Abstract
Key Clinical Message
The prognosis of patients with relapsed or refractory (R/R) BL is poor with limited response to salvage therapy, especially for patients with early relapse (<6 months) or refractory disease. Novel therapies may be promising but need refinement.
Abstract
The prognosis of patients with relapsed or refractory (R/R) BL is poor with a limited response to salvage therapy, especially for patients with early relapse (<6 months) or refractory disease. We present three cases of patients with R/R BL receiving novel therapies followed by a literature review. Amongst others, the patients received inotuzumab ozogamicin, idelalisib, ibrutinib, and CAR‐T cell therapy, however, with limited response. In literature, response to several novel agents is described; however, most promising results are seen with CAR‐T cell therapy. Concluding from case series, sequential CAR‐T cell therapy, targeting multiple B‐cell antigens, seems most promising.
Keywords: Burkitt lymphoma, cellular therapy, chemotherapy, immunotherapy, targeted therapy
1. INTRODUCTION
The introduction of intensive immunochemotherapy regimens has lead to high cure rates of Burkitt lymphoma (BL) in first‐line treatment, even in high‐risk disease. 1 The prognosis of patients with relapsed or refractory (R/R) BL is, however, poor with a limited response to salvage therapy, especially for patients with early relapse (<6 months) or refractory disease. 2 We present three cases of patients with R/R BL receiving novel therapies followed by a literature review.
2. CASE 1
A 36‐year‐old male presented with Ann Arbor stage IV BL without central nervous system (CNS) involvement. The lymphoma cells were positive for, amongst others, CD19, CD20, and CD22 (Figure 1). The patient received initial treatment with one course R‐CHOP, followed by two courses of R‐CODOX‐M/R‐IVAC, but no complete metabolic response (CMR) was seen with persistent BL confirmed by biopsy (Figure 2). Both autologous stem cells were collected and an allogeneic donor search was initiated, aiming to consolidate an anticipated response to salvage therapy with a stem cell transplant. Salvage R‐DHAP chemotherapy concurrent with 5x4Gy radiotherapy was administered, followed by disease progression. After this, the patient received two courses inotuzumab ozogamicin 3 with no safety issues but again showing progressive disease. Then, fourth line treatment with idelalisib 150 mg bid plus dexamethasone was attempted, but at this point in time molecular analysis showed a TP53 mutation and no PIK3CA mutations. With slight disease progression after 2 months and limited toxicity, gemcitabine 1000 mg/m2 was added. Unfortunately, PET‐CT imagining showed disease progression and suspicion for invasive pulmonary aspergillosis. Palliative care was offered and the patient died at home with his family.
FIGURE 1.
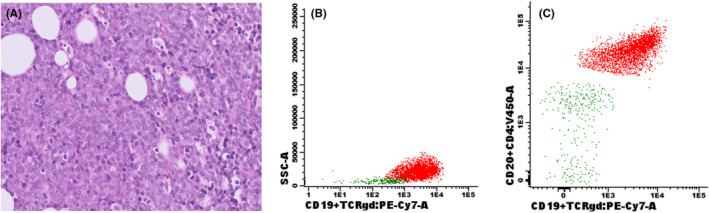
Lymph node examinations of case 1, showing: (A) Hematoxylin and eosin stain of the lymphoma cells on a lymph node biopsy, and (B) positive CD19 and (C) CD22 markers at flowcytometric examination of the aberrant B‐cell population on a lymph node aspirate.
FIGURE 2.
