Abstract
Background
Two‐dimensional speckle tracking echocardiography has been shown to correlate with microvascular dysfunction, a hallmark of hypertrophic cardiomyopathy (HCM). We hypothesized that there is an association between myocardial work and left ventricular ischemia, with incremental value to global longitudinal strain, in patients with HCM.
Methods and Results
We performed a prospective assessment of patients with HCM, undergoing 2‐dimensional speckle tracking echocardiography and stress perfusion cardiac magnetic resonance. Results were stratified according to obstructive or nonobstructive HCM and the presence of significant replacement fibrosis (late gadolinium enhancement ≥15% of left ventricular mass). Seventy‐five patients with HCM (63% men, age 55±15 years) were evaluated, 28% with obstructive HCM (mean gradient 89±60 mm Hg). Perfusion defects were found in 90.7%, involving 22.5±16.9% of left ventricular mass, and 38.7% had late gadolinium enhancement ≥15%. In a multivariable analysis, a lower global work index (r=−0.519, β‐estimate −10.822; P=0.001), lower global work efficiency (r=−0.379, β‐estimate −0.123; P=0.041), and impaired global constructive work (r=−0.532, β‐estimate −13.788; P<0.001) significantly correlated with ischemia. A segmental analysis supported these findings, albeit with lower correlation coefficients. A global work index cutoff ≤1755 mm Hg% was associated with hypoperfusion with a sensitivity of 88% and a specificity of 71%, while the best cutoff for global longitudinal strain (>−15.5%) had a sensitivity of 64% and a specificity of 57%. The association between myocardial work and perfusion defects was significant independently of late gadolinium enhancement ≥15% and obstructive HCM.
Conclusions
Impaired myocardial work was significantly correlated with the extent of ischemia in cardiac magnetic resonance, independently of the degree of left ventricular hypertrophy or fibrosis, with a higher predictive power than global longitudinal strain.
Keywords: coronary microvascular dysfunction, hypertrophic cardiomyopathy, myocardial deformation, myocardial work, strain imaging
Subject Categories: Magnetic Resonance Imaging (MRI), Echocardiography, Cardiomyopathy
Nonstandard abbreviations and acronyms
- CMD
coronary microvascular dysfunction
- GCW
global constructive work
- GLS
global longitudinal strain
- GWE
global work efficiency
- GWI
global work index
- GWW
global wasted work
- HCM
hypertrophic cardiomyopathy
- LGE
late gadolinium enhancement
- MW
myocardial work
- NORRE
Normal Reference Ranges for Echocardiography
- TTE
transthoracic echocardiogram
Clinical Perspective.
What Is New?
In hypertrophic cardiomyopathy, impaired myocardial work parameters, including lower global work index, global work efficiency, and global constructive work, were significantly correlated with the presence and extent of myocardial ischemia in a cardiac magnetic resonance perfusion assessment, regardless of the degree of left ventricular hypertrophy or fibrosis.
A global work index cutoff value of ≤1755 mm Hg% showed a higher predictive power than global longitudinal strain for the presence of myocardial ischemia, regardless of the pattern of hypertrophy.
What Are the Clinical Implications?
Myocardial work allows for an accurate evaluation of myocardial deformation independently of the afterload, and these findings suggest that myocardial work analysis may have an incremental value over a standard longitudinal strain analysis and can potentially have a role in clinical practice as a marker of ischemia.
Hypertrophic cardiomyopathy (HCM) 1 is defined by unexplained left ventricular (LV) wall thickening. In addition to ventricular hypertrophy, other pathophysiological features include diastolic dysfunction, abnormalities in the mitral valve apparatus, and the development of myocardial fibrosis. 2 , 3 , 4 , 5 Coronary microvascular dysfunction (CMD) has also been identified as a prevalent feature playing an important pathophysiological role in HCM, associated with replacement fibrosis, heart failure, and ventricular arrhythmias. 6 , 7
Two‐dimensional speckle‐tracking echocardiography provides a more accurate assessment of the cardiac performance and earlier detection of LV dysfunction when compared with LV ejection fraction 8 and has shown a correlation with the extent of fibrosis by cardiac magnetic resonance (CMR), with a possible role in risk stratification in HCM. 9 However, speckle‐tracking echocardiography parameters have several pitfalls, namely, the need for geometrical assumptions; apical foreshortening; measurement variability among operators; and, particularly, load dependency. 10
Myocardial work (MW) is a novel noninvasive technique that encompasses the evaluation of LV deformation and afterload, incorporating the arterial blood pressure and longitudinal strain acquired by speckle‐tracking echocardiography analysis. As MW accounts for dynamic pressures, it allows an enhanced assessment of systolic dysfunction, even in patients with significant changes in afterload, a hallmark of the patients with obstructive HCM. 11 While MW parameters have been associated with the extent of myocardial fibrosis in HCM, 12 , 13 it is unclear if myocardial ischemia may be linked with MW independently of the scar burden.
A new era of HCM treatment has started with the licensing and introduction of sarcomere modulators for obstructive HCM, 14 and ongoing trials are testing its effect in nonobstructive HCM with promising early results. 15 In this context, the validation of novel and more sensitive imaging biomarkers of systolic function can be relevant, for both trial design and clinical monitoring in the “real‐world” context.
In this prospective multimodality imaging study, we hypothesized that there is an association between impaired MW, evaluated by echocardiography, and LV ischemia detected by CMR in patients with HCM. Our analysis was stratified according to the presence of nonobstructive or obstructive HCM and according to the burden of replacement fibrosis, measured by late gadolinium enhancement (LGE) in CMR. A segmental analysis was also performed; due to the intraindividual heterogeneity characteristic of HCM, we hypothesized that by comparing individual LV segments, additional associations could potentially be revealed.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This prospective study enrolled consecutive adult patients with HCM with follow‐up in the dedicated cardiomyopathy units of Santa Marta Hospital (n=63) (Lisbon, Portugal) and Garcia de Orta Hospital (n=12) (Almada, Portugal), from January 2019 to May 2020 and following the principles outlined in the Declaration of Helsinki. The institutional ethics committees approved the study protocol. All patients provided written informed consent. The echocardiographic studies were performed at the Santa Marta Hospital (Lisbon, Portugal), and the CMR studies were performed at the Heart Center, Hospital da Cruz Vermelha Portuguesa (Lisbon, Portugal). Medication was unchanged in the period between CMR and echo studies, which were performed with a median time interval of 54 (interquartile range, 74) days.
