Abstract
Background
Although depression is well established as an independent risk factor for cardiovascular disease (CVD) in the nonpregnant population, this association has largely not been investigated in pregnant populations. We aimed to estimate the cumulative risk of new CVD in the first 24 months postpartum among pregnant individuals diagnosed with prenatal depression compared with patients without depression diagnosed during pregnancy.
Methods and Results
Our longitudinal population‐based study included pregnant individuals with deliveries during 2007 to 2019 in the Maine Health Data Organization's All Payer Claims Data. We excluded those with prepregnancy CVD, multifetal gestations, or no continuous health insurance during pregnancy. Prenatal depression and CVD (heart failure, ischemic heart disease, arrhythmia/cardiac arrest, cardiomyopathy, cerebrovascular disease, and chronic hypertension) were identified by International Classification of Diseases, Ninth Revision (ICD‐9)/International Classification of Diseases, Tenth Revision (ICD‐10) codes. Cox models were used to estimate hazard ratios (HRs), adjusting for potential confounding factors. Analyses were stratified by hypertensive disorder of pregnancy. A total of 119 422 pregnancies were examined. Pregnant individuals with prenatal depression had an increased risk of ischemic heart disease, arrhythmia/cardiac arrest, cardiomyopathy, and new hypertension (adjusted HR [aHR], 1.83 [95% CI, 1.20–2.80], aHR, 1.60 [95% CI, 1.10–2.31], aHR, 1.61 [95% CI, 1.15–2.24], and aHR, 1.32 [95% CI, 1.17–1.50], respectively). When the analyses were stratified by co‐occurring hypertensive disorders of pregnancy, several of these associations persisted.
Conclusions
The cumulative risk of a new CVD diagnosis postpartum was elevated among individuals with prenatal depression and persists even in the absence of co‐occurring hypertensive disorders of pregnancy. Further research to determine the causal pathway can inform postpartum CVD preventive measures.
Keywords: arrhythmia, cardiomyopathy, cerebrovascular disease, chronic hypertension, ischemic heart disease, postpartum screening, prenatal depression
Subject Categories: Preeclampsia, Hypertension, High Blood Pressure
Nonstandard Abbreviations and Acronyms
- aHR
adjusted hazard ratio
Clinical Perspective.
What Is New?
Our cohort establishes the association between prenatal depression and new cardiovascular diagnoses within 24 months postpartum.
This association has been robustly described in the nonpregnant population; however, it has not been previously demonstrated in a pregnant population.
In the entire cohort, prenatal depression was most strongly associated with ischemic heart disease, and several of these associations between prenatal depression and cardiovascular diagnosis persisted even among the pregnancies without co‐occurring hypertensive disorders of pregnancy.
What Are the Clinical Implications?
In response to the American Heart Association's call to action to implement postpartum cardiovascular disease screening, all clinicians, including cardiologists, should consider reviewing a patient's pregnancy‐specific risk factors, including prenatal depression.
Future prospective interventional studies are needed to examine possible pharmacotherapeutic and lifestyle interventions that can target comorbid prenatal depression and cardiovascular disease in the pregnant and postpartum population.
A comprehensive understanding of all the pregnancy‐specific risk factors for cardiovascular disease is needed to reduce preventable pregnancy‐related morbidity and mortality attributable to cardiovascular disease.
Cardiovascular disease (CVD) is the leading cause of pregnancy‐related mortality, with 1 in 3 deaths attributable to heart disease, primarily from cardiomyopathy, cerebrovascular disease, and other CVD conditions. 1 , 2 , 3 In addition, most pregnancy‐related deaths are considered preventable (65.8%), according to maternal mortality review committees, where preventability determination was performed. 4 Therefore, it is crucial to identify the risk factors for CVD among pregnant people that can be intervened on. Furthermore, although studies have shown an association between pregnancy complications, including hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, and fetal growth restriction, with an increased subsequent risk of CVD, little attention has been paid to perinatal mental health as a CVD risk factor. 5 , 6 , 7 , 8 , 9
Given the high rate of preventable cardiovascular morbidity and mortality among women, the American Heart Association issued a call to action to use information from pregnancy as a “physiologic stress test” to identify people at increased risk for CVD and implement cardiovascular prevention during the unique window of opportunity postpartum. 10 , 11 , 12 However, our current understanding of risk factors for future CVD among postpartum patients is not comprehensive. A gap remains in understanding how prenatal depression and overall mental health disorders, both during and outside of pregnancy, affect CVD risk. The time is now to explore the possible contribution of prenatal depression to CVD risk, especially given the increasing prevalence of prenatal depression and the robust association of depression and CVD in the nonpregnant population. 13 , 14 , 15 , 16 , 17
Therefore, in our longitudinal population‐based study, our primary objective was to estimate the cumulative risk of new CVD diagnosis in the first 24 months postpartum among pregnant individuals with prenatal depression compared with those without depression during pregnancy. Given the known association of CVD and depression outside of pregnancy, our hypothesis was that patients with prenatal depression have an increased risk of new cardiovascular diagnoses during the initial postpartum period.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Maine Health Data Organization at https://mhdo.maine.gov/tableau/data.cshtml.
A longitudinal population‐based study of both multiparous and nulliparous people with singleton live birth or stillbirth gestations was conducted to compare those diagnosed with prenatal depression against those not diagnosed with depression during pregnancy. Data from the Maine Health Data Organization's All Payer Claims Data were used and included pregnant individuals with deliveries during 2007 to 2019 that were paid for by private and public insurers in Maine. 18 The largest possible sample size in our database, 13 years of deliveries, was used to maximize power. In addition to claims for hospital inpatient and outpatient visits, these data include claims paid for Maine residents by private and public insurers for office visits, clinic visits, and prescription claims, as required by state statute and rules. 18 The International Classification of Diseases, Clinical Modification (ICD‐CM), diagnosis and procedure codes, Current Procedural Terminology codes, and the Medicare Severity Diagnosis‐Related Group classification system were used to identify deliveries during the study period.
