Abstract
Optimal perioperative fluid management is crucial, with over- or under-replacement associated with complications. There are many strategies for fluid therapy, including liberal fluid therapy (LFT), restrictive fluid therapy (RFT) and goal-directed fluid therapy (GDT), without a clear consensus as to which is better.
We aimed to find out which is the more effective fluid therapy option in adult surgical patients undergoing non-vascular abdominal surgery in the perioperative period.
This study is a systematic review and network meta-analysis (NMA) with node-splitting analysis of inconsistency, sensitivity analysis and meta-regression.
We conducted a literature search of Pubmed, Cochrane Library, EMBASE, Google Scholar and Web of Science.
Only studies comparing restrictive, liberal and goal-directed fluid therapy during the perioperative phase in major non-cardiac surgery in adult patients will be included. Trials on paediatric patients, obstetric patients and cardiac surgery were excluded. Trials that focused on goal-directed therapy monitoring with pulmonary artery catheters and venous oxygen saturation (SvO2), as well as those examining purely biochemical and laboratory end points, were excluded.
A total of 102 randomised controlled trials (RCTs) and 78 studies (12,100 patients) were included. NMA concluded that goal-directed fluid therapy utilising FloTrac was the most effective intervention in reducing the length of stay (LOS) (surface under cumulative ranking curve (SUCRA) = 91%, odds ratio (OR) = -2.4, 95% credible intervals (CrI) = -3.9 to -0.85) and wound complications (SUCRA = 86%, OR = 0.41, 95% CrI = 0.24 to 0.69). Goal-directed fluid therapy utilising pulse pressure variation was the most effective in reducing the complication rate (SUCRA = 80%, OR = 0.25, 95% CrI = 0.047 to 1.2), renal complications (SUCRA = 93%, OR = 0.23, 95% CrI = 0.045 to 1.0), respiratory complications (SUCRA = 74%, OR = 0.42, 95% CrI = 0.053 to 3.6) and cardiac complications (SUCRA = 97%, OR = 0.067, 95% CrI = 0.0058 to 0.57). Liberal fluid therapy was the most effective in reducing the mortality rate (SUCRA = 81%, OR = 0.40, 95% CrI = 0.12 to 1.5). Goal-directed therapy utilising oesophageal Doppler was the most effective in reducing anastomotic leak (SUCRA = 79%, OR = 0.45, 95% CrI = 0.12 to 1.5). There was no publication bias, but moderate to substantial heterogeneity was found in all networks.
In preventing different complications, except mortality, goal-directed fluid therapy was consistently more highly ranked and effective than standard (SFT), liberal or restricted fluid therapy. The evidence grade was low quality to very low quality for all the results, except those for wound complications and anastomotic leak.
Keywords: intraoperative fluid therapy, systematic literature review, network meta-analysis, perioperative fluid management, general anaesthesia, continuous cardiac output monitoring, goal-directed fluid therapy
Introduction and background
Introduction
Background
Perioperative fluid management is an important factor in perioperative care that contributes to long-term mortality and morbidity [1] and is frequently debated amongst perioperative physicians [2-6]. The three current options are restrictive fluid therapy (RFT), goal-directed fluid therapy (GDT) and liberal fluid therapy (LFT). LFT assists with compensatory intravascular volume expansion, meets physiological requirements and compensates for blood loss, perioperative fasting and redistribution of third space. However, overhydration may lead to tissue oedema with poor wound healing, respiratory and cardiovascular complications and delayed recovery [1]. RFT aims for zero balance, less fluid volume given, with purported reduced perioperative complications [1] and shorter hospital stays. However, it may increase the risk of renal impairment and oliguria. GDT targets various measured hemodynamic variables to optimise hemodynamic status and oxygen delivery. Multiple randomised controlled trials (RCTs) and meta-analyses have been performed comparing the two of these three fluid therapies. Previous meta-analyses concluded that a restrictive approach may be beneficial [2-5]. However, since then, there have been new RCTs on fluid therapies, such as the Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery (RELIEF) trial in 2018, which concluded that a liberal approach did not increase complication rates and a restrictive approach resulted in increased risks of renal impairment [7]. There is only one updated meta-analysis included up to the RELIEF trial [8], and other later RCTs have still not been analysed in a meta-analysis [8,9].
With the advancement in intraoperative monitoring, novel means of achieving GDT have become available. Zhao et al. [10] recently performed a network meta-analysis (NMA) that compared these different GDTs in patients undergoing all types of surgery but did not compare GDT with other fluid therapies. NMA is an emerging technique for comparing multiple interventions in a single analysis through pooling direct and indirect evidence [11]. It allows different interventions to be compared indirectly if they have also been compared to one common intervention in different RCTs. This study is the first to utilise NMA to compare the many subgroups of GDT with RFT, LFT and standard fluid therapies (SFTs) at the same time.
Objectives
We aimed to investigate which is the more effective fluid therapy strategy in adult surgical patients undergoing non-vascular abdominal surgery during the perioperative period. Is there a difference in the perioperative mortality, length of stay (LOS), complication rates and organ-specific complication rates? This study aims to compare the effectiveness of RFT versus LFT versus GDT in adult patients undergoing non-vascular abdominal surgery during the perioperative period using an NMA and systematic review of the available data.
Methods
Protocol and Registration
We utilised the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions (PRISMA-NMA) guideline [12]. This work was not registered on any systematic reviews registry.
Inclusion and Exclusion Criteria
Only RCTs comparing the effects of RFT, LFT and GDT during the perioperative phase in major non-cardiac surgery in adult patients were included. Studies of intraoperative and postoperative fluid therapy were included. To support the transitivity assumption in NMA, similar studies were selected [13]. Studies on fluid therapy in cardiothoracic surgery, orthopaedic surgery, neurosurgery and vascular surgery were excluded from the NMA but included in the systematic review. Trials on paediatric patients and obstetric patients were excluded. Only journal articles published in English were included. Articles that did not report the primary or secondary end points were excluded. Trials that focused on GDT with pulmonary artery catheters and venous oxygen saturation (SvO2), as well as those examining purely biochemical and laboratory end points, were excluded. Grey literature such as non-traditional articles including reports, audits, editorials, commentaries, conference reports and abstracts were excluded.
Information Sources and Literature Search
We conducted a literature search of Pubmed, Cochrane Library, EMBASE, Google Scholar and Web of Science. All searches were performed from December 2020 to January 2021. The databases were searched separately to allow mapping to relevant subject headings. The search strategy followed validated methods of the QUOROM statement and Cochrane collaboration [14,15]. We expanded the subject headings search to include all relevant subheadings but restricted to human studies and the English language. Search terms included multiple combinations of Medical Subject Headings (MeSH), such as fluid therapy, surgery, perioperative, operative, complications, restrictive, liberal, goal-directed, FloTrac, esophageal/oesophageal Doppler, pulse pressure variation, optimisation/optimization and others. We did not apply restrictions on publication status or date.
Study Selection and Data Collection Process
Two researchers (YX and AT) independently screened articles by their titles and abstracts to identify eligible studies. Eligible entries then proceeded to full-text review. The references of the identified articles were hand-searched to avoid missing relevant studies. Any new studies found this way had their reference list manually searched. YX and AT used a predesigned data abstraction form to record the trial characteristics, outcomes and information related to the quality of the trial. We scored each trial according to the Jadad scale [16]. Two adjudicators (LL and LW) helped resolve disagreement by consensus. The extracted data was then laid out in a systematic review table (Appendices).
Data Items
The following data were extracted from eligible studies: author name, publication year, study design (whether the randomised controlled trial was single centre or multicentre), surgery type, intervention type and control, intervention phase (preoperative, intraoperative, or postoperative), fluid protocols and hemodynamic monitors and patient number (intervention versus control). The primary outcomes were hospital mortality (both 30-day and 90-day mortality, if reported), length of stay (LOS) (days) and complication rate. When articles reported LOS in terms of median and interquartile range or range, or mean and 95% confidence intervals, the mean and standard deviation were calculated using validated formulas proposed by McGrath et al. [17], Wan et al. [18] and Higgins et al. [19]. The complication rate was reported as the number of patients having at least one complication to avoid double-counting patients and to ensure the event rate was less than the sample size. The secondary outcomes were complication severity (major or minor) and organ-specific complications, which included respiratory complications (respiratory failure, pulmonary oedema, pneumonia and pleural effusion), cardiac complications (cardiac failure, myocardial infarction and arrhythmia), wound infections (both deep and superficial were pooled into a single composite end point), renal complications (including any renal impairment and need for dialysis) and anastomotic leak.
Geometry of the Network
To avoid imbalanced distribution of studies per node and allow for meaningful comparisons between treatment options, we classified the studies based on the authors’ intentions and labels. Following the current literature, we defined RFT, which aimed for zero to negative fluid balance, with minimal physiological maintenance and minimal preloading of intravenous fluid before induction [20]. LFT was defined according to Miller’s Anesthesia [21]. There was no standard definition for SFT amongst different centres, and some studies did not specify it in their protocol. GDT was defined as a protocolised approach using hemodynamic monitors to direct volume replacement to achieve certain hemodynamic goals. We pooled interventions with only one or two studies into a single node (GDTOthers). As the pulse variability index and pulse pressure variation are based on similar concepts, these were pooled to reduce inconsistency (GDTPpv). The majority of GDT studies utilised oesophageal Doppler (GDTOD), Edwards Lifesciences Vigileo/FloTrac (GDTFlo) and lithium dilution cardiac output (LiDCO) (GDTLid). Studies with three arms were included. However, if two arms with the same GDT monitoring modality use different fluids, the data of both arms were pooled.
Risk of Bias Within Individual Studies
We used a modified Cochrane Collaboration Risk of Bias Tool (RoB2) to assess the risk of bias in randomised trials [19]. We assessed selection bias including random sequence generation and allocation concealment, performance bias such as blinding of participants and personnel, detection bias such as blinding of outcome assessment, attrition bias due to incomplete outcome data, and reporting bias due to selective reporting. The overall risk of bias was then assigned to each study and categorised as high risk, low risk or unclear.
Summary Measures
We estimated the comparative efficacy of each fluid therapy using mean difference (MD), odds ratio (OR) and 95% credible intervals (CrI). We then used the surface under cumulative ranking curve (SUCRA) to visualise the treatment hierarchy. For comparisons with significant inconsistency, we report the direct, indirect and network evidence with comments on the reliability of the evidence.
Methods of Statistical Analysis
We used RevMan 5.4 to produce a risk of bias assessment and summary as per the Cochrane guide [19]. For the NMA, we used R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), Rstudio version 1.4.1103, and the gemtc, rjags, dmetar and netmeta packages to perform the statistical analysis [22]. All statistical analysis was performed by researcher YX and corroborated by AT. We selected a Bayesian approach for better coverage and estimation of effects in terms of probabilities [23-25]. We performed Markov chain Monte Carlo (MCMC) simulations using a random effects model, and final estimates were based on stable posterior sampling after initial iterations were discarded. We assessed the simulations for convergence using trace plots and the Brooks-Gelman-Rubin statistic and assessed the density plots for normal distribution. The potential scale reduction factor (PSRF) was below 1.05 for all simulations, indicating reliability. SUCRA scores were given to each intervention using the models.
Assessment of Inconsistency and Additional Analyses
We evaluated the consistency of the network models using the node split method with significant inconsistency defined as P < 0.05. When we identified inconsistency, we reviewed the extracted data to ensure that there were no errors and examined the potential effect modifiers of the studies. We performed further sensitivity analyses to improve the robustness of the results. We used multivariate network meta-regression to analyse the remaining inconsistency. The deviance information criterion (DIC) was assessed, and we took a DIC of more than five to indicate that there were substantial differences in the models with and without the covariate [26]. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines were used to assess the quality of evidence of each network [27]. Evidence was graded as high quality, moderate quality, low quality or very low quality.
Risk of Bias Across Studies
We assessed heterogeneity for each outcome using I2 and defined I2 of 75% and greater as substantial heterogeneity, 25%-75% as moderate heterogeneity and below 25% as low heterogeneity as defined by Higgins et al. [28]. We used a comparison-adjusted funnel plot and Egger’s test to evaluate the risk of publication bias [29]. An asymmetrical funnel plot and a P-value of <0.1 on Egger’s test indicated the presence of publication bias.
Review
Results
Study Selection
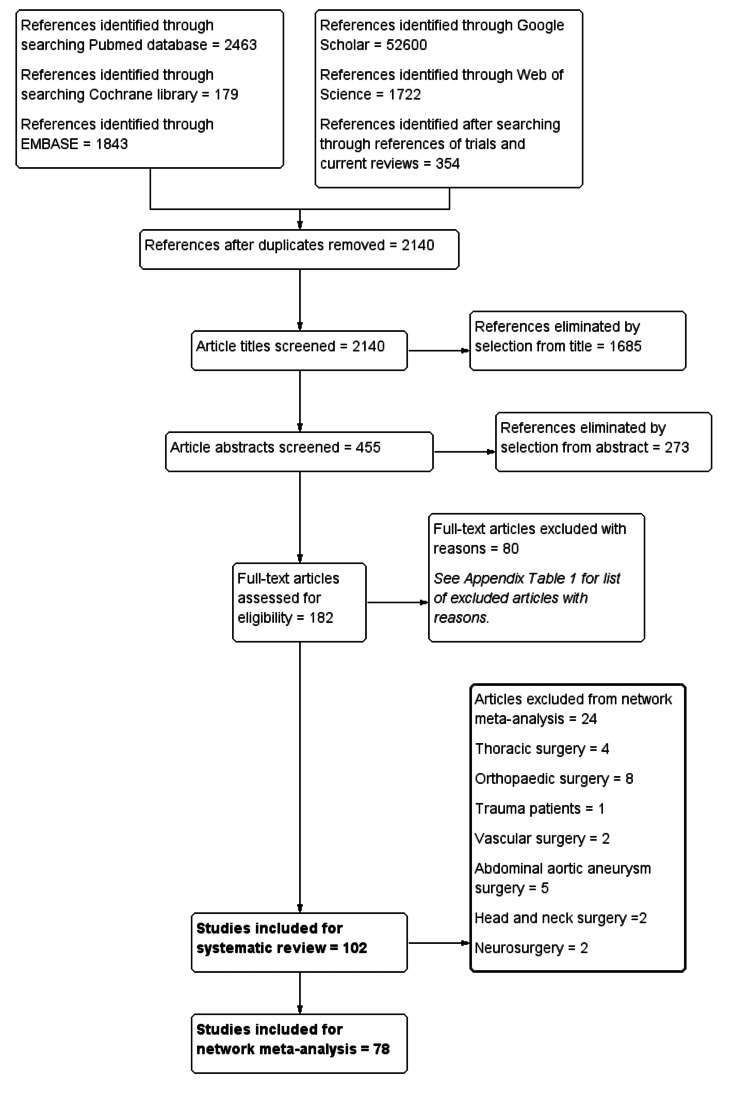
After a search and removal of duplicates, we screened 2,140 article titles and eliminated 1,685 studies. We assessed 182 articles for eligibility and included 102 articles in the systematic review [7,30-132], while 78 articles were eligible for network meta-analysis (Figure 1). The excluded studies and their reasons for exclusion, and the included studies and their characteristics can be found in the Appendices.
Figure 1. Study selection diagram.
Study Characteristics
Sixteen studies looked at RFT versus SFT, eight studies compared RFT to GDT, 10 studies compared RFT to LFT and 68 studies compared GDT to SFT. LOS, mortality and complications were reported by 85, 83 and 77 studies, respectively. In terms of secondary outcomes, wound complications, anastomotic leak, renal complications, respiratory complications and cardiac complications were reported by 64, 43, 63, 75 and 71 studies, respectively. Fifty studies were assessed to have a low risk of bias, while 52 studies had an unclear or high risk of bias. In total, there were 14,017 patients included in the systematic review, with a median number of 80 patients per study.
Summary of Network Geometry
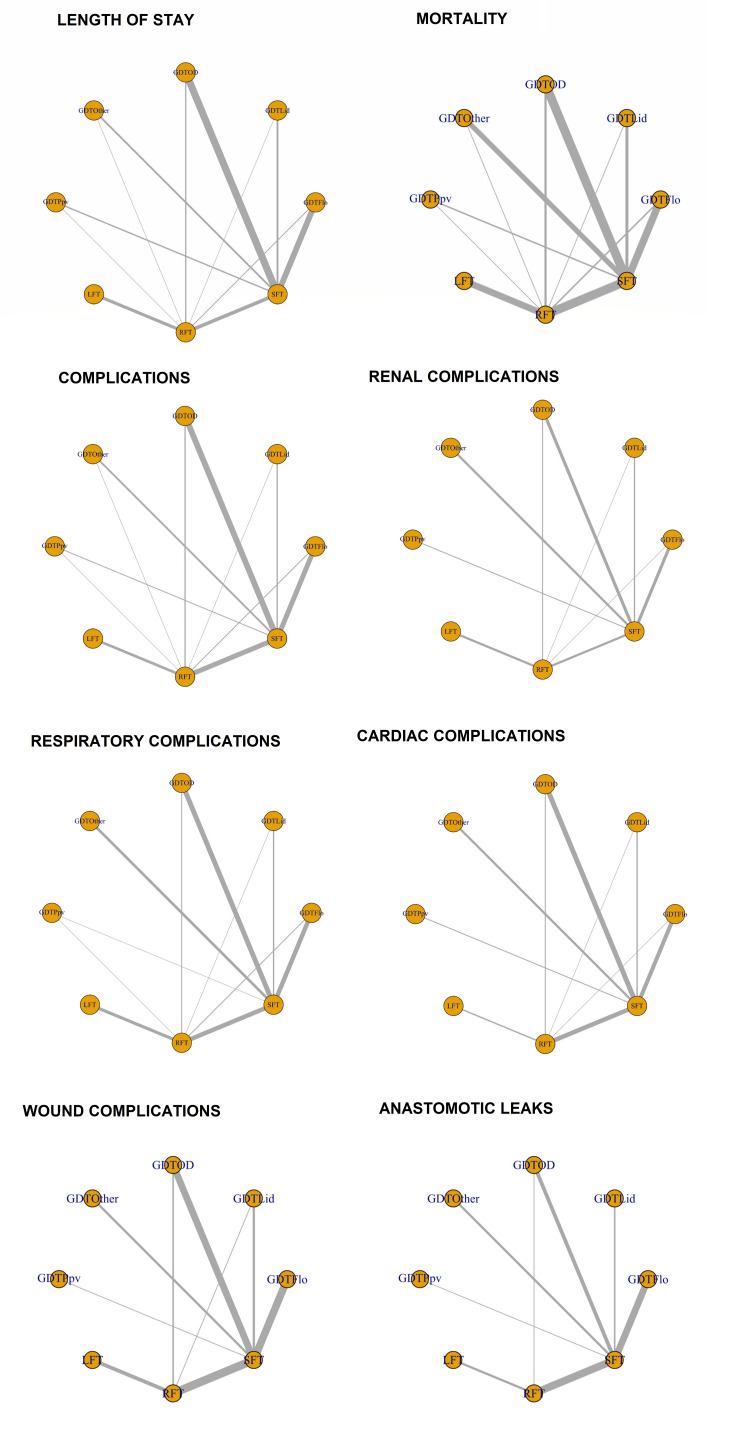
The network geometry plots for the different outcomes are compiled and shown in Figure 2. After exclusion, 63 studies (10,608 patients) that reported LOS, 64 studies (11,219 patients) that reported mortality, 58 studies (7,591 patients) that reported complication rate, 54 studies (10,221 patients) that reported respiratory complications, 38 studies (8,803 patients) that reported renal complications, 47 studies (6,847 patients) that reported cardiac complications, 45 studies (9,335 patients) that reported wound complications and 34 studies (7,957 patients) that reported anastomotic leak were included in individual NMAs. All networks contained eight nodes. GDTFlo and GDTOD studies were the most common in most of the networks. The potential scale reduction factor (PSRF) on all the network models was less than 1.05, indicating sufficient convergence and reliability of the model. The Gelman plots and convergence plots can be obtained on request from the primary author (YX).
Figure 2. Network geometry plots for the different outcomes.
