Abstract
Mouse embryonic stem (ES) cells can proliferate indefinitely in an undifferentiated state in the presence of leukemia inhibitory factor (LIF), or differentiate into all three germ layers upon removal of this factor. To determine cellular factors associated with self-renewal of undifferentiated ES cells, we used polymerase chain reaction-assisted cDNA subtraction to screen genes that are expressed in undifferentiated ES cells and down-regulated after incubating these cells in a differentiation medium without LIF for 48 h. The mRNA expression of a tetraspanin transmembrane protein, CD9, was high in undifferentiated ES cells and decreased shortly after cell differentiation. An immunohistochemical analysis confirmed that plasma membrane-associated CD9 was expressed in undifferentiated ES cells but low in the differentiated cells. Addition of LIF to differentiating ES cells reinduced mRNA expression of CD9, and CD9 expression was accompanied with a reappearance of undifferentiated ES cells. Furthermore, activation of STAT3 induced the expression of CD9, indicating the LIF/STAT3 pathway is critical for maintaining CD9 expression. Finally, addition of anti-CD9 antibody blocked ES cell colony formation and reduced cell viability. These results indicate that CD9 may play a role in LIF-mediated maintenance of undifferentiated ES cells.
INTRODUCTION
Mouse embryonic stem (ES) cells, which originally derived from inner cell mass of an early embryo named blastocyst, are able to sustain their pluripotency in in vitro cell culture (Evans and Kaufman, 1981; Martin, 1981). Undifferentiated mouse ES cells can be maintained for a long time in media containing the cytokine leukemia inhibitory factor (LIF) (Smith et al., 1988; Williams et al., 1988). Pluripotency of such cultured ES cells has been demonstrated in both in vivo and in vitro experiments. When injected into blastocysts, ES cells participate in embryonic development involving all three germ layers producing chimeric mice (Bradley et al., 1984). ES cells also form teratomas containing various mature tissues when injected into immunocompromised mice (Evans and Kaufman, 1981; Martin, 1981). In vitro, mouse ES cells start to differentiate to every possible lineage upon removal of LIF from the culture medium. The mechanism by which LIF maintains ES cells in undifferentiated state is not completely understood. The transcription factor STAT3 is a downstream target of LIF and its receptor interaction (Niwa et al., 1998; Matsuda et al., 1999). It has been shown that the activity of STAT3 is necessary and sufficient for LIF-induced self-renewal of mouse ES cells (Matsuda et al., 1999). It remains unclear, however, which genes are regulated by the STAT3 transcription factor in mouse ES cells and play actual roles in the maintenance of stem cells. Indeed, there is no generally accepted mechanism by which stem cells are maintained as undifferentiated cells. Human ES cell cultures have been recently established using mouse fibroblasts as feeder cells (Shamblott et al., 1998; Thomson et al., 1998). In contrast to mouse ES cells, LIF cannot replace such feeder cells to maintain self-renewal of human ES cells (Thomson et al., 1998). In addition, some of the adult stem cells may be maintained in a multipotent status in vitro for extended intervals (Pittenger et al., 1999), but the in vitro culture of others such as hematopoietic and neural stem cells have not yet been successfully established as homogeneous stem cell populations. Elucidating how different types of stem cells can be maintained in culture may provide important clues into the regulation of stem cell self-renewal, and may prove critical to facilitate in studies of adult stem cells. It is important to understand the molecular mechanisms of in vitro maintenance of stem cells, particularly when clinical application of such stem cells into various diseases now becomes promising (Petersen and Terada, 2001).
To identify candidate genes that play a role in stem cell maintenance, we attempted to isolate genes that were highly expressed in undifferentiated ES cells but rapidly down-regulated after cell differentiation. We used a polymerase chain reaction (PCR)-assisted cDNA subtraction method that has been successfully applied to enrich/identify genes that are selectively expressed in other cells (Seale et al., 2000; Geschwind et al., 2001; Terskikh et al., 2001). Through this method, a plasma membrane-associated molecule, CD9, was determined to be a gene selectively expressed in undifferentiated ES cells. Additionally, the potential regulation and role of CD9 in mouse ES cells were explored.
MATERIALS AND METHODS
ES Cell Culture
Mouse ES cell lines R1 (a gift from A. Nagy, Toronto, Ontario, Canada) or EB3/5 (a gift from H. Niwa, Osaka, Japan) were maintained on gelatin-coated dish in a medium containing 1000 U/ml recombinant mouse LIF (ESGRO; Chemicon International, Temecula, CA) as described previously (Hamazaki et al., 2001). In vitro ES cell differentiation was induced using a standard method as we described previously (Minamino et al., 1999; Hamazaki et al., 2001). Briefly, ES cells were washed twice with phosphate-buffered saline (PBS), and resuspended in a differentiation medium (Iscove's modified Dulbecco's medium [Invitrogen, Carlsbad, CA] containing 20% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 300 μM monothioglycerol). Cells were cultured on Petri dish in which ES cells form aggregated embryoid body. In experiments of readdition of LIF, ES cells were differentiated in the ES differentiation medium described above for initial 3 d, and LIF (or 4-hydroxy tamoxifen [ 4-HT]) was added back directly to the culture.