Prenatal depression was identified using the Mental Health Research Network International Classification of Diseases, Ninth Revision (ICD‐9)/International Classification of Diseases, Tenth Revision (ICD‐10), code list and excluded codes for depressive disorders in full remission (Table S1). 19 , 20 , 21 Prenatal depression was defined as a diagnosis date between 6 weeks of gestation and delivery date. Exclusion criteria included any diagnosis before pregnancy of the cardiovascular conditions being examined postpartum, records with implausible gestational age for stillbirth or live birth (<20 weeks), non‐Maine residents, multifetal gestation, those with implausible time to next pregnancy (<60 days), and those without health insurance during pregnancy and the first 2 months postpartum and in the month of delivery. Patients with multifetal gestation were excluded both because of their increased risk for pregnancy complications, such as preeclampsia, compared with their singleton counterparts and because of their increased risk for new and worsening mental health disorders. 22 , 23 , 24
CVD diagnosis, the primary outcome, was categorized into 6 subcategories: heart failure, ischemic heart disease, cerebrovascular disease/stroke, arrhythmia/cardiac arrest, cardiomyopathy, and chronic hypertension. We used previously published cross‐walked ICD‐9/ICD‐10 code lists and added “not otherwise specified” CVD diagnosis codes based on review by Maternal Fetal Medicine physicians (C.M.A.‐B. and H.S.L.; Table S1). 25 , 26 New‐onset chronic hypertension was defined from 43 days postpartum until 24 months postpartum because hypertension within 42 days of delivery is considered a hypertensive disorder of pregnancy. 27 , 28 A code list published by the Centers for Disease Control and Prevention was used for new‐onset chronic hypertension. 29 The remaining CVD outcomes were defined from delivery date until 24 months postpartum. Given the rarity of some of the CVD outcomes, a composite outcome of severe cardiac disease was developed that included heart failure, cerebrovascular disease/stroke, and cardiomyopathy.
Socioeconomic factors and access to care were not available in the All Payer Claims Data and, thus, were estimated by linking the data set to publicly available community‐level information. Zip code–level data on the median percentage of residents living below federal poverty level, of non‐White race, and on those who were adults with less than college educational attainment were identified from the American Community Survey 5‐year zip code files for all communities in Maine. 30 To assess access to care, information on the number of general practice and medical specialty physicians per capita from the Area Health Resources Files were linked by county Federal Information Processing Standard codes. 31
Insurance status was assessed using the All Payer Claims Data eligibility file. People were classified as Medicaid insured if they were enrolled in Medicaid during their delivery month 32 ; otherwise, they were classified as insured by commercial insurance or Medicare based on delivery month enrollment information. We compared baseline characteristics and demographics for patients who lost health insurance within 1 year postpartum versus those with at least 1 year of postpartum coverage to see if insurance coverage windows affected estimates of prepregnancy, prenatal, and postpartum medical conditions.
Statistical Analysis
To estimate the prenatal depression hazard ratios (HRs) and 95% CIs for time to first diagnosis for each of the 6 cardiovascular conditions in the first 24 months postpartum, we used Cox proportional hazard models. Models were adjusted for potential confounders, including maternal age, prepregnancy depression, prepregnancy hypertension, prepregnancy diabetes, obesity, smoking, nulliparity, pregnancy number in data set, year of delivery, Medicaid coverage during pregnancy, county‐level measures, zip code–level measures (including percentage non‐White race by zip code), co‐occurring hypertensive disorders of pregnancy, and co‐occurring gestational diabetes. Models did not adjust for the individual's race or ethnicity as this information was not included in the data set. We did not include factors that could have been consequences of prenatal depression (eg, cesarean section or postpartum depression as a result of prenatal depression) as potential confounders. We also did not include mental health conditions diagnosed during pregnancy as potential confounders, as these could have been along the causal pathway from prenatal depression to the postpartum CVD diagnosis. 33 However, as a sensitivity analysis, we further adjusted for anxiety during pregnancy, which was the most common mental health condition diagnosed during pregnancy, to see how our findings would change with adjustment for this potential intermediate.
Observations were censored on loss of health insurance coverage, start of next pregnancy, or at 24 months, whichever came first. Analyses were also stratified by co‐occurring hypertensive disorders of pregnancy to further control for the confounding effect of this condition. To estimate the cumulative risk for each of the 6 CVD conditions diagnosed in the first 24 months postpartum, we used unadjusted Cox proportional hazard models stratified by prenatal depression. To assess the proportional hazards assumption and visualize the survival curves, we used inverse probability of treatment‐weighted Cox proportional hazards models. 34
We used Structured Query Language to create the analytical files from the relational database and used SAS version 9.4 to perform the statistical analysis. We did not use missing data methods to impute diagnoses and dates when they were not recorded in the claims. This study was determined to be exempt from human subject review by the University of Southern Maine's Institutional Review Board.
RESULTS
Of the 166 053 unique pregnancies in the Maine Health Data Organization's All Payer Claims Data from 2006 to 2021, we included 119 422 pregnancies in our analysis (Figure 1). Prenatal depression prevalence was 21.6%. Baseline characteristics were similar between the groups, with the exception that some characteristics were more commonly observed in the prenatal depression group, such as use of Medicaid insurance and prepregnancy depression and anxiety (Table 1). In both groups, loss of health insurance within 1 year of delivery was common (23.7% among those with prenatal depression and 29.4% among those without prenatal depression). In the analysis of patients by insurance coverage window, there were some differences in maternal and pregnancy characteristics; for example, the prevalence of prepregnancy medical conditions was higher among those with longer insurance coverage (Table S2). The overall cumulative risk of postpartum depression in our entire cohort was 29.0%. Among those without prenatal depression, the cumulative risk was 22.5%; and among those with prenatal depression, the cumulative risk was 75.2% for postpartum depression.