Each yellow dot represents a node for each intervention. The grey lines represent direct comparisons between the interventions. The thickness of the grey line represents the number of studies and patients available for that direct comparison.
GDT, goal-directed therapy; GDTOD, GDT utilising oesophageal Doppler; GDTFlo, GDT using Vigileo/FloTrac; GDTLid, GDT utilising LiDCO; GDTPpv, GDT utilising pulse pressure variation or pulse variability index; GDTOthers, GDT utilising other technology; LFT, liberal fluid therapy; SFT, standard fluid therapy; RFT, restricted fluid therapy
Risk of Bias Within Studies
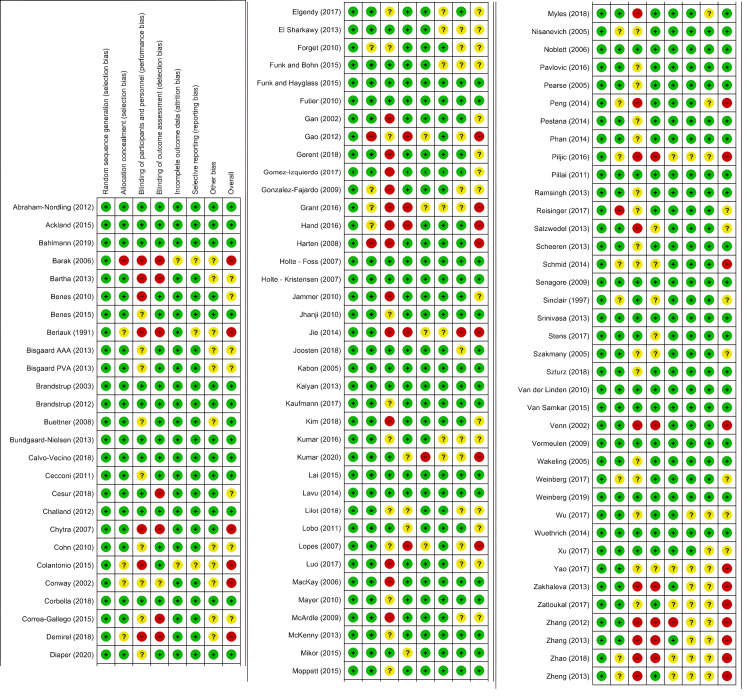
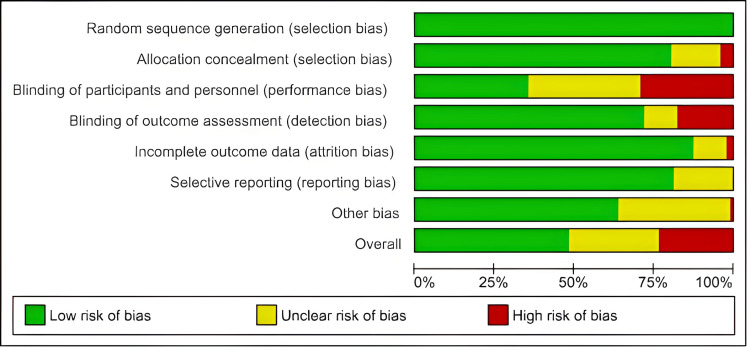
The risks of bias within the individual studies are presented in the Appendices. The risk of bias summary is presented in Figure 3, and the risk of bias graph is presented in Figure 4.
Figure 3. Risk of bias summary.
Results of risk of bias assessment representing all of the judgements in a cross-tabulation of study by entry. Green positives represent low risk of bias, yellow question marks represent unclear risk of bias and red negatives represent high risk of bias.
Figure 4. Risk of bias graph.
The proportion of studies with each of the judgements of risk of bias. Green represents ‘low risk’, red represents ‘high risk’ and yellow represents ‘unclear risk’ of bias.
Heterogeneity and Risk of Bias Across Studies
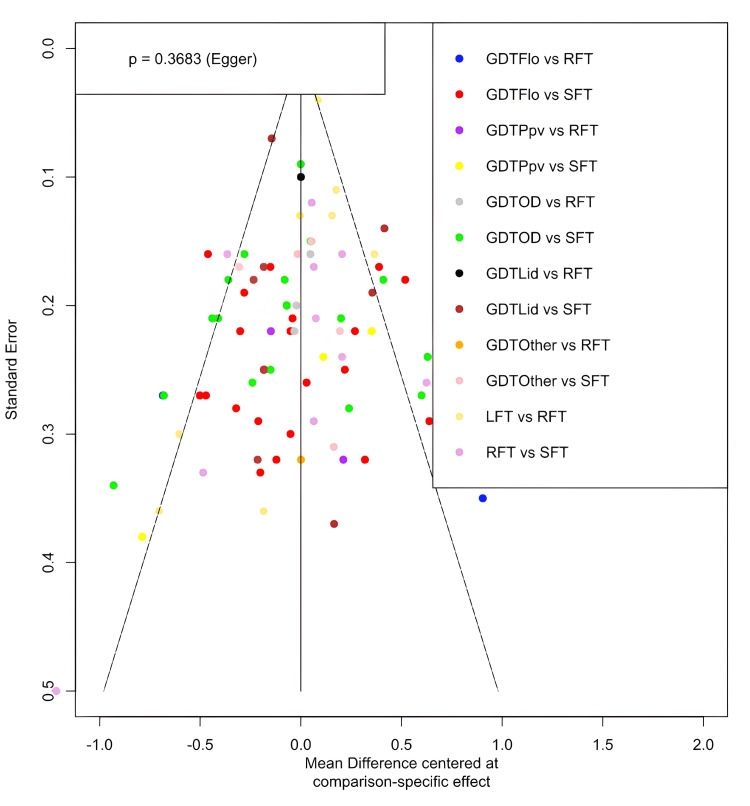
There was moderate to substantial heterogeneity in all the network meta-analyses (length of stay: I2 = 70.2%, moderate; mortality: I2 = 0%, low; complication rate: I2 = 78.7%, substantial; renal complications: I2 = 51.2%, moderate; respiratory complications: I2 = 49.9%, moderate; cardiac complications: I2 = 55.3%, moderate; wound complications: I2 = 28.5%, moderate; anastomotic leak complications: I2 = 38.9%, moderate). Therefore, random effects models were used for all the network meta-analyses. The comparison-adjusted funnel plots were found to be symmetrical. An example is produced for the length of stay (tau2 = 0.0838, tau = 0.2895). Egger’s test was P = 0.3683, suggesting a lack of publication bias (Figure 5).
Figure 5. Comparison-adjusted funnel plot for the length of stay.
A scatterplot of the standard error of each study against the mean difference of each study for the length of stay. The solid line represents the null hypothesis that the study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates. Each coloured dot represents one study.
GDT, goal-directed therapy; GDTOD, GDT utilising oesophageal Doppler; GDTFlo, GDT using Vigileo/FloTrac; GDTLid, GDT utilising LiDCO; GDTPpv, GDT utilising pulse pressure variation or pulse variability index; GDTOthers, GDT utilising other technology; LFT, liberal fluid therapy; SFT, standard fluid therapy; RFT, restricted fluid therapy
Synthesis of Results
The results of the different NMAs for the different outcome measures are summarised below (Figure 6 and Figure 7).
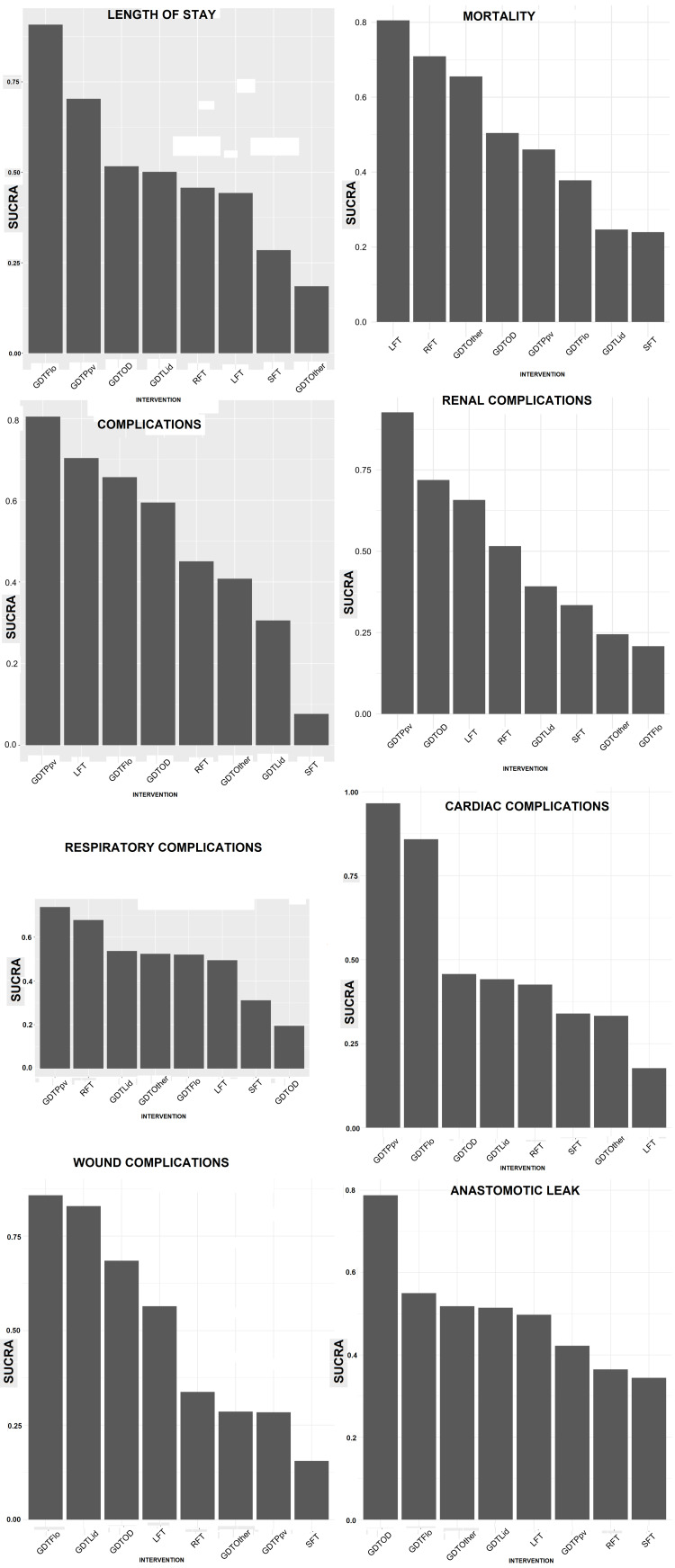
Figure 6. Graphs of SUCRA score (y-axis) for each intervention (x-axis) for each complication.
SUCRA, surface under cumulative ranking curve; goal-directed therapy, GDT; GDTOD, GDT utilising oesophageal Doppler; GDTFlo, GDT using Vigileo/FloTrac; GDTLid, GDT utilising LiDCO; GDTPpv, GDT utilising pulse pressure variation or pulse variability index; GDTOthers, GDT utilising other technology; LFT, liberal fluid therapy; SFT, standard fluid therapy; RFT, restricted fluid therapy
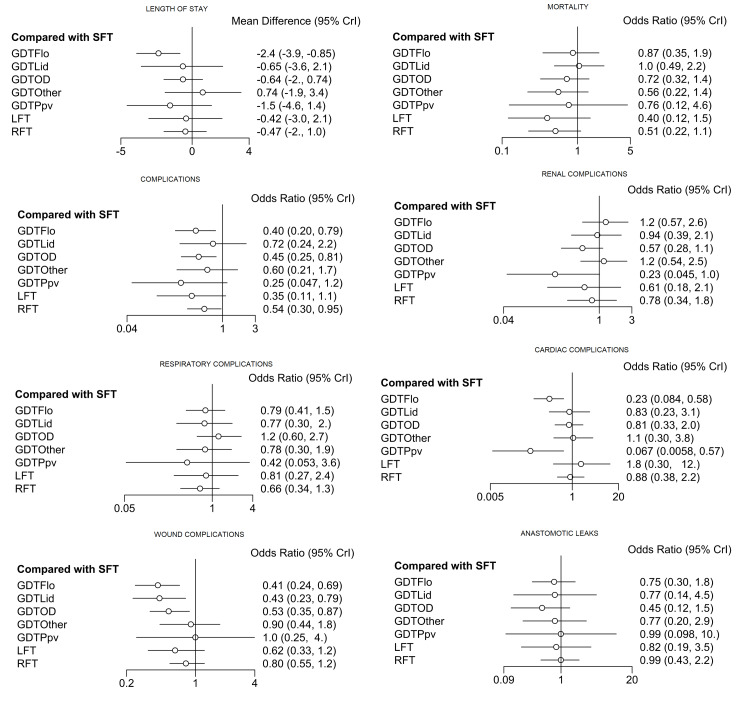
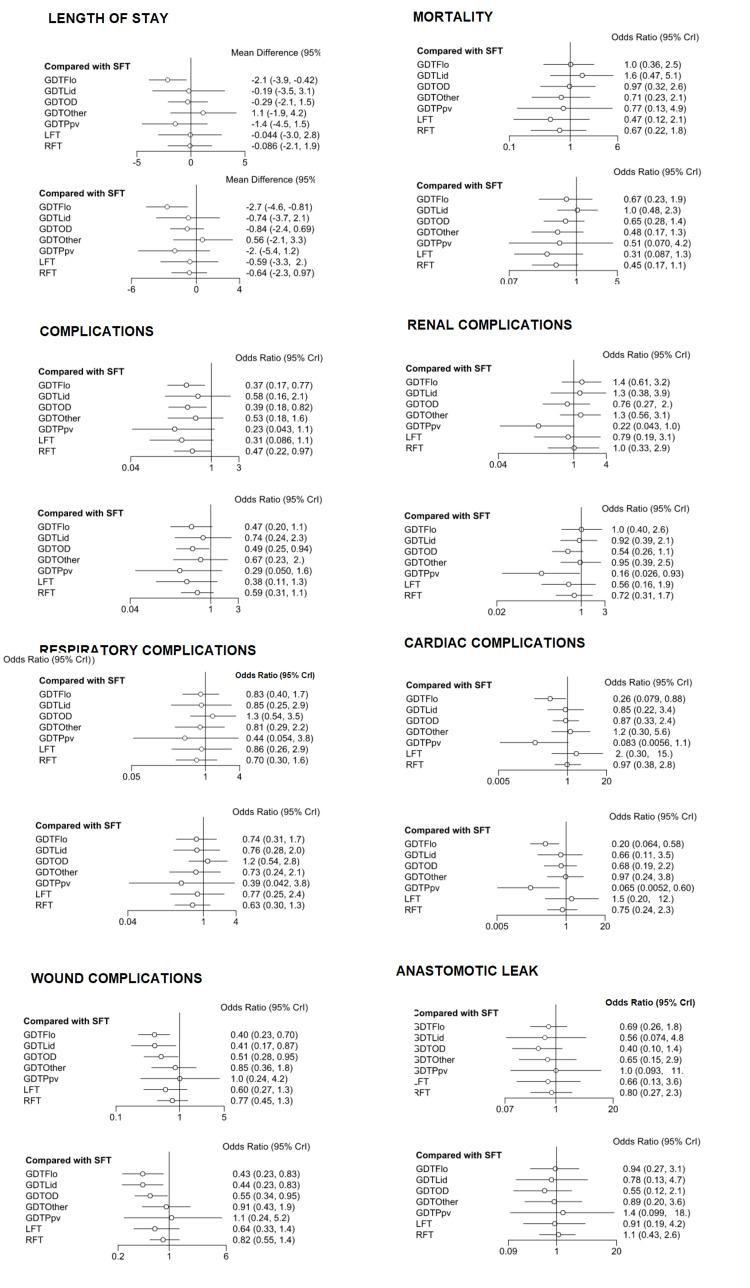
Figure 7. Relative effects forest plots.
Forest plots showing the estimated effect (mean difference) of each intervention as compared to SFT, as well as the 95% CrI for each comparison.
CrI, credible interval; GDT, goal-directed therapy; GDTOD, GDT utilising oesophageal Doppler; GDTFlo, GDT using Vigileo/FloTrac; GDTLid, GDT utilising LiDCO; GDTPpv, GDT utilising pulse pressure variation or pulse variability index; GDTOthers, GDT utilising other technology; LFT, liberal fluid therapy; SFT, standard fluid therapy; RFT, restricted fluid therapy
Length of stay: For the length of stay (LOS), the NMA suggested that GDTFlo was the most effective intervention in reducing LOS (SUCRA = 91%, OR = -2.4, 95% CrI = -3.9 to -0.85, low quality), followed by GDTPpv (SUCRA = 70%, OR = -1.5, 95% CrI = -4.6 to 1.4, very low quality) and GDTOD (SUCRA = 52%, OR = -0.64, 95% CrI = -2 to 0.74, low quality).
Mortality: LFT was the most effective intervention in reducing mortality (SUCRA = 81%, OR = 0.40, 95% CrI = 0.12 to 1.5, very low quality), followed by RFT (SUCRA = 71%, OR = 0.51, 95% CrI = 0.22 to 1.1, low quality) and GDTOther (SUCRA = 66%, OR = 0.56, 95% CrI = 0.22 to 1.4, very low quality).
Complication rate: GDTPpv was the most effective intervention in reducing complication rates (SUCRA = 80%, OR = 0.25, 95% CrI = 0.047 to 1.2, very low quality), followed by LFT (SUCRA = 70%, OR = 0.35, 95% CrI = 0.11 to 1.1, very low quality), and GDTFlo (SUCRA = 66%, OR = 0.40, 95% CrI = 0.2 to 0.79, very low quality).
Renal complications: GDTPpv was the most effective intervention in reducing renal complication (SUCRA = 93%, OR = 0.23, 95% CrI = 0.045, to 1.0, very low quality), followed by GDTOD (SUCRA = 72%, OR = 0.57, 95% CrI = 0.28 to 1.1, moderate quality) and LFT (SUCRA = 66%, OR = 0.61, 95% CrI = 0.18 to 2.1, moderate quality).
Respiratory complications: GDTPpv was the most effective intervention in reducing respiratory complications (SUCRA = 74%, OR = 0.42, 95% CrI = 0.053 to 3.6, low quality), followed by RFT (SUCRA = 68%, OR = 0.66, 95% CrI = 0.34 to 1.3, moderate quality) and GDTLid (SUCRA = 54%, OR = 0.77, 95% CrI = 0.30 to 2.0, low quality).
Cardiac complications: GDTPpv was the most effective intervention in reducing cardiac complications (SUCRA = 97.57064%, OR = 0.067, 95% CrI = 0.0058 to 0.57, very low quality), followed by GDTFlo (SUCRA = 86%, OR = 0.23, 95% CrI = 0.084 to 0.58, low quality) and GDTOD (SUCRA = 46%, OR = 0.81, 95% CrI = 0.33 to 2.0, moderate quality).
Wound complications: GDTFlo was the most effective intervention in reducing wound complications (SUCRA = 86%, OR = 0.41, 95% CrI = 0.24 to 0.69, high quality), GDTLid (SUCRA = 83%, OR = 0.43, 95% CrI = 0.23 to 0.79, high quality) and GDTOD (SUCRA = 68%, OR = 0.53, 95% CrI = 0.35 to 0.87, high quality).
Anastomotic leak: GDTOD was the most effective intervention in reducing anastomotic leaks (SUCRA = 79%, OR = 0.45, 95% CrI = 0.12 to 1.5, moderate quality), followed by GDTFlo (SUCRA = 55%, OR = 0.75, 95% CrI = 0.3 to 1.8, high quality) and GDTOther (SUCRA = 52%, OR = 0.77, 95% CrI = 0.20 to 2.9, low quality).
Exploration of Inconsistency and Additional Analyses
The following is a summary of the exploration of the inconsistency of the NMAs of the outcomes. The network summaries, full node-splitting analysis of inconsistency and the forest plots of the network meta-regression for each outcome can be obtained upon request to the primary author (YX).