PCR-assisted cDNA Subtraction
PCR-assisted cDNA subtraction was performed based on the manufacturer's manual for the PCR-Select cDNA subtraction kit (CLONTECH, Palo Alto, CA). Total RNA was prepared from undifferentiated ES cells (tester) and 48-h differentiated ES cells (driver) by using an RNA aqueous kit (Ambion, Austin, TX). The mRNA was purified using Poly (A) Pure kit (Ambion).
Cloning and Sequencing of Subtracted cDNA
A subtracted cDNA library was cloned into TOPO TA-cloning vector (Invitrogen). After transformation, insert cDNAs were amplified by colony PCR with nested PCR primers 1 and 2R provided with the PCR-Select cDNA subtraction kit (CLONTECH). Amplified PCR fragments were purified with PCR purification kit (QIAGEN, Valenica, CA) and sequenced.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared from ES cells by using an Aqueous kit (Ambion). Total RNA (2 μg) was used for cDNA synthesis by using SuperScript II first-strand synthesis system with oligo(dT) (Invitrogen). Final products of reverse transcriptase reaction were filled up to 200 μl with H2O, and 5 μl was used for each PCR reaction. PCR amplification was performed using Taq DNA polymerase (Eppendorf, Westbury, NY). The PCR reaction consisted of 25–30 cycles (specified below) of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C. Sequence of upstream and downstream primers pair and cycle numbers used for each gene were as follows: CD9 (CAGTGCTTGCTATTGGACTATG, GCCACAGCAGTCCAACGCCATA, 30), osteopontin (GCAGACACTTTCACTCCAATCG, GCCCTTTCCGTTGTTGTCCTG, 30), CD81 (CCATCCAGGAGTCCCAGTGTCT, GAGCATGGTGCTGTGCTGTGGC, 30), platelet endothelial cell adhesion molecule-1 (PECAM-1) (AGGGGACCAGCTGCACATTAGG, AGGCCGCTTCTCTTGACCACTT, 30), E-cadherin (GTCAACACCTACAACGCTGCC, CTT-GGCCTCAAAATCCAAGCC, 25), β1 integrin (AATGTTTCAGTG-CAGAGCC, ATTGGGATGATGTCGGGAC, 30), α3 integrin (AACA-GCGCTACCTCCTTCTG, GTCCTTCCGCTGAATCATGT, 30), α5 integrin (GCTGGACTGTGGTGAAGACA, CAGTCGCTGACTGGGA-AAAT, 30), α6 integrin (AGGAGTCGCGGGATATCTTT, CAGGCCTTCTCCGTCAAATA, 30), heparin binding-epidermal growth factor (HB-EGF) (GTTGGTGACCGGTGAGAGTC, TGCAAGAGGGAGTACGGAAC, 30), brachyury (AAGGAACCACCGGTCATC, GTGTGCGTCAGTGGTGTGTAATG, 30), β-actin (TTCCTTCTTGGGTATGGAAT, GAGCAATGATCTTGATCTTC, 25), Oct-4 (TGGAGAC-TTTGCAGCCTGAG, TGAATGCATGGGAGAGCCCA, 30), UTF1 (GCCAACT-CATGGGGCTATTG, CGTGGAAGAACTGAATCTGAGC, 30), FGF4 (TACTGCAACGTGGGCATCGGA, GTGGGTTACCTTCATGGTAGG, 30), Rex-1 (CGTGTAACATACACCATCCG, GAAATCCTCTTCCAGAATGG, 30), and FGF5 (AAAGTCAATGGCTCCCACGAA, CTTCAGTCTGTACTTCACTGG, 30).
For each set of PCR primers, RT-PCR without reverse transcriptase was conducted to confirm that no genomic DNA was amplified.
Immunofluorescence Staining
ES cells were cultured on gelatin-coated plate, washed once with PBS, and fixed in 3.7% formamide/PBS for 15 min at room temperature. Cells were then treated with 0.5% Triton X/PBS for 5 min and with 5% bovine serum albumin/PBS for 1 h at room temperature. Cells were further incubated with either anti-SSEA1 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), anti-mouse osteopontin (R & D Systems, Minneapolis, MN), or anti-mouse CD9 (KMC8) (BD PharMingen, San Diego, CA) for 2 h at room temperature. After four times washing with PBS, cells were incubated with anti-mouse IgG, anti-goat IgG, or anti-rat IgG antibodies conjugated to fluorescein isothiocyanate (Jackson Immunoresearch Laboratories, West Grove, PA). After four times washing with PBS, cells were mounted by Vectashield containing 4,6 diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA).
