Abstract
Inositol 1,4,5-trisphosphate (IP3) is an important second messenger in animal cells and is central to a wide range of cellular responses. The major intracellular activity of IP3 is to regulate release of Ca2+ from intracellular stores through IP3 receptors (IP3Rs). We describe a system for the transient disruption of IP3 signaling in the model organism Caenorhabditis elegans. The IP3 binding domain of the C. elegans IP3R, ITR-1, was expressed from heat shock-induced promoters in live animals. This results in a dominant-negative effect caused by the overexpressed IP3 binding domain acting as an IP3 “sponge.” Disruption of IP3 signaling resulted in disrupted defecation, a phenotype predicted by previous genetic studies. This approach also identified two new IP3-mediated processes. First, the up-regulation of pharyngeal pumping in response to food is dependent on IP3 signaling. RNA-mediated interference studies and analysis of itr-1 mutants show that this process is also IP3R dependent. Second, the tissue-specific expression of the dominant-negative construct enabled us to circumvent the sterility associated with loss of IP3 signaling through the IP3R and thus determine that IP3-mediated signaling is required for multiple steps in embryogenesis, including cytokinesis and gastrulation.
INTRODUCTION
Inositol 1,4,5-trisphosphate (IP3) is an important and widely used second messenger in animal cells. IP3 is produced by the hydrolysis of phosphatidylinositol 4,5-bisphosphate after the activation of phospholipase C (PLC). The best understood routes of PLC activation are through the stimulation of G protein-coupled receptors or tyrosine kinase-linked receptors at the cell surface, although the presence of additional isoforms of PLC suggests other possible routes (Rhee and Bae, 1997; Katan, 1998). The only known action of IP3 is to induce Ca2+ release from intracellular stores through activation of IP3 receptors (IP3Rs) (Berridge, 1993). This pathway and the Ca2+ signals derived from it are central to a wide range of cellular responses (Berridge, 1993, 1997; Clapham 1995).
Two challenges in the study of this signaling network are to unravel the different functions played by IP3 signaling in animals and to develop methods for manipulating these processes and thus advance our understanding of how the specificity of IP3 signaling is achieved. Recent genetic approaches have provided new insights into IP3 function in animals. The central role played by IP3Rs in a wide range of cellular responses is reflected by the high level of lethality resulting from gene knockout observed in both Drosophila (Acharya et al., 1997; Venkatesh and Hasan, 1997) and mice (Matsumoto et al., 1996). Nevertheless, careful analysis has enabled, for example, the functions of IP3Rs in cerebellum in mice (Inoue et al., 1998) and phototransduction in Drosophila to be studied (Acharya et al., 1997; Raghu et al., 2000). Caenorhabditis elegans is also proving to be a powerful system for understanding IP3 signaling function. IP3Rs in C. elegans are encoded by a single gene, itr-1, and are widely expressed (Baylis et al., 1999; Dal Santo et al., 1999). A genetic screen for genes that act downstream of let-23 (epidermal growth factor receptor) identified itr-1 as a component of a signaling pathway regulating ovulation (Clandidin et al., 1998). Analysis of itr-1 loss-of-function mutants (Dal Santo et al., 1999) demonstrated that itr-1 is a component of a rhythm generator that controls the defecation motor program. Thus, C. elegans is an accessible system for dissecting IP3 signaling in a whole animal.
To complement and extend these studies it is desirable to develop methods for achieving three goals. First, to be able to ablate IP3 signaling in particular cells and/or at particular points in development. Second, to be able to distinguish between IP3 signaling and its downstream effect through IP3Rs. Third, to develop systems that are sufficiently malleable that they enable detection of a broad range of phenotypes without the severe reduction in viability that results from compounding several such phenotypes. To achieve this we have taken the approach of disrupting IP3 signaling by induction of high-level expression of the IP3 binding domain of ITR-1. This domain has IP3 binding activity (Baylis et al., 1999) and should therefore act as an IP3 “sponge,” competing with endogenous targets of IP3 such as IP3Rs. We demonstrate that this approach is successful and identify previously unidentified roles for IP3 in the regulation of pharyngeal pumping and in embryogenesis.
MATERIALS AND METHODS
Production of Transgenic Animals Expressing IP3 Sponge Constructs
The region of itr-1 cDNA corresponding to residues 1–705 was amplified by polymerase chain reaction with Expand High Fidelity (Roche Applied Sciences, Indianapolis, IN), and ligated into pPD49.78 (Mello and Fire, 1995) to give the plasmid referred to as sponge. Derivatives of this plasmid, containing the mutations K579Q and R582Q (“control-sponge”) or R511C (“super-sponge”), were constructed by single-round amplification of the entire plasmid with Expand High Fidelity and mutagenic primers, and DpnI digestion of the parental strand. Restriction fragments carrying the mutations were then cloned into the corresponding region of the wild-type sponge plasmid. Constructs were microinjected into C. elegans (Mello and Fire, 1995) with the rol-6 gene marker plasmid pRF4.
In Vitro Binding Assays
The itr-1 cDNA encoding the wild-type and mutant binding domains described above were cloned into pGEX2TK (Amersham Biosciences, Piscataway, NJ), and expressed in Escherichia coli BLR (Stratagene, La Jolla, CA). Fusion protein expression and total protein lysates preparation were as described previously (Yoshikawa et al., 1996; Baylis et al., 1999). Glutathione S-transferase (GST) fusion proteins were purified as in De Waard et al. (1995), except that Triton X-100 was omitted and proteins were dialyzed against binding buffer (50 mM Tris-HCl pH 8, 1 mM 2-mercaptoethanol, 1 mM EDTA). IP3 binding assays (Yoshikawa et al., 1996) used 500 nM purified fusion protein with 5.3 nM [3H]IP3 in binding buffer containing the appropriate concentration of unlabeled IP3. Reactions were incubated at 4°C for 10 min then GST fusion proteins were collected using glutathione-agarose beads, which were washed three times with binding buffer before scintillation counting.
