Abstract
In many cells endosomal vacuoles show clathrin coats of which the function is unknown. Herein, we show that this coat is predominantly present on early endosomes and has a characteristic bilayered appearance in the electron microscope. By immunoelectron miscroscopy we show that the coat contains clathrin heavy as well as light chain, but lacks the adaptor complexes AP1, AP2, and AP3, by which it differs from clathrin coats on endocytic vesicles and recycling endosomes. The coat is insensitive to short incubations with brefeldin A, but disappears in the presence of the phosphatidylinositol 3-kinase inhibitor wortmannin. No association of endosomal coated areas with tracks of tubulin or actin was found. By quantitative immunoelectron microscopy, we found that the lysosomal-targeted receptors for growth hormone (GHR) and epidermal growth factor are concentrated in the coated membrane areas, whereas the recycling transferrin receptor is not. In addition, we found that the proteasomal inhibitor MG 132 induces a redistribution of a truncated GHR (GHR-369) toward recycling vesicles, which coincided with a redistribution of endosomal vacuole-associated GHR-369 to the noncoated areas of the limiting membrane. Together, these data suggest a role for the bilayered clathrin coat on vacuolar endosomes in targeting of proteins to lysosomes.
INTRODUCTION
The best-documented way of endocytosis is receptor-mediated uptake of ligands via clathrin-coated vesicles (reviewed in Schmid, 1997). Receptors are recruited and concentrated into clathrin-coated pits at the plasma membrane. After coated vesicle formation, the clathrin coat is removed by the concerted action of auxilin and heat shock protein 70 (Ungewickell et al., 1995). The uncoated vesicles fuse with early endosomes (EEs) in a rab5-regulated manner (Rubino et al., 2000). The highly dynamic EE consists of a vacuolar part (also known as sorting endosome) and emerging tubular extensions. The tubulovacuolar organization of EEs reflects their critical role in protein sorting. Receptors destined for degradation in lysosomes, such as epidermal growth factor receptor (EGFR) and growth hormone receptor (GHR), are incorporated into small vesicles in the lumen of the endosomal vacuole, which form by inward budding of the limiting membrane (microautophagy). Recycling receptors, such as the transferrin receptor (TfR) enter the tubular extensions of EEs and recycling endosomes (REs) from where they are routed back to the plasma membrane (Stoorvogel et al., 1987; Hopkins et al., 1994).
Despite the progress on the structural and molecular characterization of EEs, little is known about the mechanisms of intraendosomal protein sorting. It has been suggested that sorting occurs in a process of “iterative fractioning” (Dunn et al., 1989). This model, based on recycling receptors of which the ligands are released upon entry in the acidic environment of the sorting endosome, proposes that receptors rapidly and continuously enter the tubular extensions for recycling to the plasma membrane, whereas the ligands together with the bulk of the fluid phase are retained within the sorting endosome. Indeed, fluorescent lipids recycle to the plasma membrane with kinetics similar to the TfR, supporting the idea of a constitutive membrane recycling (Mayor et al., 1993). Furthermore, recycling of the TfR is independent of the presence of its cytoplasmic tail (Jing et al., 1990). Although the existence of an additional, active mechanism for sorting into REs cannot be excluded (van Dam and Stoorvogel, 2002), these findings are consistent with a model in which the recycling pathway is followed by default (Verges et al., 1999), implicating that targeting to the lysosome is signal mediated. Indeed, signal-mediated transport to the lysosomes has been described for a large number of proteins, including EGFR, GHR, interleukin 2 receptor β chain, and a number of G protein-coupled receptors (Subtil et al., 1998; Lemmon and Traub, 2000; Marchese and Benovic, 2001; Shenoy et al., 2001). For EGFR, incorporation in internal endosomal vesicles, a prerequisite for lysosomal targeting, depends on a di-leucine motif in its cytoplasmic tail as well as on its kinase activity (Felder et al., 1990; Kornilova et al., 1996; Kil et al., 1999). Recent evidence also suggests a role for the ubiquitin-proteasome system in lysosomal targeting of EGFR. The ubiquitin ligase c-Cbl, a negative regulator of EGFR, is phosphorylated upon EGFR stimulation, after which it binds to the EGFR cytoplasmic tail and mediates ubiquitination of the receptor (Galisteo et al., 1995; Levkowitz et al. 1998, 1999; Joazeiro et al., 1999). Because EGFR is degraded in the lysosome (Dunn et al., 1986), ubiquitination probably has a regulatory rather than a degradative function. Further studies are required to establish whether the interaction between c-Cbl and EGFR occurs at the plasma membrane and/or at the level of endosomes (Levkowitz et al., 1998; Stang et al., 2000; de Melker et al., 2001). The ubiquitin-proteasomal system is also involved in endocytosis of GHR. Recently, we have shown that it regulates both the initial internalization of growth hormone (GH)–GHR complexes from the plasma membrane, as well as a lysosomal sorting step at the endosomal level (Govers et al., 1997, 1999; Sachse et al., 2001; van Kerkhof et al., 2001). These data are in agreement with recent studies in yeast, demonstrating that ubiquitination of cargo proteins may serve as a signal for incorporation into the internal vesicles of the prevacuolar compartment (Katzmann et al., 2001; Reggiori and Pelham, 2001; Urbanowski and Piper, 2001).
In various morphological studies, it was found that clathrin, in addition to the plasma membrane and the trans-Golgi network (TGN), also resides on vacuolar sorting endosomes as well as on membrane buds emerging from the tubular REs (Stoorvogel et al., 1996; Dell'Angelica et al., 1998; Futter et al., 1998; Prekeris et al., 1998, 1999; de Wit et al., 1999). The role of clathrin coats on the vacuolar sorting endosomes is unknown, but it was recently shown that clathrin recruitment to endosomal vacuoles is increased upon overexpression of the hepatocyte growth factor-regulated tyrosin kinase substrate (Hrs) (Raiborg et al., 2001). The recruitment of Hrs and clathrin to these membranes depends on phosphatidylinositol 3-kinase (PtdIns 3-kinase) activity. Notably, overexpression of Hrs does not affect TfR recycling, but leads to an accumulation of EGFR in the Hrs positive endosomes, suggesting a role for phosphatidylinositol 3-phosphate (PtdIns3P) and Hrs in transport to lysosomes. Finally, high levels of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein syntaxin 7 were found in the clathrin-coated membrane domains of early endosomal vacuolar sorting endosomes (Prekeris et al., 1999).
Immunoelectron microscopy (immunoEM) provides the possibility to visualize endosomal subdomains at high morphological resolution and analyze their protein composition (Geuze et al., 1983; Klumperman et al., 1993). Herein, we have used this approach to study the distribution of various types of cargo and regulatory proteins in coated and noncoated areas of the endosome. We found that syntaxin 7 and Hrs together with the lysosome-bound GHR and EGFR are concentrated in the coated areas on the sorting endosome, whereas recycling TfR is not. Our data are consistent with a role of the endosomal clathrin coat in protein sorting toward lysosomes.
MATERIALS AND METHODS
Materials and Antibodies
GH was a gift of Eli Lilly (Indianapolis, IN). Epidermal growth factor (EGF) was purchased from Invitrogen (Carlsbad, CA). Sulfosuccinimidyl 6-(biotinamido) hexanoate for biotinylation of GH was obtained from Pierce Chemical (Rockford, IL). GH was biotinylated as described (Bentham et al., 1994). Biotinylated transferrin (Tf) was purchased from Sigma Chemical (St. Louis, MO). Brefeldin A, nocodazole, and wortmannin were purchased from Sigma Chemical, latrunculin A was purchased from Molecular Probes (Eugene, OR), and MG 132 was purchased from Calbiochem (San Diego, CA). To detect GH, cells were incubated with human GH and labeled with guinea pig polyclonal antibody against human GH (Biogenesis, Poole, Dorset, United Kingdom), or incubated with biotinylated GH and labeled with rabbit polyclonal antibody against biotin (Rockland, Gilbertsville, PA). In a previous study we showed that these two approaches yield identical data (Sachse et al., 2001). Rabbit antiserum against the cytosolic tail of rabbit GHR was described previously (Strous et al., 1996); rabbit antiserum against syntaxin 7 (Prekeris et al., 1999) and a mouse monoclonal antibody (mAb) against β-tubulin were a kind gift from Dr. R. Scheller (Stanford University, Palo Alto, CA/Genentech, South San Francisco, CA). Rabbit antiserum against clathrin-light chain was a kind gift from Dr. E. Ungewickell (Hannover Medical School, Germany). Mouse mAb against β1/β2-adaptin was a kind gift from Dr. T. Kirchhausen (Harvard University, Boston, MA). Sheep antiserum against EGFR was obtained from Invitrogen. Rabbit antiserum against γ-adaptin was obtained from Sigma Chemical. Rabbit polyclonal antiserum against biotin was obtained from Rockland. Mouse mAb against actin (clone C4) was obtained from ICN Biomedicals (Cleveland, OH). Mouse mAb against human TfR was purchased from Zymed Laboratories (South San Francisco, CA). Mouse mAb against clathrin heavy-chain was obtained from Transduction Laboratories (Lexington, KY) and polyclonal rabbit antibody against mouse IgG from DAKO (Glostrup, Denmark). The rabbit polyclonal anti-Hrs antibody was raised against a C-terminal peptide of human Hrs (PPAQGSEAQLISFD). Specificity of the antibody was confirmed in competition experiments by immunoblotting of cell extracts and immunofluorescence of cells transiently overexpressing green fluorescent protein- and hemagglutinin-tagged Hrs.