Among the 12 comparisons, inconsistency was found in GDTFlo versus RFT and GDTFlo versus SFT in LOS (P = 0.002, P = 0.002), mortality, complication rate (P = 0.007, P = 0.008), renal complications (P = 0.029, P = 0.039) and cardiac complications (P = 0.006, P = 0.007) analysis. RFT versus GDTPpv and SFT versus GDTPpv (P = 0.018, P = 0.017) were inconsistent in respiratory complication analysis. RFT versus SFT was inconsistent in mortality analysis (P = 0.05). No inconsistency was found in the analysis of wound complications and anastomotic leak.
Sensitivity analyses were performed and identified that the trials of Colantonio et al. [50] and Elgendy et al. [57] contributed to the inconsistency in the comparison of complication rate and cardiac complications. The randomized controlled trial by Zatloukal et al. [128] was the source of inconsistency in renal complications analysis. The studies of Zhang et al. [130] and Lopes et al. [87] were the sources of inconsistency in respiratory complications. After removing the inconsistency, rankings remained the same or slightly altered, except in the respiratory complication analysis. RFT was found to be the most effective treatment (71%), followed by GDTLid (59%) and GDTOther (58%).
Network meta-regression was performed and showed a minimal effect of risk of bias in all analyses (Appendices).
Discussion
Summary of Evidence
This systematic review encompassed 102 studies of 14,017 patients. Seventy-eight studies involving 12,100 patients were included in this NMA. Only randomised controlled trials (RCTs) of adult patients undergoing elective non-vascular abdominal surgery were included, partially fulfilling the transitivity assumption, and there were minimal effects on the risk of bias. There was significant heterogeneity in the studies reflecting the diversity of the results, and no publication bias was detected.
The effects of GDT for wound complications and anastomotic leak are the most reliable. The evidence is high to moderate quality, with no inconsistency, minimal imprecision and indirectness, and not affected by bias. Overall, different forms of GDT were more highly ranked as interventions for the different outcomes, except for mortality.
No specific studies could be attributed to the inconsistency in LOS and mortality networks. Hence, the evidence for LOS and mortality has to be interpreted cautiously and considered to be low quality and very low quality. This could be due to low mortality in elective abdominal surgery (266 of 11,219 patients, 2.37%). Both LFT and RFT were highly ranked, which seems counterintuitive. GDTPpv has a seemingly impressive result for complication rates, renal complications, and respiratory complications but can be exaggerated by the small number of studies [3]. This highlights that GDTPpv is lacking in research and should be explored further. Pulse pressure variation and pulse variability index are promising modalities of haemodynamic monitoring that require neither expensive nor invasive equipment. The effects of GDTFlo for cardiac complications, while significant, are likely overstated.
Regarding NMA on GDT in surgical patients, our results are consistent with the findings of Zhao et al. [10]. Compared with Zhao et al. [10], we also included LFT and RFT in the current NMA with improved transitivity by only including non-vascular abdominal surgery in adults. Our study had a broader and better-defined classification system, which was based on the haemodynamic monitor used and fluid protocol in each trial. Hence, there were more studies pooled within each stratum for analysis. Also, more outcomes were measured in the current study.
Limitations
As expected, there was significant heterogeneity, a reflection of the multiple intervention arms, with a majority of studies having small sample sizes and statistically non-significant results. The lack of standardised protocols for controls made comparisons difficult as SFT differed from centre to centre. Also, several GDT studies espoused RFT principles. Future analysis may consider classifying the arms into the actual volume of fluid received instead of the haemodynamic monitor used or the authors’ intentions. The populations and types of major non-vascular abdominal surgeries between different RCTs were different. The composite outcomes utilised may have introduced bias. Sensitivity analysis was used to identify inconsistency, but it may introduce selection bias by excluding articles with contradicting data or large effect sizes. Thus, we reported the original results of the network plot with the inconsistencies found.
Conclusions
In adult patients undergoing major non-vascular abdominal surgery, the use of different haemodynamic monitors and methods of GDT have different outcome-dependent effectiveness on the length of stay, mortality and different perioperative complications. GDT seems superior to either RFT or LFT in reducing perioperative complications, although the evidence is mostly low quality. The evidence was the strongest and most reliable in the outcomes of wound infections and anastomotic leak, graded as medium to high quality.
Acknowledgments
There was no specific source of funding or grant for the systematic review and network meta-analysis. There was no other support from the public, commercial, or not-for-profit sectors. No additional support was provided by institutional or departmental sources at Queen Elizabeth Hospital, Hong Kong. Supplementary data are available on reasonable request to Dr. Yang Xianyi, Timothy, at yangxianyi@gmail.com.
Appendices
Table 1 shows the excluded studies and their reasons for exclusion.
Table 1. Excluded studies and reasons for exclusion.
PA, pulmonary artery; RCT, randomised controlled trial; TEE, transesophageal echocardiography; ERAS, enhanced recovery after surgery; PAC, pulmonary artery catheter
| Excluded studies | Reasons for exclusion |
| Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, Cecconi M. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: a meta-analysis. Br J Anaesth. 2014 Apr;112(4):648-59. doi: 10.1093/bja/aet466. Epub 2014 Jan 10. PMID: 24413429. | Review article |
| Boland MR, Noorani A, Varty K, Coffey JC, Agha R, Walsh SR. Perioperative fluid restriction in major abdominal surgery: systematic review and meta-analysis of randomized, clinical trials. World J Surg. 2013 Jun;37(6):1193-202. doi: 10.1007/s00268-013-1987-8. PMID: 23463399. | Review article |
| Dushianthan A, Knight M, Russell P, Grocott MP. Goal-directed haemodynamic therapy (GDHT) in surgical patients: systematic review and meta-analysis of the impact of GDHT on post-operative pulmonary complications. Perioper Med (Lond). 2020 Oct 15;9:30. doi: 10.1186/s13741-020-00161-5. PMID: 33072306; PMCID: PMC7560066. | Review article |
| Giglio, M., Dalfino, L., Puntillo, F. et al. Hemodynamic goal-directed therapy and postoperative kidney injury: an updated meta-analysis with trial sequential analysis. Crit Care 23, 232 (2019). https://doi.org/10.1186/s13054-019-2516-4. | Review article |
| Pang Q, Liu H, Chen B, Jiang Y. Restrictive and liberal fluid administration in major abdominal surgery. Saudi Med J. 2017 Feb;38(2):123-131. doi: 10.15537/smj.2017.2.15077. PMID: 28133683; PMCID: PMC5329622. | Review article |
| Rahbari NN, Zimmermann JB, Schmidt T, Koch M, Weigand MA, Weitz J. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009 Apr;96(4):331-41. doi: 10.1002/bjs.6552. PMID: 19283742. | Review article |
| Som A, Maitra S, Bhattacharjee S, Baidya DK. Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trials. J Anesth. 2017 Feb;31(1):66-81. doi: 10.1007/s00540-016-2261-7. Epub 2016 Oct 13. PMID: 27738801. | Review article |
| Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM, Swierz MJ, Polak M, Wordliczek J. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012767. doi: 10.1002/14651858.CD012767.pub2. PMID: 31829446; PMCID: PMC6953415. | Review article |
| Xu C, Peng J, Liu S, Huang Y, Guo X, Xiao H, Qi D. Goal-directed fluid therapy versus conventional fluid therapy in colorectal surgery: A meta analysis of randomized controlled trials. Int J Surg. 2018 Aug;56:264-273. doi: 10.1016/j.ijsu.2018.06.034. Epub 2018 Jul 1. PMID: 29972762. | Review article |
| Zhao X, Tian L, Brackett A, Dai F, Xu J, Meng L. Classification and differential effectiveness of goal-directed hemodynamic therapies in surgical patients: A network meta-analysis of randomized controlled trials. J Crit Care. 2021 Feb;61:152-161. doi: 10.1016/j.jcrc.2020.10.031. Epub 2020 Nov 3. PMID: 33171332. | Review article |
| Zhao X, Zhang L, Brackett A, Dai F, Xu J, Meng L. Hemodynamic management and surgical site infection: Network meta-analysis of randomized controlled trials. J Clin Anesth. 2020 Dec;67:110021. doi: 10.1016/j.jclinane.2020.110021. Epub 2020 Sep 11. PMID: 32927235. | Review article |
| Ashok V, Bala I, Bharti N, Jain D, Samujh R. Effects of intraoperative liberal fluid therapy on postoperative nausea and vomiting in children-A randomized controlled trial. Paediatr Anaesth. 2017 Aug;27(8):810-815. doi: 10.1111/pan.13179. Epub 2017 Jun 6. PMID: 28585750. | Pediatric |
| Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery. A prospective, randomized clinical trial. Ann Surg. 1991 Sep;214(3):289-97; discussion 298-9. doi: 10.1097/00000658-199109000-00011. PMID: 1929610; PMCID: PMC1358649. | PA catheter, not fluid versus fluid |
| Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, Wo CJ, Rimle DA, Kram HB, Umali R, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma. 1995 May;38(5):780-7. doi: 10.1097/00005373-199505000-00018. PMID: 7760409. | Trauma resuscitation |
| Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993 Dec 8;270(22):2699-707. PMID: 7907668. | O2 delivery trial, PA catheter |
| Bloom M, Cuff G, Plichta A. Goal‐directed fluid therapy in neurosurgical cases. Journal of Neurosurgical Anesthesiology 2015;27(4):424‐5. | Abstract only |
| Calvo Vecino JM, Ripolles Melchor J, Martinez Hurtado E, Abad Gurumeta A, Casans Frances R, Serrano A. Efficacy of intraoperatory optimisation of fluids guided with transoesophageal doppler monitorisation: a multicentre randomised controlled trial. European Journal of Anaesthesiology2014; Vol. 31:13. | Abstract only |
| Chattopadhyay S, Patel A, Mittal S, Biliatis I, Christian S, Terblanche A, et al. The role of intra‐operative fluid optimisation using oesophageal doppler in advanced ovarian cancer; early postoperative recovery and fitness for discharge. International Journal of Gynecologic Cancer Volume: 23 Issue 1 (2013) ISSN: 1048-891X Online ISSN: 1525-1438 | Not an RCT |
| Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, Thevathasan T, Pieretti A, Ferrone C, Hoeft A, Scheeren TWL, Thompson BT, Kurth T, Eikermann M. Effects of Intraoperative Fluid Management on Postoperative Outcomes: A Hospital Registry Study. Ann Surg. 2018 Jun;267(6):1084-1092. doi: 10.1097/SLA.0000000000002220. PMID: 28288059. | Not an RCT |
| Concha PM, Mertz KV, Cortínez FL, Zúñiga DA, Pinedo MG. Transesophageal echocardiography to monitor fluid administration during the perioperative period. Revista Medica de Chile 2011;139(9):1157‐62. (PUBMED: 22215394). | Non-English |
| Cordero‐Rochet MJ, McCluskey SA, Minkovich L, Gilbert R. Goal directed fluid management in free flap reconstructive surgery. Canadian Journal of Anesthesia 2014;61:S75. | Abstract only |
| Donati A, Loggi S, Preiser JC, Orsetti G, Münch C, Gabbanelli V, Pelaia P, Pietropaoli P. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007 Dec;132(6):1817-24. doi: 10.1378/chest.07-0621. Epub 2007 Oct 9. PMID: 17925428. | Oxygen delivery-focused, not fluid therapy |
| Dhawan R, Shahul S, Roberts JD, Smith ND, Steinberg GD, Chaney MA. Prospective, randomized clinical trial comparing use of intraoperative transesophageal echocardiography to standard care during radical cystectomy. Annals of Cardiac Anaesthesia 2018;21(3):255‐61. (PUBMED: 30052211). | Did not report primary or secondary end points |
| Foppa C, Zakhaleva J, Tam J, Denoya PI, Bishawi M, Bergamaschi R. The impact of intravenous fluid administration on complication rates in bowel surgery with enhanced recovery protocol: a randomized controlled trial. Techniques in Coloproctology 2014;18(1):91. | Similar to the 2013 paper of Zakhaleva et al. [127] |
| Fischer M, Matsuo K, Gonen M, Grant F, Dematteo RP, D'Angelica MI, Mascarenhas J, Brennan MF, Allen PJ, Blumgart LH, Jarnagin WR. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann Surg. 2010 Dec;252(6):952-8. doi: 10.1097/SLA.0b013e3181ff36b1. PMID: 21107104. | Acute normovolemic haemodilution |
| Forget P, Lois F, De Kock M. Does the pleth variability index improve fluid management during major abdominal surgery?. Critical Care (London, England) 2009;13(Suppl 1):P204. | Poster of the 2010 paper of Forget et al. [58] |
| Forget P, Lois F, Kartheuser A, Leonard D, Remue C, Kock M. The concept of titration can be transposed to fluid management. But does it change the volumes? Randomised trial on pleth variability index during fast‐track colonic surgery. Current Clinical Pharmacology2013; Vol. 8, issue 2:110‐4. (PUBMED: 23061978). | Too similar to primary authors’ previous paper, possible overlap of patient cohort |
| Fukui K, Markstaller K, Leibundgut D, Pestel G. Timing of intraoperative fluid management by difference in pulse pressure (dPP). European Journal of Anaesthesiology 2009;45:68. | Abstract only |
| Funcke S, Saugel B, Koch C, Schulte D, Zajonz T, Sander M, et al. Individualized, perioperative, hemodynamic goal‐directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial. Trials 2018;19(1):273. (PUBMED: 29743101). | Protocol only |
| Goepfert MS, Richter HP, Zu Eulenburg C, Gruetzmacher J, Rafflenbeul E, Roeher K, von Sandersleben A, Diedrichs S, Reichenspurner H, Goetz AE, Reuter DA. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled trial. Anesthesiology. 2013 Oct;119(4):824-36. doi: 10.1097/ALN.0b013e31829bd770. PMID: 23732173. | Cardiac surgery study |
| Gottin, L., Martini, A., Menestrina, N. et al. Perioperative Fluid Administration in Pancreatic Surgery: a Comparison of Three Regimens. J Gastrointest Surg 24, 569–577 (2020). https://doi.org/10.1007/s11605-019-04166-4. | Non-randomized (prospective study) |
| Hendrix RJ, Damle A, Williams C, Harris A, Spanakis S, Lambert DH, Lambert LA. Restrictive Intraoperative Fluid Therapy is Associated with Decreased Morbidity and Length of Stay Following Hyperthermic Intraperitoneal Chemoperfusion. Ann Surg Oncol. 2019 Feb;26(2):490-496. doi: 10.1245/s10434-018-07092-y. Epub 2018 Dec 4. PMID: 30515670. | Retrospective review |
| Hughes T, Cottam S, Heaton N, Bernal W, Auzinger G, Wendon J, et al. Peri‐operative haemodynamic optimisation using Pulsioflex monitoring in Whipple's surgery. Anaesthesia 2013;68:33. | Non-randomized |
| Jammer I, Tuovila M, Ulvik A. Stroke volume variation to guide fluid therapy: is it suitable for high-risk surgical patients? A terminated randomized controlled trial. Perioper Med (Lond). 2015 Jul 22;4:6. doi: 10.1186/s13741-015-0016-x. PMID: 26203353; PMCID: PMC4511544. | Trial terminated |
| Johnson E, Nunoo R, Al‐Abbad J, Senagore A, Emery T, Dujovny N, et al. Intraoperative fluid management and its correlation with the surgical Apgar score in laparoscopic segmental colectomy. Diseases of the Colon and Rectum 2011;54(5):e200. | Abstract |
| Kapoor PM, Kakani M, Chowdhury U, Choudhury M, Lakshmy, Kiran U. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann Card Anaesth. 2008 Jan-Jun;11(1):27-34. doi: 10.4103/0971-9784.38446. PMID: 18182756. | Cardiac surgery |
| Kellman S, Roberts JD, Chaney M, Negron E. Prospective randomized clinical trial comparing routine intraoperative transesophageal echocardiography to standard care during radical cystectomy. Anesthesia and Analgesia 2014;118(5 Suppl 1):S53. | TEE study not fluid study, abstract only |
| Kulkarni R, Craske DA, Abdel‐Galil K, Hatfield A, Liu A, Pick A, et al. Haemodynamic optimisation in head and neck cancer surgery: pilot randomised controlled trial of LiDCO rapid. European Archives of Oto‐Rhino‐Laryngology 2012;269(4):1370. | Abstract only |
| Lahtinen SL, Liisanantti JH, Poukkanen MM, Laurila PA. Goal-directed fluid management in free flap surgery for cancer of the head and neck. Minerva Anestesiol. 2017 Jan;83(1):59-68. doi: 10.23736/S0375-9393.16.11451-8. Epub 2016 Oct 19. PMID: 27759740. | Retrospective analysis |
