Abstract
Background:
Children with in-utero Zika virus (ZIKV) exposure without congenital Zika syndrome (CZS) are at risk for abnormal neurodevelopment. Preschool-age outcomes for children with antenatal ZIKV exposure have not yet been established.
Methods:
Children with in-utero ZIKV exposure and nonexposed controls had neurodevelopmental evaluations at age 3–5 years in Sabanalarga, Colombia. Cases did not have CZS and were previously evaluated prenatally through age 18 months. Controls were born before ZIKV arrival to Colombia. Neurodevelopmental assessments included Pediatric Evaluation of Disability Inventory (PEDI-CAT), Behavior Rating Inventory of Executive Function (BRIEF-P), Bracken School Readiness Assessment (BSRA), and Movement Assessment Battery for Children (MABC). Family demographics and child medical history were recorded.
Results:
Fifty-five ZIKV-exposed children were evaluated at mean age 3.6 years and 70 controls were evaluated at 5.2 years. Family demographics were similar between groups. BRIEF-P t-scores were higher for cases than controls in shift and flexibility domains. Cases had lower PEDI-CAT mobility t-scores compared to controls. There was no difference in MABC between groups. In 11% of cases and 1% of controls, parents reported child mood problems.
Conclusions:
Children with in-utero ZIKV exposure without CZS may demonstrate emerging differences in executive function, mood, and adaptive mobility that require continued evaluation.
Introduction
Children exposed to the Zika virus (ZIKV) in utero during the ZIKV epidemic, have aged into early childhood. Congenital Zika Syndrome (CZS) describes the severe phenotype of infants with multiple neurological abnormalities resulting in complex ongoing medical needs.1–3 Although many children with antenatal ZIKV exposure do not have CZS at birth,4 early neurodevelopmental outcome studies suggest a risk for neurodevelopmental delays in the first two years of life.5,6
There is a need to understand the neurologic and neurodevelopmental outcomes in children with the full spectrum of congenital ZIKV exposure. Several studies demonstrated lower-than-expected developmental performance in motor and language domains, highlighting the need for follow-up in these areas.5,6 Due to uncertainty regarding neurodevelopment in ZIKV exposed infants, the Centers for Disease Control and Prevention (CDC) recommended routine follow-up through early childhood.7 Since the ZIKV epidemic occurred relatively recently from 2015–2017, long-term child outcome data have not yet been documented. Thus, the international healthcare community remains on a steep learning curve as many critical questions about ZIKV and its long-term consequences remain unanswered.
In 2016, we developed an international collaboration to evaluate ZIKV-infected pregnant women with serial prenatal imaging.8 Infants from this well-characterized Colombian cohort were seen for early neurodevelopmental evaluations.5 Building upon our prior study that found lower-than-expected multi-domain assessment scores in the children to age 18 months, we sought to determine outcomes prior to school entry. The objectives of this study were to determine whether children exposed to ZIKV in-utero who do not have CZS have abnormal neurodevelopmental outcomes at preschool age. We hypothesized that the ZIKV-exposed children would have lower multi-domain developmental assessments compared to non-ZIKV exposed controls.
Methods
Participants
We performed a prospective cohort study of ZIKV-exposed children and non-ZIKV exposed control children in Atlántico Department, Colombia. Seventy children with antenatal ZIKV exposure without CZS from our longitudinal cohort established in pregnancy were eligible and aged three to four years.8 Children were normocephalic at birth, had normal sequential fetal ultrasonography and magnetic resonance imaging examinations, no clinical findings of CZS at birth, and at least one neurodevelopmental evaluation up to 18 months of age as part of an early outcome study.5,8 Their mothers had symptomatic ZIKV infection in pregnancy, resided in an area of endemic ZIKV transmission, and had laboratory confirmation of ZIKV, thus meeting the CDC clinical criteria for probable ZIKV infection.5,8
We prospectively enrolled 70, four- and five-year-old children from Atlántico Department, Colombia without antenatal ZIKV-exposure as controls. All controls were born prior to April 1, 2016. The eligibility birth date was selected to enroll controls close in age to exposed cases but born before emergence of ZIKV circulation in Colombia, ruling out possibility of exposure to asymptomatic maternal ZIKV infection during at least the first half of gestation. Children were excluded as a control if their mother reported an infectious disease during pregnancy (i.e., Zika, Dengue, Chikungunya), if they had a chronic medical condition, history of seizures, abnormal hearing or vision not corrected by lenses, developmental concerns expressed by their caregiver, received therapy for developmental delays, had a behavioral or psychological condition, or were born preterm (≤36 weeks).