Cells
The Chinese hamster cell line ts20, containing a thermosensitive E1 enzyme (Kulka et al., 1988), was stably transfected with full-length rabbit GHR cDNA (wtGHR cells), or with cDNA encoding the truncated GHR 1-369 (GHR-369 cells) (Govers et al., 1998). Cells were grown at the permissive temperature of 30°C in Eagle's minimal essential medium (MEMα) supplemented with 4.5 g/l glucose, 10% fetal bovine serum (FBS), penicillin, streptomycin, and 0.45 mg/ml geneticin. For experiments, cells were grown in 60-mm dishes in the absence of geneticin. To increase GHR expression, 10 mM sodium butyrate was added to the cells 18 h before use. HeLa cells were cultured in DMEM containing 10% FBS, penicillin, streptomycin, and 2 mM l-glutamine.
Internalization of GH, EGF, and Uptake of Biotinylated Transferrin
To deplete growth factors, GHR-expressing cells were incubated for 1 h in MEMα containing 0.1% bovine serum albumin (BSA), after which 8 nM GH or biotinylated GH was added and cells were incubated for a further 30 or 60 min. Then cells were washed three times with MEMα containing 0.1% BSA, fixed, and processed for immunoEM as described below. When indicated, 20 μM MG 132 dissolved in ethanol or vehicle only was added 1 h before the start of the experiment. Brefeldin A (10 μg/ml) dissolved in methanol was added 10 min before fixation. Wortmannin (100 nM) dissolved in dimethyl sulfoxide was added 45 min before fixation. Nocodazole (10 μg/ml) dissolved in dimethyl sulfoxide or 4 μM latrunculin A dissolved in methanol was added 2 h or 20 min before fixation, respectively. BSA conjugated to 5-nm colloidal gold (final OD of 5 at 520 nm) was used to mark the endocytic pathway. Before use, BSA-gold was dialyzed overnight against phosphate-buffered saline (PBS) at 4°C. Cells were incubated with BSA-gold for either 5 min and then fixed or incubated for 10 min followed by a washing step and further incubation for 15 min in the absence of BSA-gold. Iron saturation of biotinylated transferrin was done as described (Stoorvogel et al., 1987). For Tf uptake, wtGHR or GHR-369 cells were incubated in MEMα containing 0.1% BSA for 1 h at permissive temperature. Biotinylated Tf was added to a final concentration of 20 μg/ml, after which cells were incubated for a further 30 min. HeLa cells were incubated overnight in DMEM containing 0.5% FBS. EGF (100 nM) was added, after which cells were incubated for a further 10 min before fixation.
ImmunoEM
Cells were fixed in 2% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer pH 7.4. To visualize GH in GHR-369 cells, 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4 was used. To preserve microtubules cells were fixed in PHEM buffer [60 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 25 mM HEPES, 2 mM MgCl2, 10 mM EGTA, pH 6.9]. Processing of cells for ultrathin cryosectioning and immunolabeling according to the protein A-gold method was done as described previously (Slot et al., 1991). In brief, fixed cells were washed with 0.02 M glycine in PBS, scraped gently from the dish in PBS containing 1% gelatin, and pelleted in 12% gelatin in PBS. The cell pellet was solidified on ice and cut into small blocks. For cryoprotection, blocks were infiltrated overnight with 2.3 M sucrose at 4°C and afterward mounted on aluminum pins and frozen in liquid nitrogen. To pick up ultrathin cryosections, a 1:1 mixture of 2.3 M sucrose and 1.8% methylcellulose was used (Liou et al., 1996).
Quantitative ImmunoEM
We defined endosomal vacuolar compartments as early or late by distinct time points of BSA-gold uptake and the number of internal vesicles that in thin sections was visible in the lumen (Table 1; Sachse et al., 2001). Because membrane continuities are not always visible in the plane of the section, a distinction between endosome-associated recycling tubules and detached REs cannot always be made. We therefore included all 40–60-nm-diameter tubulovesicular membranes within 200 nm from an endosomal vacuole in the category endosome-associated tubules, whereas such membranes at distance from endosomal vacuoles were included in the category REs. Primary endocytic vesicles were distinguished from recycling tubules and endosomes by their electron-lucent lumen and larger diameter of ∼100 nm (Sachse et al., 2001). Lysosomes were recognized by the presence of electron-dense amorphous material and/or the presence of internal membrane sheets.
Table 1.
Distribution of internalized BSA-gold over different classes of endosomes
| BSA uptake | No. of internal vesicles
|
Lysosomes | |||
|---|---|---|---|---|---|
| ≤2 | 3–5 | 6–9 | ≥10 | ||
| 5 min | 46.3 ± 7.4 | 41.3 ± 4.9 | 12.0 ± 2.0 | 0.4 ± 0.6 | 0 ± 0 |
| 10 min + 15-min chase | 14.3 ± 3.5 | 23.9 ± 3.6 | 39.5 ± 2.2 | 10.3 ± 2.3 | 12.0 ± 2.1 |
HeLa cells were incubated with BSA conjugated to 5-nm gold for the indicated time points. Numbers represent the percentage of endosomal vacuoles that were positive for BSA-gold (±SD). Note that primary endocytic vesicles are not included in these countings.
To establish the relative distributions of GH, GHR, GHR-369, Tf, TfR, and EGFR over subdomains of the EE vacuole (Tables 2, 3, and 5), areas of a grid were selected at low magnification for good ultrastructure and then scanned at a magnification of 15,000× along a linear track. All gold particles within a distance of 20 nm from the EE vacuole were considered as membrane associated and assigned to the subdomain where they were localized. For all quantitations at least three independent counting sessions were performed. The occurrence of a protein over an endosomal subdomain was expressed as percentage of total label over the limiting membrane of the EE vacuole. To measure the relative surface area of the coats (Tables 2 and 3), pictures of EE vacuoles were randomly taken. A transparent overlay displacing a squared lattice of lines 5 mm apart was placed over the pictures with a final magnification of 81,000×. The length of the limiting membrane of the vacuole was then measured by counting the number of intersections with the line lattice overlay and the percentage of the limiting membrane occupied by an endosomal subdomain was calculated. By dividing the percentage of gold label of a given protein present over an endosomal subdomain by the percentage of the limiting membrane occupied by this subdomain, the labeling surface ratio was calculated (Tables 2 and 3). When this ratio is >1.00, the labeling density of a protein in this subdomain exceeds a random distribution, indicating an enrichment. Likewise, a ratio <1.00 indicates a lower labeling density than with a random distribution over the endosomal limiting membrane. Dividing the labeling surface ratio of a coated subdomain by that of the noncoated subdomain yielded the enrichment or exclusion factor for the coated over the noncoated domain. To establish the effect of MG 132 on the distribution of GH in GHR369 cells (Table 4), for each condition three independent counting sessions were performed and the occurrence of GH over a given compartment was expressed as percentage of total label. Finally, the level of colocalization of GH with Tf in vesicular/tubular REs was established in three independent sessions, by calculating the percentage of Tf positive REs that also contained GH.
Table 2.
Distribution of TfR, Tf, GHR, and GH over the limiting membrane of early endosomal vacuoles of wtGHR cells
| Coated areas | Noncoated areas | Nondefined | |
|---|---|---|---|
| A Labeling (%) | |||
| TfR | 9.1 ± 1.7 | 77.3 ± 5.6 | 13.6 ± 4.3 |
| Tf | 8.8 ± 1.8 | 74.6 ± 1.3 | 16.6 ± 0.7 |
| GHR | 24.4 ± 4.4 | 60.7 ± 1.9 | 14.9 ± 3.1 |
| GH | 21.2 ± 4.2 | 59.7 ± 5.1 | 19.1 ± 1.3 |
| B Surface area (%) | 11.3 | 74.7 | 14.0 |
| C Labeling surface ratio | |||
| TfR | 0.8 | 1.0 | 1.0 |
| Tf | 0.8 | 1.0 | 1.2 |
| GHR | 2.2 | 0.8 | 1.1 |
| GH | 1.9 | 0.8 | 1.4 |
WtGHR cells were allowed to internalize biotinylated GH or -Tf for 30 min and labeled for biotin, TfR, or GHR. (A) Numbers represent the percentage of total gold label (±SD) of the indicated protein found over the subdomains of the limiting membrane of the EE vacuole. Areas of the membrane that were not clearly visible in the cross section are referred to as nondefined. (B) Relative contributions of coated and noncoated membrane areas to the total of the endosomal limiting membrane are indicated as percentages. (C) Labeling surface ratio of a given protein in either of the endosomal subdomains was calculated by dividing the percentage of total label in one subdomain by the percentage of membrane that is occupied by this subdomain (n = 50 endosomes). A ratio >1.00 indicates an increase and <1.00 a decrease in labeling density compared with a random distribution of a protein over the endosomal limiting membrane.
Table 3.
Relative distribution of TfR and EGFR over the limiting membrane of early endosomal vacuoles of HeLa cells
| Coated areas | Noncoated areas | Nondefined | |
|---|---|---|---|
| A Labeling (%) | |||
| TfR | 5.9 ± 2.2 | 81.7 ± 1.8 | 12.4 ± 3.9 |
| EGFR | 18.2 ± 2.4 | 70.0 ± 7.3 | 11.8 ± 5.0 |
| B Surface area (%) | 5.5 | 85.3 | 9.2 |
| C Labeling surface ratio | |||
| TfR | 1.1 | 1.0 | 1.3 |
| EGFR | 3.3 | 0.8 | 1.3 |
Hela cells were allowed to internalize EGF for 10 min and labeled for EGFR or TfR. (A) Numbers represent the percentage of total gold label (±SD) found over the subdomains of the limiting membrane of the endosomal vacuole. Areas of the membrane that were not clearly visible in the cross section and could not be judged whether they were coated or not are referred to as nondefined. (B) Relative contributions of coated and noncoated membrane areas to the total of the limiting membrane are indicated as percentages. (C) Labeling surface ratio was calculated as in Table 2 (n = 50 endosomes).