| Li H, Afzal A, Lian Q, Kramer GC, Svensen C, Prough D. Restricted fluid therapy decreases surgical blood loss ‐ a clinical study of two fluid regimens during cesarean section under spinal anesthesia. Anesthesia and Analgesia 2011;112(5 Suppl 1):291. | Abstract only |
| Liu TJ, Zhang JC, Gao XZ, Tan ZB, Wang JJ, Zhang PP, et al. Clinical research of goal‐directed fluid therapy in elderly patients with radical resection of bladder cancer. Journal of Cancer Research and Therapeutics 2018;14(Suppl 1):S173‐9. (PUBMED: 29578169). | Case-control study, non-randomized |
| Martini A, Menestrina N, Simion D, Filetici L, Schweiger V, Gottin L. Perioperative fluid administration in pancreatic surgery: comparison of three regimens. Critical Care 2009;13:S80‐1. | Interim analysis of the study of Gottin et al. |
| McArdle GT, Price G, Lewis A, Hood JM, McKinley A, Blair PH, Harkin DW. Positive fluid balance is associated with complications after elective open infrarenal abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2007 Nov;34(5):522-7. doi: 10.1016/j.ejvs.2007.03.010. Epub 2007 Sep 6. PMID: 17825590. | Retrospective cohort study |
| Mccaul, James & Sutton, D.N. & Hatfield, A. & Pick, A. & Liu, A. & Craske, D.C. (2011). P243. Randomised controlled trial of LidCO rapid goal directed therapy versus control for fluid optimisation in patients undergoing major head and neck cancer surgery. Oral Oncology - ORAL ONCOL. 47. 10.1016/j.oraloncology.2011.06.486. | Abstract only |
| McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ. 2004 Jul 31;329(7460):258. doi: 10.1136/bmj.38156.767118.7C. Epub 2004 Jul 8. Erratum in: BMJ. 2004 Aug 21;329(7463):438. PMID: 15242867; PMCID: PMC498021. | Cardiac surgery |
| Melis M, Marcon F, Masi A, Sarpel U, Miller G, Moore H, Cohen S, Berman R, Pachter HL, Newman E. Effect of intra-operative fluid volume on peri-operative outcomes after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2012 Jan;105(1):81-4. doi: 10.1002/jso.22048. Epub 2011 Jul 25. PMID: 21792977. | Not an RCT |
| Minkovich L, McCluskey SA, Djaiani G, Goldstein D, Gilbert R. Goal directed fluid therapy in surgery for head and neck cancers. Canadian Journal of Anesthesia 2012;59:1344381. | Abstract only |
| Minto G, Challand C, Sneyd JR, Mellor N, Hosie KB, Erasmus P, et al. Is the impact of intraoperative goal‐directed fluid therapy on length of stay after major elective colorectal surgery related to patients' aerobic fitness?. British Journal of Anaesthesia 2011;106(3):440. | Abstract only |
| Mintz Y, Weiss YG, Rivkind AI. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2004 Aug;240(2):386; author reply 386-8. doi: 10.1097/01.sla.0000134633.10987.87. PMID: 15273568; PMCID: PMC1356423. | Reply to the paper of Brandstrup et al. [41], correspondence |
| Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. A fast‐track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology2009; Vol. 136, issue 3:842‐7. (PUBMED: 19135997). | ERAS-focused, not comparing fluid therapy |
| Munoz CA, Rojas JLT, Bermudez OIG. Intraoperative oesophageal doppler during emergency abdominal surgery. British Journal of Anaesthesia 2012;108:ii347. | Not fluid therapy RCT |
| Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995 Apr;130(4):423-9. doi: 10.1001/archsurg.1995.01430040085019. PMID: 7535996. | Cardiac study |
| NCT03193320. Management of intraoperative fluids in ambulatory surgery [Intraoperative fluid therapy management in low‐risk patients under general anesthesia ‐ a randomized controlled trial comparing liberal, restrictive and pleth variability index (PVI)‐guided fluid administration in a day surgery setting]. https://clinicaltrials.gov/ct2/show/record/NCT03193320 (first received 15 June 2017). | Trial protocol, not yet complete or recruiting |
| Osawa EA, Rhodes A, Landoni G, Galas FR, Fukushima JT, Park CH, Almeida JP, Nakamura RE, Strabelli TM, Pileggi B, Leme AC, Fominskiy E, Sakr Y, Lima M, Franco RA, Chan RP, Piccioni MA, Mendes P, Menezes SR, Bruno T, Gaiotto FA, Lisboa LA, Dallan LA, Hueb AC, Pomerantzeff PM, Kalil Filho R, Jatene FB, Auler Junior JO, Hajjar LA. Effect of Perioperative Goal-Directed Hemodynamic Resuscitation Therapy on Outcomes Following Cardiac Surgery: A Randomized Clinical Trial and Systematic Review. Crit Care Med. 2016 Apr;44(4):724-33. doi: 10.1097/CCM.0000000000001479. PMID: 26646462. | Cardiac |
| Park S, Kim H, Koo B. Effect of goal‐directed fluid therapy using stroke volume variation in free flap reconstruction. Anesthesia and Analgesia 2016;122(5 Suppl 3):S302. | Abstract or poster |
| Peng NH, Gao T, Chen YY, Xi FC, Zhang JJ, Li N, Zhu WM, Yu WK. Restricted intravenous fluid regimen reduces fluid redistribution of patients operated for abdominal malignancy. Hepatogastroenterology. 2013 Oct;60(127):1653-9. PMID: 24627921. | Not an indexed journal, article cannot be retrieved (journal discontinued) |
| Pösö T, Winsö O, Aroch R, Kesek D. Perioperative fluid guidance with transthoracic echocardiography and pulse-contour device in morbidly obese patients. Obes Surg. 2014 Dec;24(12):2117-25. doi: 10.1007/s11695-014-1329-4. PMID: 24902655. | Non-randomized, non-blinded |
| Rath G, Mishra N, Chaturvedi A, Bithal P. Effect of goal‐directed intraoperative fluid therapy on hospital stay in patients undergoing excision of supratentorial tumors. Journal of Neurosurgical Anesthesiology 2018;30(4):421‐2. | Abstract only |
| Rinehart J, Lilot M, Lee C, Joosten A, Huynh T, Canales C, Imagawa D, Demirjian A, Cannesson M. Closed-loop assisted versus manual goal-directed fluid therapy during high-risk abdominal surgery: a case-control study with propensity matching. Crit Care. 2015 Mar 19;19(1):94. doi: 10.1186/s13054-015-0827-7. PMID: 25888403; PMCID: PMC4372998. | Case-control study, not an RCT |
| Self WH, Semler MW, Bellomo R, Brown SM, deBoisblanc BP, Exline MC, Ginde AA, Grissom CK, Janz DR, Jones AE, Liu KD, Macdonald SPJ, Miller CD, Park PK, Reineck LA, Rice TW, Steingrub JS, Talmor D, Yealy DM, Douglas IS, Shapiro NI; CLOVERS Protocol Committee and NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators. Liberal Versus Restrictive Intravenous Fluid Therapy for Early Septic Shock: Rationale for a Randomized Trial. Ann Emerg Med. 2018 Oct;72(4):457-466. doi: 10.1016/j.annemergmed.2018.03.039. Epub 2018 May 10. PMID: 29753517; PMCID: PMC6380679. | Not an RCT |
| Silversides, J.A., Perner, A. & Malbrain, M.L.N.G. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med 45, 1440–1442 (2019). https://doi.org/10.1007/s00134-019-05713-y. | Not a trial |
| Sundaram SC, Salins SR, Nandha Kumar A, Korula G. Intra‐operative fluid management in adult neurosurgical patients undergoing intracranial tumour surgery: randomised control trial comparing pulse pressure variance (PPV) and central venous pressure (CVP). Journal of Clinical and Diagnostic Research 2016;10(5):UC01‐5. (PUBMED: 27437329). | Does not assess primary or secondary end points |
| Ueno S, Tanabe G, Yamada H, Kusano C, Yoshidome S, Nuruki K, Yamamoto S, Aikou T. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery. 1998 Mar;123(3):278-86. PMID: 9526519. | Not an RCT |
| Valentine RJ, Duke ML, Inman MH, Grayburn PA, Hagino RT, Kakish HB, Clagett GP. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg. 1998 Feb;27(2):203-11; discussion 211-2. doi: 10.1016/s0741-5214(98)70351-9. PMID: 9510275. | PAC article |
| Vanakas T, Asouhidou I, Samaras A, Diminikos G, Ioannou P. Implementation of goal‐directed protocol in elderly patients undergoing femoral fracture repair. European Journal of Anaesthesiology2012; Vol. 29:67. | Abstract |
| Wang S, Wang X, Dai H, Han J, Li N, Li J. The effect of intraoperative fluid volume administration on pancreatic fistulas after pancreaticoduodenectomy. J Invest Surg. 2014 Apr;27(2):88-94. doi: 10.3109/08941939.2013.839766. PMID: 24665844. | Retrospective case-control, non-RCT |
| Wen XL, Jing GX, He P, Hou JR. Clinical study on the capacity management guided by stroke volume variation in elderly patients with laparoscopic radical gastrectomy for gastric cancer. Journal of Xi'an Jiaotong University (Medical Sciences) 2016;37(6):851‐6. | Non-English study |
| Wenkui Y, Ning L, Jianfeng G, Weiqin L, Shaoqiu T, Zhihui T, Tao G, Juanjuan Z, Fengchan X, Hui S, Weiming Z, Jie-Shou L. Restricted peri-operative fluid administration adjusted by serum lactate level improved outcome after major elective surgery for gastrointestinal malignancy. Surgery. 2010 Apr;147(4):542-52. doi: 10.1016/j.surg.2009.10.036. Epub 2009 Dec 11. PMID: 20004445. | Lactate protocol on both arms, not goal-directed therapy |
| Wilmin S, Dierick A, De Hert S, Van Der Linden P. Optimization of oxygen delivery in vascular surgery with Flotrac System, a prospective double blind randomized study. European Journal of Anaesthesiology 2009;45:38. | Abstract |
| Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999;318(7191):1099‐1103. (PUBMED: 10213716). | Inotrope versus inotrope study |
| Xiao W, Duan QF, Fu WY, Chi XZ, Wang FY, Ma DQ, et al. Goal‐directed fluid therapy may improve hemodynamic stability of parturient with hypertensive disorders of pregnancy under combined spinal epidural anesthesia for cesarean delivery and the well‐being of newborns. Chinese Medical Journal 2015;128(14):1922‐31. (PUBMED: 26168834). | Pregnant patient, caesarean section |
| Yin K, Ding J, Wu Y, Peng M. Goal-directed fluid therapy based on noninvasive cardiac output monitor reduces postoperative complications in elderly patients after gastrointestinal surgery: A randomized controlled trial. Pak J Med Sci. 2018 Nov-Dec;34(6):1320-1325. doi: 10.12669/pjms.346.15854. PMID: 30559778; PMCID: PMC6290223. | Too high risk of bias, likely fabricated data |
| Yu X, Yan J, Zhai Z, Ma X. Optimized management of fluid volume guided by PiCCO parameters in skull base tumor resection. Critical Care Medicine 2016;44(12 Suppl 1):473. | Abstract only |
| Yu Y, Dong J, Xu Z, Shen H, Zheng J. Pleth variability index-directed fluid management in abdominal surgery under combined general and epidural anesthesia. J Clin Monit Comput. 2015 Feb;29(1):47-52. doi: 10.1007/s10877-014-9567-5. Epub 2014 Feb 21. PMID: 24557584. | Did not report primary or secondary end points |
| Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative "optimization" of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery. 1997 Sep;122(3):584-92. doi: 10.1016/s0039-6060(97)90132-x. PMID: 9308617. | PAC study, not fluid therapy study |
| Zeng K, Li Y, Liang M, Gao Y, Cai H, Lin C. The influence of goal‐directed fluid therapy on the prognosis of elderly patients with hypertension and gastric cancer surgery. Drug Design, Development and Therapy2014; Vol. 8:2113‐9. (PUBMED: 25378913). | Retracted |
| Zheng L, Gu E, Peng X, Zhang L, Cao Y. Effect of goal‐directed haemodynamic management on the postoperative outcome in elderly patients with fragile cardiac function undergoing abdominal surgery. National Medical Journal of China2016; Vol. 96, issue 43:3464‐9. (PUBMED: 27903339). | Non-English study |
Table 2 shows the included studies and their characteristics.
Table 2. Characteristics and primary outcomes of included studies.
RFT, restricted fluid therapy; SFT, standard fluid therapy; LFT, liberal fluid therapy; GDT, goal-directed therapy; R-GDT, restrictive goal-directed therapy; AAA, abdominal aortic aneurysm; postop, postoperative; EBL, estimated blood loss; LR, lactated Ringer’s solution; HAES 6%, hydroxyethyl starch 6%; NS, normal saline; SV, stroke volume; PPV, pulse pressure variation; GEDI, global end-diastolic index; ITBV, intrathoracic blood volume; FTc, flow time (corrected); RCT, randomised controlled trial; TKR, total knee replacement; THR, total hip replacement; GDTPpv, GDT utilising pulse pressure variation or pulse variability index; HIPEC, hyperthermic intraperitoneal chemotherapy; GDTFlo: GDT using Vigileo/FloTrac; LiDCO, lithium dilution cardiac output; GDTLid, GDT utilising LiDCO; SVI, stroke volume index; GDTClear, GDT utilising Clearsight System; SVV, stroke volume variation; CI, cardiac index; GDTOD, GDT utilising oesophageal Doppler; PPV, pulse pressure variation; ERAS, enhanced recovery after surgery; PAC, pulmonary artery catheter; GDTPic, GDT utilising PiCCO; PVI, pleth variability index; GDTPVI: GDT utilising pleth variability index; CVP, central venous pressure; MAP, mean arterial pressure; CO, cardiac output; Scvo2, central venous oxygen saturation; GDTScv, GDT utilising Scvo2; GDTNic: GDT utilising NICOM; GDTPro: GDT utilising ProAQT; GDTNex, GDT utilising Nexfin
| Author (year) | Design of the study (country) | Type of surgery | Type of intervention versus control | Phase of intervention | Fluid protocols used (intervention versus control) | Number of patients (intervention versus control) | Age of patients (intervention versus control) | In-hospital mortality | Disability-free survival at one year | Length of hospital stay (days) |
| RFT versus SFT | ||||||||||
| Abraham-Nordling (2012) | Single-centre RCT (Sweden) | Elective colorectal surgery | RFT versus SFT | Intraoperative | RFT = 2 mL/kg/hour, D10, SFT = preloading, 5 mL/kg/hour and additional 1,000 mL of fluid, LR | RFT = 79, SFT = 82 | RFT = 68, SFT = 69 | RFT = 0, SFT = 0 | Not reported | RFT = 6 (4-8), SFT = 6 (4-8), P = 0.194 |
| Brandstrup (2003) | Multicentre RCT (Denmark) | Elective colorectal surgery | RFT versus SFT | Intraoperative | RFT = volume to volume replacement with HAES 6%, SFT = preloading, 7 mL/kg/hour for the first hour, 5 mL/kg/hour for the second and third hour, 3 mL/kg/hour following hours, NS, 2-3:1 volume replacement with NS | RFT = 69, SFT = 72 | RFT = 64, SFT = 69 | RFT = 0, SFT = 4, P = 0.12 | Not reported | RFT = 10.8 (7.53), SFT = 12.1 (10.9) |
| Cohn (2010) | Single-centre RCT (USA) | Elective colorectal surgery | RFT versus SFT | Intraoperative | RFT = 2 mL/kg/hour, 1:1 fluid replacement for blood loss, LR, SFT = preloading, 7 mL/kg/hour for the first hour, followed by 5 mL/kg/hour, 3:1 fluid replacement for EBL, LR | RFT = 18, SFT = 9 | RFT = 56, SFT = 45 | RFT = 0, SFT = 0 | Not reported | Not reported |
| Futier (2010) | Single-centre RCT (France) | Major abdominal surgery | RFT versus SFT | Intraoperative, postoperative | RFT = 6 mL/kg/hour LR, SFT = 12 mL/kg/hour LR | RFT = 36, SFT = 34 | RFT = 61.9, SFT = 60.4 | RFT = 1, SFT = 1 | Not reported | Not reported |
| Gao (2012) | Single-centre RCT (China) | Gastrointestinal surgery | RFT versus SFT | Intraoperative, postoperative | RFT = 7 mL/kg/hour followed by 5 mL/kg/hour LR, 1,000-1,500 mL/day, SFT = preloading, 12 mL/kg/hour LR, 2,000-2,500 mL/day | RFT = 93, SFT = 86 | RFT = 72, SFT = 73 | RFT = 2, SFT = 4 P = 0.353 | Not reported | Not reported |
| González-Fajardo (2009) | Single-centre RCT (Spain) | Open AAA surgery | RFT versus SFT | Postoperative | RFT = 1,500 mL/day, NS, SFT = 2,500 mL/day, dextrose and NS | RFT = 20, SFT = 20 | RFT = 65.5, SFT = 61.95 | RFT = 0, SFT = 1 | Not reported | RFT = 8.4 (7.75-9.05), SFT = 12.4 (8.68-16.12) |
| Jie (2014) | Single-centre RCT (China) | Elective colorectal surgery | RFT versus SFT | Intraoperative, postoperative | RFT = no preloading, 7 mL/kg in the first hour and then 5 mL/kg in the following hours LR, 1,000-1,500 mL/day, crystalloid, SFT = preloading, 500 mL 6% HAES, 12 mL/kg/hour LR, 2,000-2,500 mL/day, crystalloid | RFT = 89, SFT = 96 | RFT = 64.7, SFT = 65.4 | RFT = 0, SFT = 0 | Not reported | Not reported |
| Lavu (2014) | Single-centre RCT (USA) | Pancreatic surgery | RFT versus SFT | Intraoperative, postoperative | RFT = 1 mL/kg hypertonic saline, followed by 9 mL/kg/hour LR, EBL replaced 1:1, SFT = 15 mL/kg/hour LR, EBL replaced 3:1 | RFT = 131, SFT = 128 | RFT = 66.6, SFT = 68.3 | RFT = 0, SFT = 1 | Not reported | RFT = 7 (4-41), SFT = 7 (5-61) |
| Lobo (2011) | Single-centre RCT (Brazil) | High-risk elective surgery | RFT-GDT versus SFT-GDT | Intraoperative | RFT = 4 mL/kg/hour LR, LiDCO, SFT = 12 mL/kg/hour LR, LiDCO | RFT = 45, SFT = 43 | RFT = 69.2, SFT = 68.6 | RFT = 0, SFT = 2 | Not reported | RFT = 6 (4-9), SFT = 6 (4-10) |
| MacKay (2006) | Single-centre RCT (UK) | Elective colorectal surgery | RFT versus SFT | Intraoperative, postoperative | RFT = 2,000 mL/day of NS + dextrose until D1, SFT = 3,000 mL/day NS + dextrose until D3 | RFT = 37, SFT = 32 | RFT = 73.2, SFT = 72.6 | RFT = 1, SFT = 1 | Not reported | RFT = 7.2 (6.1-11.2), SFT = 7.2 (6.1-11.2), P = 0.902 |