The diagnosis of HCM was established according to current guidelines. 1 The inclusion and exclusion criteria were the same as previously described by Aguiar Rosa et al. 16 Namely, epicardial coronary artery disease was excluded with invasive coronary angiography in symptomatic patients. Asymptomatic patients aged >40 years underwent coronary computerized tomography. In asymptomatic younger patients without cardiovascular risk factors, a low likelihood of obstructive coronary artery disease was assumed.
Transthoracic Echocardiogram
All subjects underwent the same comprehensive transthoracic echocardiogram (TTE) protocol as previously described. 13 Valvular timings obtained from TTE and the instantaneous systolic pressure value were used to estimate a normalized, patient‐specific LV pressure curve. In patients with obstructive HCM, the gradient without Valsalva maneuver was added to the systolic blood pressure. MW was then used to evaluate the work during shortening in systole plus negative work during lengthening in isovolumetric relaxation (global constructive work [GCW]), the negative work during lengthening in systole plus the work performed during shortening in isovolumetric relaxation (global wasted work [GWW]), global work efficiency (GWE = GCW/[GCW+GWW]×100%), and the amount of work performed by the LV during systole (area under the pressure‐strain curve) (global work index [GWI]). 17
CMR Protocol and Analysis
All subjects underwent the same stress CMR protocol and analysis, as previously described by Aguiar Rosa et al. 16 Please see Data S1 for the detailed CMR protocol.
For perfusion assessment, the myocardium was divided into 32 subsegments (16 American Heart Association segments subdivided into an endocardial and epicardial layer). Ischemic burden for each patient was calculated as the number of involved subsegments, assigning 3% of myocardium to each subsegment. Each segment was analyzed for the presence or absence of perfusion defects by a single operator, thus avoiding interobserver variability. Artifacts were assessed by using motion correction in the perfusion sequence, and perfusion defects were required to persist for ≥3 beats throughout the stress perfusion sequence. Perfusion defects sparing the subendocardium and coincident with LGE were not considered, as subendocardial involvement is mandatory for microvascular dysfunction defects. The LGE extent was quantitatively assessed on a per‐segment basis using a signal threshold versus reference myocardium of ≥6 standard deviations. Extensive fibrosis was defined by the involvement of ≥15% of total LV mass. 5 CMR data was evaluated by 1 reader, who was blinded to the echocardiographic results.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 23.0 for Windows (IBM, Armonk, NY). Point estimates and 95% CIs are described for all mean estimates.
Descriptive statistics are presented as absolute frequency (number) and relative frequency (percentage) for categorical variables and as the mean or median for continuous variables. Normal distribution of continuous variables was verified by the Kolmogorov–Smirnov test. Correlations between continuous variables were assessed using Spearman's correlation. For the assessment of differences between 2 groups for continuous variables, Student's t test (for normally distributed variables) and the Mann–Whitney U test (for nonnormally distributed variables) were used. For the comparison among 3 groups for continuous variables, the independent‐samples Kruskal–Wallis test was used.
MW parameters were explored by a receiver operating characteristic curve to determine the optimal cutoff values for predicting perfusion defects. The best cutoff value was defined as the point combining the highest sum of sensitivity and specificity. The Hanley and McNeil test was used to assess the significance of the difference between the areas under the curves (AUCs) from 2 receiver operating characteristic curves derived from independent samples.
Univariable linear regression analyses were performed to assess the correlation between MW parameters, perfusion defects, and the burden of LGE (percentage of total LV mass). Subsequently, multivariable analyses, adjusted for potential confounders, were performed. Variables with a significant correlation in the univariate analysis (linear regression with a P value <0.05) were included in the multivariable analyses. Results were stratified according to the presence of obstructive or nonobstructive HCM and according to the presence of extensive fibrosis. A segment‐by‐segment analysis was performed to assess the correlation among MW, ischemia, and fibrosis, calculated with univariable and multivariable linear regressions.
Statistical differences with a P value <0.05 were considered significant.
Results
A total of 75 patients were enrolled. The baseline characteristics of the patients are presented in Table 1. Mean age was 55±15 years, with 47 (63%) male patients and a mean LV ejection fraction by TTE of 68%±7%. A pattern of asymmetrical septal hypertrophy was present in 46 (61%) patients, while 22 (29%) had apical hypertrophy and 7 (9%) had concentric hypertrophy. The maximal LV wall thickness was 20.4±4.2 mm. LV outflow tract obstruction was present in 21 (28%) patients (mean maximal LV outflow tract gradient of 89±60 mm Hg). A significant proportion of patients were symptomatic, as shown by a New York Heart Association class ≥II in 34 (45%) patients.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | All (n=75) | With perfusion defects (n=68) | Without perfusion defects (n=7) | P value |
|---|---|---|---|---|
| Age, y, mean±SD | 55±15 | 54±15 | 56±12 | 0.718 |
| Male sex, n (%) | 47 (63) | 41 (60) | 6 (86) | 0.186 |
| NYHA class ≥II, n (%) | 34 (45) | 31 (46) | 3 (43) | 0.890 |
| Body mass index, kg/m2, mean±SD | 29±5 | 29±5 | 28±5 | 0.805 |
| Body mass index >25 kg/m2, n (%) | 59 (79) | 55 (81) | 4 (57) | 0.162 |
| Hypertension, n (%) | 38 (51) | 36 (53) | 2 (29) | 0.262 |
| Diabetes, n (%) | 12 (16) | 12 (18) | 0 (0) | 0.589 |
| Obstructive HCM, n (%) | 21 (28) | 20 (29) | 1 (14) | 0.396 |
| HCM risk‐SCD score, %, mean±SD | 3±2 | 3±2 | 2±1 | 0.123 |
| Beta‐blockers, n (%) | 54 (72) | 50 (74) | 4 (57) | 0.392 |
| CCB, n (%) | 18 (24) | 18 (27) | 0 (0) | 0.186 |
| LVEDD by TTE, mm, mean±SD | 50±5 | 50±5 | 54±4 | 0.033 |
| MWT by TTE, mm, mean±SD | 20±4 | 21±4 | 16±1 | <0.001 |
| LVEF by TTE, %, mean±SD | 68±7 | 68±7 | 68±6 | 0.950 |
| GLS by TTE, %, mean±SD | −14±4 | −14±4 | −16±2 | 0.013 |
| LVEDV by CMR, mL, mean±SD | 120±33 | 119±34 | 131±20 | 0.208 |
| LV mass index by CMR, g/m2, mean±SD | 97±30 | 98±31 | 84±14 | 0.048 |
| LVEF by CMR, %, mean±SD | 72±8 | 72±9 | 71±5 | 0.726 |
| Segments with perfusion defects, n, mean±SD | 6±4 | 6±4 | … | … |
| Perfusion defects, % of LV mass, mean±SD | 23±17 | 23±17 | … | … |
| Number of segments with LGE, n, mean±SD | 9±5 | 9±4 | 4±3 | 0.002 |
| LGE, % of LV mass, mean±SD | 13±9 | 14±9 | 5±5 | 0.002 |
| GWI, mm Hg%, median (IQR) | 1282 (992–1621) | 1228 (967–1517) | 1768 (1396–1985) | 0.012 |
| GCW, mm Hg%, median (IQR) | 1500 (1213–1763) | 1446 (1195–1745) | 2036 (1567–2158) | 0.012 |
| GWW, mm Hg%, median (IQR) | 146 (88–220) | 150 (91–223) | 101 (28–193) | 0.167 |
| GWE, %, median (IQR) | 87 (81–93) | 86 (81–93) | 95 (91–98) | 0.005 |
CCB indicates calcium channel blocker; CMR, cardiac magnetic resonance; GCW, global constructive work; GLS, global longitudinal strain; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; HCM, hypertrophic cardiomyopathy; IQR, interquartile range; LGE, late gadolinium enhancement; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MWT, maximal wall thickness; NYHA, New York Heart Association; SCD, sudden cardiac death; and TTE, transthoracic echocardiogram.