This study received approval from the Children’s National Hospital Institutional Review Board, Washington, D.C. and the Institutional Review Committee and Independent Committee on Research Ethics (CIRCIE), Barranquilla, Colombia. Women provided written informed consent for their child’s study participation. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This study is registered on ClinicalTrials.gov, NCT04398901.
Setting
The ZIKV-exposed children were recruited by in-person contact at their home or by phone and live in or near Sabanalarga in the Atlántico Department, Colombia (Figure 1). Children were scheduled for visits between December 3, 2020, and February 18, 2021. Control children were recruited by door-to-door meetings with families in Sabanalarga by research staff. In some cases, twins or similarly aged cousins were enrolled. Visits for controls occurred between January 28, 2021, and February 18, 2021. The visits occurred at a local school due internet connection and availability of adequate space for activities.
Figure. 1. Colombia Zika Child Outcomes Study Process.
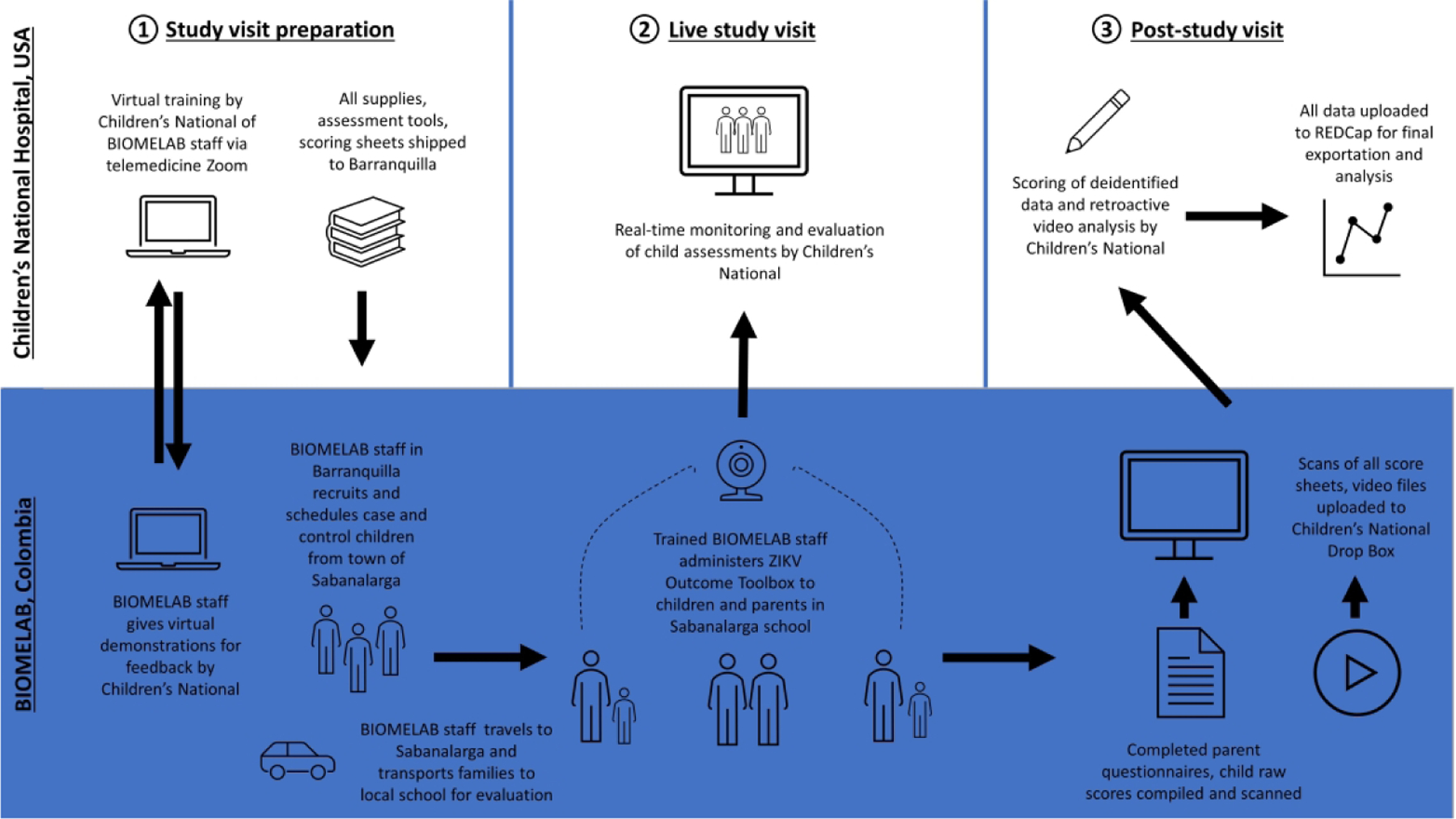
We harnessed novel telemedicine advancements to allow for remote coordination of neurodevelopmental evaluations. Live video and telehealth Zoom allowed for the U.S. team to train Colombian research staff in Barranquilla, Colombia, as well as monitor and score assessments of children on-site in Sabanalarga, Colombia.