Propidium Iodide Staining
Propidium iodide was added (final 10 μg/ml) directly to the culture medium for staining cells with low viability. After a 30-min incubation at room temperature, staining was observed under a fluorescent microscope (IX70; Olympus, Tokyo, Japan).
RESULTS
To identify genes that may be involved in maintenance of undifferentiated ES cells, we attempted to isolate genes that are highly expressed in ES cells and rapidly down-regulated after cell differentiation. Herein, we used a PCR-assisted subtraction method. The cDNA prepared from undifferentiated ES cells was subtracted by the cDNA from differentiating ES cells in a differentiation medium without LIF for 48 h. Of the subtracted cDNA clones, 304 cDNAs have been sequenced. Of these, 98 genes were cloned more than two times, and the rest were unique. Osteopontin was the most frequently cloned gene, and a total of nine cDNA clones (corresponding to a total of three different cDNA fragments) was found among the 304 sequenced genes. Osteopontin, one of the extracellular matrix proteins, has been shown to be highly expressed in ES cells and decreased after ES cell differentiation (Botquin et al., 1998). In the present screening, we also cloned other genes previously identified as ones highly expressed in ES cells and down-regulated after cell differentiation, such as Rex-1 (Hosler et al., 1989), KLF4 (Kelly and Rizzino, 2000), and stathmin (Doye et al., 1992) (one clone each). These results indicate that the method effectively identifies undifferentiated ES cell-associated genes.
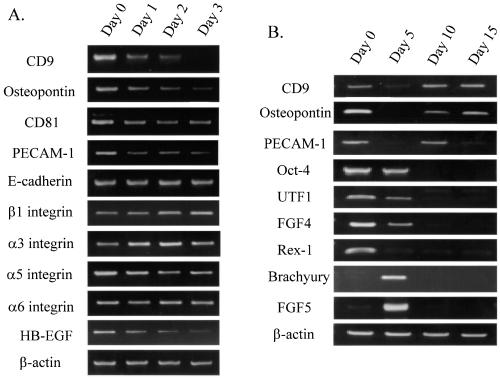
CD9 was among the genes identified, and its expression in ES cells has not been reported previously. CD9 is a type III membrane protein with four transmembrane domains (tetraspanin) and proposed to be involved in cell adhesion, migration, proliferation, and fusion (Ikeyama et al., 1993; Masellis-Smith and Shaw, 1994; Hadjiargyrou and Patterson, 1995; Maecker et al., 1997; Tachibana and Hemler, 1999). Using RT-PCR, we confirmed that the mRNA expression of CD9 was down-regulated within 24 h of ES cell differentiation (Figure 1A). The CD9 expression was further decreased until day 3 in the differentiation medium. In the same system, we also examined other cell adhesion-related molecules such as osteopontin and PECAM-1, which have been reported to be expressed in undifferentiated ES cells (Botquin et al., 1998; Robson et al., 2001). The mRNAs of both osteopontin and PECAM-1 were expressed in undifferentiated ES cells and down-regulated after differentiation as well. In contrast, the expression of other cell adhesion-related molecules including E-cadherin, and β1, α3, α5, and α6 integrins was not significantly changed throughout the time course of differentiation (days 0–3). Expression of HB-EGF, which is known to associate with CD9 (Iwamoto et al., 1994), also down-regulated during ES cell differentiation. The expression of CD81, another tetraspanin molecule closely related to CD9 (Maecker et al., 1997), was also detected in undifferentiated ES cells and modestly down-regulated after differentiation.
Figure 1.
(A) Expression pattern of CD9 and other cell adhesion-related molecules during initial ES cell differentiation. Total RNA was isolated from undifferentiated ES cells (day 0) and differentiated ES cells (days 1–3 after cultured in the differentiation medium). (B) Expression pattern of CD9 and other molecules during long-term ES cell differentiation. Total RNA was isolated from undifferentiated ES cells (day 0) and differentiated ES cells (days 5, 10, and 15 after cell differentiation). RNA was subjected to RT-PCR analysis. PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining.
