Abstract
Purpose
The WHO classification of Tumors of the Central Nervous System represents the international standard classification for brain tumors. In 2021 the 5th edition (WHO CNS5) was published, and this review summarizes the changes regarding IDH-mutant gliomas and discusses unsolved issues and future perspectives.
Methods
This review is based on the 5th edition of the WHO Blue Book of CNS tumors (WHO CNS5) and relevant related papers.
Results
Major changes include taxonomy and nomenclature of IDH-mutant gliomas. Essential and desirable criteria for classification were established considering technical developments. For the first time molecular features are not only relevant for the classification of IDH-mutant gliomas but may impact grading as well.
Conclusion
WHO CNS5 classification moves forward towards a classification which is founded on tumor biology and serves clinical needs. The rapidly increasing knowledge on the molecular landscape of IDH-mutant gliomas is expected to further refine classification and grading in the future.
Keywords: WHO, Glioma, Classification, CNS5, Molecular pathology, Neuropathology, IDH-mutant
Introduction
The WHO Classification of Tumors of the Central Nervous System from 2021 represents the 5th edition (WHO CNS5) of the international standard classification for brain tumors [1]. WHO CNS5 is a further development along the lines of the 2016 update of the 4th edition by reinforcing the role of molecular diagnostics. Many recommendations of the “Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy” (cIMPACT-NOW) have been included [2, 3]. IDH-mutant gliomas were first recognized by the WHO as distinct entities in 2016. In brief, after their initial discovery in glioblastomas arising from lower-grade precursor lesions, so called “secondary” glioblastomas in 2008 [4], it became rapidly evident that IDH-mutations are typical for diffuse lower grade astrocytomas and oligodendrogliomas [5, 6] and associate with a far better prognosis than diffuse IDH-wildtype gliomas [7] most frequently resembling glioblastomas, IDH-wildtype [8]. Furthermore, IDH-mutations invariably present with additional molecular alterations which again are prognostically important: IDH-mutations co-occur either with mutations in TP53 and ATRX associating with a less-favorable prognosis or with whole-arm 1p/19q codeletion and TERT promoter (TERTp) mutations associating with the most favorable prognosis for IDH-mutant gliomas [9–11]. Because tumors with the trias “IDH/TP53/ATRX” most commonly show an astrocytic differentiation and tumors with the trias “IDH/1p/19q codeletion/TERTp” an oligodendroglial differentiation the designations “astrocytoma” and “oligodendroglioma” were carried on by the WHO to these -by now molecularly defined- tumor types. Specific changes regarding IDH-mutant gliomas in WHO CNS5, unsolved issues and future perspectives will be reviewed and discussed in the following.
Technical development
Progress in understanding the molecular basis of brain tumors and consequential incorporation of molecular features in brain tumor classification implicates specific needs for molecular analyses. Availability of molecular analyses is fortunately increasing rapidly worldwide. Especially, the influence of DNA-methylation profiling on WHO CNS5 is substantial following the results from many studies clearly demonstrating the enormous potential of genome-wide DNA-methylation profiling for brain tumors classification [12, 13]. Beside providing a methylation profile the method additionally provides information on chromosomal copy number alterations (e.g. for the detection of 1p/19q codeletion). In fact, almost all tumor entities in WHO CNS5 exhibit specific DNA-methylation profiles including astrocytoma, IDH-mutant and oligodendroglioma, IDH-mutant and 1p/19q-codeleted. While large centers already use next-generation sequencing of DNA and RNA as well as genome-wide DNA-methylation profiling for routine diagnostics, it has to be emphasized that most diagnostically relevant molecular alterations may still be determined using more conventional techniques like Sanger sequencing (for IDH1/2 status determination), fluorescence in situ hybridization (FISH; for 1p/19q and CDKN2A/B status determination) or immunohistochemistry (for IDH1 R132H- and ATRX-testing).
Revised taxonomy and nomenclature
The novel WHO classification aimed for simplification, standardization and harmonization with classifications from other organ systems. While in the 2016 update IDH-mutant tumors comprised 5 different entities (Diffuse astrocytoma WHO grade II, IDH-mutant; Anaplastic astrocytoma WHO grade III, IDH-mutant; Glioblastoma WHO grade IV, IDH-mutant; Oligodendroglioma WHO grade II, IDH-mutant and 1p/19q-codeleted; Anaplastic oligodendroglioma WHO grade III, IDH-mutant and 1p/19q-codeleted) the novel classification recognizes just two entities, now termed “types”: “Astrocytoma, IDH-mutant” and “oligodendroglioma, IDH-mutant and 1p/19q-codeleted”. Grading is performed within these types and Arabic instead of Roman numerals are used. To avoid confusion with grading systems from other organs, the term “CNS WHO grade” is endorsed. The range of grades for astrocytomas (grades 2, 3 or 4) and oligodendrogliomas (grades 2 and 3) remains the same. Of note, the term “glioblastoma” is persevered for IDH-wildtype tumors only and the former “glioblastoma, IDH-mutant” has been renamed “Astrocytoma, IDH-mutant, CNS WHO grade 4”. Additional terms like “diffuse” or “anaplastic” are not used anymore (Table 1). To emphasize the biological differences of brain tumors typically occurring in different age groups, CNS5 features a grouping of IDH-mutant gliomas together with glioblastomas, IDH-wildtype as “adult-type” diffuse gliomas, separated from “pediatric type” low- and high-grade diffuse gliomas, even though IDH-mutant gliomas can occasionally develop in the pediatric population as well.