ZIKV Outcome Toolbox
Neurodevelopment was assessed by the ZIKV Outcome Toolbox (Table 1) containing items selected specifically for this study based on the multiple domains assessed, availability in Spanish, and possibility of observational assessments of child motor function. The Toolbox includes parental questionnaires and standardized child evaluations. The Pediatric Evaluation of Disability Inventory (PEDI-CAT) is a computer-based questionnaire that measures the functional domains of daily activities, mobility, social-cognitive, and responsibility (Pearson Q-global).9 The Behavior Rating Inventory of Executive Function (BRIEF-P) is a questionnaire to assess executive function (PAR, Inc.).10,11 Parents also completed the Parenting Stress Index (PSI-4) (PAR, Inc.), questionnaires on the family and home environment and their child’s medical history. Socioeconomic status was evaluated by parental occupation and education level. Mothers of children with ZIKV-exposure were queried about feelings of stigmatization due to a diagnosis of ZIKV in pregnancy. A research coordinator read the questions to the parent when needed based on literacy level.
Table 1:
ZIKV Outcome Toolbox
| ZIKV Outcome Toolbox item | Assessment type | Domains assessed | Time (min) | Special features |
|---|---|---|---|---|
| Socioeconomic, Medical History | Parent questionnaire | Medical health, environment, safety | 10 | Completed with help of trained team member |
| PEDI-CAT | Parent questionnaire | Daily activities Mobility Social-cognitive Responsibility | 10 | Computerized questionnaire completed with help of trained team member |
| BRIEF-P | Parent questionnaire | Executive function | 10 | Completed with help of trained team member |
| PSI-4 | Parent questionnaire | Parental stress | 10 | Completed with help of trained team member |
| BSRA-3 | In-person child assessment | School readiness | 15 | Telehealth live monitored and video-recorded |
| MABC-2 | In-person child assessment | Fine and gross motor | 20 | Telehealth live monitored and video-recorded |
Abbreviations: BRIEF-P: Behavior Rating Inventory of Executive Function – Preschool; BSRA-3: Bracken School Readiness Assessment – Third Edition; PEDI-CAT: Pediatric Evaluation of Disability Inventory Computer Adaptive Test; PSI-4: Parenting Stress Index – Fourth Edition; MABC-2: Movement Assessment Battery for Children – Second Edition
Children were evaluated by the Bracken School Readiness Assessment (BSRA) and the Movement Assessment Battery for Children-Second Edition (MABC) (Pearson). The BSRA measures a child’s knowledge of five areas: colors, letters, numbers/counting, sizes/comparison, and shapes. The MABC evaluates manual dexterity, aiming and catching, and balance. For the MABC manual dexterity items of posting coins and stringing beads, six coins and beads were used for all children. Some children refused to complete all or some parts of the assessments, in which case evaluations were not scoreable.
Child weight, height, and head circumference were measured, and body mass index (BMI) was calculated.
Telehealth Training and Observation of Child Activities
Due to the COVID-19 pandemic and inability for the U.S. study team to travel to Colombia, training was completed by Zoom telehealth (Figure 1).12 The Colombian team demonstrated learned skills by live telemedicine connection with similar aged children of their own. Telehealth connections were used during study visits for live observation of child activities and remote recording (Figure 1). Two laptops with telehealth connections captured child performance: one capturing BSRA and seated MABC activities, and the other standing MABC activities. Both laptops directly recorded video to hard drives. The videos were used for MABC and BSRA scoring, and observational assessments.