We also examined the mRNA expression of CD9 in a longer time course of ES cell differentiation, up to day 15 (Figure 1B). CD9 mRNA was expressed in undifferentiated ES cells (day 0) and down-regulated at day 5 of differentiation. The expression was increased again at day 10 and 15 of differentiation. Osteopontin and PECAM-1 also highly expressed in ES cells, temporally decreased by day 5, and increased again at day 10. Accordingly, those cell adhesion-related molecules (CD9, osteopontin, and PECAM-1) were highly expressed in undifferentiated ES cells and rapidly decreased during initial cell differentiation. However, their expression was not ES cell specific in contrast to germ cell/early embryonic cell-specific genes such as Oct-4, UTF1, FGF4, and Rex-1 (Rogers et al., 1991; Niswander and Martin, 1992; Okuda et al., 1998; Pesce et al., 1998). Oct-4, known to be required for inner cell mass formation in blastocysts (Nichols et al., 1998), was expressed until day 5 of the ES cell differentiation and eliminated by day 10. Expression of UTF1, FGF4 and Rex-1 was also eliminated by days 5–10. Markers for early mesodermal differentiation, such as brachyury and FGF5 (Haub and Goldfarb, 1991) expression, were high at day 5 and decreased thereafter in the system. In addition, contractile cardiac myocytes were observed under microscope by day 8–10, and expression of albumin mRNA was detected by day 12 as we demonstrated previously (Hamazaki et al., 2001).
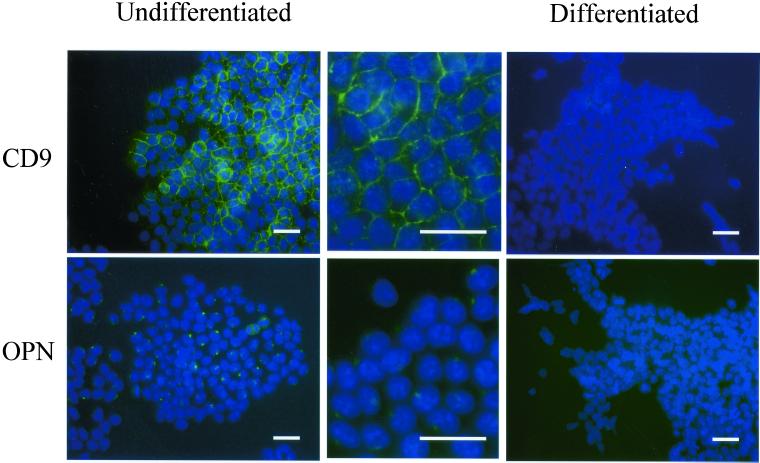
The protein expression of CD9 and osteopontin was then examined using immunofluorescence staining. CD9 was localized at cell surface of ES cells, as expected, when they were maintained in the ES maintenance medium containing LIF. However, within 5 d of cell differentiation, CD9 protein expression became almost undetectable (Figure 2). Osteopontin, which is a secreting protein, was detected at peri-nucleus, presumably endoplasmic reticulum or Golgi, in undifferentiated ES cells. The protein expression of osteopontin was similarly decreased within 5 d of ES cell differentiation.
Figure 2.
CD9 and osteopontin were selectively expressed in undifferentiated ES cells. The expression of CD9 and osteopontin in undifferentiated ES cells (day 0) or differentiated ES cells (day 5) was examined by indirect immunofluorescence analysis. Undifferentiated ES cells were maintained on gelatin-coated dish in the ES medium (left, low magnification; middle, high magnification). Differentiated ES cells were cultured for 5 d in the differentiation medium (right). Cells were fixed with 3.7% formaldehyde, permeabilized with 0.5% Triton X-100, and incubated initially with anti-CD9 antibody (1:1000 dilution) or anti-osteopontin antibody (1:1000 dilution), and subsequently with fluorescein isothiocyanate-conjugated anti-rat IgG (1:100 dilution; for CD9) or anti-goat IgG (1:100 dilution; for osteopontin). Cells were costained with 4,6 diamidino-2-phenylindole to demonstrate nuclei. Bar, 50 μm.
