Abstract
Background
For frail patients with limited life expectancy, time in hospital following transcatheter aortic valve replacement is an important measure of quality of life; however, data remain scarce. Thus, we aimed to investigate frailty and its relation to time in hospital during the first year after transcatheter aortic valve replacement.
Methods and Results
From 2008 to 2020, all Danish patients who underwent transcatheter aortic valve replacement and were alive at discharge were included. Using the validated Hospital Frailty Risk Score, patients were categorized in the low, intermediate, and high frailty groups. Time in hospital and mortality up to 1 year are reported according to frailty groups. In total, 3437 (57.6%), 2277 (38.1%), and 257 (4.3%) were categorized in the low, intermediate, and high frailty groups, respectively. Median age was ≈81 years. Female sex and comorbidity burden were incrementally higher across frailty groups (low frailty: heart failure, 24.1%; stroke, 7.2%; and chronic kidney disease, 4.5%; versus high frailty: heart failure, 42.8%; stroke, 34.2%; and chronic kidney disease, 29.2%).
In the low frailty group, 50.5% survived 1 year without a hospital admission, 10.8% were hospitalized >15 days, and 5.8% of patients died. By contrast, 26.1% of patients in the high frailty group survived 1 year without a hospital admission, 26.4% were hospitalized >15 days, and 15.6% died within 1 year. Differences persisted in models adjusted for sex, age, frailty, and comorbidity burden (excluding overlapping comorbidities).
Conclusions
Among patients undergoing transcatheter aortic valve replacement, frailty is strongly associated with time in hospital and mortality. Prevention strategies for frail patients to reduce hospitalization burden could be beneficial.
Keywords: epidemiology, frailty, mortality, rehospitalization, transcatheter aortic valve replacement
Subject Categories: Epidemiology, Quality and Outcomes, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Valvular Heart Disease, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- TAVR
transcatheter aortic valve replacement
Clinical Perspective.
What Is New?
For highly frail patients, time in hospital is significant and mortality is high; almost half of patients spent >2 weeks in hospital or died within 1 year after transcatheter aortic valve replacement.
What Are the Clinical Implications?
The Hospital Frailty Risk Score represents a tool for identification of highly frail patients.
It can be used to guide resource management for pre–transcatheter aortic valve replacement and post–transcatheter aortic valve replacement optimization to improve outcomes.
Frailty, characterized as a decreased physiological reserve and diminished resistance to stressors, is common among elderly patients undergoing transcatheter aortic valve replacement (TAVR) in everyday clinical practice. 1 , 2 Although the definitions of identifying frail patients have varied across previous studies, it is becoming an increasingly recognized risk factor for poor outcomes after TAVR, including short‐ and long‐term mortality and disabilities. 3 , 4 , 5 , 6 Consequently, with this limited life expectancy for frail patients, time in hospital represents a central parameter of patient autonomy, quality of life, and quality of care. However, data on the relation between frailty and time in hospital remain scarce.
Furthermore, as readmissions and the associated duration of hospital stays are associated with substantial societal costs, a reduction may also be associated with great societal benefits. Moreover, rehospitalizations have gained increased attention and even been included in the primary end point of a major randomized controlled trial. 7 , 8 Despite frailty's importance in patient selection and recommendations from guidelines to assess it, no universally accepted frailty assessment tool exists. 9 , 10 Assessing frailty with clinical frailty tools can be time‐consuming, may be subject to interobserver variability, and may not be standard procedure. 11
Thus, to address these gaps in knowledge, we sought to investigate the relationship between frailty and time spent in hospital and readmission after TAVR using a validated frailty risk score leveraging administrative data. 12
Methods
Because of Danish laws, the underlying data, analytical methods, and study materials of the study cannot be shared. Moreover, no informed consent from patients is required.
Data Sources
This study leveraged data from Danish nationwide registries: through a unique personal identification provided to all permanent Danish residents, it is possible to cross‐link information from the following registries at an individual level: the Danish Civil Registration System, 13 the Danish National Patient Registry, 14 and the Danish National Prescription Registry were used. 15 These registries have previously been described. 16 All registries have been extensively used for research purposes. The overall positive predictive value of cardiac diagnoses, procedures, and surgeries is high. 17 , 18
Study Design, Population, and Outcome
This was a nationwide retrospective cohort study in which all patients undergoing first‐time TAVR and surviving until discharge were identified from 2008 through 2020. As data on all hospital contacts were available through 2021, all patients included had a possibility of 1 year of full follow‐up. Thus, patients were followed up until death, emigration, 1 year of follow‐up, or December 31, 2021, whichever came first.