C. elegans Culture and Phenotypic Assays
C. elegans strains were routinely cultured at 20°C on E. coli OP50 grown on NGM plates (Brenner, 1974). Expression from the hsp16-2 promoter was induced by placing worms at 33°C for 2 h (Dixon et al., 1990). In all phenotypic assays, semisynchronized populations were produced by allowing a small number of worms to lay for 12 h then removing them from the plate. The resulting offspring were heat shocked once immediately before egg laying commenced, i.e., as young adults. For brood size and other egg laying experiments, the heat shock was repeated every 24 h for the duration of the laying period. All assays were carried out at 20°C.
Brood sizes were quantified by placing single worms on individual plates, moving them every 24 h, and counting the eggs laid. Defecation cycles were analyzed by observation with a stereomicroscope, measuring the time interval from one posterior body contraction to the next. For each genotype, young adults were heat shocked as described above and 12 animals (six each at 6 and 18 h after heat shock, or the equivalent stage for wild type) were followed for 10 continuous defecation cycles. Pharyngeal pumping was analyzed by counting pharyngeal contractions, as visualized under a stereomicroscope (Avery and Horvitz, 1989). For each genotype, young adults were heat shocked as described above and 12 animals (six each at 6 and 18 h after heat shock) were followed for five periods of 30 s. In both defecation and pharyngeal pumping experiments, no significant differences were observed between 6 and 18 h. The effects of serotonin were determined after incubation of worms on NGM plus 7.5 mM hydroxytryptamine in the absence of food (Waggoner et al., 1998).
Embryos were dissected from adults 24 h after the second heat shock, i.e., at the start of the third day of egg laying, mounted in embryo culture medium (Zwaal et al., 1996), and analyzed by four-dimensional video recording under Nomarski optics (Zwaal et al., 1996). Terminal phenotypes were observed after incubation of mounted embryos for 18 h at 15°C. Development was also observed in utero. The percentage of embryos corresponding to each of the main phenotypes was quantified from observation of terminal phenotypes. ELT-2 expression was characterized by establishing transgenic animals carrying the relevant IP3 sponge construct in the strain JM73, which carries an integrated elt-2::gfp fusion (Fukushige et al., 1998). Green fluorescent protein (GFP) was visualized using a Leica SP confocal microscope. The percentage of embryos expressing ELT-2 was quantified by observing animals ∼24 h after the second heat shock, scoring only embryos that had developed beyond the 2E cell (or equivalent) stage.
Immunofluorescence
Semisynchronized hermaphrodites were heat shocked every 24 h from the onset of laying, removed to fresh plates on day 2, and harvested in M9 on day 3. Permeabilization by freeze cracking, fixation with methanol and acetone, and antibody staining were performed as described previously (Baylis et al., 1999), by using a rabbit anti-PGL-1 polyclonal antibody (Kawasaki et al., 1998). The secondary antibody was goat anti-rabbit IgG-Alexa 488 conjugate (Molecular Probes, Eugene, OR). Propidium iodide (0.2 μg/ml) was included to stain nuclei. Images were collected using a Leica SP confocal microscope.
RNA-mediated Interference (RNAi) of itr-1
Clones of two regions of itr-1 cDNA, base pairs 136-1056 (pNG019) and 6308–7266 (pNG030), were generated in the vector pPD129.36 that contains two T7 RNA polymerase promoters flanking the insert site (Timmons et al., 2001). As a control we used pPD128.110, a derivative of pPD129.36 with a GFP cDNA insert (Timmons et al., 2001). Plasmids were transformed into E. coli HT115 (DE3) and these strains used to perform RNAi feeding experiments (Timmons et al., 2001). Five L4 hermaphrodites were moved onto each feeding plate and incubated at 20°C for 36 h then moved onto separate feeding plates and allowed to lay for 24 h at 20°C. These progeny were scored.
RESULTS
Establishing an Inducible IP3 Dominant-Negative System to Identify IP3-dependent Signaling Processes in C. elegans
To determine the functional consequences of disrupting IP3 signaling in C. elegans, we overexpressed the IP3 binding domain of the C. elegans IP3R, ITR-1, by using an inducible promoter. The IP3 binding domain of ITR-1 was placed under the control of the hsp16-2 promoter (Figure 1A) and this construct, referred to as the sponge plasmid, used to generate stable, transgenic animals. The hsp16-2 promoter, although never directing constitutive expression, directs heat shock-induced expression in a wide range of tissues, including the intestine, nerves, muscle (body wall, pharyngeal, anal, and vulval), and hypodermis, the main exception being the germ line (Stringham et al., 1992), a general feature of C. elegans transgenes. Thus, heat shocking of worms could be used to induce high-level, widespread expression of the IP3 binding domain.
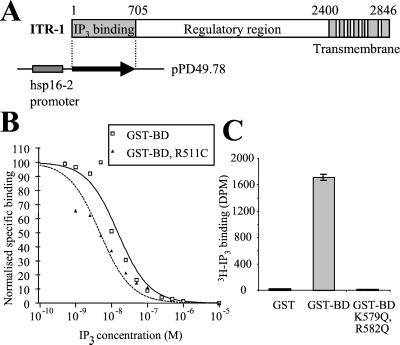
Figure 1.