Table 1.
Terminology of IDH-mutant gliomas in the WHO classification of 2016 and 2021
| WHO 2016 | WHO 2021 |
|---|---|
| Diffuse Astrocytoma WHO grade II, IDH-mutant | Astrocytoma, IDH-mutant CNS WHO grade 2 |
| Anaplastic Astrocytoma WHO grade III, IDH-mutant | Astrocytoma, IDH-mutant CNS WHO grade 3 |
| Glioblastoma WHO grade IV, IDH-mutant | Astrocytoma, IDH-mutant CNS WHO grade 4 |
| Oligodendroglioma WHO grade II, IDH-mutant and 1p/19q-codeleted WHO grade II | Oligodendroglioma, IDH-mutant and 1p/19q- codeleted CNS WHO grade 2 |
| Anaplastic Oligodendroglioma WHO grade II, IDH-mutant and 1p/19q-codeleted WHO grade III | Oligodendroglioma, IDH-mutant and 1p/19q- codeleted CNS WHO grade 3 |
Layered diagnostics, integrated diagnosis and NOS diagnoses
Introduced in the 2016 update of the WHO classification the concept of layered and integrated diagnostics is further emphasized in WHO CNS5. Diagnostics should include different layers, namely histology, WHO grading and molecular findings. The WHO conform integrated diagnosis is based on combining histological and molecular information. Respective reports should include all information, including the integrated diagnosis and the different layers it is based on. A not-otherwise-specified (NOS) diagnosis indicates that a molecular workup for a WHO conform integrated diagnoses is not available or has failed, and therefore the diagnosis is purely based on histological assessment [14]. For the first time molecular features are not only relevant for the classification of IDH-mutant gliomas but may impact grading as well.
Essential and desirable diagnostic criteria
WHO CNS5 provides essential criteria which must be present to make an integrated diagnoses and desirable criteria which clearly support a diagnosis but are not mandatory. The histological finding of “a diffusely infiltrating glioma” and the molecular finding of an IDH1/2 hotspot mutation are essential for classifying both astrocytoma and oligodendroglioma. Of note, for oligodendroglioma, the IDH-mutation analysis may not be required when DNA methylome profiling is performed and unequivocally assigns the tumor to the methylation class “oligodendroglioma, IDH-mutant and 1p/19q- codeleted”. The diagnosis of astrocytoma requires either the immunohistochemical loss of nuclear ATRX expression or detection of a pathogenic ATRX mutation or the exclusion of combined whole-arm deletions of 1p and 19q. Presence of a TP53 mutation or strong nuclear expression of p53 in > 10% of tumor cells is a desirable finding as well as a methylation profile of “astrocytoma, IDH-mutant” and an astrocytic differentiation by morphology. Diagnosis of “oligodendroglioma, IDH-mutant and 1p/19q-codeleted” requires the detection of combined whole-arm deletions of 1p and 19q. A DNA methylome profile of oligodendroglioma, IDH-mutant and 1p/19q-codeleted, a retained nuclear expression of ATRX and a TERT promoter mutation are desirable criteria (Table 2).
Table 2.
Essential and desirable criteria for the diagnosis of IDH-mutant gliomas
| Tumor type | Essential criteria | Desirable criteria | Additional altered genes |
|---|---|---|---|
| Astrocytoma, IDH-mutant | Diffusely infiltrating glioma | Astrocytic differentiation by morphology | CDKN2A, CDKN2B, CDK4, CCND2, PDGFRA, MET, MYC, MYCN, RB1, PIK3R1, PIC3CA, others |
| IDH1/2 hotspot mutation | TP53 mutation or strong nuclear expression of p53 in > 10% of tumor cells | ||
| ATRX loss or ATRX mutation OR exclusion of combined whole-arm deletions of 1p and 19q | Methylation profile of astrocytoma, IDH-mutant | ||
| Oligodendroglioma, IDH-mutant and 1p/19q-codeleted | Diffusely infiltrating glioma | Retained nuclear expression of ATRX | CDKN2A, CDKN2B, CIC, FUBP1, NOTCH1 |
| IDH1/2 hotspot mutation | TERT promoter mutation | ||
| Combined whole-arm deletions of 1p and 19q | DNA methylome profile of oligodendroglioma, IDH-mutant and 1p/19q-codeleted |
Dual-genotype and NEC diagnoses
Not elsewhere classified (NEC) diagnoses are reserved for situations in which all appropriate information regarding e.g. histology, IDH1/2-mutation, ATRX and 1p/19q status are available, but results do not match a WHO diagnosis or are conflicting regarding WHO essential criteria [14]. In respect to IDH-mutant gliomas this is of relevance for so called “dual-genotype” IDH-mutant gliomas. These are exceedingly rare and characterized by the presence of defining molecular alterations of both astrocytoma (ATRX loss/mutation and TP53 mutation) and oligodendroglioma (whole-arm 1p/19q codeletion). These features have been found either in two morphological distinct regions of the same tumor mass or as a single clone in all tumor regions [15–17]. As these cases fall out of the current WHO classification principle, a layered diagnosis providing all histological and molecular information with the addition of “NEC” is suggested.