Figure 1. Analytic sample identification.
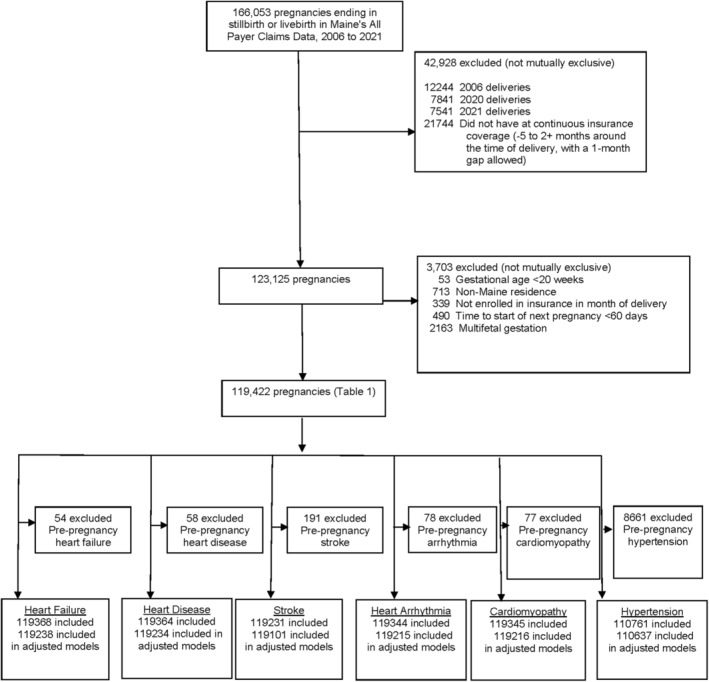
Table 1.
Baseline Characteristics, Deliveries in Maine From 2017 to 2019
| Characteristic | Total | Prenatal depression (6 wk gestation to delivery) | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| No. | No. | % | No. | % | |
| Total | 119 422 | 93 585 | 100 | 25 837 | 100 |
| Maternal age at delivery, y | |||||
| Missing | 50 | 40 | 0.0 | 10 | 0.0 |
| 15–19 | 7392 | 5294 | 5.7 | 2098 | 8.1 |
| 20–24 | 28 161 | 20 960 | 22.4 | 7201 | 27.9 |
| 25–29 | 35 761 | 28 362 | 30.3 | 7399 | 28.6 |
| 30–34 | 30 257 | 24 667 | 26.4 | 5590 | 21.6 |
| ≥35 | 17 801 | 14 262 | 15.2 | 3539 | 13.7 |
| Stillbirth | 626 | 462 | 0.5 | 164 | 0.6 |
| Gestational age at delivery | |||||
| At least 37 wk | 110 450 | 87 117 | 93.1 | 23 333 | 90.3 |
| 20 to <37 wk | 8972 | 6468 | 6.9 | 2504 | 9.7 |
| Cesarean section | 34 452 | 26 243 | 28.0 | 8209 | 31.8 |
| Delivery number in data set | |||||
| 1 | 82 459 | 65 834 | 68.3 | 16 625 | 62.3 |
| ≥2 | 40 666 | 30 599 | 31.7 | 10 067 | 37.7 |
| Health insurance coverage | |||||
| Medicaid | 66 014 | 47 119 | 50.4 | 18 895 | 73.1 |
| Private | 53 120 | 46 327 | 49.5 | 6793 | 26.3 |
| Medicare | 288 | 139 | 0.2 | 149 | 0.6 |
| Last month of continuous insurance postpartum, mo | |||||
| <6 | 23 179 | 19 025 | 20.3 | 4154 | 16.1 |
| 6–11 | 10 476 | 8513 | 9.1 | 1963 | 7.6 |
| 12–23 | 16 625 | 13 379 | 14.3 | 3246 | 12.6 |
| ≥24 | 69 142 | 52 668 | 56.3 | 16 474 | 63.8 |
| Hypertensive disorder of pregnancy | 14 637 | 10 937 | 11.7 | 3700 | 14.3 |
| Gestational diabetes | 10 101 | 7586 | 8.1 | 2515 | 9.7 |
| Anxiety during pregnancy | 15 454 | 3886 | 4.2 | 11 568 | 44.8 |
| Prepregnancy depression | 20 237 | 8859 | 9.5 | 11 378 | 44.0 |
| Prepregnancy anxiety | 15 486 | 7748 | 8.3 | 7738 | 30.0 |
| Prepregnancy hypertension* | 8661 | 6131 | 6.6 | 2530 | 9.8 |
| Prepregnancy diabetes | 3373 | 2227 | 2.4 | 1146 | 4.4 |
| Prepregnancy heart failure* | 54 | 36 | 0.0 | 18 | 0.1 |
| Prepregnancy ischemic heart disease* | 58 | 36 | 0.0 | 22 | 0.1 |
| Prepregnancy cerebrovascular disease/stroke* | 191 | 116 | 0.1 | 75 | 0.3 |
| Prepregnancy arrhythmia/cardiac arrest* | 78 | 57 | 0.1 | 21 | 0.1 |
| Any prepregnancy cardiomyopathy* | 77 | 50 | 0.1 | 27 | 0.1 |
| Zip code non‐White race, % | 5.6 | 5.5 | 0.0 | 5.9 | 0.0 |
Data source: Maine Health Data Organization's All Payer Claims Data.
Excluded from Cox regression modeling when examining specific cardiovascular disease outcome.
The cumulative risks of new diagnoses for CVD in the first 24 months postpartum were 0.2% for heart failure, ischemic heart disease, cardiomyopathy, and arrhythmia, 0.5% for stroke, and 2.1% for hypertension (Table 2). The cumulative risk of severe cardiac disease (composite of heart failure, stroke, or cardiomyopathy) was 0.8%. The cumulative risks of CVD diagnoses were higher for pregnancies complicated by prenatal depression compared with pregnancies that were not complicated by prenatal depression.