TTE and CMR were performed on all 75 patients. A total of 1200 segments were analyzed in the segmental analysis. Stress perfusion images were interpretable in all patients. LGE images were not interpretable in 1 patient due to artifact. Perfusion defects were found in 68 (90.7%) patients, in a mean 5.7±3.9 segments. The mean burden of ischemia was 22.5%±16.9% of LV mass. Only 4 (5%) patients had no LGE. The mean LGE was 12.7%±8.6% of the LV mass, with 29 (39%) patients having at least 15% of LGE (of total LV mass) and a mean 8.7±4.6 segments with fibrosis.
According to the reference values from the European Association of Cardiovascular Imaging NORRE (Normal Reference Ranges for Echocardiography) study, 18 values lower than the normal range of GWI, GWE, and GCW were found in 36 (48%), 45 (60%), and 44 (56%) patients, respectively (Table 2). Increased values of GWW were found in 14 (20%) patients. Five (7%) patients had GCW values higher than the mean reference (2232 mm Hg%).
Table 2.
Myocardial Work Parameters in HCM—Percentage of Patients Below the Reference Values (According to the EACVI NORRE Study 18 ), Stratified According to Sex and Pattern of Hypertrophy
| Myocardial work | Reference values* | Patients with impaired myocardial work parameters | ||
|---|---|---|---|---|
| Male (n=47) | Female (n=28) | All (n=75) | ||
| GWI, n (%) |
Male >1270 mm Hg% Female >1310 mm Hg% |
24 (51) | 12 (43) | 36 (48) |
| GCW, n (%) |
Male >1650 mm Hg% Female >1544 mm Hg% |
32 (68) | 12 (43) | 44 (56) |
| GWW, n (%) |
Male <238 mm Hg% Female <239 mm Hg% |
7 (15) | 7 (25) | 14 (20) |
| GWE, n (%) |
Male >90% Female >91% |
28 (60) | 17 (61) | 45 (60) |
| Myocardial work | Reference values* | Septal (n=46) | Apical (n=22) | Concentric (n=7) |
|---|---|---|---|---|
| GWI, n (%) |
Male >1270 mm Hg% Female >1310 mm Hg% |
21 (46) | 9 (41) | 6 (86) |
| GCW, n (%) |
Male >1650 mm Hg% Female >1544 mm Hg% |
29 (63) | 8 (36) | 7 (100) |
| GWW, n (%) |
Male <238 mm Hg% Female <239 mm Hg% |
8 (17) | 3 (14) | 3 (43) |
| GWE, n (%) |
Male >90% Female >91% |
28 (61) | 11 (50) | 6 (86) |
EACVI indicates European Association of Cardiovascular Imaging; GCW, global constructive work; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; HCM, hypertrophic cardiomyopathy; and NORRE, Normal Reference Ranges for Echocardiography.
As published by Manganaro et al. 18
The pattern of hypertrophy was also related to the MW parameters. The majority of patients with concentric hypertrophy presented with significantly impaired MW, with values lower than the normal range of GWI, GWE, and GCW found in 86%, 86%, and 100%, respectively, and higher values of GWW found in 43% (Table 2). A concentric pattern of hypertrophy was associated with lower GWI (P=0.029) and lower GCW (P=0.006; Table 3). An example of the GWI and GWE values obtained in patients with asymmetric septal, apical, or concentric HCM and respective LV pressure‐strain loops are presented in Figure 1.
Table 3.
Myocardial Work Parameters in HCM Stratified According to Pattern of LV Hypertrophy, Sex, LVOT Obstruction, and Severe Fibrosis (LGE ≥15%)
| Myocardial work parameters | ||||
|---|---|---|---|---|
| Septal (n=46) | Apical (n=22) | Concentric (n=7) | P value | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| GWI, mm Hg% | 1306 (1036–1583) | 1493 (1064–1703) | 1025 (624–1228) | 0.029 |
| GCW, mm Hg% | 1498 (1232–1743) | 1738 (1260–1945) | 1180 (773–1397) | 0.006 |
| GWW, mm Hg% | 143 (88–212) | 154 (71–218) | 173 (137–343) | 0.738 |
| GWE, % | 87 (83–93) | 88 (81–95) | 81 (75–87) | 0.116 |
| Male (n=47) | Female (n=28) | P value | ||
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| GWI, mm Hg% | 1228 (1025–1493) | 1357 (885–1677) | … | 0.734 |
| GCW, mm Hg% | 1441 (1203–1740) | 1638 (1330–1884) | … | 0.333 |
| GWW, mm Hg% | 143 (89–207) | 181 (76–250) | … | 0.145 |
| GWE, % | 87 (83–93) | 87 (76–94) | … | 0.622 |
| Obstructive HCM (n=21) | Nonobstructive HCM (n=54) | P value | ||
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| GWI, mm Hg% | 1190 (871–1500) | 1339 (1076–1659) | … | 0.168 |
| GCW, mm Hg% | 1443 (1170–1716) | 1548 (1241–1814) | … | 0.375 |
| GWW, mm Hg% | 156 (120–227) | 145 (82–220) | … | 0.580 |
| GWE, % | 84 (81–90) | 88 (84–94) | … | 0.104 |
| LGE≥15% (n=29) | LGE<15% (n=45) | P value | ||
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| GWI, mm Hg% | 1147 (862–1363) | 1381 (1139–1744) | … | 0.010 |
| GCW, mm Hg% | 1374 (1108–1450) | 1656 (1406–1926) | … | 0.002 |
| GWW, mm Hg% | 154 (98–229) | 146 (79–219) | … | 0.566 |
| GWE, % | 86 (79–92) | 88 (84–94) | … | 0.107 |
GCW indicates global constructive work; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricular; and LVOT, left ventricular outflow tract.