The outcome of interest was total number of days spent hospitalized: During follow‐up, all overnight hospital admissions were identified. For each patient, the total number of unique hospitalizations and the time spent hospitalized (length of stay) were summed. The secondary outcome of interest was the proportion of patients dying within 1 year of follow‐up.
Definition of Frailty, Comorbidities, and Comedication
To stratify patients in frailty groups at time of TAVR, we used The Hospital Frailty Risk Score. 12 It is a validated frailty risk assessment tool based on International Classification of Diseases, Tenth Revision (ICD‐10), codes originally developed, internally, and externally validated among older patients in the United Kingdom (Table S1 provides codes). From information on previous hospital admissions up to 10 years before date of TAVR, a score was calculated for each patient. Then, patients were categorized as low, intermediate, and high frailty risk if they scored 0 to 4, 5 to 15, and >15 points, respectively. Patients with a score ≥5 were considered frail. The Hospital Frailty Risk Score has been compared with 2 measures of frailty: the Fried Phenotype, in which a clinical frailty assessment is performed; and the Rockwood Frailty Index, consisting of a deficit accumulation that does not require a clinical evaluation. The comparison showed a fair overlap (κ scores 0.22 and 0.30, respectively). 12
As done previously, other individual comorbidities were identified from inpatient and outpatient hospital contacts registered with a primary and optional secondary diagnosis codes in a period of up to 10 years before date of TAVR (Table S2 provides codes). 19 For diabetes, a filled prescription of a glucose‐lowering drug was used as a proxy. 20 For comedication, all filled prescriptions at a Danish pharmacy in a period of up to 180 days before TAVR were identified (Table S2 provides codes). Likewise, ≥2 prescriptions filled for blood pressure–lowering drugs were used as a proxy for hypertension. 21
Statistical Analysis
Baseline characteristics are presented with counts and percentages and medians and interquartile ranges (IQRs) for categorical and continuous variables, respectively. To illustrate differences in time in hospital, patients were categorized into the following categories: “never hospitalized,” “hospitalized 1 to 14 days,” “hospitalized 15 to 28 days,” and “hospitalized >28 days.” A final category, “died,” comprised the proportion of patients dying within 1 year of follow‐up regardless of any prior admissions (ie, if a patient was hospitalized for 14 days and died subsequently, that patient was categorized as “died”). The same proportions were calculated according to frailty risk. Here, frailty groups were compared within each outcome category using the χ2 test. The 1‐year unadjusted risk of death was estimated with the reverse Kaplan‐Meier estimator. For adjusted analysis, we associated frailty groups with the composite outcome of >14 days of hospitalization or death in the first year after TAVR in a multivariable logistic regression model. The low frailty group was the reference group, and the model was adjusted for sex, age, calendar year groups of TAVR procedure, and comorbidities not reflected in the Hospital Frailty Risk Score (heart failure, diabetes, peripheral artery disease, liver disease, and chronic obstructive pulmonary disease). The additional adjustment variables were chosen to limit residual confounding. The level of statistical significance was set at 5%. Data management, analyses, figures, and tables were all done using statistical software R version 4.0.3. 22
Ethical Approval
The data responsible institution for this study was the Capital Region, and the study was performed under the approval number P‐2019‐191. In Denmark, retrospective cohort studies do not require approval from the Research Ethics Committee. To ensure data staypseudo anonymized, ranges and groups of observations with <5 patients were not reported.
Results
Population Characteristics
From 2008 to 2020, 5971 patients underwent first‐time TAVR and were discharged alive. The median age was 81 years, and 55.4% were men. Median length of stay for the TAVR procedure was 4 days (IQR, 2–7 days) and decreased to 2 days in 2020. The mean number of admissions in the year before TAVR was 1.9 (SD, 1.9), and the median was 1.0 (IQR, 1–3). The mean number of days spent hospitalized the year preceding TAVR was 9.2 days (SD, 13.6 days), and the median was 4 days (IQR, 1–12 days) (Table).