Table 5.
Distribution of GH–GHR-369 complexes in the vacuole of early endosomes in the presence or absence MG 132
| Limiting membrane
|
Lumen
|
|||
|---|---|---|---|---|
| Coated | Noncoated | Nondefined | Internal vesicles | |
| Control | 15.0 ± 3.2 | 32.5 ± 2.2 | 10.2 ± 2.6 | 42.3 ± 4.2 |
| MG 132 | 12.4 ± 2.6 | 43.5 ± 2.6 | 13.3 ± 3.1 | 30.8 ± 4.3 |
Cells were preincubated for 1 h with or without 20 μM MG 132. After addition of biotinylated GH, incubation was continued for 60 min before fixation and processing for immunoEM and labeling with anti-biotin. Numbers represent the percentage of total gold label (±SD) found over the subdomains of the endosomal vacuole.
Table 4.
Subcellular distributions of GH–GHR-369 complexes in the presence or absence MG 132
| Plasma membrane | Clathrin-coated structures | Endocytic vesicles | EE | LE | Endosome-associated tubules | Recycling endosomes | |
|---|---|---|---|---|---|---|---|
| Control | 36.1 ± 2.2 | 7.3 ± 1.4 | 3.9 ± 1.0 | 29.1 ± 1.5 | 16.3 ± 2.7 | 2.2 ± 1.1 | 5.1 ± 1.0 |
| MG 132 | 46.3 ± 6.1 | 7.7 ± 1.8 | 4.2 ± 0.7 | 17.5 ± 4.2 | 4.5 ± 1.6 | 5.6 ± 0.9 | 14.2 ± 3.0 |
Cells were preincubated for 1 h with or without 20 μM MG 132. After addition of biotinylated GH, incubation was continued for 60 min before fixation and processing for immunoEM. Cells were labeled with an antibody against biotin. Numbers represent the percentage of total gold label (±SD) found over the indicated compartments. Detailed definitions of the distinct categories are provided in MATERIALS AND METHODS.
RESULTS
Bilayered Clathrin Coat Is Present on Endosomes in Various Cell Types
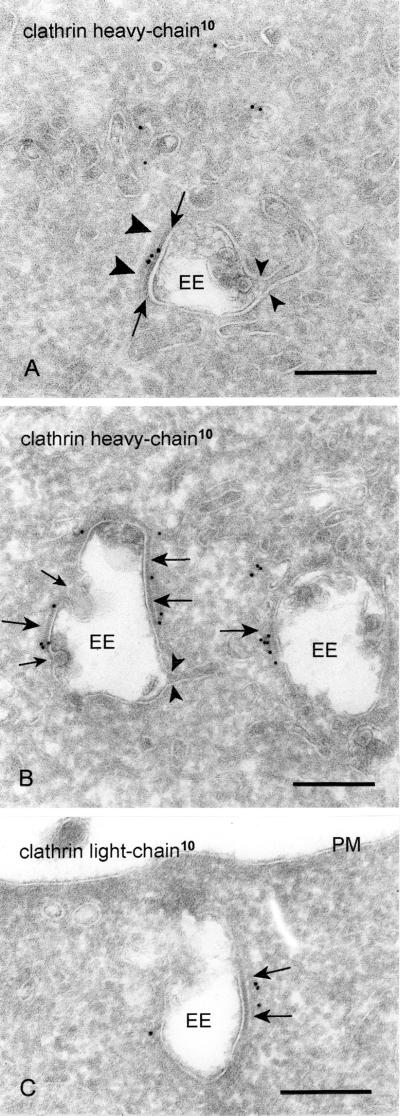
Coats on endosomal vacuoles are a general phenomenon, which we have observed in many cell types, e.g., HeLa, Chinese hamster ovary, baby hamster kidney, PC-12, HepG2, and ts-20 cells, although with different frequencies. In several recent studies it was demonstrated that these coats contain clathrin (Prekeris et al., 1998; Sorkina et al., 1999; Ramm et al., 2000). By careful examination in the electron microscope, we noticed that unlike clathrin coats at other sites in the cell, the endosomal coat consists of two layers, a thin and highly electron-dense layer closely opposed to the limiting membrane of the endosomal vacuole (Figure 1A, arrows), and a second, more fuzzy and less electron dense layer facing the cytoplasm (Figure 1A, arrowheads). A narrow, electron-lucent rim separates the two layers (Figures 1C and 2C). This characteristic bilayered appearance of the coat was apparent in ultrathin cryosections (Figure 1), as well as in conventionally prepared electron microscopy sections of osmium-fixed and Epon-embedded cells (our unpublished data). The coats were mostly flat, reminiscent in this aspect of clathrin-coated lattices at the plasma membrane. Notably, a clathrin coat with a similar morphological appearance was recently described on vacuolar endosomal intermediates in a melanoma cell line (Raposo et al., 2001). Because of the characteristic appearance of the coat, we will further refer to it as the bilayered coat. Importantly, endosomal recycling tubules always originated from noncoated areas (Figure 1), and the coat was absent from inward budding profiles (Figure 1B).
Figure 1.
Bilayered coats on endosomal vacuoles contain clathrin heavy and light chain. (A) Chinese hamster ovary cell. Clathrin heavy chain (10-nm gold) is found on the flat, coated region of an EE. The clathrin coat consists of a narrow electron-dense layer opposed to the endosomal limiting membrane (arrows) and a fuzzier layer facing the cytoplasm (arrowheads). Note that the recycling tubule emerges opposite to the coated region (small arrowheads). (B) Also in wtGHR cells, clathrin heavy chain (10-nm gold) is found in the coated areas (arrows). Note that no label is found on the inward budding vesicles (small arrows). As in A the emerging tubule has no continuity with the coated region (small arrowheads). (C) Example of an early endosome in wtGHR cells, labeled for clathrin light chain (10-nm gold) in the coated areas (arrows). PM, plasma membrane. Bars, 200 nm.
Figure 2.
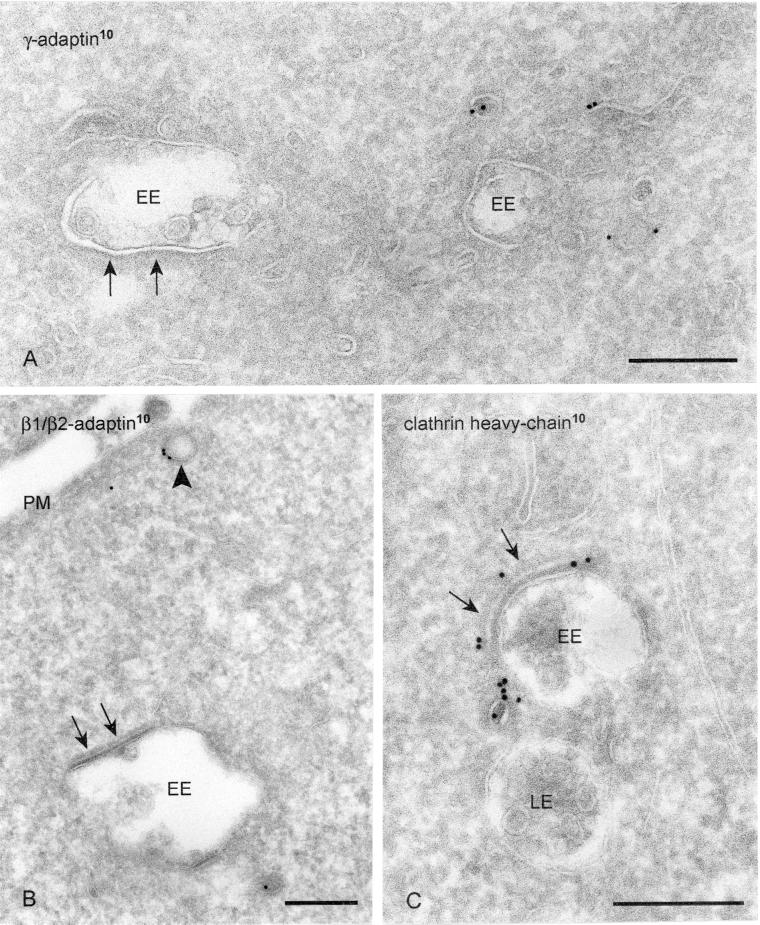
Clathrin adaptor complexes AP1 and AP2 are absent from the bilayered coats. (A) HeLa cells labeled for γ-adaptin (10-nm gold). Label is present on vesicles and tubules in vicinity to EEs but absent from the bilayered coat (arrows). (B) WtGHR cells labeled for β1/β2-adaptin (10-nm gold). Label is found at a clathrin-coated pit (arrowhead) but not in the bilayered coat (arrows). (C) WtGHR cells incubated for 10 min with 10 mM brefeldin A and labeled for clathrin heavy chain (10-nm gold). At this time point brefeldin A does not interfere with the bilayered coat (arrows). LE, late endosome; PM, plasma membrane. Bars, 200 nm.