Grading of astrocytoma
Grading of astrocytoma IDH-mutant in WHO CNS5 on the one hand retains classic morphological features for grading but on the other hand includes for the first time a molecular feature, namely the homozygous deletion of the CDKN2A and/or CDKN2B locus. CDKN2A and/or CDKN2B homozygous deletions have been shown to associate with a dismal prognosis consistently in multiple studies, justifying a grade 4 designation of affected tumors irrespective of the presence of absence of additional features [18]. CDKN2A and/or CDKN2B homozygous deletions are therefore by definition absent in grade 2 and grade 3 tumors but do enforce grade 4.
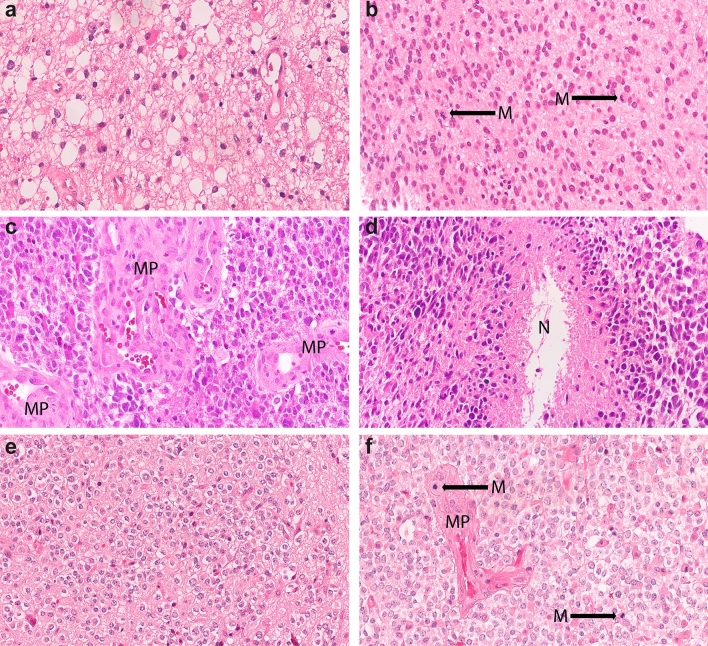
CNS WHO grade 2 tumors represent the lower end of the malignancy spectrum, showing well-developed differentiation, low to moderate cell density, mild nuclear irregularities and no or very low mitotic activity. Criteria for CNS WHO grade 3 tumors are “focal or dispersed anaplasia” and “significant mitotic activity” while microvascular proliferation, necrosis, and homozygous deletions of CDKN2A and/or CDKN2B are by definition absent (Fig. 1). Anaplasia manifests in increased cell density, increased nuclear atypia, multinucleation and abnormal mitosis. Cutoff values or thresholds for both mitotic activity and anaplasia are not established with the exception that a single mitosis in a very small biopsy (like a stereotactic biopsy) may suffice for a grade 3 designation. CNS WHO grade 4 tumors represent the high end of the malignancy spectrum and are defined by the presence of at least one of the following features: microvascular proliferation, necrosis or homozygous deletion of CDKN2A and/or CDKN2B (Table 3, Figs. 1, 2).
Fig. 1.
Histology of IDH-mutant gliomas. Astrocytoma, IDH-mutant CNS WHO grade 2 with low cellularity and mild nuclear atypia (a), grade 3 showing increased cellularity and mitotic activity (M, arrows) (b), grade 4 exhibiting microvascular proliferation (c, MP) and palisading necrosis (d, N). Oligodendroglioma, IDH-mutant and 1p/19q-codeleted CNS WHO grade 2 displaying the typical honeycomb appearance (e) and grade 3 showing microvascular proliferation (MP) and increased mitotic activity (M)
Table 3.