Table .
Baseline Characteristics According to Frailty Risk Group
| Characteristic | Frailty risk group | Total | ||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Total No. | 3437 | 2277 | 257 | 5971 |
| Frailty score, median (IQR) | 1.5 (0.0–2.6) | 6.9 (5.3–9.5) | 18.7 (16.6–22.9) | 3.2 (1.1–6.6) |
| Men, n (%) | 1904 (55.4) | 1268 (55.7) | 134 (52.1) | 3306 (55.4) |
| Age, median (IQR), y | 81 (77–85) | 82 (77–85) | 82 (76–85) | 81 (77–85) |
| Length of stay, median (IQR), d | 4 (2–7) | 4 (3–7) | 4 (2–8) | 4 (2–7) |
| Admission in preceding year, n | ||||
| Median (IQR) | 1 (1–2) | 2 (1–3) | 3 (1–4) | 1 (1–3) |
| Mean (SD) | 1.6 (1.5) | 2.3 (2.1) | 3.2 (3.1) | 1.9 (1.9) |
| Time in hospital in preceding year, d | ||||
| Median (IQR) | 3 (1–9) | 7 (2–16) | 12 (3–25) | 4 (1–12) |
| Mean (SD) | 6.9 (11.3) | 11.8 (15.1) | 17.3 (18.8) | 9.2 (13.6) |
| Comorbidities, n (%) | ||||
| Stroke/systemic embolism | 248 (7.2) | 476 (20.9) | 88 (34.2) | 812 (13.6) |
| Myocardial infarction | 359 (10.4) | 298 (13.1) | 47 (18.3) | 704 (11.8) |
| Ischemic heart disease | 1451 (42.2) | 1203 (52.8) | 142 (55.3) | 2796 (46.8) |
| Heart failure | 829 (24.1) | 789 (34.7) | 110 (42.8) | 1728 (28.9) |
| Atrial fibrillation | 1057 (30.8) | 897 (39.4) | 126 (49.0) | 2080 (34.8) |
| Peripheral artery disease | 306 (8.9) | 332 (14.6) | 58 (22.6) | 696 (11.7) |
| Previous PCI | 742 (21.6) | 598 (26.3) | 70 (27.2) | 1410 (23.6) |
| Previous CABG | 134 (3.9) | 126 (5.5) | 17 (6.6) | 277 (4.6) |
| Diabetes | 579 (16.8) | 481 (21.1) | 63 (24.5) | 1123 (18.8) |
| COPD | 355 (10.3) | 403 (17.7) | 64 (24.9) | 822 (13.8) |
| Chronic kidney disease | 156 (4.5) | 368 (16.2) | 75 (29.2) | 599 (10.0) |
| Liver disease | 50 (1.5) | 61 (2.7) | 9 (3.5) | 120 (2.0) |
| Pharmacotherapy, n (%) | ||||
| Acetylsalicylic acid | 1630 (47.4) | 1039 (45.6) | 93 (36.2) | 2762 (46.3) |
| ADP receptor antagonist | 749 (21.8) | 636 (27.9) | 76 (29.6) | 1461 (24.5) |
| Oral anticoagulants | 1033 (30.1) | 822 (36.1) | 106 (41.2) | 1961 (32.8) |
| β Blockers | 1646 (47.9) | 1186 (52.1) | 130 (50.6) | 2962 (49.6) |
| Statins | 2141 (62.3) | 1437 (63.1) | 154 (59.9) | 3732 (62.5) |
| Calcium channel blockers | 1124 (32.7) | 757 (33.2) | 67 (26.1) | 1948 (32.6) |
| Renin‐angiotensin system inhibitors | 1854 (53.9) | 1205 (52.9) | 119 (46.3) | 3178 (53.2) |
| Diuretics | 1333 (38.8) | 762 (33.5) | 56 (21.8) | 2151 (36.0) |
CABG, indicates coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; and PCI, percutaneous coronary intervention.