| McArdle (2009) | Single-centre RCT (UK) | Open AAA surgery | RFT versus SFT | Intraoperative, postoperative | RFT = no preload, 4 mL/kg/hour LR, 2,000 mL/day crystalloid + dextrose, SFT = 10 mL/kg NS preload, 12 mL/kg/hour LR, 3,000 mL/day crystalloid + dextrose | RFT = 9, SFT = 11 | RFT = 74, SFT = 75 | RFT = 0, SFT = 0 | Not reported | RFT = 7.78 (+/- 0.64), SFT = 16 (+/- 4.82), P < 0.025 |
| Piljic (2016) | Multicentre RCT (Bosnia) | Open AAA surgery | RFT versus SFT | Intraoperative, postoperative | RFT = 10 mL/kg/hour LR, postoperative 70-100 mL/hour, SFT = 15 mL/kg/hour LR, postoperative 150-200 mL/hour | RFT = 30, SFT = 30 | RFT = 68.64, SFT =69.34 | RFT = 0, SFT = 1 | Not reported | RFT = 4.33, SFT = 6.20, P = 0.035 |
| Van Samkar (2015) | Single-centre RCT (Netherlands) | Pancreatic surgery | RFT versus SFT | Intraoperative | RFT = 5 mL/kg/hour LR, SFT = 10 mL/kg/hour LR, postoperative standardized | RFT = 26, SFT = 22 | No significant difference | RFT = 1, SFT = 1 | Not reported | RFT = 12 (11-14), SFT = 10 (9-17) |
| Vermeulen (2009) | Single-centre RCT (Netherlands) | Major abdominal surgery | RFT versus SFT | Postoperative | RFT = 1,500 mL/day, SFT = 2,500 mL/day | RFT = 30, SFT = 32 | RFT = 55.5, SFT = 53.6 | RFT = 1, SFT = 0 | Not reported | RFT = 12.3 (12.7), SFT = 8.3 (4.5), P = 0.049 |
| Wuethrich (2014) | Single-centre RCT (Switzerland) | Radical cystectomy | RFT versus SFT | Intraoperative | RFT = 1 mL/kg/hour, followed by 3 mL/kg/hour LR, SFT = 6 mL/kg/hour LR | RFT = 83, SFT = 83 | RFT = 68, SFT = 69 | RFT = 0, SFT = 4 | Not reported | RFT = 15 (11-27), SFT = 17 (10-95), P = 0.01 |
| RFT versus GDT | ||||||||||
| Benes (2015) | Single-centre RCT (Czech Republic) | THR and TKR (orthopaedic surgery) | RFT versus GDTPpv versus SFT | Intraoperative | RFT = 5 mL/kg/hour crystalloid, GDT = 5 mL/kg/hour crystalloid, PPV directed, SFT = no protocol | RFT = 40, GDT = 40, SFT = 40 | RFT = 66, GDT = 68, SFT = 70 | RFT = 1, GDT = 0, SFT = 0 | Not reported | RFT = 10 (9-12.5), GDT = 10 (8.5-13.5), SFT = 10.5 (8-12), P = 0.99 |
| Brandstrup (2012) | Multicentre RCT (Denmark) | Elective colorectal surgery | RFT versus GDTOD | Intraoperative | RFT = HAES 6% to maintain zero balance, GDT = oesophageal Doppler, fluid boluses until increase in SV < 10% | RFT =79, GDT = 72 | RFT = 68.1, GDT = 66.9 | RFT = 1, GDT = 1 | Not reported | RFT = 7.66 (8.2), GDT = 8.45 (7.5), P = 0.539 |
| Colantonio (2015) | Single-centre RCT (Italy) | Major abdominal surgery with HIPEC | RFT versus GDTFlo | Intraoperative | RFT = restrictive 4-10 mL/kg/hour, GDT = FloTrac, keep CI > 2.5 L/minute/m2, 4 mL/kg/hour | RFT = 44, GDT = 42 | RFT = 57.6, GDT = 54.5 | RFT = 4, GDT = 0, P = 0.12 | Not reported | RFT = 29 (25-33), GDT = 18 (17-21) |
| Diaper (2020) | Single-centre RCT (Switzerland) | Major abdominal surgery | RFT versus GDTLid | Intraoperative | RFT = 2-4 mL/kg/hour balanced crystalloids, GDT = LiDCO monitor, 2-4 mL/kg/hour balanced crystalloids plus fluid boluses until increase in SVI < 10% | RFT = 201, GDT = 200 | RFT = 64, GDT = 65 | RFT = 1, GDT = 2 | RFT = 89.4%, GDT = 88.3% | RFT = 13 (8-19), GDT = 12 (8-20) |
| Joosten (2018) | Single-centre RCT (Belgium) | Moderate-risk abdominal surgery | RFT versus GDTClear | Intraoperative | RFT = 4 mL/kg/hour, GDT = Clearsight closed loop system, 100 mL fluid boluses, SVV < 13% and CI > 2.5 L/minute/m2 | RFT = 19, GDT = 20 | Not reported | RFT = 0, GDT = 0 | Not reported | RFT = 3 (2-5), GDT = 3 (2-6) |
| Phan (2014) | Single-centre RCT (Australia) | Elective colorectal surgery | RFT versus GDTOD | Intraoperative | RFT = 5 mL/kg/hour LR, GDT = 5 mL/kg/hour LR, with oesophageal Doppler, fluid boluses until increase in SV < 10% | RFT = 50, GDT = 50 | RFT = 65, GDT =63.1 | RFT = 1, GDT = 0 | Not reported | RFT = 6 (4-9), GDT = 6.5 (5-9) |
| Srinivasa (2013) | Single-centre RCT (New Zealand) | Elective colorectal surgery | RFT versus GDTOD | Intraoperative | RFT = 1,500 mL crystalloid, 500 mL colloid maximum, GDT = 1,500 mL crystalloid, oesophageal Doppler, fluid boluses until increase in SV < 10% | RFT = 43, GDT = 42 | RFT = 72, GDT = 69 | RFT = 0, GDT = 0 | Not reported | RFT = 5 (2-49), GDT = 6 (3-41), P = 0.570 |
| Zatloukal (2017) | Single-centre RCT (Czech Republic) | Liver resection surgery | RFT versus GDTFlo | Intraoperative | RFT = 1 mL/kg/hour, GDT = 2 mL/kg/hour with FloTrac, to maintain low flow state (CI < 2 L) | RFT = 17, GDT = 17 | RFT = 63, GDT = 59 | RFT = 0, GDT = 1, P = 0.31 | Not reported | RFT = 10 (8-14), GDT = 8 (7-11) |
| Zhang (2012) | Single-centre RCT (China) | Major abdominal surgery | RFT versus GDTPpv | Intraoperative | RFT = 4 mL/kg/hour LR, GDT = 4 mL/kg/hour LR, fluid bolus if PPV > 11% | RFT = 20, GDT = 20 | RFT = 53.3, GDT = 56.7 | RFT = 0, GDT = 0 | Not reported | RFT = 10.9 (+/- 1.2), GDT = 11.9 (+/- 1.2), P < 0.001 |
| RFT versus LFT | ||||||||||
| Barak (2006) | Single-centre RCT (Israel) | Major abdominal surgery | RFT versus LFT | Intraoperative | RFT = positive balance 0-1,000 mL, LFT = positive balance 1,000-2,000 mL | RFT = 14, LFT = 18 | RFT = 59, LFT = 65 | RFT = 0, LFT = 0 | Not reported | RFT = 16 (11-30), LFT = 17 (8-28) |
| Grant (2016) | Single-centre RCT (USA) | Pancreatectomy | RFT versus LFT | Intraoperative, postoperative | RFT = 6 mL/kg/hour with 100 mL fluid bolus, postoperative 60 mL/hour, LFT = 12 mL/kg/hour with 250 mL bolus, postoperative 125 mL/hour | RFT = 166, LFT = 164 | RFT = 65, LFT = 65 | RFT = 1, LFT = 1* (60-day mortality) | Not reported | RFT = 7 (3-34), LFT = 7 (3-57) |
| Holte, Kristensen (2007) | Single-centre RCT (Denmark) | TKR (ERAS) | RFT versus LFT | Intraoperative | RFT = no preload, 10 mL/kg/hour LR, LFT = 10 mL/kg preload, 30 mL/kg/hour LR | RFT = 24, LFT = 24 | RFT = 71.5, LFT = 71.5 | RFT = 0, LFT = 0 | Not reported | RFT = 4 (3-18), LFT = 4 (3-5) |
| Holte, Foss (2007) | Single-centre RCT (Denmark) | Colorectal surgery (ERAS) | RFT versus LFT | Intraoperative | RFT = no preload, 7 mL/kg/hour LR followed by 5 mL/kg/hour, LFT = preload 10 mL/kg followed by 18 mL/kg/hour | RFT = 16, LFT = 16 | RFT = 73.5, LFT = 76.5 | RFT = 0, LFT = 0 | Not reported | RFT = 4 (2-39), LFT = 2.5 (2-9), P = 0.03 |
| Kabon (2005) | Multicentre RCT (USA) | Colorectal surgery | RFT versus LFT | Intraoperative | RFT = 8-10 mL/kg/hour LR, LFT = 10 mL/kg preload, 16-18 mL/kg/hour | RFT = 124, LFT = 129 | RFT = 53, LFT = 52 | RFT = 0, LFT = 0 | Not reported | RFT = 7.3 +/- 4.0, LFT = 7.0 +/- 5.4 |
| Kalyan (2013) | Single-centre RCT (UK) | Abdominal surgery | RFT versus LFT | Intraoperative, postoperative | RFT = 1.5 mL/kg/hour, postoperative 1 mL/kg/hour, LFT = preload with 500 mL, 7 mL/kg/hour for the first hour followed by 5 mL/kg/hour, postoperative 1.5 mL/kg/hour | RFT = 119, LFT = 121 | RFT = 70, LFT = 70 | RFT = 2, LFT = 4 | Not reported | RFT = 8 (6-11), LFT = 8 (7-12) |
| Kumar (2020) | Single-centre RCT (India) | Robotic colorectal surgery | RFT versus LFT | Intraoperative | RFT = 2 mL/kg/hour crystalloid, LFT = 4 mL/kg/hour crystalloid | RFT = 20, LFT = 20 | RFT = 58.3, LFT = 60.3 | RFT = 0, LFT = 0 | Not reported | Not reported |
| Nisanevich (2005) | Single-centre RCT (Israel) | Abdominal surgery | RFT versus LFT | Intraoperative | RFT = 4 mL/kg/hour LR, LFT = preload with 10 mL/kg followed by 12 mL/kg/hour LR | RFT = 77, LFT = 75 | RFT = 62.8, LFT = 59.4 | RFT = 0, LFT = 0 | Not reported | RFT = 8 (6-21), LFT = 9 (7-24), P = 0.01 |
| Myles (2018) | Multicentre RCT (international) | Major abdominal surgery | RFT versus LFT | Intraoperative, postoperative | RFT = 5 mL/kg/hour, postoperative 0.8 mL/kg/hour, LFT = preload with 10 mL/kg, 8 mL/kg/hour, postoperative 1.5 mL/kg/hour | RFT = 1,490, LFT = 1,493 | RFT = 66, LFT = 66 | RFT = 31, LFT = 18 (90-day mortality), P = 0.06 | RFT = 1,223 (81.9%), LFT = 1,232 (82.3%), P = 0.61 | RFT = 6.4 (3.6-10.6), LFT = 5.6 (3.6-10.5), P = 0.26 |
| Yao (2017) | Single-centre RCT (China) | Laparoscopic cholecystectomy | RFT versus LFT | Intraoperative | RFT = 5 mL/kg/hour, LFT = 30 mL/kg/hour | RFT = 50, LFT = 50 | RFT = 46.04, LFT = 44.50 | RFT = 0, LFT = 0 | Not reported | RFT = 1, LFT = 1, P = 0.54 |
| GDT versus SFT | ||||||||||
| Ackland (2015) | Multicentre RCT (UK) | Major elective surgery | GDTLid versus SFT | Postoperative | GDT = LiDCO-guided, 1 mL/kg/hour LR, colloid bolus to keep SV increase < 10%, SFT = 1 mL/kg/hour with additional colloid bolus following standard parameter | GDT = 102, SFT = 102 | GDT = 68, SFT = 68 | GDT = 5, SFT = 5 | Not reported (Kaplan-Meier curve) | Not reported |
| Bahlmann (2019) | Multicentre RCT (Sweden) | Transthoracic oesophageal surgery | GDTFlo versus SFT | Intraoperative | GDT = 2.5 mL/kg/hour crystalloid, FloTrac-guided colloid bolus to keep SV increase < 10%, CI > 2.5 L/minute/m2, SFT = no standardized protocol | GDT = 30, SFT = 29 | GDT = 65, SFT = 66 | GDT = 1, SFT = 1 | Not reported (Kaplan-Meier curve) | GDT = 20 (15-45), SFT = 18 (15-25) |
| Bartha (2013) | Single-centre RCT (Sweden) | Proximal femur fracture surgery | GDTLid versus SFT | Intraoperative | GDT = 3 mL/kg/hour LR plus LiDCO-guided colloid bolus to keep SV increase < 10%, SFT = 3 mL/kg/hour LR | GDT = 74, SFT = 75 | GDT = 86, SFT =85 | GDT = 3, SFT = 4 | Not reported | GDT = 9 (3-20), SFT = 10 (1-38) |
| Benes (2010) | Single-centre RCT (Czech Republic) | Elective intrabdominal surgery | GDTFlo versus SFT | Intraoperative | GDT = 8 mL/kg/hour Plasmalyte, FloTrac-guided colloid bolus to keep SV increase < 10% and dobutamine to maintain CI > 2.5 L/minute/m2, SFT = 8 mL/kg/hour Plasmalyte | GDT = 60, SFT= 60 | GDT = 66.73, SFT = 66.32 | GDT = 1, SFT = 2 | Not reported | GDT = 9 (8-11.5), SFT = 10 (8-16), P = 0.0937 |
| Bisgaard (AAA) (2013) | Single-centre RCT (Denmark) | Open abdominal aortic surgery | GDTLid versus SFT | Intraoperative | GDT = LiDCO-guided colloid bolus to keep SV increase < 10%, SFT = no standardized protocol | GDT = 32, SFT = 32 | GDT = 68, SFT = 68 | GDT = 1, SFT = 0 | Not reported | GDT = 9 (8-11), SFT = 9 (8-14) |
| Bisgaard (PVA) (2013) | Single-centre RCT (Denmark) | Open lower limb vascular surgery | GDTLid versus SFT | Intraoperative | GDT = LiDCO-guided colloid bolus to keep SV increase < 10%, SFT = no standardized protocol | GDT = 20, SFT = 20 | GDT = 71, SFT = 74 | GDT = 0, SFT = 0 | Not reported | GDT = 5.5 (3-7), SFT = 6 (3.5-9), P = 0.3 |
| Buettner (2008) | Single-centre RCT (Germany) | Elective major abdominal surgery | GDTPic versus SFT | Intraoperative | GDT = PiCCOplus-guided fluid bolus to keep SPV < 10%, SFT = no standardized protocol | GDT = 40, SFT = 40 | GDT = 61, SFT = 66 | GDT = 0, SFT = 1 | Not reported | GDT = 15 (6-97), SFT = 16 (6-41) |
| Bundgaard-Nielsen (2013) | Single-centre RCT (Denmark) | Open radical prostatectomy | GDTOD versus SFT | Intraoperative, postoperative | GDT = 50 mL/kg fluid maximum with oesophageal Doppler-guided colloid bolus to keep SV increase < 10%, postoperative 3 mL/kg/hour LR, SFT = 50 mL/kg fluid maximum | GDT = 21, SFT = 21 | GDT = 63, SFT = 64 | Not reported | Not reported | GDT = 3 (3-4), SFT = 3 (2-3) |
| Calvo-Vecino (2018) | Multicentre RCT (Spain) | Low-risk patients with major abdominal surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided fluid bolus to keep SV increase < 10%, vasopressor or inotropes based on CI, SFT = 3-5 mL/kg/hour LR for laparoscopic surgery and 5-7 mL/kg/hour LR for open surgery, fluid bolus at discretion of anaesthetist | GDT = 224, SFT = 226 | GDT = 66.3, SFT = 64.2 | GDT = 10/209, SFT = 9/211, P = 0.81 | Not reported | GDT = 5 (4-10), SFT = 7 (5-12) |
| Cecconi (2011) | Single-centre RCT (Italy) | Total hip arthroplasty | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided colloid bolus to keep SV increase < 10%, SFT = preload 250 mL colloid, 10 mL/kg/hour LR | GDT = 20, SFT = 20 | GDT = 69, SFT = 63 | GDT = 0, SFT = 0 | Not reported | GDT = 10 (9-10), SFT = 10 (9-11) |
| Cesur (2018) | Single-centre RCT (Turkey) | Elective colorectal surgery | GDTPVI versus SFT | Intraoperative | GDT = 2 mL/kg/hour, PVI-guided fluid bolus to keep PVI < 13%, SFT = 4-8 mL/kg/hour | GDT = 35, SFT = 35 | GDT = 58.68, SFT = 62.31 | Not reported | Not reported | GDT = 6 (5-7), SFT = 6 (6-7) |
| Challand (2012) | Single-centre RCT (UK) | Elective colorectal surgery | GDTOD versus SFT | Intraoperative | GDT = 10 mL/kg/hour LR, oesophageal Doppler-guided colloid bolus to keep SV increase < 10%, SFT = 10 mL/kg/hour LR | GDT = 89, SFT = 90 | GDT = 66, SFT = 65.9 | GDT = 2, SFT = 2 | Not reported | GDT = 8.8 (6.0-11.9), SFT = 6.7 (4.8-13.3), P =0.09 |
| Chytra (2007) | Single-centre RCT (Czech Republic) | Emergency trauma patients | GDTOD versus SFT | Preoperative, intraoperative, postoperative | GDT = oesophageal Doppler-guided colloid bolus to keep SV increase < 10%, blood loss replaced 1:1 colloid, SFT = 1.5 mL/kg/hour LR, blood loss replaced 1:1 colloid | GDT = 80, SFT = 82 | GDT = 33, SFT = 40 | GDT = 13, SFT = 18, P = 0.43 | Not reported | GDT = 14 (8.25-21), SFT = 17.5 (11-29), P = 0.045 |
| Conway (2002) | Single-centre RCT (UK) | Abdominal surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided colloid bolus 3 mL/kg to keep SV increase < 10% and maintain corrected flow time > 0.35 seconds, SFT = no standardized protocol | GDT = 29, SFT = 28 | GDT = 66.5, SFT = 67.5 | GDT = 0, SFT = 1 | Not reported | GDT = 12 (7-103), SFT = 11 (7-30) |
| Corbella (2018) | Single-centre RCT (Canada) | Renal transplant surgery | GDTOD versus SFT | Intraoperative | GDT = preload of 500 mL crystalloid, oesophageal Doppler-guided crystalloid bolus to keep SV increase < 10%, 0.5 mL/kg/hour, SFT = no standardized protocol | GDT = 26, SFT = 24 | GDT = 58.9, SFT = 51.7 | Not reported | Not reported | GDT = 14.3 (+/-13.3), SFT = 14.1 (+/-9.0), P = 0.42 |
| Correa-Gallego (2015) | Single-centre RCT (USA) | Liver resection | R-GDTFlo versus SFT | Intraoperative | GDT = 1 mL/kg/hour crystalloid plus FloTrac-guided SVV to less than 2 standard deviations from baseline, SFT = 6 mL/kg/hour crystalloid infusion | GDT = 69, SFT = 66 | GDT = 55, SFT = 58 | GDT = 2, SFT = 0 (within 100 days) | Not reported | GDT = 7 (6-8), SFT = 6 (5-8), P = 0.34 |
| Demirel (2018) | Single-centre RCT (Turkey) | Roux-en-Y gastric bypass surgery for obesity | GDTPVI versus SFT | Intraoperative | GDT = preload 500 mL crystalloid, 2 mL/kg/hour, colloid bolus to keep PVI < 14%, SFT = preload 500 mL crystalloid, 4-8 mL/kg/hour infusion | GDT = 30, SFT = 30 | GDT = 36.33, SFT = 40.07 | Not reported | Not reported | Not reported |
| Elgendy (2017) | Single-centre RCT (Egypt) | High-risk patients in major abdominal surgery | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided colloid bolus to keep SVV < 12% and CI >2.5 L/minute/m2, crystalloid infusion rate not specified, SFT = infusion rate not specified, to meet conventional targets | GDT = 43, SFT = 43 | GDT = 58, SFT = 57 | GDT = 3, SFT = 5 | Not reported | GDT = 9.7 (+/- 1.9), SFT = 12.2 (+/- 3.5), P = 0.071 |
| El Sharkawy (2013) | Single-centre RCT (Egypt) | Liver resection | GDTOD versus SFT | Intraoperative | GDT = 6 mL/kg/hour LR infusion, oesophageal Doppler-guided colloid bolus to keep SV increase < 10%, SFT = 6 mL/kg/hour LR infusion, colloid bolus to maintain CVP, MAP and CO | GDT = 29, SFT = 30 | GDT = 48.16, SFT = 50.80 | GDT = 0, SFT = 0 | Not reported | GDT = 6.21(+/- 0.98), SFT = 7.55 (+/- 1.82), P < 0.01 |
| Forget (2010) | Single-centre RCT (Belgium) | Elective major abdominal surgery | GDTPVI versus SFT | Intraoperative | GDT = preload 500 mL crystalloid, 2 mL/kg/hour crystalloid infusion, colloid bolus to keep PVI < 13%, SFT = preload 500 mL crystalloid, 4-8 mL/kg/hour infusion | GDT = 41, SFT = 41 | GDT = 59, SFT = 61 | GDT = 2, SFT = 0 | Not reported | GDT = 15.1 (14.3), SFT = 16 (17.8), P = 0.78 |
| Funk, HayGlass (2015) | Single-centre RCT (Canada) | Open AAA surgery | GDTFlo versus SFT | Intraoperative | GDT = 3 mL/kg/hour LR infusion, FloTrac-guided colloid bolus to keep SVV < 13%, SFT = no standardized protocol | GDT = 20, SFT = 20 | GDT = 70, SFT =67 | GDT = 0, SFT = 2 | Not reported | GDT = 8 (7-13), SFT = 8 (6-12) |
| Funk, Bohn (2015) | Single-centre RCT (Canada) | Free flap surgery | GDTFlo versus SFT | Intraoperative | GDT = 5 mL/kg/hour LR, FloTrac-guided colloid bolus to keep SVV < 13%, SFT = no standardized protocol | GDT = 20, SFT = 20 | GDT = 47, SFT = 51 | Not reported | Not reported | Not reported |
| Gan (2002) | Single-centre RCT (USA) | Major elective surgery | GDTOD versus SFT | Intraoperative | GDT = 5 mL/kg/hour, oesophageal Doppler-guided colloid bolus to keep SV increase < 10% and FTc > 0.35 seconds, SFT = 5 mL/kg/hour | GDT = 50, SFT = 50 | GDT = 56, SFT = 59 | Not reported | Not reported | GDT = 5 (+/-3), SFT = 7 (+/-3), P = 0.03 |