IP3 sponge constructs used in this study. Site-directed mutagenesis was used to abolish and to increase the affinity of IP3 binding. (A) Schematic representation of an IP3R subunit, showing domain organization. (B) In vitro binding to [3H]IP3 of the following fusion proteins: GST-BD, GST fusion protein expressing the wild-type IP3 binding domain; and GST-BD R511C, derivative of GST-BD with the mutation indicated (i.e., super-sponge). Binding was measured by the inhibition of specific [3H]IP3 binding to purified fusion protein by various concentrations of IP3. Specific binding was calculated by subtraction of nonspecific binding and normalized by expression as a percentage of maximal binding. (C) IP3 binding to GST fusion proteins in vitro. GST-BD K579Q, R582Q, derivative of GST-BD with the mutations indicated (i.e., control-sponge). Error bars are SD.
The IP3 binding domain is substantial in size (705 residues), so to control for effects due to properties other than the IP3 binding activity of the IP3 sponge (or effects due to the experimental protocol itself), we made a construct, referred to as “control-sponge” in which two residues, each of which was identified as being essential for IP3 binding in mouse IP3R-1 (Yoshikawa et al., 1996), were substituted (K579Q, R582Q). These residues have no other known function.
The efficacy of the dominant-negative sponge will reflect a balance between the levels of sponge, native receptors, and IP3 in a given location. We therefore made a third construct “super-sponge” that is predicted to have an increased affinity for IP3 as a result of the substitution R511C. Substitution of the equivalent residue in the mouse type 1 receptor, R441, by glutamine results in a twofold increase in binding (Yoshikawa et al., 1996), whereas conversion to cysteine produces a fivefold increase in affinity (Hirota, personal communication). This substitution is identical to that found in the gain-of-function allele, itr-1 (sy290) (Clandinin et al., 1998).
We confirmed that the IP3 sponge constructs showed the predicted binding to IP3 in vitro, by using GST fusion proteins of these binding domains. As Figure 1B shows, the wild-type sponge exhibits properties identical to those reported previously for the ITR-1 IP3 binding domain (Baylis et al., 1999), whereas the super-sponge construct exhibits an approximately twofold increase in affinity. Figure 1C shows that the control-sponge does not bind IP3.
Induction of IP3 Sponge Disrupts Defecation, a Known IP3R-mediated Process
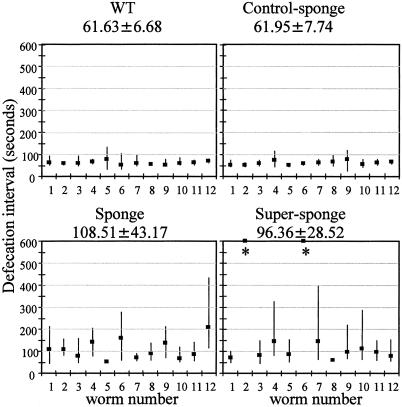
If the IP3 sponge functions to reduce IP3 signaling then at least some of its consequences should be similar to those caused by disrupting IP3R function. Defecation in C. elegans is a coordinated and regular motor program, which occurs every 45 s (Croll and Smith, 1978; Liu and Thomas, 1994). Loss-of-function mutations in itr-1 (dec-4) increase the period of, or eliminate, the defecation cycle (Dal santo et al., 1999), indicating that the cycle is regulated by the IP3R. We therefore investigated whether expression of an IP3 sponge conferred a similar effect. As Figure 2 shows, induction of either the sponge or super-sponge results in a substantial increase in mean cycle length, and also an increased variability (i.e., a decrease in rhythmicity). Some of the super-sponge worms did not defecate at all in 10-min periods of observation. These results provide evidence for the efficacy of the sponge as a device for disruption of IP3 signaling.
Figure 2.
Disruption of IP3 signaling results in increased defecation cycle length and increased variation in cycle length. Defecation was observed on food at 20°C, ∼6 h (worms 1–6) or 18 h (worms 7–12) after onset of heat shock. Worms were recorded for 10 cycles. Squares are mean; bars are minimum-maximum, for individual worms. Mean ± SD is shown above each graph. ∗, worms failed to defecate in >10 min and are not included in the mean.
Expression of an IP3 Sponge Has No Effect on Ovulation
Genetic analysis implicates itr-1 in ovulation (Clandinin et al., 1998) and itr-1 is clearly expressed in the spermatheca and uterine sheath cells (Baylis et al., 1999; Dal Santo et al., 1999; Gower et al., 2001). Furthermore, depletion of ITR-1 by RNAi results in the predicted endomitotic (Emo) phenotype (Gower and Baylis, unpublished data). Disruption of IP3 signaling is therefore predicted to result in defective ovulation. However, induction of the sponge and super-sponge under the control of the hsp16-2 promoter did not result in an Emo phenotype, and sheath cell contraction and spermathecal dilation were normal (our unpublished data). This effect may be the result of poor expression from the hsp16-2 promoter in the relevant somatic gonad cells. However, expression of the same sponge constructs under the control of the hsp16-41 promoter (in pPD49.83; Mello and Fine, 1995), which has been used to disrupt ovulation via overexpression of LFE-2 (Clandinin et al., 1998), also had no effect. In addition, inducing expression earlier in development, by commencing heat shock at the first larval stage, had no effect on brood size or ovulation (our unpublished data).
