Abstract
Aims
Cardiac conduction disease can lead to syncope, heart failure, and death. The only available therapy is pacemaker implantation, with no established prevention strategies. Research to identify modifiable risk factors has been scant.
Methods and results
Data from the Cardiovascular Health Study, a population-based cohort study of adults ≥ 65 years with annual 12-lead electrocardiograms obtained over 10 years, were utilized to examine relationships between baseline characteristics, including lifestyle habits, and conduction disease. Of 5050 participants (mean age 73 ± 6 years; 52% women), prevalent conduction disease included 257 with first-degree atrioventricular block, 99 with left anterior fascicular block, 9 with left posterior fascicular block, 193 with right bundle branch block (BBB), 76 with left BBB, and 102 with intraventricular block at baseline. After multivariable adjustment, older age, male sex, a larger body mass index, hypertension, and coronary heart disease were associated with a higher prevalence of conduction disease, whereas White race and more physical activity were associated with a lower prevalence. Over a median follow-up on 7 (interquartile range 1–9) years, 1036 developed incident conduction disease. Older age, male sex, a larger BMI, and diabetes were each associated with incident conduction disease. Of lifestyle habits, more physical activity (hazard ratio 0.91, 95% confidence interval 0.84–0.98, P = 0.017) was associated with a reduced risk, while smoking and alcohol did not exhibit a significant association.
Conclusion
While some difficult to control comorbidities were associated with conduction disease as expected, a readily modifiable lifestyle factor, physical activity, was associated with a lower risk.
Keywords: Conduction disease, Atrioventricular block, Bundle branch block, Lifestyle habits, Physical activity
Structured Graphical Abstract
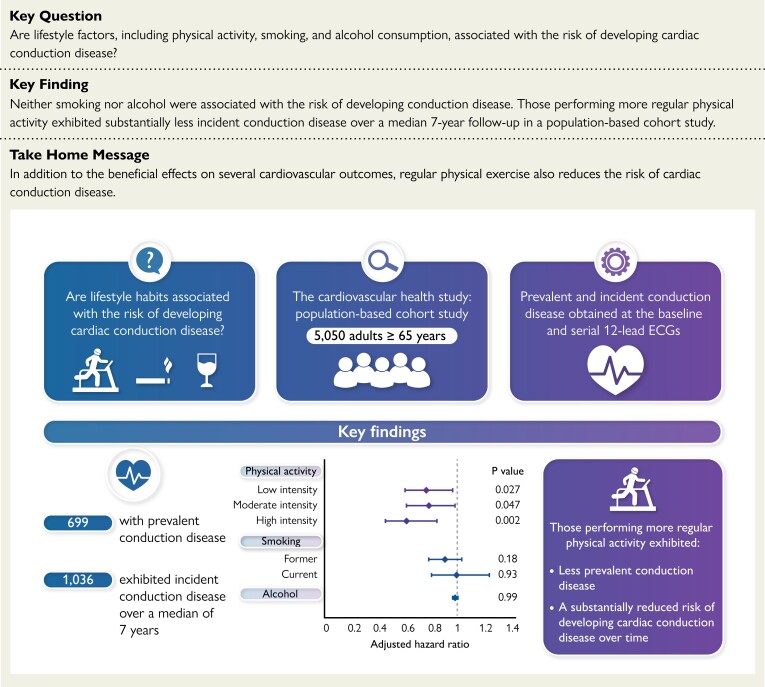
Structured Graphical Abstract.
Cross-sectional and longitudinal analyses were performed in a population-based cohort of adults 65 years and older to examine relationships between baseline characteristics, including lifestyle habits, and conduction disease. Of 5050 participants, prevalent conduction disease was detected in 699 participants. Over a median of 7 years, 1036 exhibited incident conduction disease. After adjusting for established cardiovascular risk factors, those performing more regular physical activity exhibited less prevalent conduction disease and had a substantially reduced risk of developing conduction disease over time. Neither smoking nor alcohol exhibited significant associations.
See the editorial comment for this article ‘Prevention of cardiac conduction disease: a long way to go’, by J.J. Noubiap, https://doi.org/10.1093/eurheartj/ehac752.
Cardiac conduction disease is a common condition that disrupts normal electrical depolarization of the heart and may occur at any level of the cardiac conduction system. In the worst case, progression of this disorder can lead to life-threatening rhythm disturbances.1–3 Left and right bundle branch block (LBBB and RBBB, respectively) are associated with heightened cardiovascular morbidity and mortality,4,5 and LBBB can directly result in heart failure.6 Even left anterior fascicular block (LAFB), commonly considered a benign electrocardiographic finding, has been associated with an increased risk of atrial fibrillation, congestive heart failure (CHF), and death in the absence of manifest cardiovascular disease.7
While pacemakers represent an effective solution to the end-stage of conduction disease, placement of such devices may result in complications, require serial generator changes (each associated with a heightened risk of infection), and chronic pacing may itself have deleterious effects.8–10
The prevention of cardiac conduction disease is not generally considered part of standard medical, or even cardiac, care. Before strategies to prevent this common condition can be developed, modifiable risk factors must first be identified. A few immutable characteristics have been shown to presage conduction diseases, including age and male sex,5,11,12 whereas studies investigating behaviours and lifestyle factors are scarce. We therefore sought to examine longitudinal data from the population-based Cardiovascular Health Study (CHS) to identify lifestyle factors associated with conduction disease.
Methods
Study design
The CHS is a prospective, population-based cohort study sponsored by the National Heart, Lung, and Blood Institute. Details about the CHS study have been described in detail elsewhere.13 In brief, 5201 individuals at least 65 years of age were recruited between 1989 and 1990, and an additional 687 Black individuals were recruited between 1992 and 1993, from a random sample of Medicare beneficiaries by four academic centres (Johns Hopkins University, Wake Forest University, University of Pittsburgh, and University of California, Davis) in the USA. At enrolment, all participants underwent a comprehensive examination at baseline including a detailed medical history, physical examination, 12-lead electrocardiogram (ECG), and laboratory testing. For the subsequent 10 years (1989–99), participants were followed annually with clinic visits, including ECG examinations, and semi-annually with telephone contact, after which semi-annual telephone contact was continued. Participants provided written informed consent, and the study protocol was approved by the institutional review board of each centre.