Of the 5971 patients, 3437 (57.6%), 2277 (38.1%), and 257 (4.3%) were categorized into low frailty group, intermediate frailty group, and high frailty group, respectively. The proportion of patients in the high frailty group increased during the study period from 7 of 375 (1.9%) high frailty patients in 2008 to 2010 to 140 of 3291 (4.3%) in 2017 to 2020 (Figure S1). Mean and median number of admissions in the year before TAVR increased from 1 and 1.6 in the low frailty group to 3 and 3.2 in the high frailty group. Moreover, the mean and median days in hospital in the year before TAVR increased from 3 and 6.9 in the low frailty group to 12 and 17.3 in the high frailty group. Cardiovascular and noncardiovascular comorbidity burden increased with higher frailty group (eg, history of stroke was present in 7.2% of the low frailty group versus 34.2% of the high frailty group, and history of chronic kidney disease was present in 4.5% versus 29.2%, respectively).
Time in Hospital and Mortality After TAVR
In the overall study population, 2646 of 5917 (44.3%) survived 1 full year without any readmissions, whereas the remaining 3271 of 5917 (55.7%) of patients were hospitalized at least once or died during follow‐up. Of these patients, 1284 (21.5%) were hospitalized for >2 weeks or died within 1 year of TAVR (Figure 1).
Figure 1. Overall time in hospital.
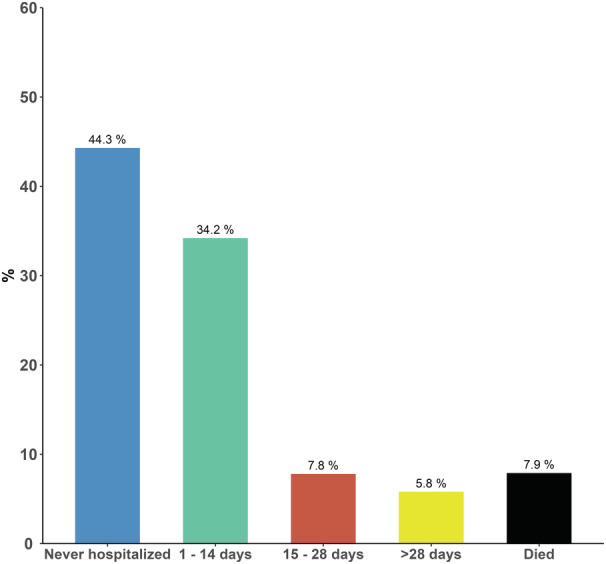
Proportion of patients who were never hospitalized, hospitalized for different time intervals, and died within 1 year of transcatheter aortic valve replacement. The figure shows that almost half of patients survived 1 year and were never hospitalized, whereas 21.5% of patients were hospitalized for >2 weeks or died within 1 year. The figure serves as a reference for the next figures, where the results are stratified on frailty group.
Time in hospital increased with higher frailty: In the low frailty group, 50.5% survived 1 year without a hospital admission, and 10.8% were hospitalized for >2 weeks. By contrast, 26.1% of patients in the high frailty group survived 1 year without a hospital admission, and 26.4% were hospitalized for >2 weeks (Figure 2). Mortality was incrementally higher according to frailty group: The 1‐year risk of death was 5.8% of patients in the low frailty group compared with 15.6% of patients in the high frailty group (Figure 3). Of the 473 patients who died within 1 year of TAVR, the median time in hospital was 17 days (IQR, 7–35 days). Time in hospital was lowest for low frailty patients who died within 1 year and highest for high frailty patients (low frailty: 14 days [IQR, 4–26 days] versus high frailty: 18 days [IQR, 9–42 days]).
Figure 2. Time in hospital according to frailty.