Figure 1. Myocardial work parameters according to different patterns of hypertrophy in hypertrophic cardiomyopathy (HCM).
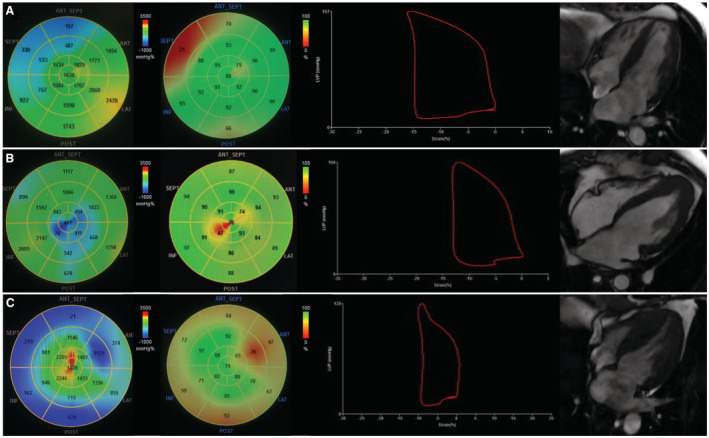
From left to right: global work index (GWI), global work efficiency (GWE), pressure‐strain loop, cardiac magnetic resonance 4‐chamber view. A, Asymmetric septal HCM. B, Apical HCM. C, concentric HCM.
Patients with apical HCM showed a slightly lower GWI in apical segments (1354±9389 mm Hg% versus 1446±645 mm Hg%, with no statistical significance [P=0.317]) and a significantly lower GWE in apical segments (84%±16% versus 92%±9%, P<0.001) compared with nonapical segments.
There were no significant sex differences in MW parameters. Patients with LGE ≥15% of total LV mass presented with significantly lower GWI (1147 [862–1363] mm Hg% versus 1381 [1139–1744]; P=0.010) and lower GCW (1374 [1108–1450] versus 1656 [1406–1926]; P=0.002) compared with patients with LGE <15%. While patients with obstructive HCM showed slightly lower values of GWI, GCW, and GWE, there was no significant statistical difference in MW parameters compared with patients with nonobstructive HCM (Table 3).
The results of univariable regression analysis for individual MW parameters associated with perfusion defects are presented in Table S1. Subsequently, a multivariable regression analysis, adjusted for potential confounders, was performed (Table 4).
Table 4.
Multivariable Linear Regression Analysis of LV Myocardial Work Parameters
| Correlation coefficient (r) | P value | β‐estimate | 95% CI | P value | |
|---|---|---|---|---|---|
| Global work index | |||||
| No. segments ischemia, n | −0.532 | <0.001 | −45.781 | −70.612 to −20.950 | <0.001 |
| Ischemia burden, % of LV mass | −0.519 | <0.001 | −10.822 | −16.772 to −4.872 | 0.001 |
| MWT | −0.471 | <0.001 | −26.773 | −48.837 to −4.710 | 0.018 |
| LV mass index | −0.558 | <0.001 | −5.759 | −10.210 to −1.308 | 0.012 |
| Concentric hypertrophy | −0.257 | 0.028 | −251.743 | −597.081 to 93.595 | 0.150 |
| No. segments LGE, n | −0.331 | 0.005 | −26.334 | −47.904 to −4.764 | 0.017 |
| LGE, % of LV mass | −0.285 | 0.015 | −13.862 | −25.073 to −2.651 | 0.016 |
| Diabetes | −0.246 | 0.036 | −198.763 | −438.733 to 41.208 | 0.103 |
| Global constructive work | |||||
| No. segments ischemia, n | −0.519 | <0.001 | −46.770 | −70.956 to −22.584 | <0.001 |
| Ischemia burden, % of LV mass | −0.532 | <0.001 | −10.342 | −15.911 to −4.772 | <0.001 |
| MWT | −0.504 | <0.001 | −32.568 | −56.703 to −8.433 | 0.009 |
| LV mass index | −0.616 | <0.001 | −6.970 | −10.222 to −3.718 | <0.001 |
| Concentric hypertrophy | −0.321 | 0.006 | −413.459 | −740.48 to −86.435 | 0.014 |
| No. segments LGE, n | −0.404 | <0.001 | −39.237 | −60.077 to −18.397 | <0.001 |
| LGE, % of LV mass | −0.372 | 0.001 | −17.466 | −28.408 to −6.525 | 0.002 |
| Global wasted work | |||||
| No. segments ischemia, n | 0.280 | 0.017 | 3.845 | −3.347 to 11.037 | 0.219 |
| Diabetes | 0.231 | 0.051 | 61.728 | −17.309 to 140.765 | 0.124 |
| Hypertension | 0.239 | 0.043 | 55.402 | −1.315 to 112.119 | 0.055 |
| Global work efficiency | |||||
| No. segments ischemia, n | −0.477 | <0.001 | −0.794 | −1.288 to −0.300 | 0.002 |
| Ischemia burden, % of LV mass | −0.379 | 0.001 | −0.123 | −0.241 to −0.005 | 0.041 |
| MWT | −0.361 | 0.002 | −0.387 | −0.865 to 0.092 | 0.111 |
| LV mass index | −0.459 | <0.001 | −0.093 | −0.160 to −0.027 | 0.007 |
| No. segments LGE, n | −0.263 | 0.026 | −0.327 | −0.727 to 0.073 | 0.107 |
| Diabetes | −0.323 | 0.006 | −7.909 | −12.845 to −2.972 | 0.002 |
LGE indicates late gadolinium enhancement; LV, left ventricular; and MWT, maximal wall thickness.