Study population
Our study population was restricted to those participants without prevalent atrial fibrillation detected on baseline ECG or a reported history of physician-diagnosed atrial fibrillation at the first encounter (n = 162) or ventricular pre-excitation on baseline ECG (n = 3). As the mechanisms underlying the development of conduction disease in the settings of CHF or previous myocardial infarction (MI) may be directly related to damage from those diseases less pertinent to direct effects of lifestyle factors in the majority of the general population, participants with a baseline history of CHF (n = 132) or MI (n = 54) were excluded from the main analyses.
Covariate ascertainment
Demographics
Age and sex were obtained from the initial assessment visit. Self-identified race was categorized as White, Black, American Indian/Alaskan native, Asian/Pacific Islander, and other. Due to a small number of non-White participants, race was dichotomized as White vs. non-White in the regression analyses. Level of education was defined according to self-reported number of educational years.
Medical conditions
Height and weight were measured at the initial assessment and used to calculate body mass index (BMI) as weight in kilograms divided by height in metres squared. Diabetes was defined as a fasting glucose level ≥ 126 mmoL/L or a reported use of an anti-hyperglycaemic medication at baseline. Hypertension was defined as seated blood pressure average ≥ 140 mmHg, a seated blood pressure average diastolic ≥ 90 mmHg, or a history of physician diagnosed hypertension combined with the use of anti-hypertensive medication. Coronary heart disease was defined as angina pectoris, previous MI, previous coronary artery bypass graft surgery, or previous percutaneous coronary intervention identified by participant self-report and confirmed by medical record verification. CHF was identified by participant self-report and was confirmed by medical record verification.14
After study enrolment, the participants were contacted twice yearly in order to obtain medical records for all hospitalizations. Incident CHF and MI events were assessed through self-report and review of International Classification of Diseases diagnostic codes from hospitalization or outpatient reports or mention of an endpoint on the hospital face sheet, discharge summary, or outpatient procedure report. Adjudication of incident CHF and MI events was performed by the CHS Events Subcommittee.15
Lifestyle habits
Prospectively and universally ascertained common habits previously associated with cardiovascular outcomes were characterized: physical activity, smoking, and alcohol consumption. Physical activity was assessed using a modified validated Minnesota Leisure-Time activity questionnaire.16 This questionnaire evaluated frequency and duration of 15 different activities during the foregoing 2 weeks. Each activity was defined as having an intensity value in metabolic equivalent task units. Usual exercise intensity was categorized into none, low, moderate, or high activity intensity based on the highest-intensity leisure-time activity reported over the prior 2 weeks, where high intensity activity was estimated to require >6 metabolic equivalent tasks.16,17 Self-reported smoking status was categorized as never, former, or current smoker. Alcohol consumption was defined as the number of alcoholic drinks usually consumed in 1 week (1 drink was defined as a 12-ounce can or bottle of beer, a 6-ounce glass of wine, or a shot of liquor).
Outcome ascertainment
The primary outcome was defined as any conduction disease, including first degree atrioventricular (AV) block, LAFB, left posterior fascicular block (LPFB), RBBB, LBBB, non-specific intra-ventricular block, Mobitz type II block, and third-degree AV block assessed from baseline and annual 12-lead ECGs recorded using MAC PC ECG Machines (Marquette Electronics, Milwaukee, WI). Standard 12-lead ECGs were recorded in all participants by strictly standardized procedures. A 10-s segment of simultaneous ECG leads was sampled at a rate of 250 samples per second. All ECGs were visually inspected for technical errors and inadequate quality, and ECG wave measurements were validated on interactive graphics terminals. The electronic ECG stored in the ECG machines were transmitted daily to a central ECG core laboratory for reading and analysis using the Novacode ECG measurement and classification system. The ECGs were initially processed with the Dalhousie ECG programme and then were later processed with the 2001 version of the GE Marquette 12-SL programme (GE Healthcare).13,18 Minnesota codes were used to define complete first-degree AV block (Minnesota code 6-3), LBBB (Minnesota code 7-1-1 and 7-1-2), RBBB (Minnesota code 7-2-1 and 7-2-2), non-specific intra-ventricular block (Minnesota code 7-4), Mobitz type II block (Minnesota code 6-2-1), and third-degree AV block (Minnesota code 6-1). A diagnosis of LAFB was defined as a QRS axis between −45° and −90° and QRS duration of <0.12 s in the absence of left ventricular hypertrophy, inferior MI, complete AV block, ventricular pre-excitation, LBBB, and artificial pacemaker on the ECG (as reported previously).7 LPFB was defined as a QRS axis between 90° and 180° and a QRS duration of <0.12 s in the absence of right ventricular hypertrophy, lateral MI, ventricular pre-excitation, LBBB, and pacemaker on the ECG.
Statistical analysis
Normally distributed continuous variables are described as means and standard deviations (SDs) and compared using unpaired, two-tailed t tests. Continuous variables without a normal distribution are presented as medians with interquartile ranges (IQRs) and are compared using Mann–Whitney tests. Categorical variables were compared using χ2 tests.
Prevalent conduction disease was described as the presence of any conduction disease on the first study ECG recording. The association between baseline covariates and prevalent conduction disease was assessed using logistic regression.
For analyses of incident conduction disease, we restricted the population to those without prevalent conduction disease. Cox proportional hazards models were used to assess the relationships between baseline covariates and incident conduction disease. Participants were censored at the time of their first ECG-diagnosed conduction disease or at the time of their last study ECG, whichever came first.
Kaplan–Meier adjusted cumulative incidence curves were constructed for conduction disease according to exercise intensity level and smoothed using randomly imputed failure times between exam visits.