Statistical Analysis
Data were entered into a Research Electronic Data Capture (REDCAP) database.13 Pearson’s Q-global reported PEDI-CAT scores based on age. The BRIEF-P, PSI-4, and MABC scores were scaled for age per test manuals. As MABC manual dexterity standard scores reflect child performance using 12 coins/beads at age 5, local norms were developed for the use of 6 coins/beads irrespective of age. Z-scores were generated from control data of 5-year-olds for “posting coins” and “threading beads”. These Z-scores were then attached to a standard score based on test manual norming. For BRIEF-P, a t-score of ≥65 was considered clinically significant. BSRA results were reported using raw scores only due to lack of Spanish edition norms. Scores were compared using Mann-Whitney or t-test depending on data’s normality, which was determined by the Kolmogorov-Smirnov test. For significant P-values, 95% confidence intervals were reported using Hodges-Lehmann method. Multiple regression evaluated associations between PEDI-CAT Mobility, BRIEF-P Shift, and BSRA Sizes and Comparison scores for cases with gestational age at ZIKV exposure, BMI, and sex. For controls, multiple regression evaluated for associations between PEDI-CAT Mobility, BRIEF-P Shift, and BSRA Sizes and Comparison scores with BMI, sex, maternal education, oldest child, and number of children in the home. Data were analyzed using SAS for Windows version 9.4 (SAS Institute Inc., Cary, NC). Two-sided test with a significance level of .05 was used.
Results
Demographics:
Fifty-five of 70 (79%) children with in utero ZIKV exposure (cases) were evaluated at a mean (SD) of 3.6 (0.4) years of age (Table 2). Fifteen cases (21%) declined participation due to COVID-19 concerns, moved out of the region, or could not be contacted. Their mothers had ZIKV during the first and second trimesters of pregnancy, with fetal ZIKV exposure at a mean of 7.9 (4.3) gestational weeks. Seventy controls without ZIKV exposure were evaluated at 5.2 (0.2) years of age.
Table 2:
Demographic, Socioeconomic, and Health Characteristics of ZIKV-exposed Children and Non-Exposed Controls
| Characteristic | ZIKV-Exposed Children (N=55) | Non-Exposed Controls (N=70) | P-value |
|---|---|---|---|
| Male sex (n [%]) | 24 (44) | 34 (49) | .594a |
| Years of age at visit (median [IQR]) | 3.8 (0.7) | 5.2 (0.4) | <.001b |
| BMI (mean [SD]) | |||
| Male | 17.0 (2.9) | 14.8 (1.1) | .002c |
| Female | 15.4 (1.7) | 15.3 (1.4) | .635c |
| Education-level mother (n [%]) | .858a | ||
| All or some primary | 5 (9) | 8 (11) | |
| All or some secondary | 41 (75) | ;48 (69) | |
| Technical school | 8 (15) | 10 (14) | |
| None | 1 (2) | 2 (3) | |
| University | 0 (0) | 0 (0) | |
| NR | 0 (0) | 2 (3) | |
| Education-level father (n [%]) | .386a | ||
| Primary school | 7 (13) | 15 (21) | |
| All or some secondary | 40 (73) | 42 (60) | |
| Technical school | 3 (6) | 3 (4) | |
| University | 0 (0) | 3 (4) | |
| None | 2 (4) | 1 (1) | |
| NR | 3 (6) | 6 (9) | |
| Most common occupation types (n) | |||
| Type 1 | Shopkeeper (12) | Taxi driver (15) | |
| Type 2 | Construction worker (8) | Various trades (15) | |
| Type 3 | Various trades (7) | Construction worker (7) | |
| Type 4 | Taxi driver (4) | Shopkeeper (4) | |
| Type 5 | Fisherman (4) | Independent worker (4) | |
| Principal sustainer (n [%]) | .537a | ||
| Mother | 5 (9) | 6 (9) | |
| Father | 40 (73) | 57 (81) | |
| Both | 2 (4) | 2 (3) | |
| Other or NR | 7 (15) | 5 (7) | |
| Child education (n [%])d | |||
| Attend daycare | 33 (60) | ||
| Attend primary school | 59 (84) | ||
| Number of children in home (median, IQR) (n=55, 69)e | 2 (1) | 3 (2) | .215b |
| Participant oldest child in home (n [%]) (n=54, 68) | 19 (35) | 25 (36) | 1a |
| Child medical history (n [%]) | |||
| Parent worried about child’s health (n=55, 65) | 12 (22) | 5 (7) | .036a |
| Vision problem (n=53, 70) | 3 (6) | ;0 (0) | .077a |
| Hearing problem (n=54, 70) | 1 (2) | 2 (3) | 1a |
| Growth problem (n=54,70) | 2 (4) | 0 (0) | .181a |
| Received therapy (n=55, 69) | 2 (4) | 1 (1) | .584a |
| Behavior problems (n=55, 67) | 4 (7) | 2 (3) | .408a |
| Mood problems (n=54, 68) | 6 (11) | 1 (1) | .043a |
Fisher’s Exact Test
Mann-Whitney U test
Student’s t-test
Sabanalarga schools using remote learning modality at the time of assessments due to the COVID-19 pandemic
sample size for individual items indicated as (n = [n ZIKV-exposed], [n controls])
Abbreviations: BMI-Body mass index; NR-No response
Questionnaires:
Most mothers (50/55, 91%) with ZIKV during pregnancy reported that worry about ZIKV did not affect interactions with their child. During pregnancy, 13 (24%) mothers said they felt different than other pregnant people due to their ZIKV diagnosis. Twelve (22%) parents of ZIKV-exposed children reported worry about their child’s health compared to 5 (7%) parents of control children, (95% CI 1.4 to 26.8, P = .03). In six (11%) cases and one (1%) control, parents reported child mood problems (95% CI 0.8 to 18.5, P = .02). There was higher report of child vision problems in cases than controls, 6% vs. 0%, respectively, (95% CI −0.6 to 11.9, P = .08) and no difference in receipt of rehabilitation therapy. Thirty-three (60%) of the ZIKV-exposed children (all < 5 years of age) were reported to be attending daycare with the remaining cared for by family members or friends. Of control participants, 59 (84%) had begun primary school at the time of their evaluation.