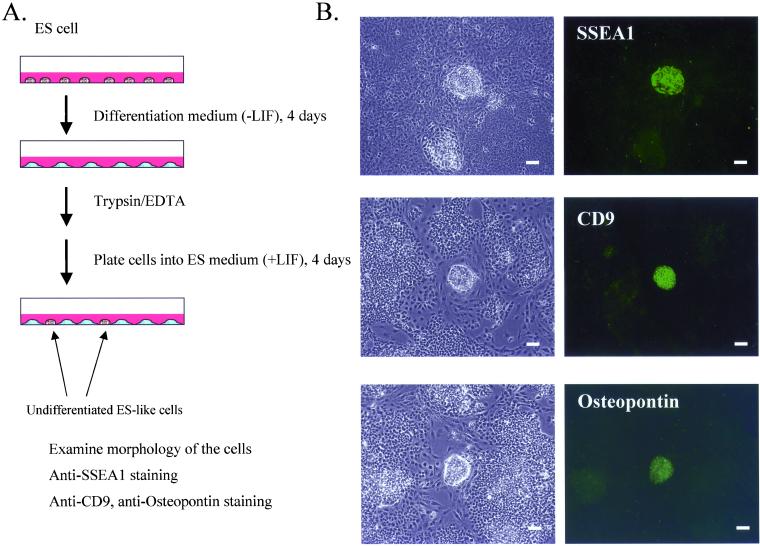
To further confirm the association of CD9 and osteopontin expression with undifferentiated status of ES cells, we examined their protein expression when LIF was added back to differentiating ES cells (Figure 3A). ES cells were incubated in the differentiation medium for 4 d, treated with trypsin, and then incubated in the ES maintenance medium containing LIF again. Most of the cells remained differentiated in this condition, but, of interest, several undifferentiated ES cell-like colonies (growing as a compact colony with tight cell-to-cell conjunctions) appeared in the culture within 4 d after switching to the LIF-containing medium (Figure 3B). These undifferentiated ES cell-like colonies were morphologically distinguishable from the other differentiated cells. Moreover, SSEA1, which is a surface marker for undifferentiated mouse ES cells (Solter and Knowles, 1978), was exclusively expressed in these compact colonies. Anti-CD9 staining revealed that these undifferentiated ES-like colonies also strongly expressed CD9. Importantly, the expression of CD9 was low or not observed in surrounding differentiated cells. Expression of osteopontin was also found exclusively in those ES-like colonies. These results indicate that CD9 expression as well as osteopontin expression is associated with the undifferentiated phenotype during early differentiation of ES cells.
Figure 3.
CD9 expression was detected exclusively in undifferentiated ES-like colonies. (A) ES cell differentiation was induced by removing LIF from the culture medium for 4 d. Cells were treated with trypsin, washed, and cultured again in the ES medium containing LIF. Within 4 d, undifferentiated ES-like colonies appeared in the culture. (B) Those mixed populations of the undifferentiated ES-like colonies and apparently differentiated cells were examined for the expression of SSEA1, CD9, and osteopontin by immunofluorescence staining as described in Figure 2 (right). Phase contrast images of the cells (left). Bar, 100 μm.
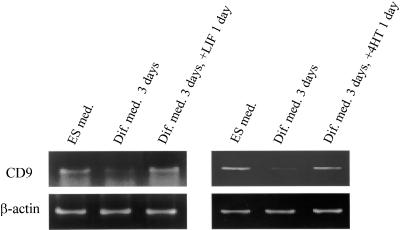
To examine whether LIF is a factor important for CD9 expression in the ES maintenance medium, we incubated ES cells in LIF-free differentiation medium for 72 h. Then, LIF was added back to the culture for additional 24 h. CD9 mRNA was increased by this readdition of LIF (Figure 4). STAT3 is known as a downstream target transcription factor of the LIF receptor-mediated signaling. By using STAT3ER (STAT3 fused to estrogen-ligand binding domain), with which STAT3 activity could be modulated by concentration of 4-HT in the medium, we examined whether STAT3 activity was sufficient for the up-regulation of CD9 expression. ES cells constitutively expressing STAT3ER were maintained with the ES maintenance medium containing LIF. After LIF removal for 3 d, 4-HT was added. As shown in Figure 4, CD9 mRNA expression was induced by addition of 4-HT. These results indicate that the LIF/STAT3 pathway is important for expression of CD9 in ES cells.
Figure 4.
CD9 expression was dependent on the LIF/STAT3 pathway. ES cells constitutively expressing STAT3ER were maintained in the ES medium containing LIF (ES med.). The cells were then cultured in the differentiation medium for 3 d (Dif. med. 3 d), and 1000 U/ml LIF or 1 μM 4-HT was added directly to the medium and cultured for another day (Dif. med. 3 d + LIF/4HT 1 d). RNA prepared from the cells was subjected to RT-PCR. PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining.