Covariates for inclusion in multivariable models included baseline demographics, established cardiovascular comorbidities, and the lifestyle factors of physical activity, smoking, and alcohol consumption.
We performed several sensitivity analyses: first, we adjusted for prevalent CHF and MI; second, we performed a time-updated analysis including incident CHF and MI (using the dates those occurred); third, we excluded those with prevalent coronary heart disease; fourth, we adjusted for prevalent atrial fibrillation; and finally, a time-updated analysis including repeated assessments of physical activity at years 5 and 9 was performed. As some anti-arrhythmic medication may induce first-degree AV block, additional sensitivity analyses including multivariable regression models adjusting for baseline use of anti-arrhythmic drugs (beta-blockers, non-dihydropyridine calcium-channel blockers, class I and III anti-arrhythmic drugs, and digoxin) and regression analyses excluding those using class I and III anti-arrhythmic drugs at baseline were further performed. We subsequently performed sensitivity analyses including incident ventricular pacing (detected from the study ECG) in the outcome as well as an analysis accounting for the competing risk of all-cause death.
Interaction analyses were performed to determine whether the effect of exercise intensity on incident conduction disease risk differed with respect to age (stratified at 75 years vs. younger).
Prevalent and incident regression analyses were repeated after restricting the outcome to infra-Hisian conduction disease alone (excluding first-degree AV block, which may represent increased atrial conduction time or slowing of AV nodal conduction rather than structural disease in the conduction system).
Although causal effects could not be concluded using these observational data, we calculated population attributable fractions (PAFs) of incident conduction disease for theoretically modifiable and lifestyle factors found to have a statistically significant association with incident conduction disease to express the magnitude of the relationships with these covariates should causal effects be present. To estimate PAFs, we used the method for cohort and cross-sectional studies recommended by Greenland and Drescher19 wherein continuous and categorical variables remodelled into binary variables: the reference for BMI was below 25 kg/m2; the reference for diabetes was the absence of the diabetes; and the reference for high exercise intensity was no exercise. The method estimates the logs of two scenario means, a baseline scenario (‘Scenario 0’) and a fantasy scenario (‘Scenario 1’), in which the exposure variable values are assumed to be set to zero, holding other predictor variables in the model constant. The log of the ratio of Scenario 1 mean to Scenario 0 mean is calculated to estimate the PAF and uses the log transformed complement of PAF (log[1-PAF]) to estimate standard errors and 95% confidence intervals (CIs).20,21 The PAFs are presented after multivariable adjustment including same baseline covariates as previously mentioned.
A two-tailed P < 0.05 was considered to be statistically significant. Statistical analyses were performed using STATA 17 (StataCorp, College Station, TX).
Results
The baseline characteristics of the 5050 participants included in the current analysis are shown in Table 1. Baseline conduction disease was comprised of 257 with first-degree AV block, 99 with LAFB, 9 with LPFB, 193 with RBBB, 76 with LBBB, and 102 with non-specific intra-ventricular block (67 had more than one manifestation of conduction disease on their baseline ECG). Participants with baseline conduction disease were older, more likely to be male, more often black, had a larger BMI, had more cardiovascular diseases, and engaged in less physical activity compared to participants without baseline conduction disease.
Table 1.
Baseline characteristics
| No conduction disease | Any conduction disease | P-value | |
|---|---|---|---|
| n = 4381 | n = 669 | ||
| Age, years | 72.4 ± 5.4 | 74.0 ± 6.2 | <0.001 |
| Sex, male, % | 1854 (42.3) | 564 (84.3) | <0.001 |
| Race | 0.021 | ||
| White, % | 3685 (84.1) | 535 (80.0) | |
| Black, % | 658 (15.0) | 132 (19.7) | |
| American Indian/Alaskan, % | 10 (0.2) | 0 (0.0) | |
| Asian/Pacific Islander, % | 4 (0.1) | 0 (0.0) | |
| Other, % | 14 (0.3) | 2 (0.3) | |
| Educational level, years, IQR | 12 (11–18) | 12 (10–18) | 0.55 |
| Mean body mass index, kg/m2 | 26.6 ± 4.7 | 27.0 ± 4.6 | 0.021 |
| Diabetes mellitus, % | 601 (13.7) | 133 (19.9) | <0.001 |
| Hypertension, % | 2486 (56.7) | 433 (64.7) | <0.001 |
| Coronary heart disease, % | 421 (9.6) | 96 (14.3) | <0.001 |
| Exercise intensity | 0.003 | ||
| No exercise, % | 338 (7.7) | 69 (10.3) | |
| Low, % | 2055 (46.9) | 328 (49.0) | |
| Moderate, % | 1532 (35.0) | 228 (34.1) | |
| High, % | 444 (10.1) | 43 (6.4) | |
| Smoking | 0.24 | ||
| Never, % | 2073 (47.3) | 312 (46.6) | |
| Former, % | 1750 (40.0) | 285 (42.6) | |
| Current, % | 544 (12.4) | 70 (10.5) | |
| Alcoho, beverages per week | 2.6 ± 7.3 | 2.2 ± 5.8 | 0.14 |
Values are n (%), mean ± SD, or median (interquartile range).
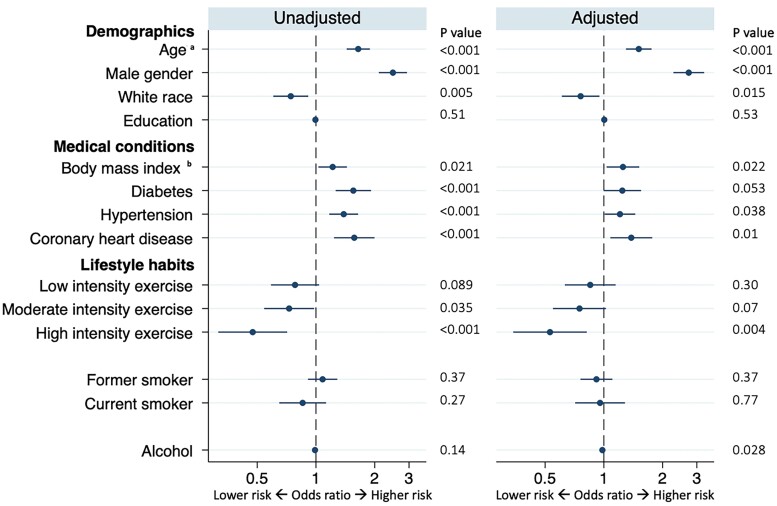
Baseline variables associated with prevalent conduction disease before and after multivariable adjustment are shown in Figure 1. After multivariable adjustment, older age, male sex, a larger BMI, hypertension, and coronary heart disease were each associated with a higher prevalence of conduction disease, whereas White race, more physical activity, and a larger alcohol consumption were associated with a lower prevalence.