Table 2.
Cumulative Risk of New Diagnosis in the First 24 Months Postpartum for Cardiovascular Condition, by Exposure Status, Deliveries in Maine From 2007 to 2019
| Variable | Total (n=119 422) | No. of events | %* | Prenatal depression (n=25 837) | % Among exposed† | % Among unexposed† |
|---|---|---|---|---|---|---|
| Heart failure | 119 368 | 202 | 0.2 | 25 819 | 0.4 | 0.2 |
| Ischemic heart disease | 119 364 | 121 | 0.2 | 25 815 | 0.3 | 0.1 |
| Cerebrovascular disease/stroke | 119 231 | 443 | 0.5 | 25 762 | 0.7 | 0.4 |
| Arrhythmia/cardiac arrest | 119 344 | 151 | 0.2 | 25 816 | 0.3 | 0.1 |
| Cardiomyopathy | 119 345 | 213 | 0.2 | 25 810 | 0.3 | 0.2 |
| Chronic hypertension (≥43 d after delivery)† | 110 761 | 1662 | 2.1 | 23 307 | 3.1 | 1.9 |
| Severe cardiac disease | 119 124 | 741 | 0.8 | 25 726 | 1.2 | 0.7 |
Data source: Maine Health Data Organization's All Payer Claims Database.
Cumulative risk by 24 months. Censoring events were loss of health insurance coverage or start of the next pregnancy, whichever was earlier. Each model excluded records with any diagnosis before pregnancy of the cardiovascular conditions being examined postpartum, and records with implausible gestational age (<20 weeks), non‐Maine residence, and multifetal gestation, those with implausible time to next pregnancy (<60 days), and those without health insurance during pregnancy and the first 2 months postpartum and in the month of delivery.
Hypertension diagnoses in the first 42 days postpartum were excluded, as these were included in the definition of hypertensive disorders of pregnancy.
Adjusted HRs (aHRs) for new diagnoses of CVD within the first 24 months postpartum among people with prenatal depression were 1.40 (95% CI, 0.99–1.98) for heart failure, 1.83 (95% CI, 1.20–2.80) for ischemic heart disease, 1.27 (95% CI, 1.00–1.60) for cerebrovascular disease/stroke, 1.60 (95% CI, 1.10–2.31) for arrhythmia/cardiac arrest, 1.61 (95% CI, 1.15–2.24) for cardiomyopathy, and 1.32 (95% CI, 1.17–1.50) for hypertension (Table 3 and Figure 2). For severe cardiac disease, the aHR was 1.39 (95% CI, 1.16–1.67). When also adjusting for anxiety during pregnancy as a sensitivity analysis, the associations between prenatal depression and arrythmia/cardiac arrest, cardiomyopathy, chronic hypertension, and severe cardiac disease all persisted but were slightly attenuated. In addition, the associations between prenatal depression and ischemic heart disease and prenatal depression and cerebrovascular disease became nonsignificant (Table S3).
Table 3.
Risk of CVD Diagnosis in the First 24 Months Postpartum for People With Prenatal Depression, Deliveries in Maine From 2007 to 2019
| Variable | Unadjusted HR (95% CI) | aHR (95% CI)* |
|---|---|---|
| Heart failure | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 2.11 (1.59–2.81) | 1.40 (0.99–1.98) |
| Ischemic heart disease | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 3.22 (2.24–4.65) | 1.83 (1.20–2.80) |
| Cerebrovascular disease/stroke | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 1.71 (1.40–2.09) | 1.27 (1.00–1.60) |
| Arrhythmia/cardiac arrest | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 2.08 (1.50–2.90) | 1.60 (1.10–2.31) |
| Cardiomyopathy | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 1.92 (1.45–2.55) | 1.61 (1.15–2.24) |
| Chronic hypertension, ≥43 d after delivery | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 1.66 (1.50–1.85) | 1.32 (1.17–1.50) |
| Severe cardiac disease | ||
| No prenatal depression | Reference | Reference |
| Prenatal depression | 1.86 (1.60–2.17) | 1.39 (1.16–1.67) |
Data source: Maine Health Data Organization's All Payer Claims Database. aHR indicates adjusted HR; CVD, cardiovascular disease; and HR, hazard ratio.
Adjusted for maternal age at time of delivery, prepregnancy depression, prepregnancy hypertension, prepregnancy diabetes, obesity, smoking, nulliparity, pregnancy number in data set, year of delivery, Medicaid coverage during pregnancy, county‐level measures, zip code–level measures, hypertensive disorders of pregnancy, and gestational diabetes.
Figure 2. Weighted adjusted* cumulative hazard curves and 95% CI bands for new diagnosis of cardiovascular disease in the first 24 months postpartum among people with and without prenatal depression, deliveries in Maine from 2007 to 2019, n=119 422.