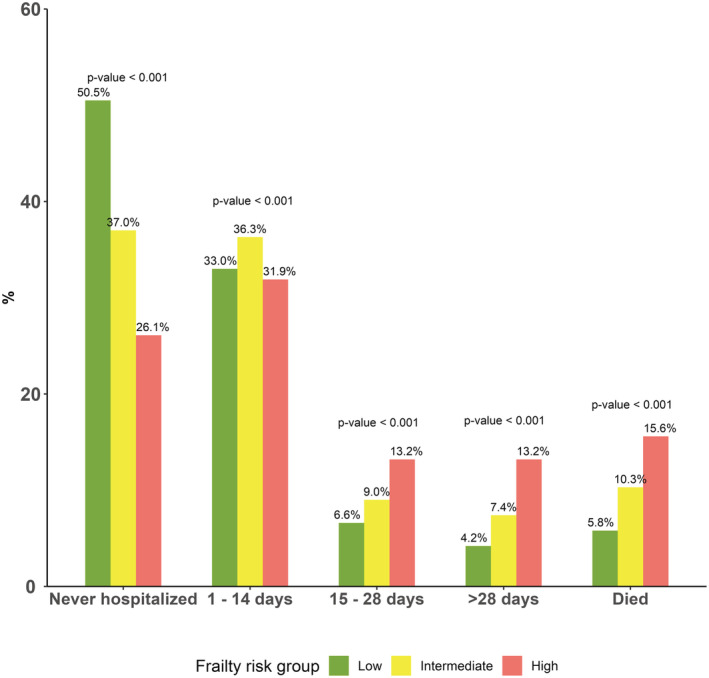
Proportion of patients who were never hospitalized, hospitalized for different intervals, and died within 1 year of transcatheter aortic valve replacement stratified on frailty groups. The percentages above bars sum to 100% for each frailty group (ie, all green bars sum to 100%). For low frailty patients, 50% survived 1 year and were never hospitalized compared with 25% of high frailty patients. In the low frailty group, 10.9% were hospitalized for >2 weeks compared with 28.1% in the high frailty group. As such, time in hospital is incrementally higher across frailty groups. The P values from χ2 test are presented above bars.
Figure 3. Risk of death.
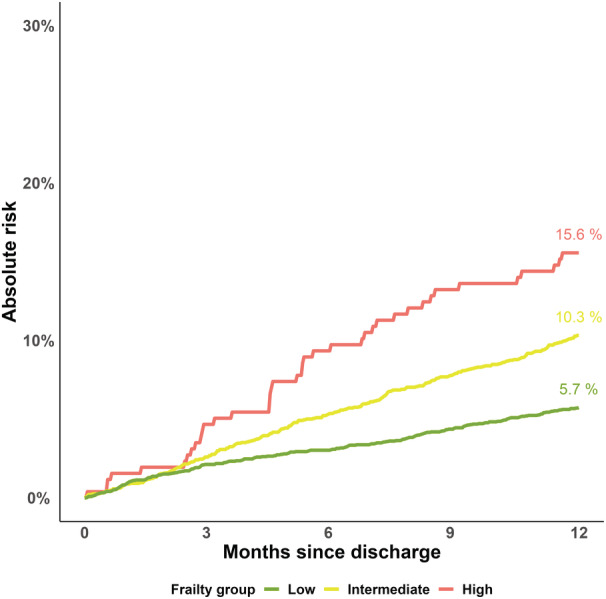
The 1‐year risk of death according to frailty. The x axis represents months since transcatheter aortic valve replacement discharge. The y axis depicts the absolute risk of death. In the low frailty group, 5.9% died within 1 year compared with 16.1% in the high frailty group. As such, mortality is incrementally higher across frailty groups.
From the multivariable logistic regression model of the composite outcome consisting of death or >14 days of hospitalization, increasing frailty group was associated with higher odds ratios of the outcome. The odds ratio was 1.65 (95% CI, 1.45–1.89) for the intermediate frailty group and 3.17 (95% CI, 2.41–4.17) for the high frailty group compared with the low frailty group.
Discussion
In this nationwide study investigating frailty and time in hospital following TAVR, the main findings were as follows: (1) The proportion of high frailty patients was only 4.3%; however, the proportion increased throughout the study period. (2) Half of patients in the low frailty risk group versus one‐quarter of patients in the high frailty risk group survived 1 year after TAVR and were never hospitalized. (3) In the low frailty risk group, 16.6% of patients were admitted for >2 weeks or died within 1 year versus 42.0% in the high frailty risk group.