The results of the ZIKV Outcome Toolbox questionnaires are in Table 3. The PEDI-CAT t-scores for cases were lower in mobility (P < .001) and responsibility (P < .001) reflecting lower reported child function in these domains, but higher in social/cognitive (P = .021) and daily activities (P < .001) compared to controls. The BRIEF-P t-scores were higher for cases than controls in shift (95% CI 0 to 8, P = .049) and flexibility (95% CI 0 to 9, P = .047), but there was no difference for percent clinically elevated (t-score ≥65). The PSI-4 score overall (95% CI 0 to 20, P = .043) and difficult child domain (95% CI 0 to 20, P = .029) were elevated for cases over controls.
Table 3:
ZIKV Outcome Toolbox Parental Questionnaires: BRIEF-P, PEDI-CAT, PSI
| Assessment | ZIKV-exposed children (N=55) | Non-exposed controls (N=70) | P-value |
|---|---|---|---|
| BRIEF-P t-score (mean [SD])/(median)[IQR] | |||
| Inhibit | 57.4 (12.0) | 56.0 (11.7) | .511a |
| Shift | 52.0 (18) | 46.0 (15) | .049b |
| Emotional control | 51.0 (15.5) | 46.0 (17) | .165b |
| Working memory | 54.1 (14.6) | 55.0 (10.5) | .689a |
| Plan/organize | 51.8 (14.5) | 52.3 (11.1) | .849a |
| ISCI | 55.2 (13.6) | 53.6 (11.5) | .186a |
| FI | 51(14.5) | 45 (18) | .047b |
| EMI | 51(17) | 48 (17.3) | .624b |
| GEC | 56(19.5) | 50 (20) | .230b |
| PEDI-CAT t-score (median [IQR]) | |||
| Daily activities | 47 (7) | 42 (7) | <.001b |
| Mobility | 48 (12) | 77 (1) | <.001b |
| Social/cognitive | 46 (12) | 38 (14) | 0.021b |
| Responsibility | 15 (29) | 60 (37) | <.001b |
| PSI (median [IQR]) | |||
| Parental distress | 46 (52) | 32 (42) | .085b |
| Parent-child dysfunctional interaction | 32 (44) | 28 (36) | .120b |
| Difficult Child | 34 (44) | 26 (32) | .029b |
| Total Score | 40 (50) | 28 (36) | .043b |
Student’s t-test (P < 0.05)
Mann-Whitney U test (P < 0.05)
Abbreviations: BRIEF-P: Behavior Rating Inventory of Executive Function – Preschool; EMI: Emergent Metacognition Index; FI: Flexibility Index; GEC: Global Executive Composite; IQR: Interquartile Range; ISCI: Inhibitory Self-Control Index; PEDI-CAT: Pediatric Evaluation of Disability Inventory – Computerized Adaptive Test; PSI: Parenting Stress Index; SD: Standard deviation
Child evaluations (Table 4):
Table 4.