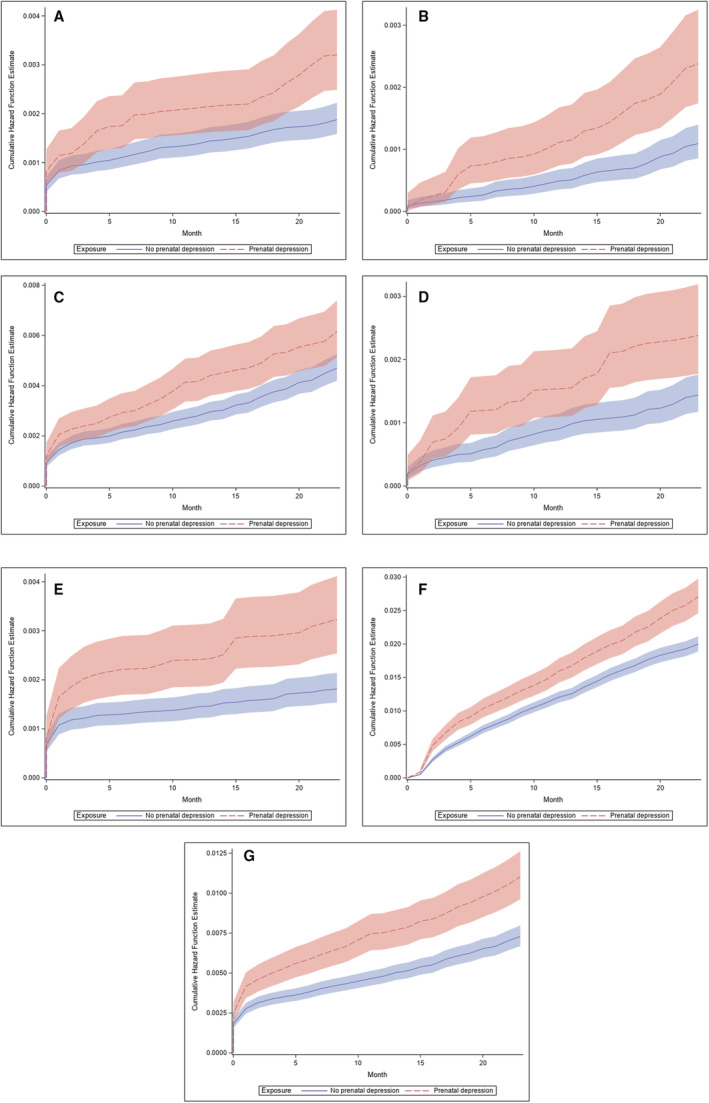
A, Heart failure. B, Ischemic heart disease. C, Cerebrovascular disease/stroke. D, Arrhythmia/cardiac arrest. E, Cardiomyopathy. F, Hypertension. G, Severe cardiac disease. * Adjusted for maternal age at time of delivery, prepregnancy depression, prepregnancy hypertension, prepregnancy diabetes, obesity, smoking, nulliparity, pregnancy number in data set, year of delivery, Medicaid coverage during pregnancy, county‐level measures, zip code–level measures, hypertensive disorders of pregnancy, and gestational diabetes.
When the analyses were stratified by co‐occurring hypertensive disorders of pregnancy, several of the associations between prenatal depression and new CVD diagnosis within 24 months postpartum persisted (Table 4). Specifically, among those without co‐occurring hypertensive disorders of pregnancy, prenatal depression was associated with an increased risk for ischemic heart disease (aHR, 1.84 [95% CI, 1.15–2.96]), cerebrovascular disease/stroke (aHR, 1.42 [95% CI, 1.09–1.86]), arrhythmia/cardiac arrest (aHR, 1.85 [95% CI, 1.26–2.72]), cardiomyopathy (aHR, 1.53 [95% CI, 1.02–2.31]), new hypertension (aHR, 1.43 [95% CI, 1.22–1.66]), and severe cardiac disease (aHR, 1.42 [95% CI, 1.15–1.75]). However, among those with co‐occurring hypertensive disorders of pregnancy, the associations between prenatal depression and cerebrovascular disease/stroke (aHR, 0.83 [95% CI, 0.52–1.33]) and arrhythmia/cardiac arrest (aHR, 0.63 [95% CI, 0.20–1.94]) were null. The association between prenatal depression and heart failure (aHR, 1.39 [95% CI, 0.80–2.42]; and aHR, 1.39 [95% CI, 0.89–2.17]) was similar among those with and without hypertensive disorders of pregnancy.
Table 4.
Risk of CVD Diagnosis in the First 24 Months Postpartum for People With Prenatal Depression, Deliveries in Maine From 2007 to 2019, Stratified by Co‐Occurring Hypertensive Disorders of Pregnancy
| CVD outcome | No hypertensive disorders of pregnancy, | aHR (95% CI)* | Hypertensive disorders of pregnancy, | aHR (95% CI)* |
|---|---|---|---|---|
| event/total | event/total | |||
| Heart failure | 125/104 622 | 77/14 616 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.39 (0.89–2.17) | 1.39 (0.80–2.42) | ||
| Ischemic heart disease | 89/104 629 | 30/14 614 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.84 (1.15–2.96) | 1.83 (0.77–4.39) | ||
| Cerebrovascular disease/stroke | 344/104 508 | 99/14 593 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.42 (1.09–1.86) | 0.83 (0.52–1.33) | ||
| Arrhythmia/cardiac arrest | 129/104 606 | 22/14 609 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.85 (1.26–2.72) | 0.63 (0.20–1.94) | ||
| Cardiomyopathy | 135/104 606 | 78/14 610 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.53 (1.02–2.31) | 1.69 (0.97–2.95) | ||
| Hypertension (≥43 d) | 1017/101 344 | 644/9293 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.43 (1.22–1.66) | 1.17 (0.97–1.43) | ||
| Severe cardiac disease | 539/104 423 | 202/14 572 | ||
| No prenatal depression | Reference | Reference | ||
| Prenatal depression | 1.42 (1.15–1.75) | 1.31 (0.94–1.84) |
Data source: Maine Health Data Organization's All Payer Claims Database. aHR indicates adjusted hazard ratio; and CVD, cardiovascular disease.
Adjusted for maternal age at time of delivery, prepregnancy depression, prepregnancy hypertension, prepregnancy diabetes, obesity, smoking, nulliparity, pregnancy number in data set, year of delivery, Medicaid coverage during pregnancy, county‐level measures, zip code–level measures, and gestational diabetes.