In a multivariable analysis (Table 4), lower values of GWI significantly correlated with a higher burden of ischemia (r=−0.519, β‐estimate −10.822; P=0.001). Likewise, impaired values of GCW were linked to a higher percentage of hypoperfusion (r −0.532, β‐estimate −13.788; P<0.001). Moreover, lower values of GWE correlated with an increased number of segments with perfusion defects (r −0.477, β‐estimate −0.794; P=0.002) and with a higher burden of ischemia (r −0.379, β‐estimate −0.123, P=0.041). In this multivariable model adjusted for potential confounders, these associations were independent of the maximal wall thickness, of the LV mass index, of the pattern of hypertrophy, and of the burden of LGE. In a univariable analysis, the presence of a higher GWW was associated with a higher number of segments with perfusion defects (r 0.280, β‐estimate 5.463; P=0.014). These associations are presented in Figure 2.
Figure 2. Correlation between myocardial work parameters and perfusion defects.
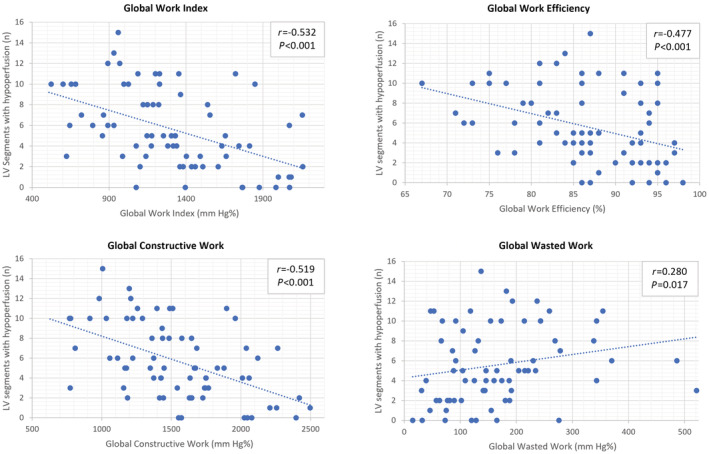
LV indicates left ventricular.
Receiver operating characteristic curve analysis (Figure S1) revealed that a GWI cutoff value of ≤1755 mm Hg% was associated with the presence of perfusion defects with a sensitivity of 88% and a specificity of 71% (AUC, 0.806; P=0.008), while the best cutoff for GLS (>−15.5%) had only a sensitivity of 64% and a specificity of 57% and did not reach statistical significance (AUC, 0.705; P=0.076). The positive predictive value of GWI ≤1755 mm Hg% was 97%, and the negative predictive value was 38%. GWI AUC values did not differ significantly between a pattern of asymmetrical septal or apical hypertrophy (AUC 0.785 versus 0.895; P=0.521).
Regarding the presence of LV outflow tract obstruction, impaired MW values did not show a significant difference in the correlation with perfusion defects in patients with obstructive HCM compared with patients with nonobstructive HCM (GWI: r −0.518 versus −0.506, P=0.953; GWE: r=−0.591 versus r=−0.317, P=0.201; Table S2 and Figure S2).
Likewise, in patients with significant fibrosis (LGE ≥15% of LV mass), there was no significant difference in the correlation of impaired MW and perfusion defects compared with patients with milder LV fibrosis (LGE <15%; GWI: r=−0.489 versus r=−0.393, P=0.632; GCW: r −0.455 versus r −0.359, P=0.643; Table S3 and Figure S3).
In a segment‐by‐segment multivariable analysis (Table 5) (univariable analysis presented in Table S4), we found that lower values of GWI (r=−0.294, β‐estimate −194.979; P<0.001) and lower values of GWE were linked to a higher burden of ischemia (r −0.242, β‐estimate −3.653; P=0.002). As in the global myocardial analysis, these associations were also independent of the wall thickness or the presence of LGE in each segment. Furthermore, the correlation between perfusion defects and segmental GWI (r=−0.294; P<0.001) was superior to that of segmental longitudinal strain (r=−0.076; P=0.014). The distribution and prevalence of perfusion defects and MW parameters in each of 16 American Heart Association segments are displayed in Figure 3.
Table 5.
Segmental Multivariable Linear Regression Analysis of LV Myocardial Work Parameters
| Correlation coefficient (r) | P value | β‐estimate | 95% CI | P value | |
|---|---|---|---|---|---|
| Global work index | |||||
| Perfusion defect | −0.294 | <0.001 | −194.979 | −289.115 to −100.843 | <0.001 |
| LGE | −0.227 | <0.001 | −193.692 | −2828.445 to −104.938 | <0.001 |
| Wall thickness ≥15 mm | −0.422 | <0.001 | −37.429 | −47.019 to −27.838 | <0.001 |
| Wall thickness 12–14 mm | −0.289 | <0.001 | −73.126 | −94.332 to −51.930 | <0.001 |
| Obstructive HCM | … | … | −124.170 | −217.892 to −30.449 | 0.009 |
| Diabetes | … | … | −162.011 | −281.331 to −42.691 | 0.008 |
| Global work efficiency | |||||
| Perfusion defect | −0.242 | <0.001 | −3.653 | −5.913 to −1.393 | 0.002 |
| LGE | −0.133 | <0.001 | −2.477 | −4.538 to −0.416 | 0.019 |
| Wall thickness ≥15 mm | −0.233 | <0.001 | −0.274 | −0.505 to −0.043 | 0.020 |
| Wall thickness 12–14 mm | −0.174 | <0.001 | −0.754 | −1.214 to −0.294 | 0.001 |
| Obstructive HCM | … | … | −2.737 | −4.912 to −0.563 | 0.014 |
| Diabetes | … | … | −5.055 | −7.670 to −2.440 | <0.001 |
HCM indicates hypertrophic cardiomyopathy; LGE: late gadolinium enhancement; and LV, left ventricular.
Figure 3. Work index (A) and work efficiency (B) median values compared with prevalence of ischemia (colors) in individual segments.