ZIKV Outcome Toolbox Child Assessments: MABC-2 and BSRA-3
| Assessment | ZIKV-exposed children (N=55) | Non-exposed controls (N=70) | P-value |
|---|---|---|---|
| MABC standard score (mean [SD])/(median [IQR])d | |||
| Manual Dexterity (n = 43, 69)a | 22.8 (7.8) | 24.3 (6.5) | .282b |
| Aiming and Catching (n=41, 69) | 20.6 (6.3) | 20.7 (4.8) | .951b |
| Balance (n= 36, 66) | 31.0 (9.8) | 32.5 (10) | .275c |
| Overall Score (n= 32, 65) | 75 (15.4) | 75 (14.8) | .992b |
| BSRA raw score (mean [SD]) (n=50, 69) | |||
| Colors (of 10) | 2.5 (3) | 6.2 (4) | -- |
| Letters (of 15) | 1.2 (2) | 1.5 (2.4) | -- |
| Numbers & Counting (of 18) | 1.2 (2.3) | 4.7 (5.5) | -- |
| Sizes & Comparisons (of 22) | 5.0 (3.4) | 7.4 (3.8) | -- |
| Shapes (of 20) | 3.9 (2.5) | 5.9 (3.1) | -- |
| Total (of 85) | 13.9 (9.8) | 25.8 (13.9) | -- |
MABC scorable sample size for each assessment indicated as (n = [n ZIKV-exposed], [n controls])
Student’s t-test (p <0.05)
Mann-Whitney U test (p <0.05)
nonparametric data represented as (median [IQR])
Abbreviations: BSRA: Bracken School Readiness Assessment; MABC: Movement Assessment Battery for Children
Twenty-three cases and five controls did not have MABC scores due to refusal of some or all MABC sections. Having a refusal was associated with younger age (95% CI 0.12 to 0.51 years, P = .002). Among the 32 cases and 65 controls who completed the MABC, there was no difference in manual dexterity, aiming and catching, balance, or total age-adjusted standard scores. By MABC score zones of green, amber, and red, the cases had 30 (94%), 0 (0%), and 2 (6%) children in each zone and the controls had 50 (77%), 6 (9%), and 9 (14%) children in each zone, respectively, and this distribution was not different between cases and controls (Fisher Exact P = .09). The BSRA scores reflect low mastery of school readiness concepts for cases and controls (Table 4).
There was no association between gestational age of ZIKV-exposure, sex, and BMI with the outcomes of PEDI-CAT Mobility, BRIEF-P Shift, and BSRA Sizes and Comparison scores by multiple regression. For the controls, the number of children in the home was associated with the BRIEF-P shift score (P = .045), but maternal education level, sex, BMI, and being the oldest child in the home were not associated with the PEDI-CAT Mobility, BRIEF-P Shift, and BSRA Sizes and Comparison scores.
Discussion
This study provides continued prospective longitudinal neurodevelopmental assessments of a well-characterized cohort of children with confirmed antenatal ZIKV exposure, without CZS at birth. These children showed overall progress in neurodevelopmental skills at preschool age and appear to be doing well. However, we detected some differences in developmental domains that require continued evaluation. Follow-up was achieved in 79% of children since the prenatal period, representing one of the longest longitudinal follow-up cohorts of ZIKV-exposed children worldwide.5,8 Compared to controls, children with antenatal ZIKV exposure showed differences in areas of executive function and parental report of mobility, responsibility, and temperament. Direct evaluation of motor skills, however, showed comparable case and control performance. While parents of ZIKV-exposed children expressed worry over their child’s health, reported child medical conditions mostly reflected common childhood conditions and injuries. The findings provide insight into neurodevelopment of children with antenatal ZIKV exposure who do not have the severe sequelae of CZS, and can guide child neurodevelopmental follow-up into school-age.
Our study is enhanced by a non-ZIKV exposed control cohort of children from the same Colombian community. Parental occupations for cases and controls were primarily in manual labor positions and there was a similar range of educational attainment, with many parents having incomplete schooling. Attention to sociodemographic similarity between cases and controls is important as home and neighborhood environments influence child development.14,15 Many studies of outcomes for infants and children with ZIKV exposure are limited by the lack of a control cohort,6,16–22 including our prior studies.5,8 During an infectious epidemic, especially when infections may be asymptomatic and laboratory diagnosis complex, the ability to identify non-exposed controls can be difficult. Any child born in Colombia during ZIKV endemicity could be ZIKV-exposed, so we included only controls with older birthdates. Controls were a mean of 1.4 years older than cases, and direct comparison of raw scores will only be possible when the cases reach the same age. However, many assessments were scaled for age, thus limiting the effect of age on our results, and highlighting our priority of selecting non-exposed over same-aged controls.
The benefits of the ZIKV Outcome Toolbox used in this study include that it can be performed in under one hour and yield results in multiple developmental domains, it is cost-effective, does not require extensive experience, and can be used in a community low-resource setting. The Toolbox worked well but required assistance of the research team for questionnaire completion since some parents had low literacy. A major advantage of the Toolbox was that the child BSRA and MABC evaluations could be recorded and livestreamed via Zoom Telehealth connections with the U.S. research team. In the future, it may be better to only include the BSRA for children aged four or older, as young age likely influenced refusals to sit for the assessment.