DISCUSSION
Pregnant individuals with prenatal depression have a higher risk of having new CVD diagnoses within 2 years of delivery compared with those without prenatal depression, even after adjusting for potential confounders. Several of these associations between prenatal depression and CVD diagnosis persisted even among the pregnancies without co‐occurring hypertensive disorders of pregnancy. Overall, of the 6 CVD outcomes we examined, the strongest association was found for prenatal depression and ischemic heart disease. Generally, the associations of prenatal depression with CVD were consistent between those with and without hypertensive disorders of pregnancy; however, there was some evidence to suggest a stronger association between prenatal depression and cerebrovascular disease/stroke and arrhythmia/cardiac arrest among those without hypertensive disorders of pregnancy. In addition, associations between prenatal depression and some CVD outcomes were attenuated after adjusting for anxiety during pregnancy, suggesting that prenatal depression alone may not increase the risk for these CVD diagnoses.
Although the associations we found for prenatal depression and CVD persisted after adjustment for potential confounders, further research to determine the causal pathway in pregnant populations is needed. For nonpregnant populations, several mechanisms have been proposed about the causal pathway. 15 One theory posits inflammation as the primary pathway between depression and CVD, with the chronic mental stress of depression leading to a sustained sympathetic activation and a proinflammatory state. 35 This is consistent with the well‐established effect of inflammation on endothelial dysfunction, a known precursor to atherosclerosis. 35 This may explain why ischemic heart disease had the strongest association with prenatal depression of the 6 CVD outcomes we examined because atherosclerosis is a major contributor to ischemic heart disease. Other causal mechanisms proposed include the effect of a mental health disorder on physical inactivity, a known contributor to cardiovascular morbidity and mortality. 36 Collection of biological data related to neurohormonal and endocrine changes related to depression could be an important next step to identify the causal pathway. Investigating these causal pathways is critical to inform clinical recommendations for postpartum management for individuals with prenatal depression.
Our findings are highly clinically relevant and timely for all providers who care for pregnant patients because prevalence is increasing for both prenatal depression and maternal cardiovascular morbidity and mortality. 3 , 13 Providers must first recognize the diagnostic challenges for new‐onset depressive symptoms during pregnancy and have a lower threshold of suspicion because there is widespread underdiagnosis of mental health disorders during pregnancy. 37 The diagnosis of depression during pregnancy may not only have critical implications for maternal mental health, but also for cardiovascular health. Therefore, system‐wide initiatives aimed at improving the diagnosis and treatment of depression during pregnancy could have downstream benefits for reducing cardiovascular morbidity for patients.
In nonpregnant populations, there is evidence of a strong graded association between depression and CVD, sudden cardiac death, and all‐cause mortality, with patients with more severe depression having higher risks of these outcomes. 14 , 15 , 16 , 17 Because nearly every major organ system undergoes significant physiologic changes during pregnancy, it is critical to examine the association of prenatal depression and CVD in pregnant individuals and not rely exclusively on the extrapolation of data in nonpregnant individuals. 38 , 39 , 40 Although one small prospective study found an association between anxiety and depression and preeclampsia, the association of prenatal depression and postpartum CVD has largely not been investigated. 41 , 42 With the growing research on shared pharmacotherapeutics to target the underlying pathophysiology of depression and CVD, 43 it is time to include the pregnant population in this research given the potential to develop a novel pharmacotherapeutic agent to both treat prenatal depression and reduce CVD postpartum. Future studies are needed to ascertain if treatment of prenatal depression with pharmacology and psychotherapy could mitigate postpartum cardiovascular risks. This could include observational studies examining antidepressants or psychotherapy as mediating factors. Findings from such studies are relevant to maternal and pediatric health given the associations between prenatal depression and adverse neonatal and pediatric outcomes. 44
Pregnant individuals in our study with prenatal depression were more likely to have Medicaid insurance, which, during the study period, was federally mandated only until 60 days postpartum. It is paramount for all patients, especially those with prenatal depression, to have access to continued health care after the so‐called “fourth trimester” (ie, the first 3 months postpartum). 45 As providers, we must respond to the American Heart Association's call to action through advocacy for continuation of insurance coverage for our patients and for continued research on postpartum cardiovascular health. The next step is to see if our study findings are reproduced in populations beyond Maine and then to develop the ideal postpartum cardiovascular screening modality. 12 Although current recommendations include CVD screening for those with known risk factors, such as hypertensive disorders of pregnancy, it is time to evaluate prenatal depression as another indication for postpartum CVD screening and implementation of preventive measures, such as diet and exercise regimens. 5 , 6 , 7 , 8 , 9 In fact, outside of pregnancy, providers are promoting exercise regimens as treatment for both depression and CVD. 46 Further research is needed to determine how to apply these approaches to our at‐risk postpartum patient population.
Our study had multiple strengths. First and foremost, it addresses a highly clinically relevant question about the contribution of prenatal depression to CVD within 24 months of delivery. It includes a large, economically diverse patient population given the distribution of patients with both Medicaid insurance and commercial private insurance and uses a robust, comprehensive database of inpatient, outpatient, office‐based, and clinic claims. In addition, our study examines the known association between prenatal depression and CVD in the general population as it applies to the pregnant population. This is warranted, as the pregnant population is too often excluded from clinical trials and inclusion of pregnant patients in research is critical to achieve equitable care for all. 47 Our prevalence of prenatal depression is consistent with prior reports that describe the period prevalence of depression from conception to delivery to be ≈20%. 13 Therefore, this implies that our study definition of prenatal depression is valid. In addition, we limited selection bias in our study design by using time‐to‐event analyses, censoring records at the month of insurance loss or start of next pregnancy, whichever came sooner. This mitigates the selection bias because all observations that met our initial inclusion criteria were included, not just those with a long duration of continuous postpartum insurance coverage. Our study results have substantial potential to inform future prospective interventional studies focused on how to improve current recommendations for postpartum screening and preventive measures.