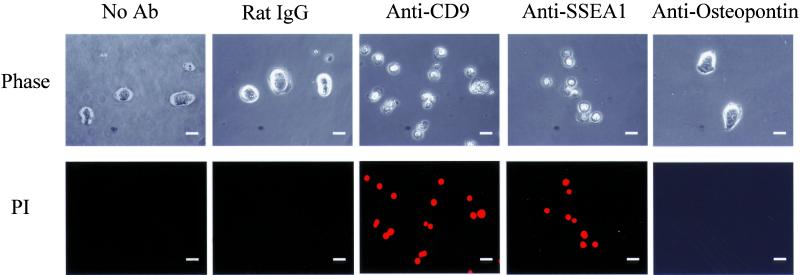
Finally, we investigated whether CD9 expression is important in undifferentiated ES cells by using a neutralizing antibody against CD9, KMC8 (Oritani et al., 1996). This antibody has been shown to block cell differentiation and cell fusion in other cell types, including myoblasts (Oritani et al., 1996; Aoyama et al., 1999; Tachibana and Hemler, 1999; Tanio et al., 1999). ES cells were incubated in the medium containing LIF in the presence of the antibody for 24 h. The control cells were incubated with the identical concentration of an isotypic control antibody containing the identical concentration of azide in stock solution. Interestingly, the ES cells cultured in the presence of the anti-CD9 antibody did not form compact ES-like colonies (Figure 5). Moreover, cells appeared to be dead within 24 h of culture in the presence of the anti-CD9 antibody. The decrease in cell viability after treatment with the anti-CD9 antibody was confirmed by propidium staining of cells (Figure 5). Anti-CD9 antibody decreased the cell viability at the concentration as low as 1 μg/ml. In contrast, neither nonspecific rat IgG2a,κ nor anti-osteopontin antibody (up to 10 μg/ml) affected the colony formation or survival of ES cells. Specific interaction between SSEA1 molecules on cell surface (LewisX determinants) is critical for cell aggregation of embryonic carcinoma cells (Eggens et al., 1989). An antibody against SSEA1, which blocks cell adhesion (Cao et al., 2001), also caused decrease in cell viability of ES cells as seen with the anti-CD9 antibody.
Figure 5.
Effect of anti-CD9 antibody on colony formation and viability of ES cells. ES cells cultured in the ES medium were washed with PBS and treated with trypsin for 10 min at 37°C in CO2 incubator. The cells were plated at the cell concentration of 2 × 103 cell/ml on gelatin-coated 24-well dish, and incubated for 24 h in ES medium without addition of any antibody, or in the presence (final concentration of 10 μg/ml) of control rat IgG2a,κ antibody (#555841; BD PharMingen), anti-CD9 antibody (KMC8, #558748; BD PharMingen), anti-SSEA1 antibody (MC-480; Developmental Studies Hybridoma Bank, University of Iowa), or anti-osteopontin antibody (#AF808; R & D Systems). Phase contrast images of cells are shown in top panels. Cell viability was monitored by staining cells with propidium iodide as described in MATERIALS AND METHODS (bottom). Bar, 20 μm.
DISCUSSION
A PCR-assisted subtraction method revealed that CD9 was among a group of genes highly expressed in ES cells but down-regulated shortly after cell differentiation. This group of genes includes several cell adhesion-related molecules such as osteopontin and PECAM-1, whereas others such as integrins β1, α3, α5, and α6 and E-cadherin were constitutively expressed during initial differentiation of ES cells. We also demonstrated that CD9 is likely under regulation of the LIF/STAT3 pathway in ES cells, which is critical for self-renewal of undifferentiated ES cells. Indeed, CD9 expression was exclusively associated with the undifferentiated phenotype of ES cells when such cells reappeared among differentiating ES cells by readdition of LIF to the culture. The expression of CD9 along with other cell adhesion-related molecules may be important for maintenance of undifferentiated ES cells. Furthermore, a blocking antibody against CD9 (KMC8) inhibited colony formation and survival of ES cells, suggesting that CD9 is playing a role in maintenance of ES cells in vitro.
A potential mechanism by which the anti-CD9 antibody reduced cell survival may be its interference with cell adhesion of ES cells as seen with anti-SSEA1 antibody (Cao et al., 2001). Although CD9 itself is not considered as a cell adhesion molecule, CD9 has been proposed to play a role in cell-extracellular matrix or cell–cell interactions as a cofactor of integrin (Rubinstein et al., 1994; Nakamura et al., 1995; Berditchevski et al., 1996). It still remains unclear, however, how the association of CD9 with integrin regulates cell–cell/cell–extracellular matrix interactions or modifies signal transduction through the integrin/integrin–ligand interaction. Osteopontin, one of the components of the extracellular matrix, can serve as a ligand of integrin (Denhardt et al., 2001), and PECAM-1 binds to integrins and enhances their function (Tanaka et al., 1992; Leavesley et al., 1994; Piali et al., 1995; Buckley et al., 1996; Newman, 1997). Collectively, integrin-associated signals may be important in self-renewal of ES cells.