Executive function is vulnerable to early life insults due to the protracted window of development of the fronto-striatal brain region,23 and encompasses working memory, planning, and organization, which are skills for academic and life success.11 At preschool-age, executive function begins to emerge. While we found higher BRIEF-P scores for the domains of shift and flexibility for cases compared to controls, these differences were not in the clinically elevated range. As children are still relatively young, evaluation at older ages is necessary. Interestingly, parents of cases reported higher child mood problems, also reflected by the PSI-4. These elements of lower executive function skills, mood and behavior problems seem to correspond with lower responsibility scores on PEDI-CAT among ZIKV-exposed cases.
Assessments of motor function have demonstrated lower scores for ZIKV-exposed children in prior studies and is an area of vulnerability due to the long maturational time course of the motor pathways.6,20,22,24 Abnormality of movement in infants with in utero ZIKV exposure may predict two-year cognitive, language, and motor outcomes.24 In our ZIKV-exposed cases, we previously showed lower mobility scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) with increasing age from 6 to 20 months, demonstrating specific concern about future neuromotor development.5 In this study we evaluated child mobility using the PEDI-CAT and MABC.5 The PEDI-CAT mobility scores were lower for cases than controls, but the average t-score was normal for both. This difference in parental reported mobility between cases and controls may reflect either lower adaptive motor skills for cases or may represent a limitation of using PEDI-CAT for inter-age comparison. Not all children completed the MABC assessment due to refusal of all or some of the test, therefore our findings may overestimate motor abilities by scoring only those who completed the evaluation. However, for those scored, the fine and gross motor MABC scores provide some reassurance that ZIKV-exposed children are progressing similarly to their neighborhood peers.
Colombian children’s exposure to early school concepts is variable and depends upon the home environment and teaching by caregivers. We found that knowledge of school readiness concepts was low in the ZIKV-exposed cases; this is likely multifactorial and cannot be ascribed to ZIKV-exposure at this time. The children have been mostly at home for the past year due to the COVID-19 pandemic and quarantines within their community. The controls, who are older, had higher knowledge of colors, numbers, and letters, likely due to more time for educational experiences. Direct comparison of raw scores on the BSRA for cases at a similar age to controls, will be necessary to understand any difference in attainment of school readiness skills.
We found that parents of the ZIKV-exposed children reported higher difficulty in parenting. The parents of both groups completed the PSI-4 questionnaire at a similar period of the SARS CoV-2 pandemic which maybe a time of increased stress for families.26 Whether the results reflect higher stress and difficulty in parenting 3–4-year-old children, the cases, versus 4–5-year-old children, the controls, or reflects more difficult children among the ZIKV-exposed, is not known at this time.
The study has several limitations. Some children did not complete all assessments and were unable to be scored. Whether the behavior of refusal for assessments represents a difference in executive function skills, self-esteem, shyness, or lack of knowledge will require future evaluations. The study did not collect current education-related effects of the SARS CoV-2 pandemic. The impact of reduced preschool experiences due to the pandemic should be considered in the context of the study results.25 As controls were older than case participants, investigators were not blinded during the child evaluations. It is unclear whether the impact of lost schooling and community closures due to the pandemic had a greater influence on one age group over the other. In addition, the exclusion criteria for controls were defined in order to preclude enrollment of children with known developmental delays so the control cohort may not be representative of the population as a whole. Additionally, it should be noted that parent-rating forms may be influenced by the parents’ perceptions or increased worry about their child’s development, due to inability to be blinded to ZIKV exposure status. In the PEDI-CAT, scores for cases are higher than those for controls in some domains. This fact may reflect an inability to compare scores between age groups, or parent reporting bias. As such, further study to allow for direct age comparison is necessary.
Conclusion
Children with in-utero ZIKV exposure without CZS appear to be making progress in neurodevelopmental skills. These children may need additional support for early executive function skills and school readiness concepts as they prepare to enter school. Longitudinal follow-up upon school entry along with more comprehensive neuropsychological assessments to evaluate cognitive and emotional development can help determine the long-term special needs of children born during the ZIKV epidemic.
Impact.
Preschool neurodevelopmental outcome in children with in-utero Zika-virus exposure is not yet known, since the Zika virus epidemic occurred in 2015–2017 and these children are only now entering school age.
This study finds that Colombian children with in-utero Zika virus exposure without congenital Zika syndrome are overall developing well, but may have emerging differences in executive function, behavior and mood, and adaptive mobility compared to children without in-utero Zika virus exposure.
Children with in-utero Zika virus exposure require continued multi-domain longitudinal neurodevelopmental evaluation through school age.