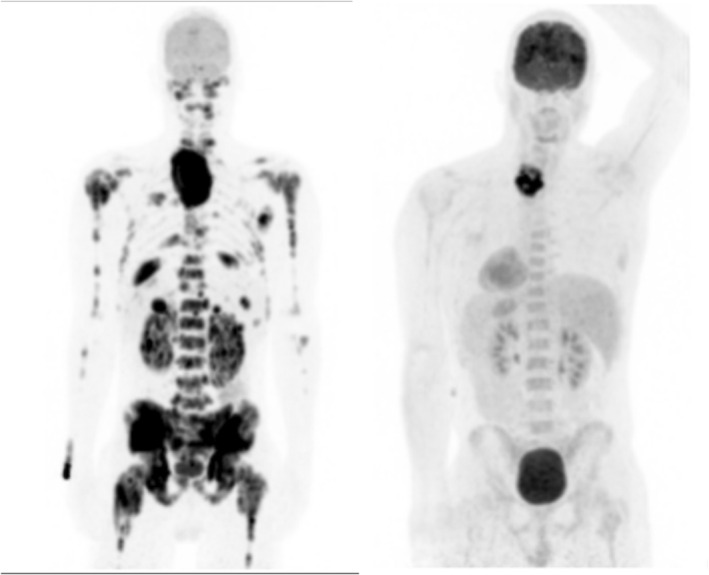
MIP images showing disease staging at diagnosis (left panel) and a residual mass following chemotherapy in the lower neck (right panel), where a biopsy proved persistent Burkitt lymphoma.
3. CASE 2
A 67‐year‐old male presented with night sweats, bone pain, and thrombocytopenia. Bone marrow examination was consistent with the diagnosis of BL. Cerebrospinal fluid (CSF) analysis showed CNS involvement. He was treated with R‐CODOX‐M/R‐IVAC with standard intrathecal MTX administrations until CSF negativity and two extra administrations of HD‐MTX, resulting in a CMR on PET‐CT. One month later, he presented with a numb chin syndrome and imaging and CSF analysis revealed relapsed BL. Salvage treatment was started with radiotherapy, dexamethasone, and one course of mitigated R‐PECC followed by idelalisib 150 mg bid. Unfortunately, disease progressed after 4 weeks and the patient died.
4. CASE 3
This 22‐year‐old male was diagnosed with stage IV CD19 positive BL with CNS involvement (positive CSF by flowcytometry). Following treatment with two courses R‐CODOX‐M/R‐IVAC and 12 intrathecal MTX administrations, PET‐CT showed a CMR. Three months later he presented with leg pain and a systemic BL relapse was biopsy proven, with peripheral nerve involvement (nn. ischiadicus and saphenus). He was treated with two courses of high dose cytarabine (2*2 g/m2 for 4 days) and radiotherapy 6 Gy on both legs (n. ischiadicus to patella), with initial good response. Disease progression was found, just before planned autologous stem transplantation. Rescue with an autologous CD19‐directed CAR‐T was planned (ARI‐0001 cells). He was bridged with R‐Gem‐Ox and ibrutinib. Lymphodepletion with fludarabine and cyclophosphamide was followed by a fractionated administration of CAR‐T cells (0.5 and 1.5 × 10e6 CAR‐T cells/kg). Infusion was delayed for several days due to an intercurrent infection. A grade 2 cytokine release syndrome was developed and resolved with tocilizumab administration. Absolute B‐cell aplasia was achieved and a peak expansion of CAR‐T cells was seen on day +14 with 48 CAR‐T cells/μL. Unfortunately, evaluation on day +30 after CAR‐T showed progression and he died shortly afterwards.
5. DISCUSSION
The best choice for salvage therapy for R/R BL has not yet been determined. 1 Many regimens have been suggested and reported, mostly by case reports or small series. The largest case series described various intensive chemotherapy regimens such as HyperCVAD, ICE, EPOCH, or MOAD, only showing responses in cases with late relapse (>6 months) following first‐line treatment. 2 Furthermore, disease responses have been reported with several treatments, including imexon, 4 doxorubicin/topotecan, 5 alisertib, 6 idelalisib, 7 ICE/ibrutinib, 8 and blinatumomab. 9 Retrospectively, both autologous and allogeneic stem cell transplant have been reported as successful consolidation therapy following initial response on salvage therapy. In that case, overall survival was 37%–53% after 3–5 years. 10 , 11 Prognosis at early relapse or refractory disease remains dismal, remaining an unmet clinical need.