Disruption of IP3 Signaling Interferes with Modulation of Pharyngeal Pumping
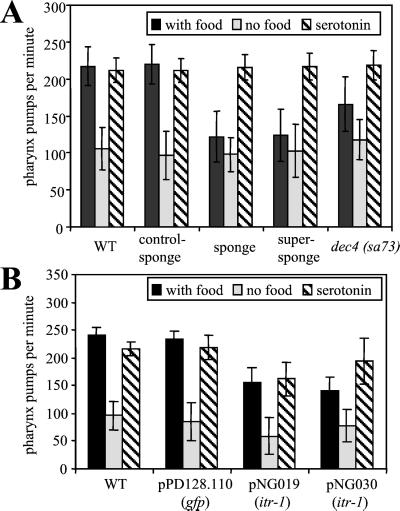
Because ITR-1 is highly expressed in the terminal bulb and isthmus of the pharynx (Baylis et al., 1999; Dal Santo et al., 1999), we tested the hypothesis that IP3 signaling has a role in pharyngeal pumping. The pharynx is a rhythmic pump that forces food into the intestine. As Figure 3A shows, animals expressing the sponge or super-sponge exhibit a dramatic decrease in the rate of pharyngeal pumping in the presence of food, as well as an increase in the variability of pumping rate. When we compared pharyngeal pumping rates in the absence of food, no significant difference was observed between the wild-type and transgenic animals expressing either active or control IP3 sponge constructs. Thus, interfering with IP3-mediated signaling does not interfere with the basal rate of pharyngeal pumping, but severely disrupts the increase in pumping rate in response to the presence of food. To determine whether this effect was due to an inability of the pharyngeal muscle to pump at the higher frequency we exposed the worms to serotonin, which induces higher rate pharyngeal pumping (Croll and Smith, 1978; Horvitz et al., 1982). This ability is unimpaired in any of the sponge-treated animals. Thus, the pharynx is able to pump at the higher rate and at least one mechanism by which serotonin induces this remains unperturbed.
Figure 3.
Disruption of IP3 signaling or IP3R expression results in slower and more erratic pharyngeal pumping. (A) Pharyngeal pumping in transgenic strains expressing IP3 sponge constructs was observed on food or in the absence of food at 20°C, ∼6 or 18 h after heat shock [wild-type and dec-4 (sa73) animals were not heat shocked]. The effects of serotonin were assayed after 1–2 h on NGM, 7.5 mM 5-hydroxytryptamine in the absence of food. Mean calculated from five observations for 12 worms. Error bars are SD. (B) Pharyngeal pumping in RNAi-treated animals. Assays were carried out as described above; pumping on food was assayed after 1–2 h on OP50. Mean calculated from five observations for 10 worms.
To ascertain whether the effect of IP3 on stimulation of pharyngeal pumping was mediated through the IP3R we analyzed the weak loss of function allele itr-1 (sa73) (Dal Santo et al., 1999). As Figure 3A shows, the rate of pumping in the presence of food is significantly reduced (p < 0.001) in these animals compared with the wild-type, although this effect is less severe than for the IP3 sponge constructs. Again, restoration of full pumping rate was observed in the presence of serotonin. To extend this observation we performed RNAi on wild-type animals to knock down itr-1. We observed the same results with two different parts of the itr-1 cDNA. Figure 3B shows that knock down of itr-1, like ablation of IP3 signaling, reduced pumping in the presence of food. Pumping rate is increased in response to serotonin, but is not fully restored to wild-type levels. These animals also display other phenotypic consequences of itr-1 knock down and are generally rather sick, which most likely explains this discrepancy. We conclude that signaling through IP3 and IP3Rs is required for the up-regulation of pharyngeal pumping in response to food.
Disruption of IP3 Signaling Results in Embryonic Arrest
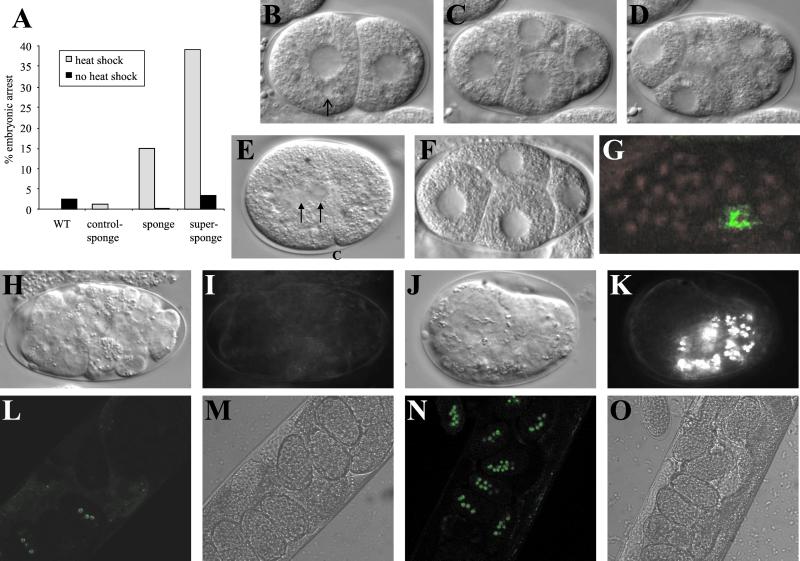
As discussed above knocking down IP3Rs in C. elegans results in sterility, at least in part due to a failure in ovulation. We exploited the fact that expression of the IP3 sponge from the hsp16-2 promoter does not disrupt ovulation to study the effect of disrupting IP3 signaling on embryonic development. Many of the eggs laid by animals expressing the sponge and especially the super-sponge are abnormal and fail to develop into larvae (Figure 4A). Indeed, the results shown underestimate this effect as the number of dead eggs increases through time. The effect is stronger in the super-sponge worms. This increase in embryonic arrest is not a side effect of starvation (resulting from the pharyngeal defects; our unpublished data). Clearly, disruption of IP3 signaling results in substantial embryonic lethality.