To establish the position of the bilayered coated endosomes within the endosomal pathway, we incubated HeLa cells with the endocytic tracer BSA conjugated to 5-nm gold for 5 or 10 min then followed by a 15-min chase in the absence of BSA-gold. While maturing from EEs to late endosomes (LEs), more internal vesicles accumulate in the lumen of the endosomal vacuole (van Deurs et al., 1993). As additional criteria to distinguish between early and late endosomal vacuoles, we therefore also classified them according to the number of internal vesicles seen in cross section. After 5 min of BSA-gold uptake, >85% of the BSA-gold–containing endosomes had less than five internal vesicles (Table 1). Only a small fraction of endosomes with six to nine or more internal vesicles was reached by the BSA-gold. Electron-dense lysosomes were invariably unlabeled. After 10-min uptake and 15-min chase, >60% of BSA-gold positive vacuoles contained more than five internal vesicles, and also lysosomes were significantly labeled. The morphological appearances of endosomes reached early or late by BSA-gold were very similar to those observed in other cell types (Klumperman et al., 1993; Kleijmeer et al., 1997; de Wit et al., 1999). We therefore defined endosomes with no more than five internal vesicles as early. It should be noted that this definition only includes the vacuolar part and not the recycling tubules. Of such EEs, >30% contained a bilayered coat in the plane of the section. In contrast, only 6.5% of the LEs contained a coat. We conclude that the bilayered coat is mostly associated with EE vacuoles. In the remainder of our study, we therefore focused on EEs, unless stated otherwise.
Clathrin Adaptor Complexes AP1, AP2, and AP3 Are Not Present in Bilayered Coats
We next performed a series of immunolabelings to see whether known coat components could be localized in the bilayered coat, including a novel clathrin homolog that does not associate with known clathrin light chains (Liu et al., 2001). As shown in Figure 1, in addition to clathrin heavy chain, clathrin light chain was readily detectable in the bilayered coat, indicating that the clathrin present in these coats is of the conventional type. Assembly of clathrin at the plasma membrane and TGN is dependent on the adaptor protein complexes AP2 and AP1, respectively. By immunoEM, γ-adaptin was found on the endosomal tubules (Futter et al., 1998; Mallard et al., 1998). By immunoprecipitation and fluorescence microscopy, both the α-adaptin (from AP2) and γ-adaptin (from AP1) subunits were localized on endosomes (Sorkina et al., 1999). Furthermore, Kornfeld and colleagues (Traub et al., 1996) localized AP2 as well as clathrin on isolated lysosomes. To see whether these adaptor complexes were present in the bilayered coats of EEs, we first used an antibody that recognized both β1 and β2-adaptin, of AP1 and AP2, respectively. Clathrin-coated pits and vesicles at the plasma membrane and in the Golgi region were clearly labeled by this antibody, whereas the bilayered endosomal coats were consistently devoid of label (Figure 2B). By using an antibody against γ-adaptin, the absence of AP1 from the bilayered coats was confirmed in PC-12 and HeLa cells (Figure 2A; our unpublished data). A third adaptor complex, AP3, has been shown to be present on endosomal tubules in A431 and PC12 cells (Dell'Angelica et al., 1998). In HeLa cells, AP3 was indeed found on tubular-vesicular profiles in close vicinity of endosomal vacuoles, but label was absent from the bilayered coat. In addition, in fibroblasts derived from mocha mice, which lack functional AP3, bilayered coats on endosomal vacuoles were still frequently observed (our unpublished data). The assembly of AP1 and AP3 on membranes depends on the small GTPase ADP-ribosylation factor 1 (ARF1) and is prevented by brefeldin A, which blocks membrane binding of ADP-ribosylation factor 1 (Robinson and Kreis, 1992; Ooi et al., 1998). When wtGHR cells were incubated for 10 min with brefeldin A the Golgi stacks had disassembled (our unpublished data), whereas the bilayered coats were still present on endosomal vacuoles and labeled positive for clathrin (Figure 2C). This observation implies that assembly of the bilayered coat is independent of ADP-ribosylation factor 1 binding.
Endosomal Bilayered Coats Disappear in Presence of PtdIns 3-Kinase Inhibitor Wortmannin
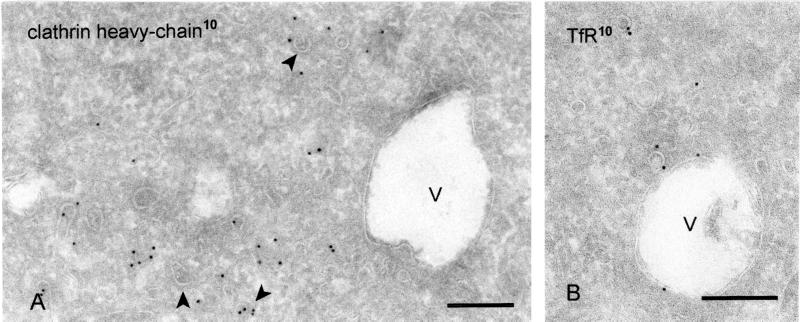
PtdIns 3-kinase metabolites are involved in the regulation of several steps of membrane traffic (Spiro et al., 1996; Christoforidis et al., 1999), including the formation of endosomal internal vesicles (Fernandez-Borja et al., 1999). Furthermore, PtdIns3P has been localized to EEs and internal vesicles of LEs (Gillooly et al., 2000). Incubation of wtGHR cells with the PtdIns 3-kinase inhibitor wortmannin for 45 min resulted in the formation of enlarged endosomal vacuoles with only few internal vesicles (Figure 3A). Importantly, wortmannin incubation resulted in a dramatic decrease of clathrin association with the endosomal vacuoles and also by morphological criteria the bilayered coat was only infrequently observed. In contrast, clathrin coats at the TGN, plasma membrane, and REs were seemingly unaffected (Figure 3A). Although the presence of TfR in the limiting membrane of wortmannin-induced vacuoles (Figure 3B) suggests that they originate from EEs, input from late endocytic compartments cannot be excluded. To quantify the effect of wortmannin on the bilayered coat we therefore took both EEs and LEs into account. In control cells, the bilayered coat covered 9% of the limiting membrane of both EEs and LEs vacuoles. After wortmannin treatment, only 3% of the vacuolar membranes were coated. Considering that the size of endosomal vacuoles increased with 17% after wortmannin treatment, these data indicate a reduction of coated membrane area. We conclude that the formation and/or maintenance of the endosomal bilayered coat depends on PtdIns 3-kinase activity.
Figure 3.
Wortmannin causes the disappearance of the bilayered coats from endosomes. WtGHR cells incubated for 45 min with 100 nM wortmannin. (A) Wortmannin induces swollen endosomal vacuoles (V) that rarely bear a visible bilayered coat and are not labeled for clathrin (10-nm gold). In contrast, association of clathrin with cytoplasmic vesicles and tubules is unperturbed (arrowheads). (B) Early endosomal marker TfR (10-nm gold) localizes to the limiting membrane of swollen endosomal vacuoles. Bars, 200 nm.
Bilayered Coats Are Not Associated with Actin or Tubulin Cytoskeleton
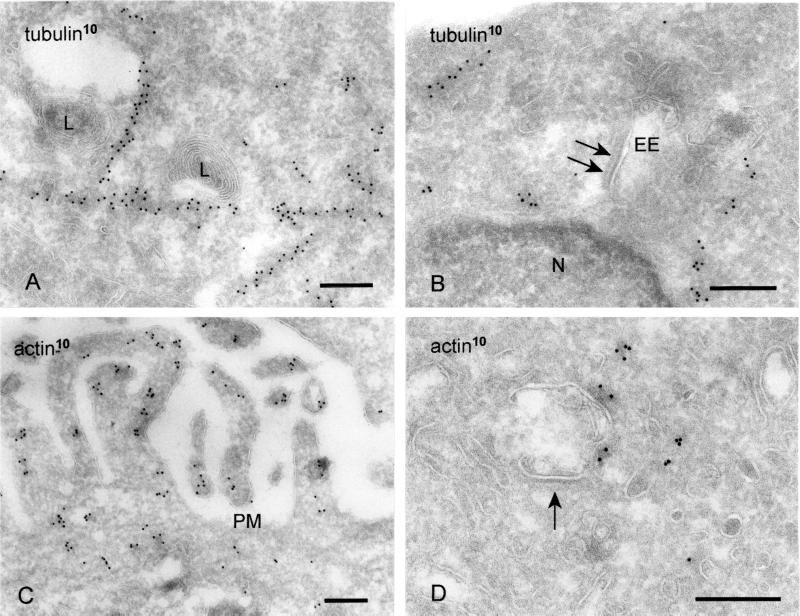
The movement and intracellular positioning of endocytic organelles depends on association with microtubules (Matteoni and Kreis, 1987; Habermann et al., 2001) and actin filaments (van Deurs et al., 1995). We therefore sought to determine whether the bilayered coat on endosomal vacuoles might function as possible anchor site for the cytoskeleton. Labeling for γ-tubulin revealed long linear patterns of gold, reflecting the longitudinal sectioning of microtubules (Figure 4, A and B). Although we frequently observed the association of LEs and lysosomes with microtubules (Figure 4A; our unpublished data), we found no linear tracks or single clusters of tubulin in close vicinity to the coated areas of EE vacuoles (Figure 4B). In addition, the microtubule-depolymerizing agent nocodazole had no influence on the presence of the bilayered coats (our unpublished data).
Figure 4.