Grading criteria for astrocytomas, IDH-mutant
| CNS WHO grade | Criteria |
|---|---|
| 2 | Well differentiated and lacks histological features of anaplasia |
| Mitotic activity is not detected or very low | |
| Microvascular proliferation, necrosis, and homozygous deletions of CDKN2A and/or CDKN2B are absent | |
| 3 | Focal or dispersed anaplasia |
| Significant mitotic activity | |
| Microvascular proliferation, necrosis, and homozygous deletions of CDKN2A and/or CDKN2B are absent | |
| 4 | Microvascular proliferation, necrosis or homozygous deletion of CDKN2A and/or CDKN2B |
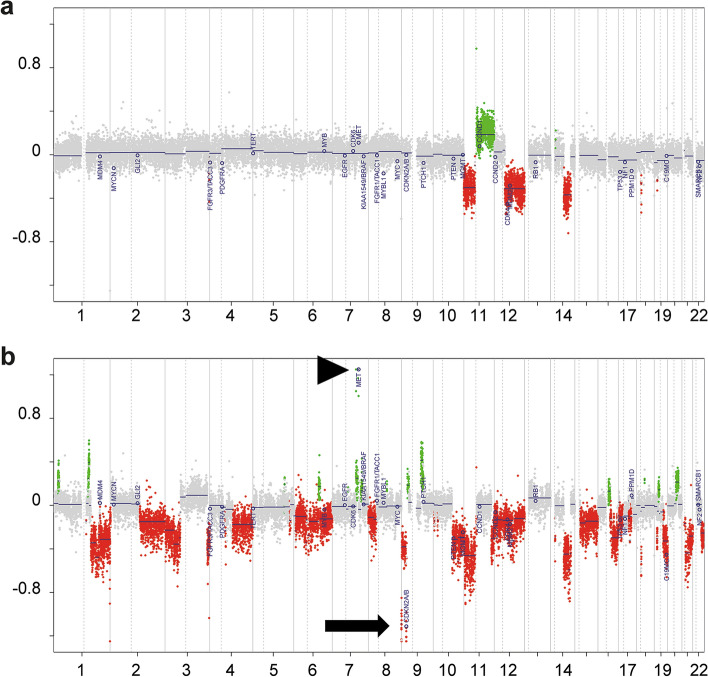
Fig. 2.
Chromosomal copy number plots from an astrocytoma, IDH-mutant at its primary manifestation corresponding to CNS WHO grade 2 (a) and at recurrence, corresponding to CNS WHO grade 4 (b). Note the overall increase of chromosomal copy number alterations (gains marked green and losses marked red) in the recurrent tumor including a homozygous CDKN2A/B (arrow) deletion and a MET amplification (arrow-head)
Grading of oligodendroglioma
Grading of oligodendroglioma, IDH-mutant and 1p/19q-codeleted is mainly based on classic histological features of malignancy, such as cellularity, cytological atypia, mitotic activity, microvascular proliferation and necrosis (Fig. 1). WHO CNS5 does not provide cutoffs for parameters and the impact of individual features remains unclear. Microvascular proliferation and brisk mitotic activity (defined as ≥ 2.5 mitoses/mm2; equating to ≥ 6 mitoses/10 HPF of 0.55 mm in diameter and 0.24 mm2 in area) have been suggested as significant prognostic indicators in some studies while in others microvascular proliferation (with or without necrosis) was more strongly associated with shorter survival than mitotic activity [19–21]. In borderline cases, WHO CNS5 endorses consideration of additional features like extend of KI-67 labeling or neuroradiological features like rapid tumor growth. Cut-off values or thresholds for these criteria are however not established. A novelty is that homozygous deletion involving the CDKN2A and/or CDKN2B locus is of relevance for the grading of oligodendroglioma as well. Presence of a homozygous CDKN2A and/or CDKN2B deletion indicates a WHO CNS grade 3. Testing may be restricted to borderline cases as homozygous CDKN2A and/or CDKN2B deletions have been found in a subset of grade 3 tumors only [22].
Developments beyond WHO CNS5
The current classification defines two types, astrocytoma and oligodendroglioma, with different grades being listed as subtypes. Recently, several studies some of which were published during the final stage of writing CNS5 or after the completion of CNS5, suggest the existence of clinically relevant (sub-)types of IDH-mutant gliomas. These may have the potential to influence future WHO classifications. Infratentorial IDH-mutant astrocytomas display several characteristics important for diagnostics, which are already mentioned in CNS5. In contrast to supratentorial IDH-mutant astrocytomas, infratentorial astrocytomas frequently harbor non-IDH1 R132H mutations such as IDH1 R132C/G and IDH2 R172S/G limiting the sensitivity of IDH1 R132H immunohistochemistry. The frequency of ATRX loss is also reduced in these tumors increasing the need for molecular testing for IDH-mutation and 1p/19q status. Clinical outcome of infratentorial IDH-mutant astrocytoma was notably worse than that of supratentorial tumors [23]. CNS5 already states that IDH-mutant tumors may occur in the setting of DNA mismatch repair deficiency syndromes and that the clinical management of respective tumors may differ from conventional sporadic cases [24, 25]. The most recent post-CNS5 study established a very poor prognosis for these tumors, comparable with that of IDH-wildtype glioblastomas. The name “Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA)” was proposed to underline their distinctiveness in pathogenesis and clinical behavior [26]. PMMRDIAs have a hypermutant genotype, often paired with microsatellite instability, an altered DNA-methylation profile and show frequent genetic inactivation of RB1- and activation of RTK/PI3K/AKT pathways. PMMRDIAs more likely occur in children and young adolescents. In CNS5 sarcomatous differentiation is described as a rare finding in grade 3 oligodendrogliomas. A post-CNS5 study suggests “oligosarcoma, IDH-mutant” as highly distinct from conventional oligodendroglioma on multiple levels including histology, epigenetic-, proteomic- and molecular profiles. Clinical outcome was significantly poorer for oligosarcomas than for grade 3 oligodendrogliomas [27].