Cell adhesion, especially interactions among cells and components of their environmental niche, is considered to be important for stem cell maintenance of hematopoietic stem cells (HSCs) (Chan and Watt, 2001). It has been demonstrated that CD9 may be involved in hematopoiesis. The KMC8 antibody against CD9 inhibited production of myeloid cells in long-term bone marrow cell cultures (Oritani et al., 1996) and expansion of erythroid progenitor cells (Aoyama et al., 1999) or osteoclasts (Tanio et al., 1999) when cocultured with stromal cells. Accordingly, CD9 might be important for colony formation or maintenance of HSCs. In this context, it is tempting to examine the expression of CD9 in adult stem cells other than HSCs, such as neuronal stem cells or liver stem cells.
CD9 is also known to associate with HB-EGF (Iwamoto et al., 1994), which is proposed to be important in cell survival under stressed conditions (Miyoshi et al., 1997; Takemura et al., 1997; Horikawa et al., 1999). Because induced expression of CD9 can potentiate the function of HB-EGF (Iwamoto et al., 1994; Higashiyama et al., 1995), CD9 may support cell survival through HB-EGF. HB-EGF mRNA was also expressed in ES cells and down-regulated during initial differentiation. It should be noted that CD9 may be involved in cell-cell fusion as well. CD9−/− female is sterile because CD9 null oocyte cannot fuse to sperm (Miyado et al., 2000; Le Naour et al., 2000), and overexpression of CD9 in myoblast-derived RD sarcoma cell facilitates the formation of multinucleated cells (Tachibana and Hemler, 1999).
Despite the potential role of CD9 in ES cells we propose herein, CD9 null mice are viable without an apparent abnormality except female infertility. One hypothesis would be that closely related tetraspanins such as CD81, which was also expressed in ES cells, may compensate function of CD9 during early embryonic development. Alternatively, CD9 may be required more stringently in in vitro maintenance of ES cells. Such a gap between in vitro ES cell maintenance and in vivo development has been observed previously with deficiencies of other molecules. The LIF/STAT3 signaling mediated by the LIF receptor/gp130 complex is critical for maintenance of ES cells in vitro; however, embryos lacking LIF, the LIF receptor, or gp130 develop normally, at least until midgestation (Stewart et al., 1992; Ware et al., 1995; Yoshida et al., 1996). Recently, Nichols et al. (2001) found that gp130−/− embryos were unable to resume embryogenesis after delayed implantation. Moreover, pluripotent cells were absent in delayed gp130−/− blastocysts, and they had reduced number of ICM cells due to apoptotic cell death. These results imply the importance of stem cell maintenance under suboptimal conditions even although it is not necessary for normal development. CD9 may be one of the factors downstream of the LIF/gp130/STAT3 pathway, critical for stem cell maintenance under such suboptimal conditions or stem cell maintenance in vitro. Maintenance of stem cells in vitro is important particularly when we consider clinical application of stem cells. Expansion of adult normal adult stem cells in vitro as a homogeneous population would facilitate application of such stem cells. The study of factors necessary for ES cell maintenance may contribute to a discovery of common mechanisms by which stem cells can be sustained as stem cells in vitro.
ACKNOWLEDGMENTS
We are indebted to Dr. Andras Nagy and Hitoshi Niwa for providing ES cell lines, Drs. Stephen Sugrue and James M. Crawford for critical reading of the manuscript, and Amy Meacham and Neal Devine for technical assistance. This work was supported by a grant from the National Institutes of Health to N.T. (DK-59699).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–01–0600. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–01–0600.