Figure 1.
Baseline characteristics associated with prevalent conduction disease. Adjusted models included all covariates listed. Circles denote odds ratios for prevalent conduction disease, and error bars denote 95% confidence intervals. Number of participants in univariable models: minimum 4988 Number of participants included in multivariable models: 4928 aInterpreted as an odds risk for every 10-year increase bInterpreted as an odds risk for every 10 kg/m2 increase.
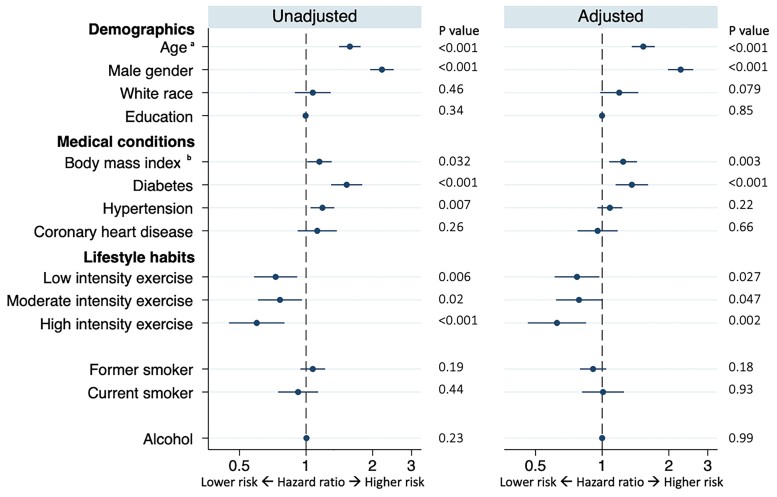
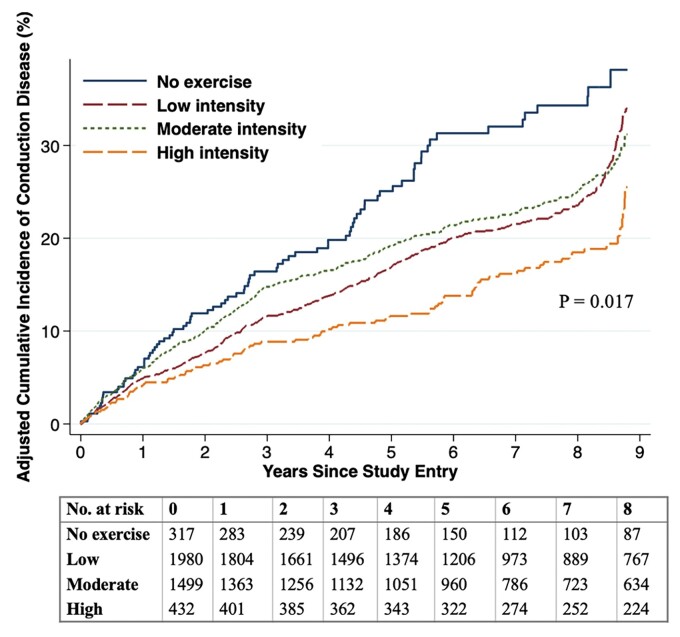
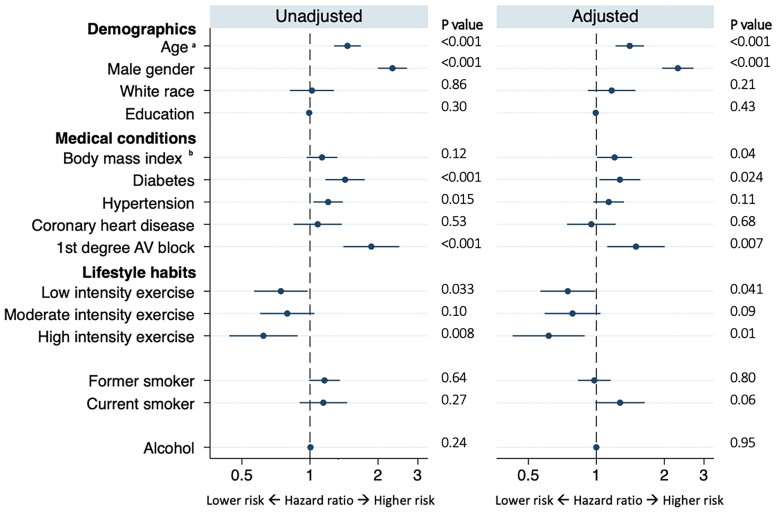
After excluding participants with conduction disease at baseline, 1036 exhibited incident conduction disease during a median follow up of 7 (IQR 1–9) years. The type of first conduction disease detected is presented in Supplementary material online, Table S1. No individuals demonstrated Mobitz type II or third-degree AV block. In both unadjusted and adjusted models, older age, male sex, a larger BMI, and diabetes mellitus were associated with a higher risk of incident conduction disease (Figure 2). Regarding lifestyle factors, while neither smoking nor alcohol consumption revealed statistically significant relationships with incident conduction disease, more physical activity was associated with a lower risk [hazard ratio (HR) 0.91, 95% CI 0.84–0.98, P = 0.017)] (Figure 2). A statistically significant linear trend between progressive increases in categories of physical activity and a lower risk of incident conduction disease was present in both unadjusted (P value for trend: P < 0.001) and adjusted models (P value for trend: P = 0.0026). Figure 3 illustrates adjusted Kaplan–Meier cumulative incidence curves for conduction disease according to each exercise intensity level. In a time-updated analysis that included assessments of physical activity at years 5 and 9, no meaningful differences were observed: time-updated high intensity exercise was associated with a 26% lower risk of incident conduction disease (HR 0.74, 95% CI 0.59–0.93, P = 0.011).