Conclusion, discussion and outlook
WHO CNS5 classification moves forward towards a classification which is based on tumor biology and serves clinical needs. New grading criteria implicate that the diagnoses of an IDH-mutant glioma which dates back many years may not be in accordance with the novel classification anymore. This should be considered for patient care and is also of importance for retrospective studies.
There is a broad consensus that grading of IDH-mutant gliomas is reasonable given the obvious diversity regarding biological aggressiveness and clinical outcome ranging from patients surviving decades to those which succumb to disease within a few years. However, it is clear that traditional grading criteria perform less well in molecularly defined tumor cohorts due to higher homogeneity. Thus, the separation of grade 2 from grade 3 astrocytomas is controversial. This is mainly due to the fact that glioblastomas, IDH-wildtype, lacking the otherwise typical histological features of microvascular proliferation and/or necrosis, were formerly annotated as anaplastic astrocytomas WHO grade III. Removing these from respective tumor cohorts revealed astrocytomas, IDH-mutant of grades 2 and 3 to display a much smaller differences in survival than previously expected. Studies on grading of diffuse astrocytic gliomas were comprehensively reviewed elsewhere [28]. In fact, while some studies still found statically significant differences others did not [29–33]. In addition, several studies aiming at defining cut-off values e.g. for proliferation or mitotic activity failed or provided inconsistent results [31, 32, 34, 35]. Including homozygous CDKN2A and/or CDKN2B deletions as grading criteria will increase the fraction of grade 4 tumors but is not expected to substantially alter the decision making for borderline cases between grade 2 and 3. Therefore, room for further improvement remains. There are several additional molecular alterations known to occur in IDH-mutant gliomas during tumor progression: e.g. amplifications of CDK4, PDGFRA, MET, MYCN, homozygous deletions of RB1, deletions of 14q, mutations in PIK3R1 or PIC3CA as well as a higher global copy number variation load [30, 31, 36–41]. Determination of the potential individual impact of these features on prognosis is complex because many of these occur concomitantly. Their prognostic value remains not as well established as for homozygous CDKN2A and/or CDKN2B deletions and currently these alterations are not of relevance for WHO grading. Additional insights into the process of tumor progression and the development of therapeutic resistance as well as more data from large molecularly and clinically well characterized cohorts will be necessary to further improve classification and grading of IDH-mutant gliomas.
Author contributions
DR: wrote the manuscript and prepared the figures and tables.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors have not disclosed any funding.
Declarations
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Board WCoTE (2021) World Health Organization Classification of Tumours of the Central Nervous System. Lyon
- 2.Louis DN, Aldape K, Brat DJ, Capper D, Ellison DW, Hawkins C, Paulus W, Perry A, Reifenberger G, Figarella-Branger D, Wesseling P, Batchelor TT, Cairncross JG, Pfister SM, Rutkowski S, Weller M, Wick W, von Deimling A. Announcing cIMPACT-NOW: the consortium to inform molecular and practical approaches to CNS tumor taxonomy. Acta Neuropathol. 2017;133:1–3. doi: 10.1007/s00401-016-1646-x. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellision DW, Figarella-Branger D, Reifenberger G, von Deimling A. WHO classification and grading of tumours of the central nervous system. Lyon: IARC Press; International Agency for Research on Cancer; 2016. [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 6.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 8.Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, Hovestadt V, Bewerunge-Hudler M, Jones DT, Schittenhelm J, Mittelbronn M, Rushing E, Simon M, Westphal M, Unterberg A, Platten M, Paulus W, Reifenberger G, Tonn JC, Aldape K, Pfister SM, Korshunov A, Weller M, Herold-Mende C, Wick W, Brandner S, von Deimling A. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130:407–417. doi: 10.1007/s00401-015-1454-8. [DOI] [PubMed] [Google Scholar]