Frailty in TAVR
Previous studies have investigated the relation of frailty on outcomes after TAVR. 5 , 23 , 24 , 25 Abugroun et al reported higher in‐hospital mortality and complications in a large sample of patients undergoing TAVR. 23 Moreover, Kundi et al found frailty to be a predictor of long‐term mortality. 5 Finally, previous studies leveraging data from trials and trial registers found higher degrees of frailty to be associated with a poor prognosis. 24 , 25 Altogether with frailty being a marker of poor clinical outcomes and high mortality, the quality of life of patients is important, especially in those with a limited life expectancy. Here, time in hospital (inversely home time) is a good measure. Yet, the aforementioned studies leverage trial data with strict inclusion criteria (eg, few patients with impaired renal function despite patients with impaired renal function constituting a large proportion of patients undergoing TAVR 24 , 25 ) or focus on short‐term clinical outcomes. 23
We found an overall prevalence of 3.8% high frailty risk patients alive at discharge, and the absolute number of high frailty risk patients increased from 7 (1.9%) in 2008 to 2010 to 129 (4.3%) in 2017 to 2020. A study by Kundi et al investigating frailty and mortality included all patients undergoing TAVR in 2016 from the Centers of Medicare and Medicaid Services Medicare Provider and Review database. 1 Using the same methods to assess frailty risk, they reported a prevalence of 8.1% high frailty risk patients. However, they included all patients undergoing TAVR, whereas our study only included patients alive at discharge (12 high frailty patients did not survive until discharge). Furthermore, our study period spanned from 2008 to 2020, and previous studies have demonstrated that the comorbidity burden of patients is decreasing over time. 16 , 26 Last, our study included all patients undergoing TAVR, whereas Kundi et al included patients in the Centers of Medicare and Medicaid Services Medicare Provider and Review database, which may altogether partially explain the difference in prevalence. Despite these differences in prevalence of high frailty risk patients, frail patients continue to undergo TAVR and the use of TAVR is also increasing. 27 Thus, frailty among patients undergoing TAVR is still important to consider.
Mortality and Time in Hospital According to Frailty Risk
Kundi et al also reported 1‐year mortality rates of 7.6% for low frailty risk patients and 30.1% for high frailty risk patients. 1 We found that 5.8% of low frailty patients died within 1 year and 15.6% of high frailty patients died within 1 year. In addition, previous studies have reported high overall rates of 1‐year rehospitalization ranging from 12.0% to 53.2%. 28 Thus, with a high 1‐year mortality rate and high overall rehospitalization rates, time in hospital is important for frail patients. Our study adds valuable information on this matter, as we focused on the time in hospital according to frailty risk; 50.5% of low frailty risk patients survived 1 year and were not hospitalized in the first year following TAVR, whereas 26.1% of high frailty risk patients survived 1 year without any hospitalizations. Notably, >15% of high frailty risk patients were in hospital for >28 days compared with 4.2% for low frailty risk patients. Not only is this easy‐to‐understand information for the physicians to convey and discuss with patients, but it also represents an area for future focus on strategies to reduce mortality and rehospitalizations (eg, optimizing pre‐TAVR condition and appropriate selection of patients). However, it is unclear what measures should be implemented to meet these needs. Currently, clinical trials investigating the effect of prehabilitation on outcomes after TAVR are underway (TAVR‐FRAILTY [Prehabilitation to Improve Functional and Clinical Outcomes in Patients With Aortic Stenosis], NCT02597985; and TAVR‐Prehab [Prehabilitation for Patients Undergoing Trans‐catheter Aortic Valve Replacement], NCT03107897).
Definition of Frailty
We used a frailty risk score based on electronic claims data, which reduces interobserver variability. 12 In addition to grading patients in a binary way into nonfrail (frailty score <5) and frail (frailty score ≥5) patients, it is possible to further categorize patients as low, intermediate, and high frailty. 12 As with other diseases, the degree of frailty may vary for patients. For our study, there was a marked difference in time in hospital between patients at intermediate frailty risk and high frailty risk, both of which are considered overall frail. For patients at intermediate frailty risk, 7.4% were hospitalized for >28 days and 10.3% died within 1 year compared with 13.2% and 15.6% for patients at high frailty risk. This suggests a more continuous spectrum of frailty rather than a binary nonfrail versus frail approach. It also offers a method to focus future strategies on smaller groups as only 257 patients were categorized as high frailty risk versus 2277 at intermediate frailty risk. Using a dichotomized system of frail versus nonfrail, the 257 patients at high frailty risk would be difficult to differentiate from the combined 2534 frail patients.