Bilayered coats are not associated with microtubules or actin (A) HeLa cells. Lysosomes (L) are associated with a linear track of tubulin (10-nm gold). (B) In contrast, tubulin is not associated with the coated areas (arrows) on EEs. (C) Actin (10-nm gold) is readily found near the plasma membrane (PM) and in microvillar protrusions. (D) When actin is found near the limiting membrane of EEs, it is not associated with coated areas (arrow). Bars, 200 nm.
Actin filaments are required for transport toward lysosomes (van Deurs et al., 1995). In addition, in Xenopus egg extracts, actin nucleation on endosomal vacuoles was demonstrated in vitro (Taunton et al., 2000). Labeling with an antibody against actin revealed actin present in the cytosol, especially in the region under the plasma membrane and in cellular protrusions (Figure 4C). In addition, label was sometimes observed near the limiting membrane of endosomal vacuoles, but not in association with the coated areas of their limiting membrane (Figure 4D). Incubation of cells with latrunculin A, which causes disassembly of actin filaments, had no effect on the occurrence of the bilayered coats (our unpublished data). In summary, these data do not yield any indication that the coated membranes provide the site of interaction of EEs with the cytoskeleton.
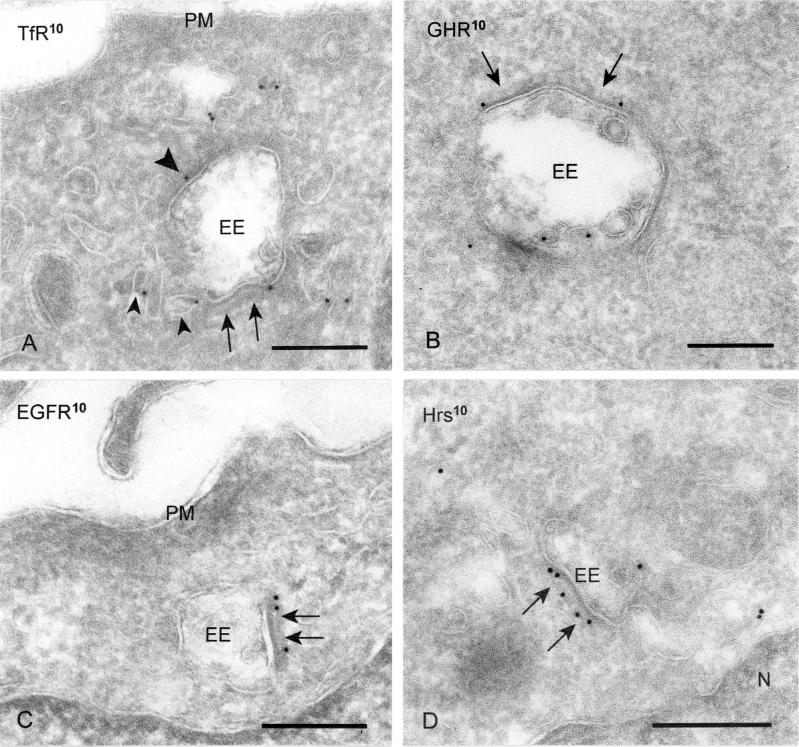
EGFR and GHR but Not TfR Are Concentrated in Bilayered Coats of Early Endosomes
To investigate a possible involvement of the bilayered coated areas in protein sorting within EEs, we next established the steady-state distribution of a recycling receptor, the TfR, and two receptors that are degraded upon ligand binding, the EGFR and GHR. As shown in many other cell types, the majority of intracellular TfR in wtGHR cells was found in REs, and only a minor amount at the limiting membrane of EE vacuoles (Figure 5A). Of this relatively low amount of label associated with the EE vacuole, 11.1% was localized on internal vesicles, which most likely represents the small percentage of TfR that is targeted to lysosomes (Omary and Trowbridge, 1981). Similar distribution patterns were found in the other cell lines used in this study. At the limiting membrane of the EE vacuoles, we found <10% of the TfR label in bilayered coated areas (Table 2). Almost similar values were obtained when the Tf–TfR complex was labeled with anti-Tf (Table 2), indicating that TfR antigenicity was not masked by the presence of the coat. Also in HeLa cells, only a very small percentage of TfR at the limiting membrane of EEs was localized in the coated areas (Table 3).
Figure 5.
EGFR, Hrs, and GHR are concentrated in the bilayered coats. (A and B) WtGHR cell incubated for 30 min with biotinylated GH. (A) TfR (10-nm gold) label occurs predominantly in REs, some of which are in vicinity of early endosomes (small arrowheads). At the limiting membrane of the EEs TfR is localized in coated (arrows) and noncoated areas (arrowhead). (B) GHR (10-nm gold) is present at the limiting membrane as well as on internal vesicles of an EE. At the limiting membrane, GHR is found in coated areas (arrows). (C and D) HeLa cells incubated for 10 min with EGF. (C) EGFR (10-nm gold) is clearly present in coated areas (arrows) of an EE. (D) Also Hrs (10-nm gold) is localized in coated areas (arrows) of EEs. PM, plasma membrane. Bars, 200 nm.
To compare protein concentrations in the coated and noncoated membrane subdomains, we divided the percentage TfR labeling present over an endosomal subdomain by the relative surface area of this domain (Tables 2 and 3). The data obtained were very similar in wtGHR and HeLa cells and revealed that the small amount of TfR at the EE vacuole distributes homogenously over coated and noncoated areas of the limiting membrane (Tables 2 and 3).
We then focused on the EGFR and GHR destined for lysosomal degradation after binding to their cognate ligands. HeLa cells were incubated for 10 min with EGF, fixed, and processed for immunolabeling with anti-EGFR. Under these conditions, the majority of EGFR was localized in EEs, i.e., both at the limiting membrane and internal vesicles (Figure 5C; our unpublished data). At marked difference with the TfR, the EGFR showed a high presence in the bilayered coated membranes (Table 3, top row). Dividing the EGFR labeling surface ratio of the coated subdomain by the noncoated subdomain revealed that the bilayered coated membranes exhibited a fourfold higher concentration than the noncoated areas (Table 3; 3.3:0.8 = 4.1). We next studied the localization of endocytosed GHR–GH complexes after 30 min of GH uptake in wtGHR cells. Like the EGFR, GHR resided on internal vesicles and the limiting membrane of the EE vacuole (Figure 5B). At the limiting membrane, GHR was often found in coated areas (Table 2). The GH-GHR complex remains associated until degradation in the lysosomes (Roupas and Herington, 1986), implicating that immunodetection of GH should result in an analogous distribution over EEs. Indeed, GH label concentrated in the coated endosomal membranes (Table 2). Finally, determination of the respective labeling surface ratios showed that the GHR-GH complex is 2.4–2.8× more concentrated in the bilayered coated areas compared with the noncoated areas of the endosomal limiting membrane (Table 2).
Together, these data show that the recycling TfR is distributed equally over the coated and noncoated membrane domains of EEs, whereas EGFR and GHR, two receptors destined for lysosomal degradation, concentrate in the coated membrane domains.
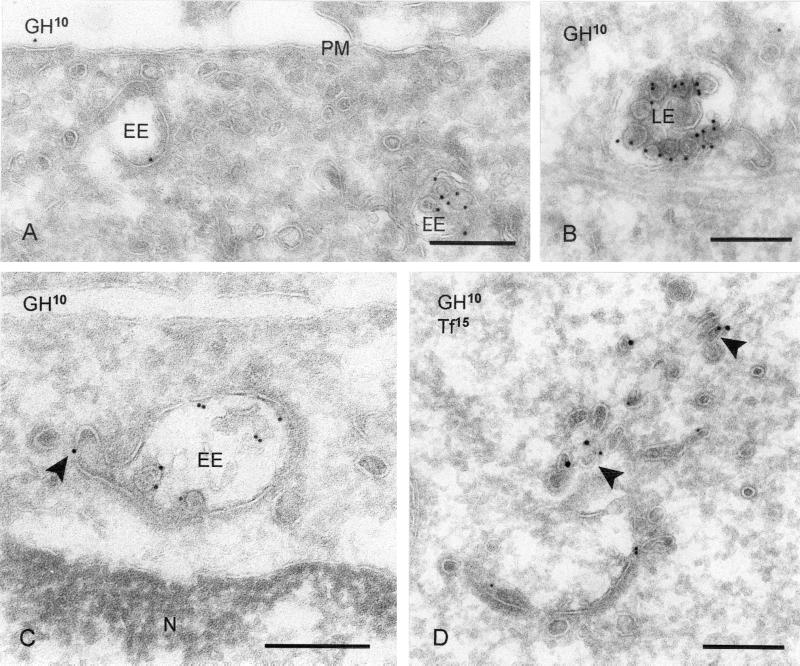
Induced Recycling of a Truncated GHR Coincides with Decreased Presence in Bilayered Coated Areas
The proteasomal system is required for the initial internalization of GHR, as well as for its sorting to lysosomes (van Kerkhof et al., 2000, 2001). In the presence of proteasomal inhibitors, internalization of the wtGHR is inhibited. In contrast, a truncated receptor, GHR-369, is still internalized under these conditions (van Kerkhof et al., 2000), but GH degradation is impaired (van Kerkhof and Strous, 2001), implicating a diminished targeting to lysosomes. This prompted us to study the intracellular distribution of GHR-369 in the presence or absence of proteasome inhibitor. Cells were incubated for 60 min with biotinylated GH, fixed, and processed for labeling with anti-biotin antibody. Under these conditions, the GHR-369–GH complex accumulated on the internal vesicles of endosomes (Figure 6, A and B), consistent with transport to lysosomes. On treatment with the proteasomal inhibitor MG 132, a first striking difference with untreated cells was the increased occurrence of GH in electron-dense tubulovesicles reminiscent of REs (Table 4 and Figure 6, C and D). Although in control cells 5.1% of total GH label was found in REs, in cells incubated with MG 132 during GH uptake, this percentage was ∼3 times higher. Concomitantly, the amount of GH label in endosome-associated tubules, which by our definition was within 200 nm of the endosomal vacuole and the likely precursors of REs, had increased as well (Table 4). In agreement with the increased recycling, the percentage of GH label in LEs dropped from 16.3 to 4.5%, upon MG 132 treatment. Coincubation with GH and Tf, and subsequent double-immunogold labeling clearly showed colocalization of GH and Tf in the electron-dense REs (Figure 6D). In control cells, 6.7% of the Tf-positive REs also labeled for GH, whereas in MG 132-treated cells this percentage amounted to 16.8%. Based on these data, we conclude that in the presence of the proteasomal inhibitor MG 132, a portion of GHR-369 is directed to REs instead of being transported to lysosomes.