- 9.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O'Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O'Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T, Jr, Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Yang L, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG, Jr, Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, McLendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ, Zhang J. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V, Mittelbronn M, Schittenhelm J, Herold-Mende C, Unterberg A, Platten M, Weller M, Wick W, Pfister SM, von Deimling A. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129:133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 12.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, Kratz A, Wefers AK, Huang K, Pajtler KW, Schweizer L, Stichel D, Olar A, Engel NW, Lindenberg K, Harter PN, Braczynski AK, Plate KH, Dohmen H, Garvalov BK, Coras R, Holsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez FJ, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Bruck W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hanggi D, Hans V, Rozsnoki S, Hansford JR, Kohlhof P, Kristensen BW, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Muhleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo NG, Driever PH, Kramm CM, Muller HL, Rutkowski S, von Hoff K, Fruhwald MC, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu CM, Perry A, Jones C, Jacques TS, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins VP, Blumcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott PA, Paulus W, Gajjar A, Robinson GW, Taylor MD, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis MA, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schuller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison DW, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, von Deimling A, Pfister SM. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capper D, Stichel D, Sahm F, Jones DTW, Schrimpf D, Sill M, Schmid S, Hovestadt V, Reuss DE, Koelsche C, Reinhardt A, Wefers AK, Huang K, Sievers P, Ebrahimi A, Scholer A, Teichmann D, Koch A, Hanggi D, Unterberg A, Platten M, Wick W, Witt O, Milde T, Korshunov A, Pfister SM, von Deimling A. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018;136:181–210. doi: 10.1007/s00401-018-1879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis DN, Wesseling P, Paulus W, Giannini C, Batchelor TT, Cairncross JG, Capper D, Figarella-Branger D, Lopes MB, Wick W, van den Bent M. cIMPACT-NOW update 1: not otherwise specified (NOS) and not elsewhere classified (NEC) Acta Neuropathol. 2018;135:481–484. doi: 10.1007/s00401-018-1808-0. [DOI] [PubMed] [Google Scholar]
- 15.Zepeda-Mendoza CJ, Vaubel RA, Zarei S, Ida CM, Matthews M, Acree S, Raghunathan A, Giannini C, Jenkins RB. Concomitant 1p/19q co-deletion and IDH1/2, ATRX, and TP53 mutations within a single clone of "dual-genotype" IDH-mutant infiltrating gliomas. Acta Neuropathol. 2020;139:1105–1107. doi: 10.1007/s00401-020-02141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasrallah MLP, Desai A, O'Rourke DM, Surrey LF, Stein JM. A dual-genotype oligoastrocytoma with histologic, molecular, radiological and time-course features. Acta Neuropathol Commun. 2020;8:115. doi: 10.1186/s40478-020-00998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barresi V, Lionti S, Valori L, Gallina G, Caffo M, Rossi S. Dual-genotype diffuse low-grade glioma: is it really time to abandon oligoastrocytoma as a distinct entity? J Neuropathol Exp Neurol. 2017;76:342–346. doi: 10.1093/jnen/nlx024. [DOI] [PubMed] [Google Scholar]
- 18.Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, Holland EC, Jenkins RB, Kleinschmidt-DeMasters B, Komori T, Kros JM, Louis DN, McLean C, Perry A, Reifenberger G, Sarkar C, Stupp R, van den Bent MJ, von Deimling A, Weller M. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139:603–608. doi: 10.1007/s00401-020-02127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannini C, Scheithauer BW, Weaver AL, Burger PC, Kros JM, Mork S, Graeber MB, Bauserman S, Buckner JC, Burton J, Riepe R, Tazelaar HD, Nascimento AG, Crotty T, Keeney GL, Pernicone P, Altermatt H. Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol. 2001;60:248–262. doi: 10.1093/jnen/60.3.248. [DOI] [PubMed] [Google Scholar]