Patient 1 was treated with inotuzumab ozogamicin (InO), which has been described as an effective salvage regimen for CD22 positive R/R B‐cell acute lymphoblastic leukemia (ALL). 3 Based on the BL CD22 positivity and the preclinical observation of InO inhibitory effects on BL cell‐lines, 12 our patient was treated with InO in the same schedule as for patients with B‐ALL. Unfortunately, disease progressed after the second course and treatment was stopped. Retrospectively, the TP53 mutation may have impeded response, as InO‐mediated cell cycle arrest seems to depend on the presence of wild‐type TP53. 13
Patients 1 and 2 received idelalisib, a PI3K delta isoform inhibitor. Activation of the PI3K/Akt/mTOR pathway, response to PI3K inhibition and increased sensitivity to dexamethasone and chemotherapy by PI3K inhibition has been demonstrated in BL cell lines. 14 , 15 Furthermore, response to idelalisib was described in one patient with BL harboring a PIK3CA H1047R mutation. 7 Based on these observations, patient 1 was initially treated with idelalisib combined with dexamethasone. Thereafter, attempting to find a more potent synergic treatment, gemcitabine was added, but unfortunately disease progressed and therapy was complicated by an opportunistic infection. In other types of cancer the PIK3CA H1047R mutation was associated with improved response rates. 7 No PIK3CA gene mutations were detected in patient 1 (this was not assessed in patient 2), possibly explaining the lack of response. PI3K pathway activation may also predict response to idelalisib treatment, but was not assessed in our patients.
Patient 3 received ibrutinib in combination with gemcitabine and oxaliplatin as bridge toward CAR‐T cell therapy. Due to the short course of this therapy a formal response evaluation was not performed. The rationale for addition of ibrutinib to chemotherapy was based on preclincial observations showing additional efficacy and a phase 1 study showing clinical feasibility. 8 , 16 The lack of efficacy may result from predominantly PI3K, and not NF‐κB, driven B‐cell receptor signaling in BL and may therefore prefer PI3K directed therapies over BTK directed therapies, such as ibrutinib. 17 , 18
Subsequently, patient 3 received a point‐of‐care anti‐CD19 CAR‐T cell product (ARI‐0001). 19 This CAR‐T cell product has been successfully applied to patients with B‐cell acute lymphoblastic leukemia and various B‐cell non‐Hodgkin lymphomas. 20 Despite an objective CAR‐T cell expansion disease progressed. Although the mechanisms have not been fully elucidated, several mechanisms of resistance to CAR‐T cell therapy in B‐cell lymphomas are suggested: Loss or low expression of the target antigen (e.g., CD19‐negative escape), low CAR‐T cell expansion or persistence due to T cell exhaustion, T cell suppressive tumor immune microenvironment, or T cell fitness. 21 Compared with other patients receiving ARI‐0001, the CAR‐T cell expansion in patient 3 was relatively low. This may have been caused due to suboptimal T cell fitness following many intensive lines of therapy and delayed infusion following lymphodepletion (12 days). Recently, responses to CAR‐T cell therapy have been reported in patients with R/R BL, with the best and most robust response rates and duration with three sequential CAR‐T infusions directed to CD19, CD20, and CD22 in children. Observations at disease relapse were loss of CD19 expression or low persistence of CAR‐T cells in peripheral blood. 22 In adults, the most promising results were also shown with sequential CAR‐T infusions (anti‐CD19 and –CD22) in six patients with refractory BL, with 50% response rate and one long‐term survivor after subsequent allogeneic stem cell transplant. In this study, higher CAR‐T cell expansion levels were seen in patients with response. A higher level of regulatory T cells (Tregs) was suggested as a possible contribution to CAR‐T cell persistence. 23 Furthermore, information about CD19 status at relapse (positive, dim, or negative) and programmed death ligand 1 or 2 (PD‐L1/L2) overexpression may help to better understand the mechanisms of relapse/refractoriness after CAR‐T therapy in BL. 22 , 23 As this was the first patient with BL that was treated with ARI‐0001, no data are available for comparison. An upcoming phase I‐II trial in 2022 that will formally include patients with BL may provide more information on the feasibility of ARI‐0001 CAR‐T therapy in BL.