| Gerent (2018) | Single-centre RCT (Brazil) | Major abdominal surgery | GDTFlo versus SFT | Postoperative | GDT = FloTrac-guided crystalloid bolus to keep CI > 2.5 L/minute/m2, SVI < 35 mL/m2, SFT = no standardized protocol | GDT = 64, SFT = 64 | GDT = 66, SFT = 68 | GDT = 14/64, SFT = 9/64, P = 0.4 | Not reported | GDT = 11 (6-19), SFT = 10 (6-15) |
| Gomez-Izquierdo (2017) | Single-centre RCT (Canada) | ERAS laparoscopic colorectal surgery | GDTOD versus SFT | Intraoperative | GDT = 1.5 mL/kg/hour LR crystalloid infusion, oesophageal Doppler-guided colloid bolus to keep SV increase < 10%, SFT = 1.5 mL/kg/hour LR crystalloid infusion | GDT = 64, SFT = 64 | GDT = 63, SFT = 61 | GDT = 0, SFT = 0 | Not reported | GDT = 4 (3-5), SFT = 4 (3-5.7), P = 0.922 |
| Hand (2016) | Single-centre RCT (USA) | Head and neck free flap surgery | GDTFlo versus SFT | Intraoperative | GDT = weight-based crystalloid infusion, FloTrac-guided fluid bolus to keep SVV < 13 and CI > 3.0, SFT = weight-based crystalloid infusion | GDT = 47, SFT = 47 | GDT = 59.1, SFT = 57.6 | Not reported | Not reported | GDT = 9.11 (5.76), SFT = 10.8 (7.65), P = 0.221 |
| Harten (2008) | Single-centre RCT (Scotland) | Emergency abdominal surgery | GDTLid versus SFT | Intraoperative | GDT = LiDCO-guided colloid bolus to keep PPV < 10%, SFT = no standardized protocol | GDT = 14, SFT = 15 | GDT = 66, SFT = 64 | GDT = 1/14, SFT = 2/15, P = 0.584 | Not reported | GDT = 17.5 (7-41), SFT = 12 (7-55), P = 0.122 |
| Jammer (2010) | Multicentre RCT (Norway) | Elective open colorectal surgery | GDTScv versus SFT | Intraoperative | GDT = preload 500 mL LR, then 1.5-2 mL/kg/hour LR, ScvO2-guided colloid bolus to keep ScvO2 > 75%, SFT = preload 1,000 mL LR, followed by 10-12 mL/kg/hour | GDT = 121, SFT = 120 | GDT = 57, SFT = 64 | GDT = 0, SFT = 0 | Not reported | GDT = 13.3, SFT = 12.6 |
| Jhanji (2010) | Single-centre RCT (UK) | Elective gastrointestinal surgery | GDTOD versus SFT | Postoperative | GDT = 1 mL/kg/hour LR with oesophageal Doppler-guided colloid bolus to keep SV increase < 10%, SFT = 1 mL/kg/hour LR with colloid bolus to keep CVP rise > 2 mmHg | GDT= 45, SFT = 45 | GDT = 68, SFT = 70 | GDT = 5/45, SFT = 6/45 | Not reported | GDT = 14 (11-26), SFT = 15 (10-26) |
| Kaufmann (2017) | Single-centre RCT (Germany) | Elective thoracic surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided crystalloid bolus to keep SV increase < 10%, SFT = conventional haemodynamic goals | GDT = 48, SFT = 48 | GDT = 65, SFT = 65 | Not reported | Not reported | GDT = 9 (5-11), SFT = 11 (9-12), P = 0.005 |
| Kim (2018) | Single-centre RCT (Korea) | Free flap reconstruction, head and neck surgery | GDTFlo versus SFT | Intraoperative | GDT = 5 mL/kg/hour crystalloid, FloTrac-guided colloid bolus to keep SVV < 12%, CI > 2.5 L/minute/m2, SFT = 5 mL/kg/hour, conventional goals | GDT = 31, SFT = 31 | GDT = 56, SFT = 56 | Not reported | Not reported | GDT = 22 (15-27), SFT = 22 (18-30) |
| Kumar (2016) | Single-centre RCT (India) | Major abdominal surgery | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided fluid bolus to keep SVV < 10%, SFT = maintain CVP 10-12 mmHg | GDT = 30, SFT = 30 | GDT = 55.3, SFT = 56.36 | Not reported | Not reported | GDT = 9.9 (+/- 2.68), SFT = 11.96 (+/- 5.15) |
| Lai (2015) | Single-centre RCT (UK) | Elective major abdominal surgery | GDTLid versus SFT | Intraoperative | GDT = LiDCO-guided colloid bolus to keep SVV < 10%, SFT = conventional goals | GDT = 109, SFT = 111 | GDT = 62.5, SFT = 63.4 | GDT = 3/109, SFT = 2/111 | Not reported | GDT = 11.8 (11.5), SFT = 9.6 (6.8) |
| Lilot (2018) | Single-centre RCT (France) | Elective abdominal surgery | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided, automated closed-loop fluid bolus to keep SVV < 13%, SFT = no standardized protocol | GDT = 24, SFT = 22 | GDT = 62.1, SFT = 68.5 | Not reported | Not reported | GDT = 13.6 (8.7), SFT = 17 (16), P = 0.361 |
| Lopes (2007) | Single-centre RCT (Brazil) | High-risk surgery | GDTPpv versus SFT | Intraoperative | GDT = PPV-guided colloid boluses to keep PPV < 10%, SFT = no standardized protocol | GDT = 17, SFT = 16 | GDT = 63, SFT = 62 | GDT = 2/17, SFT = 5/16 | Not reported | GDT = 7 (6-8.25), SFT = 17 (8-20), P < 0.01 |
| Luo (2017) | Single-centre RCT (China) | Neurosurgery | R-GDTFlo versus SFT | Intraoperative | GDT = 3 mL/kg/hour NS, FloTrac-guided colloid bolus to keep SVV < 15% and CI > 2.5, SFT = no protocol | GDT = 73, SFT = 72 | GDT = 61, SFT = 62 | GDT = 4/73, SFT = 9/72 | Not reported | GDT = 15 (7-23), SFT = 17 (9-27), P = 0.069 |
| Mayer (2010) | Single-centre RCT (Germany) | High-risk abdominal surgery | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided colloid bolus to keep SVV < 12%, SFT = conventional targets to keep CVP 8-12 mmHg | GDT = 30, SFT = 30 | GDT = 73, SFT = 72 | GDT = 2/30, SFT = 2/30 | Not reported | GDT = 15 (12-17.75), SFT = 19 (14-23.5), P = 0.006 |
| McKenny (2013) | Single-centre RCT (Ireland) | Elective gynaecological surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided colloid bolus to keep increase in SV < 10%, SFT = conventional targets | GDT = 51, SFT = 50 | GDT = 58, SFT = 58 | Not reported | Not reported | GDT = 6 (4-25), SFT = 7 (4-42), P = 0.5 |
| Mikor (2015) | Single-centre RCT (Hungary) | Major abdominal surgery | GDTScv versus SFT | Intraoperative | GDT = ScvO2-guided colloid bolus to keep ScvO2 > 75%, SFT = conventional targets | GDT = 38, SFT = 41 | GDT = 62, SFT = 62 | GDT = 1/39, SFT = 8/41, P = 0.018 | Not reported | Not reported |
| Moppett (2015) | Single-centre RCT (UK) | Hip fracture | GDTLid versus SFT | Intraoperative | GDT = LiDCO-guided colloid bolus to keep SV increase < 10%, SFT = no standardized protocol | GDT = 51, SFT = 63 | GDT = 85, SFT = 85 | GDT = 0/51, SFT = 7/63 | Not reported | GDT = 15.3 (13.8-17.2), SFT = 14.2 (12.9-15.8) |
| Noblett (2006) | Single-centre RCT (UK) | Elective colorectal resection | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided colloid bolus to keep SV increase < 10% and FTc > 350 millisecond, SFT = no standardized protocol | GDT = 51, SFT = 52 | GDT = 62.3, SFT = 67.6 | GDT = 0/51, SFT = 1/52 | Not reported | GDT = 7 (3-35), SFT = 9 (4-45), P = 0.005 |
| Pavlovic (2016) | Single-centre RCT (Switzerland) | Emergency surgery | GDTPic versus SFT | Intraoperative | GDT = 8-10 mL/kg NS preload, 3-4 mL/kg/hour crystalloid, PiCCOplus-guided colloid bolus to keep SVV < 12% or PPV < 12%, SFT = 8-10 mL/kg NS preload, 3-4 mL/kg/hour crystalloid | GDT = 20, SFT = 23 | GDT = 63, SFT = 66 | GDT = 5/20, SFT = 3/23, P = 0.540 | Not reported | GDT = 31 (17-42), SFT = 27 (13-43), P = 0.955 |
| Pearse (2014) | Multicentre RCT (UK) | Major gastrointestinal surgery | GDTLid versus SFT | Intraoperative, postoperative | GDT = LiDCO-guided colloid bolus to maintain maximal stroke volume, SFT = conventional targets, no standardized protocol | GDT = 368, SFT = 365 | GDT = 71.3, SFT = 72.2 | GDT = 12/366, SFT = 11/364 | Not reported | GDT = 10 (7-14), SFT = 11 (7-17), P = 0.05 |
| Pearse (2005) | Single-centre RCT (UK) | Major surgery | GDTLid versus SFT | Postoperative | GDT = 1.5 mL/kg/hour crystalloid, LiDCO-guided colloid bolus to maintain SV increase < 10% and DO2I > 600 mL/minute/m2, SFT = 1.5 mL/kg/hour crystalloid, maintain CVP rise of 2 mmHg | GDT = 62, SFT = 60 | GDT = 66, SFT = 68 | GDT = 6/62, SFT = 7/60 | Not reported | GDT = 17.5 (+/- 20.8), SFT = 29.5 (+/-34.8), P = 0.001 |
| Peng (2014) | Single-centre RCT (China) | Major orthopaedic surgery | GDTFlo versus SFT | Intraoperative | GDT = 5 mL/kg/hour LR, FloTrac-guided colloid bolus to maintain SVV < 10%, SFT = 5 mL/kg/hour LR, conventional targets | GDT = 40, SFT = 40 | GDT = 55, SFT = 53 | GDT = 1/40, SFT = 0/40 | Not reported | GDT = 12 (+/-3), SFT = 11 (+/-7) |
| Pestaña (2014) | Multicentre RCT (international) | Major abdominal surgery | GDTNic versus SFT | Intraoperative | GDT = NICOM-guided colloid bolus to maintain MAP > 65 mmHg and CI > 2.5L/minute/m2, SFT = no standardized protocol, conventional targets | GDT = 72, SFT = 70 | GDT = 73.5, SFT = 74 | GDT = 3/72, SFT = 4/70 | Not reported | GDT = 11.5 (8-15), SFT = 10.5 (8-16), P = 0.874 |
| Pillai (2011) | Single-centre RCT (UK) | Radical cystectomy | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided colloid bolus to keep SVV < 10% and FTc > 350 millisecond, SFT = no standardized protocol or targets | GDT = 32, SFT = 34 | GDT = 66.4, SFT = 68.5 | Not reported | Not reported | GDT = 18 (14.3-21.7), SFT = 22 (18.4-25.6), P = 0.12 |
| Ramsingh (2013) | Single-centre RCT (USA) | High-risk abdominal surgery | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided albumin bolus to keep SVV < 12%, SFT = no standardized protocol | GDT = 18, SFT = 20 | GDT = 53.5, SFT = 64.4 | Not reported | Not reported | GDT = 5.0 (3.75-8.25), SFT = 7.5 (5.25-10.75), P = 0.04 |
| Reisinger (2017) | Single-centre RCT (Netherlands) | Colorectal surgery | GDTOD versus SFT | Intraoperative, postoperative | GDT = oesophageal Doppler-guided colloid bolus to keep increase in SVI < 10%, SFT = no standardized protocol | GDT = 27, SFT = 31 | GDT = 68.6, SFT = 67.6 | GDT = 0/27, SFT = 1/31 | Not reported | GDT = 11 (4-50), SFT = 8 (5-26) |
| Salzwedel (2013) | Multicentre RCT (international) | Major abdominal surgery | GDTPro versus SFT | Intraoperative | GDT = ProAQT-guided colloid bolus to keep PPV < 10% and CI > 2.5 L/minute/m2, SFT = no standardized protocol | GDT = 79, SFT = 81 | GDT = 63, SFT = 65 | Not reported | Not reported | GDT = 11 (8), SFT = 10 (11.8), P = 0.929 |
| Scheeren (2013) | Multicentre RCT (Germany) | High-risk surgical patients | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided colloid bolus to keep SVV < 10%, SFT = conventional targets, standardized protocol | GDT = 26, SFT = 26 | GDT = 68, SFT = 73 | GDT = 0/26, SFT = 2/26 | Not reported | Not reported |
| Schmid (2014) | Single-centre RCT (Germany) | Major abdominal surgery | GDTPic versus SFT | Intraoperative, postoperative | GDT = 100 mL/hour LR, PiCCO2-guided fluid bolus to maintain GEDI > 800 and CI > 2.5, SFT = conventional targets, standardized protocol | GDT = 92, SFT = 88 | GDT = 67, SFT = 65 | GDT = 4/92, SFT = 4/88, P = 0.65 | GDT = 55/77, SFT = 61/771 | Not reported |
| Senagore (2009) | Single-centre RCT (USA) | Laparoscopic colorectal surgery (ERAS) | GDTOD versus SFT | Intraoperative | GDT = preload 5 mL/kg LR, 5 mL/kg/hour LR, oesophageal Doppler-guided colloid bolus to keep SVV < 10%, SFT = preload 5 mL/kg LR, 5 mL/kg/hour LR | GDT = 42, SFT = 22 | Not reported | GDT = 1/42, SFT = 0/22 | Not reported | GDT = 3.14, SFT = 2.7 |
| Sinclair (1997) | Single-centre RCT (UK) | Proximal femoral fracture surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided fluid bolus to keep FTc > 0.35 seconds and SVV < 10%, SFT = no standardized protocol | GDT = 20, SFT = 20 | GDT = 74, SFT = 75.5 | GDT = 1/20, SFT = 2/20 | Not reported | GDT = 12 (8-13), SFT = 20 (10-61), P < 0.05 |
| Stens (2017) | Multicentre RCT (Netherlands) | Moderate-risk abdominal surgery | GDTNex versus SFT | Intraoperative | GDT = ccNexfin-guided fluid bolus to keep PPV < 12% and CI > 2.5, MAP > 70 mmHg, SFT = keep MAP > 70 mmHg, no fluid protocol | GDT = 81, SFT= 94 | GDT = 61, SFT = 65 | GDT = 1/81, SFT = 1/94 | Not reported | GDT = 6 (1-44), SFT = 6 (1-30) |
| Szakmany (2005) | Single-centre RCT (Hungary) | Major abdominal surgery | GDTITBV versus SFT | Intraoperative | GDT = 10 mL/kg/hour LR, ITBV-guided colloid bolus to keep ITBV index 850-950 mL/m2, SFT = 10 mL/kg/hour LR, CVP-guided colloid bolus to keep CVP 8-12 mmHg | GDT = 20, SFT = 20 | GDT = 61, SFT = 59 | GDT = 2/20, SFT = 1/20 | Not reported | Not reported |
| Szturz (2018) | Single-centre RCT (Czech Republic) | Intermediate-risk open gastrointestinal surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided crystalloid bolus to keep FTc > 330 millisecond, CI > 2.5, SFT = standardized protocol, conventional targets | GDT = 71, SFT = 60 | GDT = 66, SFT = 65 | GDT = 1/71, SFT = 1/71 | Not reported | GDT = 9 (6-21), SFT = 11 (6-40), P = 0.301 |
| Van der Linden (2010) | Single-centre RCT (Belgium) | Peripheral arterial surgery | GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided colloid bolus to keep CI > 2.5 L/m2/minute, SFT = conventional targets, no fluid protocol | GDT = 40, SFT = 17 | GDT = 76, SFT = 68 | GDT = 3/40, SFT = 0/17 | Not reported | GDT = 18 (12-26), SFT = 14 (13-19) |
| Venn (2002) | Single-centre RCT (UK) | Hip fracture surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided colloid bolus to maintain SVV < 10% and FTc > 0.4 seconds, SFT = no fluid protocol, standard targets | GDT = 30, SFT = 29 | GDT = 82, SFT = 84.5 | GDT = 3/30, SFT = 2/30 | Not reported | GDT = 13.5 (10.9-17.5), SFT = 17.5 (13.9-24.4) |
| Wakeling (2005) | Single-centre RCT (UK) | Major bowel surgery | GDTOD versus SFT | Intraoperative | GDT = oesophageal Doppler-guided colloid bolus to keep SV increase < 10% and CVP rise > 3 mmHg, SFT = conventional targets, no standardized protocol | GDT = 64, SFT = 64 | GDT = 69.1, SFT = 69.6 | GDT = 0/64, SFT = 0/64 | Not reported | GDT = 10 (+/-5.75), SFT = 11.5 (+/-4.75), P = 0.031 |
| Weinberg (2017) | Single-centre RCT (Australia) | Pancreaticoduodenectomy | R-GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided crystalloid bolus to keep SVV < 20%, CI > 2 L/minute/m2, SFT = no standardized protocol | GDT = 26, SFT = 26 | GDT = 61, SFT = 68 | GDT = 0/26, SFT = 0/26 | Not reported | GDT = 9.5 (7.0-14.3), SFT = 12.5 (9-22.3), P = 0.002 |
| Weinberg (2019) | Multicentre RCT (Australia) | Major liver resection | R-GDTFlo versus SFT | Intraoperative | GDT = FloTrac-guided crystalloid bolus to keep SVV < 20% during liver transection, <15% during haemostasis and closure, SFT = low CVP < 8 mmHg, no standardized protocol | GDT = 24, SFT = 24 | GDT = 64, SFT = 61 | GDT = 0/24, SFT = 0/24 | Not reported | GDT = 7 (6-8), SFT = 8 (6-10), P = 0.17 |
| Wu (2017) | Single-centre RCT (China) | Intracranial tumour surgery | R-GDTFlo versus SFT | Intraoperative | GDT = 3 mL/kg colloid preload, 4+2+1 mL/kg/hour crystalloid, FloTrac-guided colloid bolus to keep SVV < 12% and CI > 2.5 L/minute/m2, SFT = 3 mL/kg colloid preload, 4+2+1 mL/kg/hour crystalloid, keep CVP > 8 mmHg and conventional targets | GDT = 33, SFT = 30 | GDT = 50, SFT = 50 | Not reported | Not reported | GDT = 10.4 (3.9), SFT = 12.2 (5.1) |
| Xu (2017) | Single-centre RCT (China) | Thoracic surgery with one-lung ventilation | R-GDTFlo versus SFT | Intraoperative | GDT = 4 mL/kg/hour crystalloid infusion, FloTrac-guided colloid bolus to keep SVV < 13% and CI > 2.5 L/minute/m2, SFT = 4 mL/kg/hour crystalloid infusion, conventional targets | GDT = 84, SFT = 84 | GDT = 49, SFT = 49 | Not reported | Not reported | GDT = 7 (6-8), SFT = 8 (7-9), P < 0.001 |
| Zakhaleva (2013) | Single-centre RCT (USA) | Bowel surgery (ERAS) | GDTOD versus SFT | Intraoperative | GDT = 4-8 mL/kg/hour crystalloid infusion, oesophageal Doppler colloid bolus to keep FTc > 350 millisecond and SV increase < 10%, SFT = 4-8 mL/kg/hour crystalloid infusion | GDT = 32, SFT = 40 | GDT = 57, SFT = 57 | GDT = 0, SFT = 0 | Not reported | GDT = 6 (3-30), SFT = 5 (3-16), P = 0.575 |
| Zhang (2013) | Single-centre RCT (China) | Thoracic surgery with one-lung ventilation | GDTFlo versus SFT | Intraoperative | GDT = 8 mL/kg/hour crystalloid infusion, FloTrac-guided colloid bolus to keep SVV < 11% and CI > 2.5 L, SFT = 8 mL/kg/hour crystalloid infusion, conventional targets | GDT = 30, SFT = 30 | GDT = 59.9, SFT = 61 | Not reported | Not reported | GDT = 4.5, SFT = 5 |
| Zhao (2018) | Single-centre RCT (China) | Gastrointestinal surgery | R-GDTFlo versus SFT | Intraoperative | GDT = 3 mL/kg/hour crystalloid infusion, FloTrac-guided colloid bolus to keep SVV < 13%, SFT = conventional targets, no standardized protocol | GDT = 44, SFT = 44 | GDT = 67.77, SFT = 70.41 | Not reported | Not reported | GDT = 21.93 (9.34), SFT = 26.16 (7.12) |
| Zheng (2013) | Single-centre RCT (China) | Gastrointestinal surgery | GDTFlo versus SFT | Intraoperative | GDT = 5-7 mL/kg crystalloid preload, FloTrac-guided crystalloid bolus to keep SVV < 12%, CI > 2.5 L/minute/m2, SFT = 5-7 mL/kg crystalloid preload, 4-2-1 mL/kg/hour crystalloid infusion | GDT = 30, SFT = 30 | GDT = 68, SFT = 67 | GDT = 0, SFT = 0 | Not reported | GDT = 18 (16-22.25), SFT = 22 (19-27) |
The risks of bias within the individual studies are presented in Table 3.