- 20.Figarella-Branger D, Mokhtari K, Dehais C, Jouvet A, Uro-Coste E, Colin C, Carpentier C, Forest F, Maurage CA, Vignaud JM, Polivka M, Lechapt-Zalcman E, Eimer S, Viennet G, Quintin-Roue I, Aubriot-Lorton MH, Diebold MD, Loussouarn D, Lacroix C, Rigau V, Laquerriere A, Vandenbos F, Michalak S, Sevestre H, Peoch M, Labrousse F, Christov C, Kemeny JL, Chenard MP, Chiforeanu D, Ducray F, Idbaih A, Network P. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro Oncol. 2014;16:1244–1254. doi: 10.1093/neuonc/nou047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figarella-Branger D, Mokhtari K, Dehais C, Carpentier C, Colin C, Jouvet A, Uro-Coste E, Forest F, Maurage CA, Vignaud JM, Polivka M, Lechapt-Zalcman E, Eimer S, Viennet G, Quintin-Roue I, Aubriot-Lorton MH, Diebold MD, Loussouarn D, Lacroix C, Rigau V, Laquerriere A, Vandenbos F, Michalak S, Sevestre H, Peoch M, Labrousse F, Christov C, Kemeny JL, Chenard MP, Chiforeanu D, Ducray F, Idbaih A, Delattre JY, Network P. Mitotic index, microvascular proliferation, and necrosis define 3 pathological subgroups of prognostic relevance among 1p/19q co-deleted anaplastic oligodendrogliomas. Neuro Oncol. 2016;18:888–890. doi: 10.1093/neuonc/now085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appay R, Dehais C, Maurage CA, Alentorn A, Carpentier C, Colin C, Ducray F, Escande F, Idbaih A, Kamoun A, Marie Y, Mokhtari K, Tabouret E, Trabelsi N, Uro-Coste E, Delattre JY, Figarella-Branger D, Network P. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019;21:1519–1528. doi: 10.1093/neuonc/noz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banan R, Stichel D, Bleck A, Hong B, Lehmann U, Suwala A, Reinhardt A, Schrimpf D, Buslei R, Stadelmann C, Ehlert K, Prinz M, Acker T, Schittenhelm J, Kaul D, Schweizer L, Capper D, Harter PN, Etminan N, Jones DTW, Pfister SM, Herold-Mende C, Wick W, Sahm F, von Deimling A, Hartmann C, Reuss DE. Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol. 2020;140:569–581. doi: 10.1007/s00401-020-02194-y. [DOI] [PubMed] [Google Scholar]
- 24.Dodgshun AJ, Fukuoka K, Edwards M, Bianchi VJ, Das A, Sexton-Oates A, Larouche V, Vanan MI, Lindhorst S, Yalon M, Mason G, Crooks B, Constantini S, Massimino M, Chiaravalli S, Ramdas J, Mason W, Ashraf S, Farah R, Van Damme A, Opocher E, Hamid SA, Ziegler DS, Samuel D, Cole KA, Tomboc P, Stearns D, Thomas GA, Lossos A, Sullivan M, Hansford JR, Mackay A, Jones C, Jones DTW, Ramaswamy V, Hawkins C, Bouffet E, Tabori U. Germline-driven replication repair-deficient high-grade gliomas exhibit unique hypomethylation patterns. Acta Neuropathol. 2020;140:765–776. doi: 10.1007/s00401-020-02209-8. [DOI] [PubMed] [Google Scholar]
- 25.Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, Cortes-Ciriano I, Birzu C, Geduldig JE, Pelton K, Lim-Fat MJ, Pal S, Ferrer-Luna R, Ramkissoon SH, Dubois F, Bellamy C, Currimjee N, Bonardi J, Qian K, Ho P, Malinowski S, Taquet L, Jones RE, Shetty A, Chow KH, Sharaf R, Pavlick D, Albacker LA, Younan N, Baldini C, Verreault M, Giry M, Guillerm E, Ammari S, Beuvon F, Mokhtari K, Alentorn A, Dehais C, Houillier C, Laigle-Donadey F, Psimaras D, Lee EQ, Nayak L, McFaline-Figueroa JR, Carpentier A, Cornu P, Capelle L, Mathon B, Barnholtz-Sloan JS, Chakravarti A, Bi WL, Chiocca EA, Fehnel KP, Alexandrescu S, Chi SN, Haas-Kogan D, Batchelor TT, Frampton GM, Alexander BM, Huang RY, Ligon AH, Coulet F, Delattre JY, Hoang-Xuan K, Meredith DM, Santagata S, Duval A, Sanson M, Cherniack AD, Wen PY, Reardon DA, Marabelle A, Park PJ, Idbaih A, Beroukhim R, Bandopadhayay P, Bielle F, Ligon KL. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580:517–523. doi: 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwala AK, Stichel D, Schrimpf D, Kloor M, Wefers AK, Reinhardt A, Maas SLN, Kratz CP, Schweizer L, Hasselblatt M, Snuderl M, Abedalthagafi MSJ, Abdullaev Z, Monoranu CM, Bergmann M, Pekrun A, Freyschlag C, Aronica E, Kramm CM, Hinz F, Sievers P, Korshunov A, Kool M, Pfister SM, Sturm D, Jones DTW, Wick W, Unterberg A, Hartmann C, Dodgshun A, Tabori U, Wesseling P, Sahm F, von Deimling A, Reuss DE. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol. 2021;141:85–100. doi: 10.1007/s00401-020-02243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suwala AK, Felix M, Friedel D, Stichel D, Schrimpf D, Hinz F, Hewer E, Schweizer L, Dohmen H, Pohl U, Staszewski O, Korshunov A, Stein M, Wongsurawat T, Cheunsuacchon P, Sathornsumetee S, Koelsche C, Turner C, Le Rhun E, Muhlebner A, Schucht P, Ozduman K, Ono T, Shimizu H, Prinz M, Acker T, Herold-Mende C, Kessler T, Wick W, Capper D, Wesseling P, Sahm F, von Deimling A, Hartmann C, Reuss DE. Oligosarcomas, IDH-mutant are distinct and aggressive. Acta Neuropathol. 2022;143:263–281. doi: 10.1007/s00401-021-02395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Deimling A, Ono T, Shirahata M, Louis DN. Grading of diffuse astrocytic gliomas: a review of studies before and after the advent of IDH testing. Semin Neurol. 2018;38:19–23. doi: 10.1055/s-0038-1636430. [DOI] [PubMed] [Google Scholar]