In conclusion, relapsed/refractory BL has a very poor prognosis and response to salvage therapy is limited. Following this first description of treatment with InO, additional research may address the role of TP53 mutations. Similarly, the role of PIK3CA gene mutations needs further elucidation in treatment with idelalisib. Additionally, the role of idelalisib as a possible potentiator for other (chemo)therapies may be further explored. The combination of ibrutinib with diverse chemotherapy regimens seems less promising. Finally, CAR‐T therapy may be promising, although further refinement of this therapy is needed.
AUTHOR CONTRIBUTIONS
Paulus A.F. Geerts: Conceptualization; formal analysis; investigation; methodology; supervision; validation; writing – original draft; writing – review and editing. Nils ‘t Hart: Conceptualization; investigation; writing – review and editing. Otto Visser: Conceptualization; supervision; writing – review and editing. Valentín Ortiz‐Maldonado: Conceptualization; investigation; supervision; writing – review and editing. Martine E. D. Chamuleau: Conceptualization; investigation; supervision; writing – review and editing.
FUNDING INFORMATION
The authors report receiving no funding with regard to this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
ETHICS STATEMENT
The research reported in this manuscript was performed according to the regulations of the Declaration of Helsinki.
CONSENT
Written informed consent was obtained from the patients (next of kin) to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The authors would like to thank the patients and their families for their willingness to cooperate to this manuscript. Furthermore, the authors acknowledge the pharmaceutical companies for granting the single patient requests for off‐label inotuzumab ozogamicin and ibrutinib.
Geerts PAF, ’t Hart N, Visser O, Ortiz‐Maldonado V, Chamuleau MED. Chemotherapy resistant Burkitt lymphoma: Possible novel therapies including CAR‐T cell infusion. Clin Case Rep. 2023;11:e7361. doi: 10.1002/ccr3.7361
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Crombie J, LaCasce A. The treatment of Burkitt lymphoma in adults. Blood. 2021;137(6):743‐750. doi: 10.1182/blood.2019004099 [DOI] [PubMed] [Google Scholar]
- 2. Short NJ, Kantarjian HM, Ko H, et al. Outcomes of adults with relapsed or refractory Burkitt and high‐grade B‐cell leukemia/lymphoma. Am J Hematol. 2017;92(6):E114‐E117. doi: 10.1002/ajh.24720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W. Inotuzumab Ozogamicin versus standard therapy for acute lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740‐753. doi: 10.1056/NEJMoa1509277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barr PM, Miller TP, Friedberg JW, et al. Phase 2 study of imexon, a prooxidant molecule, in relapsed and refractory B‐cell non‐Hodgkin lymphoma. Blood. 2014;124(8):1259‐1265. doi: 10.1182/blood-2014-04-570044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SM, Johnson JL, Niedzwiecki D, et al. Sequential doxorubicin and topotecan in relapsed/refractory aggressive non‐Hodgkin's lymphoma: results of CALGB 59906. Leuk Lymphoma. 2006;47(8):1511‐1517. doi: 10.1080/10428190600581385 [DOI] [PubMed] [Google Scholar]
- 6. Friedberg JW, Mahadevan D, Cebula E, et al. Phase II study of alisertib, a selective Aurora a kinase inhibitor, in relapsed and refractory aggressive B‐ and T‐cell non‐Hodgkin lymphomas. J Clin Oncol. 2014;32(1):44‐50. doi: 10.1200/JCO.2012.46.8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleckmann A, Dierks S, Schildhaus H‐U, et al. Treatment response to idelalisib in a patient with immunodeficiency‐associated Burkitt lymphoma harboring a PIK3CA H1047R mutation. Ann Hematol. 2021;100(1):277‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burke GAA, Beishuizen A, Bhojwani D, et al. Ibrutinib plus CIT for R/R mature B‐NHL in children (SPARKLE trial): initial safety, pharmacokinetics, and efficacy. Leukemia. 2020;34(8):2271‐2275. doi: 10.1038/s41375-020-0749-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duell J, Zugmaier G, Eisele F, Brüggeman M, Kufer P, Einsele H, et al. Treatment of r/r BURKITT lymphoma with BLINATUMOMAB is feasible and induced a long lasting complete remission. 2019. presented at: EHA.