Figure 4.
Disruption of IP3 signaling results in embryonic arrest. (A) Percentage of eggs laid that were arrested. (B–D) Early cell divisions generally proceed as normal. Typical embryo from an adult expressing the super-sponge. Arrow indicates an example of the large vesicle-like structures. (E) In some cases, defects are seen in cytokinesis. One-cell embryo from an adult expressing the super-sponge, showing failure of the pronuclei (arrows) to separate fully before formation of the cleavage furrow (c). (F) Typical embryo from a control-sponge transgenic adult. (G) Immunofluorescence labeling with anti-PGL-1 polyclonal antibody (green), and propidium iodide staining of DNA (red) of an embryo illustrating the most common arrest phenotype of embryos from adults expressing the super-sponge. The most commonly observed terminal phenotype (H and I) and another terminal phenotype of embryos from adults expressing the super-sponge (J and K). Nomarski images, except H and J, which are fluorescence images (showing rhabditin granules). ELT-2::GFP fluorescence (L) and corresponding transmission image of typical adult expressing the super-sponge (M). ELT-2::GFP fluorescence (N) and corresponding transmission image of typical adult expressing the control-sponge (O).
To determine the basis of the embryonic arrest we began by using three techniques for the observation of embryonic development. Using Nomarski microscopy, we 1) observed embryos in utero, 2) analyzed early cell divisions by using a four-dimensional video recording system to record embryos in multiple focal planes (Zwaal et al., 1996), and 3) characterized the terminal phenotypes of mounted embryos. This led us to identify three classes of arrested embryos.
In general, early cell divisions in embryos from adults expressing the sponge or super-sponge proceed normally (Figure 4, B–D), with no evidence of defects in the asymmetric nature of these divisions or establishment of polarity. A small proportion of embryos appears misshapen and swollen and displays difficulties in cytokinesis. The spindle appears weak and poorly anchored, and in some cases the cleavage furrow forms before the pronuclei are sufficiently separated (Figure 4E). These embryos usually arrest at the one-cell stage (9.1% of embryos observed, n = 154). This phenotype was observed despite mounting embryos in osmotically balanced medium, and was also observed in utero, so it seems unlikely that it is an indirect effect of osmotic sensitivity. Expression of the sponge or super-sponge results in the presence of large granule-free zones in the embryos produced by these transgenic worms (Figure 4, B–E). The nature of these is unknown, although they are reminiscent of the large endocytotic vesicles observed in pod-1 mutants (Rappleye et al., 1999). These are not observed in embryos from worms expressing the control-sponge (Figure 4F).
To further characterize the arrest phenotype, mounted embryos were incubated overnight at 15°C and terminal phenotypes analyzed. By far the most common phenotype (33.8%) consisted of a mass of <100 undifferentiated cells (Figure 4, H and I), with no evidence for rhabditin granules (a marker for gut differentiation; Babu, 1974; Laufer et al., 1980). This phenotype was investigated more fully, as described below. A less frequently observed phenotype (10.4%) consisted of a disorganized mass of partially differentiated cells, with evidence for pharyngeal and muscle tissue and rhabditin granules (Figure 4, J and K). The partial differentiation, and clustering of gut granules close to the posterior surface, seen in this third phenotype is similar to that observed in maternal effect mutants such as gad-1 (Knight and Wood, 1998) and emb-5 (Nishiwaki et al., 1993), where the E cells divide aberrantly and fail to migrate inwards, so that cell movements associated with gastrulation do not occur.
We further characterized the most common phenotype (Figure 4, H and I), examining whether gastrulation had occurred in these embryos. Gastrulation initiates when the two gut precursors, Ea and Ep, start to move from the ventral surface into the blastocoel. After this, as cell divisions continue, the mesodermal and germline (P4) precursors also ingress and then divide to form the three germ layers of the embryo (Sulston et al., 1983). We investigated the fate of the P lineage, via immunofluorescence labeling of P granules. As Figure 4G shows, these embryos exhibit staining in a single cell, P4, which remains at the ventral surface and consequently fails to divide to form the germ line cells Z2 and Z3. This is similar to the arrest phenotype that results from ama-1 antisense (Powell-Coffman et al., 1996).
A failure in E cell differentiation might explain the failure in gastrulation. To further define when in E-cell development the differentiation process goes awry we investigated the fate of the E lineage by using an elt-2::gfp transgene (Fukushige et al., 1999). ELT-2 is a gut cell-specific GATA family transcription factor that is first expressed at the 2E cells stage (Fukushige et al., 1998). Overall, expression of ELT-2::GFP was observed in only 35.1% of embryos in super-sponge worms, compared with 95.9% for the control-sponge (Figure 5, L–O). In agreement with our other observations, the effect is maternal, in that although some animals are indistinguishable from the wild type, others produce a very high proportion of arrested, unlabeled embryos. Thus, it appears that E-cell differentiation fails very early in the E-cell lineage. E is derived by the division of the cell EMS; in the absence of the necessary inductive signal (from P2) both derivatives of EMS take on an MS-like fate (Goldstein, 1995). We therefore tested for extra MS descendants by staining with the anti-pharyngeal muscle antibody 3NB12 (Priess and Thomson, 1987). In agreement with our visual characterization of terminal phenotypes, these embryos showed no evidence for differentiation of pharyngeal tissue.