Table 3. Risks of bias within individual studies.
Low (+), low risk of bias; high (-), high risk of bias; unclear (?), unclear risk of bias according to the relative information
RFT, restricted fluid therapy; SFT, standard fluid therapy; GDT, goal-directed therapy; LFT, liberal fluid therapy
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | Overall bias | |
| Author (year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | ||
| RFT versus SFT | ||||||||
| Abraham-Nordling (2012) | + | + | + | + | + | + | + | + |
| Brandstrup (2003) | + | + | + | + | + | + | + | + |
| Cohn (2010) | + | + | ? | + | + | + | ? | ? |
| Futier (2010) | + | + | + | + | + | + | + | + |
| Gao (2012) | + | - | ? | - | ? | + | ? | - |
| González-Fajardo (2009) | + | ? | - | + | + | + | ? | ? |
| Jie (2014) | + | + | - | - | ? | ? | - | - |
| Lavu (2014) | + | + | + | + | + | + | + | + |
| Lobo (2011) | + | + | + | ? | + | + | + | ? |
| MacKay (2006) | + | + | - | + | + | + | + | + |
| McArdle (2009) | + | + | - | + | + | + | ? | ? |
| Piljic (2016) | + | ? | - | - | ? | ? | ? | - |
| Van Samkar (2015) | + | + | + | + | + | + | + | + |
| Vermeulen (2009) | + | + | + | + | + | + | + | + |
| Wuethrich (2014) | + | + | + | + | + | + | + | + |
| RFT versus GDT | ||||||||
| Benes (2015) | + | + | ? | + | + | + | + | + |
| Brandstrup (2012) | + | + | + | + | + | + | + | + |
| Colantonio (2015) | + | ? | - | + | ? | ? | ? | - |
| Joosten (2018) | + | + | + | + | + | + | ? | + |
| Phan (2014) | + | + | ? | + | + | + | + | + |
| Srinivasa (2013) | + | + | + | + | + | + | + | + |
| Zatloukal (2017) | + | + | ? | + | ? | ? | ? | - |
| Zhang (2012) | + | + | - | - | - | ? | ? | - |
| RFT versus LFT | ||||||||
| Barak (2006) | + | - | - | - | ? | ? | ? | - |
| Grant (2016) | + | ? | - | - | ? | ? | - | - |
| Holte, Kristensen (2007) | + | + | + | + | + | + | + | + |
| Holte, Foss (2007) | + | + | + | + | + | + | + | + |
| Kabon (2005) | + | + | + | + | ? | + | + | + |
| Kalyan (2013) | + | + | + | + | + | + | + | + |
| Kumar (2020) | + | + | + | ? | - | ? | ? | - |
| Nisanevich (2005) | + | ? | ? | + | + | + | + | + |
| Myles (2018) | + | + | - | + | + | + | ? | + |
| Yao (2017) | + | + | ? | ? | ? | ? | ? | - |
| GDT versus SFT | ||||||||
| Ackland (2015) | + | + | + | + | + | + | + | + |
| Bahlmann (2019) | + | + | + | + | + | + | + | + |
| Bartha (2013) | + | + | - | - | + | + | ? | ? |
| Benes (2010) | + | + | - | + | + | + | + | ? |
| Berlauk (1991) | + | ? | - | - | + | ? | ? | - |
| Bisgaard AAA (2013) | + | + | ? | + | + | + | ? | ? |
| Bisgaard PVA (2013) | + | + | ? | + | + | + | ? | ? |
| Buettner (2008) | + | + | ? | + | + | + | ? | + |
| Bundgaard-Nielsen (2013) | + | + | + | + | + | + | + | + |
| Calvo-Vecino (2018) | + | + | + | + | + | + | + | + |
| Cecconi (2011) | + | + | ? | + | + | + | + | + |
| Cesur (2018) | + | + | + | - | + | + | + | ? |
| Challand (2012) | + | + | + | + | + | + | + | + |
| Chytra (2007) | + | + | - | - | + | + | + | - |
| Conway (2002) | + | ? | ? | ? | + | + | ? | - |
| Corbella (2018) | + | + | + | + | + | + | + | + |
| Correa-Gallego (2015) | + | + | ? | - | + | + | ? | ? |
| Demirel (2018) | + | ? | - | - | + | + | ? | - |
| Diaper (2020) | + | + | ? | + | + | + | + | + |
| Elgendy (2017) | + | + | ? | + | + | ? | + | ? |
| El Sharkawy (2013) | + | + | + | + | + | ? | ? | ? |
| Forget (2010) | + | ? | ? | + | + | + | ? | ? |
| Funk, HayGlass (2015) | + | + | + | + | + | + | + | + |
| Funk, Bohn (2015) | + | + | + | + | + | ? | ? | ? |
| Gan (2002) | + | + | - | + | + | + | + | ? |
| Gerent (2018) | + | + | - | + | + | + | + | ? |
| Gomez-Izquierdo (2017) | + | + | - | + | + | + | + | ? |
| Hand (2016) | + | ? | - | - | + | + | + | - |
| Harten (2008) | + | - | - | + | + | + | + | - |
| Jammer (2010) | + | + | - | + | + | + | + | ? |
| Jhanji (2010) | + | + | ? | + | + | + | + | + |
| Kaufmann (2017) | + | + | ? | + | + | + | + | + |
| Kim (2018) | + | + | - | + | + | + | + | ? |
| Kumar (2016) | + | + | ? | + | + | ? | ? | ? |
| Lai (2015) | + | + | + | + | + | + | + | + |
| Lilot (2018) | + | + | ? | ? | + | + | ? | ? |
| Lopes (2007) | + | + | ? | - | + | ? | ? | - |
| Luo (2017) | + | + | - | + | + | + | ? | ? |
| Mayer (2010) | + | + | ? | + | + | + | + | + |
| McKenny (2013) | + | + | ? | + | + | + | + | + |
| Mikor (2015) | + | + | + | ? | + | + | + | + |
| Moppett (2015) | + | + | ? | + | + | + | + | + |
| Noblett (2006) | + | + | + | + | + | + | + | + |
| Pavlovic (2016) | + | + | ? | + | + | + | + | + |
| Pearse (2005) | + | + | ? | + | + | + | + | + |
| Peng (2014) | + | ? | - | + | + | + | ? | - |
| Pestana (2014) | + | + | ? | + | + | + | + | + |
| Pillai (2011) | + | + | + | + | + | + | + | + |
| Ramsingh (2013) | + | + | ? | + | + | + | + | + |
| Reisinger (2017) | + | - | ? | + | + | + | + | ? |
| Salzwedel (2013) | + | + | - | ? | + | + | + | ? |
| Scheeren (2013) | + | + | ? | + | + | + | + | + |
| Schmid (2014) | + | ? | ? | ? | + | + | + | - |
| Senagore (2009) | + | + | + | + | + | + | + | + |
| Sinclair (1997) | + | ? | + | ? | + | + | + | ? |
| Stens (2017) | + | + | + | ? | + | + | + | + |
| Szakmany (2005) | + | + | ? | ? | + | + | + | ? |
| Szturz (2018) | + | + | ? | + | + | + | + | + |
| Van der Linden (2010) | + | + | + | + | + | + | + | + |
| Venn (2002) | + | + | - | - | + | + | + | - |
| Wakeling (2005) | + | + | ? | + | + | + | + | + |
| Weinberg (2017) | + | ? | ? | + | + | + | + | ? |
| Weinberg (2019) | + | + | + | + | + | + | + | + |
| Wu (2017) | + | + | ? | + | + | ? | ? | ? |
| Xu (2017) | + | + | + | + | + | + | ? | ? |
| Zakhaleva (2013) | + | + | - | - | + | ? | ? | - |
| Zhang (2013) | + | + | - | - | + | ? | ? | - |
| Zhao (2018) | + | ? | - | - | ? | ? | ? | - |
| Zheng (2013) | + | ? | - | + | ? | ? | ? | - |
Figure 8 presents network meta-regression forest plots that show the estimated effect (mean difference) of the interventions in comparison with SFT. It showed a minimal effect of risk of bias in all analyses.
Figure 8. Network meta-regression .
Network meta-regression forest plots showing the estimated effect (mean difference) of each intervention as compared to SFT, as well as the 95% CrI for each comparison for high risk of bias studies removed versus when they were included.
CrI, credible intervals; GDT, goal-directed therapy; GDTOD, GDT utilising oesophageal Doppler; GDTFlo, GDT using Vigileo/FloTrac; GDTLid, GDT utilising LiDCO; GDTPpv, GDT utilising pulse pressure variation or pulse variability index; GDTOthers, GDT utilising other technology; LFT, liberal fluid therapy; SFT, standard fluid therapy; RFT, restricted fluid therapy
The authors have declared that no competing interests exist.
References
- 1.Perioperative fluid therapy for major surgery. Miller TE, Myles PS. https://pubs.asahq.org/anesthesiology/article/130/5/825/18881/Perioperative-Fluid-Therapy-for-Major-Surgery. Anesthesiology. 2019;130:825–832. doi: 10.1097/ALN.0000000000002603. [DOI] [PubMed] [Google Scholar]
- 2.Liberal or restrictive fluid administration in fast-track colonic surgery: a randomized, double-blind study. Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, Kehlet H. Br J Anaesth. 2007;99:500–508. doi: 10.1093/bja/aem211. [DOI] [PubMed] [Google Scholar]
- 3.Liberal perioperative fluid administration is an independent risk factor for morbidity and is associated with longer hospital stay after rectal cancer surgery. Boland MR, Reynolds I, McCawley N, Galvin E, El-Masry S, Deasy J, McNamara DA. Ann R Coll Surg Engl. 2017;99:113–116. doi: 10.1308/rcsann.2016.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A restrictive dose of crystalloids in patients during laparoscopic cholecystectomy is safe and cost-effective: prospective, two-arm parallel, randomized controlled trial. Belavić M, Sotošek Tokmadžić V, Brozović Krijan A, Kvaternik I, Matijaš K, Strikić N, Žunić J. Ther Clin Risk Manag. 2018;14:741–751. doi: 10.2147/TCRM.S160778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perioperative restrictive versus goal‐directed fluid therapy for adults undergoing major non‐cardiac surgery. Wrzosek A, Jakowicka‐Wordliczek J, Zajaczkowska R, et al. http://dx.doi.org/10.1002/14651858.cd012767. Cochrane Database Syst Rev. 2017;2017:0. doi: 10.1002/14651858.CD012767.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restrictive and liberal fluid administration in major abdominal surgery. Pang Q, Liu H, Chen B, Jiang Y. Saudi Med J. 2017;38:123–131. doi: 10.15537/smj.2017.2.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Restrictive versus liberal fluid therapy for major abdominal surgery. Myles PS, Bellomo R, Corcoran T, et al. N Engl J Med. 2018;378:2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 8.Perioperative fluid restriction in abdominal surgery: a systematic review and meta-analysis. Shen Y, Cai G, Gong S, Yan J. World J Surg. 2019;43:2747–2755. doi: 10.1007/s00268-019-05091-y. [DOI] [PubMed] [Google Scholar]
- 9.Is goal-directed fluid therapy based on dynamic variables alone sufficient to improve clinical outcomes among patients undergoing surgery? A meta-analysis. Deng QW, Tan WC, Zhao BC, Wen SH, Shen JT, Xu M. Crit Care. 2018;22:298. doi: 10.1186/s13054-018-2251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Classification and differential effectiveness of goal-directed hemodynamic therapies in surgical patients: A network meta-analysis of randomized controlled trials. Zhao X, Tian L, Brackett A, Dai F, Xu J, Meng L. J Crit Care. 2021;61:152–161. doi: 10.1016/j.jcrc.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Network meta-analysis: an introduction for clinicians. Rouse B, Chaimani A, Li T. Intern Emerg Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Hutton B, Salanti G, Caldwell DM, et al. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 13.Evaluating the quality of evidence from a network meta-analysis. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. PLoS One. 2014;9:0. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: Wiley; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 16.Assessing the quality of randomized trials: reliability of the Jadad scale. Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, Laupacis A. Control Clin Trials. 1999;20:448–452. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 17.Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Stat Methods Med Res. 2020;29:2520–2537. doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. Wan X, Wang W, Liu J, Tong T. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Cochrane Handbook for systematic reviews of interventions, second edition. Hoboken, NJ: Wiley; 10.1002/9781119536604.ch8. Chapter 8: Assessing risk of bias in a randomized trial. In; pp. 205–228. [Google Scholar]
- 20.'Liberal' vs. 'restrictive' perioperative fluid therapy--a critical assessment of the evidence. Bundgaard-Nielsen M, Secher NH, Kehlet H. Acta Anaesthesiol Scand. 2009;53:843–851. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaye AD, Riopelle JM. Miller’s anesthesia, sixth edition. Philadelphia, PA: Elsevier; 2005. Intravascular fluid and electrolyte physiology; pp. 1705–1737. [Google Scholar]
- 22.Automating network meta-analysis. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Res Synth Methods. 2012;3:285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Li T, Deeks JJ. Cochrane handbook for systematic reviews of interventions version 6.3. London, UK: Cochrane; 2022. Chapter 6: Choosing effect measures and computing estimates of effect. [Google Scholar]
- 24.A comparison of Bayesian and frequentist methods in random-effects network meta-analysis of binary data. Seide SE, Jensen K, Kieser M. Res Synth Methods. 2020;11:363–378. doi: 10.1002/jrsm.1397. [DOI] [PubMed] [Google Scholar]
- 25.Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Jansen JP, Crawford B, Bergman G, Stam W. Value Health. 2008;11:956–964. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 26.Bayesian measures of model complexity and fit. Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. https://rss.onlinelibrary.wiley.com/doi/pdf/10.1111/1467-9868.00353 J R Statist Soc B. 2002;64:583–639. [Google Scholar]
- 27.A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Puhan MA, Schünemann HJ, Murad MH, et al. BMJ. 2014;349:0. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 28.Quantifying heterogeneity in a meta-analysis. Higgins JP, Thompson SG. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Graphical tools for network meta-analysis in STATA. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randomized clinical trial of fluid restriction in colorectal surgery. Abraham-Nordling M, Hjern F, Pollack J, Prytz M, Borg T, Kressner U. Br J Surg. 2012;99:186–191. doi: 10.1002/bjs.7702. [DOI] [PubMed] [Google Scholar]
- 31.Individualised oxygen delivery targeted haemodynamic therapy in high-risk surgical patients: a multicentre, randomised, double-blind, controlled, mechanistic trial. Ackland GL, Iqbal S, Paredes LG, et al. Lancet Respir Med. 2015;3:33–41. doi: 10.1016/S2213-2600(14)70205-X. [DOI] [PubMed] [Google Scholar]
- 32.Goal-directed therapy during transthoracic oesophageal resection does not improve outcome: Randomised controlled trial. Bahlmann H, Halldestam I, Nilsson L. Eur J Anaesthesiol. 2019;36:153–161. doi: 10.1097/EJA.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 33.Prolonged international normalized ratio correlates with a large intravascular fluid balance after major abdominal surgery. Barak M, Jurim O, Tal R, Katz Y. Anesth Analg. 2006;103:448–452. doi: 10.1213/01.ane.0000223677.34513.88. [DOI] [PubMed] [Google Scholar]
- 34.Randomized controlled trial of goal-directed haemodynamic treatment in patients with proximal femoral fracture. Bartha E, Arfwedson C, Imnell A, Fernlund ME, Andersson LE, Kalman S. Br J Anaesth. 2013;110:545–553. doi: 10.1093/bja/aes468. [DOI] [PubMed] [Google Scholar]
- 35.Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Benes J, Chytra I, Altmann P, et al. Crit Care. 2010;14:0. doi: 10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fluid management guided by a continuous non-invasive arterial pressure device is associated with decreased postoperative morbidity after total knee and hip replacement. Benes J, Haidingerova L, Pouska J, et al. BMC Anesthesiol. 2015;15:148. doi: 10.1186/s12871-015-0131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haemodynamic optimisation in lower limb arterial surgery: room for improvement? Bisgaard J, Gilsaa T, Rønholm E, Toft P. Acta Anaesthesiol Scand. 2013;57:189–198. doi: 10.1111/j.1399-6576.2012.02755.x. [DOI] [PubMed] [Google Scholar]
- 38.Optimising stroke volume and oxygen delivery in abdominal aortic surgery: a randomised controlled trial. Bisgaard J, Gilsaa T, Rønholm E, Toft P. Acta Anaesthesiol Scand. 2013;57:178–188. doi: 10.1111/j.1399-6576.2012.02756.x. [DOI] [PubMed] [Google Scholar]
- 39.Impact of perioperative hemodynamic optimization therapies in surgical patients: economic study and meta-analysis. Silva-Jr JM, Menezes PF, Lobo SM, et al. BMC Anesthesiol. 2020;20:71. doi: 10.1186/s12871-020-00987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Brandstrup B, Svendsen PE, Rasmussen M, et al. Br J Anaesth. 2012;109:191–199. doi: 10.1093/bja/aes163. [DOI] [PubMed] [Google Scholar]
- 41.Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Brandstrup B, Tønnesen H, Beier-Holgersen R, et al. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Influence of systolic-pressure-variation-guided intraoperative fluid management on organ function and oxygen transport. Buettner M, Schummer W, Huettemann E, Schenke S, van Hout N, Sakka SG. Br J Anaesth. 2008;101:194–199. doi: 10.1093/bja/aen126. [DOI] [PubMed] [Google Scholar]
- 43.Does goal-directed fluid therapy affect postoperative orthostatic intolerance?: a randomized trial. Bundgaard-Nielsen M, Jans Ø, Müller RG, et al. Anesthesiology. 2013;119:813–823. doi: 10.1097/ALN.0b013e31829ce4ea. [DOI] [PubMed] [Google Scholar]
- 44.Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial) Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG, et al. Br J Anaesth. 2018;120:734–744. doi: 10.1016/j.bja.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Cecconi M, Fasano N, Langiano N, Divella M, Costa MG, Rhodes A, Della Rocca G. Crit Care. 2011;15:0. doi: 10.1186/cc10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Comparison of conventional fluid management with PVI-based goal-directed fluid management in elective colorectal surgery. Cesur S, Çardaközü T, Kuş A, Türkyılmaz N, Yavuz Ö. J Clin Monit Comput. 2019;33:249–257. doi: 10.1007/s10877-018-0163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, Minto G. Br J Anaesth. 2012;108:53–62. doi: 10.1093/bja/aer273. [DOI] [PubMed] [Google Scholar]
- 48.Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: a randomized controlled trial. Chytra I, Pradl R, Bosman R, Pelnár P, Kasal E, Zidková A. Crit Care. 2007;11:0. doi: 10.1186/cc5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A prospective randomized pilot study of near-infrared spectroscopy-directed restricted fluid therapy versus standard fluid therapy in patients undergoing elective colorectal surgery. Cohn SM, Pearl RG, Acosta SM, Nowlin MU, Hernandez A, Guta C, Michalek JE. https://pubmed.ncbi.nlm.nih.gov/21265353/ Am Surg. 2010;76:1384–1392. [PubMed] [Google Scholar]
- 50.A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Colantonio L, Claroni C, Fabrizi L, et al. J Gastrointest Surg. 2015;19:722–729. doi: 10.1007/s11605-015-2743-1. [DOI] [PubMed] [Google Scholar]
- 51.Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C. Anaesthesia. 2002;57:845–849. doi: 10.1046/j.1365-2044.2002.02708.x. [DOI] [PubMed] [Google Scholar]
- 52.Cardiac output-based fluid optimization for kidney transplant recipients: a proof-of-concept trial. Corbella D, Toppin PJ, Ghanekar A, Ayach N, Schiff J, Van Rensburg A, McCluskey SA. Can J Anaesth. 2018;65:873–883. doi: 10.1007/s12630-018-1118-y. [DOI] [PubMed] [Google Scholar]
- 53.Goal-directed fluid therapy using stroke volume variation for resuscitation after low central venous pressure-assisted liver resection: a randomized clinical trial. Correa-Gallego C, Tan KS, Arslan-Carlon V, et al. J Am Coll Surg. 2015;221:591–601. doi: 10.1016/j.jamcollsurg.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Efficacy of goal-directed fluid therapy via Pleth Variability Index during laparoscopic Roux-en-Y gastric bypass surgery in morbidly obese patients. Demirel İ, Bolat E, Altun AY, Özdemir M, Beştaş A. Obes Surg. 2018;28:358–363. doi: 10.1007/s11695-017-2840-1. [DOI] [PubMed] [Google Scholar]
- 55.Goal-directed hemodynamic therapy versus restrictive normovolemic therapy in major open abdominal surgery: a randomized controlled trial. Diaper J, Schiffer E, Barcelos GK, Luise S, Schorer R, Ellenberger C, Licker M. Surgery. 2021;169:1164–1174. doi: 10.1016/j.surg.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Transoesophageal Doppler compared to central venous pressure for perioperative hemodynamic monitoring and fluid guidance in liver resection. El Sharkawy OA, Refaat EK, Ibraheem AE, et al. Saudi J Anaesth. 2013;7:378–386. doi: 10.4103/1658-354X.121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Outcome of intraoperative goal-directed therapy using Vigileo/FloTrac in high-risk patients scheduled for major abdominal surgeries: a prospective randomized trial. Elgendy MA, Esmat IM, Kassim DY. Egypt J Anaesth. 2017;33:263–269. [Google Scholar]