- 10. Sweetenham JW, Pearce R, Taghipour G, Blaise D, Gisselbrecht C, Goldstone AH. Adult Burkitt's and Burkitt‐like non‐Hodgkin's lymphoma—outcome for patients treated with high‐dose therapy and autologous stem‐cell transplantation in first remission or at relapse: results from the European Group for Blood and Marrow Transplantation. J Clin Oncol. 1996;14(9):2465‐2472. [DOI] [PubMed] [Google Scholar]
- 11. Maramattom LV, Hari PN, Burns LJ, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2013;19(2):173‐179. doi: 10.1016/j.bbmt.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22‐specific antibody‐targeted chemotherapy using inotuzumab ozogamicin (CMC‐544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240‐2245. doi: 10.1038/sj.leu.2404866 [DOI] [PubMed] [Google Scholar]
- 13. Tirro E, Massimino M, Romano C, Pennisi MS, Stella S, Vitale SR. Chk1 inhibition restores Inotuzumab Ozogamicin Citotoxicity in CD22‐positive cells expressing mutant p53. Front Oncol. 2019;9:57. doi: 10.3389/fonc.2019.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhatti M, Ippolito T, Mavis C, et al. Pre‐clinical activity of targeting the PI3K/Akt/mTOR pathway in Burkitt lymphoma. Oncotarget. 2018;9(31):21820‐21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang X, Zhou X, Wang X. Clinical development of phosphatidylinositol 3‐kinase inhibitors for non‐Hodgkin lymphoma. Biomark Res. 2013;1(1):30. doi: 10.1186/2050-7771-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu Y, Lee S, Shah T, et al. Ibrutinib significantly inhibited Bruton's tyrosine kinase (BTK) phosphorylation, in‐vitro proliferation and enhanced overall survival in a preclinical Burkitt lymphoma (BL) model. Onco Targets Ther. 2019;8(1):e1512455. doi: 10.1080/2162402X.2018.1512455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116‐120. doi: 10.1038/nature11378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Efremov DG, Turkalj S, Laurenti L. Mechanisms of B cell receptor activation and responses to B cell receptor inhibitors in B cell malignancies. Cancers (Basel). 2020;12(6):1‐33. doi: 10.3390/cancers12061396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castella M, Caballero‐Banos M, Ortiz‐Maldonado V, et al. Point‐of‐care CAR T‐cell production (ARI‐0001) using a closed semi‐automatic bioreactor: experience from an academic phase I clinical trial. Front Immunol. 2020;11:482. doi: 10.3389/fimmu.2020.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ortiz‐Maldonado V, Rives S, Castella M, Alonso‐Saladrigues A, Benítez‐Ribas D, Caballero‐Baños M. CART19‐BE‐01: a multicenter trial of ARI‐0001 cell therapy in patients with CD19(+) relapsed/refractory malignancies. Mol Ther. 2021;29(2):636‐644. doi: 10.1016/j.ymthe.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newcomb R, Jacobson C. Chimeric antigen receptor T cells for B‐cell lymphoma. Cancer J. 2021;27:107‐111. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Deng B, Hu B, et al. Sequential different B cell antigen‐targeted CAR T‐cell therapy for pediatric refractory/relapsed Burkitt lymphoma. Blood Adv. 2021;138:257. doi: 10.1182/bloodadvances.2021004557/1822478/bloodadvances.2021004557.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X, Ge T, Li T, et al. CAR19/22 T cell therapy in adult refractory Burkitt's lymphoma. Cancer Immunol Immunother. 2021;70(8):2379‐2384. doi: 10.1007/s00262-021-02850-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