Figure 6.
MG 132 induces recycling of endocytosed GHR-369. (A and B) GHR-369 cells incubated for 1 h with biotinylated GH. (A) GH (10-nm gold) is found on the plasma membrane (PM), as well as in EEs. (B) Example of GH (10-nm gold) staining on the internal vesicles of an LE. (C) GHR-369–expressing cells incubated for 1 h in the presence of MG 132 and subsequently 1 h with MG 132 plus biotinylated GH. GH (10-nm gold) is found on both the limiting membrane and internal vesicles of an EE. Note that label is present in a tubule that evolves from the vacuole (arrowhead). (D) Cells were treated as in C but GH was used instead of biotinylated ligand and in the last 30 min also biotinylated Tf was added. Double labeling for GH (10-nm gold) and biotin (15-nm gold) showed colocalization on electron-dense REs (arrowheads). N, nucleus. Bars, 200 nm.
We then focused on GHR-369 distribution in the EE vacuole. In untreated cells we found 42.3% of GH in the lumen of EE vacuoles, where it was associated with internal vesicles. At the limiting membrane, 15.0% of GH was present in the bilayered coated and 32.5% in noncoated areas (Table 5), similar to the distribution of wtGHR. In MG 132-treated cells, the amount of GH label on internal vesicles was reduced to 30.9% in agreement with the previously observed inhibition of degradation (van Kerkhof and Strous, 2001). Notably, the concomitant increase of GH at the vacuolar limiting membrane was restricted to the noncoated areas only, thus shifting the ratio of GH label at the limiting membrane toward the noncoated areas.
Hrs and Syntaxin 7 Are Concentrated in Bilayered Coated Areas of EEs
Accumulating evidence implicates that the Hrs is necessary for transport from EEs to LEs. In mammalian cells, Hrs was localized to TfR-positive EEs and phosphorylated upon activation of several growth factor receptors, including EGFR (Komada et al., 1997; Urbéet al., 2000). Membrane association depends on its FYVE-finger domain, which binds PtdIns3P. Hence, membrane association of Hrs is disturbed by wortmannin (Urbéet al., 2000). We therefore investigated the possible association of Hrs with the bilayered coats on endosomes. As shown in Figure 5D, Hrs was present in the bilayered coated areas of EEs. In HeLa cells, 50.2 ± 1.1% (average ± SD) of Hrs located at the limiting membrane of EE vacuoles was found in the bilayered coats. Determination of the labeling surface ratios revealed that Hrs was 20 times concentrated in the bilayered coats compared with noncoated areas of the endosomal limiting membrane.
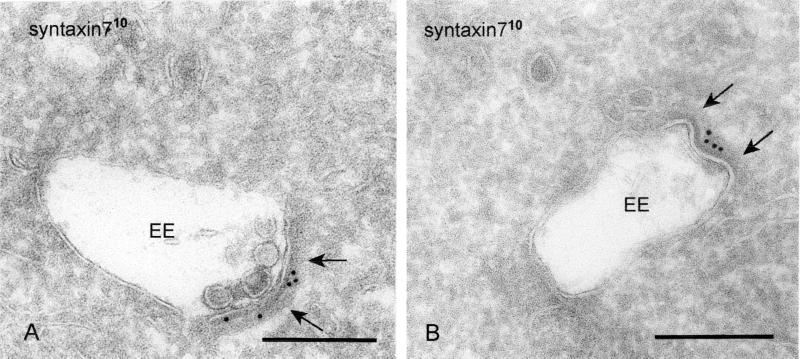
The SNARE protein syntaxin 7 has been suggested to be involved in membrane transport in the LE pathway (Mullock et al., 2000; Nakamura et al., 2000). In a previous study, we demonstrated the presence of syntaxin 7 in the bilayered clathrin-coated domains of EE vacuoles (Prekeris et al., 1998). In wtGHR cells, syntaxin 7 was largely restricted to endosomal vacuoles (Figure 7A), with only occasional label in associated vesicles and tubules; 49.7 ± 4.5% (average ± SD) of syntaxin 7 present at the limiting membrane of EE vacuoles was found in the bilayered coated areas. Calculation of the labeling surface ratios revealed a 10 times higher syntaxin 7 concentration in the bilayered coats than in the noncoated areas of the endosomal limiting membrane. Thus, in addition to receptor proteins targeted for lysosomal degradation also proteins known to be involved in late steps of endosomal trafficking are present in the coated areas on EEs.
Figure 7.
Syntaxin 7 is concentrated in the bilayered coat (A) WtGHR cells. Syntaxin 7 (10-nm gold) is largely restricted to the limiting membrane of EEs, where it accumulates in the bilayered coated areas (arrows). (B) In HeLa cells, syntaxin 7 (10-nm gold) shows a similar staining. Bars, 200 nm.
DISCUSSION
Coats on endosomal vacuoles were first documented in early morphological studies on cells of the rat adrenal medulla (Holtzman and Dominitz, 1968). Over time, however, this observation has attracted surprisingly little interest and the role of these coats in protein trafficking has remained elusive. Herein, we show that coated endosomes are a common phenomenon in several cell types. The coat has a characteristic bilayered appearance and is predominantly present on early and only occasionally on late endosomes. The most striking observation in our study is that within the endosomal limiting membrane, GHR and EGFR destined for degradation in lysosomes are concentrated in the bilayered coated areas. These represent the first example of cargo proteins that concentrate in this peculiar type of clathrin-coated membranes. In contrast, the recycling TfR is equally distributed over the vacuolar membrane. Equivalent results were obtained using antibodies against receptors and ligands and in several cell types. Together, the data strongly indicate a role for the clathrin coats of endosomal vacuoles in protein sorting to lysosomes. Moreover, we found no evidence for a role of the bilayered coats in the formation of clathrin-coated REs, nor for an association of the coated endosomal membranes with the cytoskeleton.
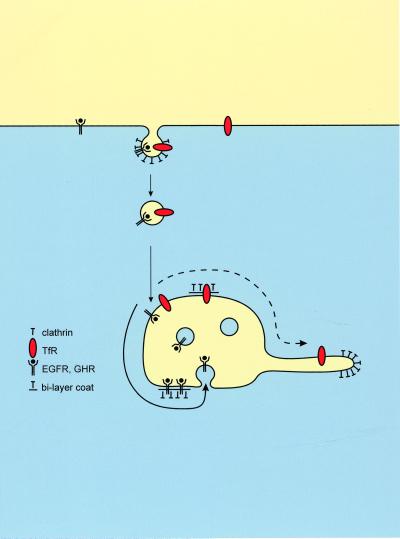
One likely explanation for our findings is that before their incorporation into internal endosomal vesicles the receptor–ligand complexes are concentrated in the bilayered coated areas of EEs. The notion that TfR is not excluded from coated membrane areas suggests that proteins may freely enter these areas. Concentration would then depend on an active retention rather than an active recruitment of cargo proteins. This concentration by retention in addition might serve to stop proteins from entering evolving recycling tubules, which constitutes up to 95% of the membrane traffic at the EE stage (Draye at al., 1988). A model illustrating this hypothesis is given in Figure 8.
Figure 8.
Converging lines of evidence suggest a model, in which an interaction between coat components and down-regulated receptors results in retention in the bilayered coats. This retention concentrate lysosomal routed proteins and segregates them from recycling proteins. Recycling proteins that are not retained in the bilayered coats pass through the endosomal vacuole and follow the massive bulk flow into recycling endosomes (dashed bold line). In contrast, retention in the coats (continuous bold line) of proteins routed to lysosomes would precede incorporation in internal endosomal vesicles and subsequent transport to lysosomes.