- 58.Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Forget P, Lois F, de Kock M. Anesth Analg. 2010;111:910–914. doi: 10.1213/ANE.0b013e3181eb624f. [DOI] [PubMed] [Google Scholar]
- 59.Goal-directed fluid therapy for microvascular free flap reconstruction following mastectomy: a pilot study. Funk D, Bohn J, Mutch W, Hayakawa T, Buchel EW. Plast Surg (Oakv) 2015;23:231–234. doi: 10.4172/plastic-surgery.1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Funk DJ, HayGlass KT, Koulack J, Harding G, Boyd A, Brinkman R. Crit Care. 2015;19:247. doi: 10.1186/s13054-015-0974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Futier E, Constantin JM, Petit A, et al. Arch Surg. 2010;145:1193–1200. doi: 10.1001/archsurg.2010.275. [DOI] [PubMed] [Google Scholar]
- 62.Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Gan TJ, Soppitt A, Maroof M, et al. Anesthesiology. 2002;97:820–826. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Restricted intravenous fluid regimen reduces the rate of postoperative complications and alters immunological activity of elderly patients operated for abdominal cancer: a randomized prospective clinical trail. Gao T, Li N, Zhang JJ, et al. World J Surg. 2012;36:993–1002. doi: 10.1007/s00268-012-1516-1. [DOI] [PubMed] [Google Scholar]
- 64.Effect of postoperative goal-directed therapy in cancer patients undergoing high-risk surgery: a randomized clinical trial and meta-analysis. Gerent AR, Almeida JP, Fominskiy E, et al. Crit Care. 2018;22:133. doi: 10.1186/s13054-018-2055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: a randomized controlled trial. Gómez-Izquierdo JC, Trainito A, Mirzakandov D, et al. Anesthesiology. 2017;127:36–49. doi: 10.1097/ALN.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 66.Effect of postoperative restrictive fluid therapy in the recovery of patients with abdominal vascular surgery. González-Fajardo JA, Mengibar L, Brizuela JA, Castrodeza J, Vaquero-Puerta C. Eur J Vasc Endovasc Surg. 2009;37:538–543. doi: 10.1016/j.ejvs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Prospective randomized controlled trial of liberal vs restricted perioperative fluid management in patients undergoing pancreatectomy. Grant F, Brennan MF, Allen PJ, et al. Ann Surg. 2016;264:591–598. doi: 10.1097/SLA.0000000000001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Intraoperative goal-directed hemodynamic management in free tissue transfer for head and neck cancer. Hand WR, Stoll WD, McEvoy MD, et al. Head Neck. 2016;38 Suppl 1:0–80. doi: 10.1002/hed.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Effect of intraoperative fluid optimisation on renal function in patients undergoing emergency abdominal surgery: a randomised controlled pilot study (ISRCTN 11799696) Harten J, Crozier JE, McCreath B, Hay A, McMillan DC, McArdle CS, Kinsella J. Int J Surg. 2008;6:197–204. doi: 10.1016/j.ijsu.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double-blind study. Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H. Anesth Analg. 2007;105:465–474. doi: 10.1213/01.ane.0000263268.08222.19. [DOI] [PubMed] [Google Scholar]
- 71.Does central venous oxygen saturation-directed fluid therapy affect postoperative morbidity after colorectal surgery? A randomized assessor-blinded controlled trial. Jammer I, Ulvik A, Erichsen C, Lødemel O, Ostgaard G. Anesthesiology. 2010;113:1072–1080. doi: 10.1097/ALN.0b013e3181f79337. [DOI] [PubMed] [Google Scholar]
- 72.Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Crit Care. 2010;14:0. doi: 10.1186/cc9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perioperative restricted fluid therapy preserves immunological function in patients with colorectal cancer. Jie HY, Ye JL, Zhou HH, Li YX. World J Gastroenterol. 2014;20:15852–15859. doi: 10.3748/wjg.v20.i42.15852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Personalized versus protocolized fluid management using noninvasive hemodynamic monitoring (Clearsight system) in patients undergoing moderate-risk abdominal surgery. Joosten A, Raj Lawrence S, Colesnicenco A, et al. Anesth Analg. 2019;129:0. doi: 10.1213/ANE.0000000000003553. [DOI] [PubMed] [Google Scholar]
- 75.Supplemental intravenous crystalloid administration does not reduce the risk of surgical wound infection. Kabon B, Akça O, Taguchi A, et al. Anesth Analg. 2005;101:1546–1553. doi: 10.1213/01.ANE.0000180217.57952.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Randomized clinical trial of fluid and salt restriction compared with a controlled liberal regimen in elective gastrointestinal surgery. Kalyan JP, Rosbergen M, Pal N, et al. Br J Surg. 2013;100:1739–1746. doi: 10.1002/bjs.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oesophageal Doppler guided goal-directed haemodynamic therapy in thoracic surgery - a single centre randomized parallel-arm trial. Kaufmann KB, Stein L, Bogatyreva L, et al. Br J Anaesth. 2017;118:852–861. doi: 10.1093/bja/aew447. [DOI] [PubMed] [Google Scholar]
- 78.Effect of goal-directed haemodynamic therapy in free flap reconstruction for head and neck cancer. Kim HJ, Kim EJ, Lee HJ, et al. Acta Anaesthesiol Scand. 2018;62:903–914. doi: 10.1111/aas.13100. [DOI] [PubMed] [Google Scholar]
- 79.Effect of liberal versus restrictive fluid therapy on intraoperative lactate levels in robot- assisted colorectal surgery. Kumar L, Kumar K, Sandhya S, Koshy DM, Ramamurthi KP, Rajan S. Indian J Anaesth. 2020;64:599–604. doi: 10.4103/ija.IJA_401_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Outcomes associated with stroke volume variation versus central venous pressure guided fluid replacements during major abdominal surgery. Kumar L, Rajan S, Baalachandran R. J Anaesthesiol Clin Pharmacol. 2016;32:182–186. doi: 10.4103/0970-9185.182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Randomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitness. Lai CW, Starkie T, Creanor S, et al. Br J Anaesth. 2015;115:578–589. doi: 10.1093/bja/aev299. [DOI] [PubMed] [Google Scholar]
- 82.The HYSLAR trial: a prospective randomized controlled trial of the use of a restrictive fluid regimen with 3% hypertonic saline versus lactated Ringers in patients undergoing pancreaticoduodenectomy. Lavu H, Sell NM, Carter TI, et al. Ann Surg. 2014;260:445–453. doi: 10.1097/SLA.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 83.Effect of goal-directed fluid therapy on the prognosis of elderly patients with hypertension receiving plasmakinetic energy transurethral resection of prostate. Liang M, Li Y, Lin L, et al. https://e-century.us/files/ijcem/10/1/ijcem0040487.pdf Int J Clin Exp Med. 2017;10:1290–1296. [Google Scholar]
- 84.Comparison of cardiac output optimization with an automated closed-loop goal-directed fluid therapy versus non standardized manual fluid administration during elective abdominal surgery: first prospective randomized controlled trial. Lilot M, Bellon A, Gueugnon M, et al. J Clin Monit Comput. 2018;32:993–1003. doi: 10.1007/s10877-018-0106-7. [DOI] [PubMed] [Google Scholar]
- 85.Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 86.Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Lobo SM, Ronchi LS, Oliveira NE, et al. Crit Care. 2011;15:0. doi: 10.1186/cc10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO Jr, Michard F. Crit Care. 2007;11:0. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goal-directed fluid restriction during brain surgery: a prospective randomized controlled trial. Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Ann Intensive Care. 2017;7:16. doi: 10.1186/s13613-017-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O'Dwyer PJ. Br J Surg. 2006;93:1469–1474. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 90.Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Crit Care. 2010;14:0. doi: 10.1186/cc8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Preliminary results of a prospective randomized trial of restrictive versus standard fluid regime in elective open abdominal aortic aneurysm repair. McArdle GT, McAuley DF, McKinley A, Blair P, Hoper M, Harkin DW. Ann Surg. 2009;250:28–34. doi: 10.1097/SLA.0b013e3181ad61c8. [DOI] [PubMed] [Google Scholar]
- 92.A randomised prospective trial of intra-operative oesophageal Doppler-guided fluid administration in major gynaecological surgery. McKenny M, Conroy P, Wong A, et al. Anaesthesia. 2013;68:1224–1231. doi: 10.1111/anae.12355. [DOI] [PubMed] [Google Scholar]
- 93.Continuous central venous oxygen saturation assisted intraoperative hemodynamic management during major abdominal surgery: a randomized, controlled trial. Mikor A, Trásy D, Németh MF, et al. BMC Anesthesiol. 2015;15:82. doi: 10.1186/s12871-015-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LiDCO-based fluid management in patients undergoing hip fracture surgery under spinal anaesthesia: a randomized trial and systematic review. Moppett IK, Rowlands M, Mannings A, Moran CG, Wiles MD. Br J Anaesth. 2015;114:444–459. doi: 10.1093/bja/aeu386. [DOI] [PubMed] [Google Scholar]
- 95.Effect of intraoperative fluid management on outcome after intraabdominal surgery. Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Anesthesiology. 2005;103:25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Noblett SE, Snowden CP, Shenton BK, Horgan AF. Br J Surg. 2006;93:1069–1076. doi: 10.1002/bjs.5454. [DOI] [PubMed] [Google Scholar]
- 97.Impact of early haemodynamic goal-directed therapy in patients undergoing emergency surgery: an open prospective, randomised trial. Pavlovic G, Diaper J, Ellenberger C, Frei A, Bendjelid K, Bonhomme F, Licker M. J Clin Monit Comput. 2016;30:87–99. doi: 10.1007/s10877-015-9691-x. [DOI] [PubMed] [Google Scholar]
- 98.Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Crit Care. 2005;9:0–93. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. Pearse RM, Harrison DA, MacDonald N, et al. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 100.Goal-directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Peng K, Li J, Cheng H, Ji FH. Med Princ Pract. 2014;23:413–420. doi: 10.1159/000363573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: a prospective, randomized, multicenter, pragmatic trial: POEMAS Study (PeriOperative goal-directed thErapy in Major Abdominal Surgery) Pestaña D, Espinosa E, Eden A, et al. Anesth Analg. 2014;119:579–587. doi: 10.1213/ANE.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 102.A randomised controlled trial of fluid restriction compared to oesophageal Doppler-guided goal-directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Phan TD, D'Souza B, Rattray MJ, Johnston MJ, Cowie BS. Anaesth Intensive Care. 2014;42:752–760. doi: 10.1177/0310057X1404200611. [DOI] [PubMed] [Google Scholar]
- 103.Restrictive versus standard fluid regimen in Elective minilaparotomy abdominal aortic repair-prospective randomized controlled trial. Piljic D, Petricevic M, Piljic D, Ksela J, Robic B, Klokocovnik T. Thorac Cardiovasc Surg. 2016;64:296–303. doi: 10.1055/s-0035-1548736. [DOI] [PubMed] [Google Scholar]
- 104.A double-blind randomized controlled clinical trial to assess the effect of Doppler optimized intraoperative fluid management on outcome following radical cystectomy. Pillai P, McEleavy I, Gaughan M, et al. J Urol. 2011;186:2201–2206. doi: 10.1016/j.juro.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 105.Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. Ramsingh DS, Sanghvi C, Gamboa J, Cannesson M, Applegate RL 2nd. J Clin Monit Comput. 2013;27:249–257. doi: 10.1007/s10877-012-9422-5. [DOI] [PubMed] [Google Scholar]
- 106.Doppler-guided goal-directed fluid therapy does not affect intestinal cell damage but increases global gastrointestinal perfusion in colorectal surgery: a randomized controlled trial. Reisinger KW, Willigers HM, Jansen J, Buurman WA, Von Meyenfeldt MF, Beets GL, Poeze M. Colorectal Dis. 2017;19:1081–1091. doi: 10.1111/codi.13923. [DOI] [PubMed] [Google Scholar]
- 107.Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Salzwedel C, Puig J, Carstens A, et al. Crit Care. 2013;17:0. doi: 10.1186/cc12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. Scheeren TW, Wiesenack C, Gerlach H, Marx G. J Clin Monit Comput. 2013;27:225–233. doi: 10.1007/s10877-013-9461-6. [DOI] [PubMed] [Google Scholar]
- 109.Can goal-directed hemodynamic management improve renal outcome after major non-cardiac surgery? Schmid S, Heim M, Kapfer B, Blobner M, Kochs E, Jungwirth B. Eur J Anaesthesiol. 2014;31:57. [Google Scholar]
- 110.Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Dis Colon Rectum. 2009;52:1935–1940. doi: 10.1007/DCR.0b013e3181b4c35e. [DOI] [PubMed] [Google Scholar]
- 111.Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. Sinclair S, James S, Singer M. BMJ. 1997;315:909–912. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Br J Surg. 2013;100:66–74. doi: 10.1002/bjs.8940. [DOI] [PubMed] [Google Scholar]
- 113.The added value of cardiac index and pulse pressure variation monitoring to mean arterial pressure-guided volume therapy in moderate-risk abdominal surgery (COGUIDE): a pragmatic multicentre randomised controlled trial. Stens J, Hering JP, van der Hoeven CW, et al. Anaesthesia. 2017;72:1078–1087. doi: 10.1111/anae.13834. [DOI] [PubMed] [Google Scholar]
- 114.Effects of volumetric vs. pressure-guided fluid therapy on postoperative inflammatory response: a prospective, randomized clinical trial. Szakmany T, Toth I, Kovacs Z, Leiner T, Mikor A, Koszegi T, Molnar Z. Intensive Care Med. 2005;31:656–663. doi: 10.1007/s00134-005-2606-4. [DOI] [PubMed] [Google Scholar]
- 115.Multi-parametric functional hemodynamic optimization improves postsurgical outcome after intermediate risk open gastrointestinal surgery: a randomized controlled trial. Szturz P, Folwarczny P, Kula R, Neiser J, Ševčík P, Benes J. Minerva Anestesiol. 2019;85:244–254. doi: 10.23736/S0375-9393.18.12467-9. [DOI] [PubMed] [Google Scholar]
- 116.A randomized controlled trial comparing an intraoperative goal-directed strategy with routine clinical practice in patients undergoing peripheral arterial surgery. Van der Linden PJ, Dierick A, Wilmin S, Bellens B, De Hert SG. Eur J Anaesthesiol. 2010;27:788–793. doi: 10.1097/EJA.0b013e32833cb2dd. [DOI] [PubMed] [Google Scholar]
- 117.Intraoperative fluid restriction in pancreatic surgery: a double blinded randomised controlled trial. van Samkar G, Eshuis WJ, Bennink RJ, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0140294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Br J Anaesth. 2002;88:65–71. doi: 10.1093/bja/88.1.65. [DOI] [PubMed] [Google Scholar]
- 119.Intravenous fluid restriction after major abdominal surgery: a randomized blinded clinical trial. Vermeulen H, Hofland J, Legemate DA, Ubbink DT. Trials. 2009;10:50. doi: 10.1186/1745-6215-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, Fleming SC. Br J Anaesth. 2005;95:634–642. doi: 10.1093/bja/aei223. [DOI] [PubMed] [Google Scholar]
- 121.Restrictive intraoperative fluid optimisation algorithm improves outcomes in patients undergoing pancreaticoduodenectomy: a prospective multicentre randomized controlled trial. Weinberg L, Ianno D, Churilov L, et al. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0183313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goal directed fluid therapy for major liver resection: a multicentre randomized controlled trial. Weinberg L, Ianno D, Churilov L, et al. Ann Med Surg (Lond) 2019;45:45–53. doi: 10.1016/j.amsu.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goal-directed fluid management based on the auto-calibrated arterial pressure-derived stroke volume variation in patients undergoing supratentorial neoplasms surgery. Wu J, Ma Y, Wang T, Xu G, Fan L, Zhang Y. https://www.researchgate.net/publication/320699386_Goal-directed_fluid_management_based_on_the_auto-calibrated_arterial_pressure-derived_stroke_volume_variation_in_patients_undergoing_supratentorial_neoplasms_surgery Int J Clin Exp Med. 2017;10:3106–3114. [Google Scholar]
- 124.Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: a randomized clinical trial. Wuethrich PY, Burkhard FC, Thalmann GN, Stueber F, Studer UE. Anesthesiology. 2014;120:365–377. doi: 10.1097/ALN.0b013e3182a44440. [DOI] [PubMed] [Google Scholar]
- 125.Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: a randomized controlled trial. Xu H, Shu SH, Wang D, Chai XQ, Xie YH, Zhou WD. J Thorac Dis. 2017;9:2992–3004. doi: 10.21037/jtd.2017.08.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Comparison of postoperative pain and residual gas between restrictive and liberal fluid therapy in patients undergoing laparoscopic cholecystectomy. Yao L, Wang Y, Du B, Song J, Ji F. Surg Laparosc Endosc Percutan Tech. 2017;27:346–350. doi: 10.1097/SLE.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 127.The impact of intravenous fluid administration on complication rates in bowel surgery within an enhanced recovery protocol: a randomized controlled trial. Zakhaleva J, Tam J, Denoya PI, Bishawi M, Bergamaschi R. Colorectal Dis. 2013;15:892–899. doi: 10.1111/codi.12180. [DOI] [PubMed] [Google Scholar]
- 128.Comparison of absolute fluid restriction versus relative volume redistribution strategy in low central venous pressure anesthesia in liver resection surgery: a randomized controlled trial. Zatloukal J, Pradl R, Kletecka J, Skalicky T, Liska V, Benes J. Minerva Anestesiol. 2017;83:1051–1060. doi: 10.23736/S0375-9393.17.11824-9. [DOI] [PubMed] [Google Scholar]
- 129.Goal-directed fluid optimization based on stroke volume variation and cardiac index during one-lung ventilation in patients undergoing thoracoscopy lobectomy operations: a pilot study. Zhang J, Chen CQ, Lei XZ, Feng ZY, Zhu SM. Clinics (Sao Paulo) 2013;68:1065–1070. doi: 10.6061/clinics/2013(07)27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Intraoperative fluid management in open gastrointestinal surgery: goal-directed versus restrictive. Zhang J, Qiao H, He Z, Wang Y, Che X, Liang W. Clinics (Sao Paulo) 2012;67:1149–1155. doi: 10.6061/clinics/2012(10)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.The accuracy and effectiveness of goal directed fluid therapy in plateau-elderly gastrointestinal cancer patients: a prospective randomized controlled trial. Zhao G, Peng P, Zhou Y, Li J, Jiang H, Shao J. https://www.semanticscholar.org/paper/The-accuracy-and-effectiveness-of-goal-directed-in-Zhao-Peng/1ecec94cd32352065fba31814bcdc344877e322e Int J Clin Exp Med. 2018;11:8516–8522. [Google Scholar]
- 132.Goal-directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. Zheng H, Guo H, Ye JR, Chen L, Ma HP. World J Surg. 2013;37:2820–2829. doi: 10.1007/s00268-013-2203-6. [DOI] [PubMed] [Google Scholar]