Figure 2.
Baseline characteristics associated with incident conduction disease. Adjusted models included all covariates listed. Circles denote hazard ratios for incident conduction disease, and error bars denote 95% confidence intervals. Number of participants in univariable models: minimum 4185 Number of participants included in multivariable models: 4133 aInterpreted as a hazard for every 10-year increase bInterpreted as a hazard for every 10 kg/m2 increase.
Figure 3.
Cumulative incidence of conduction disease by baseline regular exercise intensity. Adjusted Kaplan–Meier cumulative incidence curves for conduction disease according to exercise intensity level. The curves were smoothed using randomly imputed failure times between exam visits and adjusted for age, gender, race, education, body mass index, diabetes, hypertension, coronary heart disease, smoking, and alcohol.
In sensitivity analyses, no meaningful differences were observed when adjusting for prevalent CHF and MI (Supplementary material online, Table S2), in time-updated analyses including incident CHF and MI (Supplementary material online, Table S3), nor when adjusting for prevalent atrial fibrillation in multivariable regression analyses (Supplementary material online, Table S4). When excluding those with prevalent coronary heart disease (n = 517), only those performing high exercise intensity exhibited a lower risk of developing incident conduction disease (Supplementary material online, Table S5).
When adjusting for use of all anti-arrhythmic drugs at baseline (n = 1208) in multivariable regression models and when excluding those on class I and III anti-arrhythmic drugs (n = 92) in unadjusted and adjusted regression models, no substantive differences were observed (Supplementary material online, Tables S6 and S7). Similar results persisted when including incident ventricular pacing in the outcome (Supplementary material online, Table S8) and when considering all-cause death as a competing risk (Supplementary material online, Table S9). Supplementary material online, Tables S10 and S11 show the numbers of deaths at each ECG timepoint and loss to follow-up before the last possible ECG or death, respectively.
No meaningful differences were observed when the analyses were stratified by age ≥ 75 years vs. younger (Supplementary material online, Table S12; P for interaction = 0.84).
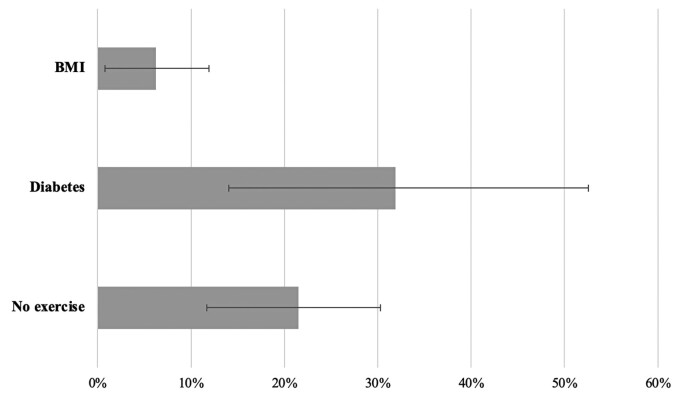
When examining non-immutable covariates that were significantly associated with incident conduction disease and assuming causal effects between modifiable predictors and incident conduction disease were present, reduced physical activity would explain more than a fifth and diabetes almost a third of the conduction disease experienced by this population (Figure 4).
Figure 4.
Population attributable fractions for incident conduction disease. Each bar represents the estimated population attributable fraction of incident conduction disease for every baseline modifiable characteristic associated with incident conduction disease after multivariable adjustment. Multivariable adjustment included age, gender, race, education, body mass index, diabetes, hypertension, coronary heart disease, physical activity, smoking, and alcohol. Error bars denote 95% confidence intervals.
Prevalent infra-Hisian disease (including any fascicular block, bundle branch block, or non-specific intraventricular block) was detected in 469 participants and was associated with older age, male sex, a larger BMI, diabetes, hypertension, and coronary heart disease in unadjusted analysis, whereas White race and high exercise intensity were associated with lower prevalence. Except for coronary heart disease, they all remained associated with prevalent infra-Hisian disease after multivariable adjustment (Supplementary material online, Table S13). A total of 714 exhibited incident infra-Hisian disease during a median follow-up time on 8 (IQR 1–9) years. After multivariable adjustment, older age, male sex, a larger BMI, diabetes, and first-degree AV block at baseline were associated with a higher risk of incident infra-Hisian disease, whereas more physical activity was associated with a lower risk (Figure 5).
Figure 5.
Characteristics associated with incident infra-Hisian disease. Risk factors associated with incident infra-Hisian disease. Multivariable adjustment included all covariates listed. Circles denote hazard ratios for prevalent conduction disease and error bars denote 95% confidence intervals. aInterpreted as a hazard for every 10-year increase bInterpreted as a hazard for every 10 kg/m2 increase.
Discussion
While some immutable characteristics, including older age and male sex, were associated with conduction disease as expected, a readily modifiable lifestyle behaviour, physical activity, was associated with a lower risk even after adjusting for baseline cardiovascular disease and time-updated CHF and MI (Structured Graphical Abstract). Additional less readily modifiable risk factors associated with incident conduction disease included a larger BMI and diabetes. In contrast, other lifestyle factors previously commonly associated with other cardiovascular diseases, such as cigarette smoking and alcohol consumption, did not exhibit a significant association with incident conduction disease.