Recently, a morphological identical bilayered coat was found on endosomal vacuoles in the melanoma cell line MNT-1 (Raposo et al., 2001). It was suggested that these coated endosomal intermediates might be the precursor organelles for melanosomes. Our data are consistent with this model but in addition suggest a more general function of the coats in endocytic protein transport. Morphologically, the endosomal coat differs from previously identified clathrin coats present on the TGN, plasma membrane, and REs by its characteristic bilayered appearance. The endosomal coats also differ from other clathrin coats by their protein composition. We found no indications for the presence of the known adaptor–protein complexes AP1, AP2, or AP3 in the bilayered coat, which is consistent with immunofluorescence data of Raiborg et al. (2001). Although these data do not exclude that the bilayer coats may contain low concentrations or an altered form of these adaptor proteins, these observations clearly set this coat apart from other clathrin-coated membranes in the cell. The lack of AP2 labeling convincingly distinguished the bilayered coats from clathrin coats on primary endocytic vesicles, whereas the absence of AP1 and AP3 illustrates the difference with the clathrin coats on REs and TGN (Dell'Angelica et al., 1998; Futter et al., 1998; Mallard et al., 1998). Furthermore, the coat was not sensitive to a short incubation with brefeldin A, which was sufficient for disassembly of the Golgi complex, suggesting that assembly of the coat is independent of ARF1, although we cannot rule out that ARF1 binding at the endosomal vacuole may be mediated by a brefeldin A-insensitive guanine-nucleotide exchange factor. Thus, our study combined with those of others now suggests that clathrin on endosomes may act in two ways: 1) as a flat lattice on the endosomal vacuole, possibly mediating the retention of lysosome-directed proteins (Figure 8); and 2) in the form of buds on recycling tubules, mediating the transport of recycling proteins (Stoorvogel et al., 1996; Futter et al., 1998; Mallard et al., 1998; van Dam and Stoorvogel, 2002).
Unlike wtGHR, GHR-369 is endocytosed from the plasma membrane independently of the ubiquitin-proteasome system. We found that under control conditions, uptake of GH by GHR-369 cells was similar to wtGHR cells and resulted in equivalent distributions of wild-type and truncated GHR over the coated and noncoated subdomains of EEs. However, upon interference with the ubiquitin-proteasome system by MG 132 treatment, the distribution of GHR-369 partially shifted from the degradative to the recycling pathway, as concluded from an increased localization in REs where it colocalized with Tf. These findings are in agreement with data showing that in the presence of MG 132, GH breakdown is impaired in GHR-369 cells (van Kerkhof and Strous, 2001). On MG 132 treatment, within EE vacuoles the percentage of GHR-369 label on internal vesicles decreased, which is consistent with the decline in lysosomal breakdown. Importantly, the concomitant relative increase in label at the limiting membrane was found in the noncoated domains only (Table 5). Thus, MG 132 resulted in a shift in the relative distribution of GHR-369 over the endosomal limiting membrane from coated to noncoated regions. According to our model (Figure 8), this shift is explained by a reduced retention of GHR-369 in the coated areas, resulting in an increased flow to noncoated membranes and subsequently the REs. Indeed, MG 132 treatment resulted in a minor decrease in the percentage of label found in coated regions of EE vacuoles, but this was not statistically significant (Table 5). An alternative or additional explanation may therefore be that under control conditions the binding sites for the receptors in the coats are saturated. If MG 132 affects the incorporation of GHR-369 into internal endosomal vesicles at a step downstream of the retention in the coat, this will result in higher levels of recycling receptors, which then will fail to bind to the already saturated coat. Clearly, these speculative explanations have to be addressed in future experiments. In addition, it should be kept in mind that the MG 132 block on sorting is not complete, as is also indicated by the fact that some GHR–GH complexes still reach the LE.
The finding that MG 132 causes GHR-369 redistribution to REs indicates that sorting into internal endosomal vesicles is dependent on the ubiquitin-proteasome system. This is in agreement with our previous findings that lysosomal degradation of GHR depends on an active ubiquitin system and the presence of the so-called UbE-motif in the GHR cytoplasmic tail, which in addition is required for ubiquitination and initial internalization of the receptor (van Kerkhof et al., 2001). Also the entry of EGFR into the LE pathway depends on the ubiquitin system, specifically by the action of the ubiquitin ligase c-Cbl, which mediates ubiquitination and subsequent down-regulation of activated EGFR (Levkowitz et al., 1998, 1999). The molecular details of ubiquitin-mediated lysosomal sorting in mammalian cells are only beginning to emerge. However, in yeast, recent data suggest that ubiquitin serves as a signal for sorting into the late endosomal pathway via the so-called endosomal sorting complex required for transport, consisting of a subset of Vps class E proteins: Vps23, Vps28, and Vps37 (Katzmann et al., 2001). Ubiquitinated cargo is recognized by endosomal sorting complex required for transport, which initiates cargo entry into internal vesicles. Before entry into vesicles, ubiquitin is removed from the cargo by the deubiquitinating enzyme Doa4, which is recruited by class E Vps proteins (Katzmann et al., 2001). Proteins of the class E protein family are required for protein sorting to the yeast prevacuolar multivesicular body (Odorizzi et al., 1998) and have several identified orthologs in higher mammals, indicating that the molecular mechanism for lysosomal sorting is conserved. In this respect, the high enrichment of Hrs in the endosomal bilayered coats is of importance, because Hrs has a high similarity with the class E protein Vps27 (Piper et al., 1995). Recently, it was shown that the clathrin-binding motif of Hrs is necessary for the recruitment of clathrin to EEs in Hrs-overexpressing cells (Raiborg et al., 2001). The association of Hrs with the endosomal membrane is dependent on the interaction of its FYVE-finger domain with PtdIns3P (Urbéet al., 2000). In agreement with immunofluorescence data by Raiborg et al. (2001), we found that incubation with the PtdIns 3-kinase inhibitor wortmannin resulted in a dissociation of clathrin from endosomal vacuoles. We found a severe reduction in the percentage of membrane that was covered by a bilayered coat. Importantly, our data show that clathrin remained associated to other intracellular membranes, emphasizing the special character of the endosomal clathrin coat. In addition to clathrin, Hrs associates with the Hrs binding protein, which contains a Src homology 3 domain (Takata et al., 2000) that binds to the deubiquitinating enzyme UBPY (Kato et al., 2000). Deletion of the Src homology 3 domain inhibits degradation of the platelet-derived growth factor (Takata et al., 2000). Thus, one could envision that if an endosomal sorting complex also exists in mammalian cells, it may be located in the bilayered coated areas of the endosomal vacuole.
In a previous study, we found that the SNARE protein syntaxin 7 prominently labeled endosomal coated areas (Prekeris et al., 1999). Herein, we show that syntaxin 7 is 10 times concentrated in the coated areas compared with the noncoated endosomal membranes. The role of syntaxin 7 in endosomal trafficking is unclear. It was localized on both early (Wong et al., 1998; Prekeris et al., 1999) and late endosomal structures (Mullock et al., 2000; Nakamura et al., 2000; Ward et al., 2000) and found in a complex together with the late endosomal SNAREs Vamp8, syntaxin 8, and vti1b (Prekeris et al., 1999; Antonin et al., 2000; Mullock et al., 2000). The presence of high concentrations of a SNARE protein in the endosomal coat suggests a role in membrane fusion events, but at which step is not known. Because syntaxin 7 is involved in LE traffic or may function in endosome-lysosome fusion (Mullock et al., 2000), it can be envisioned that syntaxin 7 in EEs is concentrated in the coated areas to act at a downstream fusion event. Indeed, it is generally thought that disassembly of a clathrin coat is necessary to expose SNAREs and other fusion machinery proteins for interaction with their binding partners. A function of the clathrin coat might therefore be to prevent the interaction of syntaxin 7 with its cognate SNAREs at the level of EEs.
Syntaxin 7, together with clathrin and Hrs, appears in high labeling densities in the coat. Yet, on the internal vesicles only the cargo proteins EGFR and GHR were detected and none of these transport machinery proteins. It could be reasoned that coat proteins are immediately degraded when entering the internal of EEs. However, this is unlikely because the environment of EEs is only mildly acidic. The consistent absence of coat proteins therefore may indicate that the bilayered coat is removed before inward budding or, alternatively, that internal vesicles originate from noncoated areas of the membrane (Figure 8). In this context, it is interesting to note that Hrs is phosphorylated in response to EGF and that this phosphorylation event is dependent both on the internalization of the EGFR from the plasma membrane to the endosome and translocation of Hrs from the cytosol to the EE membrane (Urbéet al., 2000). Once phosphorylated, Hrs appears to dissociate from the endosomal membrane because phosphorylated Hrs is almost exclusively found in the cytosol (Urbéet al., 2000). Hence, it is tempting to speculate that the disassembly of the EE clathrin coat may at least in part be triggered by phosphorylation of Hrs, resulting in the dissociation of Hrs from the EE membrane. The concentrated cargo, encompassing the EGFRs, could then be incorporated into the internal vesicles by a yet to be identified mechanism.
In summary, our data provide evidence that the clathrin coat on EE vacuoles is structurally and morphologically distinct from all other known clathrin coats and suggest a role for this coat in the sorting and the retention of proteins destined for incorporation into internal endosomal vesicles, a unprecedented mode of clathrin function.
ACKNOWLEDGMENTS
We thank Rene Scriwanek and Marc van Peski for excellent photographic work; T. Kirchhausen, R. Scheller, and E. Ungewickell for kind gifts of antibodies; Ann de Maziere, Jürgen Gent, Georg Ramm, Julia Schantl, and Peter van Kerkhof for helpful discussions; and Hans Geuze for critical reading of the manuscript. This work was supported by a grant of the Netherlands Organization for Scientific Research (NWO-902-23-192), a European Union Network grant (ERBFMRXCT96-0026), and by grants from the National Institutes of Health (HL-59150 and NS-37525). Sylvie Urbé is funded by the North West Cancer Research Fund.
Abbreviations used:
- EE
early endosome
- EGFR
epidermal growth factor receptor
- GHR
growth hormone receptor
- Hrs
hepatocyte growth factor-regulated tyrosine kinase substrate
- LE
late endosome
- PtdIns 3-kinase
phosphatidyl-inositol 3-kinase
- TfR
transferrin receptor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–10–0525. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–10–0525.