REFERENCES
- Aoyama K, Oritani K, Yokota T, Ishikawa J, Nishiura T, Miyake K, Kanakura Y, Tomiyama Y, Kincade PW, Matsuzawa Y. Stromal cell CD9 regulates differentiation of hematopoietic stem/progenitor cells. Blood. 1999;93:2586–2594. [PubMed] [Google Scholar]
- Botquin V, Hess H, Fuhrmann G, Anastassiadis C, Gross MK, Vriend G, Scholer HR. New POU dimer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by Oct-4 and Sox-2. Genes Dev. 1998;1998 12:2073–2090. doi: 10.1101/gad.12.13.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins) Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Cao Z, Zhao Z, Mohan R, Alroy J, Stanley P, Panjwani N. Role of the LewisX glycan determinant in corneal epithelial cell adhesion and differentiation. J Biol Chem. 2001;276:21714–21723. doi: 10.1074/jbc.M009672200. [DOI] [PubMed] [Google Scholar]
- Chan JY-H, Watt SM. Adhesion receptors on hematopoietic progenitor cells. Br J Hematol. 2001;112:541–557. doi: 10.1046/j.1365-2141.2001.02439.x. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye V, Kellermann O, Buc-Caron MH, Sobel A. High expression of stathmin in multipotential teratocarcinoma and normal embryonic cells versus their early differentiated derivatives. Differentiation. 1992;50:89–96. doi: 10.1111/j.1432-0436.1992.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Eggens I, Fenderson B, Toyokuni T, Dean B, Stroud M, Hakomori S. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem. 1989;264:9476–9484. [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, et al. A genetic analysis of neural progenitor differentiation. Neuron. 2001;29:325–339. doi: 10.1016/s0896-6273(01)00209-4. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- Hadjiargyrou M, Patterson PH. An anti-CD9 monoclonal antibody promotes adhesion and induces proliferation of Schwann cells in vitro. J Neurosci. 1995;15:574–583. doi: 10.1523/JNEUROSCI.15-01-00574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haub O, Goldfarb M. Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development. 1991;112:397–406. doi: 10.1242/dev.112.2.397. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa M, Higashiyama S, Nomura S, Kitamura Y, Ishikawa M, Taniguchi N. Upregulation of endogenous heparin-binding EGF-like growth factor and its role as a survival factor in skeletal myotubes. FEBS Lett. 1999;459:100–104. doi: 10.1016/s0014-5793(99)01213-2. [DOI] [PubMed] [Google Scholar]
- Hosler BA, LaRosa GJ, Grippo JF, Gudas LJ. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989;9:5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama S, Koyama M, Yamaoko M, Sasada R, Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993;177:1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DL, Rizzino A. DNA microarray analyses of genes regulated during the differentiation of embryonic stem cells. Mol Reprod Dev. 2000;56:113–123. doi: 10.1002/(SICI)1098-2795(200006)56:2<113::AID-MRD1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Oliver JM, Swart BW, Berndt MC, Haylock DN, Simmons PJ. Signals from platelet/endothelial cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human CD34+ hemopoietic progenitor cells. J Immunol. 1994;153:4673–4683. [PubMed] [Google Scholar]
- Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masellis-Smith A, Shaw AR. CD9-regulated adhesion. Anti-CD9 monoclonal antibody induce pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. J Immunol. 1994;152:2768–2777. [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL, Terada N. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci USA. 1999;96:15127–15132. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyado K, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Higashiyama S, Nakagawa T, Hayashi N, Taniguchi N. Membrane-anchored heparin-binding epidermal growth factor-like growth factor acts as a tumor survival factor in a hepatoma cell line. J Biol Chem. 1997;272:14349–14355. doi: 10.1074/jbc.272.22.14349. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell-cell contact sites. J Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339. doi: 10.1242/dev.128.12.2333. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda A, et al. UTF1, a novel transcriptional coactivator expressed in pluripotent embryonic stem cells and extra-embryonic cells. EMBO J. 1998;17:2019–2932. doi: 10.1093/emboj/17.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oritani K, Wu X, Medina K, Hudson J, Miyake K, Gimble JM, Burstein SA, Kincade PW. Antibody ligation of CD9 modifies production of myeloid cells in long-term cultures. Blood. 1996;87:2252–2261. [PubMed] [Google Scholar]
- Pesce M, Gross MK, Scholer HR. In line with our ancestors: Oct-4 and the mammalian germ. Bioessays. 1998;20:722–732. doi: 10.1002/(SICI)1521-1878(199809)20:9<722::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Terada N. Stem cells: A journey into a new frontier. J Am Soc Nephrol. 2001;12:1773–1780. doi: 10.1681/ASN.V1281773. [DOI] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Robson P, Stein P, Zhou B, Schultz RM, Baldwin HS. Inner cell mass-specific expression of a cell adhesion molecule (PECAM-1/CD31) in the mouse blastocyst. Dev Biol. 2001;234:317–329. doi: 10.1006/dbio.2001.0274. [DOI] [PubMed] [Google Scholar]
- Rogers MB, Hosler BA, Gudas LJ. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Le Naour F, Billard M, Prenant M, Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol. 1994;24:3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Natl Acad Sci USA. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Tachibana I, Hemler ME. Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J Cell Biol. 1999;146:893–904. doi: 10.1083/jcb.146.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura T, Kondo S, Homma T, Sakai M, Harris RC. The membrane-bound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. J Biol Chem. 1997;272:31036–31042. doi: 10.1074/jbc.272.49.31036. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanio Y, Yamazaki H, Kunisada T, Miyake K, Hayashi SL. CD9 molecule expressed on stromal cells is involved in osteoclastogenesis. Exp Hematol. 1999;27:853–859. doi: 10.1016/s0301-472x(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Terskikh AV, Easterday MC, Li L, Hood L, Kornblum HI, Geschwind DH, Weissman IL. From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs. Proc Natl Acad Sci USA. 2001;98:7652–7653. doi: 10.1073/pnas.131200898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Ware CB, et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Yoshida K, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]