To our knowledge, this is the first study to investigate the association between physical activity and incident conduction disease. The results showed an inverse association between exercise intensity level and the risk of conduction disease, but only those performing high exercise intensity exhibited a significantly lower risk of incident conduction disease across all sensitivity analyses. More broadly, the relationship between more physical activity and lower risks of cardiovascular disease and all-cause mortality is well-established.22,23 The mechanisms underlying the protective effects of physical activity on cardiovascular risk remain largely unknown but might be explained by the deceleration of the atherosclerotic process, including improvement of endothelial dysfunction, and decreased systemic inflammation.24,25 As cardiac conduction disease arises from fibrosis and fibrosis often stems from an initial inflammatory process,26 it would appear similar mechanisms could explain a protective effect on the development of conduction disease among the more physically active. Some degree of the favourable relationships observed could be explained by beneficial effects of physical activity on mediating risk factors, including diabetes and larger BMI,27,28 both of which showed increased risk of conduction disease in the current study. As the multivariable analyses included adjusting for these possible mediators, the overall reductions in conduction disease related to more physical activity may in fact have been under-estimated. While we cannot conclude that causal effects regarding physical activity and conduction disease were present in this observational study, we conducted calculations to report the magnitude of the relationship that might be clinically meaningful to clinicians, patients, and the lay public should causal relationships in fact exist. Under those assumptions, these data suggest that successfully encouraging physical activity might help mitigate a substantial proportion of conduction disease in the general population.
While the absence of an observed relationship between alcohol and conduction disease is consistent with the previous literature,12 the negative findings regarding smoking, a major predictor of ischaemic heart disease and tissue fibrosis,29,30 are somewhat surprising. However, previous studies investigating the effects of smoking on conduction abnormalities have produced varied results: whereas some studies have found that nicotine exposure expedites AV nodal conduction,31 others suggest that cigarette smoking has limited effects on the His-Purkinje system conduction and intra-ventricular conduction.32 In addition, while we previously reported a correlation between smoking and conduction disease in a different cohort at high risk of cardiovascular disease,33 the current study cohort represented the general population. Finally, smoking was based on self-report, and individuals may have underreported their smoking history which, given an actual association between smoking and incident conduction disease was present, would likely distort the results towards the null hypothesis.
Several traditional risk factors identified in the current analysis recapitulated previous work on the subject. Heightened risk among older individuals and men in particular has been consistently observed.5,11,33 The observed association between diabetes and a higher risk of conduction disease also fits with previous studies.12,33 Kerola et al.12 found that higher fasting glucose levels were associated with incident AV block, with 11% of the risk of AV block possibly attributed to an elevated fasting glucose level. Once again, potential mechanisms linking diabetes and conduction disease may be related to inflammation and fibrosis as well as enhanced risks associated with mediators such as coronary artery disease and MI.34,35
To our surprise, given the known mechanistic links between hypertension and fibrosis in the cardiovascular system,36–38 hypertension did not appear to predict incident conduction disease. However, the point estimates favoured an increased risk of incident disease among those with hypertension, and participants with hypertension exhibited a greater odds of prevalent conduction disease. There are several potential explanations for the negative findings regarding incident disease: we may have had inadequate power to detect a statistically significant relationship, the effects of hypertension may occur early (hence those destined for the disease already manifested prevalent disorders), and adverse consequences of hypertension may have been somewhat mitigated by anti-hypertensive treatment.
Because the PR interval is comprised of intra-atrial conduction time, AV nodal conduction time (itself modulated by dynamic autonomic, particularly parasympathetic, tone), and intra-Hisian conduction, it may not represent the same processes generally attributed to fibrosis of the cardiac conduction system that can lead to bundle branch blocks, complete heart block, and other adverse cardiovascular outcomes.1 We therefore performed an analysis examining only infra-Hisian conduction disease. Importantly, this yielded nearly identical results as the overall analyses, most notably demonstrating again that more physical activity was associated with a lower risk of infra-Hisian disease. Therefore, the relationships observed pertain to risk factors for structural adverse remodelling of the cardiac conduction system and are not simply reflective of, for example, larger or diseased atria or more vagal tone influencing the AV node.
Our study brings several strengths: to our knowledge, this is the first population-based study to evaluate the association between common lifestyle factors and the incidence of conduction diseases. Previous studies examining similar relationships have been limited to single conduction abnormalities,5,12,39,40 study populations restricted to men,4,41 or only included patients with high cardiovascular risks.33,42 The study population, assembled from a random sample of Medicare beneficiaries, should not result in selection bias that is commonly present in studies using medical records relying on individuals seeking medical care. Comprehensive baseline covariates, including lifestyle habits, were prospectively ascertained in a uniform method according to predefined protocols, and incident outcomes were obtained over years of longitudinal follow-up.
It is important to acknowledge several limitations. As mentioned, the observational design of the study prevents us from drawing conclusions regarding causal relationships. While it is possible the associations observed represented effects on overall cardiovascular health rather than direct consequences on the conduction system, those with baseline CHF and MI were excluded from the main analyses, and a sensitivity analysis adjusting for time-updated CHF and MI did not meaningfully change the results. The study also had some loss to follow-up, though it appears unlikely that imperfect retention would result in a spurious false positive relationship between more physical activity and less incident conduction disease. Known predictors of concomitant ventricular conduction abnormalities, such as atrial fibrillation, CHF, and MI, were excluded from the study population; however, sensitivity analyses showed no meaningful differences when including those predictors in multivariable analyses. Further, most of the primary exposures, including the lifestyle factors, were based on ascertainment from self-reported questionnaires at baseline, which might induce recall bias. The baseline assessments may not have reflected actual behaviours over the years of follow-up, but previous assessments suggest that most of the measured covariates remain fairly stable within individuals over time43–45 and analyses in the current study examining physical activity patterns over several years also revealed consistent results. The current study did not cover all lifestyle habits such as dietary patterns, which could also potentially impact the development of conduction disease. Physical activity was limited to a composite exercise intensity score and did not consider other measurements such as walking pace or overall walking score. Moreover, we did not fully ascertain all conditions that may have reduced the ability to perform physical activity, such as neurologic disorders. However, those comorbidities do not have an established association with conduction disease. Importantly, we did not assess every possible cause of AV block, such as autoimmune conditions, thyroid dysfunction, or Lyme disease. To our knowledge, such factors would not be expected to result in infra-Hisian disease independent of AV block, which constituted the majority of our outcome of interest. In addition, we did not address all known causes of cardiac conduction disease, such as valvular procedures or sleep apnoea. Of note, the current study was not designed to elucidate the mechanisms responsible for the observed associations, and some exposures, such as sleep apnoea, could potentially be mediators and not true confounders (i.e. those more physically active could consequently experience less sleep apnoea, which would then be along the causal pathway towards conduction disease). Finally, as the study only included individuals above the age of 65 years, primarily of White race, generalization of our findings to younger individuals and those of other racial and ethnic groups should be made with caution.