- 29.Franceschi E, Tosoni A, Bartolini S, Minichillo S, Mura A, Asioli S, Bartolini D, Gardiman M, Gessi M, Ghimenton C, Giangaspero F, Lanza G, Marucci G, Novello M, Silini EM, Zunarelli E, Paccapelo A, Brandes AA. Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur J Cancer. 2020;137:10–17. doi: 10.1016/j.ejca.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Cimino PJ, Zager M, McFerrin L, Wirsching HG, Bolouri H, Hentschel B, von Deimling A, Jones D, Reifenberger G, Weller M, Holland EC. Multidimensional scaling of diffuse gliomas: application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathol Commun. 2017;5:39. doi: 10.1186/s40478-017-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, Koelsche C, Wefers A, Reinhardt A, Huang K, Sievers P, Shimizu H, Nanjo H, Kobayashi Y, Miyake Y, Suzuki T, Adachi JI, Mishima K, Sasaki A, Nishikawa R, Bewerunge-Hudler M, Ryzhova M, Absalyamova O, Golanov A, Sinn P, Platten M, Jungk C, Winkler F, Wick A, Hanggi D, Unterberg A, Pfister SM, Jones DTW, van den Bent M, Hegi M, French P, Baumert BG, Stupp R, Gorlia T, Weller M, Capper D, Korshunov A, Herold-Mende C, Wick W, Louis DN, von Deimling A. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. doi: 10.1007/s00401-018-1849-4. [DOI] [PubMed] [Google Scholar]
- 32.Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, Armstrong TS, Sulman EP, Cahill DP, Vera-Bolanos E, Yuan Y, Reijneveld JC, Ylstra B, Wesseling P, Aldape KD. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, Sahm F, Koelsche C, Korshunov A, Olar A, Hartmann C, Reijneveld JC, Wesseling P, Unterberg A, Platten M, Wick W, Herold-Mende C, Aldape K, von Deimling A. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129:867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoda RA, Marxen T, Longo L, Ene C, Wirsching HG, Keene CD, Holland EC, Cimino PJ. Mitotic index thresholds do not predict clinical outcome for IDH-mutant astrocytoma. J Neuropathol Exp Neurol. 2019;78:1002–1010. doi: 10.1093/jnen/nlz082. [DOI] [PubMed] [Google Scholar]
- 35.Duregon E, Bertero L, Pittaro A, Soffietti R, Ruda R, Trevisan M, Papotti M, Ventura L, Senetta R, Cassoni P. Ki-67 proliferation index but not mitotic thresholds integrates the molecular prognostic stratification of lower grade gliomas. Oncotarget. 2016;7:21190–21198. doi: 10.18632/oncotarget.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang RR, Shi ZF, Zhang ZY, Chan AK, Aibaidula A, Wang WW, Kwan JSH, Poon WS, Chen H, Li WC, Chung NY, Punchhi G, Chu WC, Chan IS, Liu XZ, Mao Y, Li KK, Ng HK. IDH mutant lower grade (WHO Grades II/III) astrocytomas can be stratified for risk by CDKN2A, CDK4 and PDGFRA copy number alterations. Brain Pathol. 2020;30:541–553. doi: 10.1111/bpa.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T, Motomura K, Ohka F, Shiina S, Yamamoto T, Nagata Y, Yoshizato T, Mizoguchi M, Abe T, Momii Y, Muragaki Y, Watanabe R, Ito I, Sanada M, Yajima H, Morita N, Takeuchi I, Miyano S, Wakabayashi T, Ogawa S, Natsume A. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20:66–77. doi: 10.1093/neuonc/nox132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips JJ, Aranda D, Ellison DW, Judkins AR, Croul SE, Brat DJ, Ligon KL, Horbinski C, Venneti S, Zadeh G, Santi M, Zhou S, Appin CL, Sioletic S, Sullivan LM, Martinez-Lage M, Robinson AE, Yong WH, Cloughesy T, Lai A, Phillips HS, Marshall R, Mueller S, Haas-Kogan DA, Molinaro AM, Perry A. PDGFRA amplification is common in pediatric and adult high-grade astrocytomas and identifies a poor prognostic group in IDH1 mutant glioblastoma. Brain Pathol. 2013;23:565–573. doi: 10.1111/bpa.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li KK, Shi ZF, Malta TM, Chan AK, Cheng S, Kwan JSH, Yang RR, Poon WS, Mao Y, Noushmehr H, Chen H, Ng HK. Identification of subsets of IDH-mutant glioblastomas with distinct epigenetic and copy number alterations and stratified clinical risks. Neurooncol Adv. 2019;1:vdz015. doi: 10.1093/noajnl/vdz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cimino PJ, Holland EC. Targeted copy number analysis outperforms histologic grading in predicting patient survival for WHO grades II/III IDH-mutant astrocytomas. Neuro Oncol. 2019;21:819–821. doi: 10.1093/neuonc/noz052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakimoto H, Tanaka S, Curry WT, Loebel F, Zhao D, Tateishi K, Chen J, Klofas LK, Lelic N, Kim JC, Dias-Santagata D, Ellisen LW, Borger DR, Fendt SM, Vander Heiden MG, Batchelor TT, Iafrate AJ, Cahill DP, Chi AS. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20:2898–2909. doi: 10.1158/1078-0432.CCR-13-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]