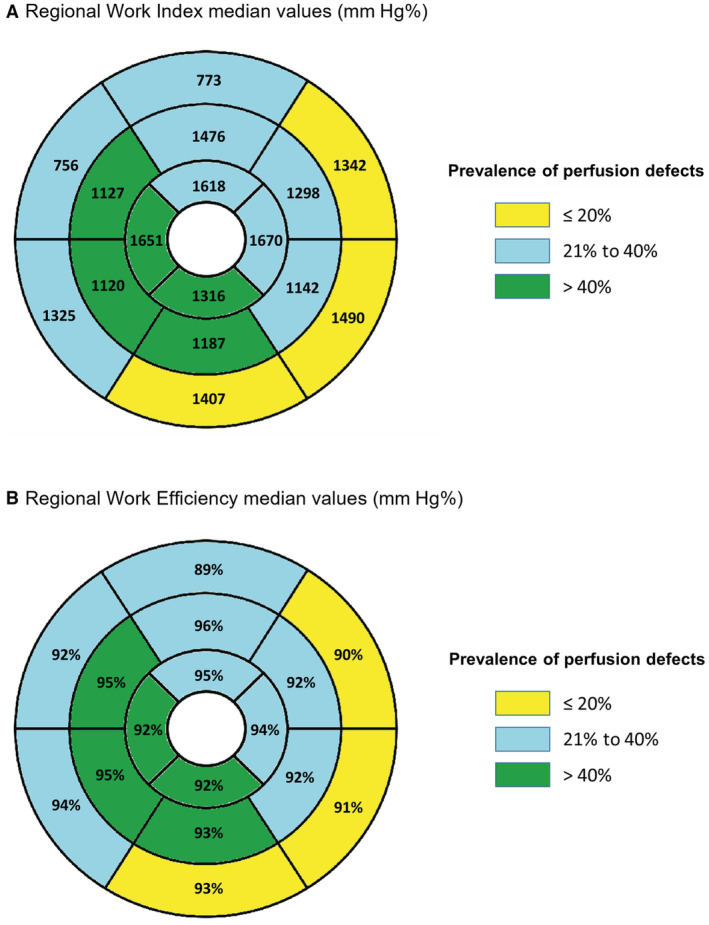
There was a significant correlation between the presence of LGE and impaired GWI (r=−0.227, β‐estimate −193.692; P<0.001) and impaired GWE (r=−0.133, β‐estimate −2.477; P=0.019). Lower values of GWI (β‐estimate −124.170; P=0.009) and GWE (β‐estimate −2.737; P=0.014) were correlated with obstructive HCM (Table 5).
Discussion
In a cohort of patients with HCM, our results revealed an association between CMD and worse LV performance assessed by MW parameters, including a lower GWI, GWE, and GCW.
MW parameters have proven prognostic value in several conditions, including cardiac amyloidosis, 19 acute coronary syndromes, 20 reverse remodeling with sacubitril‐valsartan, 21 LV dyssynchrony before cardiac resynchronization therapy, 22 and in cardio‐oncology patients. 23
In patients with nonbstructive HCM, impaired MW indices were previously shown to correlate with maximal LV wall thickness, diastolic dysfunction and associated with worse long‐term outcomes, 24 with a proposed cutoff of GCW <1730 mm Hg%. In our cohort of patients with HCM, the majority (n=50, 67%) presented with a GCW under this cutoff value.
There are scarce published data regarding patients with obstructive HCM, in which the assessment of MW requires adding the LV outflow tract gradient (without Valsalva maneuver) to the systolic blood pressure. 13 As the estimation of the LV outflow tract gradient may introduce further interoperator variability, this fact poses an added difficulty in the evaluation of MW. In addition, HCM is characterized by intraindividual phenotypic heterogeneity of different LV segments. 25
In our study, MW parameters were significantly reduced in patients with HCM, in comparison with the published reference values, 18 with around half the patients presenting impaired GWI and GWE values and 60% impaired GWE values. As expected, patients without perfusion defects had lower LV mass, less burden of fibrosis, and lower GLS (reflecting higher deformation). In this study, we show that these patients are also characterized by higher values of GWI, GCW, and GWE—indicating higher LV deformation. No differences were noted regarding LV ejection fraction (either measured by TTE or CMR) in patients with ischemia compared with patients with no perfusion defects, which might be in keeping with the limitations of LV ejection fraction on the evaluation of LV performance in HCM. 26
While the role of MW, particularly GCW, as a tool for assessing LV fibrosis has been previously documented, 12 , 13 this is the first study, to the best of our knowledge, to uncover the association between MW parameters and the extent of myocardial ischemia. Importantly, this association was independent of the degree of LV hypertrophy, the pattern of hypertrophy, and the burden of replacement fibrosis.
Microvascular dysfunction is a cardinal feature of HCM, playing a transversal role through its pathophysiology, including tissue characteristics and clinical manifestations, as we have previously described. 16 , 25 , 27 Furthermore, coronary microvascular rarefaction and defects in arteriolar control of coronary flow have been described in patients with HCM, contributing to impaired capillary supply and coronary reserve. 28 , 29 An increased burden of ischemia has been linked to LV hypertrophy, higher values of native T1 mapping and more extensive replacement fibrosis and diffuse tissue abnormalities. 16 , 25 Increased severity of ischemia was associated with higher incidence of atrial fibrillation, worse functional capacity, and worse outcomes, including progressive heart failure. 16 , 27 Diabetes was associated with CMD in our cohort, which is in keeping with published evidence showing that CMD is prevalent in patients with type 2 diabetes and a strong independent predictor of cardiovascular mortality in this population. 30 , 31
Among the assessed MW parameters, GWI and GCW showed the highest correlation with CMD, and a GWI cutoff of ≤1755 mm Hg% had a high accuracy to predict ischemia in HCM. This finding is in line with previously published data, as impaired MW has been shown to be correlated with worse outcomes and extent of fibrosis. 13 , 24 While lower GWE significantly correlated with CMD, the association was weaker. Nevertheless, impaired GWE was the individual MW parameter observed in a higher percentage of patients in our cohort (n=45; 60%), underscoring the impairment in LV systolic performance in HCM despite the characteristic hypercontractility. Although an increased GWW (compared with reference values) was present in 20% of enrolled patients with HCM, GWW revealed only a minor relationship with ischemia.
MW allows for an accurate evaluation of myocardial deformation independently of the afterload by assessing contractility, taking into account noninvasive blood pressure measurements, unlike the assessment of GLS. 11 Therefore, a possible advantage of the use of MW indices compared with GLS is the reduction of overdiagnosis of LV subclinical dysfunction of patients with HCM and arterial hypertension or other causes of increased afterload. In our cohort, a GWI cutoff value of ≤1755 mm Hg% showed a higher predictive power than GLS for the presence of perfusion defects, and the latter did not reach statistical significance. This finding suggests that MW analysis may have an incremental value over a standard longitudinal strain analysis and can potentially have a role in clinical practice as a marker of ischemia. Furthermore, the predictive value of MW was similar regardless of the pattern of hypertrophy.