REFERENCES
- Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 2000;19:6453–6464. doi: 10.1093/emboj/19.23.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham J, Aplin R, Norman MR. Histochemical detection of binding sites for human growth hormone using biotinylated ligand. J Histochem Cytochem. 1994;42:103–107. doi: 10.1177/42.1.8263321. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- de Melker AA, van Der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane, and remains receptor associated throughout the endocytic route. J Cell Sci. 2001;114:2167–2178. doi: 10.1242/jcs.114.11.2167. [DOI] [PubMed] [Google Scholar]
- de Wit H, Lichtenstein Y, Geuze HJ, Kelly RB, van der Sluijs P, Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol Biol Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draye JP, Courtoy PJ, Quintart J, Baudhuin P. A quantitative model of traffic between plasma membrane and secondary lysosomes: evaluation of inflow, lateral diffusion, and degradation. J Cell Biol. 1988;107:2109–2115. doi: 10.1083/jcb.107.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WA, Connolly TP, Hubbard AL. Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes: receptor pathway. J Cell Biol. 1986;102:24–36. doi: 10.1083/jcb.102.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galisteo ML, Dikic I, Batzer AG, Langdon WY, Schlessinger J. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem. 1995;270:20242–20245. doi: 10.1074/jbc.270.35.20242. [DOI] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJ, Lodish HF, Schwartz AL. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoEM during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers R, ten Broeke T, van Kerkhof P, Schwartz AL, Strous GJ. Identification of a novel ubiquitin conjugation motif, required for ligand-induced internalization of the growth hormone receptor. EMBO J. 1999;18:28–36. doi: 10.1093/emboj/18.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers R, van Kerkhof P, Schwartz AL, Strous GJ. Di-leucine-mediated internalization of ligand by a truncated growth hormone receptor is independent of the ubiquitin conjugation system. J Biol Chem. 1998;273:16426–16433. doi: 10.1074/jbc.273.26.16426. [DOI] [PubMed] [Google Scholar]
- Govers R, van Kerkhof P, Schwartz AL, Strous GJ. Linkage of the ubiquitin-conjugating system and the endocytic pathway in ligand-induced internalization of the growth hormone receptor. EMBO J. 1997;16:4851–4858. doi: 10.1093/emboj/16.16.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann A, Schroer TA, Griffiths G, Burkhardt JK. Immunolocalization of cytoplasmic dynein and dynactin subunits in cultured macrophages: enrichment on early endocytic organelles. J Cell Sci. 2001;114:229–240. doi: 10.1242/jcs.114.1.229. [DOI] [PubMed] [Google Scholar]
- Holtzman E, Dominitz R. Cytochemical studies of lysosomes, golgi apparatus and endoplasmic reticulum in secretion and protein uptake by adrenal medulla cells of the rat. J Histochem Cytochem. 1968;16:320–336. doi: 10.1177/16.5.320. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing SQ, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- Kato M, Miyazawa K, Kitamura N. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J Biol Chem. 2000;275:37481–37487. doi: 10.1074/jbc.M007251200. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting cimplex, ESCRT-1. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Kil SJ, Hobert M, Carlin C. A leucine-based determinant in the epidermal growth factor receptor juxtamembrane domain is required for the efficient transport of ligand-receptor complexes to lysosomes. J Biol Chem. 1999;274:3141–3150. doi: 10.1074/jbc.274.5.3141. [DOI] [PubMed] [Google Scholar]
- Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Geuze HJ. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J, Hille A, Veenendaal T, Oorschot V, Stoorvogel W, von Figura K, Geuze HJ. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Masaki R, Yamamoto A, Kitamura N. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J Biol Chem. 1997;272:20538–20544. doi: 10.1074/jbc.272.33.20538. [DOI] [PubMed] [Google Scholar]
- Kornilova E, Sorkina T, Beguinot L, Sorkin A. Lysosomal targeting of epidermal growth factor receptors via a kinase-dependent pathway is mediated by the receptor carboxyl-terminal residues 1022–1123. J Biol Chem. 1996;271:30340–30346. doi: 10.1074/jbc.271.48.30340. [DOI] [PubMed] [Google Scholar]
- Kulka RG, Raboy B, Schuster R, Parag HA, Diamond G, Ciechanover A, Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J Biol Chem. 1988;263:15726–15731. [PubMed] [Google Scholar]
- Lemmon SK, Traub LM. Sorting in the endosomal system in yeast and animal cells. Curr Opin Cell Biol. 2000;12:457–466. doi: 10.1016/s0955-0674(00)00117-4. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Liu SH, Towler MC, Chen E, Chen CY, Song W, Apodaca G, Brodsky FM. A novel clathrin homolog that co-distributes with cytoskeletal components functions in the trans-Golgi network. EMBO J. 2001;20:272–284. doi: 10.1093/emboj/20.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock BM, et al. Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and Is required for late endosome-lysosome fusion. Mol Biol Cell. 2000;11:3137–3153. doi: 10.1091/mbc.11.9.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Yamamoto A, Wada Y, Futai M. Syntaxin 7 mediates endocytic trafficking to late endosomes. J Biol Chem. 2000;275:6523–6529. doi: 10.1074/jbc.275.9.6523. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Dell'Angelica EC, Bonifacino JS. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Trowbridge IS. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981;256:12888–12892. [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Yang B, Oorschot V, Klumperman J, Scheller RH. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol Biol Cell. 1999;10:3891–3908. doi: 10.1091/mbc.10.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Gronvold Bache K, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. EMBO J. 2001;20:5008–5021. doi: 10.1093/emboj/20.17.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm G, Pond L, Watts C, Stoorvogel W. Clathrin-coated lattices and buds on MHC class II compartments do not selectively recruit mature MHC-II. J Cell Sci. 2000;113:303–313. doi: 10.1242/jcs.113.2.303. [DOI] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152:809–823. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 2001;17:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Roupas P, Herington AC. Growth hormone receptors in cultured adipocytes: a model to study receptor regulation. Mol Cell Endocrinol. 1986;47:81–90. doi: 10.1016/0303-7207(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Rubino M, Miaczynska M, Lippe R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol Chem. 2000;275:3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- Sachse M, van Kerkhof P, Strous GJ, Klumperman J. The ubiquitin-dependent endocytosis motif is required for efficient incorporation of growth hormone receptor in clathrin-coated pits but not clathrin-coated lattices. J Cell Sci. 2001;114:3943–3952. doi: 10.1242/jcs.114.21.3943. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Bild A, Tebar F, Sorkin A. Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J Cell Sci. 1999;112:317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang E, Johannessen LE, Knardal SL, Madshus IH. Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J Biol Chem. 2000;275:13940–13947. doi: 10.1074/jbc.275.18.13940. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Geuze HJ, Strous GJ. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987;104:1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous GJ, van Kerkhof P, Govers R, Ciechanover A, Schwartz AL. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J. 1996;15:3806–3812. [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Rocca A, Dautry-Varsat A. Molecular characterization of the signal responsible for the targeting of the interleukin 2 receptor beta chain toward intracellular degradation. J Biol Chem. 1998;273:29424–29429. doi: 10.1074/jbc.273.45.29424. [DOI] [PubMed] [Google Scholar]
- Takata H, Kato M, Denda K, Kitamura N. A hrs binding protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors. Genes Cells. 2000;5:57–69. doi: 10.1046/j.1365-2443.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Bannykh SI, Rodel JE, Aridor M, Balch WE, Kornfeld S. AP-2-containing clathrin coats assemble on mature lysosomes. J Cell Biol. 1996;135:1801–1814. doi: 10.1083/jcb.135.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Urbanowski JL, Piper RC. Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole. Traffic. 2001;2:622–630. doi: 10.1034/j.1600-0854.2001.20905.x. [DOI] [PubMed] [Google Scholar]
- Urbé S, Mills IG, Stenmark H, Kitamura N, Clague MJ. Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol Cell Biol. 2000;20:7685–7692. doi: 10.1128/mcb.20.20.7685-7692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam EM, Stoorvogel W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol Biol Cell. 2002;13:169–182. doi: 10.1091/mbc.01-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol. 1995;66:309–323. [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–24. [PubMed] [Google Scholar]
- van Kerkhof P, dos Santos CM, Sachse M, Klumperman J, Bu G, Strous GJ. Proteasome inhibitors block a late step in lysosomal transport of selected membrane but not soluble proteins. Mol Biol Cell. 2001;12:2556–2566. doi: 10.1091/mbc.12.8.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P, Strous GJ. The ubiquitin-proteasome pathway regulates lysosomal degradation of the growth hormone receptor and its ligand. Biochem Soc Trans. 2001;29:488–493. doi: 10.1042/bst0290488. [DOI] [PubMed] [Google Scholar]
- van Kerkhof P, Govers R, Alves dos Santos CM, Strous GJ. Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J Biol Chem. 2000;275:1575–1580. doi: 10.1074/jbc.275.3.1575. [DOI] [PubMed] [Google Scholar]
- Verges M, Havel RJ, Mostov KE. A tubular endosomal fraction from rat liver: biochemical evidence of receptor sorting by default. Proc Natl Acad Sci USA. 1999;96:10146–10151. doi: 10.1073/pnas.96.18.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Pevsner J, Scullion MA, Vaughn M, Kaplan J. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol Biol Cell. 2000;11:2327–2333. doi: 10.1091/mbc.11.7.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Hong W. Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J Biol Chem. 1998;273:375–380. doi: 10.1074/jbc.273.1.375. [DOI] [PubMed] [Google Scholar]