Conclusions
Those performing more regular physical activity experienced less cardiac conduction disease. This common condition associated with worse cardiovascular outcomes and overall mortality that can only be treated in the most severe examples with an invasive and permanent artificial pacemaker may be mitigated by lifestyle behaviour readily available to the great majority of individuals.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the support from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Neurological Disorders and Stroke (NINDS) and the contributions of the research institutions, study investigators, research staff, and study participants in creating this resource for biomedical research.
Contributor Information
Emilie K Frimodt-Møller, Division of Cardiology, University of California San Francisco, 505 Parnassus Ave, M1180B, San Francisco, CA 94143, USA; Department of Cardiology, Herlev and Gentofte Hospital, University of Copenhagen, Copenhagen, Denmark.
Elsayed Z Soliman, Department of Cardiovascular Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Jorge R Kizer, Division of Cardiology, University of California San Francisco, 505 Parnassus Ave, M1180B, San Francisco, CA 94143, USA.
Eric Vittinghoff, Division of Cardiology, University of California San Francisco, 505 Parnassus Ave, M1180B, San Francisco, CA 94143, USA.
Bruce M Psaty, Cardiovascular Health Research Unit, Departments of Medicine, Epidemiology, and Health Systems and Population Health, University of Washington, Washington, WA 98195-9458, USA.
Tor Biering-Sørensen, Department of Cardiology, Herlev and Gentofte Hospital, University of Copenhagen, Copenhagen, Denmark; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
John S Gottdiener, Division of Cardiology, University of Maryland, Baltimore, MD 21201, USA.
Gregory M Marcus, Division of Cardiology, University of California San Francisco, 505 Parnassus Ave, M1180B, San Francisco, CA 94143, USA.
Supplementary data
Supplementary data is available at European Heart Journal online.
Funding
The CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006 and grants U01HL080295 and U01HL130114 from the NHLBI, with additional contribution from the NINDS. Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Data availability
The data used for this study were provided by the Cardiovascular Health Study (CHS) (https://chs-nhlbi.org/) with permission, and the authors therefore do not have the authority to share the study data with investigators outside the University of California, San Francisco. Investigators can submit an application to obtain the data directly from the CHS (https://chs-nhlbi.org/sites/chs-nhlbi.org/files/page/CHS%20Data_Request_Form%20v8_2020.pdf) using their established processes.
References
- 1. Davies M, Harris A. Pathological basis of primary heart block. Heart 1969;31:219–226. 10.1136/hrt.31.2.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein AD, Parsonnet V. Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol 2001;24:842–855. 10.1046/j.1460-9592.2001.00842.x [DOI] [PubMed] [Google Scholar]
- 3. Smits J, Veldkamp M, Wilde A. Mechanisms of inherited cardiac conduction disease. Europace 2005;7:122–137. 10.1016/j.eupc.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 4. Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation 1998;98:2494–2500. 10.1161/01.CIR.98.22.2494 [DOI] [PubMed] [Google Scholar]
- 5. Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen city heart study. Eur Heart J 2013;34:138–146. 10.1093/eurheartj/ehs291 [DOI] [PubMed] [Google Scholar]
- 6. Sze E, Daubert JP. Left bundle branch block-induced left ventricular remodeling and its potential for reverse remodeling. J Interv Card Electrophysiol 2018;52:343–352. 10.1007/s10840-018-0407-2 [DOI] [PubMed] [Google Scholar]
- 7. Mandyam MC, Soliman EZ, Heckbert SR, Vittinghoff E, Marcus GM. Long-term outcomes of left anterior fascicular block in the absence of overt cardiovascular disease. JAMA 2013;309:1587–1588. 10.1001/jama.2013.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 2011;32:991–998. 10.1093/eurheartj/ehq497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uslan DZ. Permanent pacemaker and implantable cardioverter defibrillator infection. Arch Intern Med 2007;167:669. 10.1001/archinte.167.7.669 [DOI] [PubMed] [Google Scholar]
- 10. Palmisano P, Accogli M, Zaccaria M, Luzzi G, Nacci F, Anaclerio M, et al. . Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. EP Europace 2013;15:531–540. 10.1093/europace/eus337 [DOI] [PubMed] [Google Scholar]
- 11. Schneider JF. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979;90:303–310. 10.7326/0003-4819-90-3-303 [DOI] [PubMed] [Google Scholar]
- 12. Kerola T, Eranti A, Aro AL, Haukilahti MA, Holkeri A, Junttila MJ, et al. . Risk factors associated with atrioventricular block. JAMA Netw Open 2019;2:e194176. 10.1001/jamanetworkopen.2019.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. . The cardiovascular health study: design and rationale. Ann Epidemiol 1991;1:263–276. 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 14. Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, et al. . Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Ann Epidemiol 1995;5:270–277. 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 15. Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, et al. . Study of cardiovascular health outcomes in the era of claims data. Circulation 2016;133:156–164. 10.1161/CIRCULATIONAHA.115.018610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755. 10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 17. Soares-Miranda L, Siscovick DS, Psaty BM, Longstreth WT, Mozaffarian D. Physical activity and risk of coronary heart disease and stroke in older adults. Circulation 2016;133:147–155. 10.1161/CIRCULATIONAHA.115.018323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soliman EZ, Prineas RJ, Case LD, Russell G, Rosamond W, Rea T, et al. . Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart 2011;97:1597–1601. 10.1136/hrt.2010.215871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993;49:865. 10.2307/2532206 [DOI] [PubMed] [Google Scholar]