DISCUSSION
We describe the successful application of a system for regulated, transient disruption of IP3 signaling in a whole animal. When the IP3 binding domain of the IP3R is overexpressed under the control of an inducible promoter in C. elegans, defecation, a previously identified putative IP3-mediated process, is disrupted and new roles for IP3 signaling are identified.
In contrast to the analysis of mutations in IP3Rs the IP3 sponge has the potential to disrupt IP3 signaling through any target, and thus to detect IP3-dependent processes that are not mediated through IP3Rs. Other proteins are known to bind IP3, for example, phospholipase Cδ and p130 (Yoshida et al., 1994), although functions for the IP3 binding of such proteins remain unknown. Of the IP3-dependent phenotypes analyzed in this article the evidence that the regulation of defecation and pharyngeal pumping also involve IP3Rs is strong (Dal Santo et al., 1999; this study). The evidence is less clear-cut for the roles of IP3 in embryogenesis, because disruption of IP3R expression results in sterility.
The efficiency with which the sponge reduces IP3 signaling may be influenced by a number of variables. The concentration of the sponge proteins themselves will depend on the efficiency of expression, which may vary depending on the particular transgenic line, cell or subcellular locale, or developmental stage. Furthermore, the efficacy of the sponge may depend on its binding affinity and also the concentration of IP3. Thus, the current application of this technique is not quantitative and cannot be regarded as a method for ablating all IP3 signaling; nevertheless, its utility is demonstrated by the novel phenotypes identified.
IP3 and Defecation Rhythm
We used the previously identified role of IP3Rs in the rhythm generator underlying the defecation cycle to assess the effectiveness of the IP3 sponge as a means of ablating IP3 function. Ablating IP3 signaling with the sponge clearly disrupts this process, supporting the model (Dal Santo et al., 1999) that IP3 plays a role in regulating this process. Comparison of the effects of the sponge and loss-of-function alleles of itr-1 (Dal Santo et al., 1999) allows some insight into the effectiveness of the sponge in the cells regulating this process. itr-1 (n2559) is a severe allele and animals are sterile and do not defecate, whereas itr-1 (sa73) is a weak allele with relatively normal fertility, disrupted but functional defecation (Dal Santo et al., 1999), and moderately disrupted pharyngeal pumping (this study). In terms of defecation, expression of the sponge or super-sponge from the hsp16-2 promoter has an effect that is more disruptive than sa73 and in some cases as extensive as n2559. Overall, however, sponge disruption is not as extreme as the severe allele. Thus, a range of effectiveness, presumably reflecting the variables discussed above, is observed.
IP3 and Ovulation
Clandidin et al. (1998) demonstrated that itr-1 lies downstream of let-23 in the regulation of ovulation. Gain-of-function alleles of itr-1 suppress lin-3 sterility. It is therefore surprising that IP3 sponge expression (from hsp16-2 or hsp16-41 promoters) does not result in sterility. One possible explanation is inadequate expression in the cells responsible, although Clandinin and colleagues used the hsp16-41 promoter to successfully disrupt ovulation by overexpression of lfe-2. Another possibility is that this mechanism is mediated through IP3Rs but not IP3. Several lines of evidence suggest this may not be true. First, Clandinin et al. (1998) clearly showed a role for lfe-2, IP3 3-kinase, which converts IP3 to inositol 1,3,4,5-tetrakisphosphate, IP4. The ability to alter ovulation by altering lfe-2 may result from alterations in IP3 levels. However, IP4 is also an active signaling molecule (Irvine and Schell, 2001) and it cannot be ruled out that changes in lfe-2 function through IP4. We have performed RNAi on phospholipase Cγ, the predicted mediator of IP3 production by let-23. These animals have an Emo phenotype, suggesting that PLCγ and presumably IP3 are required for ovulation (our unpublished data). We believe the most likely explanation is that the local sponge concentration is insufficient. Sponge levels in these cells may be lower than elsewhere; IP3 levels may be too high to disrupt; or IP3 signals and associated signaling components may localized, with the sponge lacking the information required for localization such that its local concentration is insufficient.
Role of IP3 Signaling in Pharynx
The pharynx of C. elegans is a good model for understanding behavioral control of a rhythmic physiological process. Results from analysis of sponge expression, RNAi, and the itr-1 (sa73) mutant demonstrate that the IP3 signaling pathway is required for the up-regulation of pharyngeal pumping in response to food. Both sponge expression and RNAi severely reduce this effect. This is without any significant effect on either basal rates of pharyngeal pumping or the ability of the pharynx to pump when stimulated by exogenous serotonin (although RNAi worms do show some reduction in the effect of serotonin).
IP3Rs are expressed both in the muscle cells of the terminal bulb and isthmus and in the nervous system (Baylis et al., 1999). That expression of an IP3 sponge does not interfere with basal pharyngeal pumping is perhaps not surprising, given that this is thought to be largely intrinsic to the muscle, where the IP3R would not be expected to respond sufficiently rapidly to play a central role. Although pumping per se is not dependent on function of the pharyngeal nervous system these neurons are required for the proper control of feeding behavior. Specifically, the neuron MC is central to the regulation of pumping rate in response to food (Avery and Horvitz, 1989; Raizen et al., 1995). Based on the role that we have demonstrated in increasing pumping rate in response to food, it appears that IP3R signaling plays a role in the behavioral modulation of pumping rate. The observation that the effect of serotonin is not altered is interesting. Serotonin stimulates pumping rate via at least two mechanisms (Avery and Thomas, 1997), one through stimulation of MC, the other through a direct action on the pharyngeal muscle. Our results suggest that at least one of these is not affected by ablating IP3 signaling but that nevertheless the normal stimulation of pharyngeal pumping in response to food is disrupted. One explanation is that the direct effect of serotonin on the pharynx remains intact. Alternatively, the mechanism ablated in IP3 sponge worms may be an additional, serotonin-independent mechanism. We are currently using sponge expression from cell-specific promoters (Gower et al., 2001) to dissect this process further.