Moreover, the Hospital Frailty Risk Score focuses not only on chronic diseases, as other comorbidity index scores largely do (eg, the Charlson Index and the Elixhauser Comorbidity Index). 29 It also incorporates nonmedical conditions, such as unspecified fall, superficial injury of the head, fractures, and infections, all of which award substantial points to the individual's frailty risk. 12 However, it does not capture other areas, such as walking assistance devices.
Strengths and Limitations
A major strength of this study was the method of frailty assessment with the Hospital Frailty Risk Score in combination with robust underlying data. The Hospital Frailty Risk Score is easy to implement as the data are collected routinely and many countries use the ICD‐10 system; however, the performance of the model relies on the quality of the claims data used. The Danish National Patient Registry is complete and suitable for large‐scale epidemiological research. 30 Moreover, with the unique personal identification number used to cross‐link information from the other registries used, it is possible to observe patients from birth until emigration or death, limiting a potential underestimation of frailty because of a lack of data registration.
This study had limitations: the Hospital Frailty Risk Score assigns the different diagnoses a prespecified score. However, as stated in the original article, the severity of the diseases may vary (eg, the severity of ICD‐10 code N18: chronic renal failure). Furthermore, the Hospital Frailty Risk Score was not developed specifically for patients undergoing TAVR. Data on time spent in skilled nursing facilities were not available in the registers, which could have contributed to reflect home time rather than time in hospital. 31 However, the incidence of nursing home admission is low and comparable to that of the general population in Denmark. 32
Conclusions
Time in hospital for frail patients is significant, and rehospitalizations are common in the first year after TAVR. Frailty assessed with the Hospital Frailty Risk Score was associated with time in hospital and mortality and should be used for patient selection to take days outside the hospital into account when 1‐year life expectancy is estimated before referral for a TAVR. Prevention strategies for frail patients to reduce hospitalization burden are warranted.
Sources of Funding
The current study received funding in the form of external, independent grants from “Torben og Alice Frimodts Fond,” “Dagmar Marshalls Fond,” “Eva og Henry Frænkels Mindefond,” and “Snedkermester Sophus Jacobsen og Hustru Astrid Jacobsens Fond.” None of the funds had any influence on the conceptualization, data gathering, or writing of the manuscript.
Disclosures
Dr Butt reports advisory board honoraria from Bayer. Dr Køber reports speaker honorarium from Novo Nordisk, Novartis, AstraZeneca, Bayer, and Boehringer. Dr Olesen reports speaker honoraria or consultancy fees from Bayer, Bristol‐Myers Squibb, and Pfizer. Dr Fosbøl reports an independent research grant from Novo Nordisk Foundation. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figure S1
This article was sent to Saket Girotra, MD, SM, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029264
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Kundi H, Popma JJ, Reynolds MR, Strom JB, Pinto DS, Valsdottir LR, Shen C, Choi E, Yeh RW. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J. 2019;40:2231–2239. doi: 10.1093/eurheartj/ehz187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodríguez‐Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko‐Zajko W, Gonzalez‐Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition‐consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 4. Kiani S, Stebbins A, Thourani VH, Forcillo J, Vemulapalli S, Kosinski AS, Babaliaros V, Cohen D, Kodali SK, Kirtane AJ, et al. The effect and relationship of frailty indices on survival after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2020;13:219–231. doi: 10.1016/j.jcin.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 5. Kundi H, Valsdottir LR, Popma JJ, Cohen DJ, Strom JB, Pinto DS, Shen C, Yeh RW. Impact of a claims‐based frailty indicator on the prediction of long‐term mortality after transcatheter aortic valve replacement in Medicare beneficiaries. Circ Cardiovasc Qual Outcomes. 2018;11:e005048. doi: 10.1161/CIRCOUTCOMES.118.005048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoenenberger AW, Moser A, Bertschi D, Wenaweser P, Windecker S, Carrel T, Stuck AE, Stortecky S. Improvement of risk prediction after transcatheter aortic valve replacement by combining frailty with conventional risk scores. JACC Cardiovasc Interv. 2018;11:395–403. doi: 10.1016/j.jcin.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 8. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 9. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger E v, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71.doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 10. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, de Bonis M, de Paulis R, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2021;2021:1–72. doi: 10.1093/ejcts/ezac209 [DOI] [Google Scholar]