- 20. Kiwanuka N, Ssetaala A, Ssekandi I, Nalutaaya A, Kitandwe PK, Ssempiira J, et al. . Population attributable fraction of incident HIV infections associated with alcohol consumption in fishing communities around lake Victoria, Uganda. PLoS One 2017;12:e0171200. 10.1371/journal.pone.0171200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brady AR. Adjusted population attributable fractions from logistic regression. Stata Technical Bulletin 1998;7:8–12. [Google Scholar]
- 22. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. . 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019;140:e596–e646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 24. Cheng SJ, Yu HK, Chen YC, Chen CY, Lien WC, Yang PY, et al. . Physical activity and risk of cardiovascular disease among older adults. Int J Gerontol 2013;7:133–136. 10.1016/j.ijge.2013.03.001 [DOI] [Google Scholar]
- 25. Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol 2002;22:1869–1876. 10.1161/01.ATV.0000036611.77940.F8 [DOI] [PubMed] [Google Scholar]
- 26. Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210. 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LaMonte MJ, Blair SN. Physical activity, cardiorespiratory fitness, and adiposity: contributions to disease risk. Curr Opin Clin Nutr Metab Care 2006;9:540–546. 10.1097/01.mco.0000241662.92642.08 [DOI] [PubMed] [Google Scholar]
- 28. Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes Mellitus. N Engl J Med 1991;325:147–152. 10.1056/NEJM199107183250302 [DOI] [PubMed] [Google Scholar]
- 29. Barry J, Mead K, Nabel EG, Rocco MB, Campbell S, Fenton T, et al. . Effect of smoking on the activity of ischemic heart disease. JAMA 1989;261:398–402. 10.1001/jama.1989.03420030072032 [DOI] [PubMed] [Google Scholar]
- 30. Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, et al. . Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 2007;93:1056–1063. 10.1136/hrt.2005.087171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Irfan AB, Arab C, DeFilippis AP, Lorkiewicz P, Keith RJ, Xie Z, et al. . Smoking accelerates atrioventricular conduction in humans concordant with increased dopamine release. Cardiovasc Toxicol 2021;21:169–178. 10.1007/s12012-020-09610-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bekheit S, Fletcher E. The effects of smoking on myocardial conduction in the human heart. Am Heart J 1976;91:712–720. 10.1016/S0002-8703(76)80536-4 [DOI] [PubMed] [Google Scholar]
- 33. Dewland TA, Soliman EZ, Davis BR, Magnani JW, Yamal JM, Piller LB, et al. . Effect of the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) on conduction system disease. JAMA Intern Med 2016;176:1085–1092. 10.1001/jamainternmed.2016.2502 [DOI] [PubMed] [Google Scholar]
- 34. le Feuvre C, Jacqueminet S, Barthelemy O. Myocardial ischemia: a silent epidemic in type 2 diabetes patients. Future Cardiol 2011;7:183–190. 10.2217/fca.10.127 [DOI] [PubMed] [Google Scholar]
- 35. Nesto RW, Phillips RT. Asymptomatic myocardial ischemia in diabetic patients. Am J Med 1986;80:40–47. 10.1016/0002-9343(86)90451-1 [DOI] [PubMed] [Google Scholar]
- 36. Querejeta R, Lopez B, Gonzalez A, Sanchez E, Larman M, Ubago JL M, et al. . Increased collagen type I synthesis in patients with heart failure of hypertensive origin. Circulation 2004;110:1263–1268. 10.1161/01.CIR.0000140973.60992.9A [DOI] [PubMed] [Google Scholar]
- 37. Díez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens 2007;9:546–550. 10.1111/j.1524-6175.2007.06626.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med 2005;2:209–216. 10.1038/ncpcardio0158 [DOI] [PubMed] [Google Scholar]
- 39. Alventosa-Zaidin M, Guix Font L, Benitez Camps M, Roca Saumell C, Pera G, et al. . Right bundle branch block: prevalence, incidence, and cardiovascular morbidity and mortality in the general population. Eur J Gen Pract 2019;25:109–115. 10.1080/13814788.2019.1639667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider JF. Newly acquired right bundle-branch block: the Framingham study. Ann Intern Med 1980;92:37–44. 10.7326/0003-4819-92-1-37 [DOI] [PubMed] [Google Scholar]
- 41. Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. Eur Heart J 2005;26:2300–2306. 10.1093/eurheartj/ehi580 [DOI] [PubMed] [Google Scholar]
- 42. Pollari F, Großmann I, Vogt F, Kalisnik JM, Cuomo M, Schwab J, et al. . Risk factors for atrioventricular block after transcatheter aortic valve implantation: a single-centre analysis including assessment of aortic calcifications and follow-up. EP Europace 2019;21:787–795. 10.1093/europace/euy316 [DOI] [PubMed] [Google Scholar]
- 43. Knott CS, Bell S, Britton A. The stability of baseline-defined categories of alcohol consumption during the adult life-course: a 28-year prospective cohort study. Addiction 2018;113:34–43. 10.1111/add.13949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schliep KC, Schisterman EF, Mumford SL, Perkins NJ, Ye A, Pollack AZ, et al. . Validation of different instruments for caffeine measurement among premenopausal women in the BioCycle study. Am J Epidemiol 2013;177:690–699. 10.1093/aje/kws283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, et al. . Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology 2010;51:201–209. 10.1002/hep.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this study were provided by the Cardiovascular Health Study (CHS) (https://chs-nhlbi.org/) with permission, and the authors therefore do not have the authority to share the study data with investigators outside the University of California, San Francisco. Investigators can submit an application to obtain the data directly from the CHS (https://chs-nhlbi.org/sites/chs-nhlbi.org/files/page/CHS%20Data_Request_Form%20v8_2020.pdf) using their established processes.