Limitations include the lack of race and ethnicity information about the individuals in our data set, which would have been helpful for informing efforts to improve equity. Patient‐reported data on physical inactivity would have also been helpful to include as a potential confounder, given the known association of physical inactivity with both depression and CVD. 36 In addition, although our overall sample size was large, given the rarity of the 6 CVD outcomes, the number of CVD diagnosis events was small, especially in the analysis stratified by hypertensive disorders of pregnancy. The data we used omitted claims since January 2014 related to substance use disorder, in response to a regulation from the Substance Abuse and Mental Health Services Administration 18 ; this could have led to some of the study population with prenatal depression being incorrectly classified as not having prenatal depression because of missing claims. In addition, some private plans are exempt from submitting claims to the All Payer Claims Data (eg, certain self‐funded plans and plans with <$2 million in annual premium), leading to incomplete capture of deliveries in Maine paid for by private insurers. 18 We used a standard code list from recent peer‐reviewed publications to identify prenatal depression; however, these codes were not validated against the clinical diagnosis of depression. 20 , 21 In addition, there is a possibility that the ICD 9 and 10 codes do not accurately reflect all diagnoses, particularly for people without continuous insurance coverage or those who did not visit a health care provider for their medical condition. Last, our population is from a single state, reducing generalizability to patients across the United States. However, because Maine is the state with the greatest percentage of its residents living in rural areas, 48 our results are likely generalizable to other rural states. Therefore, further research is needed to evaluate this association of prenatal depression with CVD in other populations.
In conclusion, the cumulative risk of being diagnosed with a new cardiovascular condition within 2 years of delivery is greater among people with prenatal depression, even after adjusting for potential confounders. Further research to confirm our findings and identify the causal pathway for these associations is critical to inform recommendations for cardiovascular screening and prevention during the postpartum period for patients with prenatal depression.
Sources of Funding
The research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R15HD101793.
Disclosures
Kristin Palmsten receives research contracts from AbbVie, GSK (formerly GlaxoSmithKline), and Sanofi that are unrelated to this study.
Supporting information
Tables S1–S3
Acknowledgments
We thank the Maine Health Data Organization, which is responsible for the State of Maine's All Payer Claims Data. We used the Maine Health Data Organization's All Payer Claims Data as authorized under Data Request Number 2021040501. Each author has made substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; drafted the article and revised it critically for important intellectual content; and given final approval of the version to be published. Each author certifies that she has participated sufficiently in the work to believe in its overall validity and to take public responsibility for appropriate portions of its content.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028133
For Sources of Funding and Disclosures, see page 10.
References
- 1. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy‐related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366–373. doi: 10.1097/AOG.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis NL, Smoots AN, Goodman DA. Pregnancy‐Related Deaths: Data from 14 US Maternal Mortality Review Committees, 2008‐2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 5. Rich‐Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fraser A, Nelson SM, Macdonald‐Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wenger NK. Recognizing pregnancy‐associated cardiovascular risk factors. Am J Cardiol. 2014;113:406–409. doi: 10.1016/j.amjcard.2013.08.054 [DOI] [PubMed] [Google Scholar]
- 8. Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O'Brien S, Chew‐Graham CA, Verma G, Kadam UT, Mamas MA. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e007809. doi: 10.1161/JAHA.117.007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith GN, Louis JM, Saade GR. Pregnancy and the postpartum period as an opportunity for cardiovascular risk identification and management. Obstet Gynecol. 2019;134:851–862. doi: 10.1097/AOG.0000000000003363 [DOI] [PubMed] [Google Scholar]
- 10. Brown HL, Smith GN. Pregnancy complications, cardiovascular risk factors, and future heart disease. Obstet Gynecol Clin North Am. 2020;47:487–495. doi: 10.1016/j.ogc.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 11. Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council . Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 12. Mehta LS, Sharma G, Creanga AA, Hameed AB, Hollier LM, Johnson JC, Leffert L, McCullough LD, Mujahid MS, Watson K, et al. Call to action: maternal health and saving mothers: a policy statement from the American Heart Association. Circulation. 2021;144:e251–e269. doi: 10.1161/CIR.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 13. Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db [DOI] [PubMed] [Google Scholar]
- 14. Meng R, Yu C, Liu N, He M, Lv J, Guo Y, Bian Z, Yang L, Chen Y, Zhang X, et al. Association of depression with all‐cause and cardiovascular disease mortality among adults in China. JAMA Netw Open. 2020;3:e1921043. doi: 10.1001/jamanetworkopen.2019.21043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462 [DOI] [PubMed] [Google Scholar]
- 16. Shi S, Liu T, Liang J, Hu D, Yang B. Depression and risk of sudden cardiac death and arrhythmias: a meta‐analysis. Psychosom Med. 2017;79:153–161. doi: 10.1097/PSY.0000000000000382 [DOI] [PubMed] [Google Scholar]
- 17. Rajan S, McKee M, Rangarajan S, Bangdiwala S, Rosengren A, Gupta R, Kutty VR, Wielgosz A, Lear S, AlHabib KF, et al. Association of symptoms of depression with cardiovascular disease and mortality in low‐, middle‐, and high‐income countries. JAMA Psychiatry. 2020;77:1052–1063. doi: 10.1001/jamapsychiatry.2020.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Statute and Rules . Maine Health Data Organization. Accessed March 7, 2022, https://mhdo.maine.gov/rules.htm