The independent association of MW parameters and ischemia was also shown in a segmental analysis, although with an inferior correlation compared with the global LV assessment, which may be explained by the inherent intraindividual phenotypic heterogeneity of LV segments in HCM. However, while there was no association of MW parameters and obstructive HCM in a global LV assessment, by performing a segment‐by‐segment evaluation, we uncovered an independent correlation between impaired GWI and GWE and obstructive HCM.
In the evaluation of scar, we found that MW indices were correlated with the severity of LV fibrosis. Patients with LGE ≥15% of LV mass presented with significantly impaired GWI and GCW comparing to patients with milder fibrosis (LGE <15%). The relationship between MW and the extent of myocardial fibrosis is currently not well established, with 1 retrospective study including 82 patients with nonobstructive HCM showing that GCW was significantly reduced and associated with the extent of qualitative assessment of LGE, albeit without quantification of the percentage of LGE. 12 Further studies have reproduced this correlation, with a GCW cutoff of ≤1550 mm Hg% being proposed as potentially useful in clinical practice to detect significant LV fibrosis. 13 In our current study, there was no significant difference between the correlation of MW and CMD in patients with significant fibrosis versus patients with milder fibrosis.
In the subgroup of patients with concentric HCM, a higher proportion showed significantly impaired MW parameters, in comparison with patients with asymmetric septal or apical HCM, maybe reflecting the widespread tissue abnormalities in patients with concentric HCM versus the predominantly focal hypertrophy in other hypertrophy patterns.
As novel pathophysiology‐directed medications are introduced and given the observed association with other imaging parameters of clinical relevance and the sensitive measurement of contractility it provides, future studies should test the usefulness of myocardial work as an early imaging biomarker for the assessment of the effect of sarcomere modulators.
Future validation of the MW parameters' prognostic capabilities regarding clinical manifestations, arrhythmic events, risk stratification, and long‐term outcomes is crucial to evaluate its clinical utility.
Limitations
Our study has some limitations that should be acknowledged. One is the relatively small population. The MW indices were performed in resting conditions, while ischemia was evaluated during stress CMR. This is in line with most studies on MW, which assess indices in resting conditions, and currently there are no robust data to support its use with vasodilator stress. Additionally, CMR and echocardiography studies were conducted with a median time interval of 54 (interquartile range, 74) days, and although therapy was unchanged in the period between studies, this might have led to minor differences in blood pressure or heart rate measurements. Perfusion defects, a surrogate for myocardial ischemia, were evaluated through a semiquantitative visual analysis of ischemia in 32 segments, as used in previous studies comparing stress CMR with invasive evaluation of fractional flow reserve. 32 While the adopted method is readily available and easily applicable, it relies on visual assessment and the total of LV assessed is 96% (3% for each segment). Significant replacement fibrosis was defined as LGE ≥15% of LV mass (as proposed by Chan et al 5 ), but there is currently no universal consensus on the percentage of LGE that defines significant. Six (8%) enrolled patients were in atrial fibrillation, which may contribute to further heterogeneity in MW indices assessment.
In our cohort, there was a significantly high burden of perfusion defects and replacement fibrosis. While consecutive patients were enrolled, this may be partly due to selection bias. Thus, the findings in our study may not be applicable to a general HCM population. As knowledge on MW continues to expand, the MW indices reference values are still not entirely defined.
Conclusions
In HCM, impaired MW parameters, including lower GWI, GWE, and GCW, were significantly correlated with the presence and extent of myocardial ischemia in a CMR perfusion assessment, regardless of the degree of LV hypertrophy or fibrosis, and GWI showed a higher predictive power than GLS, regardless of the pattern of hypertrophy. A segmental analysis supported these findings.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1
Tables S1–S4
Figures S1–S3
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028857
For Sources of Funding and Disclosures, see page 11.
This article was sent to Erik B. Schelbert, MD, MS, Associate Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1. Elliott P, Anastasakis A, Borger M, Borggrefe M, Cecchi F, Charron P, Hagege A, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2015;121:7–57. doi: 10.1093/eurheartj/ehu284 [DOI] [Google Scholar]
- 2. O'Hanlon R, Grasso A, Roughton M, Moon J, Clark S, Wage R, Webb J, Kulkarn M, Dawson D, Sulaibeekh L, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 3. Adabag A, Maron B, Appelbaum E, Harrigan C, Buros J, Gibson C, Lesser J, Hanna C, Udelson J, Manning W, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1313. doi: 10.1016/j.jacc.2007.11.071 [DOI] [PubMed] [Google Scholar]
- 4. Maron M, Appelbaum E, Harrigan C, Buros J, Gibson C, Hanna C, Lesser J, Udelson J, Manning W, Maron B. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184–191. doi: 10.1161/CIRCHEARTFAILURE.108.768119 [DOI] [PubMed] [Google Scholar]
- 5. Chan R, Maron B, Olivotto I, Pencina M, Assenza G, Haas T, Lesser J, Gruner C, Crean A, Rakowski H, et al. Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–449. doi: 10.1161/CIRCULATIONAHA.113.007094 [DOI] [PubMed] [Google Scholar]
- 6. Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol. 2000;31:988–998. doi: 10.1053/hupa.2000.16659 [DOI] [PubMed] [Google Scholar]
- 7. Barbosa A, Almeida J, Guerreiro C, Teixeira P, Ladeiras Lopes R, Dias Ferreira N, Sousa O, Braga P. Late gadolinium enhancement location assessed by magnetic resonance and arrhythmogenic risk in hypertrophic cardiomyopathy. Rev Port Cardiol. 2020;39:615–621. doi: 10.1016/j.repce.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 8. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334 [DOI] [PubMed] [Google Scholar]
- 9. Barbosa A, Dias Ferreira N, Martins O'Neill C, Ruivo C, Cruz I, Rocha Lopes L. Impaired myocardial deformation assessed by cardiac magnetic resonance is associated with increased arrhythmic risk in hypertrophic cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2020;73:849–851. doi: 10.1016/j.rec.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 10. Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Circulation. 2000;102:1158–1164. doi: 10.1161/01.CIR.102.10.1158 [DOI] [PubMed] [Google Scholar]