Role of IP3 Signaling in Embryogenesis
Our results demonstrate that disruption of IP3 signaling interferes with multiple events during embryogenesis. We observe three classes of arrested embryos: 1) embryos in which cytokinesis is defective and which usually arrest at the one cell stage; 2) embryos that form a ball of <100 cells, with no apparent differentiation of cell types and no signs of gastrulation; and 3) embryos that have undergone some, disorganized differentiation. Using the IP3 sponge has enabled us to circumvent the sterility conferred by ablating IP3 receptors. However, interpretation of the data is made complex by the fact that the sponge levels are unlikely to be constant over time, between animals or between embryos. This may partially explain why we see three different phenotypes. First, our results indicate roles for IP3 signaling in cytokinesis or events required for it, this seems to be true only in the first cell division, suggesting a specific role for this signal in these very early events. Parallels can be drawn with other systems. In zebrafish and humans, the distribution of both Ca2+ and IP3Rs is highly dynamic during this period, but IP3Rs are particularly highly localized to cleavage furrows, polar bodies, and points of contact between blastomeres (Creton et al., 1998; Goud et al., 1999). Lithium inhibition of the phosphoinositide pathway in sea urchin embryos results in arrest at cytokinesis (Becchetti and Whitaker, 1997).
The major phenotype observed is that described in 2 above. Our results suggest that IP3 signaling plays a role in early E-cell differentiation. The failure of the E cells to differentiate inevitably leads to a failure in gastrulation. The failure in E-cell differentiation is probably part of a more widespread failure in cell differentiation as suggested by the lack of observable differentiation and the lack of MS cell derivatives. Perhaps the most similar phenotype to this is that which results from blocking embryonic transcription by ama-1 antisense (Powell-Coffman et al., 1996). One possibility is therefore that an IP3-mediated calcium signal is required to permit embryonic transcription. Alternatively, IP3 signaling may play a fundamental role in the signals required for cell determination. The third class of embryos develop to a later stage and the defect in these embryos remains unclear, although by analogy to gad-1 (Knight and Wood, 1998) and emb-5 (Nishiwaki et al., 1993) mutants this may suggest a failure in cell migrations associated with gastrulation, as a result of aberrant E-cell division.
It is unclear whether these functions are mediated through IP3Rs. Itr-1 (n2559) and RNAi-treated animals are sterile. During RNAi treatment, a few viable and affected worms are produced. We have not observed dead embryos in these escapees (regardless of whether RNAi treatment is by feeding or microinjection; Gower and Baylis, unpublished data). Nor have we observed any disruption of embryogenesis in the sa73 weak loss-of-function allele of itr-1. Thus, it appears that by definition any fertile animal has substantial IP3R function; therefore, in both the itr-1 (sa73) and RNAi fertile offspring, the lack of effect may merely reflect limited disruption of ITR-1 function. Using the currently available tools, we are therefore unable to test the hypothesis that IP3 functions in embryogenesis in an ITR-1–independent manner. We are currently attempting to generate systems in which this will be possible.
Use of the IP3 sponge has enabled the identification of new IP3-mediated processes in C. elegans. Despite this, others may well remain to be identified. In particular, IP3Rs are expressed in the excretory cell and neurons and no known functional correlates have yet been found for these sites of expression. The application of the IP3 sponge leads to new tools for dissecting these processes in C. elegans and other animals.
ACKNOWLEDGMENTS
We are grateful to P. Zipperlen and J. Ahringer for help in analyzing embryos and for antibodies. The IP3 sponge was so-named by K. Mikoshiba. We thank A. Fire for heat-shock and RNAi feeding vectors; J. McGhee for JM73; S. Strome for the anti-PGL-1 antibody; the Caenorhabditis Genetic Center for C. elegans strains; and K. Mikoshiba, T. Furuichi, F. Yoshikawa, and J. Hirota for helpful discussions and for communicating unpublished data. This work was supported by the Medical Research Council (United Kingdom) (to H.A.B., S.L., and D.S.W.) and the Biotechnology and Biological Sciences Research Council (United Kingdom) (to N.J.D.G.). H.A.B. is a Medical Research Council Senior Fellow.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0422. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–08–0422.