Funding:
This work was supported by the Thrasher Research Fund. SBM has additional funding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD102445, and a contract with the U.S. Centers for Disease Control and Prevention, for work on Zika virus separate from this study. CP receives support from National Center for Advancing Translational Sciences, Grant KL2TR001424.
Footnotes
Competing Interests: The authors have no competing interests relevant to this article to disclose.
Consent Statement: All participating families provided written informed consent in their native language and could decline participation in the study at any time.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Alves LV, Paredes CE, Silva GC, Mello JG, Alves JG. Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: a case series study. BMJ Open 2018;8(7):e021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo AS, et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol 2016;73(12):1407–1416. [DOI] [PubMed] [Google Scholar]
- 3.Moore CA, et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr 2017;171(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoen B, et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N Engl J Med 2018;378(11):985–994. [DOI] [PubMed] [Google Scholar]
- 5.Mulkey SB, et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr 2020;174(3):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen-Saines K, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019;25(8):1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adebanjo T, et al. Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection - United States, October 2017. MMWR Morb Mortal Wkly Rep 2017;66(41):1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulkey SB, et al. Sequential Neuroimaging of the Fetus and Newborn With In Utero Zika Virus Exposure. JAMA Pediatr 2019;173(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haley SM, et al. Accuracy and precision of the Pediatric Evaluation of Disability Inventory computer-adaptive tests (PEDI-CAT). Dev Med Child Neurol 2011;53(12):1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia Fernandez T, Gonzalez-Pienda JA, Rodriguez Perez C, Alvarez Garcia D, Alvarez Perez L. Psychometric characteristics of the BRIEF scale for the assessment of executive functions in Spanish clinical population. Psicothema 2014;26(1):47–52. [DOI] [PubMed] [Google Scholar]
- 11.Krivitzky LS, Walsh KS, Fisher EL, Berl MM. Executive functioning profiles from the BRIEF across pediatric medical disorders: Age and diagnosis factors. Child Neuropsychol 2016;22(7):870–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulkey SB, et al. Harnessing the power of telemedicine to accomplish international pediatric outcome research during the COVID-19 pandemic. J Telemed Telecare 2021, Dec 28. doi: 10.1177/1357633X211063166. [DOI] [PMC free article] [PubMed]
- 13.Harris PA, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci 2009;13(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strickhouser JE, Sutin AR. Family and neighborhood socioeconomic status and temperament development from childhood to adolescence. J Pers 2020;88(3):515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abtibol-Bernardino MR, et al. Neurological Findings in Children without Congenital Microcephaly Exposed to Zika Virus in Utero: A Case Series Study. Viruses 2020;12(11). [DOI] [PMC free article] [PubMed]
- 17.Cavalcante TB, et al. Congenital Zika syndrome: Growth, clinical, and motor development outcomes up to 36 months of age and differences according to microcephaly at birth. Int J Infect Dis 2021;105:399–408. [DOI] [PubMed] [Google Scholar]
- 18.Cranston JS, et al. Association Between Antenatal Exposure to Zika Virus and Anatomical and Neurodevelopmental Abnormalities in Children. JAMA Netw Open 2020;3(7):e209303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faical AV, et al. Neurodevelopmental delay in normocephalic children with in utero exposure to Zika virus. BMJ Paediatr Open 2019;3(1):e000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes Moreira ME, et al. Neurodevelopment in Infants Exposed to Zika Virus In Utero. N Engl J Med 2018;379(24):2377–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marban-Castro E, et al. Zika virus infection in pregnant travellers and impact on childhood neurodevelopment in the first two years of life: A prospective observational study. Travel Med Infect Dis 2021;40:101985. [DOI] [PubMed] [Google Scholar]
- 22.Pecanha PM, et al. Neurodevelopment of children exposed intra-uterus by Zika virus: A case series. PLoS One 2020;15(2):e0229434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makris N, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex 2007;17(6):1364–1375. [DOI] [PubMed] [Google Scholar]
- 24.Einspieler C, et al. Association of Infants Exposed to Prenatal Zika Virus Infection With Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw Open 2019;2(1):e187235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grupo Banco Mundial. Impactos de la Crisis del COVID-19 en la Educación y Respuestas de Política en Colombia [Impacts of the COVID-19 Crisis on Education and Policy Responses in Colombia] World Bank. July 24, 2020.. Accessed November 16, 2021. https://thedocs.worldbank.org/en/doc/641601599665038137-0090022020/original/ColombiaCOVIDeducationfinal.pdf
- 26.Brown SM, Doom JR, Lechuga-Pena S, Watamura SE, Koppels T. Stress and parenting during the global COVID-19 pandemic. Child Abuse Negl 2020;110(Pt 2):104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