- 11. Allen C, Patterson T, Redwood S, Prendergast B. Frailty in patients undergoing transcatheter aortic valve replacement: frequently measured, seldom managed. JACC Cardiovasc Interv. 2020;13:232–234. doi: 10.1016/j.jcin.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 12. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 14. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 15. Pottegard A, Schmidt SAJ, Wallach‐Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strange JE, Sindet‐Pedersen C, Gislason GH, Torp‐Pedersen C, Kragholm KH, Lundahl C, Fosbøl EL, Butt JH, Køber L, Søndergaard L, et al. Temporal trends in utilization of transcatheter aortic valve replacement and patient characteristics: a nationwide study. Am Heart J. 2022;243:140–146. doi: 10.1016/j.ahj.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 17. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adelborg K, Sundbøll J, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiac examination, procedure and surgery codes in the Danish National Patient Registry: a population‐based validation study. BMJ Open. 2016;6:e012817. doi: 10.1136/bmjopen-2016-012817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strange JE, Fosbøl EL, Sindet‐Pedersen C, Havers‐Borgersen E, Køber L, Gislason GH, Olesen JB. Mortality at one year after transcatheter aortic valve replacement—relation of age and comorbidities. Int J Cardiol Heart Vasc. 2022;43:101157. doi: 10.1016/j.ijcha.2022.101157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carstensen B, Kristensen JK, Marcussen MM, Borch‐Johnsen K. The National Diabetes Register. Scand J Public Health. 2011;39:58–61. doi: 10.1177/1403494811404278 [DOI] [PubMed] [Google Scholar]
- 21. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen A‐MS, Gislason GH, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R‐project.org/ [Google Scholar]
- 23. Abugroun A, Daoud H, Hallak O, Abdel‐Rahman ME, Klein LW. Frailty predicts adverse outcomes in older patients undergoing transcatheter aortic valve replacement (TAVR): from the National Inpatient Sample. Cardiovasc Revasc Med. 2022;34:56–60. doi: 10.1016/j.carrev.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 24. Strom JB, Xu J, Orkaby AR, Shen C, Song Y, Charest BR, Kim DH, Cohen DJ, Kramer DB, Spertus JA, et al. Role of frailty in identifying benefit from transcatheter versus surgical aortic valve replacement. Circ Cardiovasc Qual Outcomes. 2021;14:14. doi: 10.1161/CIRCOUTCOMES.121.008566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnold SV, Zhao Y, Leon MB, Sathananthan J, Alu M, Thourani VH, Smith CR, Mack MJ, Cohen DJ. Impact of frailty and prefrailty on outcomes of transcatheter or surgical aortic valve replacement. Circ Cardiovasc Interv. 2022;15:15. doi: 10.1161/CIRCINTERVENTIONS.121.011375 [DOI] [PubMed] [Google Scholar]
- 26. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, et al. STS‐ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595 [DOI] [PubMed] [Google Scholar]
- 27. Durko AP, Osnabrugge RL, van Mieghem NM, Milojevic M, Mylotte D, Nkomo VT, Pieter KA. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635–2642. doi: 10.1093/eurheartj/ehy107 [DOI] [PubMed] [Google Scholar]
- 28. Sukul D, Bach DS. Readmissions after transcatheter aortic valve implantation. What are they doing right? How can we do better? Eur Heart J. 2017;38:2218–2220. doi: 10.1093/eurheartj/ehx252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 30. Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, Sørensen HT. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mentias A, Keshvani N, Desai MY, Kumbhani DJ, Sarrazin MV, Gao Y, Kapadia S, Peterson ED, Mack M, Girotra S, et al. Risk‐adjusted, 30‐day home time after transcatheter aortic valve replacement as a hospital‐level performance metric. J Am Coll Cardiol. 2022;79:132–144. doi: 10.1016/j.jacc.2021.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strange JE, Sindet‐Pedersen C, Holt A, Andersen MP, Torp‐Pedersen C, Køber L, Gislason GH, Olesen JB, Fosbøl EL. Nursing home admission following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2023;16:179–188. doi: 10.1016/j.jcin.2022.10.051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