- 11. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, Haugaa KH, Opdahl A, Fjeld JG, Gjesdal O, et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: a non‐invasive index of myocardial work. Eur Heart J. 2012;33:724–733. doi: 10.1093/eurheartj/ehs016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galli E, Vitel E, Schnell F, le Rolle V, Hubert A, Lederlin M, Donal E. Myocardial constructive work is impaired in hypertrophic cardiomyopathy and predicts left ventricular fibrosis. Echocardiography. 2019;36:74–82. doi: 10.1111/echo.14210 [DOI] [PubMed] [Google Scholar]
- 13. Gonçalves AV, Rosa SA, Branco L, Galrinho A, Fiarresga A, Lopes LR, Thomas B, Baquero L, Carmo MM, Ferreira RC. Myocardial work is associated with significant left ventricular myocardial fibrosis in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2021;37:2237–2244. doi: 10.1007/s10554-021-02186-3 [DOI] [PubMed] [Google Scholar]
- 14. Olivotto I, Oreziak A, Barriales‐Villa R, Abraham T, Masri A, Garcia‐Pavia P, Saberi S, Lakdawala N, Wheeler M, Owens A, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 15. Edelberg JM, Sehnert AJ, Mealiffe ME, del Rio CL, McDowell R. The impact of mavacamten on the pathophysiology of hypertrophic cardiomyopathy: a narrative review. Am J Cardiovasc Drugs. 2022;22:497–510. doi: 10.1007/s40256-022-00532-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aguiar Rosa S, Thomas B, Fiarresga A, Papoila A, Alves M, Pereira R, Branco G, Cruz I, Rio P, Baquero L, et al. The impact of ischemia assessed by magnetic resonance on functional, arrhythmic and imaging features of hypertrophic cardiomyopathy. Front Cardiovasc Med. 2021;8:761860. doi: 10.3389/fcvm.2021.761860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Gjesdal O, Edvardsen T, Smiseth OA. Assessment of wasted myocardial work: a novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol. 2013;305:H996–H1003. doi: 10.1152/ajpheart.00191.2013 [DOI] [PubMed] [Google Scholar]
- 18. Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, Cimino S, Go YY, Bernard A, Kacharava G, et al. Echocardiographic reference ranges for normal non‐invasive myocardial work indices: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2019;20:582–590. doi: 10.1093/ehjci/jey188 [DOI] [PubMed] [Google Scholar]
- 19. Roger‐Rollé A, Cariou E, Rguez K, Fournier P, Lavie‐Badie Y, Blanchard V, Roncalli J, Galinier M, Carrié D, Lairez O. Can myocardial work indices contribute to the exploration of patients with cardiac amyloidosis? Open Heart. 2020;7:e001346. doi: 10.1136/openhrt-2020-001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boe E, Russell K, Eek C, Eriksen M, Remme EW, Smiseth OA, Skulstad H. Non‐invasive myocardial work index identifies acute coronary occlusion in patients with non‐ST‐segment elevation‐acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 2015;16:1247–1255. doi: 10.1093/ehjci/jev078 [DOI] [PubMed] [Google Scholar]
- 21. Valentim Gonçalves A, Galrinho A, Pereira‐da‐Silva T, Branco L, Rio P, Timóteo AT, Abreu J, Soares RM, Feliciano J, Moreira RI, et al. Myocardial work improvement after sacubitril–valsartan therapy. J Cardiovasc Med. 2020;21:223–230. doi: 10.2459/JCM.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 22. van der Bijl P, Vo NM, Mv K, Mertens B, Ajmone Marsan N, Delgado V, Bax JJ. Prognostic implications of global, left ventricular myocardial work efficiency before cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2019;20:1388–1394. doi: 10.1093/ehjci/jez095 [DOI] [PubMed] [Google Scholar]
- 23. Vaz Ferreira V, Mano TB, Cardoso I, Coutinho Cruz M, Moura Branco L, Almeida‐Morais L, Timóteo A, Galrinho A, Castelo A, Garcia Brás P, et al. Myocardial work brings new insights into left ventricular remodelling in cardio‐oncology patients. Int J Environ Res Public Health. 2022;19:2826. doi: 10.3390/ijerph19052826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hiemstra YL, van der Bijl P, el Mahdiui M, Bax JJ, Delgado V, Marsan NA. Myocardial work in nonobstructive hypertrophic cardiomyopathy: implications for outcome. J Am Soc Echocardiogr. 2020;33:1201–1208. doi: 10.1016/j.echo.2020.05.010 [DOI] [PubMed] [Google Scholar]
- 25. Garcia Brás P, Aguiar Rosa S, Thomas B, Fiarresga A, Cardoso I, Pereira R, Branco G, Cruz I, Baquero L, Cruz Ferreira R, et al. Associations between perfusion defects, tissue changes and myocardial deformation in hypertrophic cardiomyopathy, uncovered by a cardiac magnetic resonance segmental analysis. Rev Port Cardiol. 2022;41:559–568. doi: 10.1016/j.repc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 26. Haland TF, Hasselberg NE, Almaas VM, Dejgaard LA, Saberniak J, Leren IS, Berge KE, Haugaa KH, Edvardsen T. The systolic paradox in hypertrophic cardiomyopathy. Open Heart. 2017;4:e000571. doi: 10.1136/openhrt-2016-000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguiar Rosa S, Rocha Lopes L, Branco L, Galrinho A, Fiarresga A, Thomas B, Brás P, Gonçalves A, Cardoso I, Papoila A, et al. Blunted coronary flow velocity reserve is associated with impairment in systolic function and functional capacity in hypertrophic cardiomyopathy. Int J Cardiol. 2022;359:61–68. doi: 10.1016/j.ijcard.2022.04.032 [DOI] [PubMed] [Google Scholar]
- 28. Johansson B, Mörner S, Waldenström A, Stål P. Myocardial capillary supply is limited in hypertrophic cardiomyopathy: a morphological analysis. Int J Cardiol. 2008;126:252–257. doi: 10.1016/j.ijcard.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 29. Yoshida Y, Shimizu I, Minamino T. Capillaries as a therapeutic target for heart failure. J Atheroscler Thromb. 2022;29:971–988. doi: 10.5551/jat.RV17064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kibel A, Selthofer‐Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, Gros M. Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res. 2017;45:1901–1929. doi: 10.1177/0300060516675504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, Catalini C, Gjeloshi K, Albanese G, Di Martino A, et al. Coronary microvascular dysfunction in diabetes mellitus: pathogenetic mechanisms and potential therapeutic options. Biomedicines. 2022;10:2274. doi: 10.3390/biomedicines10092274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choudhury L, Rosen S, Patel D, Nihoyannopoulos P, Camici P. Coronary vasodilator reserve in primary and secondary left ventricular hypertrophy. A study with positron emission tomography. Eur Heart J. 1997;18:108–116. doi: 10.1093/oxfordjournals.eurheartj.a015090 116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
Figures S1–S3