- 19. Publicly‐available resources from the Mental Health Research Network. Accessed April 22, 2022, https://github.com/MHResearchNetwork/MHRN‐Central
- 20. Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164:1515–1520. doi: 10.1176/appi.ajp.2007.06111893 [DOI] [PubMed] [Google Scholar]
- 21. Avalos LA, Raine‐Bennett T, Chen H, Adams AS, Flanagan T. Improved perinatal depression screening, treatment, and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917–925. doi: 10.1097/AOG.0000000000001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wenze SJ, Battle CL, Tezanos KM. Raising multiples: mental health of mothers and fathers in early parenthood. Arch Womens Ment Health. 2015;18:163–176. doi: 10.1007/s00737-014-0484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Day MC, Barton JR, O'Brien JM, Istwan NB, Sibai BM. The effect of fetal number on the development of hypertensive conditions of pregnancy. Obstet Gynecol. 2005;106:927–931. doi: 10.1097/01.AOG.0000182578.82926.9c [DOI] [PubMed] [Google Scholar]
- 24. Sibai BM, Hauth J, Caritis S, Lindheimer MD, MacPherson C, Klebanoff M, VanDorsten JP, Landon M, Miodovnik M, Paul R, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal‐Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938–942. doi: 10.1016/S0002-9378(00)70350-4 [DOI] [PubMed] [Google Scholar]
- 25. Cartus AR, Jarlenski MP, Himes KP, James AE, Naimi AI, Bodnar LM. Adverse cardiovascular events following severe maternal morbidity. Am J Epidemiol. 2022;191:126–136. doi: 10.1093/aje/kwab208 [DOI] [PubMed] [Google Scholar]
- 26. Ackerman CM, Platner MH, Spatz ES, Illuzzi JL, Xu X, Campbell KH, Smith GN, Paidas MJ, Lipkind HS. Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization. Am J Obstet Gynecol. 2019;220:582.e1–582.e11. doi: 10.1016/j.ajog.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 27. Magee L, von Dadelszen P. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. 2013;Cd004351. doi: 10.1002/14651858.CD004351.pub3 [DOI] [PubMed] [Google Scholar]
- 28. Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S, Shahul SS, Thadhani R, Seely EW, Karumanchi SA, et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726–1733. doi: 10.1161/CIRCULATIONAHA.115.015721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Data on selected pregnancy complications in the United States. Centers for Disease Control and Prevention. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion; 2022. Accessed January 12, 2022. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy‐complications‐data.htm [Google Scholar]
- 30. American Community Survey . United States Census Bureau. Accessed January 31, 2019. https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml
- 31. Area Health Resource File . Health Resources & Services Administration. Accessed March 25, 2019. data.hrsa.gov.
- 32. Medicaid and CHIP income eligibility limits for pregnant women as a percent of the federal poverty level. Kaiser Family Foundation; 2022. Accessed March 7, 2022. https://www.kff.org/health‐reform/state‐indicator/medicaid‐and‐chip‐income‐eligibility‐limits‐for‐pregnant‐women‐as‐a‐percent‐of‐the‐federal‐poverty‐level/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D [Google Scholar]
- 33. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 35. Halaris A. Inflammation‐associated co‐morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45–70. doi: 10.1007/7854_2016_28 [DOI] [PubMed] [Google Scholar]
- 36. Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134:e262–e279. doi: 10.1161/CIR.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 37. Faisal‐Cury A, Levy RB, Azeredo CM, Matijasevich A. Prevalence and associated risk factors of prenatal depression underdiagnosis: a population‐based study. Int J Gynaecol Obstet. 2021;153:469–475. doi: 10.1002/ijgo.13593 [DOI] [PubMed] [Google Scholar]
- 38. Ajjimaporn A, Somprasit C, Chaunchaiyakul R. A cross‐sectional study of resting cardio‐respiratory and metabolic changes in pregnant women. J Phys Ther Sci. 2014;26:779–782. doi: 10.1589/jpts.26.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bobrowski RA. Pulmonary physiology in pregnancy. Clin Obstet Gynecol. 2010;53:285–300. doi: 10.1097/GRF.0b013e3181e04776 [DOI] [PubMed] [Google Scholar]
- 40. ACOG Practice Bulletin No. 211: critical care in pregnancy. Obstet Gynecol. 2019;133:e303–e319. doi: 10.1097/AOG.0000000000003241 [DOI] [PubMed] [Google Scholar]
- 41. Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:487–490. doi: 10.1016/s0029-7844(99)00602-x [DOI] [PubMed] [Google Scholar]
- 42. Nicholson L, Lecour S, Wedegärtner S, Kindermann I, Böhm M, Sliwa K. Assessing perinatal depression as an indicator of risk for pregnancy‐associated cardiovascular disease. Cardiovasc J Afr. 2016;27:119–122. doi: 10.5830/CVJA-2015-087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chávez‐Castillo M, Nava M, Ortega Á, Rojas M, Núñez V, Salazar J, Bermúdez V, Rojas‐Quintero J. Depression as an Immunometabolic disorder: exploring shared pharmacotherapeutics with cardiovascular disease. Curr Neuropharmacol. 2020;18:1138–1153. doi: 10.2174/1570159X18666200413144401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tuovinen S, Lahti‐Pulkkinen M, Girchenko P, Lipsanen J, Lahti J, Heinonen K, Reynolds RM, Hämäläinen E, Kajantie E, Laivuori H, et al. Maternal depressive symptoms during and after pregnancy and child developmental milestones. Depress Anxiety. 2018;35:732–741. doi: 10.1002/da.22756 [DOI] [PubMed] [Google Scholar]
- 45. Tully KP, Stuebe AM, Verbiest SB. The fourth trimester: a critical transition period with unmet maternal health needs. Am J Obstet Gynecol. 2017;217:37–41. doi: 10.1016/j.ajog.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 46. Bueno‐Antequera J, Munguía‐Izquierdo D. Exercise and depressive disorder. Adv Exp Med Biol. 2020;1228:271–287. doi: 10.1007/978-981-15-1792-1_18 [DOI] [PubMed] [Google Scholar]
- 47. Committee Opinion No. 646: ethical considerations for including women as research participants. Obstet Gynecol. 2016;127:e100–e107. doi: 10.1097/AOG.0000000000001150 [DOI] [PubMed] [Google Scholar]
- 48. Growth in urban population outpaces rest of nation, Census Bureau Reports . United States Census Bureau. Accessed January 31, 2019. https://www.census.gov/newsroom/releases/archives/2010_census/cb12‐50.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