REFERENCES
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–887. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- Avery L, Thomas JH. Feeding and Defecation. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. pp. 679–716. [PubMed] [Google Scholar]
- Babu P. Biochemical genetics of Caenorhabditis elegans. Mol Gen Genet. 1974;135:39–44. [Google Scholar]
- Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1) J Mol Biol. 1999;294:467–476. doi: 10.1006/jmbi.1999.3229. [DOI] [PubMed] [Google Scholar]
- Becchetti A, Whitaker M. Lithium blocks cell cycle transitions in the first cell cycles of sea urchin embryos, an effect rescued by myo-inositol. Development. 1997;124:1099–1107. doi: 10.1242/dev.124.6.1099. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium: two interacting second messengers. Am J Nephrol. 1997;17:1–11. doi: 10.1159/000169064. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Creton R, Speksnijder JE, Jaffe LF. Patterns of free calcium in zebrafish embryos. J Cell Sci. 1998;111:1613–1622. doi: 10.1242/jcs.111.12.1613. [DOI] [PubMed] [Google Scholar]
- Croll NA, Smith JM. Integrated behavior in the feeding phase of C. elegans (Nematoda) J Zool. 1978;184:507–517. [Google Scholar]
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the α1-β anchoring site in voltage-dependent Ca2+ channels. J Biol Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- Dixon DK, Jones D, Candido EP. The differentially expressed 16-kD heat shock genes of Caenorhabditis elegans exhibit differential changes in chromatin structure during heat shock. DNA Cell Biol. 1990;9:177–191. doi: 10.1089/dna.1990.9.177. [DOI] [PubMed] [Google Scholar]
- Fukushige T, Hawkins MG, McGhee JD. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol. 1998;198:286–302. [PubMed] [Google Scholar]
- Fukushige T, Hendzel MJ, Bazett-Jones DP, McGhee JD. Direct visualization of the elt-2 gut-specific GATA factor binding to a target promoter inside the living Caenorhabditis elegans embryo. Proc Natl Acad Sci USA. 1999;96:11883–11888. doi: 10.1073/pnas.96.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. An analysis of the response to gut induction in the C. elegans embryo. Development. 1995;121:1227–1236. doi: 10.1242/dev.121.4.1227. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Van Oostveldt P, Dhont M. Presence and dynamic redistribution of type 1 inositol 1,4,5-trisphosphate receptors in human oocytes and embryos during in-vitro maturation, fertilization and early cleavage divisions. Mol Human Reprod. 1999;5:441–451. doi: 10.1093/molehr/5.5.441. [DOI] [PubMed] [Google Scholar]
- Gower NJD, Temple GR, Schein JE, Marra M, Walker DS, Baylis HA. Dissection of the promoter region of the inositol 1,4,5-trisphosphate receptor gene. itr-1, in C. elegans: a molecular basis for cell-specific expression of IP3R isoforms. J Mol Biol. 2001;306:145–157. doi: 10.1006/jmbi.2000.4388. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode C. elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF, Schell MJ. Back in the water: the return of the inositol phosphates. Nat Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. BBA-Mol Cell Biol L. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Shim Y-H, Kirchner J, Kaminker J, Wood WB, Strome S. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–645. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- Knight JK, Wood WB. Gastrulation initiation in Caenorhabditis elegans requires the function of a gad-1, which encodes a protein with WD repeats. Dev Biol. 1998;198:253–265. [PubMed] [Google Scholar]
- Laufer JS, Bazzicalupo P, Wood WB. Segregation of developmental potential in embryos of Caenorhabditis elegans. Cell. 1980;19:569–577. doi: 10.1016/s0092-8674(80)80033-x. [DOI] [PubMed] [Google Scholar]
- Liu DWC, Thomas JH. Regulation of a periodic motor program in C. elegans. J Neurosci. 1994;14:1953–1962. doi: 10.1523/JNEUROSCI.14-04-01953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. London: Academic Press; 1995. pp. 452–482. [Google Scholar]
- Nishiwaki K, Sano T, Miwa J. emb-5, a gene required for the correct timing of gut precursor cell division during gastrulation in Caenorhabditis elegans, encodes a protein similar to the yeast nuclear protein spt-6. Mol Gen Genet. 1993;239:313–322. doi: 10.1007/BF00276929. [DOI] [PubMed] [Google Scholar]
- Powell-Coffman JA, Knight JK, Wood WB. Onset of C. elegans gastrulation is blocked by inhibition of embryonic transcription with an RNA polymerase antisense RNA. Dev Biol. 1996;178:472–483. doi: 10.1006/dbio.1996.0232. [DOI] [PubMed] [Google Scholar]
- Priess JR, Thomson JN. Cellular interactions in early C. elegans embryos. Cell. 1987;48:241–250. doi: 10.1016/0092-8674(87)90427-2. [DOI] [PubMed] [Google Scholar]
- Raghu P, Colley NJ, Webel R, James T, Hasan G, Danin M, Selinger Z, Hardie RC. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol Cell Neurosci. 2000;15:429–445. doi: 10.1006/mcne.2000.0846. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Paredez AR, Smith CW, McDonald KL, Aroian RV. The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode Caenorhabditis elegans. Genes Dev. 1999;13:2838–51. doi: 10.1101/gad.13.21.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode C. elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Hasan G. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr Biol. 1997;7:500–509. doi: 10.1016/s0960-9822(06)00221-1. [DOI] [PubMed] [Google Scholar]
- Waggoner LE, Tong-Zhou G, Schafer RW, Schafer WR. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron. 1998;21:203–214. doi: 10.1016/s0896-6273(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kanematsu T, Watanabe Y, Koga T, Ozaki S, Iwanaga S, Hirata M. D-myo-inositol 1,4,5-trisphosphate-binding proteins in rat brain membranes. J Biochem. 1994;115:973–980. doi: 10.1093/oxfordjournals.jbchem.a124447. [DOI] [PubMed] [Google Scholar]
- Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- Zwaal RR, Ahringer J, van Luenen HGAM, Rushforth A, Anderson P, Plasterk HA. G proteins are required for spatial orientation of early cell cleavages in C. elegans embryos. Cell. 1996;86:619–629. doi: 10.1016/s0092-8674(00)80135-x. [DOI] [PubMed] [Google Scholar]