Set al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 22. Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AVet al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2016;9:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakamoto N, Kawamura Y, Sato N, Nimura A, Matsuki M, Yamauchi Aet al. Late gadolinium enhancement on cardiac magnetic resonance represents the depolarizing and repolarizing electrically damaged foci causing malignant ventricular arrhythmia in hypertrophic cardiomyopathy. Heart Rhythm 2015;12:1276–84. [DOI] [PubMed] [Google Scholar]